Impaired Upper Airway Muscle Function with Excessive or Deficient Dietary Intake of Selenium in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethical Approval
2.3. Experimental Animals, Dietary Interventions and Chronic Intermittent Hypoxia
2.4. Ex Vivo Sternohyoid Muscle Function
2.5. Muscle Histology
2.5.1. Tissue Preparation
2.5.2. Laminin Immunofluorescence
2.6. Biochemical Assays
2.6.1. Western Blotting
2.6.2. Determination of Total Selenium in Plasma
2.7. Statistical Analysis
3. Results
3.1. Feeding Rats with Selenium-Deficient or -Excessive Diets Results in Corresponding Changes in Serum Selenium Content
3.2. Dietary Deficiency or Excess of Selenium Does Not Alter Body Mass or Organ Parameters
3.3. Dietary Selenium Deficiency or Excess Both Influence Rat Sternohyoid Muscle Ex Vivo Performance
3.4. Dietary Se Levels Do Not Alter SH Muscle Fibre Size
3.5. Dietary Se Levels Do Not Alter Glutathione-Dependent Antioxidant Systems in Rat SH Muscle
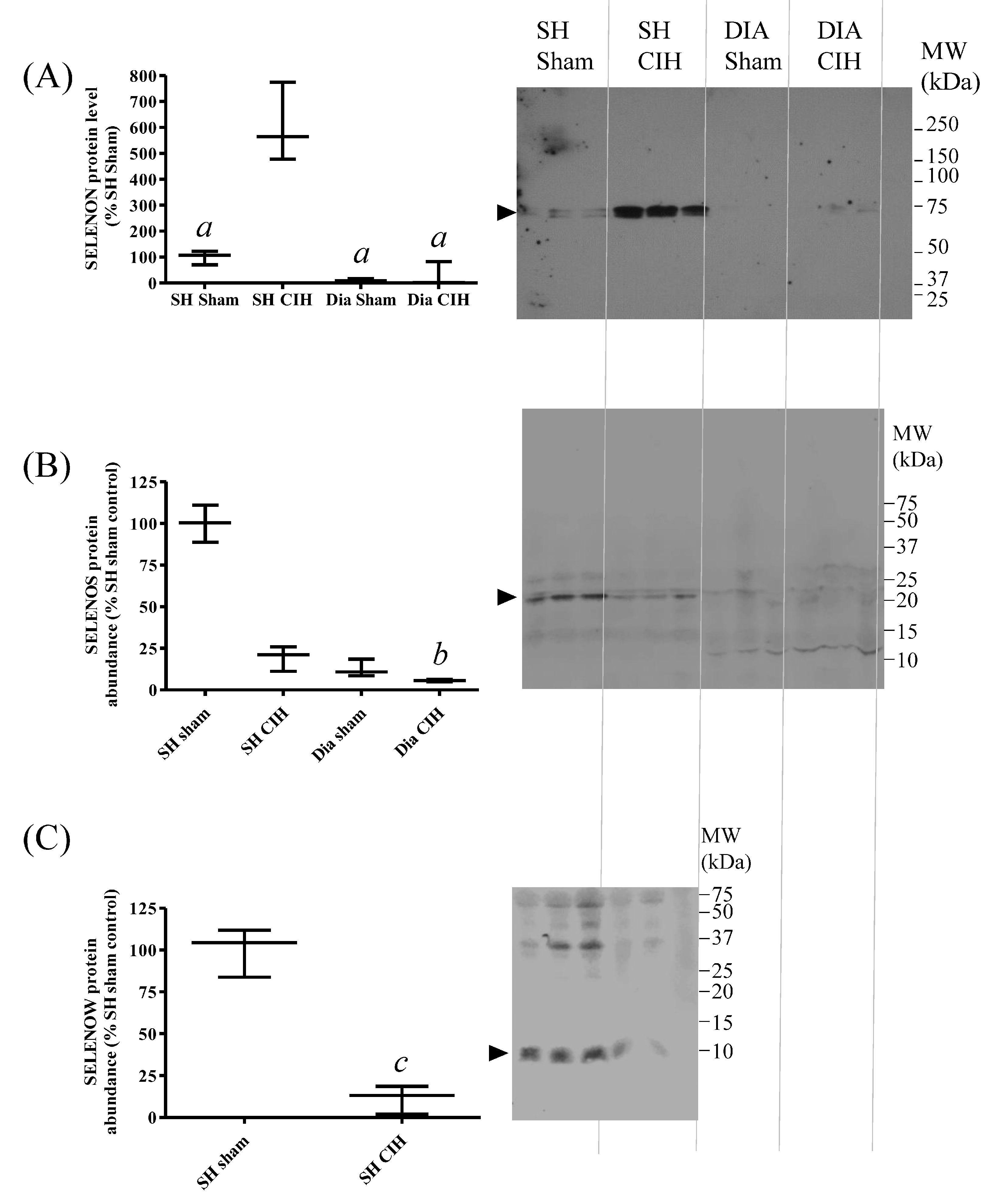
3.6. Abundance of SELENON and SELENOS Proteins in Rat SH Muscle Are Not Altered by Dietary Se, Whereas SELENOW Is Increased by Excessive Se Intake
3.7. SH Muscle SELENON Is Increased and SELENOW Protein Is Decreased in a Rat Model of Obstructive Sleep Apnoea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Mansukhani, M.P.; Kolla, B.P.; Somers, V.K. Hypertension and Cognitive Decline: Implications of Obstructive Sleep Apnea. Front. Cardiovasc. Med. 2019, 6, 96. [Google Scholar] [CrossRef]
- Casale, M.; Pappacena, M.; Rinaldi, V.; Bressi, F.; Baptista, P.; Salvinelli, F. Obstructive sleep apnea syndrome: From phenotype to genetic basis. Curr. Genom. 2009, 10, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H.; German Nutrition Society (DGE). Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Arshad, M.A.; Ebeid, H.M.; Hassan, F. Revisiting the Effects of Different Dietary Sources of Selenium on the Health and Performance of Dairy Animals: A Review. Biol. Trace Elem. Res. 2021, 199, 3319–3337. [Google Scholar] [CrossRef]
