Effects and Mechanisms of Lutein on Aging and Age-Related Diseases
Abstract
1. Introduction
2. Effects and Mechanisms of Lutein on Aging
2.1. Epidemiological Studies
2.2. Experimental Studies
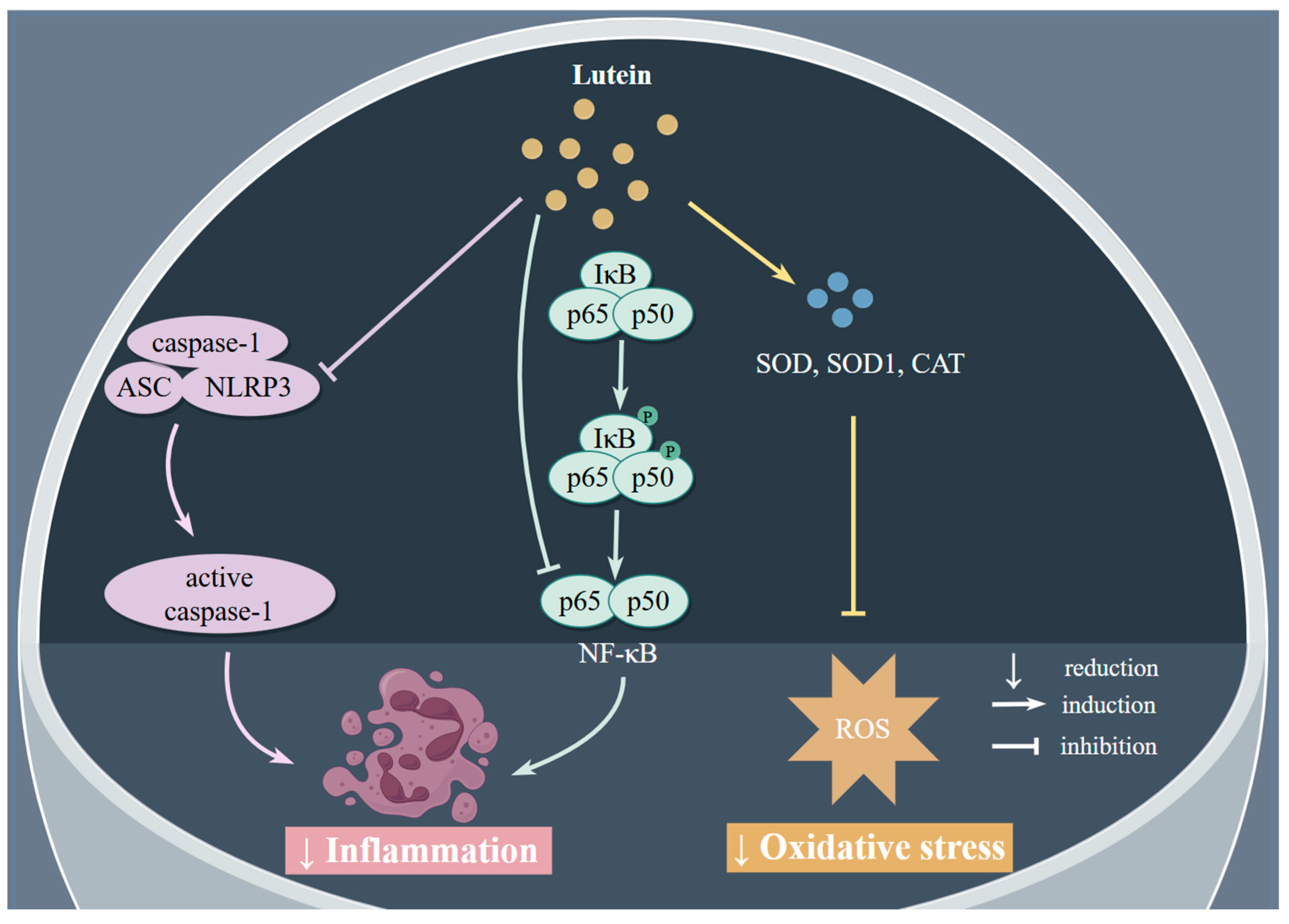
2.2.1. Antioxidant Effects
2.2.2. Anti-Inflammatory Effects
| Study Type | Model | Dose and Duration | Effects and Mechanisms | Ref. |
|---|---|---|---|---|
| Aging | ||||
| In vivo | D. melanogaster, wild-type, Oregon-R-C | 0.03, and 0.1 mg lutein/mL | ↑ mean lifespan ↓ MDA ↑ antioxidant enzyme activities ↑ expression of SOD1, SOD2, and CAT | [44] |
| In vivo | Swiss albino mice | 5, 50, 100, and 250 mg/kg b.wt for 15 days | ↑ survival time ↑ TAC in lung, brain, and liver ↑ CAT activity ↑ glutathione in brain and lung ↓ MDA protected liver and kidney function | [45] |
| In vivo | C. elegans | 1, 10, 100, 250, and 500 µg/L for 30 min | ↓ ROS ↓ CAT ↓ survival loss | [46] |
| In vivo | C. elegans | 10 and 100 µM | ↑ survival rate ↑ lifespan ↓ ROS ↑ CAT, neuroligin 1 | [47] |
| In vitro and in vivo | Mesenchymal stem cells | 10, 20, 30, 50, and 100 µM | ↑ growth rate, cell proliferation, cell viability ↓ SA-β-gal-positive cells ↓ p21, p16, and p53 ↑ expression of Clock gene ↓ TNF-α, IL-1β, and IL-6 ↓ NF-κB and NLRP3 ↓ ROS, MDA | [49] |
| AMD | ||||
| In vitro | H2O2-induced ARPE-19 cells | 5, 10, and 20 µM for 3 days | ↓ ROS ↓ production of SA-β-gal ↓ G2 arrest ↑ HO-1 and NQO1 ↑ activation of Nrf2 ↓ p53–p21 pathway | [50] |
| In vitro | H2O2-induced ARPE-19 cells | 0, 2.5, 5, 10, 20, and 40 µM for 24 h | ↓ omega-6 PUFA oxidation ↓ pro-inflammatory HETE ↓ Isop ↓ transcriptional regulation of GPx and NFE2L2 | [51] |
| In vivo | Male Wistar rats | 39 nmol/d for 8 weeks | ↓ VEGF ↑ SOD2 ↓ abnormalities in ganglion cell and diabetic retina ↓ mRNA expression of Hif1α and Xbp1 | [52] |
| In vivo | Male SD rats | 25, 50, and 100 mg/kg body weight for 30 days | Attenuated decrease in electroretinogram a-wave and b-wave amplitudes and thinning of photoreceptor cell layer caused by apoptosis ↓ light-induced oxidative stress ↓ inflammatory cytokine levels ↑ expression of BCO2 | [53] |
| In vitro | Human primary corneal epithelial cells (HCE-F) | 50, 100, and 250 µM | ↓ ROS ↓ apoptotic cell death ↑ Nrf2, ratio of Nrf2/Keap1 ↓ Keap1 | [54] |
| In vitro | NIH/3T3 Swiss albino mouse fibroblast cells | 0, 0.01, 0.1, 1, and 10 µM lutein for 6 h | ↓ ROS | [55] |
| In vivo | Abca4(−/−)/orAbca4(−/−)/Bco2(−/−) double-knockout C57BL/6 mice | 1 g/kg of diet for 3 months | ↓ A2E and iso-A2E ↑ visual performance | [56] |
| In vitro | Rat Muller cells | 2.5, 5, 10, and 20 µM for 24 h | ↑ cell viability ↓ cell apoptosis ↑ Bcl-2/Bax ratio ↓ caspase-3 ↓ LC3II ↓ autophagosome formation ↑ p-mTOR/mTOR | [57] |
| In vitro | ARPE cells | 0.1, 0.5, 1.5, and 10 µM for 24 h | ↓ expression of TXNIP, CXCL8, BAX, CASP1 ↑ expression of BCL2 | [58] |
| In vitro | ARPE-19 cells | 1 µM | ↓ ERS ↑ IRE1-XBP1 pathway ↑ ATF6 ↑ ATF4 | [59] |
| Cataract | ||||
| In vitro | Human lens epithelial cells | 5 µM for 48 h | ↓ protein carbonyl ↓ MDA ↓ DNA damage ↑ GSH and GSH: GSSG ratio ↓ H2O2-induced cell death | [60] |
| In vivo and in vitro | Shumiya cataract rats and human lens epithelial cells | In vivo: 2 mg/kg body weight for 3 weeks In vitro: 5, 10 µM for 48 h | ↓ mRNA levels of peroxiredoxin 6 and catalase in both models | [61] |
| In vitro | Human lens epithelial cells | 2 mmol/L for 4 h | ↓ JNK, p38 ↓ lipid peroxidation | [62] |
| In vivo | Type 1 diabetic rat | Short-term: 10 mg/kg body weight for 29 days Long-term: 0.4 mg/kg body weight for 69 days | ↓ N-epsilon-(carboxymethyl)lysine ↓ N-epsilon-(carboxyethyl)lysine | [63] |
| AD | ||||
| In vivo | Male Wistar rats | 5 mg/kg body weight daily for 1 month | ↓ MDA ↓ total oxidative status ↑ TAC ↑ passive avoidance learning, spatial memory in Morris water maze and Barnes maze tests, and cognitive memory | [64] |
| In vivo | Zebrafish/female mice | Zebrafish: 0.93, 1.56 mg/L for 10 days Mice: 285, 668 mg/kg for 10 days | ↑ escape spatial learning and memory ↓ brain AChE activity ↑ glutathione ↑ activity of SOD | [65] |
| In vivo | Wistar rats | 100 mg/kg for 8 weeks | ↓ MDA ↑ antioxidant enzyme activities ↑ Nrf2 and HO-1 ↓ NF-κB | [66] |
| In vitro | Cerebrovascular endothelial cells | 0.8 µM for 12 h | ↑ cell viability ↓ ROS and lipid peroxides ↓ NF-κB ↑ Nrf2, NQO1, and HO-1 ↓ apoptosis | [67] |
| In vitro | BV-2 cells | 2.5, 5, 7.5, and 10 ng/µL for 24 h | ↓ ROS ↓ IL-1β, TNF-α ↑ IL-4 | [68] |
| In vitro | SH-SY5Y cells | 2.5, 5, 7.5, and 10 ng/µL for 24 h | ↓ ROS ↓ CAT activity ↓ TNF-α, IL-6, IL-8 ↓ HAMP ↓ Glu-induced accumulation of iron ↓ lipoxygenases | [69] |
| In vitro | Rat PC-12 cells | 0.2, 2, 20, and 200 µM for 2 h | ↑ cell viability ↓ ROS ↓ apoptosis ↑ Bcl-2 ↓ active caspase-3/7 level ↓ MAPK pathways (pERK1/2, p-p38, p-JNK) | [70] |
| In vivo | Male C57BL/6 mice | 5, 10, and 20 mg/kg body weight/day for 7 days | ↓ loss of nigral dopaminergic neurons ↑ striatal dopamine level ↓ MPTP-induced mitochondrial dysfunction ↓ oxidative stress and motor abnormalities ↓ MPTP-induced neuronal damage/apoptosis ↓ pro-apoptotic markers (Bax, caspases-3, 8,9) ↑ anti-apoptotic marker (Bcl-2) | [71] |
| In vitro | PC12 cells | 5, 10, 20 µM for 2 h | ↓ oxidative damage and apoptosis ↓ caspase-3, caspase-9, Baxc-caspase-3 ↑ Bcl-2/Bax ratio, Bcl-2 ↑ PI3K, Akt PI3K inhibitor abolished protective effect of lutein | [72] |
| In vitro | PC12 cells | 20 µM for 2 h | ↓ H2O2-mediated growth inhibition and morphological changes ↓ mRNA expression of AMAD10 and Bax ↓ phosphorylation of JNK1/2 | [73] |
| In vitro | SH-SY5Y cells | 0.1, 1, and 10 µM for 24 h | ↑ glutathione ↓ ROS Protected against mitochondrial uncoupling | [74] |
| In vivo | C. elegans | 1 µM for 6 days | ↓ neurodevelopmental deficits Restored mitochondrial dysfunction-induced neuroligin expression | [75] |
| In vivo | Female Sprague-Dawley rats | 50 or 100 mg/kg for 14 days | ↑ body weight ↑ neurobehavioral alterations ↑ attenuated oxidative stress ↑ mitochondrial enzyme complex activities of rat brain Neuroprotective effect | [76] |
| In vitro | SH-SY5Y cells | 5 µM for 72 h | ↑ differentiation of SH-SY5Y cells ↑ pAkt ↑ microtubule-associated protein 2 ↑ ROS ↑ glucose consumption, rates of glycolysis ↑ respiratory activity of mitochondrial complexes ↑ acetyl-CoA, PDH expression, HK activity | [77] |
| In vitro | 10, 20, and 50 µM for 24 h | ↓ Aβ fibril formation | [78] | |
