TSG Extends the Longevity of Caenorhabditis elegans by Targeting the DAF-16/SKN-1/SIR-2.1-Mediated Mitochondrial Quality Control Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Lifespan Assay
2.3. Phenotype Assays
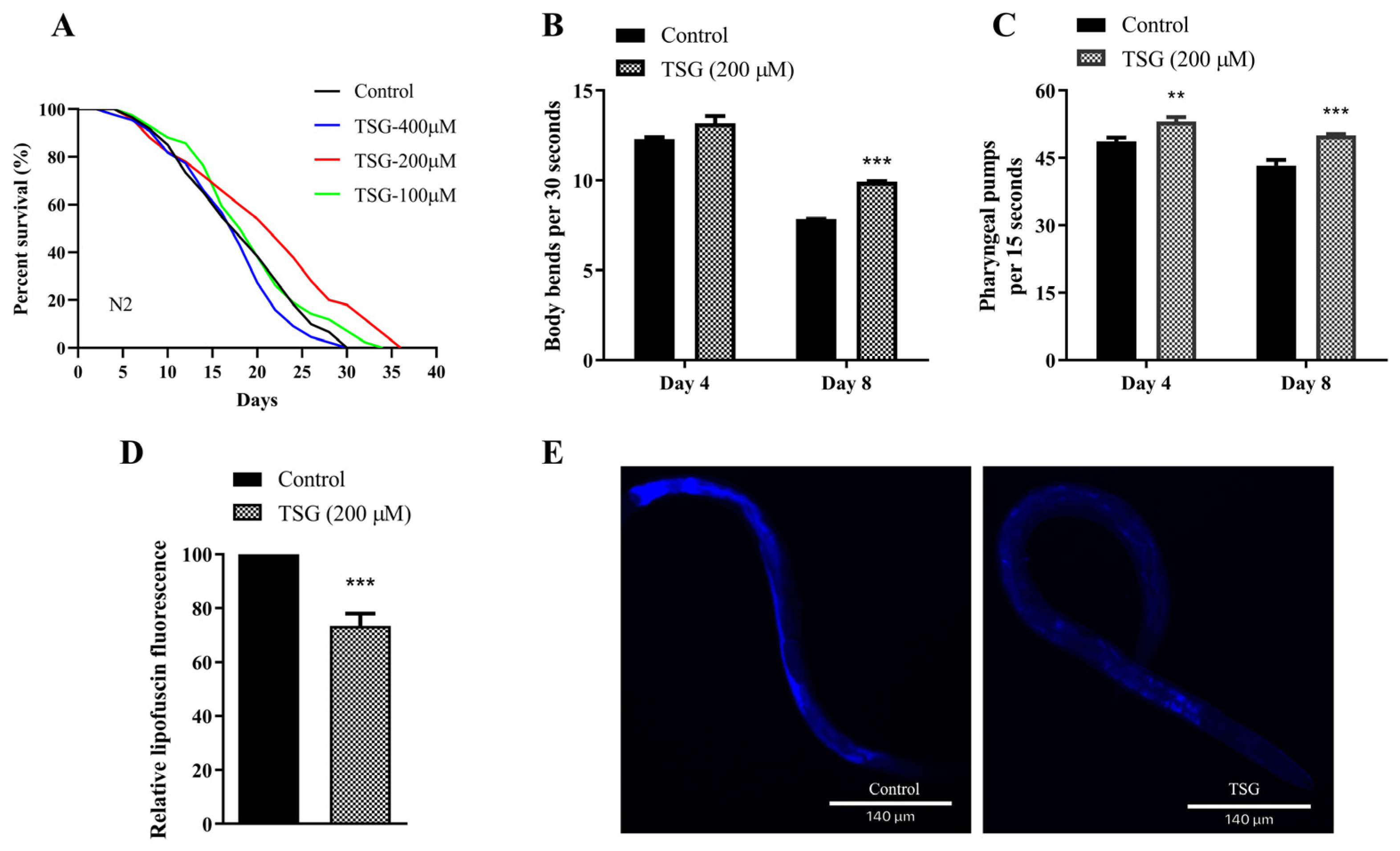
2.3.1. Body Bending
2.3.2. Pharyngeal Pumping
2.3.3. Lipofuscin Assay
2.4. Stress Resistance Assays
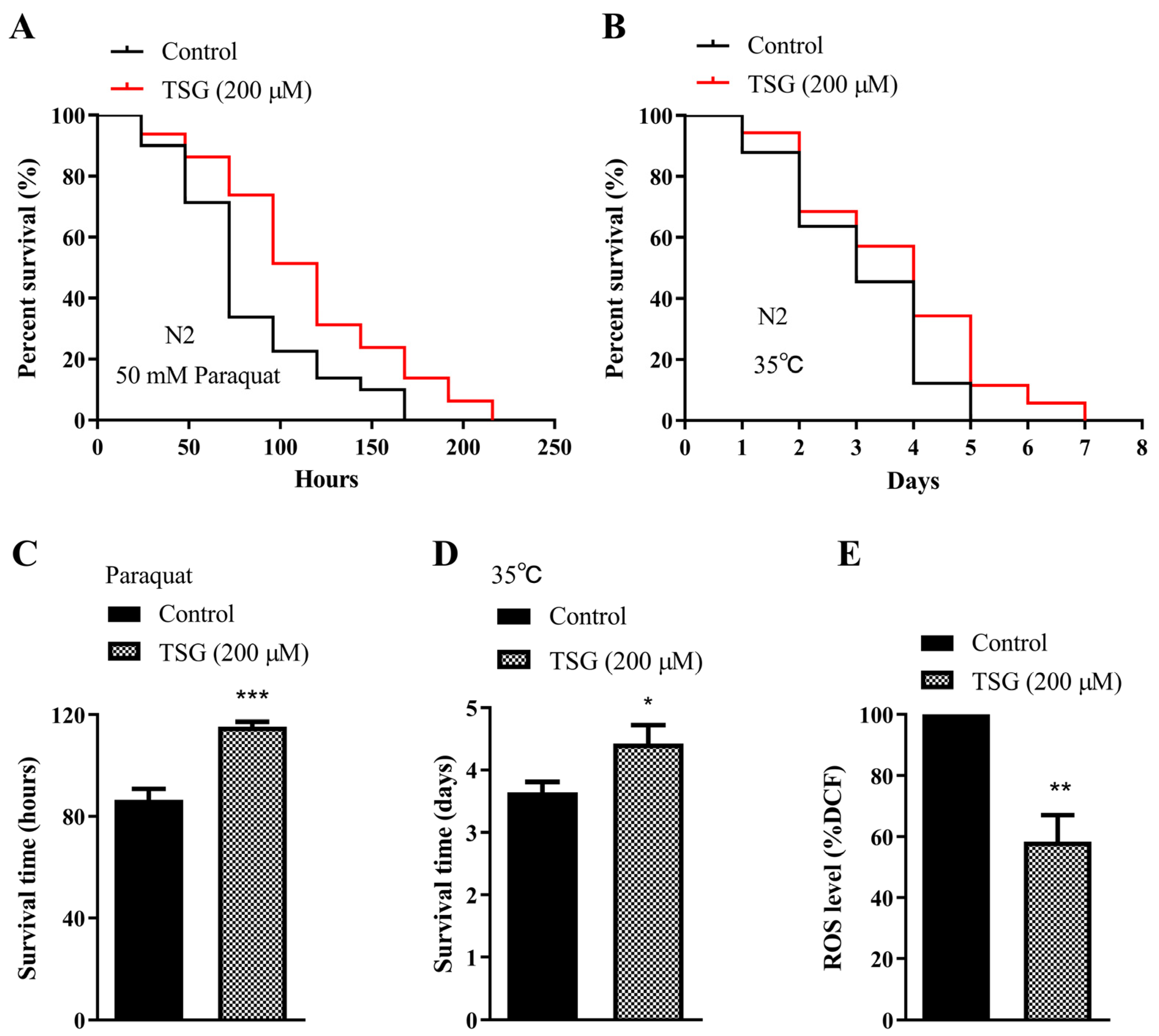
2.4.1. Paraquat or Rotenone Stress Resistance
2.4.2. Thermotolerance Assay
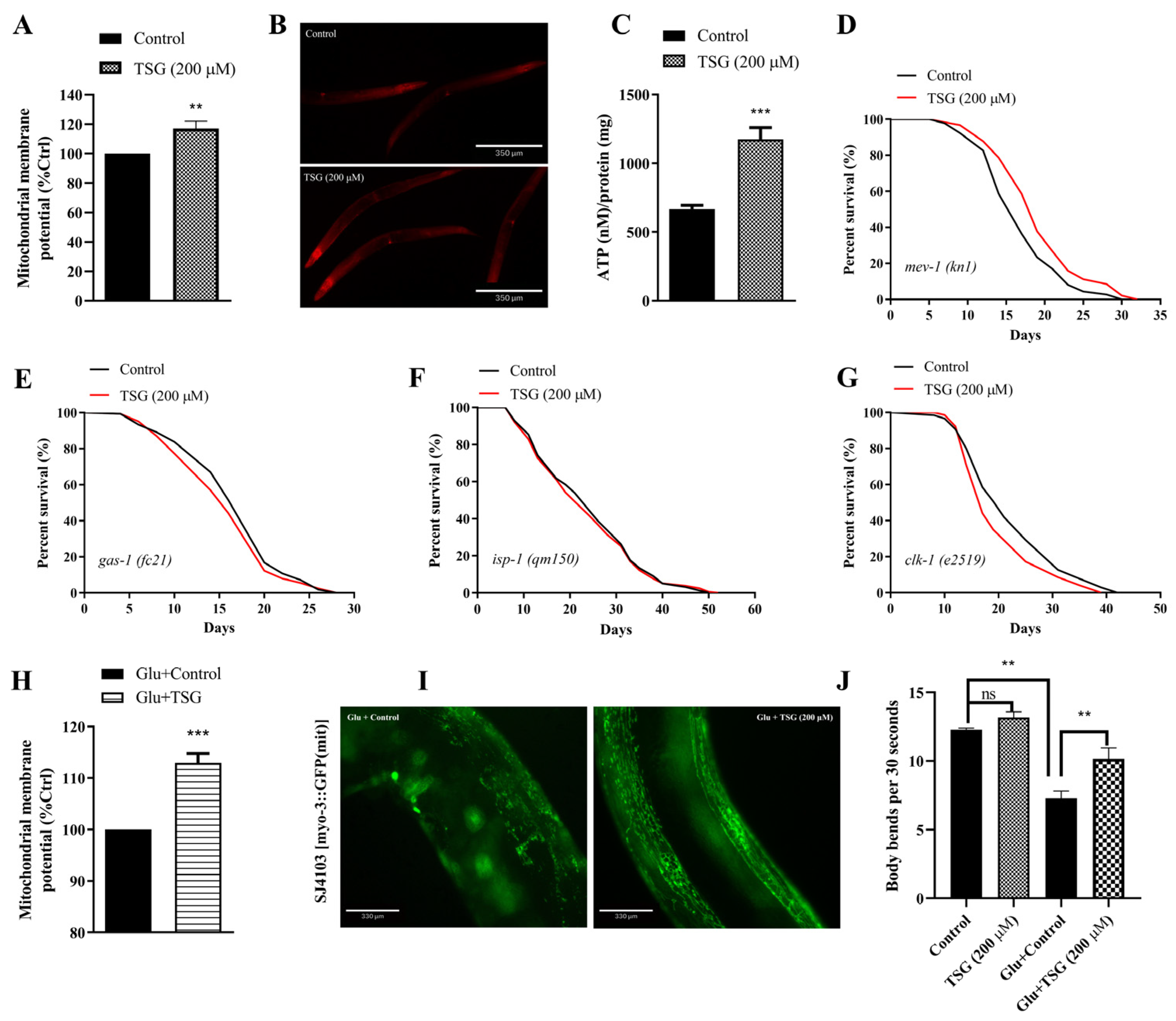
2.4.3. High-Glucose Stress Assay
2.5. ROS Assay
2.6. Fluorescence Assay
2.7. DAF-16 and SKN-1 Nuclear Translocation Assays
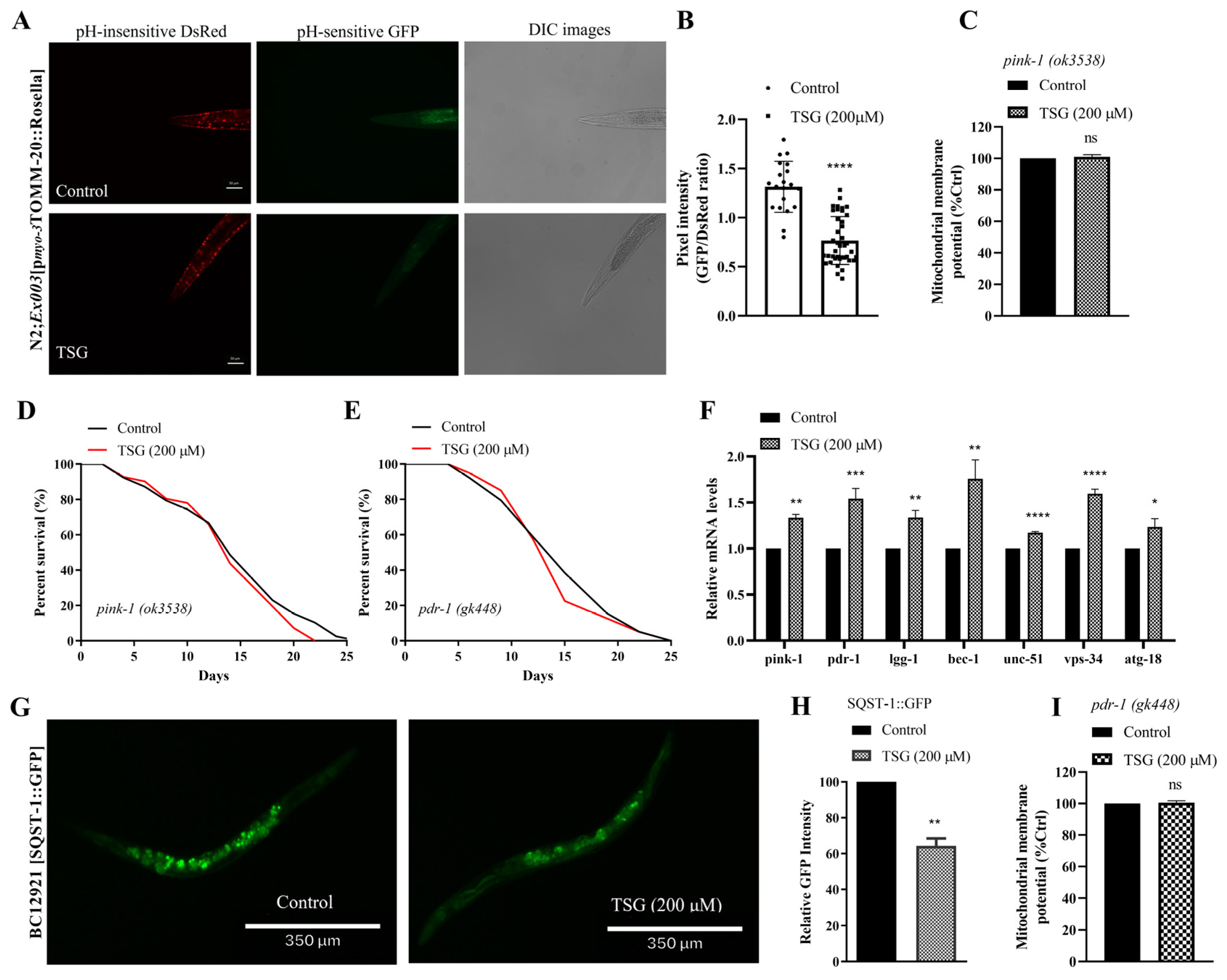
2.8. Quantitative RT-PCR Assay
2.9. Paralysis Assay
2.10. Chemotaxis Assay
2.11. Serotonin Sensitivity Assay
2.12. Behavior Analysis of Transgenic C. elegans Expressing Tau Protein
2.12.1. Locomotion Speed Assay
2.12.2. Liquid Thrashing Assay
2.13. Western Blotting
2.14. RNA Interference (RNAi) Assay
2.15. Mitochondrial Membrane Potential (ΔΨ) Assay
2.16. ATP Level Assay
2.17. NAD+ Level Assay
2.18. Mitochondrial DNA (mtDNA) Copy Number Assay
2.19. Statistical Analysis
3. Results
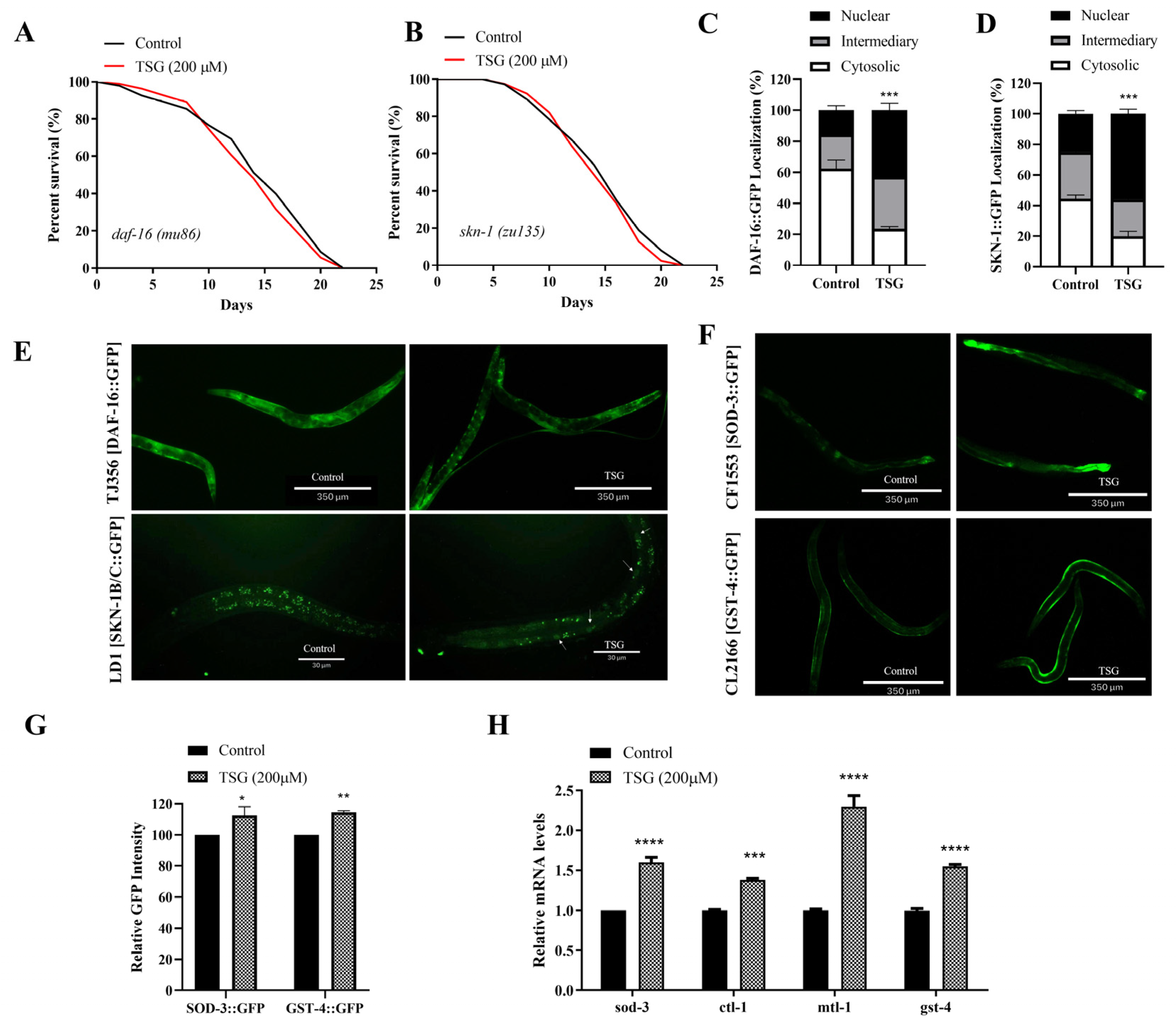
3.1. TSG Extended the Lifespan and Improved the Health Status of C. elegans
