Dietary Organic Zinc Supplementation Modifies the Oxidative Genes via RORγ and Epigenetic Regulations in the Ileum of Broiler Chickens Exposed to High-Temperature Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Birds, Husbandry, and Diet
2.2. Antioxidant Indexes
2.3. ROS Levels Assay
2.4. Hepatic Complexes I, III, and V Activities and ATP Content Assay
2.5. Intestinal Morphometry
2.6. Total RNA Isolation and Real-Time qPCR
2.7. Western Blotting Analysis
2.8. ChIP-qPCR Measurement
2.9. Statistical Analysis
3. Results
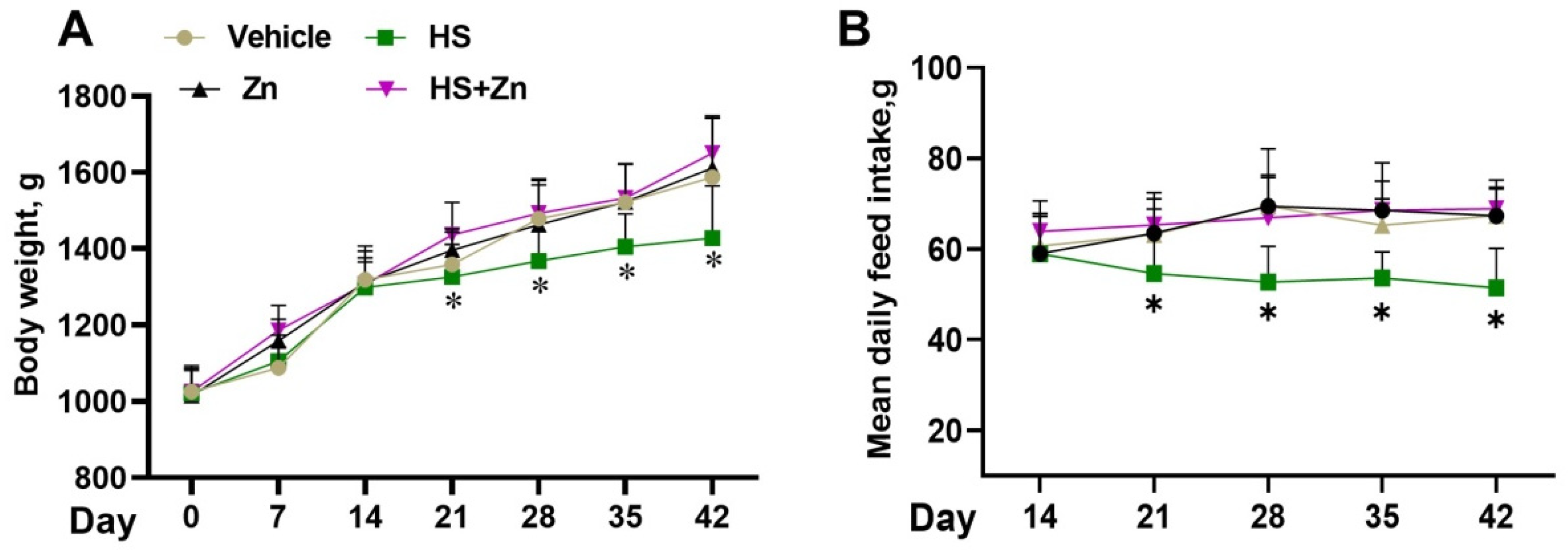
3.1. Growth Performance
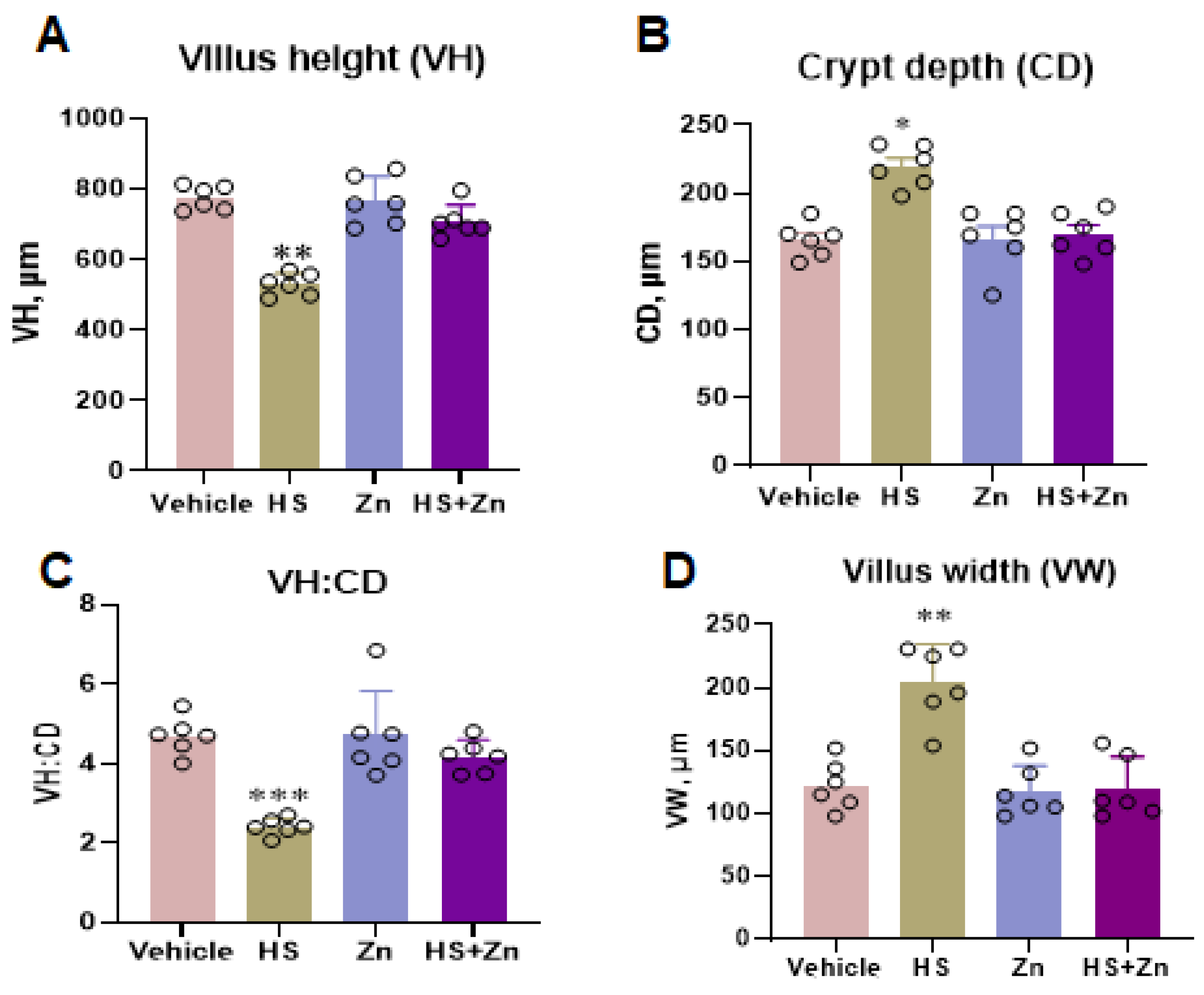
3.2. Intestinal Morphology
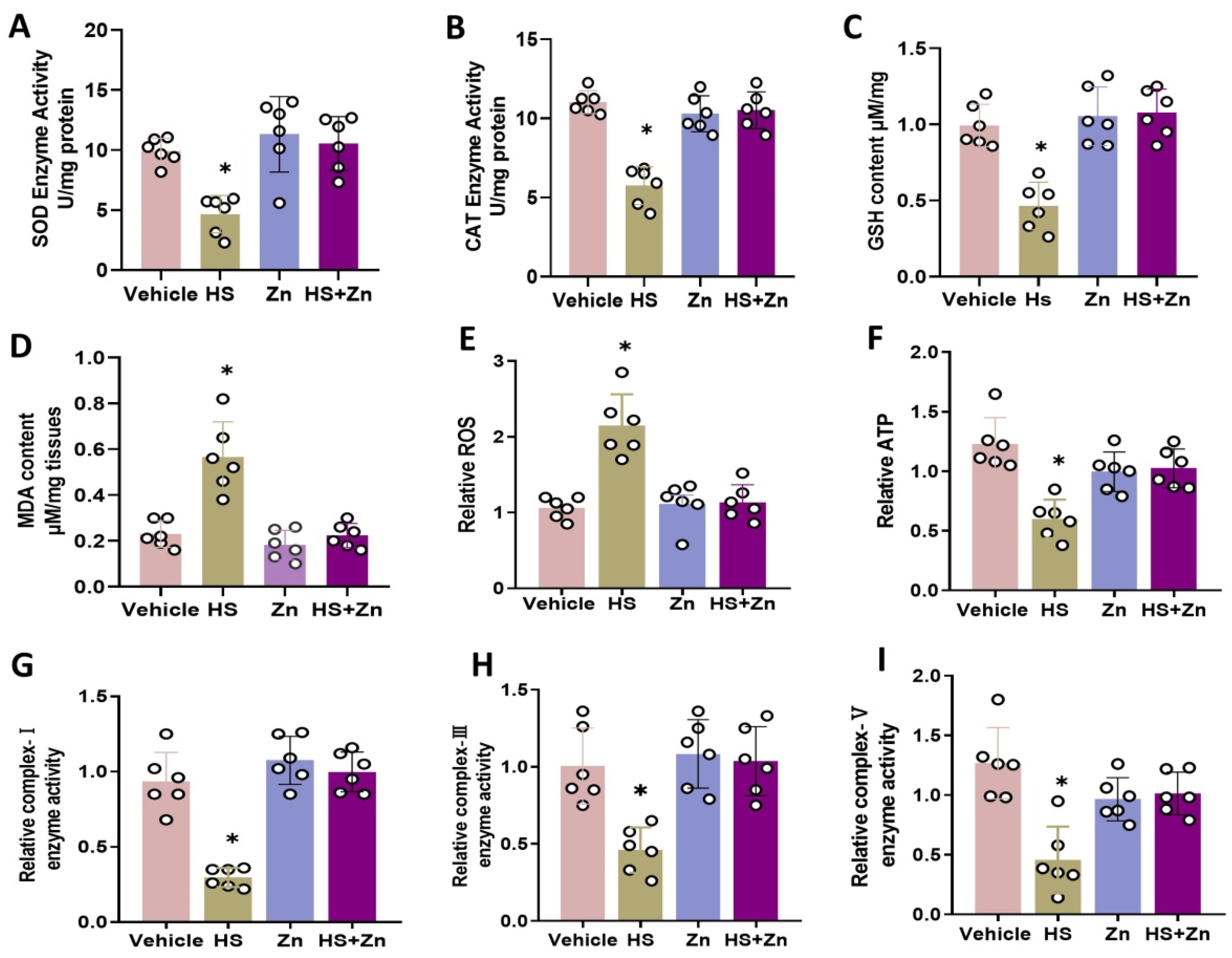
3.3. Oxidative Stress in the Ileal of Broiler Chickens
3.4. Ileal Gene Expression
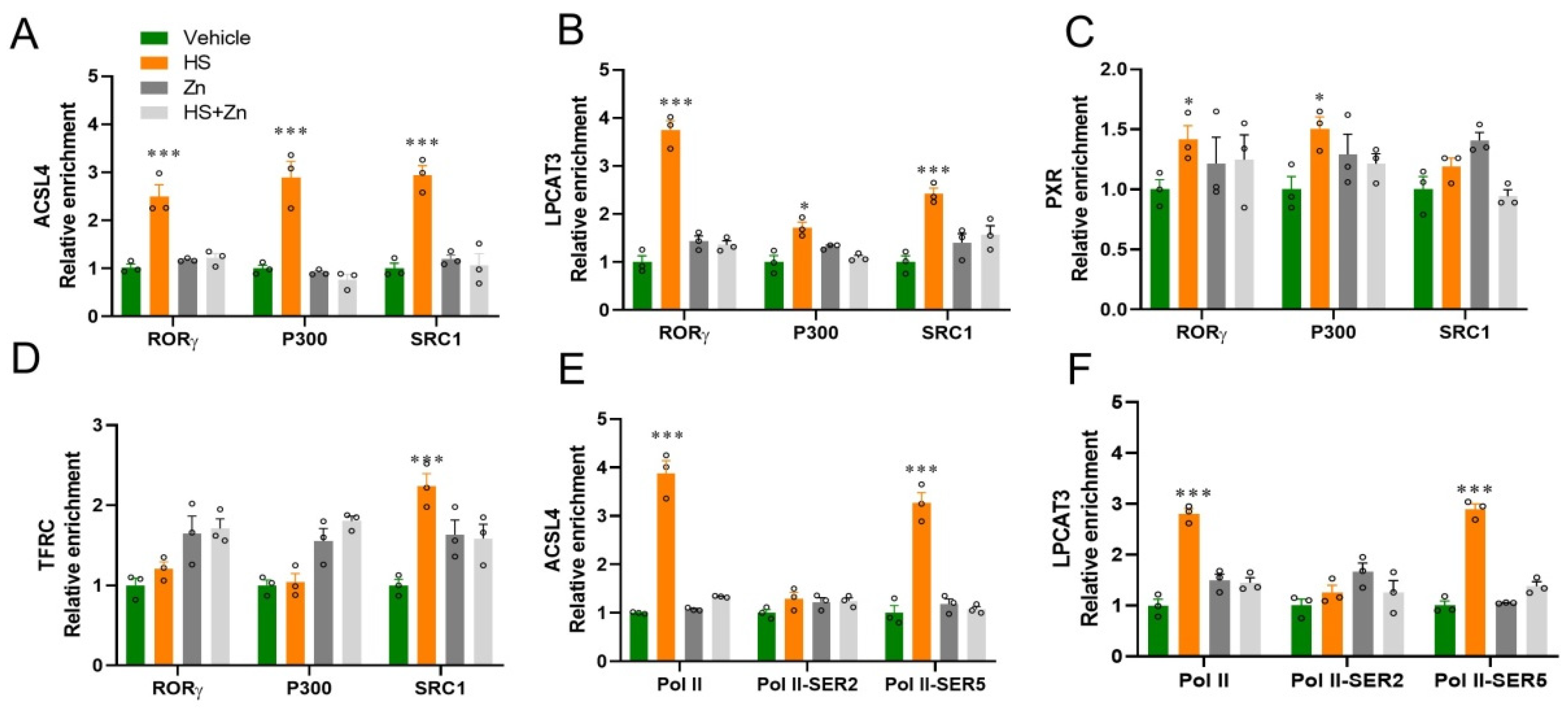
3.5. Histone Modifications Facilitate the Transcriptional Suppression of Antioxidants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, J. Heat stress: Impact on livestock well-being and productivity and mitigation strategies to alleviate the negative effects. Anim. Prod. Sci. 2018, 58, 1404–1413. [Google Scholar] [CrossRef]
- Cheng, M.; McCarl, B.; Fei, C. Climate Change and Livestock Production: A Literature Review. Atmosphere 2022, 13, 140. [Google Scholar] [CrossRef]
- Tang, L.P.; Liu, Y.L.; Zhang, J.X.; Ding, K.N.; Lu, M.H.; He, Y.M. Heat stress in broilers of liver injury effects of heat stress on oxidative stress and autophagy in liver of broilers. Poult. Sci. 2022, 101, 102085. [Google Scholar] [CrossRef]
- Hu, P.; Li, K.; Peng, X.; Yao, T.; Zhu, C.; Gu, H.; Liu, H.-Y.; Sun, M.-A.; Hu, Y.; Ennab, W.; et al. Zinc intake ameliorates intestinal morphology and oxidative stress of broiler chickens under heat stress. Front. Nutr. 2024, 14, 1308907. [Google Scholar] [CrossRef]
- Saracila, M.; Panaite, T.D.; Mironeasa, S.; Untea, A.E. Dietary Supplementation of Some Antioxidants as Attenuators of Heat Stress on Chicken Meat Characteristics. Agriculture 2021, 11, 638. [Google Scholar] [CrossRef]
- Nawaz, A.H.; Wang, F.; Jiao, Z.; Zhang, W.; Zheng, J.H.; Sun, J.; Zhu, Z.; Lin, S.; Zhang, L.; Lin, J. Differential responses to heat stress in normal and dwarf chickens: Implications for meat quality and immune function. Annal. Anim. Sci. 2024, 0. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Xing, T.; Li, J.; Zhang, L.; Jiang, Y.; Gao, F. Transcriptome analysis reveals the mechanism of chronic heat stress on meat quality of broilers. J. Anim. Sci. Biot. 2022, 13, 110. [Google Scholar] [CrossRef]
- Baumgard, L.; Rhoads, R. 268 Heat Stress Impacts on Cattle Production. J. Anim. Sci. 2023, 101, 60–61. [Google Scholar] [CrossRef]
- Shehata, A.M.; Saadeldin, I.M.; Tukur, H.A.; Habashy, W.S. Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress. Animals 2020, 10, 2407. [Google Scholar] [CrossRef]
- Song, J.; Lei, X.; Luo, J.; Everaert, N.; Zhao, G.; Wen, J.; Yang, Y. The effect of Epigallocatechin-3-gallate on small intestinal morphology, antioxidant capacity and anti-inflammatory effect in heat-stressed broilers. Anim. Physiol. Anim. Nutr. 2019, 103, 1030–1038. [Google Scholar] [CrossRef]