| In vivo | Wistar rats | 50 mg/kg for 14 days | Reversed memory deficit ↓ activity of AChE | [79] |
| PD | ||||
| In vivo | Rotenone-induced Drosophila melanogaster | 6 µM for 7 days | ↑ survival rate ↑ dopamine levels ↑ tyrosine hydroxylase ↑ activity of AchE ↑ SOD, CAT activity ↓ thiobarbituric acid reactive substances and glutathione S-transferase | [80] |
| In vivo | Male C57BL/6 mice | 5, 10, and 20 mg/kg body weight/day for 7 days | ↓ loss of nigral dopaminergic neurons ↑ striatal dopamine level ↓ MPTP-induced mitochondrial dysfunction ↓ oxidative stress and motor abnormalities ↓ MPTP-induced neuronal damage/apoptosis ↓ pro-apoptotic markers (Bax, caspases-3, 8,9) ↑ anti-apoptotic marker (Bcl-2) | [71] |
| Osteoporosis | ||||
| In vivo | Ovariectomized Wistar rats | 50 mg/kg for 4 weeks | ↓ serum lipid peroxide and glutathione ↓ femur tissue lipid peroxide and ROS ↑ CAT, SOD, GST, GPx ↓ IL-6, IL-8, TNF-α ↓ NF-κB, IL-6, NFATc1 ↑ Nrf2, NQO1, HO-1 | [81] |
| In vitro | Primary rat chondrocytes | 1 µM for 24 h | Protective effect against cytotoxicity ↓ oxidative stress ↑ SOD, CAT, GST, GPx ↑ Nrf2, HO-1, and NQO1 | [82] |
| In vivo and in vitro | Newborn and 5- and 6-week-old ddy mice Primary osteoblastic cells, bone marrow cells | In vitro: 3 and 10 µM for 14 days In vivo: 66 mg/d for 4 weeks | ↑ formation of mineralized bone nodules ↓ 1α, 25-dihydroxy vitamin D3-induced bone resorption ↓ 1α, 25-dihydroxy vitamin D3-induced osteoclast formation ↓ RANKL ↑ osteoclast formation ↓ femoral bone mass in cortical bone in vivo | [83] |
| In vitro | Primary osteoblastic cells | 3, 10, and 30 µM for 24 h | ↓ expression of RANKL in osteoblasts ↓ IL-1-induced osteoclast formation and bone resorption ↓ macrophage differentiation into osteoclasts ↓ mature osteoclast survival ↑ bone formation (↑ BMP2 ↓ Sclerostin) | [84] |
| In vitro | Mononuclear cells of mouse bone marrow | 10−8, 10−7, and 10−6 mol/L for 7 days | ↓ number of osteoclast cells ↓ TRAP activity ↓ percentage of bone surface ↑ expression of RANK ↓ osteoclast differentiation in vitro | [85] |
| In vitro | Femoral diaphyseal and femoral metaphyseal tissues of male Wistar rats | 10−8–10−6 M for 48 h | ↓ metaphyseal alkaline phosphatase activity | [86] |
3. Effects and Mechanisms of Lutein on Age-Related Diseases
3.1. Lutein and Age-Related Macular Degeneration (AMD)
3.1.1. Epidemiological Studies
3.1.2. Experimental Studies
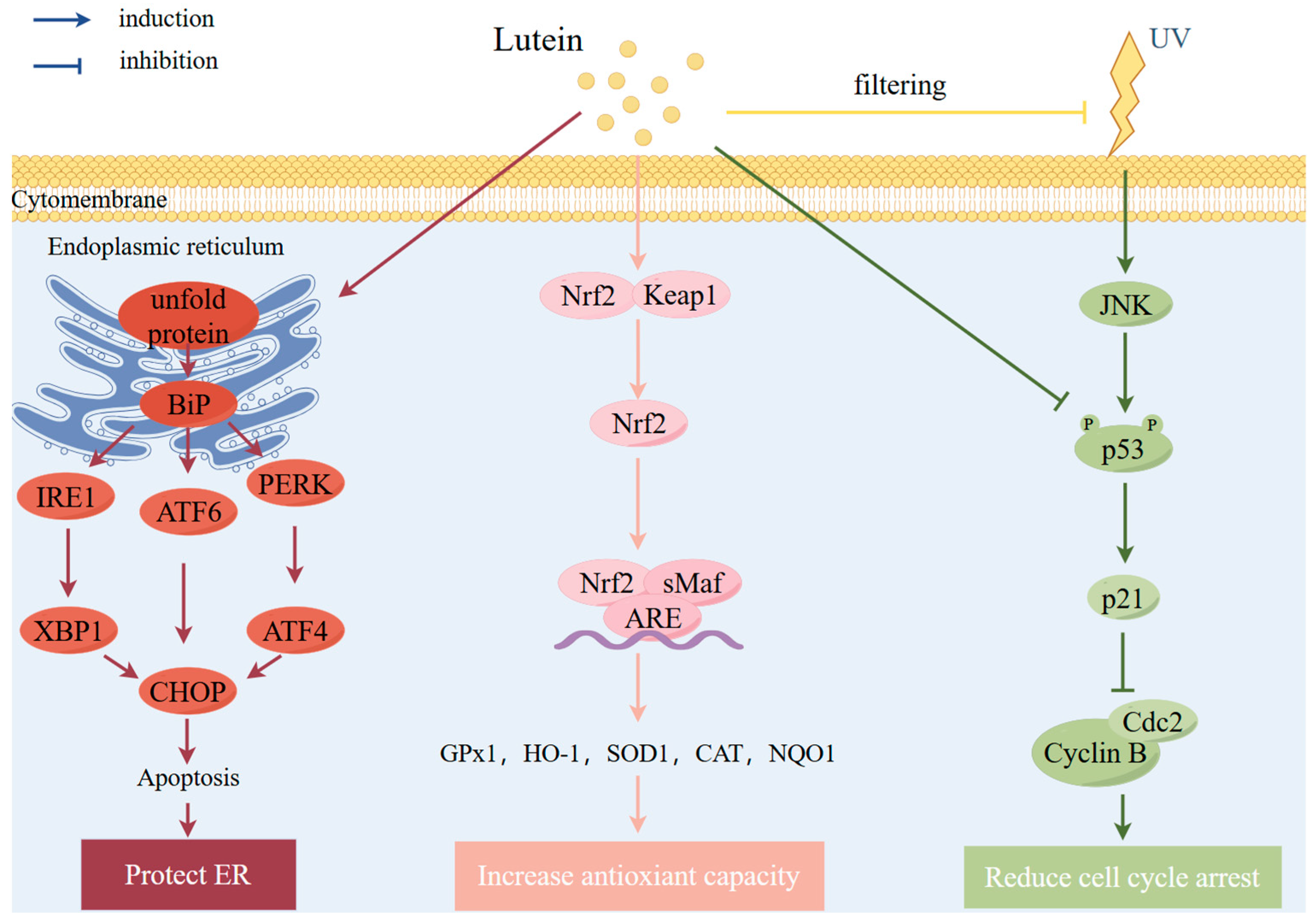
Antioxidant Effects
Protective Effects against Light Irradiation
Other Mechanisms
3.1.3. Clinical Trials
3.2. Lutein and Age-Related Cataracts
3.2.1. Epidemiological Studies
3.2.2. Experimental Studies
3.2.3. Clinical Trials
3.3. Lutein and Alzheimer’s Disease
3.3.1. Epidemiological Studies
3.3.2. Experimental Studies
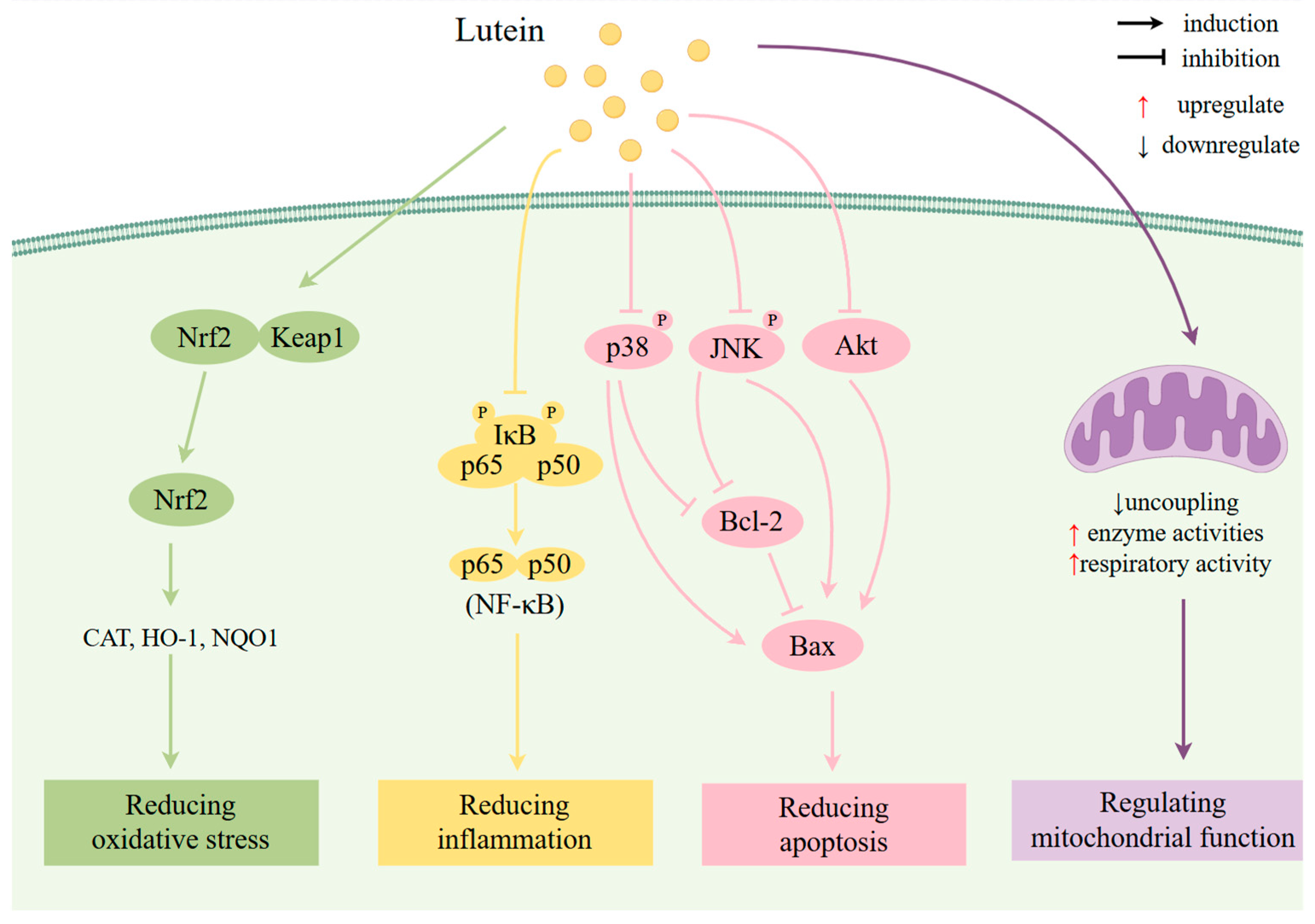
Antioxidant Effects
Anti-Inflammatory Effects
Anti-Apoptosis Effects
Regulation of Mitochondrial Function
Other Mechanisms
3.3.3. Clinical Trials
3.4. Lutein and Parkinson’s Disease
3.4.1. Epidemiological Studies
3.4.2. Experimental Studies
3.5. Lutein and Osteoporosis
3.5.1. Epidemiological Studies
3.5.2. Experimental Studies
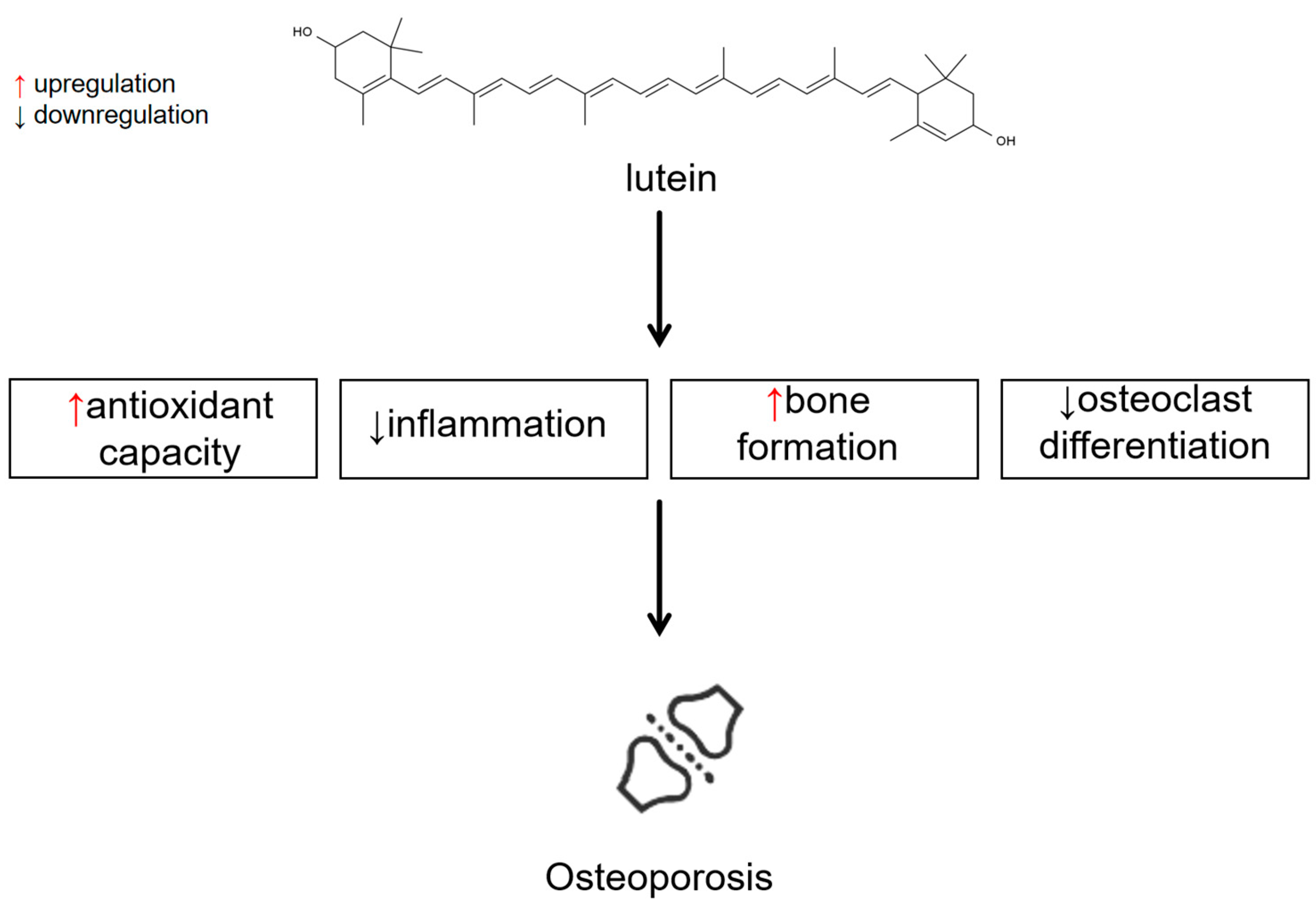
Antioxidant Effects and Anti-Inflammatory Effects
Other Mechanisms
4. Bioavailability Improvement in Lutein
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kirkwood, T.B.L.; Austad, S.N. Why do we age? Nature 2000, 408, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cai, J.P.; Zhang, L.Q.; Sun, N.; Cui, J.; Wang, H.; Yang, J.F. The mechanism of RNA oxidation involved in the development of heart failure. Free Radic. Res. 2019, 53, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Samani, N.J.; van der Harst, P. Biological ageing and cardiovascular disease. Heart 2008, 94, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K.; Knudtson, M.D.; Meuer, S.M.; Swift, M.; Gangnon, R.E. Fifteen-year cumulative incidence of age-related macular degeneration. Ophthalmology 2007, 114, 253–262. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, B.S.; Gasek, N.S.; Zhou, Y.Y.; Cohn, R.L.; Martin, D.E.; Zuo, W.L.; Flynn, W.F.; Guo, C.; Jellison, E.R.; et al. Targeting p21Cip1 highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab. 2022, 34, 186. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.K.; Ren, Y.K.; Wang, Y.Y.; Fang, J.R.; Yue, H.; Ma, S.S.; Guan, F.X. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Mitra, S.; Rauf, A.; Tareq, A.; Jahan, S.; Emran, T.B.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef]
- López-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Marsche, G.; Freudenberger, P.; Schallert, M.; Toeglhofer, A.M.; Nagl, C.; Schmidt, R.; Launer, L.J.; Schmidt, H. Association between higher plasma Lutein, zeaxanthin, and vitamin C concentrations and longer telomere length: Results of the Austrian Stroke Prevention Study. J. Am. Geriatr. Soc. 2014, 62, 222–229. [Google Scholar] [CrossRef]
- He, X.K.; Yin, X.; Chen, X.; Chen, X.L. Aging and antioxidants: The impact of dietary carotenoid intakes on soluble klotho levels in aged adults. Front. Endocrinol. 2023, 14, 1283722. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, X.T.; Zhuang, W.; Xia, L.; Chen, Y.; Wang, Y.; Wu, C.C.; Rao, Z.Y.; Du, L.; Zhao, R.; et al. Green leafy vegetable and lutein intake and multiple health outcomes. Food Chem. 2021, 360, 130145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fan, Y.H.; Li, J.; Wang, J.Q.; Kong, L.Y.; Wang, L.A.; Li, Z.F.; Ma, M.; Shi, X.; Liu, S.J.; et al. The associations of plasma carotenoids and vitamins with risk of age-related macular degeneration: Results from a matched case-control study in China and meta-Analysis. Front. Nutr. 2022, 9, 745390. [Google Scholar] [CrossRef]
- Tsika, C.; Tsilimbaris, M.K.; Makridaki, M.; Kontadakis, G.; Plainis, S.; Moschandreas, J. Assessment of macular pigment optical density (MPOD) in patients with unilateral wet age-related macular degeneration (AMD). Acta Ophthalmol. 2011, 89, E573–E578. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Cougnard-Grégoire, A.; Korobelnik, J.F.; Schalch, W.; Etheve, S.; Rougier, M.B.; Féart, C.; Samieri, C.; Delyfer, M.N.; Delcourt, C. Plasma lutein, a nutritional biomarker for development of advanced age-related macular degeneration: The Alienor Study. Nutrients 2021, 13, 2047. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.A.; Li, J.; Fan, Y.H.; Li, Z.F.; Ma, M.; Liu, S.J.; Li, B.Y.; Shi, J.; Li, C.; et al. Dietary vitamins, carotenoids and their sources in relation to age-related macular degeneration risk in China: A population-based case-control study. Br. J. Nutr. 2023, 129, 1804–1811. [Google Scholar] [CrossRef]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol. 2015, 133, 1415–1424. [Google Scholar] [CrossRef]