3.2. TSG Protected against Oxidative Stress in C. elegans
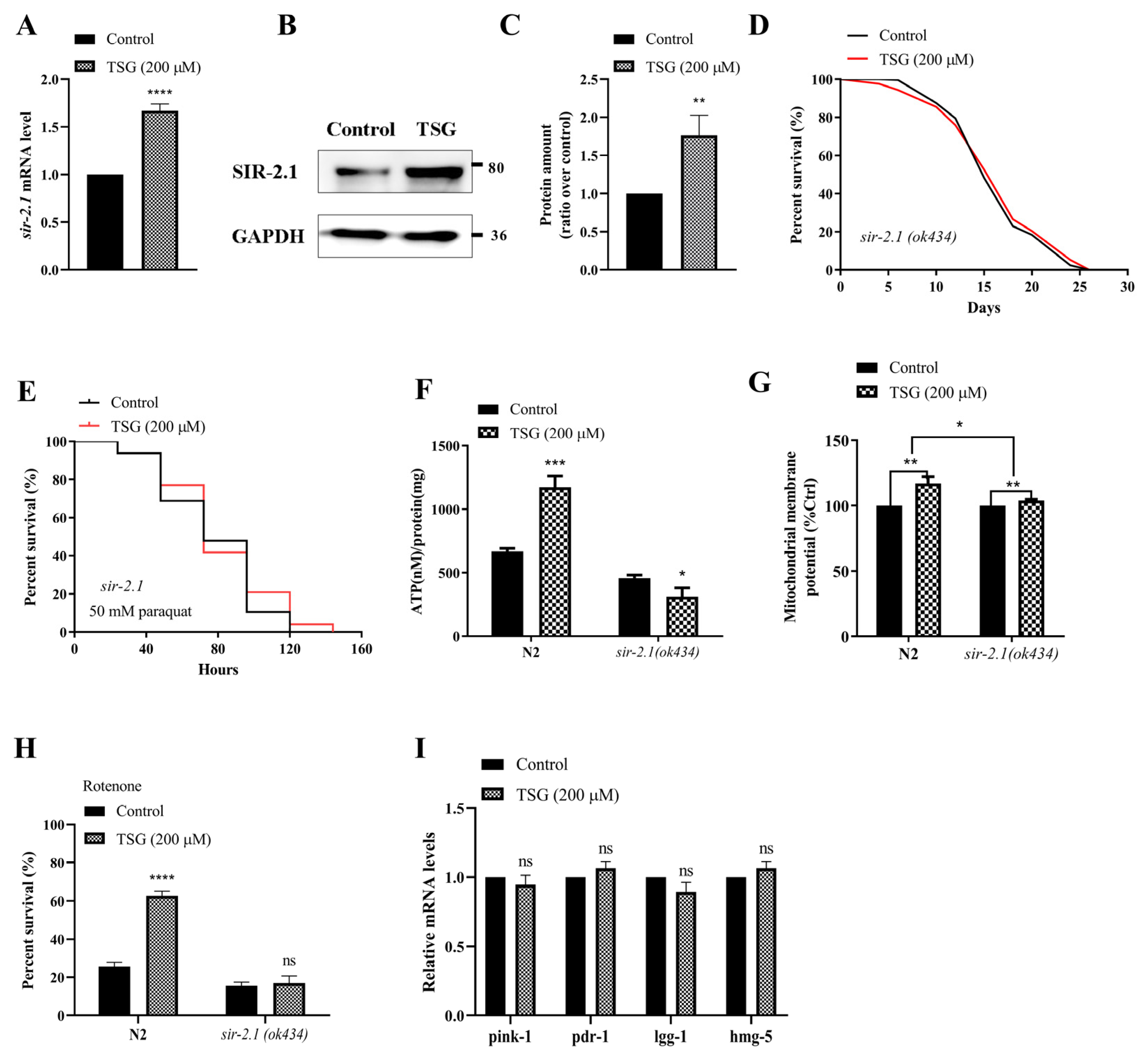
3.3. TSG Improved Mitochondrial Function
3.4. TSG Improves Mitochondrial Function by Inducing Mitophagy and Stimulating Mitochondrial Biogenesis
3.5. TSG Modulated DAF-16/FOXO, SKN-1/Nrf2 and SIR-2.1/SIRT1 to Activate Mitochondrial Biogenesis and Mitophagy
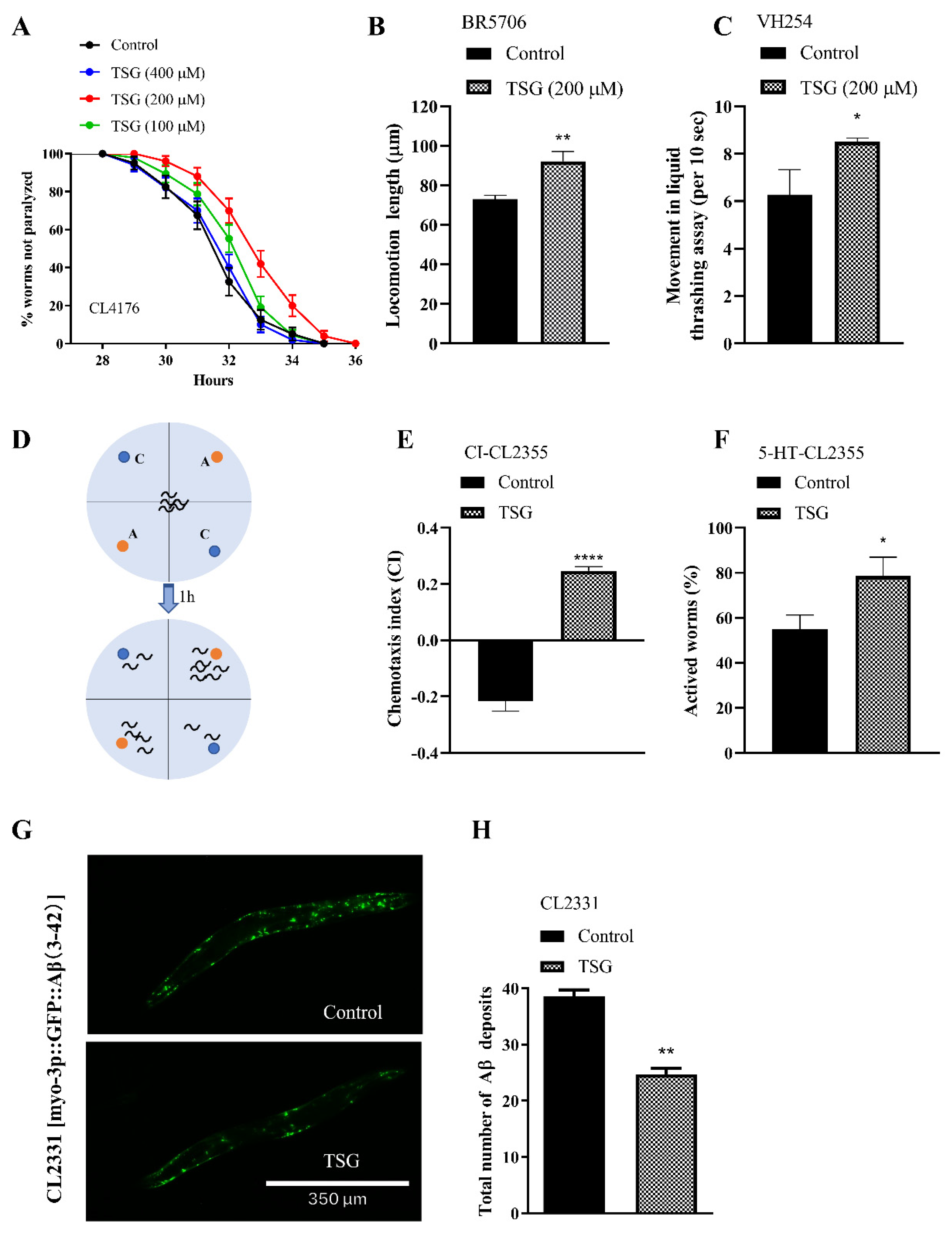
3.6. TSG Protected against Aβ- or Tau-Induced Toxicity
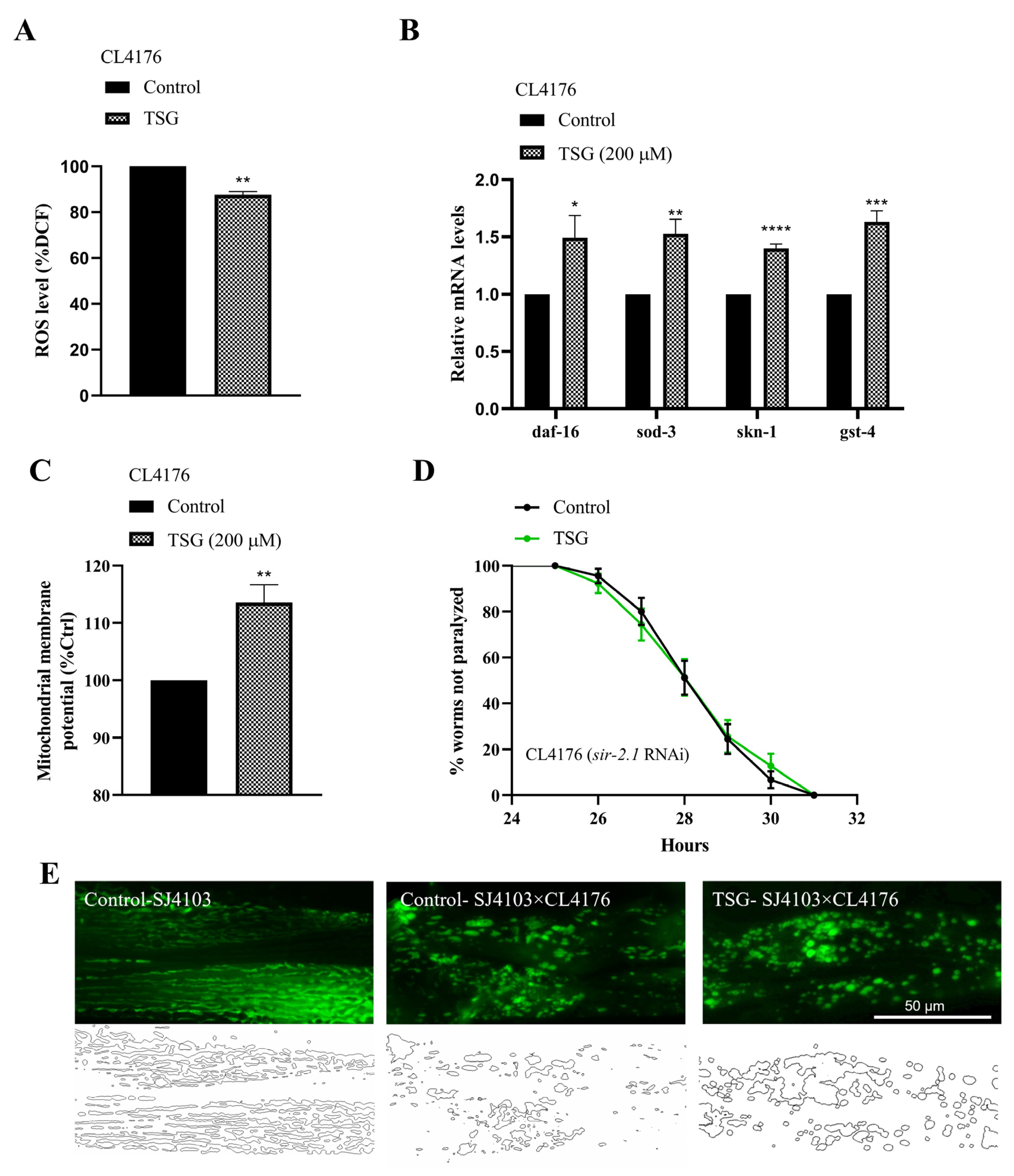
3.7. TSG Inhibited Aβ-Induced Toxicity through Antioxidant Activity and Improving Mitochondrial Function
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TSG | 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside; |
| C. elegans | Caenorhabditis elegans; |
| ETC | electron transfer chain; |
| MQC | mitochondrial quality control; |
| UPRmt | mitochondrial unfolded protein response; |
| PMT-E | ethanol fraction of Polygonum multiflorum Thunb; |
| Aβ | β-amyloid; |
| NGM | nematode growth media; |
| CGC | Caenorhabditis Genetics Center; |
| CI | chemotaxis index; |
| ΔΨ | mitochondrial membrane potential; |
| UPRER | reticulum unfolded protein response; |
| AD | Alzheimer’s disease. |
