Computational Insights into the Radical Scavenging Activity and Xanthine Oxidase Inhibition of the Five Anthocyanins Derived from Grape Skin
Abstract
:1. Introduction
2. Computational Section
2.1. Thermodynamic Parameters
2.2. Kinetic Parameters
2.3. Molecular Docking Studies
3. Results and Discussion
3.1. Conformational Analysis
3.2. Analysis of Radical Scavenging Reactions
3.2.1. HAT Mechanism
3.2.2. SET-PT Mechanism
3.2.3. SPLET Mechanism
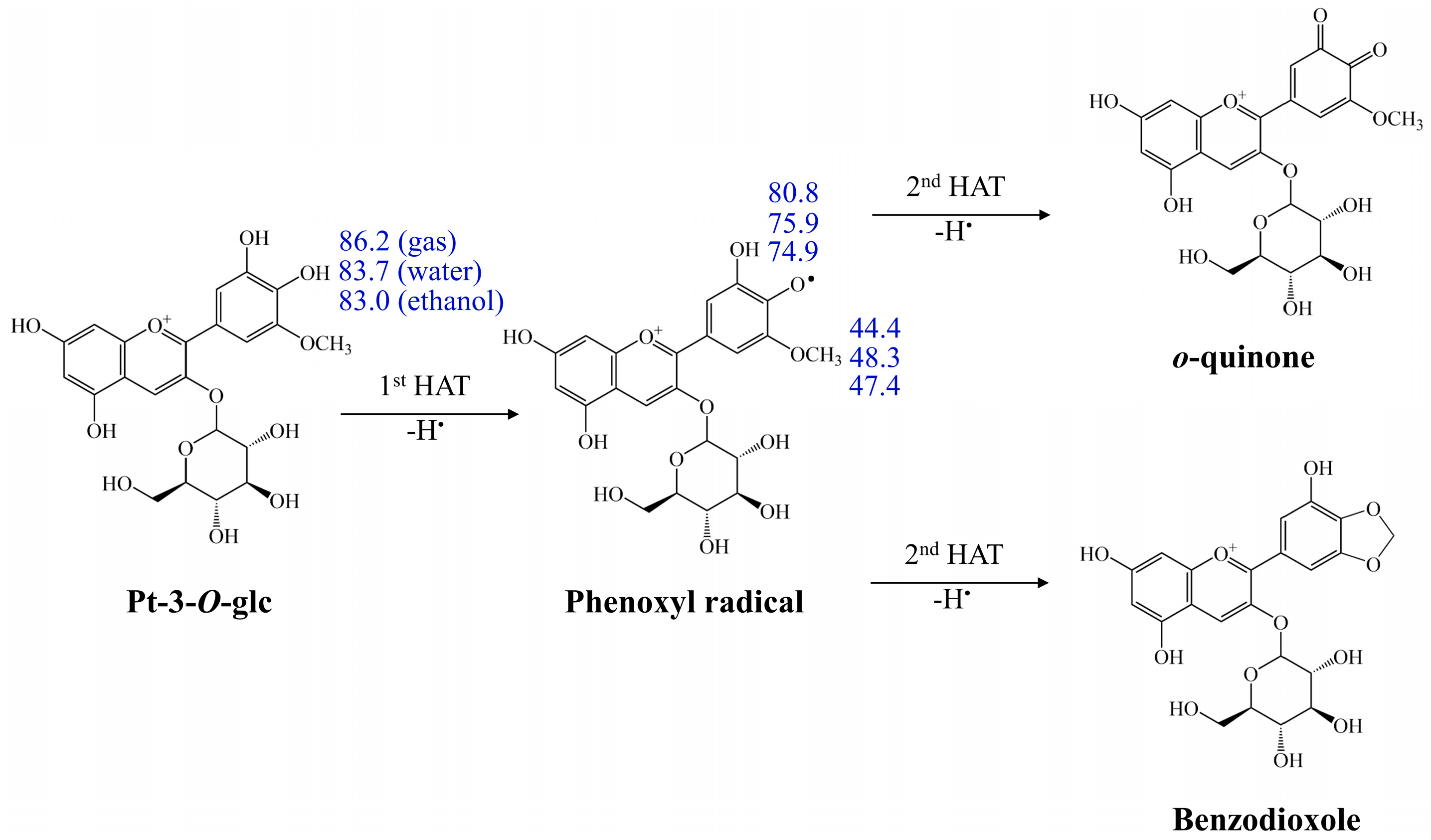
3.2.4. Double HAT Mechanism
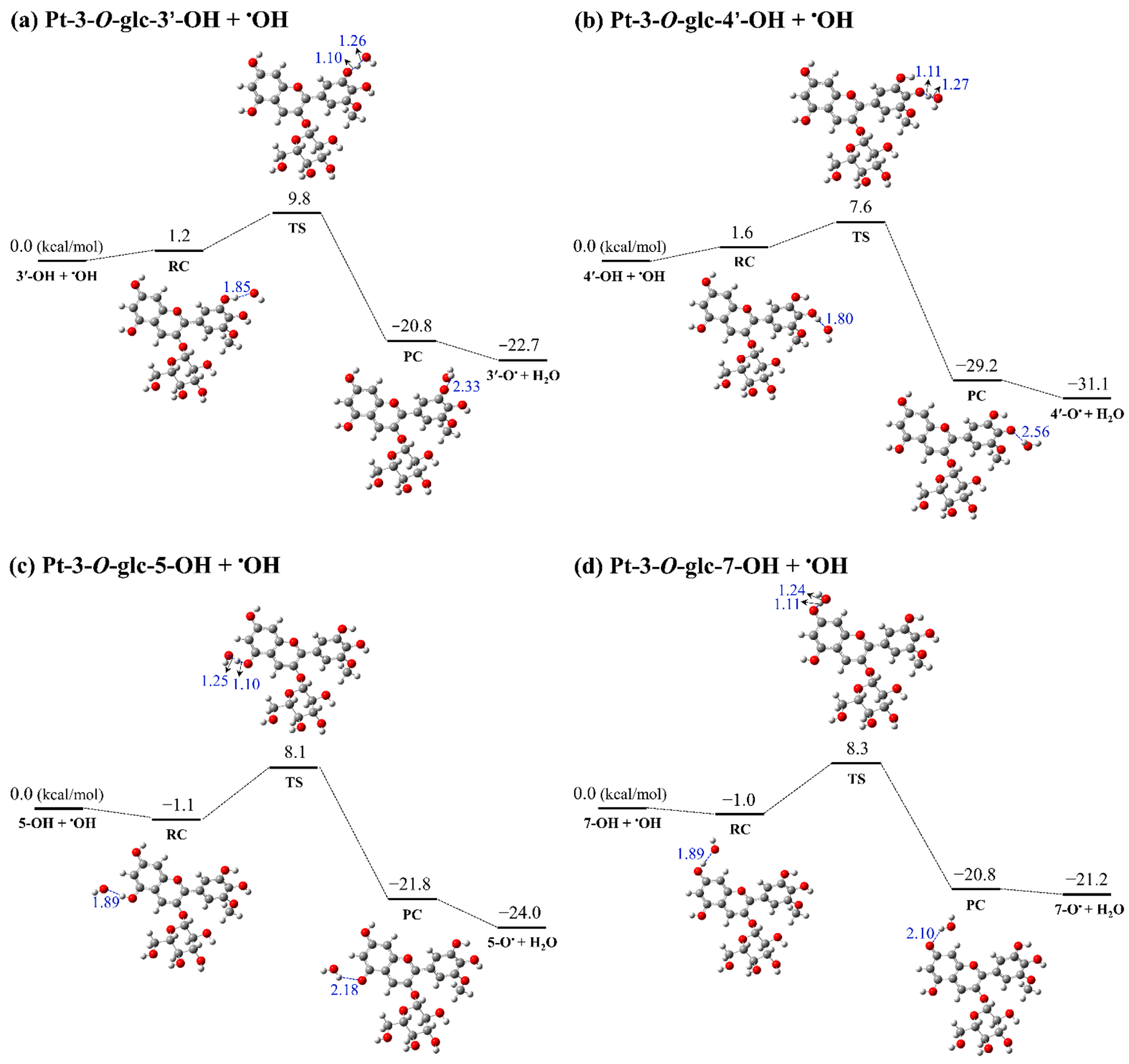
3.3. Kinetic Study
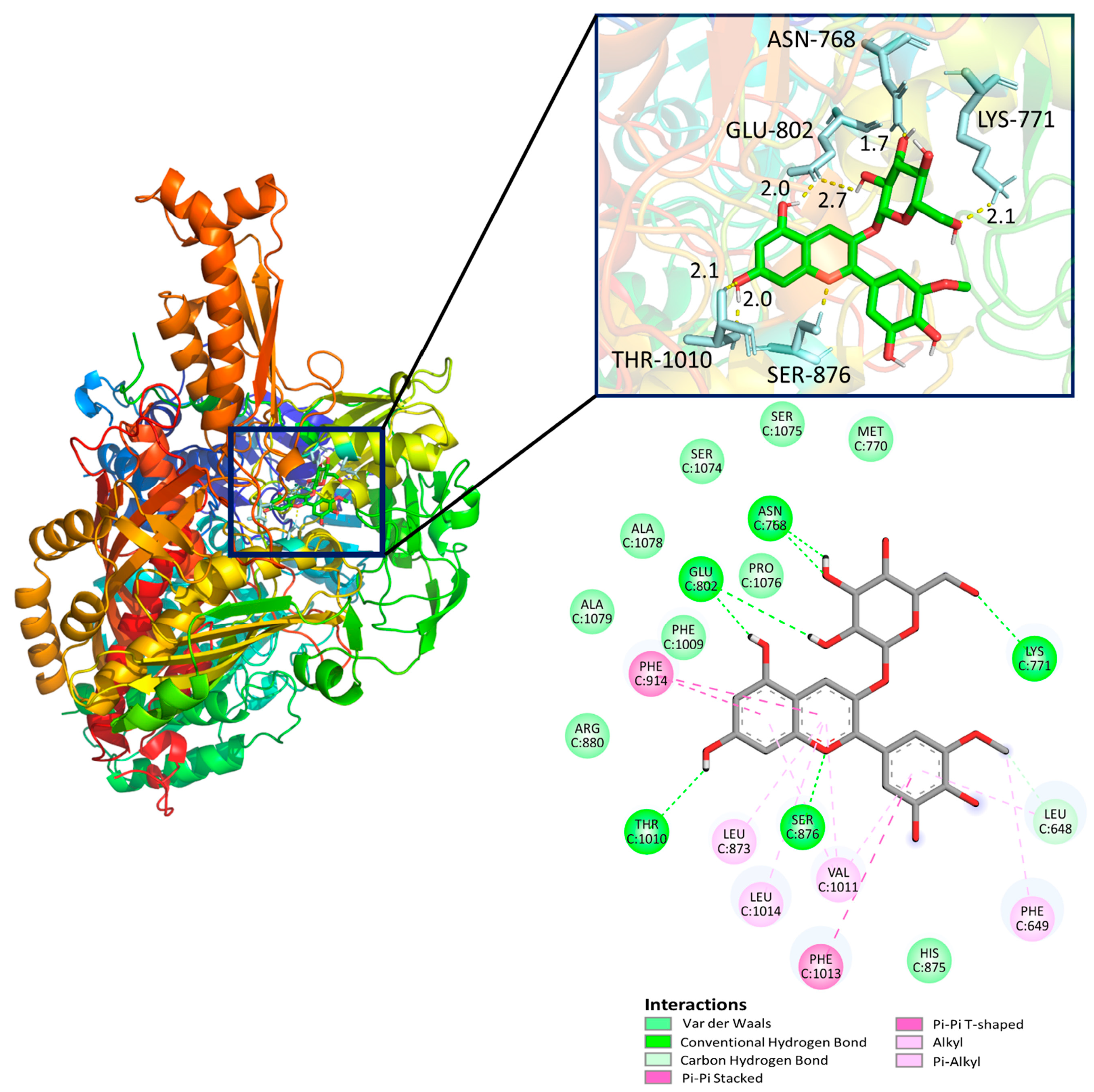
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Radical medicine: Treating ageing to cure disease. Nat. Rev. Mol. Cell Biol. 2005, 6, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Bonekamp, N.A.; Völkl, A.; Fahimi, H.D.; Schrader, M. Reactive oxygen species and peroxisomes: Struggling for balance. BioFactors 2009, 35, 346–355. [Google Scholar] [CrossRef]
- Okamoto, K.; Kusano, T.; Nishino, T. Chemical nature and reaction mechanisms of the molybdenum cofactor of xanthine oxidoreductase. Curr. Pharm. Design 2013, 19, 2606–2614. [Google Scholar] [CrossRef]
- Dangles, O. Antioxidant activity of plant phenols: Chemical mechanics and biological significance. Curr. Org. Chem. 2012, 16, 692–714. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Rio, D.D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Mateus, N.; Freitas, V.D. Anthocyanins. Plant pigments and beyond. J. Agric. Food Chem. 2014, 62, 6879–6884. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Marianne, L.-C.; Lucía, A.-G.; de Jesúsc, M.-S.M.; Leonardoa, H.-M.E.; Mendoza-Sáncheza, M. Optimization of the green extraction process of antioxidants derived from grape pomace. Sustain. Chem. Pharm. 2024, 37, 101396. [Google Scholar] [CrossRef]