- Stevens, R.; Hannah, B.; Richard, C. Dietary analysis and nutritional behaviour in people with and without age-related macular disease. Clin. Nutr. ESPEN 2015, 10, e112–e117. [Google Scholar] [CrossRef]
- Karppi, J.; Laukkanen, J.A.; Kurl, S. Plasma lutein and zeaxanthin and the risk of age-related nuclear cataract among the elderly Finnish population. Br. J. Nutr. 2012, 108, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.F.; Chylack, L.T.; Hankinson, S.E.; Khu, P.M.; Rogers, G.; Friend, J.; Tung, W.; Wolfe, J.K.; Padhye, N.; Willett, W.C.; et al. Long-term nutrient intake and early age-related nuclear lens opacities. Arch. Ophthalmol. 2001, 119, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Yu, R.B.; Liu, R.; Hao, Z.X.; Han, C.C.; Zhu, Z.H.; Ma, L. Association between lutein and zeaxanthin status and the risk of cataract: A meta-analysis. Nutrients 2014, 6, 452–465. [Google Scholar] [CrossRef]
- Ma, L.; Hao, Z.X.; Liu, R.R.; Yu, R.B.; Shi, Q.; Pan, J.P. A dose-response meta-analysis of dietary lutein and zeaxanthin intake in relation to risk of age-related cataract. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 63–70. [Google Scholar] [CrossRef]
- Jiang, H.; Yin, Y.; Wu, C.R.; Liu, Y.; Guo, F.; Li, M.; Ma, L. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am. J. Clin. Nutr. 2019, 109, 43–54. [Google Scholar] [CrossRef]
- Feart, C.; Letenneur, L.; Helmer, C.; Samieri, C.; Schalch, W.; Etheve, S.; Delcourt, C.; Dartigues, J.F.; Barberger-Gateau, P. Plasma carotenoids are inversely associated with dementia risk in an elderly French cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Dorey, C.K.; Gierhart, D.; Fitch, K.A.; Crandell, I.; Craft, N.E. Low xanthophylls, retinol, lycopene, and tocopherols in grey and white matter of brains with Alzheimer’s disease. J. Alzheimer’s Dis. 2023, 94, 1–17. [Google Scholar] [CrossRef]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Suzuki, T.; Arai, H.; Miyazawa, T. Significance of lutein in red blood cells of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2012, 28, 593–600. [Google Scholar] [CrossRef]
- Wang, W.; Shinto, L.; Connor, W.E.; Quinn, J.F. Nutritional biomarkers in Alzheimer’s disease: The association between carotenoids, n-3 fatty acids, and dementia severity. J. Alzheimer’s Dis. 2008, 13, 31–38. [Google Scholar] [CrossRef]
- Min, J.Y.; Min, K.B. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer’s disease mortality in older adults. Dement. Geriatr. Cogn. Disord. 2014, 37, 246–256. [Google Scholar] [CrossRef]
- Mullan, K.; Cardwell, C.R.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Plasma antioxidant status in patients with Alzheimer’s disease and cognitively intact elderly: A meta-analysis of case-control studies. J. Alzheimer’s Dis. 2018, 62, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.Y.; Shi, H.X.; Wang, K.; Wang, X.G.; Yu, N.; Guo, B.S. The associations of plasma/serum carotenoids with Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2021, 82, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Wang, Y.M.; Buchman, A.S.; Holland, T.M.; Bennett, D.A.; Morris, M.C. Dietary antioxidants associated with slower progression of parkinsonian signs in older adults. Nutr. Neurosci. 2022, 25, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Ying, A.F.; Khan, S.; Wu, Y.; Jin, A.Z.; Wong, A.S.Y.; Tan, E.K.; Yuan, J.M.; Koh, W.P.; Tan, L.C.S. Dietary antioxidants and risk of Parkinson’s disease in the Singapore Chinese Health Study. Mov. Disord. 2020, 35, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Gorell, J.M.; Rybicki, B.A.; Sanders, K.; Peterson, E.L. Adult nutrient intake as a risk factor for Parkinson’s disease. Int. J. Epidemiol. 1999, 28, 1102–1109. [Google Scholar] [CrossRef]
- Talebi, S.; Ghoreishy, S.M.; Jayedi, A.; Travica, N.; Mohammadi, H. Dietary antioxidants and risk of Parkinson’s disease: A systematic review and dose-response meta-analysis of observational studies. Adv. Nutr. 2022, 13, 1493–1504. [Google Scholar] [CrossRef]
- Hayhoe, R.P.G.; Lentjes, M.A.H.; Mulligan, A.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Carotenoid dietary intakes and plasma concentrations are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Br. J. Nutr. 2017, 117, 1439–1453. [Google Scholar] [CrossRef]
- Dai, Z.L.; Wang, R.W.; Ang, L.W.; Low, Y.L.; Yuan, J.M.; Koh, W.P. Protective effects of dietary carotenoids on risk of hip fracture in men: The Singapore Chinese Health Study. J. Bone Miner. Res. 2014, 29, 408–417. [Google Scholar] [CrossRef]
- Kan, B.; Guo, D.J.; Yuan, B.M.; Vuong, A.M.; Jiang, D.P.; Zhang, M.M.; Cheng, H.T.; Zhao, Q.Q.; Li, B.B.; Feng, L.J.; et al. Dietary carotenoid intake and osteoporosis: The National Health and Nutrition Examination Survey, 2005–2018. Arch. Osteoporos. 2022, 17, 2. [Google Scholar] [CrossRef]
- Shahriarpour, Z.; Nasrabadi, B.; Hejri-Zarifi, S.; Shariati-Bafghi, S.E.; Yousefian-Sanny, M.; Karamati, M.; Rashidkhani, B. Oxidative balance score and risk of osteoporosis among postmenopausal Iranian women. Arch. Osteoporos. 2021, 16, 43. [Google Scholar] [CrossRef]
- Bovier, E.R.; Hammond, B.R. The macular carotenoids lutein and zeaxanthin are related to increased bone density in young healthy adults. Foods 2017, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Hannan, M.T.; Blumberg, J.; Cupples, L.A.; Kiel, D.P.; Tucker, K.L. Inverse association of carotenoid intakes with 4-y change in bone mineral density in elderly men and women: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2009, 89, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Cao, W.T.; Liu, J.; Cao, Y.; Su, Y.X.; Chen, Y.M. Greater serum carotenoid concentration associated with higher bone mineral density in Chinese adults. Osteoporosis Int. 2016, 27, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Han, S.K.; Wang, H.; Wang, T.T. Lutein extends the lifespan of Drosophila melanogaster. Arch. Gerontol. Geriatr. 2014, 58, 153–159. [Google Scholar] [CrossRef]
- Vasudeva, V.; Tenkanidiyoor, Y.S.; Peter, A.J.; Shetty, J.; Lakshman, S.P.; Fernandes, R.; Patali, K.A. Radioprotective efficacy of lutein in ameliorating electron beam radiation-induced oxidative Injury in Swiss albino mice. Iran. J. Med. Sci. 2018, 43, 41–51. [Google Scholar]