- Wasti, S.; Sah, N.; Mishra, B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef]
- Cheng, K.; Zhang, M.; Huang, X.; Zheng, X.; Song, Z.; Zhang, L.; Wang, T. An evaluation of natural and synthetic vitamin E supplementation on growth performance and antioxidant capacity of broilers in early age. Can. J. Anim. Sci. 2017, 98, 187–193. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Oladokun, S.; Adewole, D.I. Biomarkers of heat stress and mechanism of heat stress response in Avian species: Current insights and future perspectives from poultry science. J. Therm. Biol. 2022, 110, 103332. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M.; Toyomizu, M. Mechanisms underlying the Effects of Heat Stress on Intestinal Integrity, Inflammation, and Microbiota in Chickens. J. Poult. Sci. 2023, 60, 2023021. [Google Scholar] [CrossRef]
- Gouda, A.; Tolba, S.; Mahrose, K.; Felemban, S.G.; Khafaga, A.F.; Khalifa, N.E.; Jaremko, M.; Moustafa, M.; Alshaharni, M.O.; Algopish, U.; et al. Heat shock proteins as a key defense mechanism in poultry production under heat stress conditions. Poult. Sci. 2024, 103, 103537. [Google Scholar] [CrossRef]
- Wasti, S.; Sah, N.; Singh, A.K.; Lee, C.N.; Jha, R.; Mishra, B. Dietary supplementation of dried plum: A novel strategy to mitigate heat stress in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Liang, R.R.; Lin, H.; Zhu, L.X.; Zhang, Y.M.; Mao, Y.W.; Dong, P.C.; Niu, L.B.; Zhang, M.H.; Luo, X. Effect of acute heat stress and slaughter processing on poultry meat quality and postmortem carbohydrate metabolism. Poult. Sci. 2017, 96, 738–746. [Google Scholar] [CrossRef]
- Tallentire, C.W.; Leinonen, I.; Kyriazakis, I. Breeding for efficiency in the broiler chicken: A review. Agron. Sustain. Dev. 2016, 36, 66. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Physiological alterations of poultry to the high environmental temperature. J. Therm. Biol. 2018, 76, 101–106. [Google Scholar] [CrossRef]
- Hu, R.; He, Y.; Arowolo, M.A.; Wu, S.; He, J. Polyphenols as Potential Attenuators of Heat Stress in Poultry Production. Antioxidants 2019, 8, 67. [Google Scholar] [CrossRef]
- Jin, J.; Xue, M.; Tang, Y.; Zhang, L.; Hu, P.; Hu, Y.; Cai, D.; Luo, X.; Sun, M.-a. Effects of Zinc Source and Level on the Intestinal Immunity of Xueshan Chickens under Heat Stress. Animals 2023, 13, 3025. [Google Scholar] [CrossRef]
- Sahin, K.; Sahin, N.; Kucuk, O.; Hayirli, A.; Prasad, A. Role of dietary zinc in heat-stressed poultry: A review. Poult. Sci. 2009, 88, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Muniyappan, M.; Chen, N.; Liu, Y.; Kim, I.H. The Effect of Dietary Glucose Oxidase Supplementation on Production Performance, Egg Quality and Nutrient Digestibility in Laying Hens. Braz. J. Poult. Sci. 2022, 24, 001–008. [Google Scholar] [CrossRef]