- Abouelenein, D.; Mustafa, A.M.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Vittori, S. Phenolic and nutritional profiles, and antioxidant activity of grape pomaces and seeds from Lacrima di Morro d’Alba and Verdicchio varieties. Food Biosci. 2023, 53, 102808. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.J.; Chen, Y.Y.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Torres, S. Electrochemical behaviour and antioxidant capacity of anthocyanins from Chilean red wine, grape and raspberry. Food Chem. 2010, 121, 44–48. [Google Scholar] [CrossRef]
- Nichenametla, S.N.; Taruscio, T.G.; Barney, D.L.; Exon, J.H. A review of the effects and mechanisms of polyphenolics in cancer. Crit. Rev. Food Sci. Nutr. 2006, 46, 161–183. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes, P.-H.M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Xu, H.; Liu, X.; Yan, Q.; Yuan, F.; Gao, Y. A novel copigment of quercetagetin for stabilization of grape skin anthocyanins. Food Chem. 2015, 166, 50–55. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; López-Roca, J.M.; Ros-García, J.M.; Gómez-Plaza, E. Anthocynin fingerprint of grapes: Environmental and genetic variations. J. Sci. Food Agric. 2006, 86, 1460–1467. [Google Scholar] [CrossRef]
- Gui, H.; Dai, J.; Tian, J.; Jiang, Q.; Zhang, Y.; Ren, G.; Song, B.; Wang, M.; Saiwaidoula, M.; Dong, W.; et al. The isolation of anthocyanin monomers from blueberry pomace and their radical-scavenging mechanisms in DFT study. Food Chem. 2023, 418, 135872. [Google Scholar] [CrossRef]