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Andreux, P.A.; Houtkooper, R.H.; Auwerx, J. Pharmacological approaches to restore mitochondrial function. Nat. Rev. Drug Discov. 2013, 12, 465–483. [Google Scholar] [CrossRef]
- Jang, J.Y.; Blum, A.; Liu, J.; Finkel, T. The role of mitochondria in aging. J. Clin. Investig. 2018, 128, 3662–3670. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J. Biological Effects of Tetrahydroxystilbene Glucoside: An Active Component of a Rhizome Extracted from Polygonum multiflorum. Oxidative Med. Cell Longev. 2018, 2018, 3641960. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.L.; Chen, X.Y.; Cao, J.J.; Cui, X.H.; Wang, H.B. Polygonum multiflorum Thunb extract extended the lifespan and healthspan of Caenorhabditis elegans via DAF-16/SIR-2.1/SKN-1. Food Funct. 2021, 12, 8774–8786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Qin, Z.C.; Sun, Y.Y.; Chen, Y.S.; Chen, W.B.; Wang, H.G.; An, D.; Sun, D.; Liu, Y.Q. Genistein Promotes Anti-Heat Stress and Antioxidant Effects via the Coordinated Regulation of IIS, HSP, MAPK, DR, and Mitochondrial Pathways in Caenorhabditis elegans. Antioxidants 2023, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, E.; Goodarzi, M.; Saremi, B.; Lin, R.; Mirzaei, H. Hydralazine targets cAMP-dependent protein kinase leading to sirtuin1/5 activation and lifespan extension in C. elegans. Nat. Commun. 2019, 10, 4905. [Google Scholar] [CrossRef]