- Raisbeck, M.F. Selenosis. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 465–480. [Google Scholar] [CrossRef]
- Koller, L.D.; Exon, J.H. The two faces of selenium-deficiency and toxicity--are similar in animals and man. Can. J. Vet. Res. 1986, 50, 297–306. [Google Scholar]
- Muth, O.H.; Oldfield, J.E.; Remmert, L.F.; Schubert, J.R. Effects of Selenium and Vitamin E on White Muscle Disease. Science 1958, 128, 1090. [Google Scholar] [CrossRef]
- Nakane, T.; Asayama, K.; Kodera, K.; Hayashibe, H.; Uchida, N.; Nakazawa, S. Effect of selenium deficiency on cellular and extracellular glutathione peroxidases: Immunochemical detection and mRNA analysis in rat kidney and serum. Free Radic. Biol. Med. 1998, 25, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Andrade; Anzueto; Napier; Levine; Lawrence; Jenkinson; Maxwell. Effects of selenium deficiency on diaphragmatic function after resistive loading. Acta Physiol. Scand. 1998, 162, 141–148. [Google Scholar] [CrossRef]
- Robberecht, H.; De Bruyne, T.; Davioud-Charvet, E.; Mackrill, J.; Hermans, N. Selenium Status in Elderly People: Longevity and Age-Related Diseases. Curr. Pharm. Des. 2019, 25, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Ferrucci, L.; Sun, K.; Walston, J.; Fried, L.P.; Varadhan, R.; Guralnik, J.M.; Semba, R.D. Low serum selenium concentrations are associated with poor grip strength among older women living in the community. BioFactors 2007, 29, 37–44. [Google Scholar] [CrossRef]
- Perri, G.; Mendonça, N.; Jagger, C.; Walsh, J.; Eastell, R.; Mathers, J.C.; Hill, T.R. Dietary Selenium Intakes and Musculoskeletal Function in Very Old Adults: Analysis of the Newcastle 85+ Study. Nutrients 2020, 12, 2068. [Google Scholar] [CrossRef] [PubMed]