- Li, J.; Shi, C.; Shen, D.; Han, T.; Wu, W.; Lyu, L.; Li, W. Composition and antioxidant activity of anthocyanins and non-anthocyanin flavonoids in blackberry from different growth stages. Foods 2022, 11, 2902. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Zhang, L.; An, L.; Chen, R.; Liu, Y.; Luo, Q.; Li, Y.; Wang, H.; Xue, Y. Computational study on the antioxidant property of coumarin-fused coumarins. Food Chem. 2020, 304, 125446. [Google Scholar] [CrossRef]
- Xue, Y.; Teng, Y.; Chen, M.; Li, Z.; Wang, G. Antioxidant activity and mechanism of avenanthramides: Double H+/e− processes and role of the catechol, guaiacyl, and carboxyl groups. J. Agric. Food Chem. 2021, 69, 7178–7189. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A.; Teena Rose, K.S.; Jacob, J.M.; Varatharaj, R.; Shashikala, K.; Janardanan, D. Curcumin analogues with improved antioxidant properties: A theoretical exploration. Food Chem. 2022, 373, 131499. [Google Scholar] [CrossRef] [PubMed]
- Amića, A.; Markovićb, Z.; Kleinc, E.; Dimitrić Markovićd, J.M.; Milenković, D. Theoretical study of the thermodynamics of the mechanisms underlying antiradical activity of cinnamic acid derivatives. Food Chem. 2018, 246, 481–489. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Zhou, Y.; Guo, R.; Fu, Z.-M.; Chen, D.-F. Structure-antioxidant activity relationship of ferulic acid derivatives: Effect of ester groups at the end of the carbon side chain. LWT-Food Sci. Technol. 2020, 120, 108932. [Google Scholar] [CrossRef]
- Shang, Y.; Li, X.; Li, Z.; Zhou, J.; Qu, L.; Chen, K. Theoretical study on the radical scavenging activity and mechanism of four kinds of Gnetin molecule. Food Chem. 2022, 378, 131975. [Google Scholar] [CrossRef]
- Lu, X.-Q.; Qin, S.; Li, J. Radical scavenging capability and mechanism of three isoflavonoids extracted from Radix Astragali: A theoretical study. Molecules 2023, 28, 5039. [Google Scholar] [CrossRef]
