Impacts of Plu kaow (Houttuynia cordata Thunb.) Ethanolic Extract on Diabetes and Dyslipidemia in STZ Induced Diabetic Rats: Phytochemical Profiling, Cheminformatics Analyses, and Molecular Docking Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Preparation of Plu kaow Extract
2.2. Chemicals and Reagents
2.3. Total Phenolic Content (TPC)
2.4. Antioxidant Assays
2.5. Detection of Metabolites Using LC-MS/MS
2.6. Structural Annotation Using MetFrag Webservice
2.7. Metabolite Annotation Using Sirius (v. 5.6.)
2.8. In Silico Analysis
2.9. Hemolytic Activity of Plu kaow Ethanolic Extracts
2.10. Animals
2.11. Diet for the Animals
2.12. Experimental Design
| Group 1: | Untreated healthy control rats (HC) |
| Group 2: | Untreated diabetic control rats (DC) |
| Group 3: | Healthy rats treated with PK ethanolic extract (500 mg/kg/day in 1 mL water) (HPK) |
| Group 4: | Diabetic rats treated with PK ethanolic extract (500 mg/kg/day in 1 mL water) (DPK) |
| Group 5: | Diabetic rats treated with glibenclamide (5 mg/kg/day in 1 mL water) (DD) |
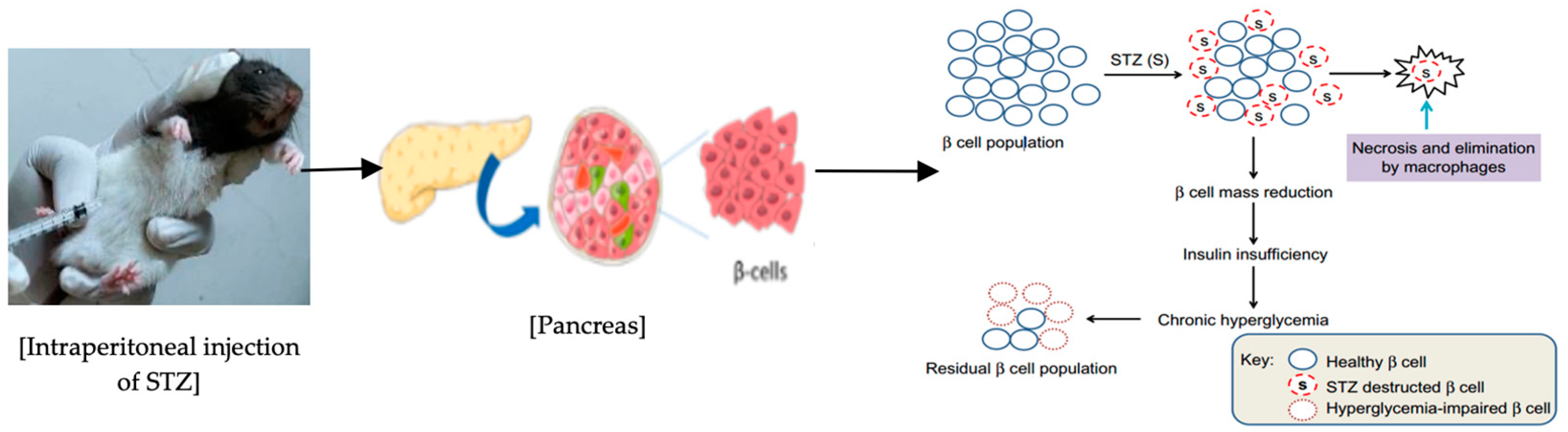
2.13. Induction of Diabetes in Rats
2.14. Collection of Blood and Determination of Biochemical Parameters
2.15. Histological Investigation
2.16. Statistical Analysis
2.17. Ethical Issues
3. Results
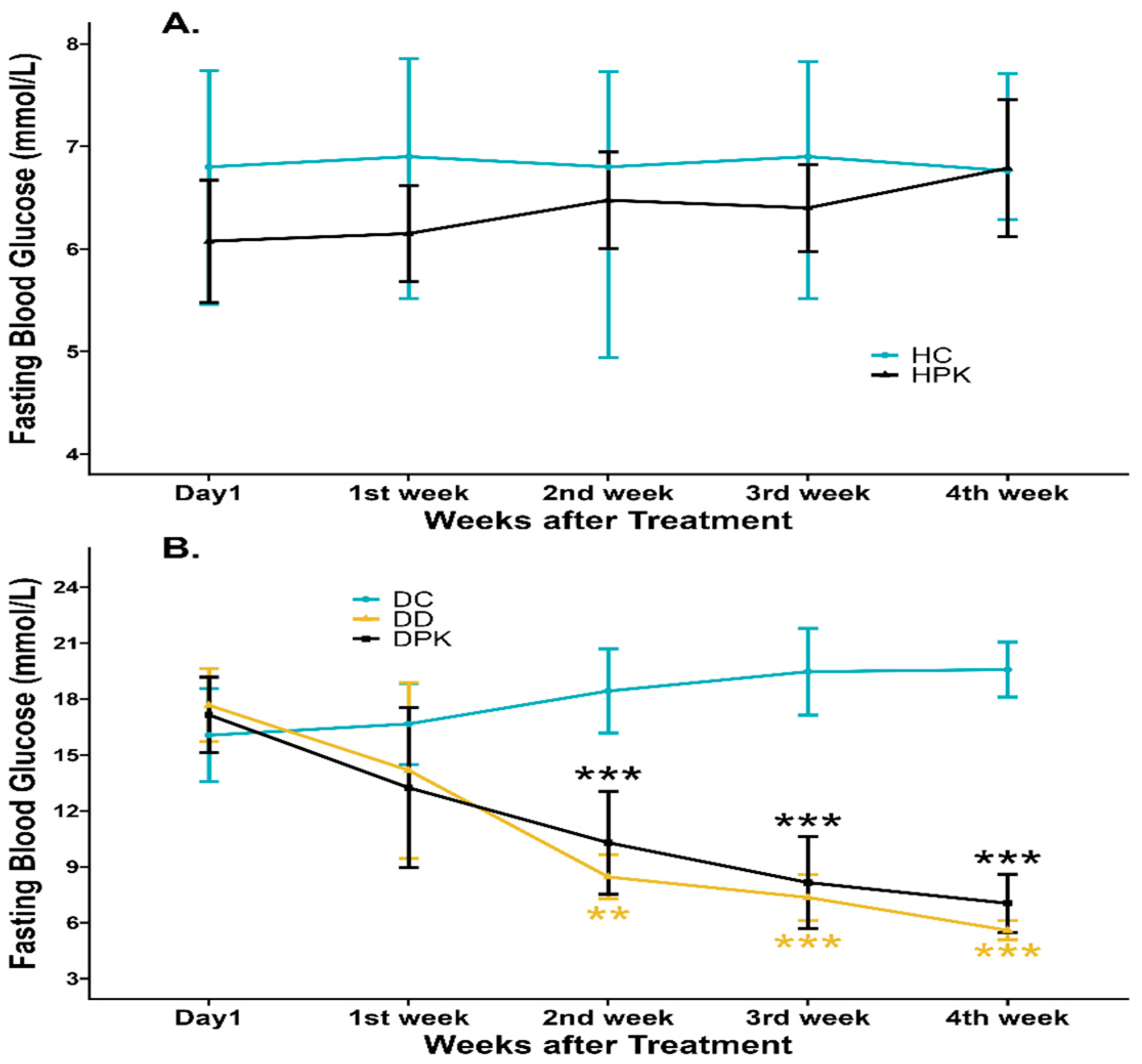
3.1. Effect of Plu kaow on Hyperglycemia of Diabetic and Normal Rats
3.2. Evaluation of the Effect of Plu kaow Ethanolic Extract on Dyslipidemia
3.3. Effect of Plu kaow Ethanolic Extract on Hepatic, Cardiac, and Renal Function Markers Activity
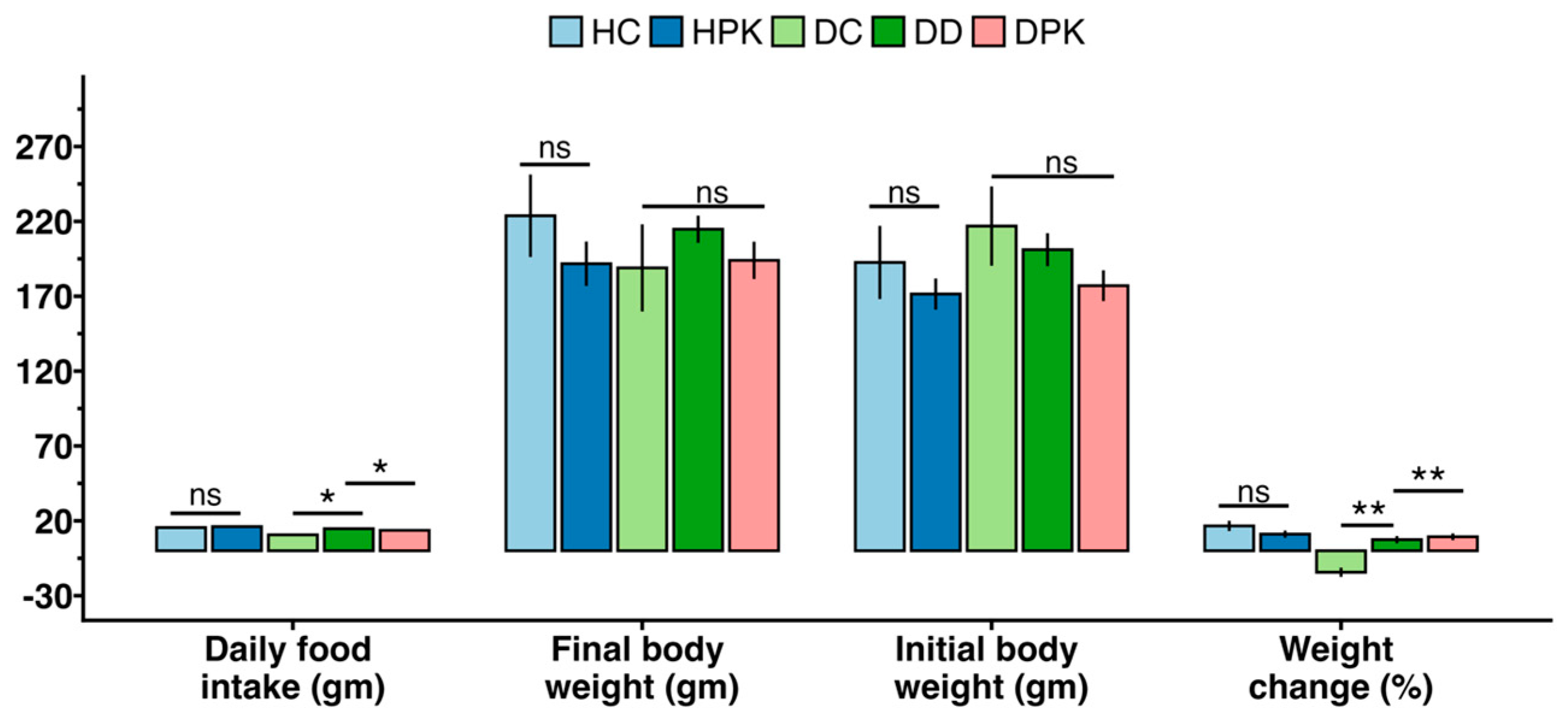
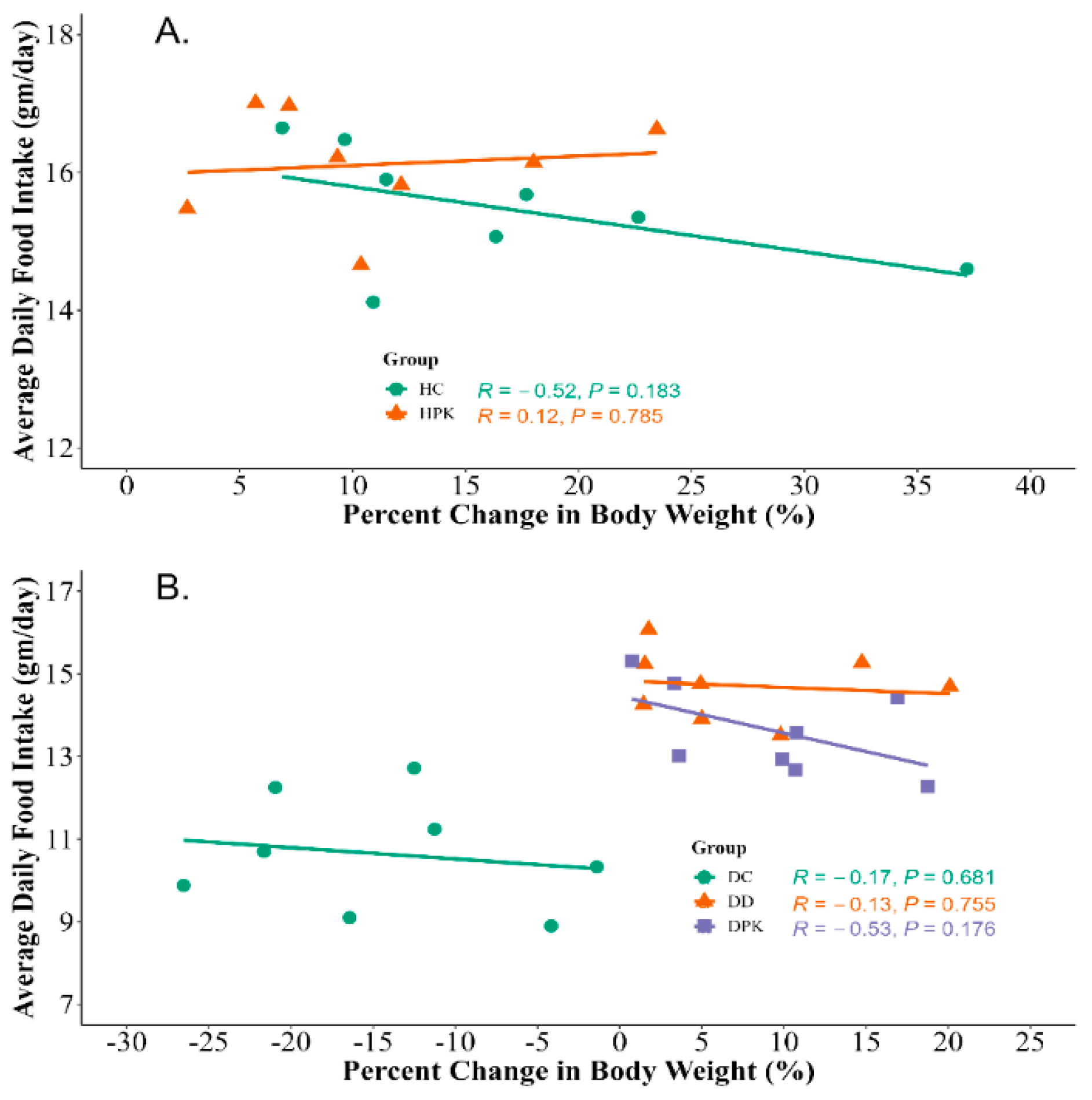
3.4. Effect of Plu kaow Extract on Body Weights and Food Consumption
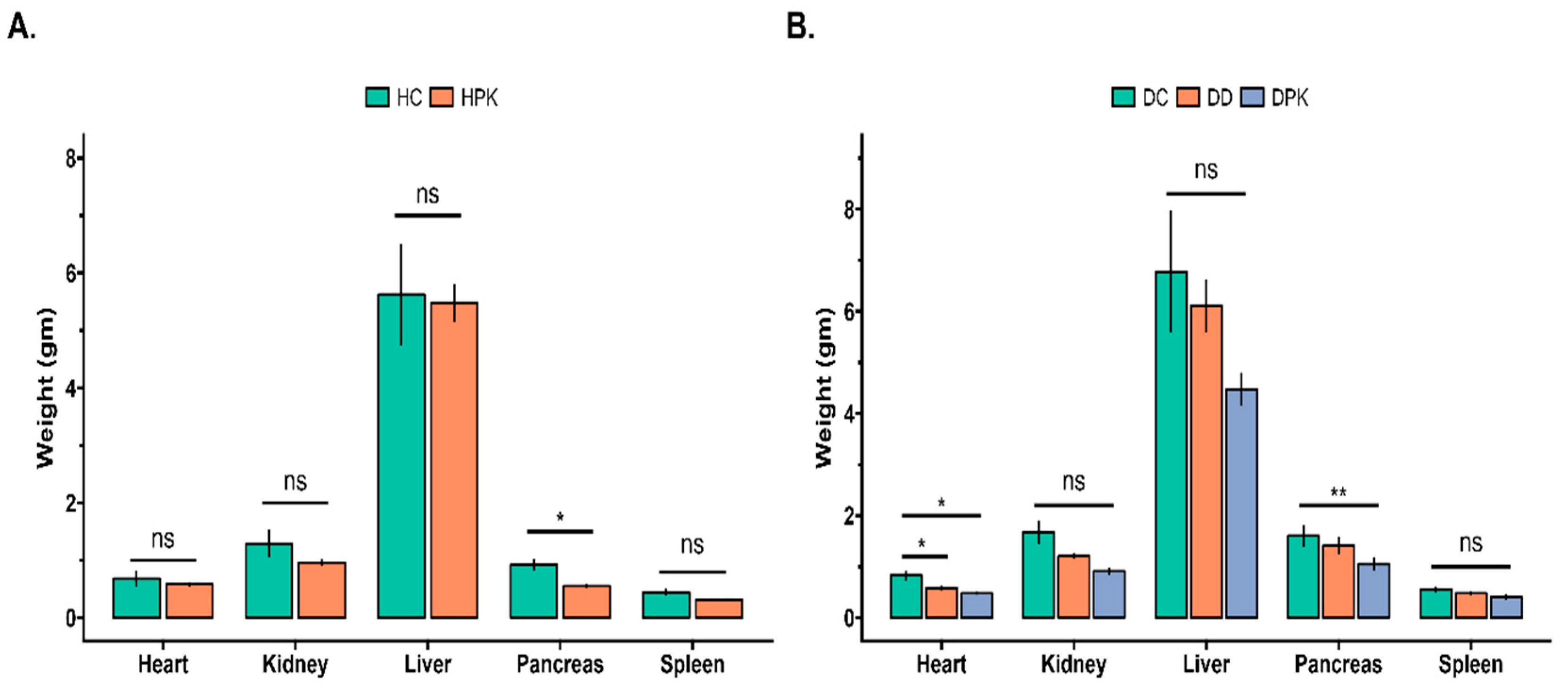
3.5. Effect of PK Extract on Organs Relative Weight of Liver, Kidney, Heart, Spleen, and Pancreas
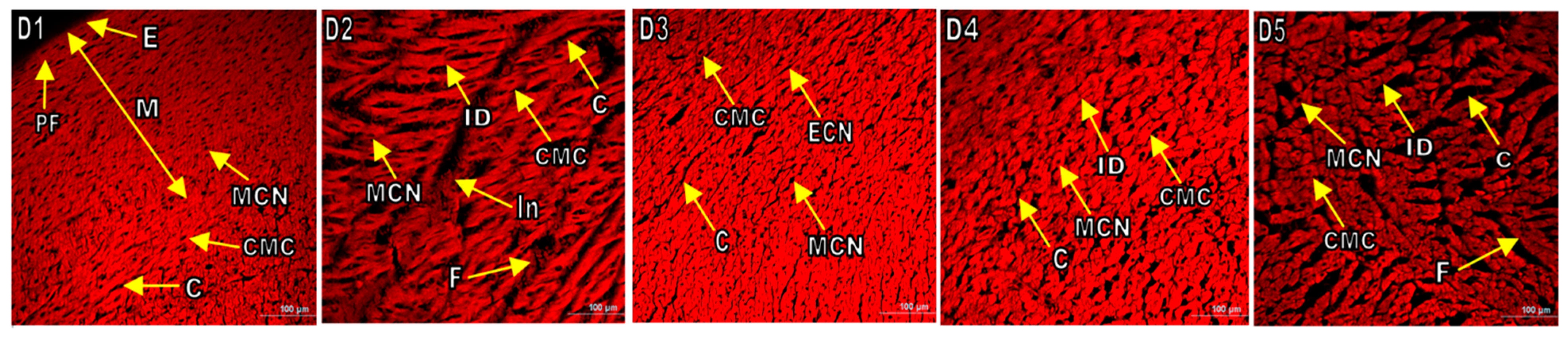
3.6. Histopathological Examination of Various Organs of Experimental Rats
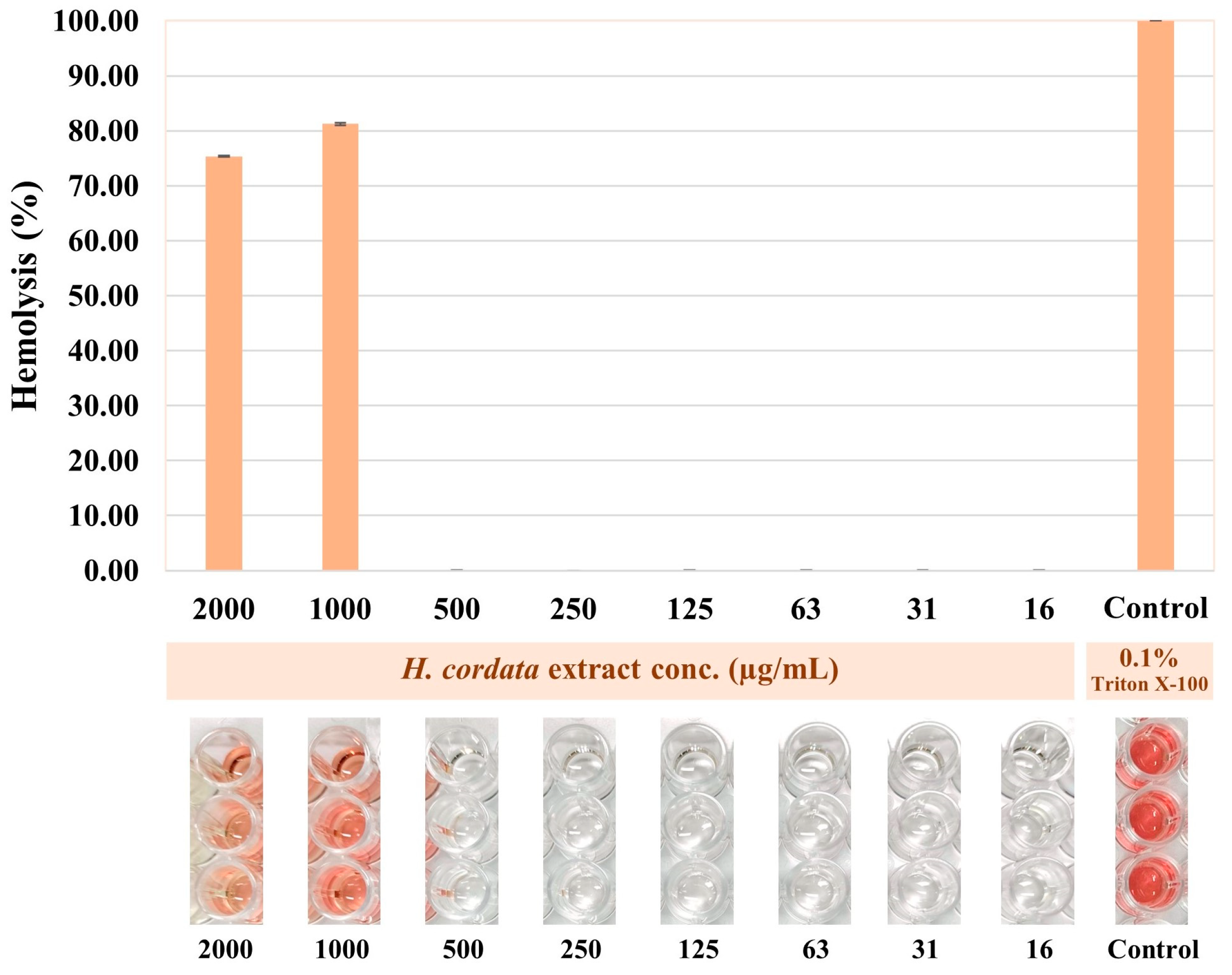
3.7. Hemolytic Activity of Plu kaow Ethanolic Extract
3.8. Antioxidant Activity of Plu kaow Ethanolic Extract
3.9. Molecular Docking
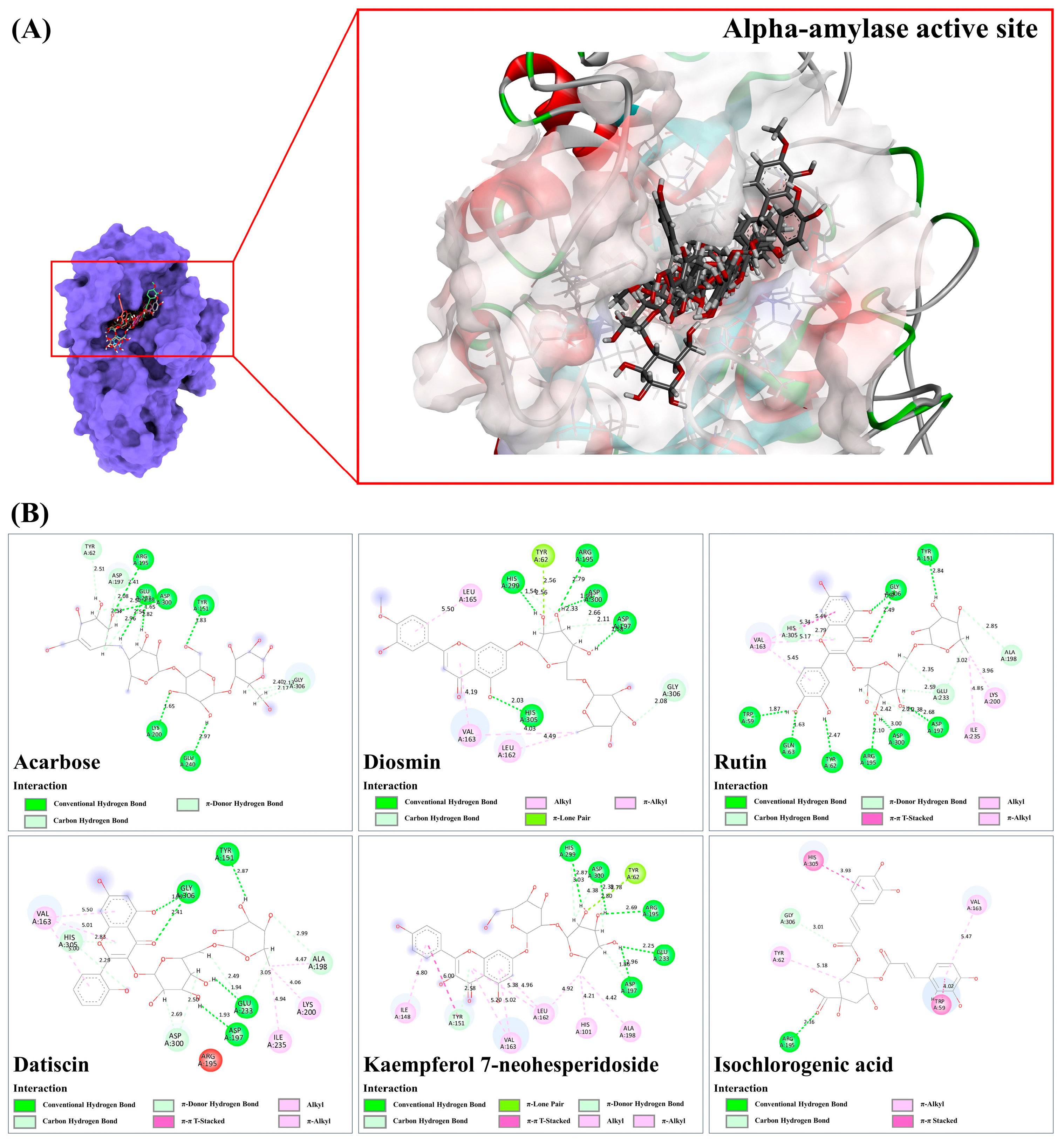
3.9.1. Alpha-Amylase
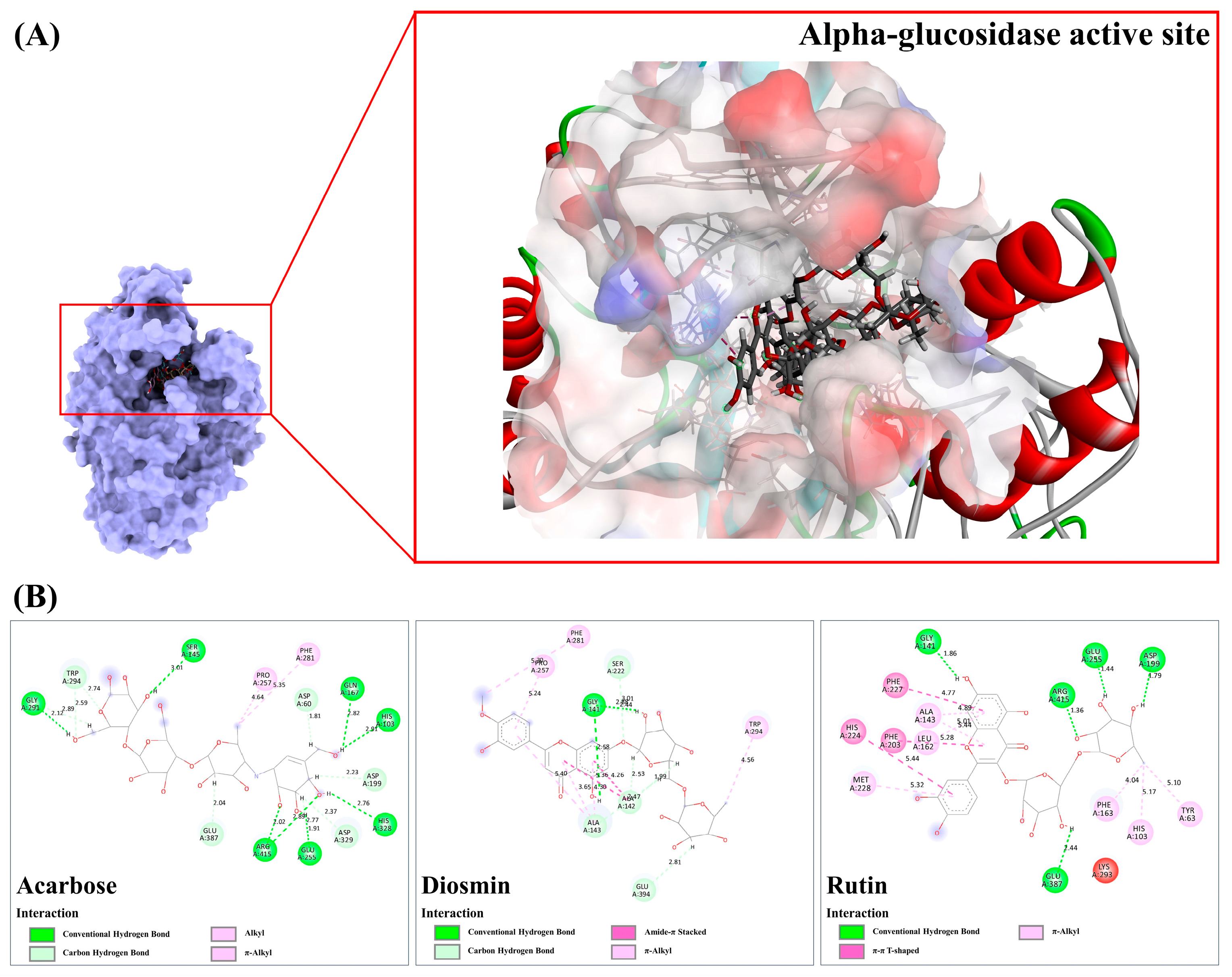
3.9.2. Alpha-Glucosidase
3.9.3. Sulfonylurea Receptor (SUR)
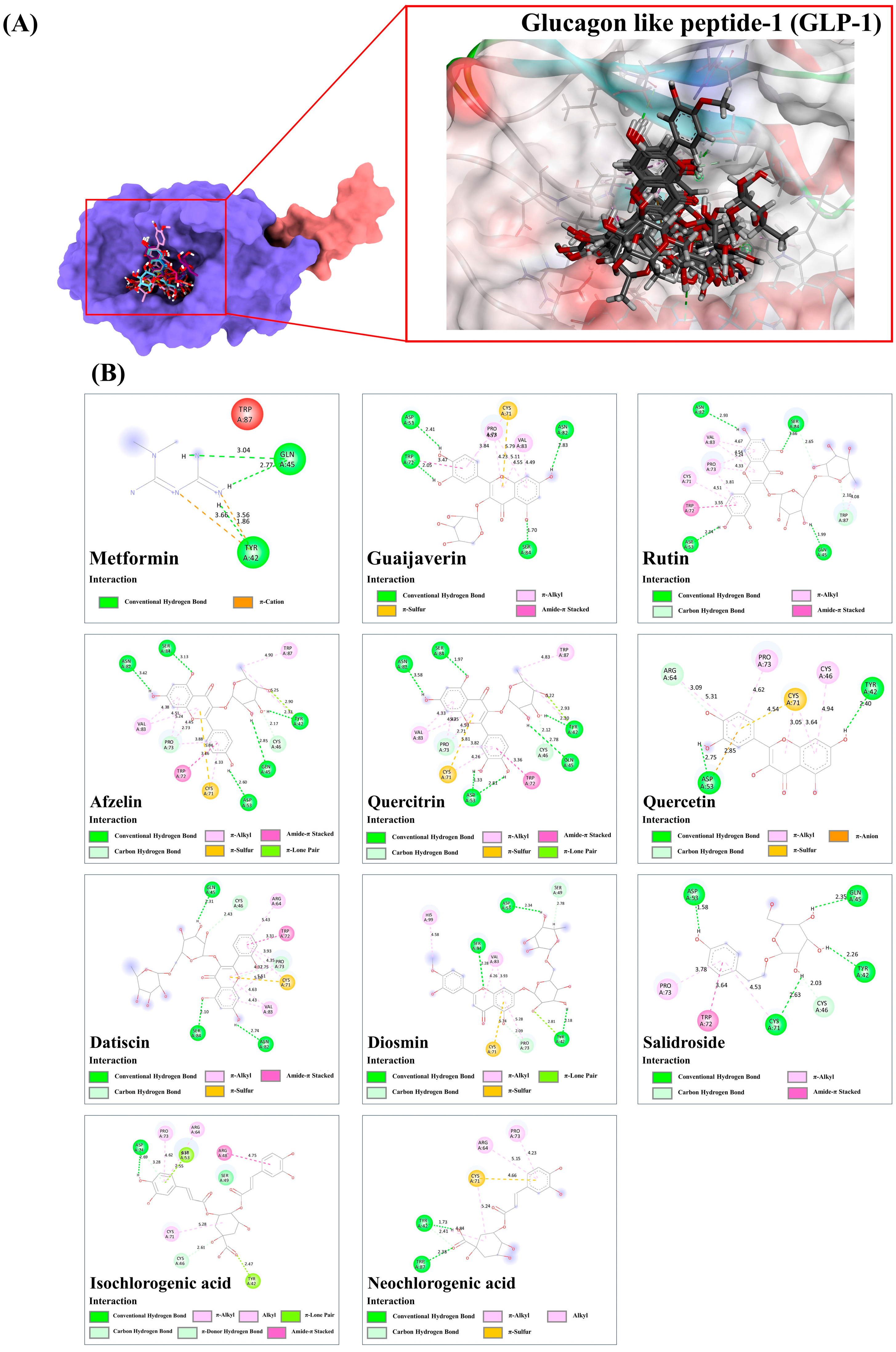
3.9.4. Glucagon Like Peptide-1 (GLP-1)
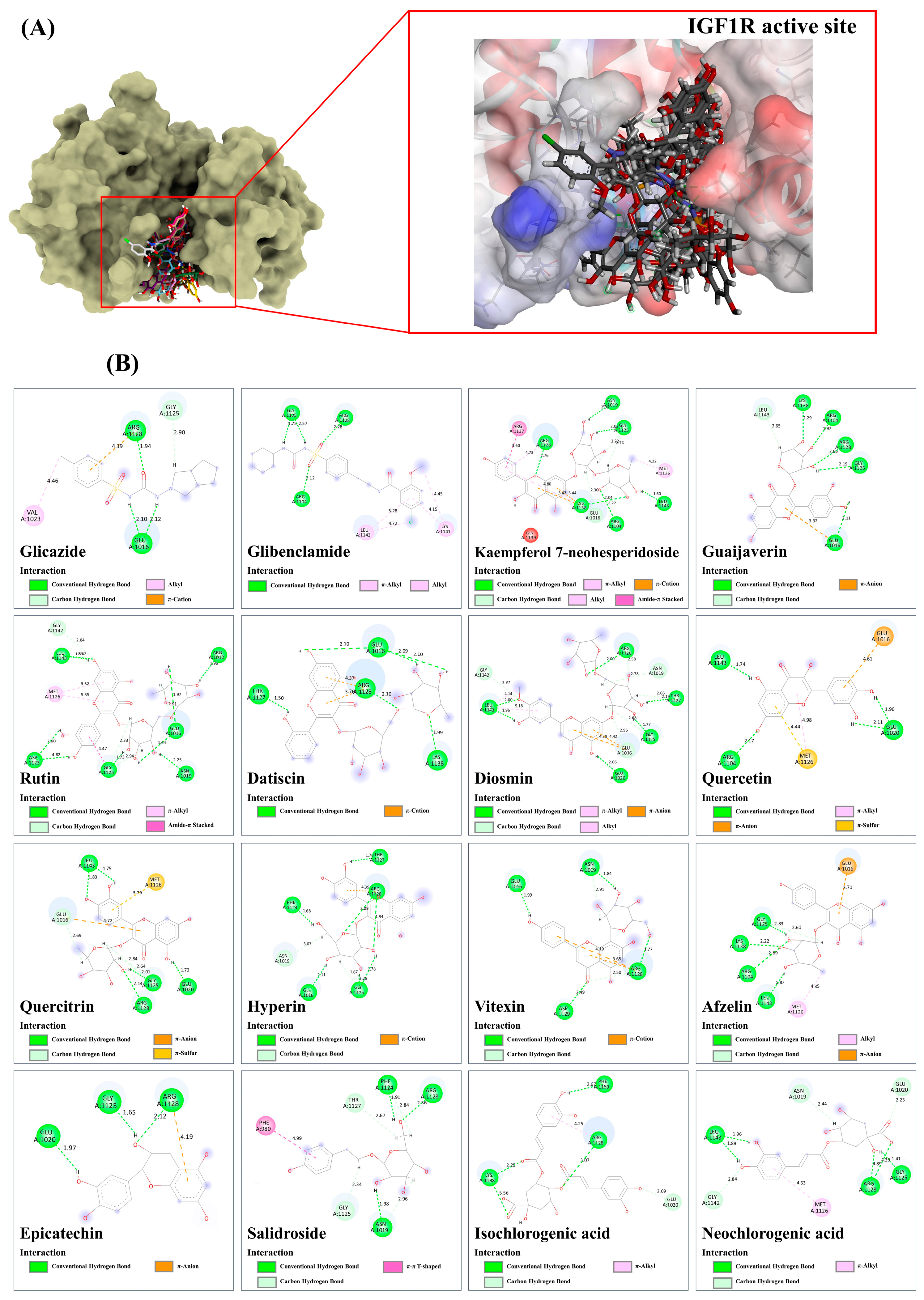
3.9.5. Insulin-like Growth Factor 1 Kinase (IGF1R)
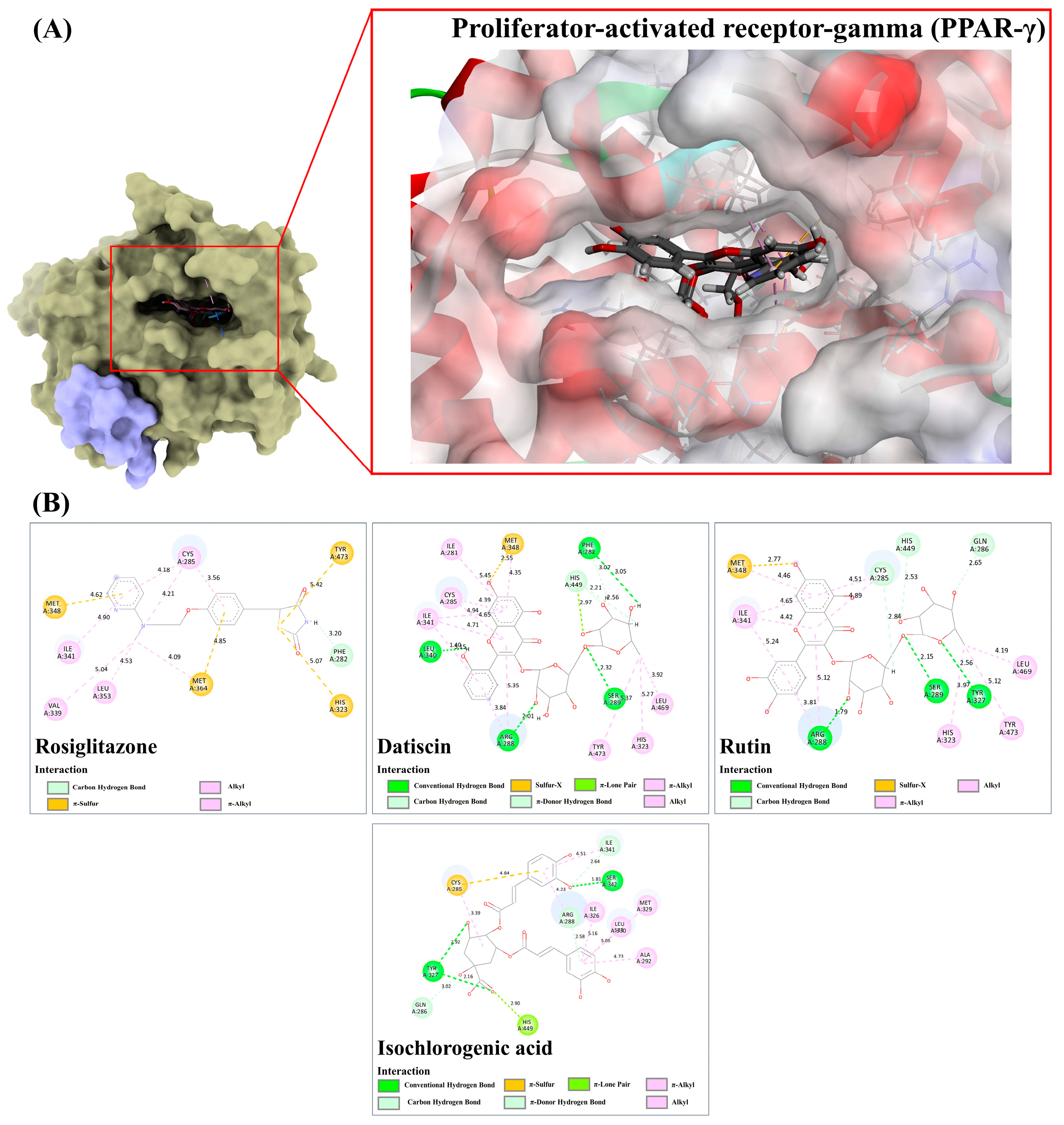
3.9.6. Peroxisome Proliferator-Activated Receptor-Gamma (PPAR-γ)
3.9.7. Glycogen Phosphorylase-1 (GP-1)
3.10. LC-MS/MS-Based Phytochemical Profiling of the Plu kaow Ethanolic Extract with Structural Annotation Using MetFrag Web Service and Sirius Tandem with CSI:FingerID and CANOPUS
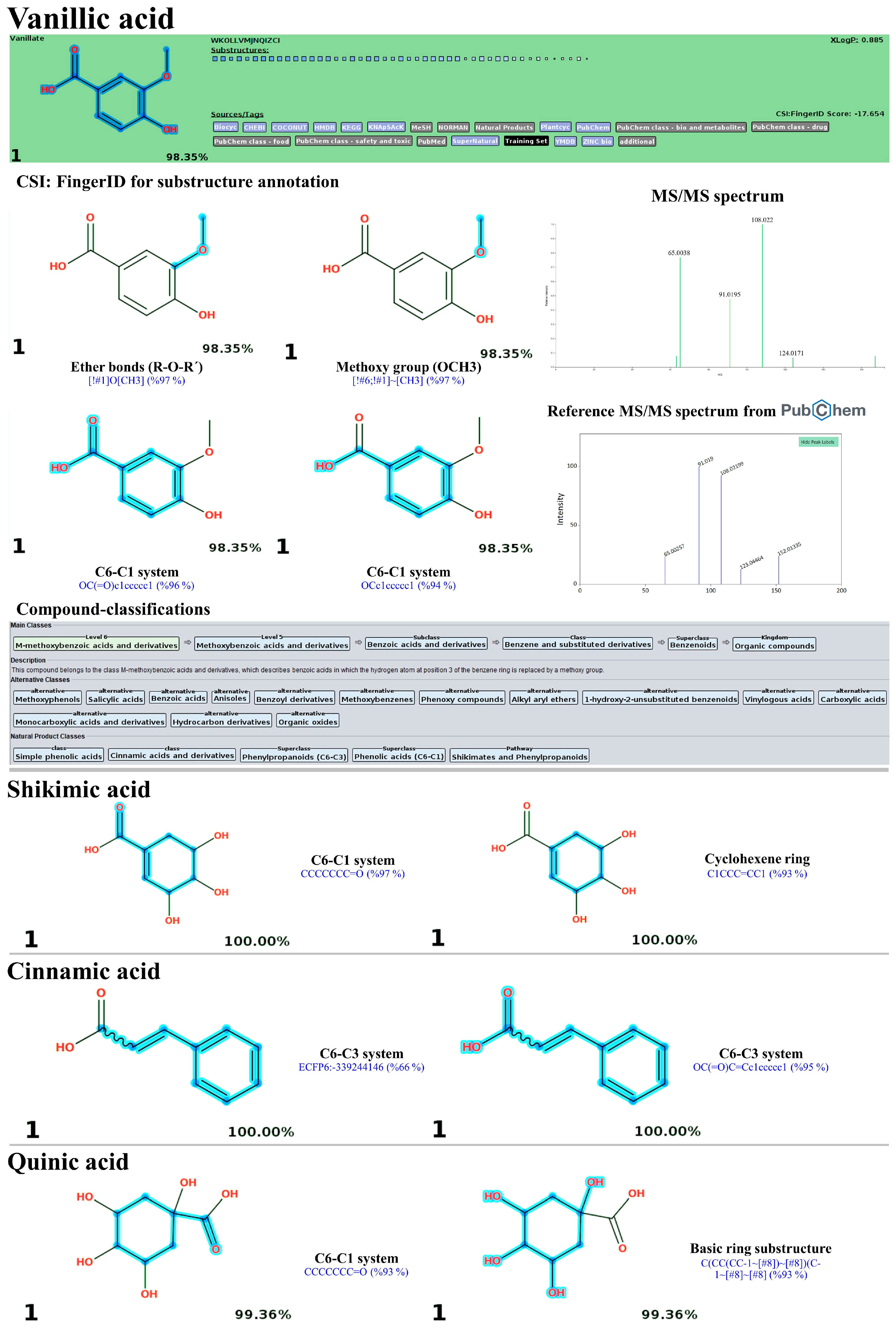
3.10.1. Simple Phenolics
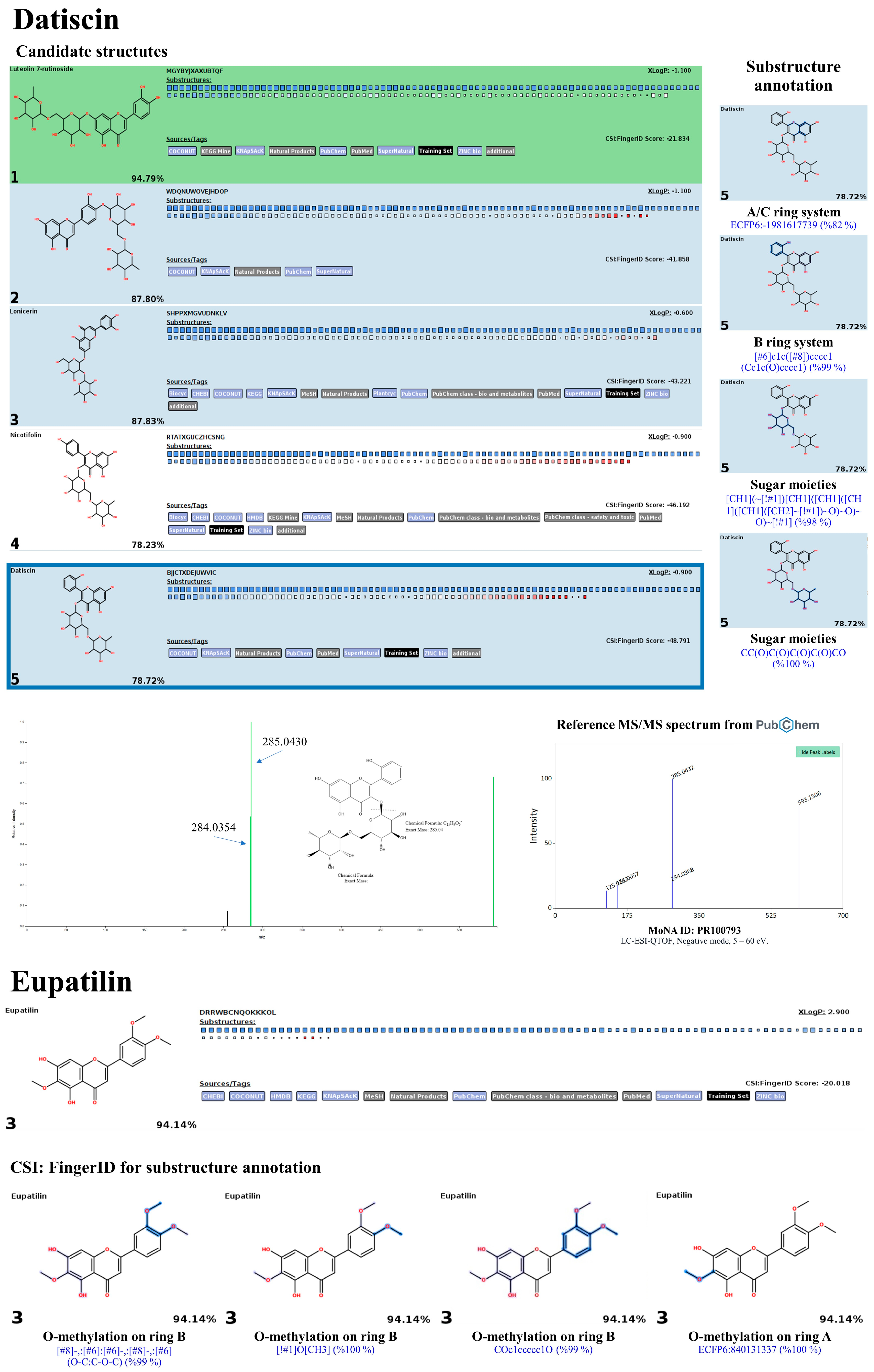
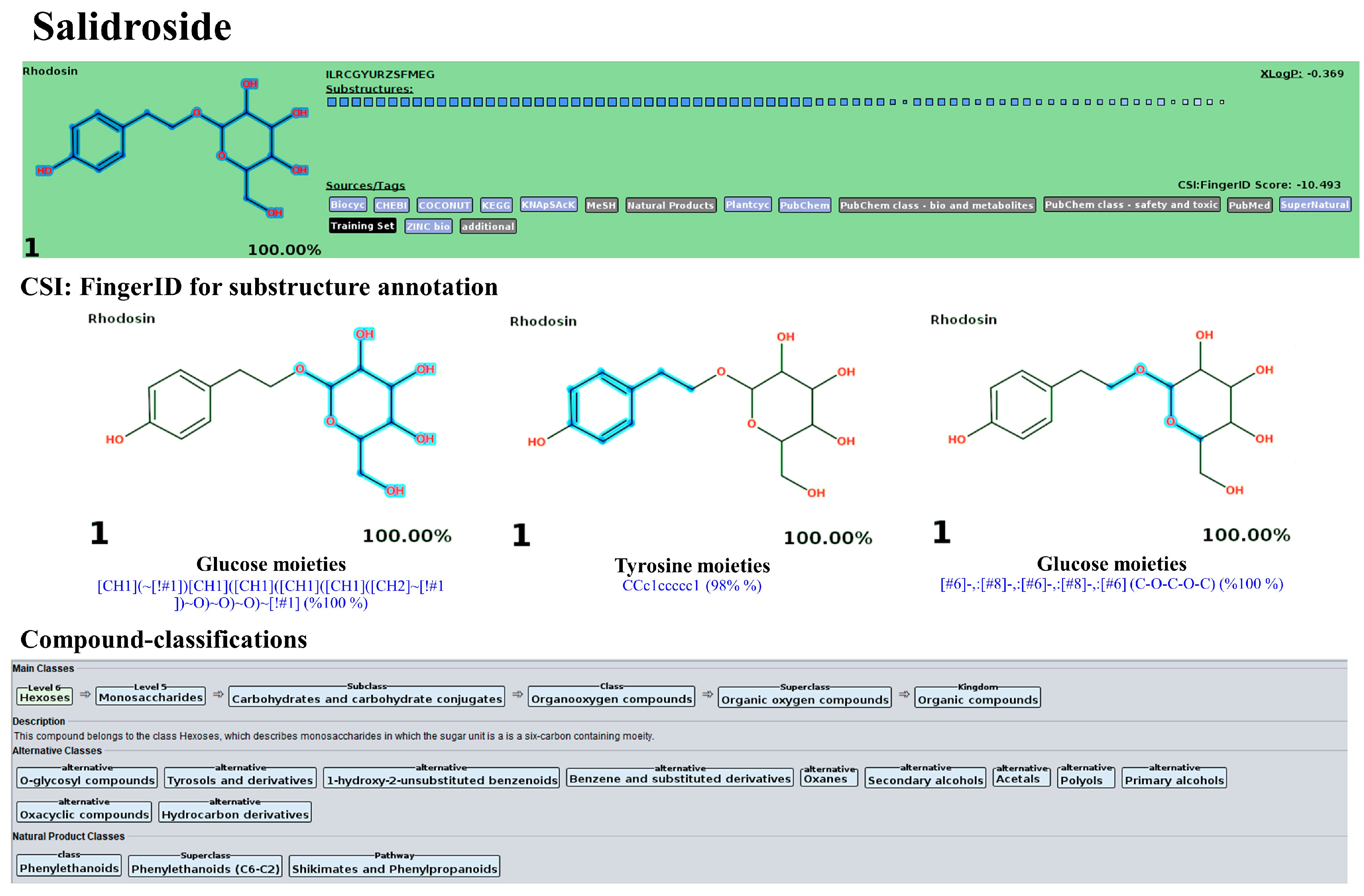
3.10.2. Flavonoids and Glycoside Analogues
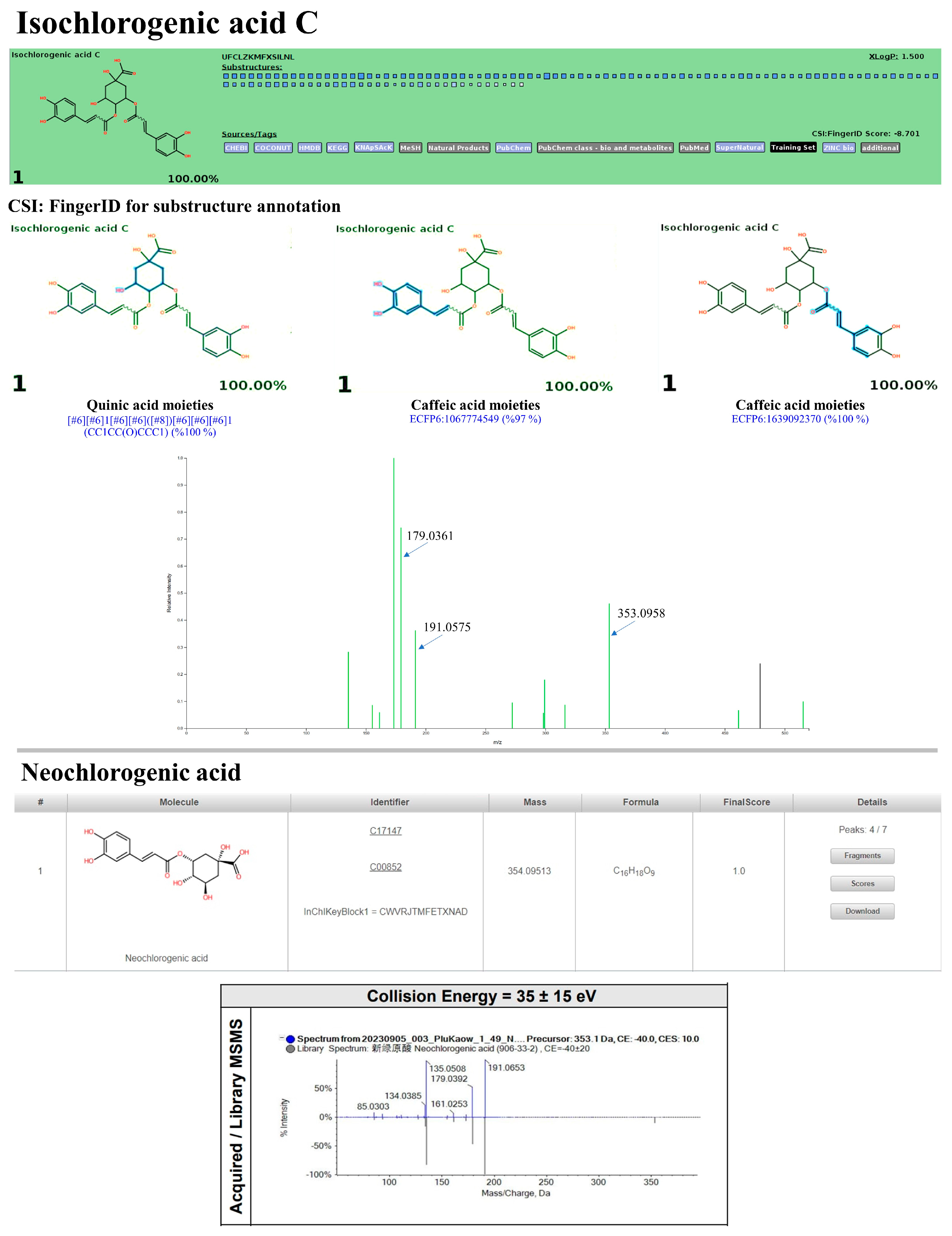
3.10.3. Non-Flavonoid Substances
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Abdul Basith Khan, M.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vergès, B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef]
- Jialal, I.; Singh, G. Management of diabetic dyslipidemia: An update. World J. Diabetes 2019, 10, 280. [Google Scholar] [CrossRef]
- Hirano, T. Abnormal lipoprotein metabolism in diabetic nephropathy. Clin. Exp. Nephrol. 2014, 18, 206–209. [Google Scholar] [CrossRef]
- Bonilha, I.; Zimetti, F.; Zanotti, I.; Papotti, B.; Sposito, A.C. Dysfunctional high-density lipoproteins in type 2 diabetes mellitus: Molecular mechanisms and therapeutic implications. J. Clin. Med. 2021, 10, 2233. [Google Scholar] [CrossRef]
- Lorenzo, C.; Hartnett, S.; Hanley, A.J.; Rewers, M.J.; Wagenknecht, L.E.; Karter, A.J.; Haffner, S.M. Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: The insulin resistance atherosclerosis study. J. Clin. Endocrinol. Metab. 2013, 98, 1622–1630. [Google Scholar] [CrossRef]
