Abstract
Frontotemporal dementia (FTD) causes progressive neurodegeneration in the frontal and temporal lobes, leading to behavioral, cognitive, and language impairments. With no effective treatment available, exploring new therapeutic approaches is critical. Recent research highlights the transcription factor Nuclear Factor erythroid-derived 2-like 2 (NRF2) as vital in limiting neurodegeneration, with its activation shown to mitigate FTD-related processes like inflammation. Dimethyl fumarate (DMF), an NRF2 activator, has demonstrated neuroprotective effects in a TAU-dependent FTD mouse model, reducing neurodegeneration and inflammation. This suggests DMF repositioning potential for FTD treatment. Until now, no trial had been conducted to analyze the effect of DMF on TDP-43-dependent FTD. In this study, we aimed to determine the potential therapeutic efficacy of DMF in a TDP-43-related FTD mouse model that exhibits early cognitive impairment. Mice received oral DMF treatment every other day from presymptomatic to symptomatic stages. By post-natal day (PND) 60, an improvement in cognitive function is already evident, becoming even more pronounced by PND90. This cognitive enhancement correlates with the neuroprotection observed in the dentate gyrus and a reduction in astrogliosis in the stratum lacunosum-moleculare zone. At the prefrontal cortex (PFC) level, a neuroprotective effect of DMF is also observed, accompanied by a reduction in astrogliosis. Collectively, our results suggest a potential therapeutic application of DMF for patients with TDP-43-dependent FTD.
1. Introduction
Frontotemporal dementia (FTD) is a progressive neurodegenerative disease affecting 4.1 per 100,000 individuals, typically between the ages of 45 and 65. Clinically, FTD patients exhibit personality, cognitive, behavioral, and language disturbances due to the degeneration of the frontal and temporal lobes, followed by hippocampal atrophy [1]. While Alzheimer’s disease (AD) is the most common form of dementia, FTD is the second leading cause in adults and the most common in those under 65. The etiology of FTD is complex and heterogeneous, with approximately 50–60% of cases being sporadic and the rest having a familial origin, linked to mutations in genes such as MAPT, TARDBP, GRN, C9orf72, and VCP. FTD is characterized by toxic, ubiquitin-positive protein aggregates [2]. Based on the predominant protein, FTD is classified into subtypes, mainly FTD-TAU and FTD-TDP-43, each affecting about 45% of patients.
TDP-43 is encoded by TARDBP and is a nuclear RNA/DNA-binding protein essential for RNA metabolism, including stability, transport, transcription, and processing. In FTD, TDP-43 mislocalizes from the nucleus to cytoplasmic inclusions, suggesting that either loss of normal function or gain of toxic properties play a key role in the disease’s pathogenesis. There are currently no approved treatments to slow FTD progression; existing strategies focus on symptom management. However, pharmacologically modulating TAU and TDP-43 proteins could be promising. The transcription factor NRF2 has emerged as crucial in limiting neurodegeneration, affecting processes like proteostasis, oxidative stress, and neuroinflammation. NRF2 levels are typically low due to different regulatory pathways. The primary mechanism regulating NRF2’s transcriptional activity involves its binding to the E3 ligase adapter Kelch-like ECH-associated protein 1 (KEAP1). This interaction presents NRF2 for ubiquitination by Cullin 3 and RING-box protein 1 (CUL3/RBX1), leading to its subsequent degradation by the proteasome [3,4,5]. Under normal homeostatic conditions, NRF2 levels are very low. However, modifications of key cysteine residues in KEAP1 by electrophiles or reactive oxygen species (ROS) cause conformational changes that prevent KEAP1 from targeting NRF2 for degradation. This results in the accumulation of newly synthesized NRF2, which can then translocate to the nucleus and bind to an enhancer sequence, known as the antioxidant response element (ARE), in the promoter regions of NRF2 target genes [6]. Together with members of the small musculo-aponeurotic fibrosarcoma (MAF) family, NRF2 recruits additional components of the transcriptional machinery. Another mechanism for modulating NRF2 involves the E3 ligase adapter β-transducin repeat-containing E3 ubiquitin-protein ligase (β-TrCP), which presents NRF2 to a CUL1/RBX1 complex [7,8]. This leads to an alternative pathway for the ubiquitin-dependent proteasome degradation of NRF2. Thus, NRF2 is subject to multiple levels of regulation, in addition to the numerous pathways modulated downstream of its activation.
Among NRF2 activators, dimethyl fumarate (DMF) has shown neuroprotective effects and is already approved for relapsing-remitting multiple sclerosis as Tecfidera, despite some gastrointestinal side effects. In our research group, we have previously shown that in FTD-TAU models, hippocampal cells overexpressing the human mutant TAUP301L protein produce the chemokine fractalkine CXCL1. This chemokine activates AKT in microglia, inhibits glycogen synthase kinase-3β (GSK-3β), and upregulates the transcription factor NRF2/NFE2L2 and its target genes, including heme oxygenase 1 (Hmox1) [9]. Additionally, DMF treatment mitigated neurodegenerative features in our FTD-TAU mouse model, reducing TAU hyperphosphorylation, neurodegeneration, neuroinflammation, and oxidative stress, supporting the repurposing of DMF for FTD treatment [10]. These results clearly demonstrated the importance of pharmacological modulation of NRF2 in FTD-TAU. However, regarding TDP-43 protein associated with FTD, evidence for NRF2 transcription factor involvement in this pathology is still limited. Recently, our group reported that in a transgenic FTD model with TDP-43 protein overexpression in forebrain neurons (FTD-TDP-43), there was only a significantly decreased expression of the NRF2-dependent enzyme NQO1 in the prefrontal cortex (PFC) of these mice, which cannot be attributed to alterations in the NRF2 pathway [11]. However, in these mice, we cannot rule out a neuroprotective effect of the NRF2 inducer, DMF, on the neurodegeneration and neuroinflammation observed in mice overexpressing TDP-43. Therefore, this study focuses on analyzing the potential beneficial effects of DMF treatment in the prefrontal cortex and hippocampus of FTD-TDP-43 mice.
2. Materials and Methods
2.1. FTD Mouse Model: CaMKII-TDP-43
All experiments were conducted with male animals that had PND90 as the endpoint age, with FVB-N background FVB-N/CaMKII-TDP43 transgenic and non-transgenic mice bred in our animal facilities from initial breeders provided by Dr. Che-Kun J Sheen (The PhD Program for Neural Regenerative Medicine, Taipei Medical University, Taiwan) and previously characterized [12]. This model is based on the overexpression of the TDP-43 protein under the control of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) promoter to specifically and selectively overexpress TDP-43 in forebrain neurons. CaMKII-TDP-43 male mice and their wildtype animals were generated from homozigotic breeding. Animals were housed in the animal facilities of Complutense University (CAI-Animalario, Faculty of Medicine, Complutense University, ref. ES280790000086) under controlled photoperiod (08:00–20:00 light) and temperature (22 ± 1 °C) and with free access to a standard diet and water. DMF (100 mg/kg) (Merck Sigma-Aldrich, Buchs, Switzerland) was suspended in 0.8% methocel (Sigma-Aldrich) and given by oral gavage. This dose was established based on previous results from our group, where neuroprotective effects were observed in other neurodegenerative disease mouse models [10,13]. We did not detect significant weight loss, hair loss, or other gross alterations in the DMF-treated mice during the administration period.
2.2. Behavioral Analysis
To evaluate recognition memory, the novel object recognition (NOR) test was performed as described [14]. Briefly, on the first and second day, mice were allowed to explore an empty open field (an opaque methacrylate box (50 × 50 × 50 cm) with a base covered with sawdust) for 10 min to habituate to the field. On the third day, mice were placed in the open field with two identical objects to explore for 10 min. On the fourth day, mice were placed in the open field with one of the objects replaced by a new object and explored for 10 min. Time spent exploring either the novel object (TN) or the familiar object (TF) was recorded. Differences were represented as a discrimination index (DI = (TN − TF)/(TN + TF)). The test was performed at two different times: first, at an early symptomatic stage (PND60), and second, 24 h before sacrifice (PND90), with 11–12 animals from each experimental group.
2.3. Randomization and Blinding
Animals were randomized for treatment. Data collection and evaluation of all experiments were performed blindly of the group identity. The data and statistical analysis are presented in line with the recommendations on experimental design and analysis in pharmacology [15].
2.4. Analysis of mRNA Levels via QUANTITATIVE Real-Time PCR
Total RNA extraction, reverse transcription, and quantitative polymerase chain reaction (qPCR) were performed as detailed in previous articles [11] Primer sequences are shown in Table 1. Data were analyzed using the 2−ΔΔCT method, with normalization of the raw data based on the geometric mean of Tbp, Actb, and Gapdh (Merck Sigma-Aldrich, Darmstadt, Germany), encoding housekeeping proteins. All PCR amplifications were performed in triplicate.

Table 1.
List of primers used in this study.
2.5. Immunofluorescence on Mouse Tissues
Immunofluorescence assays were performed on 30-µm thick coronal brain sections at the hippocampus and prefrontal cortex (PFC). The protocol followed was previously described [16]. Primary and secondary antibodies are described in Table 2. The area of the dentate gyrus from mice treated with vehicle or DMF stained with DAPI or anti-CALBINDIN D28K was analyzed by the ImageJ program. A total of three images per side and condition were analyzed as follows. The images were transformed into 16-bit with the ImageJ program. Then, with the “Free Hand Selection” tool of the ImagenJ program, we manually selected only the dentate gyrus area of each image stained with DAPI or anti-CALBINDIN D28K. The dimension of the dentate gyrus inside the selected area was quantified using the “Measure” tool in the ImageJ program and the raw results, measured in inches, were represented.

Table 2.
List of antibodies used in this study.