- Goff, J.P. Invited review: Mineral absorption mechanisms, mineral interactions that affect acid–base and antioxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef]
- Skibsted, L.H. Mineral nutrient interaction: Improving bioavailability of calcium and iron. Food Sci. Biotechnol. 2016, 25, 1233–1241. [Google Scholar] [CrossRef]
- Corte-Real, J.; Bohn, T. Interaction of divalent minerals with liposoluble nutrients and phytochemicals during digestion and influences on their bioavailability—A review. Food Chem. 2018, 252, 285–293. [Google Scholar] [CrossRef]
- Rakhra, G.; Masih, D.; Vats, A.; Vijay, A.; Ashraf, M.Z.; Singh, S.N. Study of Metal-Metal Interactions and Their Biomarkers Using an Intestinal Human Cell Line. Biol. Trace Elem. Res. 2020, 195, 95–104. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Li, X.; Zhao, D.; Qin, S.; Shi, Z.; Wang, Z. Dietary zinc supplementation in breeding pigeons improves the carcass traits of squabs through regulating antioxidant capacity and myogenic regulatory factor expression. Poult. Sci 2023, 102, 102809. [Google Scholar] [CrossRef]
- Chand, N.; Naz, S.; Khan, A.; Khan, S.; Khan, R.U. Performance traits and immune response of broiler chicks treated with zinc and ascorbic acid supplementation during cyclic heat stress. Int. J. Biom. 2014, 58, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, A.R.; Lean, I.J.; Stevenson, M.A.; Socha, M.T. Effects of feeding organic trace minerals on milk production and reproductive performance in lactating dairy cows: A meta-analysis. J. Dairy Sci. 2010, 93, 4239–4251. [Google Scholar] [CrossRef] [PubMed]
- Ringseis, R.; Eder, K. Heat stress in pigs and broilers: Role of gut dysbiosis in the impairment of the gut-liver axis and restoration of these effects by probiotics, prebiotics and synbiotics. J. Anim. Sci. Biotechnol. 2022, 13, 126. [Google Scholar] [CrossRef]
- Savaris, V.D.L.; Broch, J.; de Souza, C.; Rohloff Junior, N.; de Avila, A.S.; Polese, C.; Kaufmann, C.; de Oliveira Carvalho, P.L.; Pozza, P.C.; Vieites, F.M.; et al. Effects of vitamin A on carcass and meat quality of broilers. Poult. Sci. 2021, 100, 101490. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, H.; rahman, n.; Mohamad, R.; Zaidan, U.; Arshad, M.; Samsudin, A.A. Effects of dietary zinc oxide nanoparticles supplementation on broiler growth performance, zinc retention, liver health status, and gastrointestinal microbial load. J. Trace Elem. Miner. 2023, 4, 100072. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Wang, W.; Yang, L.; Zhu, Y. The Role of Zinc in Poultry Breeder and Hen Nutrition: An Update. Biol. Trace Elem. Res. 2019, 192, 308–318. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Desmet, S.J.; Thommis, J.; Vanderhaeghen, T.; Vandenboorn, E.M.F.; Clarisse, D.; Li, Y.; Timmermans, S.; Fijalkowska, D.; Ratman, D.; Van Hamme, E.; et al. Crosstalk interactions between transcription factors ERRα and PPARα assist PPARα-mediated gene expression. Mol. Metab. 2024, 84, 101938. [Google Scholar] [CrossRef]
