10-Gingerol Increases Antioxidant Enzymes and Attenuates Lipopolysaccharide-Induced Inflammation by Modulating Adipokines in 3T3-L1 Adipocytes
Abstract
:1. Introduction
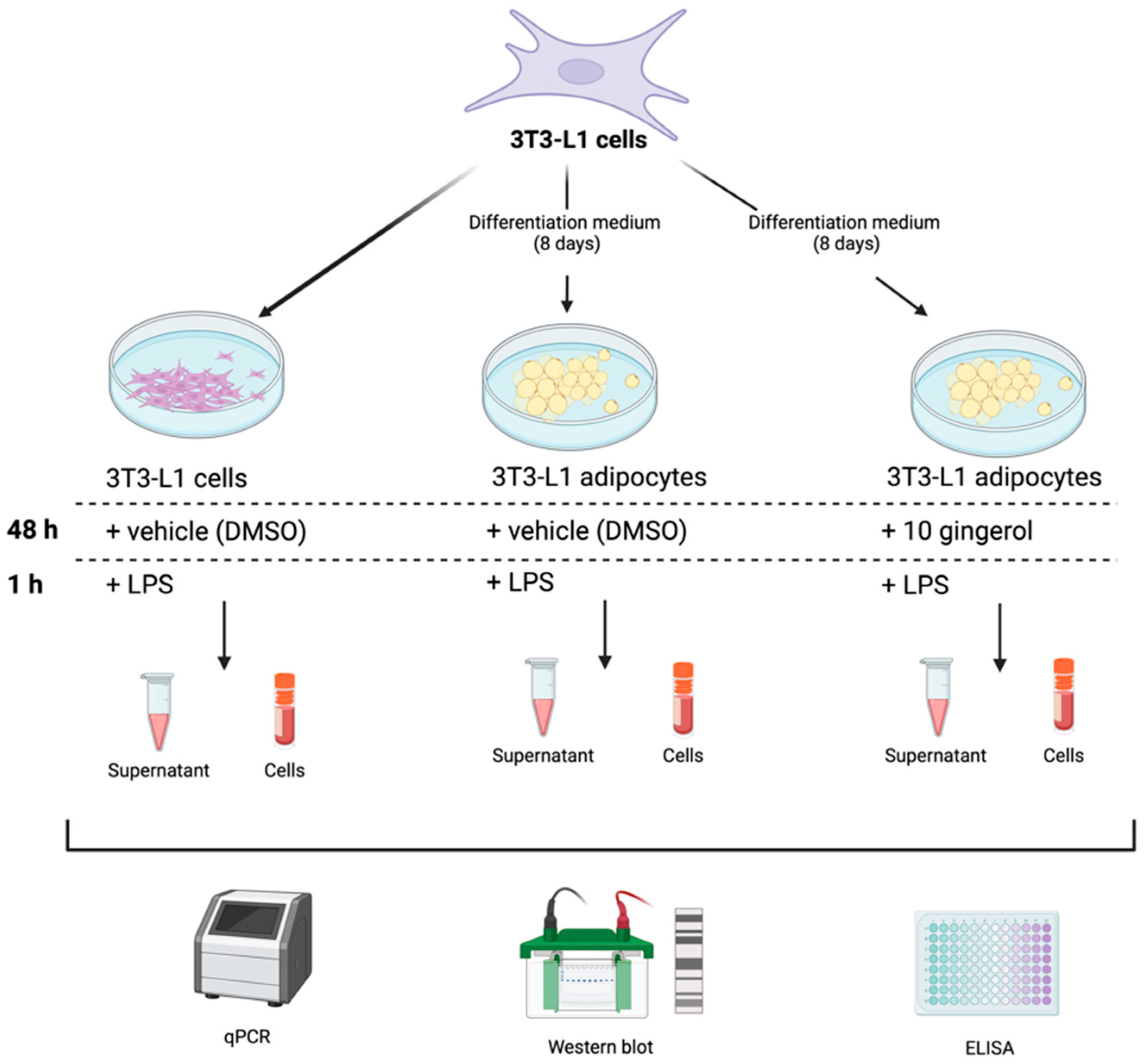
2. Materials and Methods
2.1. 10-Gingerol Preparation
2.2. Antioxidant and Free Radical Scavenging Activities of 10-Gingerol