- Kashyap, S.R.; Osme, A.; Ilchenko, S.; Golizeh, M.; Lee, K.; Wang, S.; Bena, J.; Previs, S.F.; Smith, J.D.; Kasumov, T. Glycation reduces the stability of ApoAI and increases HDL dysfunction in diet-controlled type 2 diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 388–396. [Google Scholar] [CrossRef]
- Nandini, H.S.; Naik, P.R. Antidiabetic, antihyperlipidemic and antioxidant effect of Vincamine, in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2019, 843, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Kumar, A.; Iqbal, H.; Verma, R.K.; Chanda, D.; Chauhan, A.; et al. Chemical Composition and Allelopathic, Antibacterial, Antifungal, and Antiacetylcholinesterase Activity of Fish-mint (Houttuynia cordata Thunb.) from India. Chem. Biodivers. 2017, 14, e1700189. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Meng, P.H.; Jiang, D.W.; Han, Z.M.; Wang, Z.H.; Tan, G.F.; Zhang, J. Comprehensive assessment of Houttuynia cordata Thunb., an important medicinal plant and vegetable. Agronomy 2022, 12, 2582. [Google Scholar] [CrossRef]
- Li, A.; Li, S.; Wu, X.; Lu, H.; Huang, M.; Gu, R.; Wei, L.; He, A. Influence of light intensity on the yield and quality of Houttuynia cordata. Plant Prod. Sci. 2015, 18, 522–528. [Google Scholar] [CrossRef]
- Fotev, Y.V.; Kukushkina, T.A.; Chankina, O.V.; Belousova, V.P. Houttuynia (Houttuynia cordata thunb.)–new vegetable and medicinal crop for Russia (morphological features and biochemical composition). Veg. Crop. Russ. 2018, 5, 57–61. [Google Scholar] [CrossRef]
- Dong, X.Y.; Yang, L.P. Systematic analysis of components and contents in Houttuynia cordata Thunb. Tradit. Med. Res. 2017, 2, 176–188. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, X.; Hu, Q.; Xiao, X.; Jiang, J.; Ma, X.; Wu, M. Houttuynia cordata Thunb: An ethnopharmacological review. Front. Pharmacol. 2021, 12, 714694. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, H.; Chen, D.; Zheng, J.; Yin, L.; Zou, J.; Zhang, Y.; Deng, K.; Xiao, M.; Meng, L.; et al. Comparison of Essential Oils of Houttuynia cordata Thunb. from Different Processing Methods and Harvest Seasons Based on GC-MS and Chemometric Analysis. Int. J. Anal. Chem. 2021, 2021, 8324169. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.C.; Su, C.R.; Ku, Y.C.; Wu, T.S. The constituents and their bioactivities of Houttuynia cordata. Chem. Pharm. Bull. 2009, 57, 1227–1230. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.G. Bioactive components and functional properties of Hottuynia cordata and its applications. Pharm. Biol. 2009, 47, 1154–1161. [Google Scholar] [CrossRef]
- Pradhan, S.; Rituparna, S.; Dehury, H.; Dhall, M.; Singh, Y.D. Nutritional profile and pharmacological aspect of Houttuynia cordata Thunb. and their therapeutic applications. Pharmacol. Res. -Mod. Chin. Med. 2023, 29, 100311. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.R.; Choi, Y.H.; Shin, J.Y.; Kim, C.D.; Kang, N.G.; Park, B.C.; Lee, S. Quercitrin stimulates hair growth with enhanced expression of growth factors via activation of MAPK/CREB signaling pathway. Molecules 2020, 25, 4004. [Google Scholar] [CrossRef]
- Laldinsangi, C. The therapeutic potential of Houttuynia cordata: A current review. Heliyon 2022, 8, e10386. [Google Scholar] [CrossRef] [PubMed]
- Kahksha; Alam, O.; Al-Keridis, L.A.; Khan, J.; Naaz, S.; Alam, A.; Ashraf, S.A.; Alshammari, N.; Adnan, M.; Beg, M.A. Evaluation of Antidiabetic Effect of Luteolin in STZ Induced Diabetic Rats: Molecular Docking, Molecular Dynamics, In Vitro and In Vivo Studies. J. Funct. Biomater. 2023, 14, 126. [Google Scholar] [CrossRef]
- Wang, H.Y.; Bao, J.L. Effect of Houttuynia cordata aetherolea on adiponectin and connective tissue growth factor in a rat model of diabetes mellitus. J. Tradit. Chin. Med. 2012, 32, 58–62. [Google Scholar] [CrossRef]