- Augusti, P.R.; Brasil, A.V.; Souto, C.; Göethel, G.; Rios, A.D.; Emanuelli, T.; Bürger, M.E.; Garcia, S.C. Microcystin-LR exposure induces oxidative damage in Caenorhabditis elegans: Protective effect of lutein extracted from marigold flowers. Food Chem. Toxicol. 2017, 109, 60–67. [Google Scholar] [CrossRef]
- Maglioni, S.; Arsalan, N.; Hamacher, A.; Afshar, S.; Schiavi, A.; Beller, M.; Ventura, N. High content C. elegans screen identifies natural compounds impacting mitochondria-lipid homeostasis and promoting healthspan. Cells 2022, 11, 100. [Google Scholar]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Tao, Z.; Jun, Y.; Yaqin, L.; Jie, Q.; Shaobin, W.; Dezhi, L.; Junhui, C.; Zheng, W.V. Lutein shows a protective effect against the aging of mesenchymal stem cells by downregulating inflammation. Int. Immunopharmacol. 2023, 116, 109749. [Google Scholar] [CrossRef]
- Chae, S.Y.; Park, S.Y.; Park, G. Lutein protects human retinal pigment epithelial cells from oxidative stress-induced cellular senescence. Mol. Med. Rep. 2018, 18, 5182–5190. [Google Scholar] [CrossRef]
- Leung, H.H.; Galano, J.-M.; Crauste, C.; Durand, T.; Lee, J.C.-Y. Combination of lutein and zeaxanthin, and DHA regulated polyunsaturated fatty acid oxidation in H2O2-stressed retinal cells. Neurochem. Res. 2020, 45, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Sharavana, G.; Baskaran, V. Lutein downregulates retinal vascular endothelial growth factor possibly via hypoxia inducible factor 1 alpha and X-box binding protein 1 expression in streptozotocin induced diabetic rats. J. Funct. Food. 2017, 31, 97–103. [Google Scholar] [CrossRef]
- Yang, J.; Li, D.K.; Zhang, Y.Q.; Zhang, L.; Liao, Z.Y.; Aihemaitijiang, S.M.Y.; Hou, Y.M.; Zhan, Z.J.; Xie, K.; Zhang, Z.F. Lutein protected the retina from light induced retinal damage by inhibiting increasing oxidative stress and inflammation. J. Funct. Food. 2020, 73, 104107. [Google Scholar] [CrossRef]
- Cristaldi, M.; Anfuso, C.D.; Spampinato, G.; Rusciano, D.; Lupo, G. Comparative efficiency of lutein and astaxanthin in the protection of human corneal epithelial cells in vitro from blue-violet light photo-oxidative damage. Appl. Sci.Basel 2022, 12, 1268. [Google Scholar] [CrossRef]
- Kuo, C.M.; Yang, Y.C.; Zhang, W.X.; Wu, J.X.; Chen, Y.T.; Lin, C.H.; Lin, M.W.; Lin, C.S. A Low-Cost Fertilizer Medium Supplemented with Urea for the Lutein Production of Chlorella sp. and the Ability of the Lutein to Protect Cells against Blue Light Irradiation. Bioeng. Basel 2023, 10, 594. [Google Scholar] [CrossRef]
- Arunkumar, R.; Gorusupudi, A.; Li, B.X.; Blount, J.D.; Nwagbo, U.; Kim, H.J.; Sparrow, J.R.; Bernstein, P.S. Lutein and zeaxanthin reduce A2E and iso-A2E levels and improve visual performance in Abca4−/−/Bco2−/−double knockout mice. Exp. Eye Res. 2021, 209, 108680. [Google Scholar] [CrossRef]
- Fung, F.K.C.; Law, B.Y.K.; Lo, A.C.Y. Lutein attenuates both apoptosis and autophagy upon cobalt (II) chloride-induced hypoxia in rat Muller cells. PLoS ONE 2016, 11, e0167828. [Google Scholar] [CrossRef]
- Strzalka-Mrozik, B.; Madej, M.; Kurowska, N.; Kruszniewska-Rajs, C.; Kimsa-Dudek, M.; Adamska, J.; Gola, J.M. Changes in the expression profile of pyroptosis-related genes in senescent retinal pigment epithelial cells after lutein treatment. Curr. Issues Mol. Biol. 2023, 45, 1500–1518. [Google Scholar] [CrossRef]
- Shivarudrappa, A.H.; Sharan, K.; Ponesakki, G. Lutein activates downstream signaling pathways of unfolded protein response in hyperglycemic ARPE-19 cells. Eur. J. Pharmacol. 2022, 914, 174663. [Google Scholar] [CrossRef]
- Gao, S.S.; Qin, T.Y.; Liu, Z.Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.Y.O.; Yeum, K.J.; Taylor, A.; Blumberg, J.B.; Liu, Y.Z.; et al. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar]
- Ishida, H.; Shibata, T.; Shibata, S.; Tanaka, Y.; Sasaki, H.; Kubo, E. Lutein plus water chestnut (Trapa bispinosa Roxb.) extract inhibits the development of cataracts and induces antioxidant gene expression in lens epithelial cells. BioMed Res. Int. 2020, 2020, 9204620. [Google Scholar] [CrossRef] [PubMed]
- Chitchumroonchokchai, C.; Bomser, J.A.; Glamm, J.E.; Failla, M.L. Xanthophylls and α-tocopherol decrease UVB-induced lipid peroxidation and stress signaling in human lens epithelial cells. J. Nutr. 2004, 134, 3225–3232. [Google Scholar] [CrossRef]
- Kinoshita, S.; Sugawa, H.; Nanri, T.; Ohno, R.I.; Shirakawa, J.I.; Sato, H.; Katsuta, N.; Sakake, S.; Nagai, R. Trapa bispinosa Roxb. and lutein ameliorate cataract in type 1 diabetic rats. J. Clin. Biochem. Nutr. 2020, 66, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Komaki, S.; Salehi, I.; Raoufi, S.; Golipoor, Z.; Kourosh-Arami, M.; Komaki, A. Investigation of the protective effects of lutein on memory and learning using behavioral methods in a male rat model of Alzheimer’s disease. J. Funct. Food. 2022, 99, 105319. [Google Scholar] [CrossRef]
- Patel, C.; Patel, P.; Sarkar, D.; Bulsara, J.; Soni, A.; Isapure, K.; Acharya, S. Neuroprotective effect of lutein in scopolamine-induced Alzheimer’s disease in mice and zebrafish. Rev. Bras. Farmacogn.Braz. J. Pharmacogn. 2021, 31, 762–771. [Google Scholar] [CrossRef]
- Orhan, C.; Erten, F.; Er, B.; Tuzcu, M.; Sahin, N.; Kursun, Ö.; Juturu, V.; Sahin, K. Lutein/zeaxanthin isomers regulate neurotrophic factors and synaptic plasticity in trained rats. Turk. J. Med. Sci. 2021, 51, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, W.H.; Zhao, J.S.; Meng, F.Z.; Wang, H. Lutein protects against β-amyloid peptide-induced oxidative stress in cerebrovascular endothelial cells through modulation of Nrf-2 and NF-κb. Cell Biol. Toxicol. 2017, 33, 57–67. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Agócs, A.; Deli, J. Lutein exerts antioxidant and anti-inflammatory effects and influences iron utilization of BV-2 microglia. Antioxidants 2021, 10, 363. [Google Scholar] [CrossRef]
- Pap, R.; Edina, P.; Gergely, J.; Katalin, S.; Tamas, N.; Attila, A.; Jozsef, D. Lutein decreases inflammation and oxidative stress and prevents iron accumulation and lipid peroxidation at glutamate-induced neurotoxicity. Antioxidants 2022, 11, 2269. [Google Scholar] [CrossRef]
- Singhrang, N.; Tocharus, C.; Thummayot, S.; Sutheerawattananonda, M.; Tocharus, J. Protective effects of silk lutein extract from Bombyx mori cocoons on β-Amyloid peptide-induced apoptosis in PC12 cells. Biomed. Pharmacother. 2018, 103, 582–587. [Google Scholar] [CrossRef]