2.2.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.2.2. Ferric Reducing Antioxidant Power (FRAP)
2.2.3. DPPH Radical Scavenging Assay
2.3. Cell Culture
2.3.1. Culture and Differentiation of 3T3-L1 Cells
2.3.2. 3T3-L1 Cell Treatments
2.4. Antioxidant Enzyme Expression
2.4.1. mRNA Expression by Quantitative Polymerase Chain Reaction (qPCR)
2.4.2. Western Blot Analysis
2.5. Adipokine and Pro-Inflammatory Cytokine Concentrations
2.6. Statistical Analysis
3. Results
3.1. 10-Gingerol Showed Low Antioxidant and Free Radical Scavenging Activity
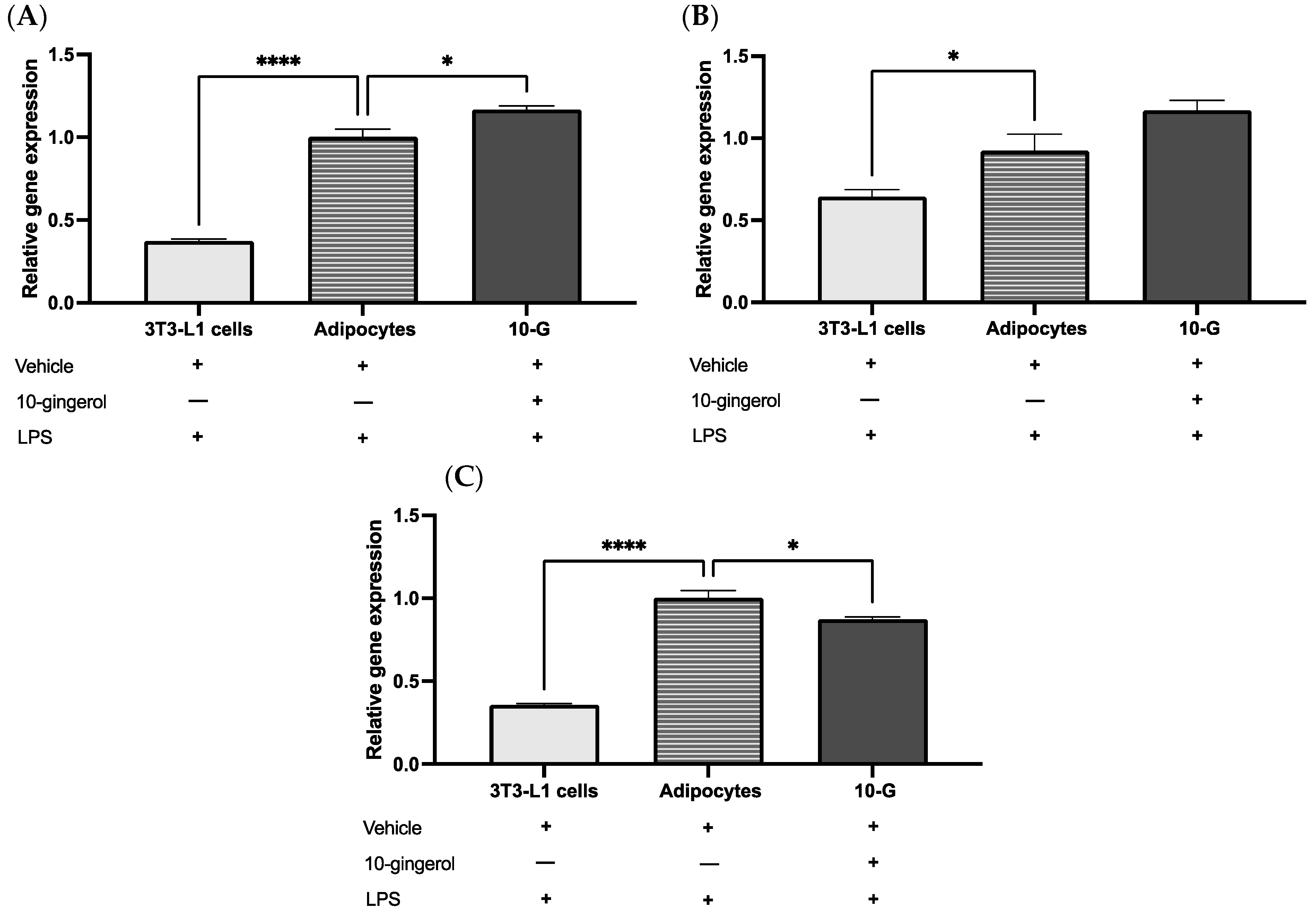
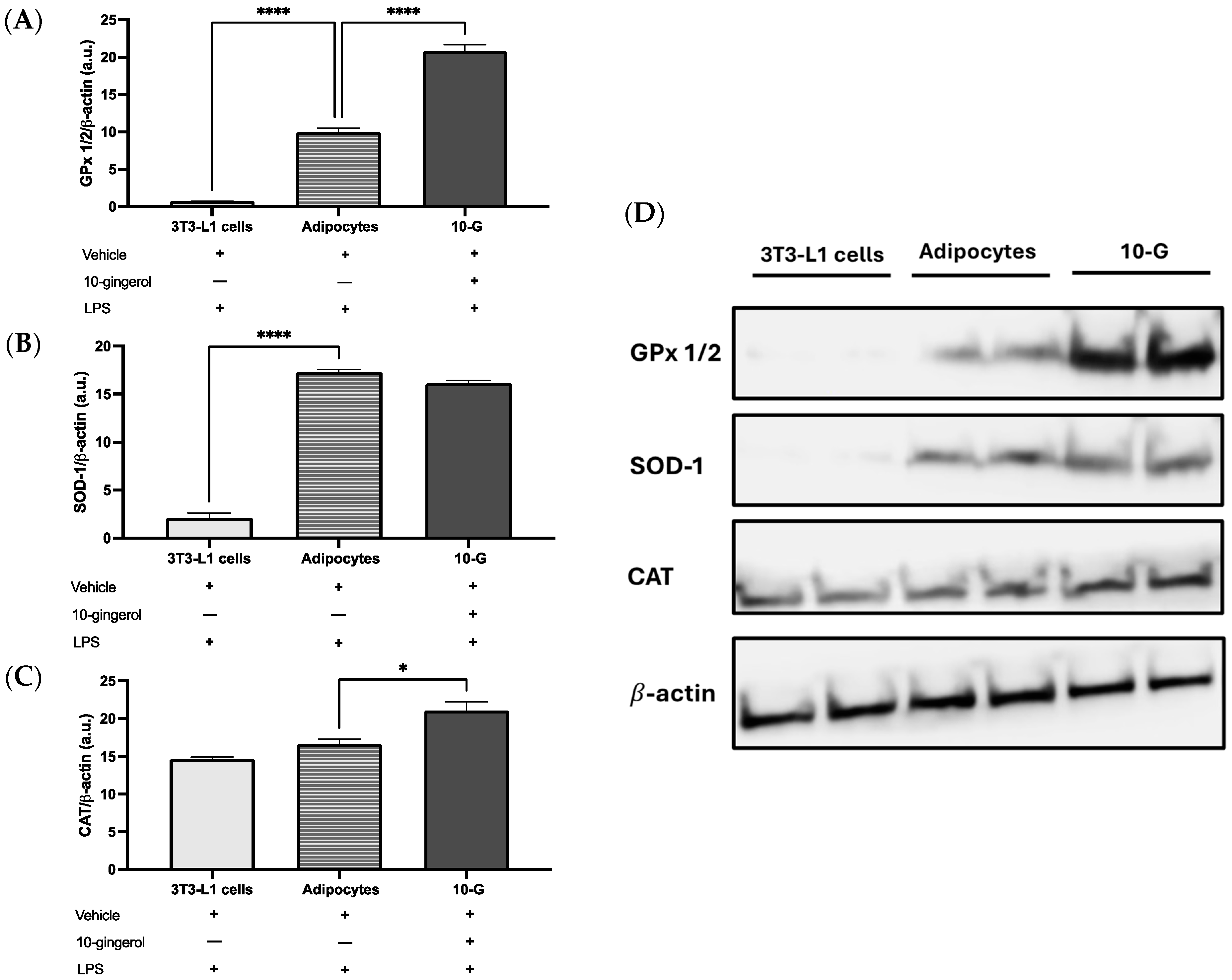