2.6. Stereological Analysis of Microgliosis and Astrogliosis
Cell counts were performed every eight sections (30 µm-thick) using Fiji Software v1.54f in three sections of the hippocampus or PFC. The error coefficient attributable to the sampling was calculated according to Gundersen and Jensen (1987) [17], and values ≤0.10 were accepted. (n = 4–5 animals per experimental group).
2.7. Statistical Analyses
Data are presented as the mean ± SEM. To determine the statistical test to be used, we employed GraphPad Instat 3, which includes the analysis of the data to a normal distribution via the Kolmogorov–Smirnov test. In addition, statistical assessments of differences between groups were analyzed (GraphPad Prism 8 by Dotmatics, San Diego, CA, USA) by performing an unpaired Student’s t-tests. A one-way ANOVA with post-hoc Newman–Keuls test was used.
3. Results
In the FTD CaMKII-TDP-43 mouse model, overexpression of the TDP-43 protein in neurons of the prefrontal cortex (PFC) and hippocampus has been shown to replicate disease-specific changes, including cognitive impairments, pathological mislocalization of TDP-43, and increased gliosis [12,14,18]. Therefore, we aim to investigate whether repositioning DMF treatment in this transgenic model for TDP-43-dependent FTD could modulate the disease’s degenerative progression.
3.1. DMF Treatment Alleviates Cognitive Impairment Caused by TDP-43
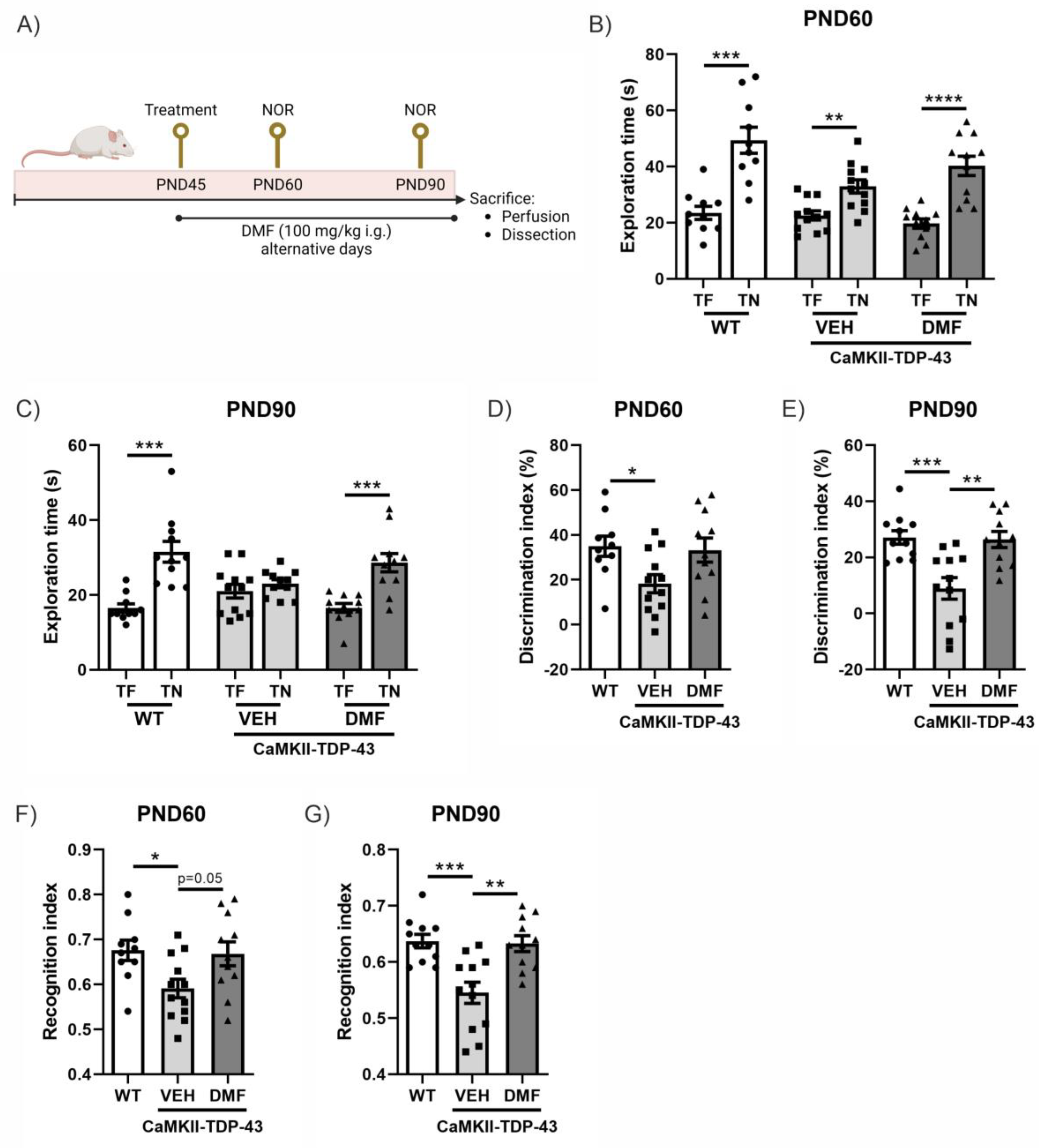
In this murine model of FTD associated with TDP-43, it has been previously described that cognitive impairment already occurs at PND60 and is exacerbated at PND90 [14,18]. Therefore, we analyzed the effect of DMF treatment at these two ages (Figure 1A). CaMKII-TDP-43 animals showed lower exploration times compared to the WT animals at both ages (Figure 1B,C). This resulted in an important decrease in the discrimination index (Figure 1D,E) and preference index (Figure 1F,G) in CaMKII-TDP-43-VEH mice compared to WT animals. Treatment with DMF produced a significant improvement in CaMKII-TDP-43-DMF animals, both in terms of the discrimination index (Figure 1D,E) and the preference index (Figure 1F,G), highlighting the treatment’s potential to ameliorate cognitive deficits in this animal model.
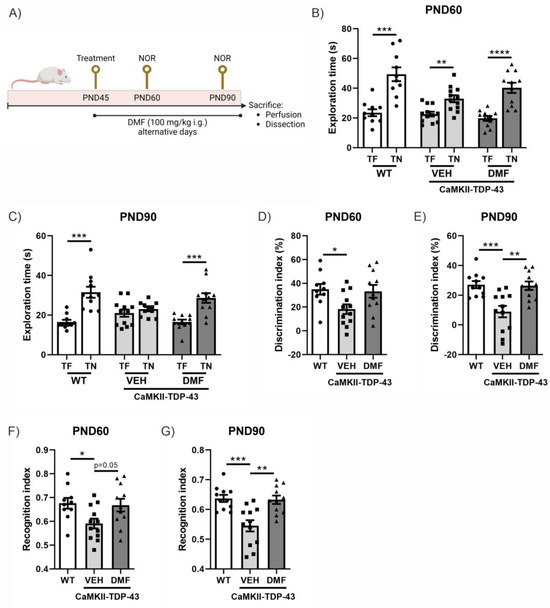
Figure 1.
Response in the Novel Object Recognition (NOR) test of CAMKII-TDP-43 and WT mice at PND60 and PND90 after a chronic i.g. administration of DMF (100 mg/kg) from PND45 up to PND90 (A) Timeline representation of the experimental design: At PND45, we started the treatments (VEH, or DMF 100 mg/kg, i.g., respectively). At PND60, we performed the first NOR analysis (four consecutive days), and at PND90, before sacrifice, we performed another NOR test. (B,C) Analysis of the exploration time of familiar object (TF) and novel object (TN) at PND60 and PND90. (D,E) Analysis of the discrimination index at PND60 and PND90. (F,G) Analysis of the recognition index at PND60 and PND90. Bars indicate the mean of n = 10–12 samples ± SEM. Asterisks show significant differences with * p < 0.05, ** p < 0.01, *** p < 0.005, **** p < 0.001 comparing each group according to a one-way ANOVA followed by Tukey’s post-test.
3.2. Neuroprotective Effect of DMF on the Granular Layer of the Hippocampus
We examined whether DMF treatment had crossed the blood-brain barrier and activated the transcription factor NRF2 pathway in the hippocampus (Figure S1) of CaMKII-TDP-43 mice by determining the mRNA levels of various NRF2-dependent enzymes. Since the mice were sacrificed 24 h after the last treatment dose, we observed only a modest induction of late-kinetic NRF2-dependent enzymes, such as Nqo1 (Figure S1C) and Gpx1 (Figure S1D), compared to the vehicle-treated CaMKII-TDP-43 mice. We confirmed that TDP-43 overexpression decreases mRNA levels of Nqo1 in transgenic mice, and that DMF treatment normalizes and even increases Nqo1 expression to baseline levels.