- Petit, N. Selenoprotein N: An endoplasmic reticulum glycoprotein with an early developmental expression pattern. Hum. Mol. Genet. 2003, 12, 1045–1053. [Google Scholar] [CrossRef]
- Villar-Quiles, R.N.; von der Hagen, M.; Métay, C.; Gonzalez, V.; Donkervoort, S.; Bertini, E.; Castiglioni, C.; Chaigne, D.; Colomer, J.; Cuadrado, M.L.; et al. The clinical, histologic, and genotypic spectrum of SEPN1 -related myopathy: A case series. Neurology 2020, 95, e1512–e1527. [Google Scholar] [CrossRef]
- Zhang, S.; Lei, L.; Fan, Z.; Su, S.; Duo, J.; Luan, Q.; Lu, Y.; Di, L.; Wang, M.; Da, Y. Delayed Respiratory Insufficiency and Extramuscular Abnormalities in Selenoprotein N-Related Myopathies. Front. Neurol. 2021, 12, 766942. [Google Scholar] [CrossRef]
- Rederstorff, M.; Castets, P.; Arbogast, S.; Lainé, J.; Vassilopoulos, S.; Beuvin, M.; Dubourg, O.; Vignaud, A.; Ferry, A.; Krol, A.; et al. Increased Muscle Stress-Sensitivity Induced by Selenoprotein N Inactivation in Mouse: A Mammalian Model for SEPN1-Related Myopathy. PLoS ONE 2011, 6, e23094. [Google Scholar] [CrossRef]
- Jurynec, M.J.; Xia, R.; Mackrill, J.J.; Gunther, D.; Crawford, T.; Flanigan, K.M.; Abramson, J.J.; Howard, M.T.; Grunwald, D.J. Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. Proc. Natl. Acad. Sci. USA 2008, 105, 12485–12490. [Google Scholar] [CrossRef] [PubMed]
- Chernorudskiy, A.; Varone, E.; Colombo, S.F.; Fumagalli, S.; Cagnotto, A.; Cattaneo, A.; Briens, M.; Baltzinger, M.; Kuhn, L.; Bachi, A.; et al. Selenoprotein N is an endoplasmic reticulum calcium sensor that links luminal calcium levels to a redox activity. Proc. Natl. Acad. Sci. USA 2020, 117, 21288–21298. [Google Scholar] [CrossRef]
- Bodnár, D.; Ruzsnavszky, O.; Oláh, T.; Dienes, B.; Balatoni, I.; Ungvári, É.; Benkő, I.; Babka, B.; Prokisch, J.; Csernoch, L.; et al. Dietary selenium augments sarcoplasmic calcium release and mechanical performance in mice. Nutr. Metab. 