- Ma, Q.; Wei, R.; Wang, Z.; Liu, W.; Sang, Z.; Li, Y.; Huang, H. Bioactive alkaloids from the aerial parts of Houttuynia cordata. J. Ethnopharmacol. 2017, 195, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Prasad, S.K.; Hemalatha, S. In vitro study on glucose utilization capacity of bioactive fractions of Houttuynia cordata in isolated rat hemidiaphragm and its major phytoconstituent. Adv. Pharmacol. Pharm. Sci. 2016, 2016, 2573604. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Klamrak, A.; Nabnueangsap, J.; Narkpuk, J.; Saengkun, Y.; Janpan, P.; Nopkuesuk, N.; Chaveerach, A.; Teeravechyan, S.; Rahman, S.S.; Dobutr, T.; et al. Unveiling the Potent Antiviral and Antioxidant Activities of an Aqueous Extract from Caesalpinia mimosoides Lamk: Cheminformatics and Molecular Docking Approaches. Foods 2023, 13, 81. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Van, L.V.; Pham, E.C.; Nguyen, C.V.; Duong, N.T.; Le Thi, T.V.; Truong, T.N. In vitro and in vivo antidiabetic activity, isolation of flavonoids, and in silico molecular docking of stem extract of Merremia tridentata (L.). Biomed. Pharmacother. 2022, 146, 112611. [Google Scholar] [CrossRef] [PubMed]
- Alinezhad, H.; Azimi, R.; Zare, M.; Ebrahimzadeh, M.A.; Eslami, S.; Nabavi, S.F.; Nabavi, S.M. Antioxidant and antihemolytic activities of ethanolic extract of flowers, leaves, and stems of Hyssopus officinalis L. Var. angustifolius. Int. J. Food Prop. 2013, 16, 1169–1178. [Google Scholar] [CrossRef]
- Rahman, S.S.; Reja, M.M.; Islam, M.R.; Islam, M.M.; Rouf, S.M.; Rahman, M.H. Proximate nutrient analysis of elephant apple (Dillenia indica) fruit and its hypoglycemic, and hypolipidemic potentials in alloxan-induced diabetic rats. Food Humanit. 2023, 1, 1355–1361. [Google Scholar] [CrossRef]
- Latifi, E.; Mohammadpour, A.A.; Fathi, B.; Nourani, H. Antidiabetic and antihyperlipidemic effects of ethanolic Ferula assa-foetida oleo-gum-resin extract in streptozotocin-induced diabetic wistar rats. Biomed. Pharmacother. 2019, 110, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.S.; Salauddin, H.M.; Rahman, M.; Muhsin, M.M.; Rouf, S.M. Nutritional composition and antidiabetic effect of germinated endosperm (Borassus flabellifer), tuber (Amorphophallus paeoniifolius) and their combined impact on rats. Biochem. Biophys. Rep. 2021, 25, 100917. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, L.J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 2, 181–188. [Google Scholar] [CrossRef]
- Madhuri, K.; Naik, P.R. Ameliorative effect of borneol, a natural bicyclic monoterpene against hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic Wistar rats. Biomed. Pharmacother. 2017, 96, 336–347. [Google Scholar] [CrossRef]
- Ibrahim, M.; Parveen, B.; Zahiruddin, S.; Gautam, G.; Parveen, R.; Khan, M.A.; Gupta, A.; Ahmad, S. Analysis of polyphenols in Aegle marmelos leaf and ameliorative efficacy against diabetic mice through restoration of antioxidant and anti-inflammatory status. J. Food Biochem. 2022, 46, e13852. [Google Scholar] [CrossRef]
- Kumar, M.; Prasad, S.K.; Krishnamurthy, S.; Hemalatha, S. Antihyperglycemic Activity of Houttuynia cordata Thunb. in Streptozotocin-Induced Diabetic Rats. Adv. Pharmacol. Pharm. Sci. 2014, 2014, 809438. [Google Scholar] [CrossRef]
- Shanak, S.; Saad, B.; Zaid, H. Metabolic and epigenetic action mechanisms of antidiabetic medicinal plants. Evid. -Based Complement. Altern. Med. 2019, 2019, 3583067. [Google Scholar] [CrossRef]
- Date, K.; Satoh, A.; Iida, K.; Ogawa, H. Pancreatic α-amylase controls glucose assimilation by duodenal retrieval through N-glycan-specific binding, endocytosis, and degradation. J. Biol. Chem. 2015, 290, 17439–17450. [Google Scholar] [CrossRef] [PubMed]
- Donley, V.R.; Hiskett, E.K.; Kidder, A.C.; Schermerhorn, T. ATP-sensitive potassium channel (K ATP channel) expression in the normal canine pancreas and in canine insulinomas. BMC Vet. Res. 2005, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Sesti, G.; Sciacqua, A.; Cardellini, M.; Marini, M.A.; Maio, R.; Vatrano, M.; Succurro, E.; Lauro, R.; Federici, M.; Perticone, F. Plasma concentration of IGF-I is independently associated with insulin sensitivity in subjects with different degrees of glucose tolerance. Diabetes Care 2005, 28, 120–125. [Google Scholar] [CrossRef]