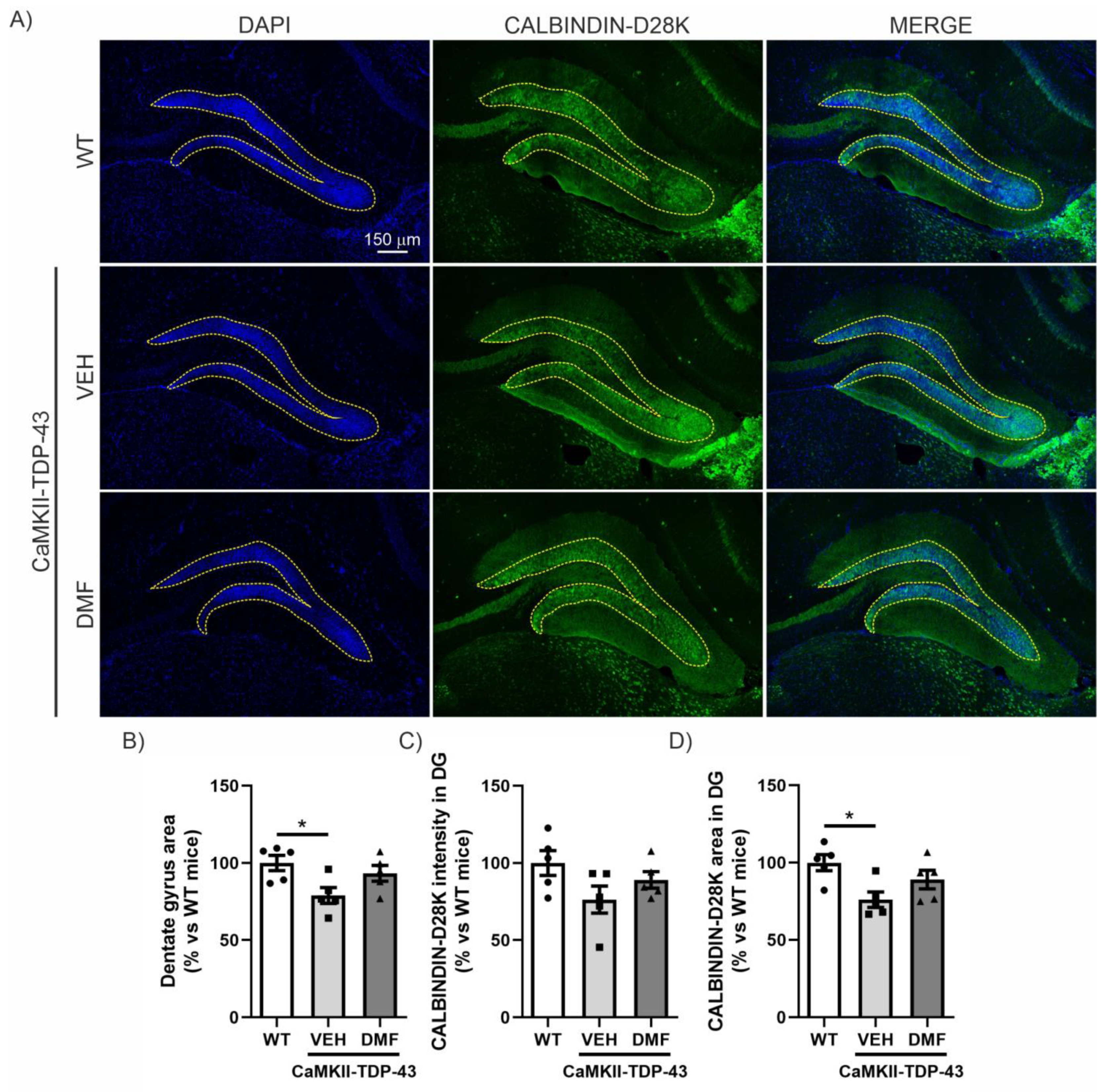
Within the hippocampus, the dentate gyrus (DG) is believed to contribute to the formation of new episodic memories [19], spontaneous exploration of novel environments, and other functions. Therefore, we investigated whether there was a correlation between the data obtained from the NOR (Figure 1) and structural alterations in the DG. DAPI staining, which binds strongly to adenine-thymine rich regions in DNA, indicated that TDP-43 overexpression induced partial loss of the granular cell layer in CaMKII-TDP-43 mice (Figure 2A,B). However, this loss was partially attenuated by the treatment with DMF, as shown in the area quantification (Figure 2B). Regarding synaptic plasticity, we evaluated the expression levels of CALBINDIN-D28K, a member of the calcium-binding protein superfamily, that, in the hippocampus, has been found in glutamatergic neurons, including mature granule cells in the DG [20]. This protein localizes to axonal boutons and dendritic spines and dynamically modulates synaptic plasticity; reduced CALBINDIN-D28K levels correlate with impaired hippocampus-dependent memory [21]. Immunofluorescence analysis of the DG showed reduced CALBINDIN-D28K expression levels (Figure 2A–C) and area (Figure 2A–D) in the granular layer of CaMKII-TDP-43 mice (Figure 2A–C) compared to WT animals, and the treatment with DMF was able to partially recover it. These results suggest that treatment with DMF provides protection against TDP-43-induced neurodegeneration.
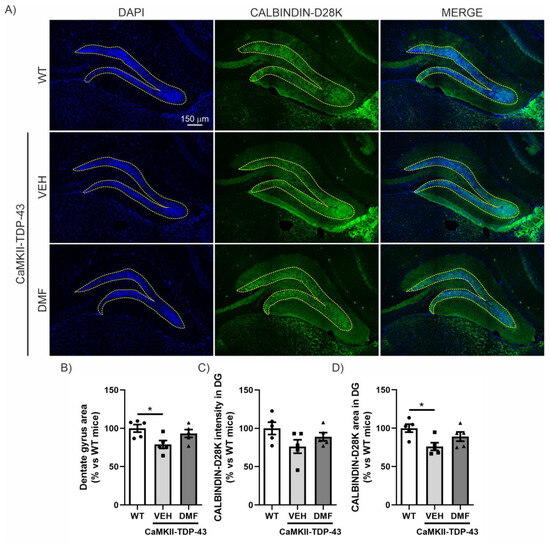
Figure 2.
DMF has a neuroprotective effect on the granular cell layer of the dentate gyrus. (A) Immunofluorescence staining of DAPI and CALBINDIN-D28K of 30 μm-thick sections of the dentate gyrus of the hippocampus from WT, and CAMKII-TDP-43 mice treated with vehicle or DMF. (B) Quantification of the area stained with DAPI in the dentate gyrus from WT, and CAMKII-TDP-43 mice treated with vehicle or DMF. (C) Quantification of the intensity of the area stained with CALBINDIN-D28K in the dentate gyrus from WT, and CAMKII-TDP-43 mice treated with vehicle or DMF. (D) Quantification of the area stained with CALBINDIN-D28K in the dentate gyrus from WT, and CAMKII-TDP-43 mice treated with vehicle or DMF. Bars indicate the mean of n = 4–5 samples ± SEM. Asterisks show significant differences with * p < 0.05 comparing each group according to a one-way ANOVA followed by Tukey’s post-test.
Regarding other areas of the hippocampus, such as CA1, CA2, and CA3, we also conducted an analysis of the neurodegeneration process by counting the number of neurons and by assessing CALBINDIN-D28K levels. In the CA1 area, we observed that CaMKII-TDP-43 mice have a slight neuronal loss compared to WT mice, and DMF treatment only has a subtle effect (Figure S2A–C). In the CA2 area, we did not observe any neuronal damage effects due to TDP-43 overexpression (Figure S2D–F). Finally, in the CA3 area, there is a slight decrease in both the number of neurons and CALBINDIN-D28K levels in CaMKII-TDP-43 mice, and DMF treatment does not appear to have any effect (Figure S3). Taken together, these results suggest that the CaMKII-TDP-43 mice exhibit cognitive impairment associated with degeneration of the DG, and that DMF treatment can mitigate this degenerative condition, having a positive impact on their cognition.
3.3. DMF Treatment Partially Reverses Astrogliosis but Not Microgliosis in the Hippocampus of CaMKII-TDP-43 Mice
One of the main characteristics of FTD-TDP-43 is neuroinflammation, which involves reactive astrogliosis and microgliosis accompanied by an imbalance between pro-inflammatory cytokines and anti-inflammatory (homeostatic) factors [22,23].