- Zhong, J.; He, X.; Gao, X.; Liu, Q.; Zhao, Y.; Hong, Y.; Zhu, W.; Yan, J.; Li, Y.; Li, Y.; et al. Hyodeoxycholic acid ameliorates nonalcoholic fatty liver disease by inhibiting RAN-mediated PPARα nucleus-cytoplasm shuttling. Nat. Commun. 2023, 14, 5451. [Google Scholar] [CrossRef]
- Jin, L.; Martynowski, D.; Zheng, S.; Wada, T.; Xie, W.; Li, Y. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORgamma. Mol. Endocrinol. 2010, 24, 923–929. [Google Scholar] [CrossRef]
- Raichur, S.; Lau, P.; Staels, B.; Muscat, G.E.O. Retinoid-related orphan receptor γ regulates several genes that control metabolism in skeletal muscle cells: Links to modulation of reactive oxygen species production. J. Mol. Endocrinol. 2007, 39, 29–44. [Google Scholar] [CrossRef]
- Gu, H.; Hu, P.; Zhao, Y.; Liu, Y.; Wang, Y.T.; Ahmed, A.A.; Liu, H.Y.; Cai, D. Nuclear Receptor RORα/γ: Exciting Modulators in Metabolic Syndrome and Related Disorders. Front. Nutr. 2022, 9, 925267. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, W.; Li, C. Recent Advances in Genetic and Epigenetic Modulation of Animal Exposure to High Temperature. Front. Genet. 2020, 11, 653. [Google Scholar] [CrossRef]
- Ma, L.; Li, C.; Yin, H.; Huang, J.; Yu, S.; Zhao, J.; Tang, Y.; Yu, M.; Lin, J.; Ding, L.; et al. The Mechanism of DNA Methylation and miRNA in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 9360. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef]
- Marinova, Z.; Leng, Y.; Leeds, P.; Chuang, D.M. Histone deacetylase inhibition alters histone methylation associated with heat shock protein 70 promoter modifications in astrocytes and neurons. Neuropharmacology 2011, 60, 1109–1115. [Google Scholar] [CrossRef]
- Corso-Díaz, X.; Jaeger, C.; Chaitankar, V.; Swaroop, A. Epigenetic control of gene regulation during development and disease: A view from the retina. Prog. Retin. Eye Res. 2018, 65, 1–27. [Google Scholar]
- Klosin, A.; Casas, E.; Hidalgo-Carcedo, C.; Vavouri, T.; Lehner, B. Transgenerational transmission of environmental information in C. elegans. Science 2017, 356, 320–323. [Google Scholar] [CrossRef]
- Zheng, H.-T.; Zhuang, Z.-X.; Chen, C.-J.; Liao, H.-Y.; Chen, H.-L.; Hsueh, H.-C.; Chen, C.-F.; Chen, S.-E.; Huang, S.-Y. Effects of acute heat stress on protein expression and histone modification in the adrenal gland of male layer-type country chickens. Sci. Rep. 2021, 11, 6499. [Google Scholar] [CrossRef]
- Xiao, C.; Kong, L.; Pan, X.; Zhu, Q.; Song, Z.; Everaert, N. High Temperature-Induced Oxidative Stress Affects Systemic Zinc Homeostasis in Broilers by Regulating Zinc Transporters and Metallothionein in the Liver and Jejunum. Oxid. Med. Cell. Long. 2022, 2022, 1427335. [Google Scholar] [CrossRef]
- Gu, H.; Liu, Y.; Zhao, Y.; Qu, H.; Li, Y.; Ahmed, A.A.; Liu, H.Y.; Hu, P.; Cai, D. Hepatic Anti-Oxidative Genes CAT and GPX4 Are Epigenetically Modulated by RORγ/NRF2 in Alphacoronavirus-Exposed Piglets. Antioxidants 2023, 12, 1305. [Google Scholar] [CrossRef]