2016, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Fodor, J.; Al-Gaadi, D.; Czirják, T.; Oláh, T.; Dienes, B.; Csernoch, L.; Szentesi, P. Improved Calcium Homeostasis and Force by Selenium Treatment and Training in Aged Mouse Skeletal Muscle. Sci. Rep. 2020, 10, 1707. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-G.; Zhou, J.-C.; Zhao, H.; Lei, X.-G.; Xia, X.-J.; Gao, G.; Wang, K.-N. Enhanced water-holding capacity of meat was associated with increased Sepw1 gene expression in pigs fed selenium-enriched yeast. Meat Sci. 2011, 87, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.P.; Ream, W.; Whanger, P.D. Selenoprotein W gene regulation by selenium in L8 cells. Biometals 2002, 15, 411–420. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Park, Y.H.; Lee, J.H.; Hong, J.-H.; Kim, I.Y. Selenoprotein W enhances skeletal muscle differentiation by inhibiting TAZ binding to 14-3-3 protein. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 1356–1364. [Google Scholar] [CrossRef]
- Yao, H.; Fan, R.; Zhao, X.; Zhao, W.; Liu, W.; Yang, J.; Sattar, H.; Zhao, J.; Zhang, Z.; Xu, S. Selenoprotein W redox-regulated Ca2+ channels correlate with selenium deficiency-induced muscles Ca2+ leak. Oncotarget 2016, 7, 57618–57632. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, K.J.; Jang, J.K.; Jeon, Y.H.; Ko, K.Y.; Kwon, J.H.; Lee, S.-R.; Kim, I.Y. Selenoprotein S-dependent Selenoprotein K Binding to p97(VCP) Protein Is Essential for Endoplasmic Reticulum-associated Degradation. J. Biol. Chem. 2015, 290, 29941–29952. [Google Scholar] [CrossRef]
- Turanov, A.A.; Shchedrina, V.A.; Everley, R.A.; Lobanov, A.V.; Yim, S.H.; Marino, S.M.; Gygi, S.P.; Hatfield, D.L.; Gladyshev, V.N. Selenoprotein S is involved in maintenance and transport of multiprotein complexes. Biochem. J. 2014, 462, 555–565. [Google Scholar] [CrossRef]