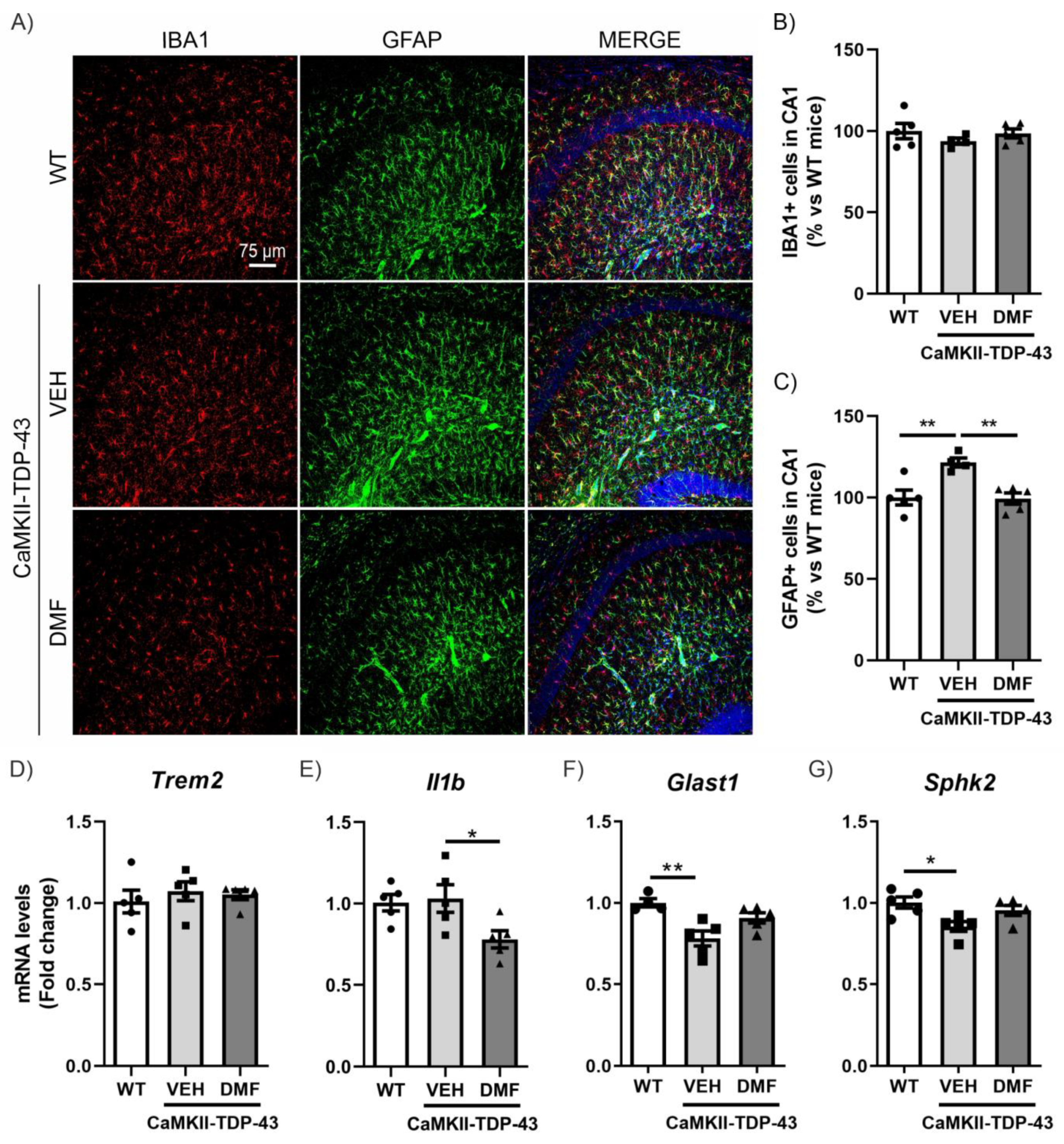
To specifically analyze neuroinflammatory processes, we conducted assessments of microgliosis and astrogliosis using immunofluorescence (IF). Microgliosis was analyzed using the IBA1 marker (ionized calcium-binding adapter molecule 1). As shown in Figure 3A,B, we did not observe TDP-43 protein overexpression to result in differences in IBA1-positive cells in the CA1 region of the hippocampus and saw no effects from the DMF treatment. These results were corroborated with the analysis of the mRNA expression levels of Trem2 and Il1b (Figure 3D,E), two markers of microglial function. Triggering receptor expressed on myeloid cells 2 (Trem2) is a myeloid cell-specific gene found in brain microglia. It is essential for microglia-mediated synaptic refinement, regulating microglial function and promoting the clearance of neurotoxic substances and abnormal proteins [24,25]. Our results showed no differences between CaMKII-TDP-43-VEH and WT mice (Figure 3D). Furthermore, we did not observe differences in the expression levels of Il1b (Figure 3E), which is widely used as pro-inflammatory marker, indicating that the overexpression of TDP-43 does not induce pro-inflammatory activation of microglia. Indeed, we observed that DMF treatment reduced its expression.
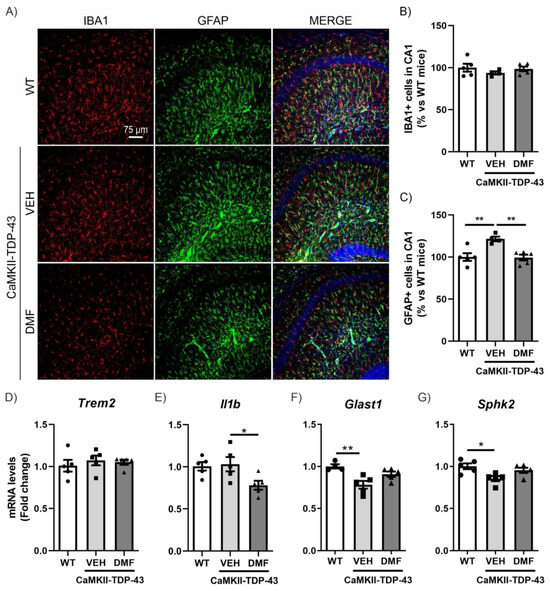
Figure 3.
DMF treatment modulates the astrogliosis observed in CAMKII-TDP-43 mice at the hippocampus. (A) Immunofluorescence of IBA1 (red) and GFAP (green), microglial and astrocytic markers, respectively, of 30 μm-thick sections in the CA1-hippocampus of mice treated with VEH or DMF, n = 4–5 samples ± SEM. Quantification of number of microglial (B) and astrocytes (C) cells at the CA1 area of mice treated with VEH or DMF, n = 4–5 samples ± SEM. RT-qPCR determination of mRNA levels of Trem2 (D), Il1b (E), Glast1 (F), and Sphk2 (G) genes at the hippocampus of mice treated with VEH or DMF, n = 4–5 samples ± SEM. The asterisks represent the difference in significance: * p < 0.05, ** p < 0.01, comparing each group according to a one-way ANOVA followed by Tukey’s post-test.
Next, we determined astrogliosis through analyzing Glial Fibrillary Acidic Protein (GFAP)-positive cells selectively in the CA1 region of the hippocampus. We observed a significant increase in CaMKII-TDP-43-VEH mice (Figure 3A–C), particularly in the stratum lacunosum-moleculare zone, which was completely reversed by DMF treatment, aligning with the anti-inflammatory effect of DMF reported previously [10]. This hippocampal layer is important as it serves as a link between the entorhinal cortex (EC) and the CA1 hippocampus, and is involved in memory processes, correlating with our NOR results. Additionally, we analyzed the mRNA expression levels of Glast1 and Sphk2 to explore this aspect in more detail. Glutamate aspartate transporter 1 (GLAST1) is highly expressed in membranes of astrocytic processes, preferentially around excitatory synapses [26], and it has been described that its down-regulation is a consequence of glutamate-induced neuronal death or the reduction of synaptic activity [27]. Our results are in line with this idea, since in CaMKII-TDP-43-VEH mice we observed a decrease in Glast1 mRNA levels compared to WT mice (Figure 3F). Furthermore, treatment with DMF prevents this decrease, corroborating the neuroprotective effect of the compound. Additionally, Sphk2 expression is highest in the brain and is related to anti-inflammatory processes [28,29,30]. CaMKII-TDP-43-VEH mice showed decreased Sphk2 mRNA levels compared to WT mice (Figure 3G), and these levels were normalized in CaMKII-TDP-43-DMF animals. These data suggest that DMF treatment reduces astrogliosis in CaMKII-TDP-43 mice. We also determined microgliosis and astrogliosis processes in other hippocampal areas, such as CA2-CA3 and the dentate gyrus (DG). In all cases, we did not observe microgliosis or astrogliosis by IF (Figure S4).
3.4. DMF Treatment Prevents Degeneration of Layer V in the PFC in CaMKII-TDP-43 Mice
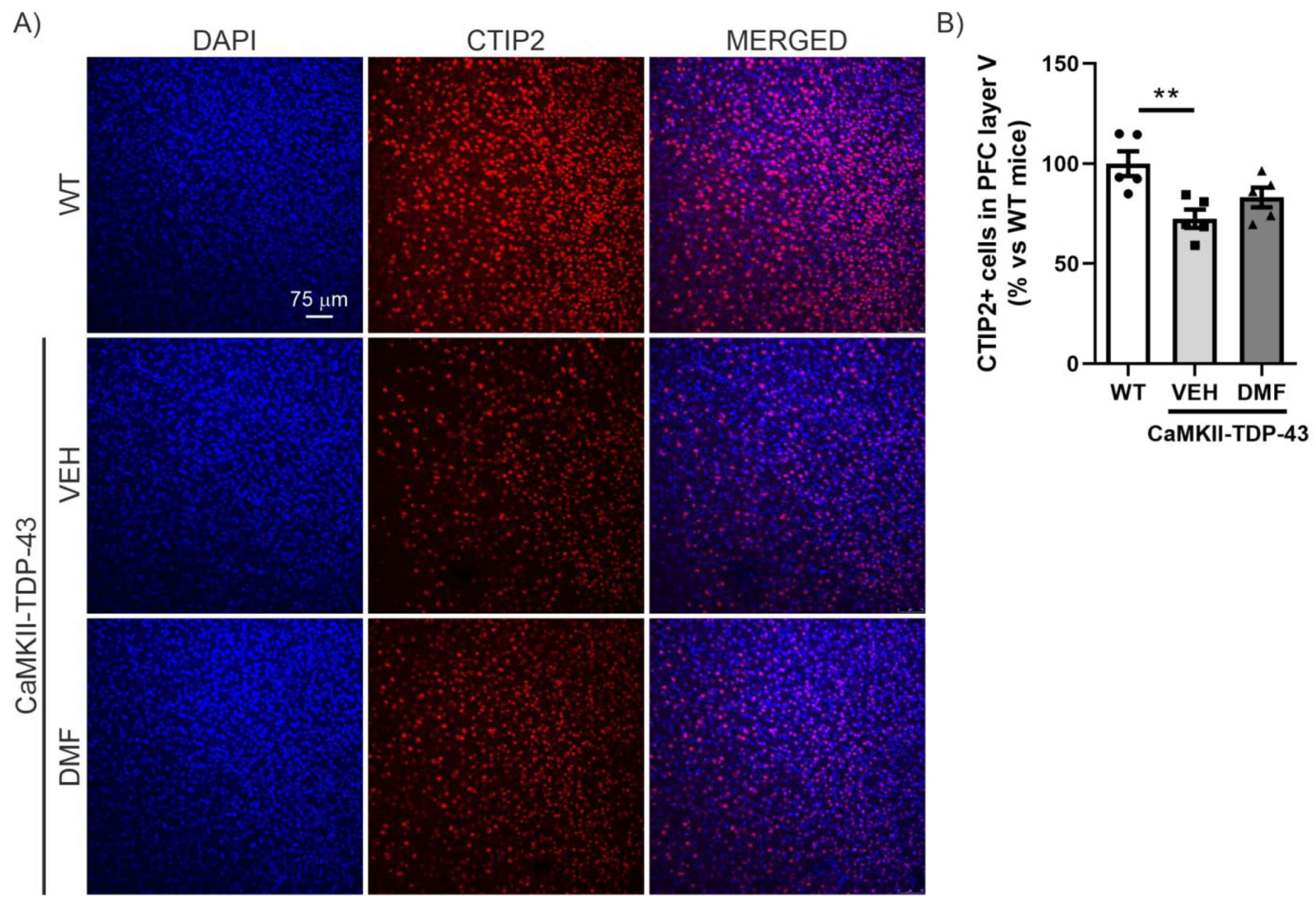
We then analyzed the prefrontal cortex (PFC) area, which is the main area associated with neurodegeneration in FTD, where a reduction in CTIP2-positive neurons has previously been described [14,18]. As shown in Figure 4, there is a noticeable reduction in the number of CTIP2-positive neurons in CaMKII-TDP-43-VEH mice compared to WT mice. Treatment with DMF prevents this significant neuronal loss, although the levels do not fully reach those observed in WT mice (Figure 4A,B).