- McCormack, J.G.; Westergaard, N.; Kristiansen, M.; Brand, C.L.; Lau, J. Pharmacological approaches to inhibit endogenous glucose production as a means of anti-diabetic therapy. Curr. Pharm. Des. 2001, 7, 1451–1474. [Google Scholar] [CrossRef]
- Garber, A.J. Long-acting glucagon-like peptide 1 receptor agonists: A review of their efficacy and tolerability. Diabetes care 2011, 34 (Suppl. S2), S279. [Google Scholar] [CrossRef] [PubMed]
- Kota, B.P.; Huang, T.H.; Roufogalis, B.D. An overview on biological mechanisms of PPARs. Pharmacol. Res. 2005, 51, 85–94. [Google Scholar] [CrossRef]
- Pokharkar, O.; Lakshmanan, H.; Zyryanov, G.V.; Tsurkan, M.V. Antiviral potential of Antillogorgia americana and elisabethae natural products against nsp16–nsp10 complex, nsp13, and nsp14 proteins of sars-cov-2: An in silico investigation. Microbiol. Res. 2023, 14, 993–1019. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Agius, L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem. J. 2008, 414, 1–8. [Google Scholar] [CrossRef]
- Wolf, S.; Schmidt, S.; Müller-Hannemann, M.; Neumann, S. In silico fragmentation for computer assisted identification of metabolite mass spectra. BMC Bioinform. 2010, 11, 1–2. [Google Scholar] [CrossRef]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching molecular structure databases with tandem mass spectra using CSI: FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef]
- Karonen, M.; Pihlava, J.M. Identification of Oxindoleacetic Acid Conjugates in Quinoa (Chenopodium quinoa Willd.) Seeds by High-Resolution UHPLC-MS/MS. Molecules 2022, 27, 5629. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J. SIRIUS SB. 4: A rapid tool for turning tandem mass spectra into metabolite structure information. 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Heikkilä, E.; Hermant, A.; Thevenet, J.; Bermont, F.; Kulkarni, S.S.; Ratajczak, J.; Santo-Domingo, J.; Dioum, E.H.; Canto, C.; Barron, D.; et al. The plant product quinic acid activates Ca2+-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. Br. J. Pharmacol. 2019, 176, 3250–3263. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Chang, D.; Nammi, S.; Bensoussan, A.; Bilinski, K.; Roufogalis, B.D. Interactions between antidiabetic drugs and herbs: An overview of mechanisms of action and clinical implications. Diabetol. Metab. Syndr. 2017, 9, 59. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A.; Singh, H.; Kaur, S.; Arora, S.; Singh, B. Protective effect of vanillic acid against diabetes and diabetic nephropathy by attenuating oxidative stress and upregulation of NF-κB, TNF-α and COX-2 proteins in rats. Phytother. Res. 2022, 36, 1338–1352. [Google Scholar] [CrossRef]
- Lakshmi, B.S.; Sujatha, S.; Anand, S.; Sangeetha, K.N.; Narayanan, R.B.; Katiyar, C.; Kanaujia, A.; Duggar, R.; Singh, Y.; Srinivas, K.; et al. Cinnamic acid, from the bark of Cinnamomum cassia, regulates glucose transport via activation of GLUT4 on L6 myotubes in a phosphatidylinositol 3-kinase-independent manner. J. Diabetes 2009, 1, 99–106. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, C.S.; Son, K.H. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci. Biotechnol. Biochem. 2000, 64, 2458–2461. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Huang, D.; Chen, S.; Xia, Y.; Zhu, S. The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α-amylase and α-glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol. 2015, 24, 1103–1110. [Google Scholar] [CrossRef]
- El-Bassossy, T.A.; Ahmed, F.A. In vitro anti-diabetic effect and molecular docking study of Phlomis aurea components as diabetic enzymes inhibitor. Egypt. J. Chem. 2024; in press. [Google Scholar] [CrossRef]
- Dubey, K.; Dubey, R.; Gupta, R.; Gupta, A. Exploration of diosmin to control diabetes and its complications-an in vitro and in silico approach. Curr. Comput. -Aided Drug Des. 2021, 17, 307–313. [Google Scholar] [CrossRef]