- Liu, H.Y.; Gu, H.; Li, Y.; Hu, P.; Yang, Y.; Li, K.; Li, H.; Zhang, K.; Zhou, B.; Wu, H.; et al. Dietary Conjugated Linoleic Acid Modulates the Hepatic Circadian Clock Program via PPARα/REV-ERBα-Mediated Chromatin Modification in Mice. Front. Nutr. 2021, 8, 711398. [Google Scholar] [CrossRef]
- Li, K.; Li, H.; Zhang, K.; Zhang, J.; Hu, P.; Li, Y.; Gu, H.; Liu, H.Y.; Yang, Z.; Cai, D. Orphan Nuclear Receptor RORγ Modulates the Genome-Wide Binding of the Cholesterol Metabolic Genes during Mycotoxin-Induced Liver Injury. Nutrients 2021, 13, 2539. [Google Scholar] [CrossRef] [PubMed]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of Feeding Different Postbiotics Produced by Lactobacillus plantarum on Growth Performance, Carcass Yield, Intestinal Morphology, Gut Microbiota Composition, Immune Status, and Growth Gene Expression in Broilers under Heat Stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Abuajamieh, M.; Abdelqader, A.; Irshaid, R.; Hayajneh, F.M.F.; Al-Khaza’leh, J.M.; Al-Fataftah, A.R. Effects of organic zinc on the performance and gut integrity of broilers under heat stress conditions. Arch. Anim. Breed. 2020, 63, 125–135. [Google Scholar] [CrossRef]
- Mack, L.A.; Felver-Gant, J.N.; Dennis, R.L.; Cheng, H.W. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult. Sci. 2013, 92, 285–294. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef]
- Chaudhary, A.; Mishra, P.; Amaz, S.A.; Mahato, P.L.; Das, R.; Jha, R.; Mishra, B. Dietary supplementation of microalgae mitigates the negative effects of heat stress in broilers. Poult. Sci. 2023, 102, 102958. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhu, H.; Ma, T.; Yan, Z.; Zhang, Y.; Geng, Y.; Zhu, Y.; Shi, Y. Effect of chronic cyclic heat stress on the intestinal morphology, oxidative status and cecal bacterial communities in broilers. J. Therm. Biol. 2020, 91, 102619. [Google Scholar] [CrossRef]
- Nanto-Hara, F.; Kikusato, M.; Ohwada, S.; Toyomizu, M. Heat Stress Directly Affects Intestinal Integrity in Broiler Chickens. J. Poult. Sci. 2020, 57, 284–290. [Google Scholar] [CrossRef]
- Tran, H.-L.; Chen, Y.-S.; Hung, H.-W.; Shih, B.-L.; Lee, T.-Y.; Yen, C.-H.; Lin, J.-B. Diet Supplementation with Prinsepiae Nux Extract in Broiler Chickens: Its Effect on Growth Performance and Expression of Antioxidant, Pro-Inflammatory, and Heat Shock Protein Genes. Animals 2024, 14, 73. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.K.; Hur, Y.B. Hematological parameters and antioxidant responses in olive flounder Paralichthys olivaceus in biofloc depend on water temperature. J. Therm. Biol. 2019, 82, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Sevcikova, M.; Modrá, H.; Slaninova, A.; Svobodova, Z. Metals as a cause of oxidative stress in fish: A review. Vet. Med. 2011, 56, 537–546. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, J.C. Effects of sub-chronic exposure to lead (Pb) and ascorbic acid in juvenile rockfish: Antioxidant responses, MT gene expression, and neurotransmitters. Chemosphere 2017, 171, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.S.; Zhao, W.; Spitz, D.R.; Robbins, M.E. Inhibiting catalase activity sensitizes 36B10 rat glioma cells to oxidative stress. Free Radic. Biol. Med. 2007, 42, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.-L. SLC7A11 as a Gateway of Metabolic Perturbation and Ferroptosis Vulnerability in Cancer. Antioxidants 2022, 11, 2444. [Google Scholar] [CrossRef]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef]