- Ogawa, T.; Kodera, Y.; Hirata, D.; Blackwell, T.K.; Mizunuma, M. Natural thioallyl compounds increase oxidative stress resistance and lifespan in Caenorhabditis elegans by modulating SKN-1/Nrf. Sci. Rep. 2016, 6, 21611. [Google Scholar] [CrossRef]
- Dostal, V.; Link, C.D. Assaying β-amyloid toxicity using a transgenic C. elegans model. J. Vis. Exp. 2010, 44, e2252. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.; Butko, P.; Christen, Y.; Lambert, M.P.; Klein, W.L.; Link, C.D.; Luo, Y. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J. Neurosci. 2006, 26, 13102–13113. [Google Scholar] [CrossRef]
- Jadiya, P.; Chatterjee, M.; Sammi, S.R.; Kaur, S.; Palit, G.; Nazir, A. Sir-2.1 modulates ‘calorie-restriction-mediated’ prevention of neurodegeneration in Caenorhabditis elegans: Implications for Parkinson’s disease. Biochem. Biophys. Res. Commun. 2011, 413, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Rolland, S.G. How to analyze mitochondrial morphology in healthy cells and apoptotic cells in Caenorhabditis elegans. Methods Enzymol. 2014, 544, 75–98. [Google Scholar] [PubMed]