3.2. 10-Gingerol Modulates the Expression of Antioxidant Enzymes in 3T3-L1 Adipocytes Exposed to LPS
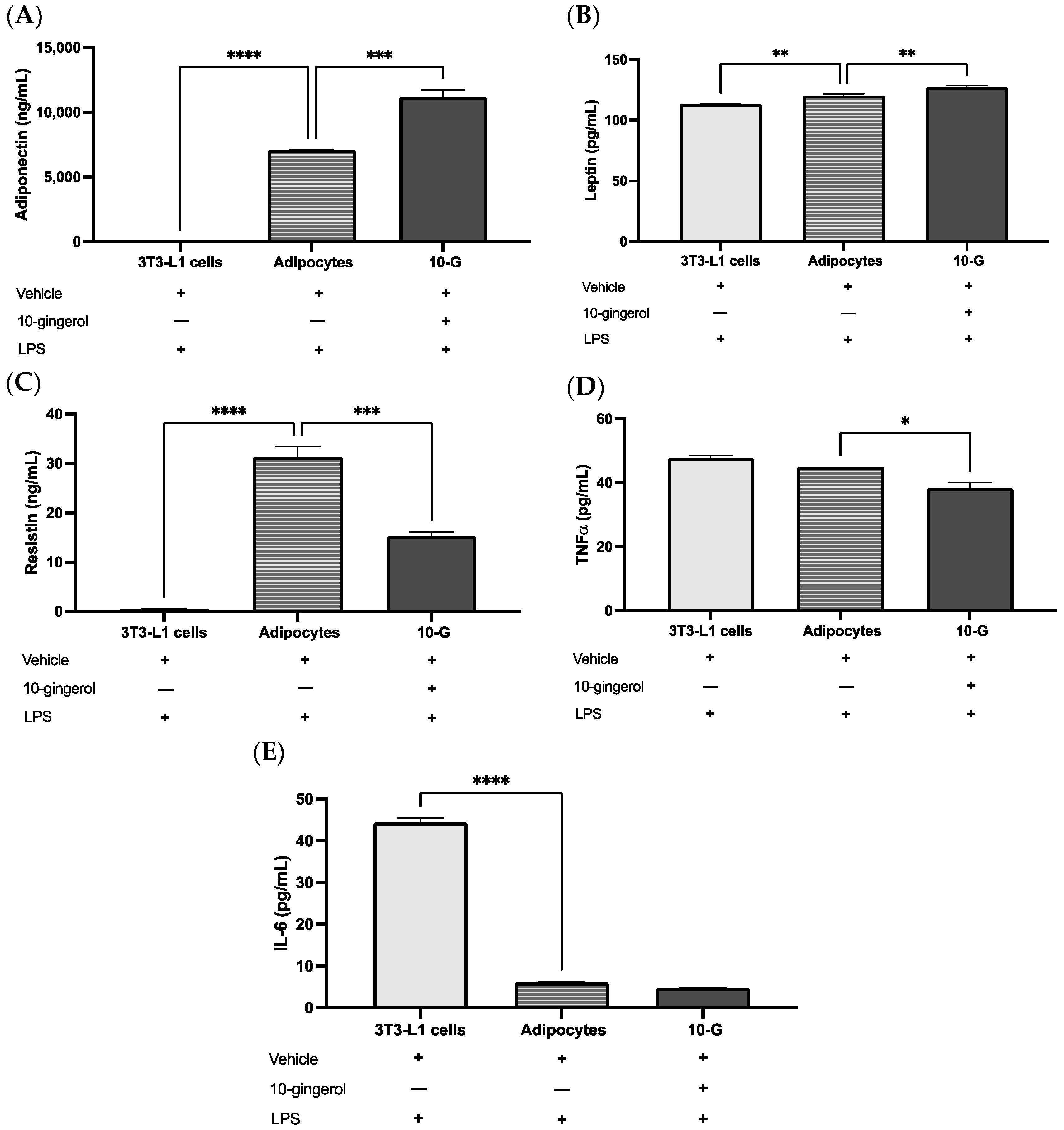
3.3. 10-G Ameliorates LPS-Induced Inflammation by Regulating Adipocytokines in 3T3-L1 Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 5 July 2024).