- Addinsall, A.B.; Wright, C.R.; Shaw, C.S.; McRae, N.L.; Forgan, L.G.; Weng, C.-H.; Conlan, X.A.; Francis, P.S.; Smith, Z.M.; Andrikopoulos, S.; et al. Deficiency of selenoprotein S, an endoplasmic reticulum resident oxidoreductase, impairs the contractile function of fast-twitch hindlimb muscles. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 315, R380–R396. [Google Scholar] [CrossRef] [PubMed]
- Addinsall, A.B.; Wright, C.R.; Kotsiakos, T.L.; Smith, Z.M.; Cook, T.R.; Andrikopoulos, S.; van der Poel, C.; Stupka, N. Impaired exercise performance is independent of inflammation and cellular stress following genetic reduction or deletion of selenoprotein S. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 318, R981–R996. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.R.; Allsopp, G.L.; Addinsall, A.B.; McRae, N.L.; Andrikopoulos, S.; Stupka, N. A Reduction in Selenoprotein S Amplifies the Inflammatory Profile of Fast-Twitch Skeletal Muscle in the mdx Dystrophic Mouse. Mediat. Inflamm. 2017, 2017, 7043429. [Google Scholar] [CrossRef] [PubMed]
- DeKok, H.J.M. Case Report: The medical treatment of obstructive sleep apnoea syndrome (OSAS) with Selenium. Med. Hypotheses 2005, 65, 817–818. [Google Scholar] [CrossRef]
- Chen, P.-C.; Guo, C.-H.; Tseng, C.-J.; Wang, K.-C.; Liu, P.-J. Blood trace minerals concentrations and oxidative stress in patients with obstructive sleep apnea. J. Nutr. Health Aging 2013, 17, 639–644. [Google Scholar] [CrossRef]
- Saruhan, E.; Sertoglu, E.; Unal, Y.; Bek, S.; Kutlu, G. The role of antioxidant vitamins and selenium in patients with obstructive sleep apnea. Sleep Breath 2021, 25, 923–930. [Google Scholar] [CrossRef]
- Matsuda, A.; Kimura, M.; Itokawa, Y. Influence of selenium deficiency on vital functions in rats. Biol. Trace Elem. Res. 1998, 61, 287–301. [Google Scholar] [CrossRef]