- Nataraj, J.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M. Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutr. Neurosci. 2016, 19, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, H.; Liu, G.S.; Kang, J.; Wang, B.A.; Wang, J.L.T.; Li, J.; Wang, H. Lutein attenuated methylglyoxal-induced oxidative damage and apoptosis in PC12 cells via the PI3K/Akt signaling pathway. J. Food Biochem. 2022, 46, e14382. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, X.; Lian, F.Z.; Yang, J.; Xi, X.R. Combination of Lutein and DHA alleviate H2O2- induced cytotoxicity in PC12 cells by regulating the MAPK pathway. J. Nutr. Sci. Vitaminol. 2021, 67, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Ademowo, O.S.; Dias, I.H.K.; Diaz-Sanchez, L.; Sanchez-Aranguren, L.; Stahl, W.; Griffiths, H.R. Partial mitigation of oxidized phospholipid-mediated mitochondrial dysfunction in neuronal cells by oxocarotenoids. J. Alzheimer’s Dis. 2020, 74, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Maglioni, S.; Schiavi, A.; Melcher, M.; Brinkmann, V.; Luo, Z.R.; Laromaine, A.; Raimundo, N.; Meyer, J.N.; Distelmaier, F.; Ventura, N. Neuroligin-mediated neurodevelopmental defects are induced by mitochondrial dysfunction and prevented by lutein in C. elegans. Nat. Commun. 2022, 13, 2620. [Google Scholar] [CrossRef]
- Binawade, Y.; Jagtap, A. Neuroprotective effect of lutein against 3-nitropropionic acid-induced Huntington’s disease-like symptoms: Possible behavioral, biochemical, and cellular alterations. J. Med. Food 2013, 16, 934–943. [Google Scholar] [CrossRef]
- Xie, K.; Ngo, S.; Rong, J.; Sheppard, A. Modulation of mitochondrial respiration underpins neuronal differentiation enhanced by lutein. Neural Regen. Res. 2019, 14, 87–99. [Google Scholar] [CrossRef]
- Katayama, S.; Ogawa, H.; Nakamura, S. Apricot carotenoids possess potent anti-amyloidogenic activity in vitro. J. Agric. Food Chem. 2011, 59, 12691–12696. [Google Scholar] [CrossRef]
- Geiss, J.M.T.; Sagae, S.C.; Paz, E.D.R.; de Freitas, M.L.; Souto, N.S.; Furian, A.F.; Oliveira, M.S.; Guerra, G.P. Oral administration of lutein attenuates ethanol-induced memory deficit in rats by restoration of acetylcholinesterase activity. Physiol. Behav. 2019, 204, 121–128. [Google Scholar] [CrossRef]
- Fernandes, E.J.; Poetini, M.R.; Barrientos, M.S.; Bortolotto, V.C.; Araujo, S.M.; Musachio, E.A.S.; De Carvalho, A.S.; Leimann, F.V.; Gonçalves, O.H.; Ramborger, B.P.; et al. Exposure to lutein-loaded nanoparticles attenuates Parkinson’s model-induced damage in Drosophila melanogaster: Restoration of dopaminergic and cholinergic system and oxidative stress indicators. Chem.Biol. Interact. 2021, 340, 109431. [Google Scholar] [CrossRef]
- Li, H.T.; Huang, C.H.; Zhu, J.; Gao, K.H.; Fang, J.; Li, H.Z. Lutein suppresses oxidative stress and inflammation by Nrf2 activation in an osteoporosis rat model. Med. Sci. Monit. 2018, 24, 5071–5075. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.Q.; Jiang, P.F.; Gao, Y.Z. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch. Med. Sci. 2018, 14, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Tominari, T.; Hirata, M.; Watanabe, K.; Matsumoto, C.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Lutein enhances bone mass by stimulating bone formation and suppressing bone resorption in growing mice. Biol. Pharm. Bull. 2017, 40, 716–721. [Google Scholar] [CrossRef]
- Tominari, T.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Lutein, a carotenoid, suppresses osteoclastic bone resorption and stimulates bone formation in cultures. Biosci. Biotechnol. Biochem. 2017, 81, 302–306. [Google Scholar] [CrossRef]
- Pei, L.; Cui, J. Effect of lutein on the differentiation of osteoclast induced from bone marrow in mice. J. Nanjing Norm. Univ. Nat. Sci. 2008, 31, 100–103. [Google Scholar]
- Yamaguchi, M.; Uchiyama, S. Effect of carotenoid on calcium content and alkaline phosphatase activity in rat femoral tissues in vitro: The unique anabolic effect of β-cryptoxanthin. Biol. Pharm. Bull. 2003, 26, 1188–1191. [Google Scholar] [CrossRef]
- Guymer, R.H.; Campbell, T.G. Age-related macular degeneration. Lancet 2023, 401, 1459–1472. [Google Scholar] [CrossRef]
- Panova, I.G.; Tatikolov, A.S.; Sukhikh, G.T. Correlation between the content of albumin and carotenoids in human vitreous body during prenatal development. Bull. Exp. Biol. Med. 2007, 144, 681–683. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Kizawa, Y.; Sekikawa, T.; Kageyama, M.; Tomobe, H.; Kobashi, R.; Yamada, T. Effects of anthocyanin, astaxanthin, and lutein on eye functions: A randomized, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2021, 69, 77–90. [Google Scholar] [CrossRef]
- Li, B.X.; Rognon, G.T.; Mattinson, T.; Vachali, P.P.; Gorusupudi, A.; Chang, F.Y.; Ranganathan, A.; Nelson, K.; George, E.W.; Frederick, J.M.; et al. Supplementation with macular carotenoids improves visual performance of transgenic mice. Arch. Biochem. Biophys. 2018, 649, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Stringham, J.M.; Garcia, P.V.; Smith, P.A.; Hiers, P.L.; McLin, L.N.; Kuyk, T.K.; Foutch, B.K. Macular Pigment and Visual Performance in Low-Light Conditions. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2459–2468. [Google Scholar] [CrossRef]
- Stringham, J.M.; Garcia, P.V.; Smith, P.A.; McLin, L.N.; Foutch, B.K. Macular pigment and visual performance in glare: Benefits for photostress recovery, disability glare, and visual discomfort. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7406–7415. [Google Scholar] [CrossRef]
- Kushwah, N.; Bora, K.; Maurya, M.; Pavlovich, M.C.; Chen, J. Oxidative stress and antioxidants in age-related macular degeneration. Antioxidants 2023, 12, 1379. [Google Scholar] [CrossRef]
- Contín, M.A.; Benedetto, M.M.; Quinteros-Quintana, M.L.; Guido, M.E. Light pollution: The possible consequences of excessive illumination on retina. Eye 2016, 30, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Pipis, A.; Touliou, E.; Pillunat, L.E.; Augustin, A.J. Effect of the blue filter intraocular lens on the progression of geographic atrophy. Eur. J. Ophthalmol. 2015, 25, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, T.; Negishi, K.; Miyake, S.; Hirasawa, M.; Osada, H.; Kurihara, T.; Tsubota, K.; Ozawa, Y. Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo. Exp. Eye Res. 2015, 132, 48–51. [Google Scholar] [CrossRef]
- Yu, M.Z.; Beight, C. Lutein and zeaxanthin isomers ameliorate photoreceptor degeneration in Pde6rd10 mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 971. [Google Scholar]