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative Stress: The Nexus of Obesity and Cognitive Dysfunction in Diabetes. Front. Endocrinol. 2023, 14, 1134025. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose Tissue in Control of Metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and Metabolic Health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- De Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial Dysfunction in Obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Kasperczyk, S.; Osadnik, T.; Pawlas, N. Oxidative Stress in Association with Metabolic Health and Obesity in Young Adults. Oxid. Med. Cell Longev. 2021, 2021, 9987352. [Google Scholar] [CrossRef]
- Foncea, R.; Carvajal, C.; Almarza, C.; Leighton, F. Endothelial Cell Oxidative Stress and Signal Transduction. Biol. Res. 2000, 33, 89–96. [Google Scholar] [CrossRef]
- Bowie, A.; O’Neill, L.A.J. Oxidative Stress and Nuclear Factor-κB Activation. Biochem. Pharmacol. 2000, 59, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Korac, B.; Kalezic, A.; Pekovic-Vaughan, V.; Korac, A.; Jankovic, A. Redox Changes in Obesity, Metabolic Syndrome, and Diabetes. Redox Biol. 2021, 42, 101887. [Google Scholar] [CrossRef]
- Antioxidants and Phytochemicals—A Scoping Review for Nordic Nutrition Recommendations 2023|Food & Nutrition Research. Available online: https://foodandnutritionresearch.net/index.php/fnr/article/view/10324 (accessed on 5 July 2024).
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Tzeng, T.-F.; Liu, I.-M. 6-Gingerol Prevents Adipogenesis and the Accumulation of Cytoplasmic Lipid Droplets in 3T3-L1 Cells. Phytomedicine 2013, 20, 481–487. [Google Scholar] [CrossRef]
- Foods|Free Full-Text|Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Available online: https://www.mdpi.com/2304-8158/8/6/185 (accessed on 5 July 2024).
- Preciado-Ortiz, M.E.; Martinez-Lopez, E.; Rodriguez-Echevarría, R.; Perez-Robles, M.; Gembe-Olivarez, G.; Rivera-Valdés, J.J. 10-Gingerol, a Novel Ginger Compound, Exhibits Antiadipogenic Effects without Compromising Cell Viability in 3T3-L1 Cells. Biomed. Rep. 2023, 19, 105. [Google Scholar] [CrossRef]
- Suk, S.; Kwon, G.T.; Lee, E.; Jang, W.J.; Yang, H.; Kim, J.H.; Thimmegowda, N.R.; Chung, M.-Y.; Kwon, J.Y.; Yang, S.; et al. Gingerenone A, a Polyphenol Present in Ginger, Suppresses Obesity and Adipose Tissue Inflammation in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2017, 61, 1700139. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Zhang, Y. Research Progress on Chemical Constituents of Zingiber officinale Roscoe. BioMed Res. Int. 2019, 2019, 5370823. [Google Scholar] [CrossRef]
- Baliga, M.S.; Haniadka, R.; Pereira, M.M.; D’Souza, J.J.; Pallaty, P.L.; Bhat, H.P.; Popuri, S. Update on the Chemopreventive Effects of Ginger and Its Phytochemicals. Crit. Rev. Food Sci. Nutr. 2011, 51, 499–523. [Google Scholar] [CrossRef]
- Yücel, Ç.; Karatoprak, G.Ş.; Açıkara, Ö.B.; Akkol, E.K.; Barak, T.H.; Sobarzo-Sánchez, E.; Aschner, M.; Shirooie, S. Immunomodulatory and Anti-Inflammatory Therapeutic Potential of Gingerols and Their Nanoformulations. Front. Pharmacol. 2022, 13, 902551. [Google Scholar] [CrossRef]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-Gingerol, 8-Gingerol, 10-Gingerol, and 6-Shogaol and Conjugate Metabolites in Healthy Human Subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rojas, B.; Gómez-Sierra, T.; Medina-Campos, O.N.; Hernández-Juárez, J.; Hernández-Cruz, P.A.; Gallegos-Velasco, I.B.; Pérez-Cervera, Y.; Pedraza-Chaverri, J. Antioxidant Activity of Glucosamine and Its Effects on ROS Production, Nrf2, and O-GlcNAc Expression in HMEC-1 Cells. Curr. Res. Toxicol. 2023, 5, 100128. [Google Scholar] [CrossRef]