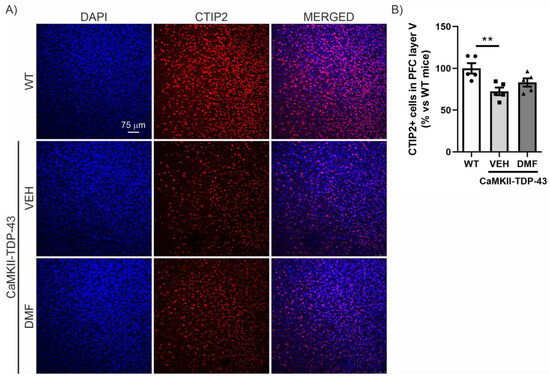
Figure 4.
TDP-43 overexpression decreases CTIP2+ neurons and DMF treatment partially reverses this effect in layer V of the cortex. (A) Immunofluorescence of CTIP2 (marker of corticospinal motor neurons and other projection neurons in layer V) (red) and DAPI (blue) of 30 μm-thick sections of the mPFC of mice treated with VEH or DMF, n = 5 samples ± SEM. (B) Quantification of number of CTIP2+ cells at the layer V of the mPFC of mice treated with VEH or DMF, n= 4–5 samples ± SEM. The asterisks represent the difference in significance: ** p < 0.01, comparing each group according to a one-way ANOVA followed by Tukey’s post-test.
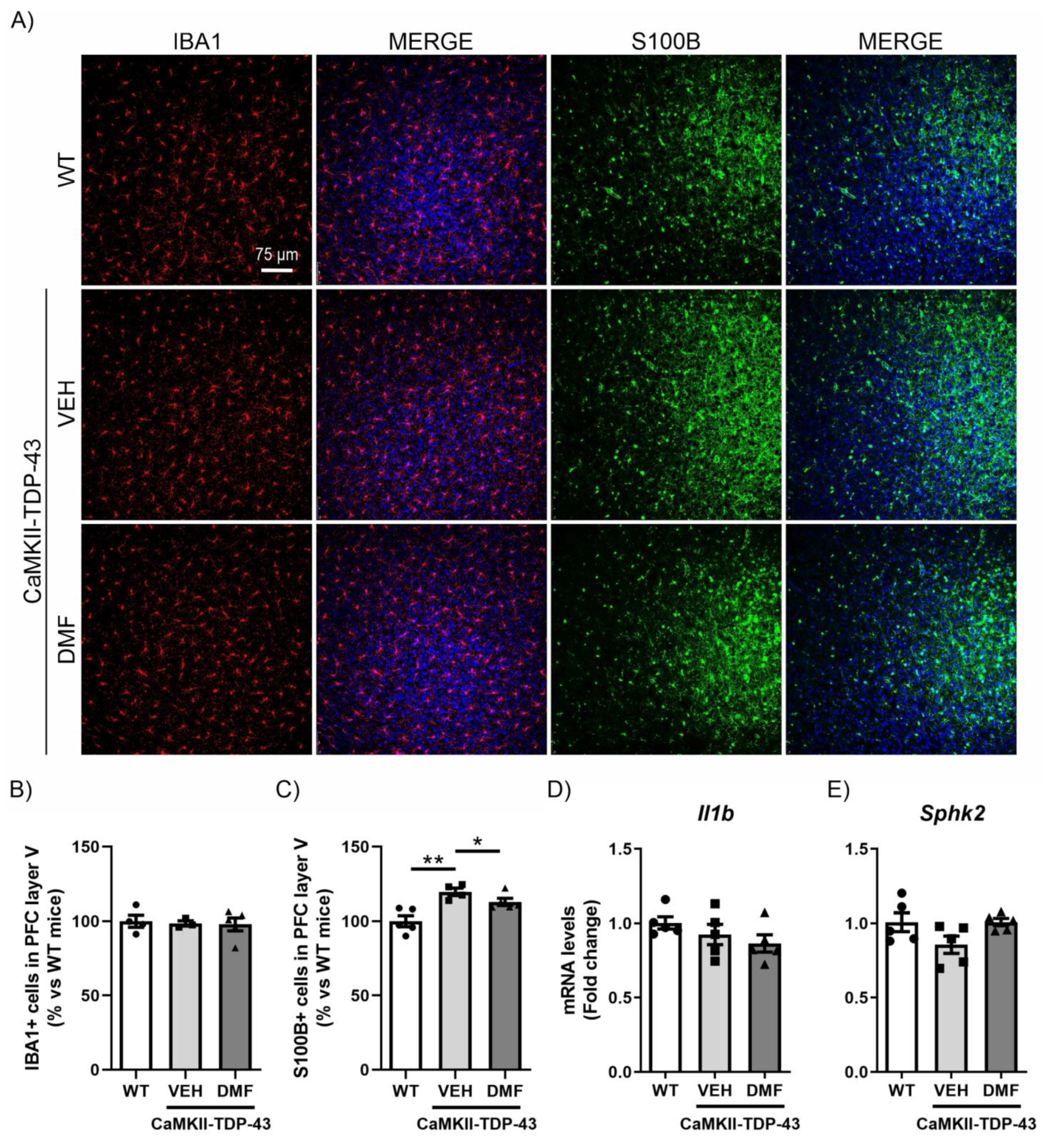
3.5. DMF Treatment Avoids Astrogliosis of Layer V in the PFC in CaMKII-TDP-43 Mice
Similar to the study conducted in the hippocampus, we analyzed the inflammatory process in the PFC. We observed that TDP-43 protein overexpression did not produce differences in the number of IBA1-positive cells in layer V of the PFC (Figure 5A,B), and no DMF effects were noticed. For astrogliosis analysis in the PFC, we used the S100-B marker (S100 calcium-binding protein B), a marker of astrocytes particularly recommended for this structure [31]. This choice is due to the different morphological characteristics of astrocytes depending on their brain region, with S100-B immunoreactivity being more intense in the PFC while GFAP immunoreactivity is more pronounced in the hippocampus [14]. IF analysis of S100-B-positive cells, specifically in layer V of the PFC, showed a significant increase in CaMKII-TDP-43-VEH mice (Figure 5A–C), which was partially reversed by DMF treatment. Here, we also analyzed the mRNA expression levels of the pro-inflammatory marker Il1b and the anti-inflammatory marker Sphk2, finding that the effects were subtler than those observed in the hippocampus (Figure 5D,E).
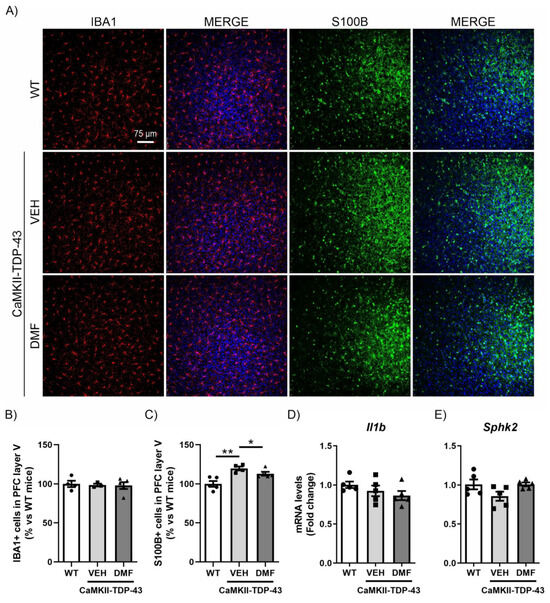
Figure 5.
The overexpression of TDP-43 induces astrogliosis in layer V of the mPFC and treatment with DMF partially reverses this effect. (A) Immunofluorescence of IBA1 (red) and S100B (green), microglial and astrocytic markers, respectively, of 30 μm-thick sections in the layer V of the mPFC of mice treated with VEH or DMF, n = 4–5 samples ± SEM. Quantification of number of microglial (B) and astrocyte (C) cells at the layer V of the mPFC of mice treated with VEH or DMF, n = 4–5 samples ± SEM. RT-qPCR determination of mRNA levels of Il1b (D) and Sphk2 (E) genes in the same area, n = 4–5 samples ± SEM. The asterisks represent the difference in significance: * p < 0.05, ** p < 0.01, comparing each group according to a one-way ANOVA followed by Tukey’s post-test.
4. Discussion
Currently, there is no cure or specific treatment for FTD. Medications used to treat or slow down AD do not seem to be effective for individuals with FTD, and some may even exacerbate FTD symptoms. Therefore, this work tackles this challenge by repurposing the drug DMF for TDP-43-dependent FTD, which has been shown to slow neurodegeneration in TAU-dependent FTD. This study presents the first evidence that DMF treatment can prevent cognitive decline, neurodegeneration, and astrogliosis in a TDP-43-dependent FTD model. Although further studies at other molecular levels are necessary, our results suggest that DMF treatment could be a promising candidate for slowing down TDP-43-dependent FTD.