- Ryu, W.; Park, C.W.; Kim, J.; Lee, H.; Chung, H. The Bcl-2/Bcl-xL inhibitor ABT-263 attenuates retinal degeneration by selectively inducing apoptosis in senescent retinal pigment epithelial cells. Mol. Cells 2023, 46, 420–429. [Google Scholar] [CrossRef]
- Huang, Y.M.; Dou, H.L.; Huang, F.F.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. BioMed Res. Int. 2015, 119, 22902297. [Google Scholar] [CrossRef]
- Ma, L.; Yan, S.F.; Huang, Y.M.; Lu, X.R.; Qian, F.; Pang, H.L.; Xu, X.R.; Zou, Z.Y.; Dong, P.C.; Xiao, X.; et al. Effect of Lutein and Zeaxanthin on Macular Pigment and Visual Function in Patients with Early Age-related Macular Degeneration. Ophthalmology 2012, 119, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.J.; Makridaki, M.; van der Veen, R.L.P.; Carden, D.; Parry, N.R.A.; Berendschot, T.T. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: The CLEAR study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.; Ferris, F.L.; Elman, M.; Antoszyk, A.; Ruby, A.; Orth, D.; Bressler, S.; et al. Sperduto, and Areds Age-Related Eye Dis Study. Lutein plus zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA J. Am. Med. Assoc. 2013, 309, 2005–2015. [Google Scholar]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L. Dietary nutrient intake and progression to late age-related macular degeneration in the age-related eye disease studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef]
- Weigert, G.; Kaya, S.; Pemp, B.; Sacu, S.; Lasta, M.; Werkmeister, R.M.; Dragostinoff, N.; Simader, C.; Garhofer, G.; Schmidt-Erfurth, U.; et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8174–8178. [Google Scholar] [CrossRef]
- Olmedilla, B.; Granado, F.; Blanco, I.; Vaquero, M. Lutein, but not α-tocopherol, supplementation improves visual function in patients with age-related cataracts: A 2-y double-blind, placebo-controlled pilot study. Nutrition 2003, 19, 21–24. [Google Scholar] [CrossRef]
- Olmedilla, B.; Granado, F.; Blanco, I.; Vaquero, M.; Cajigal, C. Lutein in patients with cataracts and age-related macular degeneration: A long-term supplementation study. J. Sci. Food Agric. 2001, 81, 904–909. [Google Scholar] [CrossRef]
- Hayashi, R.; Hayashi, S.; Arai, K.; Sakai, M.; Okamoto, H.; Chikuda, M. The gender-differentiated antioxidant effects of a lutein-containing supplement in the aqueous humor of patients with senile cataracts. Exp. Eye Res. 2014, 129, 5–12. [Google Scholar] [CrossRef]
- Chew, E.Y.; SanGiovanni, J.P.; Ferris, F.L.; Wong, W.T.; Agron, E.; Clemons, T.E.; Sperduto, R.; Danis, R.; Chandra, S.R.; Blodi, B.A.; et al. Lutein/zeaxanthin for the treatment of age-related cataract AREDS2 randomized trial report No. 4. JAMA Ophthalmol. 2013, 131, 843–850. [Google Scholar] [CrossRef]
- Nolan, J.M.; Loskutova, E.; Howard, A.; Mulcahy, R.; Moran, R.; Stack, J.; Bolger, M.; Coen, R.F.; Dennison, J.; Akuffo, K.O.; et al. The impact of supplemental macular carotenoids in Alzheimer’s disease: A randomized clinical trial. J. Alzheimer’s Dis. 2015, 44, 1157–1169. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. The effects of lutein and zeaxanthin supplementation on cognitive function in adults with self-reported mild cognitive complaints: A randomized, double-blind, placebo-controlled study. Front. Nutr. 2022, 9, 843512. [Google Scholar] [CrossRef]
- Ademowo, O.S.; Dias, H.K.I.; Milic, I.; Devitt, A.; Moran, R.; Mulcahy, R.; Howard, A.N.; Nolan, J.M.; Griffiths, H.R. Phospholipid oxidation and carotenoid supplementation in Alzheimer’s disease patients. Free Radic. Biol. Med. 2017, 108, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, V.M.; Beyer, E.C. Oxidative stress, lens gap junctions, and cataracts. Antioxid. Redox Signal. 2009, 11, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Abdollahi, M.; Raman, T.; Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Nabavi, S.M. Lutein and cataract: From bench to bedside. Crit. Rev. Biotechnol. 2016, 36, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.J.; Shang, F.; Schalch, W.; Russell, R.M.; Taylor, A. Fat-soluble nutrient concentrations in different layers of human cataractous lens. Curr. Eye Res. 1999, 19, 502–505. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Hernández, A.E.; García, E. Mesenchymal stem cell therapy for Alzheimer’s disease. Stem Cells Int. 2021, 2021, 7834421. [Google Scholar] [CrossRef]
- Ribaya-Mereado, J.D.; Blumberg, J.B. Lutein and zeaxanthin and their potential roles in disease prevention. J. Am. Coll. Nutr. 2004, 23, 567S–587S. [Google Scholar] [CrossRef]
- Manochkumar, J.; Doss, C.G.P.; El-Seedi, H.R.; Efferth, T.; Ramamoorthy, S. The neuroprotective potential of carotenoids in vitro and in vivo. Phytomedicine 2021, 91, 153676. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Mechanisms of dieases: Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s disease: Current progress in molecular signaling and therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Obulesu, M.; Lakshmi, M.J. Apoptosis in Alzheimer’s disease: An understanding of the physiology, pathology and therapeutic avenues. Neurochem. Res. 2014, 39, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimer’s Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Kametani, F.; Hasegawa, M. Reconsideration of amyloid hypothesis and Tau hypothesis in Alzheimer’s disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Lakey-Beitia, J.; Kumar, D.J.; Hegde, M.L.; Rao, K.S. Carotenoids as novel therapeutic molecules against neurodegenerative disorders: Chemistry and molecular docking analysis. Int. J. Mol. Sci. 2019, 20, 5553. [Google Scholar] [CrossRef] [PubMed]
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.R.; Terry, D.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and zeaxanthin influence brain function in older adults: A randomized controlled trial. J. Int. Neuropsychol. Soc. 2018, 24, 77–90. [Google Scholar] [CrossRef]
- Iyer, S.; Bhat, I.; Sheshappa, M.B. Lutein and the underlying neuroprotective promise against neurodegenerative diseases. Mol. Nutr. Food Res. 2024, 68, 2300409. [Google Scholar] [CrossRef]
- Kulczynski, B.; Sidor, A.; Brzozowska, A.; Gramza-Michalowska, A. The role of carotenoids in bone health-a narrative review. Nutrition 2023, 119, 112306. [Google Scholar] [CrossRef]
- Kopec, R.E.; Failla, M.L. Recent advances in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophiles. J. Food Compos. Anal. 2018, 68, 16–30. [Google Scholar] [CrossRef]