- Chen, Y.; Geng, S.; Liu, B. Three common caffeoylquinic acids as potential hypoglycemic nutraceuticals: Evaluation of α-glucosidase inhibitory activity and glucose consumption in HepG2 cells. J. Food Biochem. 2020, 44, e13361. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Kori, M.L. Structure–activity relationship and therapeutic benefits of flavonoids in the management of diabetes and associated disorders. Pharm. Chem. J. 2021, 54, 1106–1125. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Roy, A.; Dinda, S. Dietary plant flavonoids in prevention of obesity and diabetes. Adv. Protein Chem. Struct. Biol. 2020, 120, 159–235. [Google Scholar] [CrossRef]
- Brás, N.F.; Neves, R.P.; Lopes, F.A.; Correia, M.A.; Palma, A.S.; Sousa, S.F.; Ramos, M.J. Combined in silico and in vitro studies to identify novel antidiabetic flavonoids targeting glycogen phosphorylase. Bioorganic Chem. 2021, 108, 104552. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Girón, F.; Pérez-Garrido, A.; Pereira, A.S.; Gabaldón-Hernández, J.A.; Verardo, V.; Segura Carretero, A.; Pérez-Sánchez, H. Fishing the targets of bioactive compounds from Psidium guajava L. leaves in the context of diabetes. Int. J. Mol. Sci. 2023, 24, 5761. [Google Scholar] [CrossRef]
- Ali, M.; Hassan, M.; Ansari, S.A.; Alkahtani, H.M.; Al-Rasheed, L.S.; Ansari, S.A. Quercetin and Kaempferol as Multi-Targeting Antidiabetic Agents against Mouse Model of Chemically Induced Type 2 Diabetes. Pharmaceuticals 2024, 17, 757. [Google Scholar] [CrossRef]
- Proença, C.; Ribeiro, D.; Freitas, M.; Carvalho, F.; Fernandes, E. A comprehensive review on the antidiabetic activity of flavonoids targeting PTP1B and DPP-4: A structure-activity relationship analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4095–4151. [Google Scholar] [CrossRef]
- Lee, L.C.; Hou, Y.C.; Hsieh, Y.Y.; Chen, Y.H.; Shen, Y.C.; Lee, I.J.; Shih, M.C.; Hou, W.C.; Liu, H.K. Dietary supplementation of rutin and rutin-rich buckwheat elevates endogenous glucagon-like peptide 1 levels to facilitate glycemic control in type 2 diabetic mice. J. Funct. Foods 2021, 85, 104653. [Google Scholar] [CrossRef]
- Gaballah, H.H.; Zakaria, S.S.; Mwafy, S.E.; Tahoon, N.M.; Ebeid, A.M. Mechanistic insights into the effects of quercetin and/or GLP-1 analogue liraglutide on high-fat diet/streptozotocin-induced type 2 diabetes in rats. Biomed. Pharmacother. 2017, 92, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Soni, R.; Sharma, M.; Chatterjee, S.; Parihar, N.; Mukarram, M.; Kale, R.; Sayyed, A.A.; Behera, S.K.; Khairnar, A. Chlorogenic acid: A polyphenol from coffee rendered neuroprotection against rotenone-induced Parkinson’s disease by GLP-1 secretion. Mol. Neurobiol. 2022, 59, 6834–6856. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef]
- Hajiaghaalipour, F.; Khalilpourfarshbafi, M.; Arya, A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int. J. Biol. Sci. 2015, 11, 508. [Google Scholar] [CrossRef]
- Mehta, V.; Malairaman, U. Flavonoids: Prospective strategy for the management of diabetes and its associated complications. In Handbook of Research on Advancing Health Education through Technology; IGI Global: Hershey, PA, USA, 2016; pp. 286–328. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Y.; Sheng, M.; Gao, M.; Guo, W.; Zhang, Z.; Wang, D.; Wu, X.; Li, J.; Chen, Y.; et al. Selective PPARγ modulator diosmin improves insulin sensitivity and promotes browning of white fat. J. Biol. Chem. 2023, 299, 103059. [Google Scholar] [CrossRef]
- Cai, Y.; Fan, C.; Yan, J.; Tian, N.; Ma, X. Effects of rutin on the expression of PPARγ in skeletal muscles of db/db mice. Planta Medica 2012, 78, 861–865. [Google Scholar] [CrossRef]
- Lokhande, K.B.; Ballav, S.; Thosar, N.; Swamy, K.V.; Basu, S. Exploring conformational changes of PPAR-Ɣ complexed with novel kaempferol, quercetin, and resveratrol derivatives to understand binding mode assessment: A small-molecule checkmate to cancer therapy. J. Mol. Model. 2020, 26, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.L.; Chiou, J.S.; Chen, C.J.; Liang, W.M.; Tsai, F.J.; Wu, Y.C.; Lin, T.H.; Liao, C.C.; Huang, S.M.; Chou, C.H.; et al. Effect of Chinese herbal medicine therapy on risks of overall, diabetes-related, and cardiovascular diseases-related mortalities in Taiwanese patients with hereditary hemolytic anemias. Front. Pharmacol. 2022, 13, 891729. [Google Scholar] [CrossRef]