- Burns, D.P.; Drummond, S.E.; Bolger, D.; Coiscaud, A.; Murphy, K.H.; Edge, D.; O’Halloran, K.D. N-acetylcysteine Decreases Fibrosis and Increases Force-Generating Capacity of mdx Diaphragm. Antioxidants 2019, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Lemaire, P.; Lewis, P.; McDonald, F.B.; Lucking, E.; Hogan, S.; Sheehan, D.; Healy, V.; O’Halloran, K.D. Chronic intermittent hypoxia increases rat sternohyoid muscle NADPH oxidase expression with attendant modest oxidative stress. Front. Physiol. 2015, 6, 15. [Google Scholar] [CrossRef]
- Katarzyna, B.; Taylor, R.M.; Szpunar, J.; Lobinski, R.; Sunde, R.A. Identification and determination of selenocysteine, selenosugar, and other selenometabolites in turkey liver. Metallomics 2020, 12, 758–766. [Google Scholar] [CrossRef]
- Hybsier, S.; Schulz, T.; Wu, Z.; Demuth, I.; Minich, W.B.; Renko, K.; Rijntjes, E.; Köhrle, J.; Strasburger, C.J.; Steinhagen-Thiessen, E.; et al. Sex-specific and inter-individual differences in biomarkers of selenium status identified by a calibrated ELISA for selenoprotein P. Redox Biol. 2017, 11, 403–414. [Google Scholar] [CrossRef]
- Crespo, A.M.; Neve, J.; Pinto, R.E. Plasma and liver selenium levels in the rat during supplementation with 0.5, 2, 6, and 15 ppm selenium in drinking water. Biol. Trace Elem. Res. 1993, 38, 139–147. [Google Scholar] [CrossRef]
- Burns, D.P.; O’Halloran, K.D. Evidence of hypoxic tolerance in weak upper airway muscle from young mdx mice. Respir. Physiol. Neurobiol. 2016, 226, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, N.; Anan, Y.; Hashimoto, Y.; Tokoro, N.; Mizuno, R.; Hayashi, S.; Yamamoto, S.; Shimada, K.-I.; Kamata, S.; Ishii, I. Dietary selenium deficiency or selenomethionine excess drastically alters organ selenium contents without altering the expression of most selenoproteins in mice. J. Nutr. Biochem. 2019, 69, 120–129. [Google Scholar] [CrossRef]
- Elhodaky, M.; Diamond, A.M. Selenium-Binding Protein 1 in Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3437. [Google Scholar] [CrossRef]