- Bhat, I.; Madhura, R.J.; Badanthadka, M.; Mamatha, B.S. Cow ghee as an efficient carrier to improve oral bioavailability of lutein. Food Chem. 2022, 389, 133046. [Google Scholar] [CrossRef]
- Nidhi, B.; Mamatha, B.S.; Baskaran, V. Olive oil improves the intestinal absorption and bioavailability of lutein in lutein-deficient mice. Eur. J. Nutr. 2014, 53, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.; Baskaran, V.; Mamatha, B.S. Influence of fatty acids in edible oils on lutein micellization and permeation in a simulated digestion model. Food Biosci. 2022, 46, 101423. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Olmedilla-Alonso, B.; Blanco-Navarro, I.; Pérez-Sacristán, B. Lutein bioavailability from lutein ester-fortified fermented milk: In vivo and in vitro study. J. Nutr. Biochem. 2010, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Prashanth, K.V.H.; Baskaran, V. Promising interaction between nanoencapsulated lutein with low molecular weight chitosan: Characterization and bioavailability of lutein in vitro and in vivo. Food Chem. 2013, 141, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Kamil, A.; Smith, D.E.; Blumberg, J.B.; Astete, C.; Sabliov, C.; Chen, C.Y.O. Bioavailability and biodistribution of nanodelivered lutein. Food Chem. 2016, 192, 915–923. [Google Scholar] [CrossRef]
- Shanmugam, S.; Park, J.-H.; Kim, K.S.; Piao, Z.Z.; Yong, C.S.; Choi, H.-G.; Woo, J.S. Enhanced bioavailability and retinal accumulation of lutein from self-emulsifying phospholipid suspension (SEPS). Int. J. Pharm. 2011, 412, 99–105. [Google Scholar] [CrossRef]
| Study Type | Participants | Effects | Ref |
|---|---|---|---|
| Aging | |||
| Cross-sectional cohort study | 2007 Australian individuals aged 45 to 86 | Independently associated with leukocyte telomere length β = 0.079, p = 0.03, adjusted for age and sex β = 0.107, p = 0.01, additional adjustment for BMI and VO2max β = 0.12, p = 0.006, further adjustment for vascular risk factors | [12] |
| Cross-sectional study | 5056 American elderly people over the age of 60 years from NHANES | Significantly correlated with increased S-klotho concentration (β = 15.44, p < 0.01) | [13] |
| Umbrella review | 29 outcomes in 24 systematic reviews and meta-analyses | Had beneficial effects on age-related cataracts, age-related macular degeneration | [14] |
| AMD | |||
| Matched case–control study | 164 cases of AMD and 164 controls | Associated with decreased risk of AMD (OR = 0.21, 95% CI = 0.05–0.84) | [15] |
| Meta-analysis | 9 studies | Participants in highest category had 47% lower risk of developing AMD (OR = 0.53, 95% CI = 0.40–0.72, p < 0.001; I2 = 43.3%, p heterogeneity = 0.079) | [15] |
| Cross-sectional study | 34 patients with unilateral wet AMD and 33 patients with bilateral dry AMD | Patients with unilateral wet AMD had significantly higher levels of MPOD in their fellow eye but had lower levels compared with patients with bilateral dry AMD (0.58 versus 0.48, p = 0.026) | [16] |
| Cohort study | 609 participants | Participants with higher plasma lutein had reduced risk for incident advanced AMD in fully adjusted model (HR = 0.63 per 1 SD increase (95% CI = 0.41–0.97), p = 0.03) | [17] |
| Case–control study | 260 AMD cases and 260 matched controls | Lutein was associated with lower AMD risk (OR = 0.30, 95% CI = 0.10–0.88) comparing extreme quartiles | [18] |
| Cohort study | 63,443 women and 38,603 men (Nurse study) | Pooled relative risk comparing extreme quintiles (HR = 0.59; 95% CI = 0.48–0.73; p for trend < 0.001) | [19] |
| Case–control study | 158 participants with AMD and 50 participants without AMD | No significant difference between AMD and non-AMD group | [20] |
| Cataracts | |||
| Cross-sectional study | 1689 subjects aged 61–80 years | Lutein was associated with lower nuclear cataract risk (RR = 0.58, 95% CI = 0.35–0.98, p = 0.041) | [21] |
| Cohort study | 478 women without diabetes aged 53 to 73 | Lutein intake was inversely associated with risk of nuclear opacification, comparing each quintile | [22] |
| Meta-analysis | 1 cohort study and 7 cross-sectional studies | Lutein concentration in blood was inversely associated with risk of nuclear cataracts (pooled RRs = 0.63, 95% CI = 0.49–0.77) | [23] |
| Meta-analysis | 6 cohort studies | Lutein and zeaxanthin intake was inversely associated with risk of nuclear cataracts (RR = 0.75, 95% CI = 0.65–0.85); every 300 µg/d increase in dietary lutein and zeaxanthin intake was linked to 3% decrease in risk of nuclear cataracts | [24] |
| Meta-analysis | 8 RCTs and 12 cohort studies | Dietary lutein/zeaxanthin was inversely correlated with risk of age-related cataracts (RR = 0.81, 95% CI = 0.75–0.89, p < 0.001), and dose–response analysis found that every 10 mg/d increase in dietary lutein and zeaxanthin intake was linked to 26% decrease in risk of age-related cataracts | [25] |
| AD | |||
| Cohort study | 1092 older participants without dementia | Lutein was associated with decreased risk of all-cause AD (HR = 0.759, 95% CI = 0.600–0.960, p = 0.021, for +1 SD) | [26] |
| Cross-sectional study | 21 AD brains and 10 healthy brains | AD brains had significantly lower levels of lutein (p = 0.04) | [27] |
| Case–control study | 28 control subjects (age: 74.1 ± 1.3 years) and 28 patients with AD (age: 72.5 ± 1.4 years) | Concentrations of RBC lutein in AD patients were significantly lower than in control subjects. (p < 0.001) Inverse relationship was seen between RBC lutein and antioxidant concentrations (p < 0.05) in AD patients | [28] |
| Case–control study | 36 AD subjects and 10 control subjects | Lutein was significantly correlated with MMSE | [29] |
| Cohort study | 6958 participants aged older than 50 years | Lutein was associated with lower risk of AD mortality (HR = 0.43, 95% CI = 0.22–0.85), highest quartile compared to lowest quartile | [30] |
| Meta-analysis | 52 case–control studies | AD patients had significantly lower plasma levels of lutein (p = 0.01, I2 = 88%) | [31] |
| Meta-analysis | 16 studies, with 10,633 participants | AD Patients had significantly lower plasma/serum levels of lutein (SMD = −0.86, 95% CI = −1.67 to −0.05, p = 0.04) | [32] |
| PD | |||
| Cohort study | 682 participants without Parkinson’s disease | Lutein/zeaxanthin intake was inversely associated with rate of progressive Parkinsonian signs (β = −0.05, 95% CI = −0.09 to −0.02) | [33] |