- Qaid, M.M.; Abdelrahman, M.M. Role of insulin and other related hormones in energy metabolism—A review. Cogent Food Agric. 2016, 2, 1267691. [Google Scholar] [CrossRef]
- Ali, N.; Diamond, D.M.; Rice, S.M. Cardiovascular disease and its association with insulin resistance and cholesterol. In Ketogenic; Academic Press: Cambridge, MA, USA, 2023; pp. 205–236. [Google Scholar] [CrossRef]
- Sobczak, I.S.A.; Blindauer, A.C.; Stewart, J.A. Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef]
- Kim, M.J.; Sim, D.Y.; Lee, H.M.; Lee, H.J.; Kim, S.H. Hypolipogenic effect of shikimic acid via inhibition of MID1IP1 and phosphorylation of AMPK/ACC. Int. J. Mol. Sci. 2019, 20, 582. [Google Scholar] [CrossRef]
- Kang, H.; Koppula, S. Houttuynia cordata attenuates lipid accumulation via activation of AMP-activated protein kinase signaling pathway in HepG2 cells. Am. J. Chin. Med. 2014, 42, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Al-Obaidi, M.M.; Shahid, N.; Noordin, M.I.; Looi, C.Y.; Wong, W.F.; Khaing, S.L.; Mustafa, M.R. Synergistic effect of quercetin and quinic acid by alleviating structural degeneration in the liver, kidney and pancreas tissues of STZ-induced diabetic rats: A mechanistic study. Food Chem. Toxicol. 2014, 71, 183–196. [Google Scholar] [CrossRef] [PubMed]
- McLeish, M.J.; Kenyon, G.L. Relating Structure to Mechanism in Creatine Kinase. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 1–20. [Google Scholar] [CrossRef]
- Loubani, M.; Fowler, A.; Standen, N.B.; Galiñanes, M. The effect of gliclazide and glibenclamide on preconditioning of the human myocardium. Eur. J. Pharmacol. 2005, 515, 142–149. [Google Scholar] [CrossRef]
- Bati, K.; Baeti, P.B.; Gaobotse, G.; Kwape, T.E. Leaf extracts of Euclea natalensis A.D.C ameliorate biochemical abnormalities in high-fat-low streptozotocin-induced diabetic rats through modulation of the AMPK-GLUT4 pathway. Egypt. J. Basic Appl. Sci. 2024, 11, 232–252. [Google Scholar] [CrossRef]
- Galic, S.; Loh, K.; Murray-Segal, L.; Steinberg, G.R.; Andrews, Z.B.; Kemp, B.E. AMPK signaling to acetyl-CoA carboxylase is required for fasting- and cold-induced appetite but not thermogenesis. Elife 2018, 7, e32656. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, L.; Guo, X.; Tao, H.; Liu, Y.; Liu, X.; Zhang, Y.; Meng, X. The potential of herbal drugs to treat heart failure: The roles of Sirt1/AMPK. J. Pharm. Anal. 2023, 14, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Bose, S.; Lim, S.K.; Ansari, A.; Chin, Y.W.; Choi, H.S.; Kim, H. Houttuynia cordata facilitates metformin on ameliorating insulin resistance associated with gut microbiota alteration in OLETF rats. Genes 2017, 8, 239. [Google Scholar] [CrossRef] [PubMed]







| Healthy Control | Diabetes | ||||||
|---|---|---|---|---|---|---|---|
| HC | HPK | p | DC | DD | DPK | p | |
| Total Cholesterol (mg/dL) | 89.5 ± 9.1 | 94.7 ± 13.5 | ns | 154.0 ± 6.9 | 82.6 ± 5.6 *** | 89.4 ± 5.9 *** | <0.001 |
| Triglyceride (mg/dL) | 81.5 ± 11.7 | 59.1 ± 4.1 | ns | 171.3 ± 10.7 | 41.4 ± 11.0 *** | 52.4 ± 11.0 *** | <0.001 |
| HDL (mg/dL) | 38.3 ± 9.5 | 60.0 ± 12.4 | ns | 24.2 ± 2.7 | 45.4 ± 2.7 *** | 43.9 ± 2.5 *** | <0.001 |
| LDL (mg/dL) | 39.4 ± 3.3 | 29.7 ± 4.8 | ns | 103.1 ± 10.3 | 29.9 ± 5.4 *** | 35.2 ± 5.2 *** | <0.001 |
| VLDL (mg/dL) | 12.9 ± 2.2 | 9.2 ± 1.9 | ns | 32.6 ± 2.7 | 8.4 ± 2.4 *** | 10.3 ± 2.2 *** | <0.001 |
| TC/HDL ratio | 3.1 ± 0.5 | 1.6 ± 0.14 | <0.05 | 7.4 ± 1.3 | 2.0 ± 0.3 *** | 2.3 ± 0.3 *** | <0.001 |
| LDL/HDL ratio | 1.7 ± 0.4 | 0.62 ± 0.15 | ns | 4.6 ± 0.6 | 0.74 ± 0.18 *** | 0.90 ± 0.18 *** | <0.001 |
| Healthy Control | Diabetes | ||||||
|---|---|---|---|---|---|---|---|
| HC | HPK | p | DC | DD | DPK | p | |
| SGPT (U/L) | 78.2 ± 9.4 | 93.5 ± 12.3 | ns | 106.4 ± 19.2 | 47.7 ± 8.8 ** | 77.8 ± 12.9 * | * |
| SGOT (U/L) | 115.1 ± 17.1 | 86.8 ± 23.8 | ns | 144.3 ± 27.8 | 29.8 ± 7.1 *** | 69.6 ± 18.6 * | * |
| AST/ALT Ratio | 1.74 ± 0.27 | 1.39 ± 0.45 | ns | 1.5 ± 0.29 | 0.85 ± 0.23 | 1.0 ± 0.23 | ns |