- Du, Y.; Chai, Y.; Zheng, X.; Zheng, Y. Theoretical study on the multiple free radical scavenging reactions of pyranoanthocyanins. Antioxidants 2024, 13, 33. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Stepanić, V.; Trošelj, K.G.; Lučić, B.; Marković, Z.; Amić, D. Bond dissociation free energy as a general parameter for flavonoid radical scavenging activity. Food Chem. 2013, 141, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Amić, A.; Lučić, B.; Stepanić, V.; Marković, Z.; Marković, S.; Dimitrić Marković, J.M.; Amić, D. Free radical scavenging potency of quercetin catecholic colonic metabolites: Thermodynamics of 2H+/2e− processes. Food Chem. 2017, 218, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Xu, M.; Gong, D.; Zhang, G. Mechanism of flavonoids inhibiting xanthine oxidase and alleviating hyperuricemia from structure–activity relationship and animal experiments: A review. Food Front. 2023, 4, 1643–1665. [Google Scholar] [CrossRef]
- Xie, J.; Cui, H.; Xu, Y.; Xie, L.; Chen, W. Delphinidin-3-O-sambubioside: A novel xanthine oxidase inhibitor identified from natural anthocyanins. Food Qual. Saf. 2021, 5, fyaa038. [Google Scholar] [CrossRef]
- Lu, T. Molclus Program. Beijing Kein Research Center for Natural Science. Version 1.9.9.9. 2022. Available online: https://www.keinsci.com/research/molclus.html (accessed on 16 October 2023).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Glendening, P.E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO 6.0. Available online: https://nbo6.chem.wisc.edu/ (accessed on 16 October 2023).
- Fukui, K. The path of chemical reactions-the IRC approach. Acc. Chem. Res. 1981, 14, 363. [Google Scholar] [CrossRef]
- Bartmess, J.E. Thermodynamics of the electron and the proton. J. Phys. Chem. 1994, 98, 6420–6424. [Google Scholar] [CrossRef]