- Sunde, R.A. Gene Set Enrichment Analysis of Selenium-Deficient and High-Selenium Rat Liver Transcript Expression and Comparison With Turkey Liver Expression. J. Nutr. 2021, 151, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Godwin, K.O.; Edwardly, J.; Fuss, C.N. Retention of 45Ca in rats and lambs associated with the onset of nutritional muscular dystrophy. Aust. J. Biol. Sci. 1975, 28, 457–464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moghadaszadeh, B.; Rider, B.E.; Lawlor, M.W.; Childers, M.K.; Grange, R.W.; Gupta, K.; Boukedes, S.S.; Owen, C.A.; Beggs, A.H. Selenoprotein N deficiency in mice is associated with abnormal lung development. FASEB J. 2013, 27, 1585–1599. [Google Scholar] [CrossRef]
- Yeh, J.Y.; Vendeland, S.C.; Gu, Q.; Butler, J.A.; Ou, B.R.; Whanger, P.D. Dietary selenium increases selenoprotein W levels in rat tissues. J. Nutr. 1997, 127, 2165–2172. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.-L.; Huang, X.; Bo, S.; Rihua, W.; Li, S.; Xu, S. Dietary selenium regulation of transcript abundance of selenoprotein N and selenoprotein W in chicken muscle tissues. Biometals 2012, 25, 297–307. [Google Scholar] [CrossRef]
- Gao, J.; Nie, W.; Wang, F.; Guo, Y. Maternal Selenium Supplementation Enhanced Skeletal Muscle Development Through Increasing Protein Synthesis and SelW mRNA Levels of their Offspring. Biol. Trace Elem. Res. 2018, 186, 238–248. [Google Scholar] [CrossRef] [PubMed]
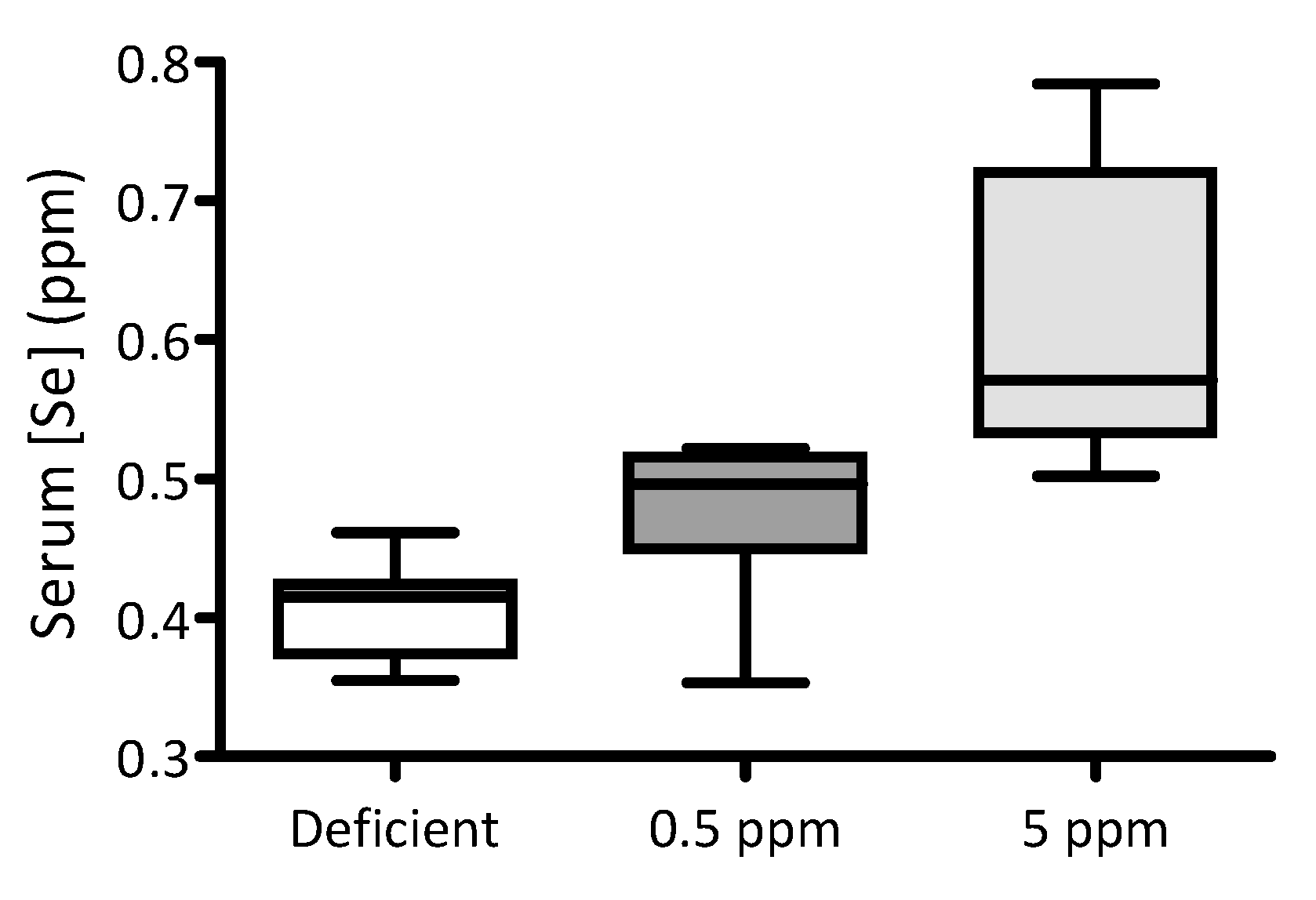

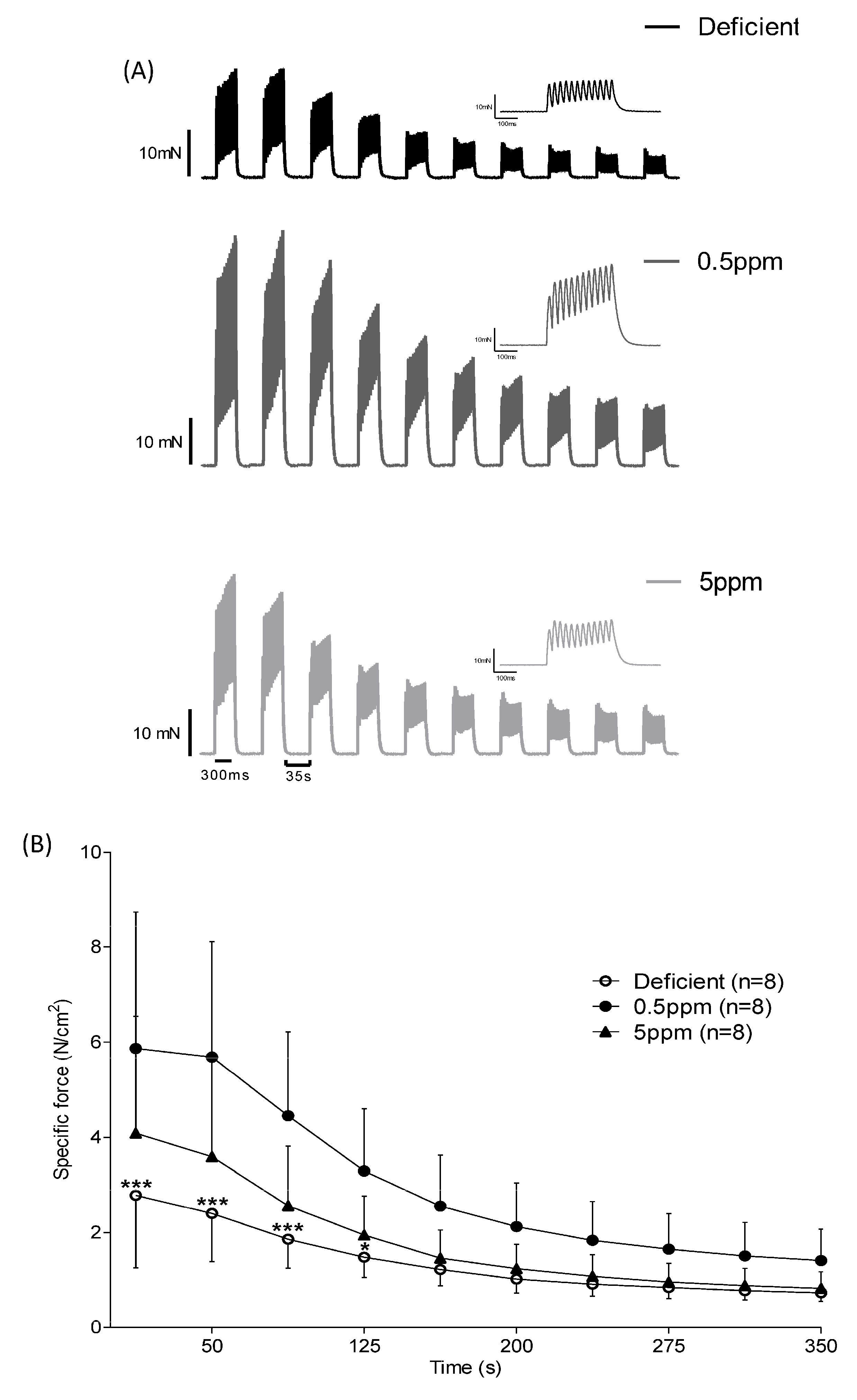



| Deficient (n = 10) | 0.5 ppm (n = 10) | 5 ppm (n = 10) | p-Value | |
|---|---|---|---|---|
| Body mass (g) | 377.9 ± 26.0 | 387.9 ± 35.9 | 380.9 ± 30.1 | 0.8472 |
| Tibia length (cm) | 4.0 ± 0.1 | 3.9 ± 0.1 | 4.0 ± 0.1 | 0.5324 |
| Tibia mass (mg) | 558.3 ± 50.2 | 552.1 ± 61.1 | 579.6 ± 33.9 | 0.4410 |
| RV (mg) | 166.5 ± 21.0 | 172.2 ± 18.5 | 162.0 ± 22.5 | 0.5573 |
| LV (mg) | 629.7 ± 76.1 | 670.3 ± 68.9 | 629.7 ± 52.1 | 0.3044 |
| Spleen (mg) | 696.7 ± 109.0 | 701.6 ± 72.0 | 673.9 ± 91.9 | 0.7834 |