- Ignacio-Mejía, I.; Contreras-García, I.J.; Mendoza-Torreblanca, J.G.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; García-Cruz, M.E.; Romo-Mancillas, A.; Gómez-Manzo, S.; Bandala, C.; Sánchez-Mendoza, M.E.; et al. Evaluation of the Antioxidant Activity of Levetiracetam in a Temporal Lobe Epilepsy Model. Biomedicines 2023, 11, 848. [Google Scholar] [CrossRef] [PubMed]
- Jumina, J.; Siswanta, D.; Zulkarnain, A.K.; Triono, S.; Priatmoko, P.; Yuanita, E.; Fatmasari, N.; Nursalim, I. Development of C-Arylcalix [4]Resorcinarenes and C-Arylcalix [4]Pyrogallolarenes as Antioxidant and UV-B Protector. Indones. J. Chem. 2019, 19, 273–284. [Google Scholar] [CrossRef]
- Alliin, a Garlic (Allium sativum) Compound, Prevents LPS-Induced Inflammation in 3T3-L1 Adipocytes—Quintero-Fabián—2013—Mediators of Inflammation—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1155/2013/381815 (accessed on 5 July 2024).
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yap, V.L.; Tan, L.F.; Rajagopal, M.; Wiart, C.; Selvaraja, M.; Leong, M.Y.; Tan, P.L. Evaluation of Phytochemicals and Antioxidant Potential of a New Polyherbal Formulation TC-16: Additive, Synergistic or Antagonistic? BMC Complement. Med. Ther. 2023, 23, 93. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant Capacity of Vegetables, Spices and Dressings Relevant to Nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Antioxidant Activities, Total Phenolics and Flavonoids Content in Two Varieties of Malaysia Young Ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Liew, S.S.; Ho, W.Y.; Yeap, S.K.; Sharifudin, S.A.B. Phytochemical Composition and in Vitro Antioxidant Activities of Citrus Sinensis Peel Extracts. PeerJ 2018, 6, e5331. [Google Scholar] [CrossRef] [PubMed]
- Tinello, F.; Zannoni, S.; Lante, A. Antioxidant Properties of Soybean Oil Supplemented with Ginger and Turmeric Powders. Appl. Sci. 2020, 10, 8438. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Ayustaningwarno, F.; Anjani, G.; Ayu, A.M.; Fogliano, V. A Critical Review of Ginger’s (Zingiber officinale) Antioxidant, Anti-Inflammatory, and Immunomodulatory Activities. Front. Nutr. 2024, 11, 1364836. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Fang, F.; Kang, I. Ginger (Zingiber officinale) Attenuates Obesity and Adipose Tissue Remodeling in High-Fat Diet-Fed C57BL/6 Mice. Int. J. Environ. Res. Public Health 2021, 18, 631. [Google Scholar] [CrossRef]
- Limón-Pacheco, J.; Gonsebatt, M.E. The Role of Antioxidants and Antioxidant-Related Enzymes in Protective Responses to Environmentally Induced Oxidative Stress. Mutat. Res. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Integrated Chromatographic Approach for the Discovery of Gingerol Antioxidants from Dracocephalum Heterophyllum and Their Potential Targets—Analytical Methods (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2022/ay/d2ay01282k (accessed on 5 July 2024).