It is important to consider that DMF functions as a prodrug, rapidly converting to monomethyl fumarate (MMF) through hydrolysis upon entering the body [32]. Both DMF and MMF trigger the activation of the NRF2 pathway. The primary chemical characteristic of DMF and MMF is their electrophilic nature, stemming from a double bond between reactive carbons known as Michael acceptors [33,34]. This structure makes DMF and MMF susceptible to nucleophilic attack via Michael addition reactions. Consequently, these compounds can form covalent bonds with cysteine thiol groups in various proteins and with glutathione (GSH). Despite their similarities, DMF and MMF exhibit some distinct properties. Notable differences exist in their reactivity with GSH and in their S-alkylation mechanisms. Specifically, MMF demonstrates a significantly slower reaction rate compared to DMF [33]. Although both compounds have similar antioxidant capacities, the main difference lies in the modulation of the pro-inflammatory pathway regulated by the transcription factor NF-κB. In this case, DMF has been described as capable of exerting anti-inflammatory effects through its covalent modulation of p65 [35], while MMF cannot reproduce this effect [36]. These different modes of action are important when establishing the signaling pathways involved in the beneficial effects of DMF/MMF in our TDP-43 FTD model, which we will discuss further below. Previous studies by our group demonstrated a significant decrease only in Nqo1 mRNA expression levels measured by qPCR and confirmed by RNAscope in this TDP-43 FTD model [11]. This mRNA reduction also reflected a protein level decrease of approximately 50%. These findings indicated that TDP-43 overexpression in the transgenic mice did not induce significant changes in NRF2 antioxidant signaling, although it did significantly decrease NQO1 levels. Recently, NQO1 has been described as an RNA-binding protein involved in translation control [37]. Given that TDP-43 regulates RNA metabolism and trafficking and interacts with several proteins involved in RNA processing, it cannot be ruled out that TDP-43 overexpression specifically modulates NQO1. In this study, we observed a significant decrease in Nqo1 mRNA levels, and DMF treatment not only normalized them but even elevated them above those of WT or CaMKII-TDP-43-VEH mice, enhancing the protective effect of this enzyme. Considering that there are hardly any studies analyzing the role of NQO1 in the context of wild-type TDP-43 protein, it will be interesting to delve deeper into this aspect in future experiments.
Our data show that TDP-43 overexpression induces astrogliosis but not microgliosis in the PFC and stratum lacunosum-moleculare. This may be due to the different roles of these cell types. Astrocytes are highly specialized glial cells responsible for the trophic and metabolic support of neurons. They are involved in ionic homeostasis, regulation of cerebral blood flow and metabolism, modulation of synaptic activity through neurotransmitter uptake and recycling, and maintenance of the blood-brain barrier [38,39,40]. In contrast, microglia coordinate the removal of synaptic connections, maintenance of brain homeostasis through the regulation of neuronal function, and clearance of protein aggregates throughout life [41,42,43]. In this mouse model, we observed not only an increase in the number of astrocytes but also changes in their morphology and in the expression of astrocytic glutamate transporters, GLAST-1, indicating that the overexpression of TDP-43 induces dysfunctional astrocytes. It has been described that astrocytes exhibit vulnerability to the TDP-43 protein. On the one hand, the dysregulation of TDP-43 in astrocytes leads to cognitive impairment [44], and on the other hand, the loss of TDP-43 in astrocytes results in changes in status of microglia, astrocytes, and oligodendrocytes [45]. These findings suggest different responses between astrocytes and neurons in relation to the TDP-43 protein [46]. This is very important in relation to NRF2 signaling, as it has been reported that astrocytes exhibit poor mitochondrial respiration along with high ROS production, while neurons display high mitochondrial respiration and low ROS production [47,48]. Consequently, it is plausible that the NRF2 signaling pathway is predominantly involved in astrocytes and microglia, and its disruption is linked to changes in the neuroinflammatory process [9,13,49]. However, there is ongoing debate in the field regarding whether NRF2 activation is exclusive to glial cells or if neurons may also participate [50]. Furthermore, our data indicate that DMF treatment significantly reduces astrogliosis in the PFC and stratum lacunosum-moleculare, possibly by enhancing the expression of anti-inflammatory genes such as SPHK2, providing further evidence of the neuroprotective effect of this compound against TDP-43-dependent FTD. Regarding microgliosis, we did not observe any changes associated with the overexpression of TDP-43. It is important to mention that these results have been obtained in males, and the same experiment needs to be conducted in females to determine the effectiveness of DMF treatment regardless of sex in order to extrapolate it to patients.
Regarding oxidative stress/ROS and its implication in TDP-43-dependent FTD, there are no studies identifying a direct relationship, although it is speculated that there may be an increase, similar to what is observed in TAU-dependent FTD [9,10,51]. Therefore, it would be valuable to conduct further studies that delve into this aspect.
Although the NRF2 pathway is one of the main mechanisms of DMF action, this compound also has NRF2-independent effects. For instance, DMF has been described as an inhibitor of GASDERMIN D [52,53], which is involved in the pyroptosis process associated with microglia [54]. The modulation of GASDERMIN D may mediate some of the anti-inflammatory effects of DMF. Unpublished results from our group indicate that pyroptosis is not induced in this TDP-43 overexpression model, suggesting that the anti-inflammatory effect we observe is not due to the inhibition of this pathway.
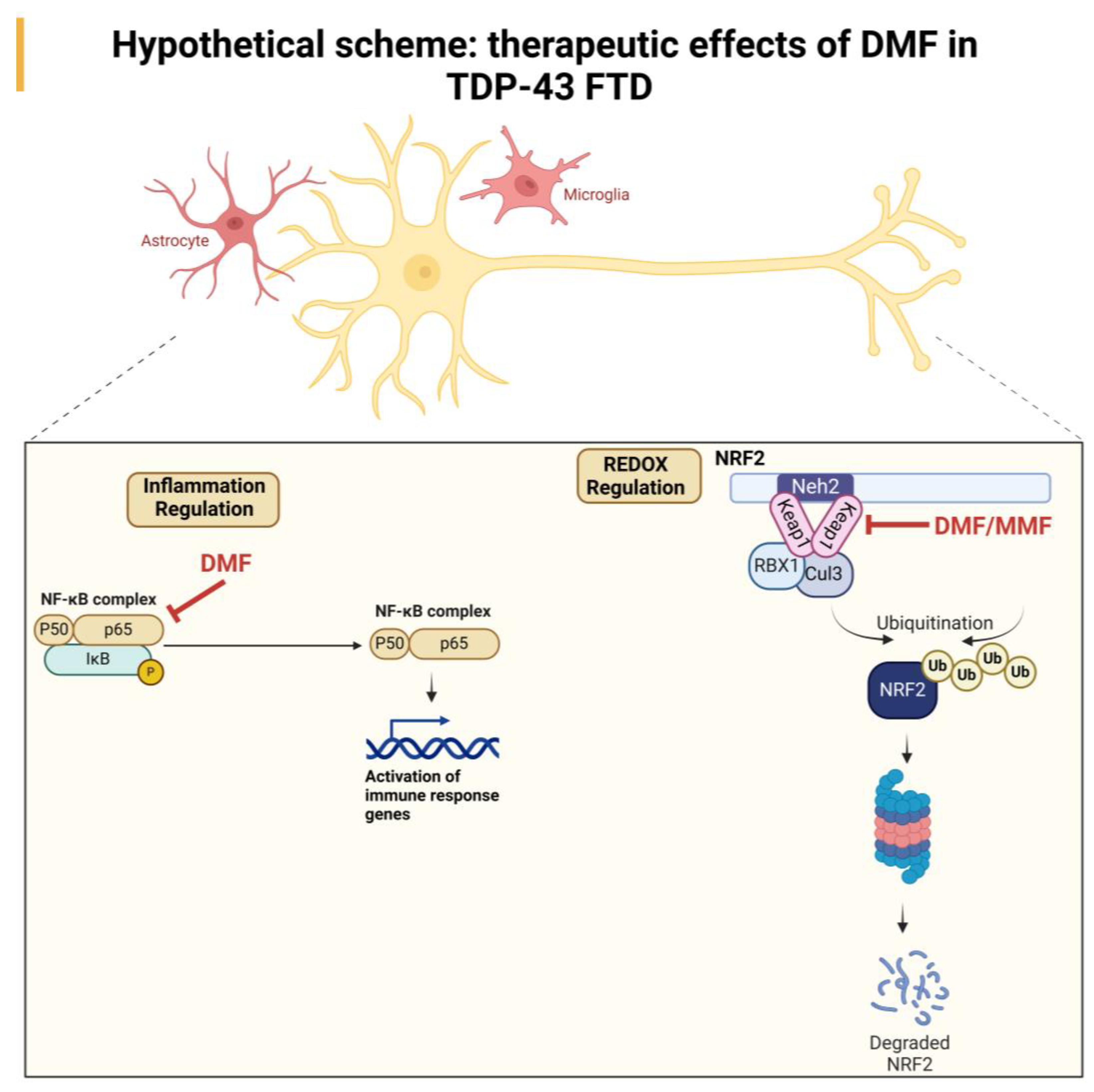
All these data indicate that the neuroprotective effect of DMF treatment may be due to a combination of antioxidant and anti-inflammatory effects mediated by the direct action of DMF or its metabolite MMF. A hypothetical scheme that shows the ROS/antioxidant/anti-inflammatory effect in the therapeutic effect of DMF is included in Figure 6.