| ALP (U/L) | 193.6 ± 16.4 | 283.9 ± 24.9 | ns | 480.9 ± 117.0 | 255.6 ± 44.9 | 340.9 ± 36.4 | ns |
| Sample | Total Phenolic Content | ABTS | DPPH |
|---|---|---|---|
| (GAE)/g | IC50 (mg/mL) | ||
| Plu kaow ethanolic extract | 84.87 | 0.44 | 0.16 |
| Gallic acid | - | 0.03 | 0.003 |
| Quercetin | - | 0.04 | 0.018 |
| Compound | PubChem (CID) | Binding Score/RMSD (Å) | ||||||
|---|---|---|---|---|---|---|---|---|
| α-Amylase/1.5606 Å * | α-Glucosidase/1.8760 Å ** | SUR/0.8158 Å * | GLP-1/1.3217 Å * | GP/0.7958 Å * | IGF1R/0.2632 Å *** | PPAR-γ/0.6223 Å * | ||
| Cinnamic acid | 44539 | 35.49 | 19.50 | 27.33 | 33.28 | 35.31 | 35.17 | 35.14 |
| Kaempferol 7-neohesperidoside | 5483905 | 67.74 | 42.87 | 46.65 | 54.59 | 66.83 | 67.50 | 54.63 |
| Luteolin | 5280455 | 60.65 | 38.05 | 41.15 | 54.25 | 52.27 | 47.14 | 54.12 |
| Epicatechin | 72276 | 55.31 | 35.60 | 36.37 | 55.20 | 52.43 | 51.66 | 57.95 |
| Quercetin | 5280343 | 62.41 | 39.36 | 37.98 | 55.22 | 54.64 | 51.49 | 53.62 |
| Quercitrin | 5280459 | 58.79 | 42.89 | 45.05 | 62.91 | 65.78 | 54.08 | 62.83 |
| Salidroside | 159278 | 54.12 | 37.22 | 38.32 | 53.71 | 54.73 | 55.50 | 51.81 |
| Vanillic acid | 8468 | 39.42 | 21.48 | 31.64 | 32.01 | 38.06 | 37.20 | 38.77 |
| Neochlorogenic acid | 5280633 | 53.04 | 40.97 | 40.38 | 53.99 | 59.52 | 53.71 | 63.04 |
| Vitexin | 5280441 | 54.15 | 45.04 | 44.75 | 50.85 | 60.50 | 51.67 | 70.74 |
| Quinic acid | 6508 | 44.20 | 25.17 | 24.86 | 30.32 | 39.60 | 41.99 | 40.01 |
| Isochlorogenic acid C | 5315832 | 69.77 | 38.06 | 38.36 | 54.92 | 55.98 | 57.23 | 60.76 |
| Guaijaverin | 5481224 | 55.19 | 44.91 | 45.23 | 62.65 | 73.78 | 62.01 | 65.34 |
| Rutin | 5280805 | 70.95 | 50.75 | 48.85 | 61.30 | 59.71 | 66.67 | 77.94 |
| Afzelin | 5316673 | 59.14 | 41.19 | 44.18 | 61.91 | 63.93 | 53.45 | 62.40 |
| Hyperin | 133568467 | 62.06 | 45.35 | 50.10 | 58.82 | 77.11 | 60.11 | 55.13 |
| Datiscin | 10054207 | 71.30 | 44.79 | 53.97 | 54.77 | 68.60 | 61.36 | 73.93 |
| Shikimic acid | 8742 | 39.01 | 24.59 | 25.84 | 31.69 | 34.82 | 39.21 | 38.12 |
| Diosmin | 5281613 | 75.28 | 50.43 | 50.99 | 55.08 | 84.12 | 67.66 | 67.96 |
| Apigenin | 5280443 | 55.86 | 37.62 | 35.90 | 53.47 | 51.83 | 44.83 | 51.25 |
| Eupatilin | 5273755 | 61.45 | 38.53 | 37.26 | 49.05 | 55.86 | 43.68 | 63.09 |
| Rosiglitazone | 77999 | - | - | - | - | - | - | 72.76 |
| Acarbose | 9811704 | 66.44 | 49.78 | - | - | - | - | - |
| Luteolin † | 5280455 | 37.37 | - | - | - | - | ||
| Glibencamide (Glyburide) | 3488 | - | - | 50.76 | - | - | 61.03 | - |
| Metformin | 4091 | - | 37.25 | - | - | - | ||
| Nojirimycin tetrazole (NTZ) | 41684 | - | - | - | - | 45.32 | - | - |
| Glicazide | 3475 | - | - | - | - | 45.93 | - | |
| No. | UPLC-ESI (-)-QTOF-MS/MS |  |  | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Retention Time (min) | Metabolite | Predicted Neutral Formula (Sirius Score %) * | Neutral mass *** | Theoretical (m/z) [M-H]− † | Exp. Mass (m/z) | Mass Error (ppm) ‡ | Library Score (%) # | Rank/DB (F1 Score) | Rank/DB Training (YES/NO) § | |
| 1 | 0.76 | Quinic acid | C7H12O6 (99%) | 192.06 | 191.0556 | 191.0593 | 19.58 | 89.8 | 1st/KEGG (1.0) | 1st/All Databases (YES) |
| 2 | 0.65 | Shikimic acid | C7H10O5 (100%) | 174.05 | 173.0450 | 173.0450 | 0 | 100 | 4th/NORMAN (0.7462) | 1st/All Databases (YES) |
| 3 | 1.88 | Neochlorogenic acid | C16H18O9 (100%) | 354.10 | 353.0873 | 353.0955 | 23.34 | 97.7 | 1st/NORMAN (1.0) | ND. (NO) |
| 4 | 3.06 | Vanillic acid | C8H8O4 (99.63%) | 168.04 | 167.0344 | 167.0362 | 10.57 | 91.7 | 6th/KEGG (0.7846) | 1st/All Databases (YES) |
| 5 | 3.78 | Salidroside | C14H20O7 (97.17%) | 300.12 | 299.1131 | 299.1150 | 6.42 | 98.8 | 1st/KEGG (1.0) | 1st/All Databases (YES) |
| 6 | 4.18 | Epicatechin | C15H14O6 (100%) | 290.08 | 289.0712 | 289.0851 | 48.03 | 90.6 | 1st/NORMAN (1.0) | 1st/All Databases (YES) |
| 7 | 4.85 | Isochlorogenic acid C | C25H24O12 (90.93%) | 516.13 | 515.1190 | 515.1216 | 5.13 | 86.5 | 7th/PubChem (0.9858) | 1st/All Databases (YES) |
| 8 | 4.90 | Diosmin | C28H32O15 (N.D) ** | 608.17 | 607.1663 | 607.1352 | −51.22 | 63.3 | 1st/KEGG (1.0) | ND. (NO) |