- Abdurrahim, A.E.; Mazurak, V.C.; Chen, L. Gingerols Synergize with Anthocyanins to Induce Antioxidant Activity in Vitro. Front. Nutr. 2023, 10, 1229015. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Sadeghi, E.; Agah, S.; Fazelian, S.; Rahimlou, M.; Kern, F.G.; Heshmati, S.; Omidi, A.; Persad, E.; Heshmati, J. Effect of Ginger (Zingiber officinale) Supplementation on Oxidative Stress Parameters: A Systematic Review and Meta-Analysis. J. Food Biochem. 2021, 45, e13612. [Google Scholar] [CrossRef]
- Scheja, L.; Heeren, J. The Endocrine Function of Adipose Tissues in Health and Cardiometabolic Disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef]
- Kim, B.; Kim, H.-J.; Cha, Y.-S. The Protective Effects of Steamed Ginger on Adipogenesis in 3T3-L1 Cells and Adiposity in Diet-Induced Obese Mice. Nutr. Res. Pract. 2021, 15, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Brahma Naidu, P.; Uddandrao, V.V.S.; Ravindar Naik, R.; Suresh, P.; Meriga, B.; Begum, M.S.; Pandiyan, R.; Saravanan, G. Ameliorative Potential of Gingerol: Promising Modulation of Inflammatory Factors and Lipid Marker Enzymes Expressions in HFD Induced Obesity in Rats. Mol. Cell. Endocrinol. 2016, 419, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, S.; Munika, E.; Wulandari, E.T.; Ferdinal, F.; Purwaningsih, E.H.; Wuyung, P.E.; Louisa, M.; Soetikno, V. 6-Gingerol Ameliorates Weight Gain and Insulin Resistance in Metabolic Syndrome Rats by Regulating Adipocytokines. Saudi Pharm. J. 2023, 31, 351–358. [Google Scholar] [CrossRef]
- Isa, Y.; Miyakawa, Y.; Yanagisawa, M.; Goto, T.; Kang, M.-S.; Kawada, T.; Morimitsu, Y.; Kubota, K.; Tsuda, T. 6-Shogaol and 6-Gingerol, the Pungent of Ginger, Inhibit TNF-α Mediated Downregulation of Adiponectin Expression via Different Mechanisms in 3T3-L1 Adipocytes. Biochem. Biophys. Res. Commun. 2008, 373, 429–434. [Google Scholar] [CrossRef]
- Jolad, S.D.; Lantz, R.C.; Chen, G.J.; Bates, R.B.; Timmermann, B.N. Commercially Processed Dry Ginger (Zingiber officinale): Composition and Effects on LPS-Stimulated PGE2 Production. Phytochemistry 2005, 66, 1614–1635. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rafie, A.R.; Hamama, A.; Siddiqui, R.A. Immature Ginger Reduces Triglyceride Accumulation by Downregulating Acyl CoA Carboxylase and Phosphoenolpyruvate Carboxykinase-1 Genes in 3T3-L1 Adipocytes. Food Nutr. Res. 2023, 67, 9126. [Google Scholar] [CrossRef]
- Lago, F.; Gómez, R.; Gómez-Reino, J.J.; Dieguez, C.; Gualillo, O. Adipokines as Novel Modulators of Lipid Metabolism. Trends Biochem. Sci. 2009, 34, 500–510. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Lee, G.-H.; Peng, C.; Jeong, S.-Y.; Park, S.-A.; Lee, H.-Y.; Hoang, T.-H.; Kim, J.; Chae, H.-J. Ginger Extract Controls mTOR-SREBP1-ER Stress-Mitochondria Dysfunction through AMPK Activation in Obesity Model. J. Funct. Foods 2021, 87, 104628. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. Int. Sch. Res. Not. 2013, 2013, 139239. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tsuchiya, H.; Hama, S.; Kajimoto, K.; Kogure, K. Resistin Affects Lipid Metabolism during Adipocyte Maturation of 3T3-L1 Cells. FEBS J. 2013, 280, 5884–5895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Iikuni, N.; Lam, Q.L.K.; Lu, L.; Matarese, G.; La Cava, A. Leptin and Inflammation. Curr. Immunol. Rev. 2008, 4, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Pirzgalska, R.M.; Pereira, M.M.A.; Kubasova, N.; Barateiro, A.; Seixas, E.; Lu, Y.-H.; Kozlova, A.; Voss, H.; Martins, G.G.; et al. Sympathetic Neuro-Adipose Connections Mediate Leptin-Driven Lipolysis. Cell 2015, 163, 84–94. [Google Scholar] [CrossRef]