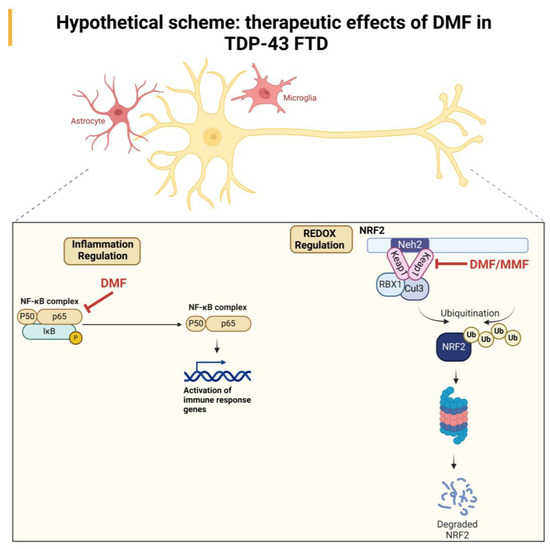
Figure 6.
Hypothetical scheme of the neuroprotective effect of DMF treatment in a TDP-43-dependent FTD model. Our results suggest an NRF2-dependent effect mediated by the actions of DMF and MMF at the antioxidant level and an anti-inflammatory effect, which may be primarily mediated by DMF.
Another aspect that must also be considered is the possibility that DMF treatment may modulate the expression or localization of the TDP-43 protein. Preliminary studies (which we have not yet published) suggest that DMF treatment does not influence the phosphorylation levels of TDP-43, but we cannot rule out that the treatment may modulate the expression or localization of TDP-43 or other proteins upstream or downstream of the signaling pathways in which TDP-43 is involved. This point could be of vital importance for the clinical relevance of DMF treatment for FTD. This will need to be analyzed in future experiments.
However, DMF treatment has several side-effects in humans. It has been reported that the capsule formulation caused adverse reactions such as flushing and gastrointestinal (GI) issues. The GI events included symptoms such as abdominal pain (both general and upper), diarrhea, and nausea. For the tablet formulation, the most frequently reported adverse reactions were GI events, followed by flushing and lymphopenia [55]. Therefore, other DMF derivatives, such as diroximel fumarate (Vumerity), and other NRF2 activators, such as omaveloxolone, which are currently being used in clinical practice, are now being analyzed [56,57,58].
5. Conclusions
This research indicates that DMF therapy mitigates cognitive decline, neurodegeneration, and excessive astrocyte activation in the PFC and stratum lacunosum-moleculare caused by TDP-43 overexpression in a mouse model of FTD. However, further studies are needed to fully elucidate the mechanisms underlying these beneficial effects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox13091072/s1, Figure S1: Induction of the NRF2 pathway by DMF in the hippocampus; Figure S2: CALBINDIN-D28K levels in the CA1 and CA2 regions of the hippocampus; Figure S3: CALBINDIN-D28K levels in the CA3 region of the hippocampus; Figure S4: Analysis of microgliosis and astrogliosis in the CA2-CA3 and dentate gyrus of the hippocampus.
Author Contributions
Conceptualization, I.L.-B., J.F.-R. and E.D.L.; methodology, I.S.-L., A.B.-A., R.M.-B., C.R.-C. and I.L.-B.; validation, I.S.-L., A.B.-A., R.M.-B., C.R.-C. and I.L.-B.; formal analysis, I.S.-L., A.B.-A., R.M.-B., C.R.-C. and I.L.-B.; resources, I.L.-B., J.F.-R. and E.D.L.; writing—original draft preparation, I.L.-B.; writing—review and editing, all the authors; supervision, I.L.-B., J.F.-R. and E.D.L.; project administration, I.L.-B., J.F.-R. and E.D.L.; funding acquisition, I.L.-B., J.F.-R. and E.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported MINECO (PID2019-105600RB-I00 and PID2022-137065OB-I00 to I.L.-B. and PID2021-128906OB-I00 to E.D.L./J.F.-R.) and ISCiii CIBERNED (CB06/05/0089 to I.L.-B./E.D.L./J.F.-R.).
Institutional Review Board Statement
The animal study protocol was approved by the ethical committes of our University (Complutense University of Madrid) and the regulatory institution (protocol code PROEX 201.8/22 and date of approval 18 November 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors want to acknowledge the laboratory of “Microscopía Confocal (SIdI-UAM)” and the “Instituto de Investigaciones Biomédicas Sols-Morreale” Confocal service, for their help with the Confocal microscope.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leroy, M.; Bertoux, M.; Skrobala, E.; Mode, E.; Adnet-Bonte, C.; Le Ber, I.; Bombois, S.; Cassagnaud, P.; Chen, Y.; Deramecourt, V.; et al. Characteristics and progression of patients with frontotemporal dementia in a regional memory clinic network. Alzheimer’s Res. Ther. 2021, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; Seeley, W.W.; Boxer, A.L.; Hillis, A.E.; Knopman, D.S.; Ljubenov, P.A.; Miller, B.; Piguet, O.; Rademakers, R.; Whitwell, J.L.; et al. Frontotemporal lobar degeneration. Nat. Rev. Dis. Primers 2023, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.I.; Padmanabhan, B.; Kobayashi, A.; Shang, C.; Hirotsu, Y.; Yokoyama, S.; Yamamoto, M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol. Cell. Biol. 2007, 27, 7511–7521. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes. Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Cuadrado, A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 2015, 88, 147–157. [Google Scholar] [CrossRef]
- Hayes, J.D.; Chowdhry, S.; Dinkova-Kostova, A.T.; Sutherland, C. Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of β-TrCP and GSK-3. Biochem. Soc. Trans. 2015, 43, 611–620. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Innamorato, N.G.; Jaworski, T.; Rabano, A.; Kugler, S.; Van Leuven, F.; Cuadrado, A. Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain 2014, 137, 78–91. [Google Scholar] [CrossRef]
- Cuadrado, A.; Kugler, S.; Lastres-Becker, I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018, 14, 522–534. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; de Lago, E.; Martínez, A.; Fernández-Ruiz, J. New Statement about NRF2 in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Biomolecules 2022, 12, 1200. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.J.; Yang, C.H.; Fang, Y.H.; Cho, K.H.; Chien, W.L.; Wang, W.T.; Wu, T.W.; Lin, C.P.; Fu, W.M.; Shen, C.K. Elevated expression of TDP-43 in the forebrain of mice is sufficient to cause neurological and pathological phenotypes mimicking FTLD-U. J. Exp. Med. 2010, 207, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Garcia-Yague, A.J.; Scannevin, R.H.; Casarejos, M.J.; Kugler, S.; Rabano, A.; Cuadrado, A. Repurposing the NRF2 Activator Dimethyl Fumarate as Therapy Against Synucleinopathy in Parkinson’s Disease. Antioxid. Redox Signal. 2016, 25, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Santos-García, I.; Rodríguez-Cueto, C.; Villegas, P.; Piscitelli, F.; Lauritano, A.; Shen, C.J.; Di Marzo, V.; Fernández-Ruiz, J.; de Lago, E. Preclinical investigation in FAAH inhibition as a neuroprotective therapy for frontotemporal dementia using TDP-43 transgenic male mice. J. Neuroinflamm. 2023, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018, 175, 987–993. [Google Scholar] [CrossRef]
- Galán-Ganga, M.; Rodríguez-Cueto, C.; Merchán-Rubira, J.; Hernández, F.; Ávila, J.; Posada-Ayala, M.; Lanciego, J.L.; Luengo, E.; Lopez, M.G.; Rábano, A.; et al. Cannabinoid receptor CB2 ablation protects against TAU induced neurodegeneration. Acta Neuropathol. Commun. 2021, 9, 90. [Google Scholar] [CrossRef]
- Gundersen, H.J.; Jensen, E.B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987, 147, 229–263. [Google Scholar] [CrossRef]
- Gonzalo-Consuegra, C.; Santos-García, I.; García-Toscano, L.; Martín-Baquero, R.; Rodríguez-Cueto, C.; Wittwer, M.B.; Dzygiel, P.; Grether, U.; de Lago, E.; Fernández-Ruiz, J. Involvement of CB(1) and CB(2) receptors in neuroprotective effects of cannabinoids in experimental TDP-43 related frontotemporal dementia using male mice. Biomed. Pharmacother. 2024, 174, 116473. [Google Scholar] [CrossRef]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef]
- Li, J.T.; Xie, X.M.; Yu, J.Y.; Sun, Y.X.; Liao, X.M.; Wang, X.X.; Su, Y.A.; Liu, Y.J.; Schmidt, M.V.; Wang, X.D.; et al. Suppressed Calbindin Levels in Hippocampal Excitatory Neurons Mediate Stress-Induced Memory Loss. Cell Rep. 2017, 21, 891–900. [Google Scholar] [CrossRef]
- Palop, J.J.; Jones, B.; Kekonius, L.; Chin, J.; Yu, G.Q.; Raber, J.; Masliah, E.; Mucke, L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc. Natl. Acad. Sci. USA 2003, 100, 9572–9577. [Google Scholar] [CrossRef]
- Bright, F.; Werry, E.L.; Dobson-Stone, C.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kassiou, M.; et al. Neuroinflammation in frontotemporal dementia. Nat. Rev. Neurol. 2019, 15, 540–555. [Google Scholar] [CrossRef]
- Lemprière, S. Neuroinflammation predicts cognitive decline in FTD. Nat. Rev. Neurol. 2023, 19, 258. [Google Scholar] [CrossRef]