- Ma, T.; Du, J.; Zhang, Y.; Wang, Y.; Wang, B.; Zhang, T. GPX4-independent ferroptosis-a new strategy in disease’s therapy. Cell Death Discov. 2022, 8, 434. [Google Scholar] [CrossRef]
- Kuwata, H.; Hara, S. Role of acyl-CoA synthetase ACSL4 in arachidonic acid metabolism. Prostaglandins Other Lipid Mediat. 2019, 144, 106363. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; Shehata, A.M.; Khidr, R.E.; Paswan, V.K.; Ibrahim, N.S.; El-Ghoul, A.A.; Aldhumri, S.A.; Gabr, S.A.; Mesalam, N.M.; Elbaz, A.M.; et al. Nutritional manipulation to combat heat stress in poultry—A comprehensive review. J. Therm. Biol. 2021, 98, 102915. [Google Scholar] [CrossRef]
- Reed, A.; Ichu, T.A.; Milosevich, N.; Melillo, B.; Schafroth, M.A.; Otsuka, Y.; Scampavia, L.; Spicer, T.P.; Cravatt, B.F. LPCAT3 Inhibitors Remodel the Polyunsaturated Phospholipid Content of Human Cells and Protect from Ferroptosis. ACS Chem. Biol. 2022, 17, 1607–1618. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin mediates monochromatic light-induced proliferation of T/B lymphocytes in the spleen via the membrane receptor or nuclear receptor. Poult. Sci. 2020, 99, 4294–4302. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Xin, Z.; Li, Y.; Wang, X.; Hu, Y.; Liu, H.; Cai, D. Time-restricted feeding downregulates cholesterol biosynthesis program via RORγ-mediated chromatin modification in porcine liver organoids. J. Anim. Sci. Biotechnol. 2020, 11, 106. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, H.K.; Shin, J.H.; Lee, J.K. Up-down regulation of HO-1 and iNOS gene expressions by ethyl pyruvate via recruiting p300 to Nrf2 and depriving It from p65. Free Radic. Biol. Med. 2013, 65, 468–476. [Google Scholar] [CrossRef]
- Sun, Z.; Chin, Y.E.; Zhang, D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell. Biol. 2009, 29, 2658–2672. [Google Scholar] [CrossRef]
- Yore, M.A.; Im, D.; Webb, L.K.; Zhao, Y.; Chadwick, J.G., Jr.; Molenda-Figueira, H.A.; Haidacher, S.J.; Denner, L.; Tetel, M.J. Steroid receptor coactivator-2 expression in brain and physical associations with steroid receptors. Neuroscience 2010, 169, 1017–1028. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, Y.; Jia, S.; Zhao, S.; Gu, B.; Lu, P.; He, Y.; Liu, R.X.; Wang, J.; Ning, G.; et al. SRC1 Deficiency in Hypothalamic Arcuate Nucleus Increases Appetite and Body Weight. J. Mol. Endocrinol. 2018, 62, 37–46. [Google Scholar] [CrossRef]
- Louet, J.F.; Chopra, A.R.; Sagen, J.V.; An, J.; York, B.; Tannour-Louet, M.; Saha, P.K.; Stevens, R.D.; Wenner, B.R.; Ilkayeva, O.R.; et al. The coactivator SRC-1 is an essential coordinator of hepatic glucose production. Cell Metab. 2010, 12, 606–618. [Google Scholar] [CrossRef]