- Cong, W.; Wang, P.; Qu, Y.; Tang, J.; Bai, R.; Zhao, Y.; Chunying, C.; Bi, X. Evaluation of the influence of fullerenol on aging and stress resistance using Caenorhabditis elegans. Biomaterials 2015, 42, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.A.; Coppotelli, G. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 2015, 521, 525–528. [Google Scholar] [CrossRef]
- Davinelli, S.; De Stefani, D.; De Vivo, I.; Scapagnini, G. Polyphenols as Caloric Restriction Mimetics Regulating Mitochondrial Biogenesis and Mitophagy. Trends Endocrinol. Metab. TEM 2020, 31, 536–550. [Google Scholar] [CrossRef]
- Connell, N.J.; Houtkooper, R.H.; Schrauwen, P. NAD(+) metabolism as a target for metabolic health: Have we found the silver bullet? Diabetologia 2019, 62, 888–899. [Google Scholar] [CrossRef]
- Ning, Z.; Li, Y.; Liu, D.; Owoicho Orgah, J.; Zhu, J.; Wang, Y.; Zhu, Y. Tetrahydroxystilbene Glucoside Delayed Senile Symptoms in Old Mice via Regulation of the AMPK/SIRT1/PGC-1α Signaling Cascade. Gerontology 2018, 64, 457–465. [Google Scholar] [CrossRef]
- Yu, N.; Pasha, M.; Chua, J.J.E. Redox changes and cellular senescence in Alzheimer’s disease. Redox Biol. 2024, 70, 103048. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.Y.W.; Wai, T.; Simonsen, A. Quality control of the mitochondrion. Dev. Cell 2021, 56, 881–905. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Yamano, K. Molecular mechanisms and physiological functions of mitophagy. Embo J. 2021, 40, e104705. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Bai, Y.; Sun, X.; Fu, R.; Liu, L.; Liu, M.; Li, Z.; Huang, X. Resveratrol Reestablishes Mitochondrial Quality Control in Myocardial Ischemia/Reperfusion Injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1α Pathway. Molecules 2022, 27, 5545. [Google Scholar] [CrossRef]
- Fang, E.F.; Waltz, T.B.; Kassahun, H.; Lu, Q.; Kerr, J.S.; Morevati, M.; Fivenson, E.M.; Wollman, B.N.; Marosi, K.; Wilson, M.A.; et al. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 2017, 7, 46208. [Google Scholar] [CrossRef]
- Wang, H.; Webster, P.; Chen, L.; Fisher, A.L. Cell-autonomous and non-autonomous roles of daf-16 in muscle function and mitochondrial capacity in aging C. elegans. Aging 2019, 11, 2295–2311. [Google Scholar] [CrossRef]
- Yuan, Y.; Cruzat, V.F.; Newsholme, P.; Cheng, J.; Chen, Y.; Lu, Y. Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech. Ageing Dev. 2016, 155, 10–21. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Hoeflich, A. Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion 2013, 13, 755–761. [Google Scholar] [CrossRef]