- Tagliatti, E.; Desiato, G.; Mancinelli, S.; Bizzotto, M.; Gagliani, M.C.; Faggiani, E.; Hernández-Soto, R.; Cugurra, A.; Poliseno, P.; Miotto, M.; et al. Trem2 expression in microglia is required to maintain normal neuronal bioenergetics during development. Immunity 2024, 57, 86–105.e109. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.; Wang, H.; Yang, K.; Luan, J.; Wang, S. TREM2: Potential therapeutic targeting of microglia for Alzheimer’s disease. Biomed. Pharmacother. 2023, 165, 115218. [Google Scholar] [CrossRef] [PubMed]
- Achicallende, S.; Bonilla-Del Río, I.; Serrano, M.; Mimenza, A.; Lekunberri, L.; Anaut-Lusar, I.; Puente, N.; Gerrikagoitia, I.; Grandes, P. GLAST versus GFAP as astroglial marker for the subcellular study of cannabinoid CB(1) receptors in astrocytes. Histochem. Cell Biol. 2022, 158, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Perego, C.; Vanoni, C.; Bossi, M.; Massari, S.; Basudev, H.; Longhi, R.; Pietrini, G. The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. J. Neurochem. 2000, 75, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Weigert, A.; von Knethen, A.; Thomas, D.; Faria, I.; Namgaladze, D.; Zezina, E.; Fuhrmann, D.; Petcherski, A.; Heringdorf, D.M.Z.; Radeke, H.H.; et al. Sphingosine kinase 2 is a negative regulator of inflammatory macrophage activation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1235–1246. [Google Scholar] [CrossRef]
- Standoli, S.; Rapino, C.; Di Meo, C.; Rudowski, A.; Kämpfer-Kolb, N.; Volk, L.M.; Thomas, D.; Trautmann, S.; Schreiber, Y.; Meyer Zu Heringdorf, D.; et al. Sphingosine Kinases at the Intersection of Pro-Inflammatory LPS and Anti-Inflammatory Endocannabinoid Signaling in BV2 Mouse Microglia Cells. Int. J. Mol. Sci. 2023, 24, 8508. [Google Scholar] [CrossRef]
- Liu, H.; Sugiura, M.; Nava, V.E.; Edsall, L.C.; Kono, K.; Poulton, S.; Milstien, S.; Kohama, T.; Spiegel, S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 2000, 275, 19513–19520. [Google Scholar] [CrossRef]
- Aguilera-Portillo, G.; Rangel-López, E.; Villeda-Hernández, J.; Chavarría, A.; Castellanos, P.; Elmazoglu, Z.; Karasu, Ç.; Túnez, I.; Pedraza, G.; Königsberg, M.; et al. The Pharmacological Inhibition of Fatty Acid Amide Hydrolase Prevents Excitotoxic Damage in the Rat Striatum: Possible Involvement of CB1 Receptors Regulation. Mol. Neurobiol. 2019, 56, 844–856. [Google Scholar] [CrossRef]
- Majkutewicz, I. Dimethyl fumarate: A review of preclinical efficacy in models of neurodegenerative diseases. Eur. J. Pharmacol. 2022, 926, 175025. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Ak, M.; Mrowietz, U. Reactivity of dimethyl fumarate and methylhydrogen fumarate towards glutathione and N-acetyl-l-cysteine—Preparation of S-substituted thiosuccinic acid esters. Bioorganic Med. Chem. 2007, 15, 333–342. [Google Scholar] [CrossRef]
- Piroli, G.G.; Manuel, A.M.; Patel, T.; Walla, M.D.; Shi, L.; Lanci, S.A.; Wang, J.; Galloway, A.; Ortinski, P.I.; Smith, D.S.; et al. Identification of Novel Protein Targets of Dimethyl Fumarate Modification in Neurons and Astrocytes Reveals Actions Independent of Nrf2 Stabilization*[S]. Mol. Cell. Proteom. 2019, 18, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, I.; Siklos, M.I.; Calderon-Gierszal, E.L.; El-Shennawy, L.; Georgieva, G.; Thayer, E.N.; Thatcher, G.R.J.; Frasor, J. Dimethyl Fumarate Inhibits the Nuclear Factor κB Pathway in Breast Cancer Cells by Covalent Modification of p65 Protein*. J. Biol. Chem. 2016, 291, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Gillard, G.O.; Collette, B.; Anderson, J.; Chao, J.; Scannevin, R.H.; Huss, D.J.; Fontenot, J.D. DMF, but not other fumarates, inhibits NF-κB activity in vitro in an Nrf2-independent manner. J. Neuroimmunol. 2015, 283, 74–85. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Panda, A.C.; Huynh, P.; Peaden, R.; Navas-Enamorado, I.; Bastian, P.; Lehrmann, E.; Diaz-Ruiz, A.; Ross, D.; et al. Novel RNA-binding activity of NQO1 promotes SERPINA1 mRNA translation. Free Radic. Biol. Med. 2016, 99, 225–233. [Google Scholar] [CrossRef]
- Allen, N.J.; Barres, B.A. Glia—More than just brain glue. Nature 2009, 457, 675–677. [Google Scholar] [CrossRef]
- Lee, H.-G.; Wheeler, M.A.; Quintana, F.J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef]
- Gradisnik, L.; Velnar, T. Astrocytes in the central nervous system and their functions in health and disease: A review. World J. Clin. Cases 2023, 11, 3385–3394. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Lopes-Araújo, A.; Santos-Sacramento, L.; Takeda, P.Y.; Anthony, D.C.; Malva, J.O.; Crespo-Lopez, M.E. What Do Microglia Really Do in Healthy Adult Brain? Cells 2019, 8, 1293. [Google Scholar] [CrossRef] [PubMed]
- Licht-Murava, A.; Meadows, S.M.; Palaguachi, F.; Song, S.C.; Jackvony, S.; Bram, Y.; Zhou, C.; Schwartz, R.E.; Froemke, R.C.; Orr, A.L.; et al. Astrocytic TDP-43 dysregulation impairs memory by modulating antiviral pathways and interferon-inducible chemokines. Sci. Adv. 2023, 9, eade1282. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.Y.T.; Agrawal, I.; Ho, W.Y.; Yen, Y.C.; Pinter, A.J.; Liu, J.; Phua, Q.X.C.; Koh, K.B.; Chang, J.C.; Sanford, E.; et al. Loss of TDP-43 in astrocytes leads to motor deficits by triggering A1-like reactive phenotype and triglial dysfunction. Proc. Natl. Acad. Sci. USA 2020, 117, 29101–29112. [Google Scholar] [CrossRef]
- Smethurst, P.; Risse, E.; Tyzack, G.E.; Mitchell, J.S.; Taha, D.M.; Chen, Y.R.; Newcombe, J.; Collinge, J.; Sidle, K.; Patani, R. Distinct responses of neurons and astrocytes to TDP-43 proteinopathy in amyotrophic lateral sclerosis. Brain 2020, 143, 430–440. [Google Scholar] [CrossRef]
- Lopez-Fabuel, I.; Le Douce, J.; Logan, A.; James, A.M.; Bonvento, G.; Murphy, M.P.; Almeida, A.; Bolaños, J.P. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc. Natl. Acad. Sci. USA 2016, 113, 13063–13068. [Google Scholar] [CrossRef]
- Vicente-Gutierrez, C.; Bonora, N.; Jimenez-Blasco, D.; Lopez-Fabuel, I.; Bates, G.; Murphy, M.P.; Almeida, A.; Bolaños, J.P. Abrogating mitochondrial ROS in neurons or astrocytes reveals cell-specific impact on mouse behaviour. Redox Biol. 2021, 41, 101917. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Ulusoy, A.; Innamorato, N.G.; Sahin, G.; Rábano, A.; Kirik, D.; Cuadrado, A. α-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson’s disease. Hum. Mol. Genet. 2012, 21, 3173–3192. [Google Scholar] [CrossRef]
- Liddell, J.R. Are Astrocytes the Predominant Cell Type for Activation of Nrf2 in Aging and Neurodegeneration? Antioxidants 2017, 6, 65. [Google Scholar] [CrossRef]
- Esteras, N.; Kopach, O.; Maiolino, M.; Lariccia, V.; Amoroso, S.; Qamar, S.; Wray, S.; Rusakov, D.A.; Jaganjac, M.; Abramov, A.Y. Mitochondrial ROS control neuronal excitability and cell fate in frontotemporal dementia. Alzheimers Dement. 2022, 18, 318–338. [Google Scholar] [CrossRef] [PubMed]
- Bandharam, N.; Lockey, R.F.; Kolliputi, N. Pyroptosis Inhibition in Disease Treatment: Opportunities and Challenges. Cell Biochem. Biophys. 2023, 81, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Abbott, D.W. Chemical modulation of gasdermin D activity: Therapeutic implications and consequences. Semin. Immunol. 2023, 70, 101845. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wan, T.; Gao, X.; Fu, M.; Duan, Y.; Shen, X.; Guo, W. Microglia Pyroptosis: A Candidate Target for Neurological Diseases Treatment. Front. Neurosci. 2022, 16, 922331. [Google Scholar] [CrossRef] [PubMed]
- Dimethyl Fumarate. Drugs and Lactation Database (LactMed®); National Institute of Child Health and Human Development: Bethesda, MD, USA, 2006.
- Amoroso, R.; Maccallini, C.; Bellezza, I. Activators of Nrf2 to Counteract Neurodegenerative Diseases. Antioxidants 2023, 12, 778. [Google Scholar] [CrossRef]
- Gunther, K.; Lynch, D.R. Pharmacotherapeutic strategies for Friedreich Ataxia: A review of the available data. Expert. Opin. Pharmacother. 2024, 25, 529–539. [Google Scholar] [CrossRef]
- Hynes, S.M.; Goldsberry, A.; Henneghan, P.D.; Murai, M.; Shinde, A.; Wells, J.A.; Wu, L.; Wu, T.; Zahir, H.; Khan, S. Relative Bioavailability of Omaveloxolone When Capsules Are Sprinkled Over and Mixed in Applesauce Compared With Administration as Intact Omaveloxolone Capsules: A Phase 1, Randomized, Open-Label, Single-Dose, Crossover Study in Healthy Adults. J. Clin. Pharmacol. 2024. Early View. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).