- Beacon, T.H.; Delcuve, G.P.; López, C.; Nardocci, G.; Kovalchuk, I.; van Wijnen, A.J.; Davie, J.R. The dynamic broad epigenetic (H3K4me3, H3K27ac) domain as a mark of essential genes. Clin. Epigenetics 2021, 13, 138. [Google Scholar] [CrossRef]
- Vahedi, G.; Takahashi, H.; Nakayamada, S.; Sun, H.W.; Sartorelli, V.; Kanno, Y.; O’Shea, J.J. STATs shape the active enhancer landscape of T cell populations. Cell 2012, 151, 981–993. [Google Scholar] [CrossRef]
- Picavet, L.W.; Samat, A.A.K.; Calis, J.; Nijhuis, L.; Scholman, R.; Mokry, M.; Tough, D.F.; Prinjha, R.K.; Vastert, S.J.; van Loosdregt, J. CBP/P300 Inhibition Impairs CD4+ T Cell Activation: Implications for Autoimmune Disorders. Biomedicines 2024, 12, 1344. [Google Scholar] [CrossRef]
- Kersten, S. Integrated physiology and systems biology of PPARα. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef]
- Sun, J.; Bian, Y.; Ma, Y.; Ali, W.; Wang, T.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z.; Zou, H. Melatonin alleviates cadmium-induced nonalcoholic fatty liver disease in ducks by alleviating autophagic flow arrest via PPAR-α and reducing oxidative stress. Poult. Sci. 2023, 102, 102835. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.; Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005, 25, 317–340. [Google Scholar] [CrossRef] [PubMed]
- van Raalte, D.H.; Li, M.; Pritchard, P.H.; Wasan, K.M. Peroxisome proliferator-activated receptor (PPAR)-alpha: A pharmacological target with a promising future. Pharm. Res. 2004, 21, 1531–1538. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, R.; Niu, J.; Yang, S.; Ma, H.; Zhao, S.; Li, H. Molecular basis for hierarchical histone de-β-hydroxybutyrylation by SIRT3. Cell Discov. 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R.; Xu, W.; Delcuve, G.P. Histone H3K4 trimethylation: Dynamic interplay with pre-mRNA splicing. Biochem. Cell Biol. 2015, 94, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.S.; Zhao, D.; Lin, T.; Gu, B.; Pal, K.; Wu, S.J.; Alam, H.; Lv, J.; Yun, K.; Gopalakrishnan, V.; et al. MLL4 Is Required to Maintain Broad H3K4me3 Peaks and Super-Enhancers at Tumor Suppressor Genes. Mol. Cell 2018, 70, 825–841.e826. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, Z.; Wu, D.; Zhang, L.; Lin, X.; Su, J.; Rodriguez, B.; Xi, Y.; Xia, Z.; Chen, X.; et al. Broad H3K4me3 is associated with increased transcription elongation and enhancer activity at tumor-suppressor genes. Nat. Genet. 2015, 47, 1149–1157. [Google Scholar] [CrossRef]
- Tsusaka, T.; Oses-Prieto, J.A.; Lee, C.; DeFelice, B.C.; Burlingame, A.L.; Goldberg, E.L. Non-specific recognition of histone modifications by H3K9bhb antibody. iScience 2023, 26, 107235. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, W.; Shan, C.; Li, B.; Liu, J.; Xing, H.; Xu, Q.; Cui, B.; Zhu, W.; Chen, J.; et al. β-hydroxybutyrate inhibits ferroptosis-mediated pancreatic damage in acute liver failure through the increase of H3K9bhb. Cell Rep. 2022, 41, 111847. [Google Scholar] [CrossRef]