- Sayed, S.; Ahmed, M.; El-Shehawi, A.; Alkafafy, M.; Al-Otaibi, S.; El-Sawy, H.; Farouk, S.; El-Shazly, S. Ginger Water Reduces Body Weight Gain and Improves Energy Expenditure in Rats. Foods 2020, 9, 38. [Google Scholar] [CrossRef]
- Hong, K.H.; Um, M.Y.; Ahn, J.; Ha, T.Y. 6-Gingerol Ameliorates Adiposity and Inflammation in Adipose Tissue in High Fat Diet-Induced Obese Mice: Association with Regulating of Adipokines. Nutrients 2023, 15, 3457. [Google Scholar] [CrossRef]
- Gualillo, O.; Eiras, S.; Lago, F.; Diéguez, C.; Casanueva, F.F. Elevated Serum Leptin Concentrations Induced by Experimental Acute Inflammation. Life Sci. 2000, 67, 2433–2441. [Google Scholar] [CrossRef]
- Coppack, S.W. Pro-Inflammatory Cytokines and Adipose Tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative Antioxidant and Anti-Inflammatory Effects of [6]-Gingerol, [8]-Gingerol, [10]-Gingerol and [6]-Shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-C.; Chang, K.-S.; Lin, C.-C. Anti-Neuroinflammatory Capacity of Fresh Ginger Is Attributed Mainly to 10-Gingerol. Food Chem. 2013, 141, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.-J.; Ok, S.; Jun, M.; Jeong, W.-S. 6-Shogaol-Rich Extract from Ginger up-Regulates the Antioxidant Defense Systems in Cells and Mice. Molecules 2012, 17, 8037–8055. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Kim, M.H.; Hong, J.; Kim, S.-H.; Yang, W.M. Dried Ginger (Zingiber officinalis) Inhibits Inflammation in a Lipopolysaccharide-Induced Mouse Model. Evid.-Based Complement. Altern. Med. 2013, 2013, 914563. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preciado-Ortiz, M.E.; Martínez-López, E.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Rodríguez-Echevarría, R.; Reyes-Pérez, S.D.; Rivera-Valdés, J.J. 10-Gingerol Increases Antioxidant Enzymes and Attenuates Lipopolysaccharide-Induced Inflammation by Modulating Adipokines in 3T3-L1 Adipocytes. Antioxidants 2024, 13, 1093. https://doi.org/10.3390/antiox13091093
Preciado-Ortiz ME, Martínez-López E, Pedraza-Chaverri J, Medina-Campos ON, Rodríguez-Echevarría R, Reyes-Pérez SD, Rivera-Valdés JJ. 10-Gingerol Increases Antioxidant Enzymes and Attenuates Lipopolysaccharide-Induced Inflammation by Modulating Adipokines in 3T3-L1 Adipocytes. Antioxidants. 2024; 13(9):1093. https://doi.org/10.3390/antiox13091093
Chicago/Turabian StylePreciado-Ortiz, María Elizabeth, Erika Martínez-López, José Pedraza-Chaverri, Omar Noel Medina-Campos, Roberto Rodríguez-Echevarría, Samantha Desireé Reyes-Pérez, and Juan José Rivera-Valdés. 2024. "10-Gingerol Increases Antioxidant Enzymes and Attenuates Lipopolysaccharide-Induced Inflammation by Modulating Adipokines in 3T3-L1 Adipocytes" Antioxidants 13, no. 9: 1093. https://doi.org/10.3390/antiox13091093