| 9 | 5.01 | Rutin | C27H30O16 (100%) | 610.15 | 609.1455 | 609.1544 | 14.61 | 96.0 | 2nd/NORMAN (0.0) | 1st/All Databases (YES) |
| 10 | 5.07 | Vitexin | C21H20O10 (100%) | 432.11 | 431.0987 | 431.1025 | 10.84 | 96.3 | 3th/NORMAN (1.0) | 1st/All Databases (YES) |
| 11 | 5.85 | Afzelin | C21H20O10 (94.77%) | 432.11 | 431.0987 | 431.10015 | 5.39 | 95.2 | 1st/KEGG (1.0) | 1st/All Databases (YES) |
| 12 | 5.18 | Hyperin | C21H20O12 (100%) | 464.10 | 463.0877 | 463.0958 | 17.59 | 99.5 | 1st/KEGG (1.0) | 1st/All Databases (YES) |
| 13 | 5.29 | Datiscin | C27H30O15 (79.27%) | 594.16 | 593.1507 | 593.1549 | 7.17 | 99.4 | ND. | 5th/All Databases (YES) |
| 14 | 5.40 | Guaijaverin | C20H18O11 (100%) | 434.08 | 433.0771 | 433.0804 | 7.64 | 95.6 | 1st/NORMAN (1.0) | 1st/All Databases (YES) |
| 15 | 5.57 | Quercitrin | C21H20O11 (100%) | 448.10 | 447.0927 | 447.1004 | 17.13 | 99.5 | 1st/NORMAN (1.0) | 1st/All Databases (YES) |
| 16 | 6.35 | Kaempferol-7-O-neohesperidroside | C27H30O15 (N.D.) ** | 594.16 | 593.1507 | 593.1339 | −28.24 | 100 | 1st/NORMAN (1.0) | ND. (NO) |
| 17 | 6.41 | Cinnamic acid | C9H8O2 (99.95%) | 148.05 | 147.0446 | 147.0460 | 9.49 | 95.3 | 1st/KEGG (1.0) | 1st/All Databases (YES) |
| 18 | 6.52 | Luteolin | C15H10O6 (95.80%) | 286.05 | 285.0399 | 285.0419 | 6.96 | 94.8 | 1st/KEGG (1.0) | 1st/All Databases (YES) |
| 19 | 6.58 | Quercetin | C15H10O7 (92.71%) | 302.04 | 301.0348 | 301.0373 | 8.21 | 97.5 | 1st/NORMAN (1.0) | 1st/All Databases (YES) |
| 20 | 7.08 | Apigenin | C15H10O5 (98.89%) | 270.05 | 269.0450 | 269.0475 | 9.29 | 99.3 | 1st/KEGGs (1.0) | 1st/All Databases (YES) |
| 21 | 8.42 | Eupatilin | C18H16O7 (100%) | 344.09 | 343.0818 | 343.0839 | 6.18 | 98.9 | 3rd/KEGG (0.988) | 3rd/All Databases 2nd/KEGG 1st/HMDB |
| Mechanism of Action | Target Compounds of PK Containing Anti-Diabetic Properties | References |
|---|---|---|
| Inhibition of α-amylase activity | Kaempferol 7-neohesperidoside, isochlorogenic acid, rutin, datiscin, diosmin | [59,60,61,62,63,64] |
| Inhibition of α-glucosidase activity | Datiscin, diosmin, rutin, isochlorogenic acid | [59,60,61,62,63,64] |
| Regulation of sulfonylurea receptor (SUR) | Diosmin, datiscin, hyperin | [63,65,66] |
| Inhibition of Glycogen phosphorylase | Kaempferol, glycosidic flavonoids, rutin, hyperin, datiscin, guaijaverin, afzelin, and diosmin | [67,68,69,70] |
| Release of glucagon-like peptide-1 (GLP-1) | Guaijaverin, afzelin, rutin, quercitrin, salidroside, isochlorogenic acid C, neochlorogenic acid | [71,72,73,74] |
| Modulation of insulin-like growth factor 1 (IGF1R) kinase | Kaempferol 7-neohesperidoside, guaijaverin, rutin, datiscin, diosmin | [75,76,77] |
| Inhibition of Peroxisome proliferator-activated receptor-gamma (PPAR-γ) | Rutin, kaempferol, datiscin, diosmin | [78,79,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.S.; Klamrak, A.; Nopkuesuk, N.; Nabnueangsap, J.; Janpan, P.; Choowongkomon, K.; Daduang, J.; Daduang, S. Impacts of Plu kaow (Houttuynia cordata Thunb.) Ethanolic Extract on Diabetes and Dyslipidemia in STZ Induced Diabetic Rats: Phytochemical Profiling, Cheminformatics Analyses, and Molecular Docking Studies. Antioxidants 2024, 13, 1064. https://doi.org/10.3390/antiox13091064
Rahman SS, Klamrak A, Nopkuesuk N, Nabnueangsap J, Janpan P, Choowongkomon K, Daduang J, Daduang S. Impacts of Plu kaow (Houttuynia cordata Thunb.) Ethanolic Extract on Diabetes and Dyslipidemia in STZ Induced Diabetic Rats: Phytochemical Profiling, Cheminformatics Analyses, and Molecular Docking Studies. Antioxidants. 2024; 13(9):1064. https://doi.org/10.3390/antiox13091064
Chicago/Turabian StyleRahman, Shaikh Shahinur, Anuwatchakij Klamrak, Napapuch Nopkuesuk, Jaran Nabnueangsap, Piyapon Janpan, Kiattawee Choowongkomon, Jureerut Daduang, and Sakda Daduang. 2024. "Impacts of Plu kaow (Houttuynia cordata Thunb.) Ethanolic Extract on Diabetes and Dyslipidemia in STZ Induced Diabetic Rats: Phytochemical Profiling, Cheminformatics Analyses, and Molecular Docking Studies" Antioxidants 13, no. 9: 1064. https://doi.org/10.3390/antiox13091064