- O’Neill, C.; Kiely, A.P.; Coakley, M.F.; Manning, S.; Long-Smith, C.M. Insulin and IGF-1 signalling: Longevity, protein homoeostasis and Alzheimer’s disease. Biochem. Soc. Trans. 2012, 40, 721–727. [Google Scholar] [CrossRef]
- Lehrbach, N.J.; Ruvkun, G. Endoplasmic reticulum-associated SKN-1A/Nrf1 mediates a cytoplasmic unfolded protein response and promotes longevity. eLife 2019, 8, e44425. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Lee, H.G.; Camins, A.; Pallàs, M.; Casadesus, G.; Smith, M.A.; Zhu, X. The sirtuin pathway in ageing and Alzheimer disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2011, 10, 275–279. [Google Scholar] [CrossRef]
- Solis, G.M.; Petrascheck, M. Measuring Caenorhabditis elegans life span in 96 well microtiter plates. J. Vis. Exp. 2011, 49, e2496. [Google Scholar]
- Kato, Y.; Miyaji, M.; Zhang-Akiyama, Q.M. FUdR extends the lifespan of the short-lived AP endonuclease mutant in Caenorhabditis elegans in a fertility-dependent manner. Genes Genet. Syst. 2017, 91, 201–207. [Google Scholar] [CrossRef]
- Kim, J.Y.; Le, T.A.N.; Lee, S.Y.; Song, D.G.; Hong, S.C.; Cha, K.H.; Lee, J.W. 3,3′-Diindolylmethane Improves Intestinal Permeability Dysfunction in Cultured Human Intestinal Cells and the Model Animal Caenorhabditis elegans. J. Agric. Food Chem. 2019, 67, 9277–9285. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Liu, W. Crotamiton derivative JM03 extends lifespan and improves oxidative and hypertonic stress resistance in Caenorhabditis elegans via inhibiting OSM-9. eLife 2022, 11, e72410. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Wei, C.; Gao, Y.; Chen, X.; Zhong, K.; Li, Y.; Yang, Z.; Gao, Y.; Wang, H. TSG Extends the Longevity of Caenorhabditis elegans by Targeting the DAF-16/SKN-1/SIR-2.1-Mediated Mitochondrial Quality Control Process. Antioxidants 2024, 13, 1086. https://doi.org/10.3390/antiox13091086
Sun M, Wei C, Gao Y, Chen X, Zhong K, Li Y, Yang Z, Gao Y, Wang H. TSG Extends the Longevity of Caenorhabditis elegans by Targeting the DAF-16/SKN-1/SIR-2.1-Mediated Mitochondrial Quality Control Process. Antioxidants. 2024; 13(9):1086. https://doi.org/10.3390/antiox13091086
Chicago/Turabian StyleSun, Menglu, Congmin Wei, Yehui Gao, Xinyan Chen, Kaixin Zhong, Yingzi Li, Zhou Yang, Yihuai Gao, and Hongbing Wang. 2024. "TSG Extends the Longevity of Caenorhabditis elegans by Targeting the DAF-16/SKN-1/SIR-2.1-Mediated Mitochondrial Quality Control Process" Antioxidants 13, no. 9: 1086. https://doi.org/10.3390/antiox13091086