| Cohort study | 63,257 men and women aged 45 to 74 years | No association between lutein consumption and risk of Parkinson’s disease | [34] |
| Case–control study | 126 Parkinson’s disease cases and 432 controls | Higher lutein intake was associated with higher Parkinson’s disease risk, comparing extreme quartiles (OR = 2.52, 95% CI = 1.32–4.84) | [35] |
| Meta-analysis | 6 cohort studies, 2 nested case–control studies, and 6 case–control studies | Lutein intake was positively associated with risk of Parkinson’s disease (RR = 1.86, 95% CI = 1.20, 2.88) in case–control studies; no dose–response correlation was found between lutein intake and risk of Parkinson’s disease | [36] |
| Osteoporosis | |||
| Cohort study | EPIC-Norfolk, n = 25,439 | Lutein had positive trends in BUA bone density for women across quintiles (p = 0.01); lutein was associated with lower risk for wrist fracture in women across quintiles (p = 0.022) | [37] |
| Cohort study | 63,257 men and women (age: 45–74 years) | Dietary lutein/zeaxanthin was negatively correlated with men’s risk of hip fractures (p = 0.049) | [38] |
| Cohort study | 4820 NHANCES participants | Dietary lutein/zeaxanthin intake was associated with reduced risk of osteoporosis (OR for quintile 5 vs. 1 = 0.53; 95% CI = 0.30–0.94; p for trend = 0.076) | [39] |
| Cross-sectional study | 151 postmenopausal Iranian women aged 50–85 years old | Highest tertile of OBS had lower risk of lumbar spine osteoporosis than those in lowest tertile (OR = 0.14; 95% CI = 0.04–0.45; p = 0.001) | [40] |
| Cross-sectional study | 63 subjects (females, n = 39; males, n = 24; average age = 22.5 years old) | MPOD was positively correlated with proximal femur and lumbar spine’s bone density (p < 0.05) | [41] |
| Cohort study | 5209 men and women aged 28–62 years old | No cross-sectional correlations between dietary lutein/zeaxanthin intake and BMD. Dietary lutein/zeaxanthin intake was inversely related to 4-year change in trochanter BMD in elderly men (p for trend = 0.008) | [42] |
| Cross-sectional study | 1898 women and 933 men aged 59.6 years | No significant association between serum lutein/zeaxanthin level and BMD | [43] |
| Study Type | Subjects | Substance and Dose | Duration | Effects | Ref. |
|---|---|---|---|---|---|
| AMD | |||||
| Randomized, double-blinded, placebo-controlled trial | 112 early AMD patients | 10 mg or 20 mg lutein, or a combination of lutein (10 mg) and zeaxanthin (10 mg) | 2 years | ↑ serum lutein concentration and MPOD ↑ contrast sensitivity | [100] |
| Randomized, double-blinded, placebo-controlled trial | Participants with probable AMD who were 50 to 79 years of age (n = 108) | 10 mg or 20 mg lutein, or a combination of lutein (10 mg) and zeaxanthin (10 mg) | 2 years | ↑ MPOD ↑ contrast sensitivity | [101] |
| Randomized, double-blind, placebo-controlled, two-center trial | 72 patients (mean age 70.5 ± 8.7) | 10 mg lutein | 4 months | ↑ MPOD ↑ visual acuity in the subgroup that had worse visual acuity | [102] |
| Multicenter, randomized, double-blinded, placebo-controlled phase 3 study | 4203 participants aged 50 to 85 years at risk for progression to advanced AMD | Lutein (10 mg) + zeaxanthin (2 mg), or DHA (350 mg) + EPA (650 mg), or combination of lutein + zeaxanthin and DHA + EPA, or placebo. | Median follow-up = 5 years | No significant reduction in progression to advanced AMD | [103] |
| Randomized (2:1), placebo-controlled, double-masked parallel group study | 126 patients with AMD | In months 1 to 3, dose was 20 mg lutein once daily, and in months 4 to 6, dose was 10 mg lutein once daily | 6 months | ↑ MPOD No significant effect of lutein supplementation on VA or macular function; significant correlation was found between increase in MPOD after 6 months and increase in MDLT and VA after 6 months | [105] |
| Cataract | |||||
| Randomized, double-blind, controlled clinical trial | 17 patients clinically diagnosed with age-related cataracts | 15 mg lutein, three times a week | 2 years | ↑ serum concentrations of lutein ↑ visual acuity and glare sensitivity | [106] |
| Clinical trial | 10 subjects diagnosed with cataracts or age-related macular degeneration | 12 mg of all-trans-lutein, 3 mg of 13/15-cis-lutein, and 3.3 mg of α-tocopherol | 26 months on average | ↑ serum concentration of lutein ↑ visual acuity and glare sensitivity | [107] |
| Clinical trial | 40 patients with cataracts | Multiple antioxidants, including 6 mg lutein | 6 weeks | ↑ superoxide scavenging activity ↑ H2O2 ↓ hydroperoxides | [108] |
| Multicenter, double-blind clinical trial | 4203 participants, aged 50 to 85 years | Lutein/zeaxanthin for 10 mg/2 mg | 4.7 years on average | ↓ risk of progression to cataract surgery | [109] |
| AD | |||||
| Randomized, double-blind, controlled clinical trial | 31 AD patients and 31 control subjects | 10 mg meso-zeaxanthin, 10 mg lutein, and 2 mg zeaxanthin per day | 6 months | No significant changes in any of cognitive function outcome variables measured | [110] |
| Randomized, double-blind, placebo-controlled trial | 90 volunteers aged 40–75 years | 10 mg of lutein and 2 mg of zeaxanthin | 6 months | ↑ visual episodic memory ↑ visual learning | [111] |
| Randomized, double-blind, placebo-controlled trial | AD patients (n = 21) and healthy age-matched control subjects (n = 16) | 10 mg meso-zeaxanthin, 10 mg lutein, and 2 mg zeaxanthin | 6 months | Novel oxidized phospholipid biomarker POVPC levels of AD patients were not different compared to healthy controls No significant effect on cognitive performance | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Cheng, J.; Xiong, R.; Chen, H.; Huang, S.; Li, H.; Pang, J.; Zhang, X.; Zhu, H. Effects and Mechanisms of Lutein on Aging and Age-Related Diseases. Antioxidants 2024, 13, 1114. https://doi.org/10.3390/antiox13091114
Ye J, Cheng J, Xiong R, Chen H, Huang S, Li H, Pang J, Zhang X, Zhu H. Effects and Mechanisms of Lutein on Aging and Age-Related Diseases. Antioxidants. 2024; 13(9):1114. https://doi.org/10.3390/antiox13091114
Chicago/Turabian StyleYe, Jialu, Jin Cheng, Ruogu Xiong, Haoqi Chen, Siyu Huang, Huabin Li, Jinzhu Pang, Xuguang Zhang, and Huilian Zhu. 2024. "Effects and Mechanisms of Lutein on Aging and Age-Related Diseases" Antioxidants 13, no. 9: 1114. https://doi.org/10.3390/antiox13091114
APA StyleYe, J., Cheng, J., Xiong, R., Chen, H., Huang, S., Li, H., Pang, J., Zhang, X., & Zhu, H. (2024). Effects and Mechanisms of Lutein on Aging and Age-Related Diseases. Antioxidants, 13(9), 1114. https://doi.org/10.3390/antiox13091114