- Terranova, C.J.; Stemler, K.M.; Barrodia, P.; Jeter-Jones, S.L.; Ge, Z.; de la Cruz Bonilla, M.; Raman, A.; Cheng, C.W.; Allton, K.L.; Arslan, E.; et al. Reprogramming of H3K9bhb at regulatory elements is a key feature of fasting in the small intestine. Cell Rep. 2021, 37, 110044. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, D.; Chung, D.; Tang, Z.; Huang, H.; Dai, L.; Qi, S.; Li, J.; Colak, G.; Chen, Y.; et al. Metabolic Regulation of Gene Expression by Histone Lysine β-Hydroxybutyrylation. Mol. Cell 2016, 62, 194–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Liu, X.; Feng, L.; Gong, Z.; Koppula, P.; Sirohi, K.; Li, X.; Wei, Y.; Lee, H.; et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 2018, 20, 1181–1192. [Google Scholar] [CrossRef]
- Wei, L.; Yang, X.; Wang, J.; Wang, Z.; Wang, Q.; Ding, Y.; Yu, A. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer’s disease through the NFκB signaling pathway. J. Neuroinflamm. 2023, 20, 208. [Google Scholar] [CrossRef]
- Pan, L.; Feng, F.; Wu, J.; Fan, S.; Han, J.; Wang, S.; Yang, L.; Liu, W.; Wang, C.; Xu, K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol. Res. 2022, 181, 106270. [Google Scholar] [CrossRef]



| Item | Basal Diet |
|---|---|
| Ingredient (%) | |
| Corn | 76.27 |
| Soybean meal | 19.50 |
| Soybean oil | 1.38 |
| DL-Met | 0.12 |
| L-Lys | 0.13 |
| CaHPO4·2H2O | 0.79 |
| CaCO3 | 1.15 |
| NaCl | 0.30 |
| Micronutrients 1 | 0.26 |
| Cornstarch + zinc | 0.10 |
| Nutrient levels composition | |
| ME, Kcal/kg | 3037 |
| Crude protien % | 15.31 |
| Lys, % | 0.81 |
| Met, % | 0.36 |
| L-Thr, % | 0.57 |
| Try, % | 0.16 |
| Met+Cys, % | 0.60 |
| Ca, % | 0.69 |
| P, % | 0.45 |
| Nonphytate P, % | 0.22 |
| Zinc mg/kg | 18.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, S.Y.; Muniyappan, M.; Huang, H.; Ennab, W.; Liu, H.-Y.; Ahmed, A.A.; Sun, M.-a.; Dessie, T.; Kim, I.H.; Hu, Y.; et al. Dietary Organic Zinc Supplementation Modifies the Oxidative Genes via RORγ and Epigenetic Regulations in the Ileum of Broiler Chickens Exposed to High-Temperature Stress. Antioxidants 2024, 13, 1079. https://doi.org/10.3390/antiox13091079
Adam SY, Muniyappan M, Huang H, Ennab W, Liu H-Y, Ahmed AA, Sun M-a, Dessie T, Kim IH, Hu Y, et al. Dietary Organic Zinc Supplementation Modifies the Oxidative Genes via RORγ and Epigenetic Regulations in the Ileum of Broiler Chickens Exposed to High-Temperature Stress. Antioxidants. 2024; 13(9):1079. https://doi.org/10.3390/antiox13091079
Chicago/Turabian StyleAdam, Saber Y., Madesh Muniyappan, Hao Huang, Wael Ennab, Hao-Yu Liu, Abdelkareem A. Ahmed, Ming-an Sun, Tadelle Dessie, In Ho Kim, Yun Hu, and et al. 2024. "Dietary Organic Zinc Supplementation Modifies the Oxidative Genes via RORγ and Epigenetic Regulations in the Ileum of Broiler Chickens Exposed to High-Temperature Stress" Antioxidants 13, no. 9: 1079. https://doi.org/10.3390/antiox13091079