| TA (mg) | 782.3 ± 113.0 | 770.6 ± 69.8 | 785.5 ± 51.0 | 0.9112 |
| EDL (mg) | 175.2 ± 18.5 | 168.4 ± 14.1 | 171.5 ± 20.3 | 0.7118 |
| Sol (mg) | 161.7 ± 15.6 | 162.8 ± 25.5 | 160.1 ± 8.11 | 0.9678 |
| Testes (mg) | 1769 ± 171.5 | 1864 ± 131.7 | 1814 ± 156.8 | 0.4192 |
| Thyroid (mg) | 16.6 ± 2.0 | 15.4 ± 3.7 | 18.5 ± 6.9 | 0.3768 |
| Deficient (n = 8) | 0.5 ppm (n = 8) | 5 ppm (n = 8) | p-Value | |
|---|---|---|---|---|
| CT (ms) | 13.7 ± 0.8 | 14.8 ± 0.9 | 15.4 ± 1.5 | 0.0064 a,b |
| ½RT (ms) | 8.4 ± 1.1 | 8.8 ± 1.2 | 9.5 ± 2.0 | 0.6921 |
| Pt (N/cm2) | 3.4 ± 1.2 | 5.9 ± 2.0 | 4.3 ± 1.8 | 0.0292 a |
| Po (N/cm2) | 24.2 ± 7.6 | 39.8 ± 13.8 | 28.2 ± 11.3 | 0.0285 a |
| Vmax (Lo/s) | 8.0 ± 2.2 | 8.7 ± 0.9 | 8.7 ± 2.4 | 0.6929 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burns, D.P.; Drummond, S.E.; Wölfel, S.; Murphy, K.H.; Szpunar, J.; O’Halloran, K.D.; Mackrill, J.J. Impaired Upper Airway Muscle Function with Excessive or Deficient Dietary Intake of Selenium in Rats. Antioxidants 2024, 13, 1080. https://doi.org/10.3390/antiox13091080
Burns DP, Drummond SE, Wölfel S, Murphy KH, Szpunar J, O’Halloran KD, Mackrill JJ. Impaired Upper Airway Muscle Function with Excessive or Deficient Dietary Intake of Selenium in Rats. Antioxidants. 2024; 13(9):1080. https://doi.org/10.3390/antiox13091080
Chicago/Turabian StyleBurns, David P., Sarah E. Drummond, Stefanie Wölfel, Kevin H. Murphy, Joanna Szpunar, Ken D. O’Halloran, and John J. Mackrill. 2024. "Impaired Upper Airway Muscle Function with Excessive or Deficient Dietary Intake of Selenium in Rats" Antioxidants 13, no. 9: 1080. https://doi.org/10.3390/antiox13091080
APA StyleBurns, D. P., Drummond, S. E., Wölfel, S., Murphy, K. H., Szpunar, J., O’Halloran, K. D., & Mackrill, J. J. (2024). Impaired Upper Airway Muscle Function with Excessive or Deficient Dietary Intake of Selenium in Rats. Antioxidants, 13(9), 1080. https://doi.org/10.3390/antiox13091080