- Rimarcík, J.; Lukes, V.; Klein, E.; Ilcin, M. Study of the solvent effect on the enthalpies of homolytic and heterolytic N–H bond cleavage in p-phenylenediamine and tetracyano-p-phenylenediamine. J. Mol. Struc Theochem 2010, 952, 25–30. [Google Scholar] [CrossRef]
- Markovic, Z.; Tosovic, J.; Milenkovic, D.; Markovic, S. Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput. Theor. Chem. 2016, 1077, 11–17. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Canneaux, S.; Bohr, F.; Henon, E. KiSThelP: A program to predict thermodynamic properties and rate constants from quantum chemistry results. J. Comput. Chem. 2014, 35, 82–93. [Google Scholar] [CrossRef]
- Wigner, E. On the quantum correction for thermodynamic equilibrium. Phys. Rev. 1932, 40, 749–759. [Google Scholar] [CrossRef]
- Cao, H.; Hall, J.; Hille, R. Substrate orientation and specificity in xanthine oxidase: Crystal structures of the enzyme in complex with indole-3-acetaldehyde and guanine. Biochemistry 2014, 53, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System, Version 2.6.0a0; Schrödinger, LLC.: New York, NY, USA, 2024.
- Dudek, A.; Spiegel, M.; Strugała-Danak, P.; Gabrielska, J. Analytical and theoretical studies of antioxidant properties of chosen anthocyanins; A structure-dependent relationships. Int. J. Mol. Sci. 2022, 23, 5432. [Google Scholar] [CrossRef]
- Pottachola, S.; Kaniyantavida, A.; Karuvanthodiyil, M. DFT study of structure and radical scavenging activity of natural pigment delphinidin and derivatives. In Density Functional Theory-Recent Advances, New Perspectives and Applications; IntechOpen: London, UK, 2021; Chapter 16. [Google Scholar] [CrossRef]
- Ma, Y.; Feng, Y.; Diao, T.; Zeng, W.; Zuo, Y. Experimental and Theoretical Study on Antioxidant Activity of the Four Anthocyanins. J. Mol. Struct. 2020, 1204, 127509. [Google Scholar] [CrossRef]
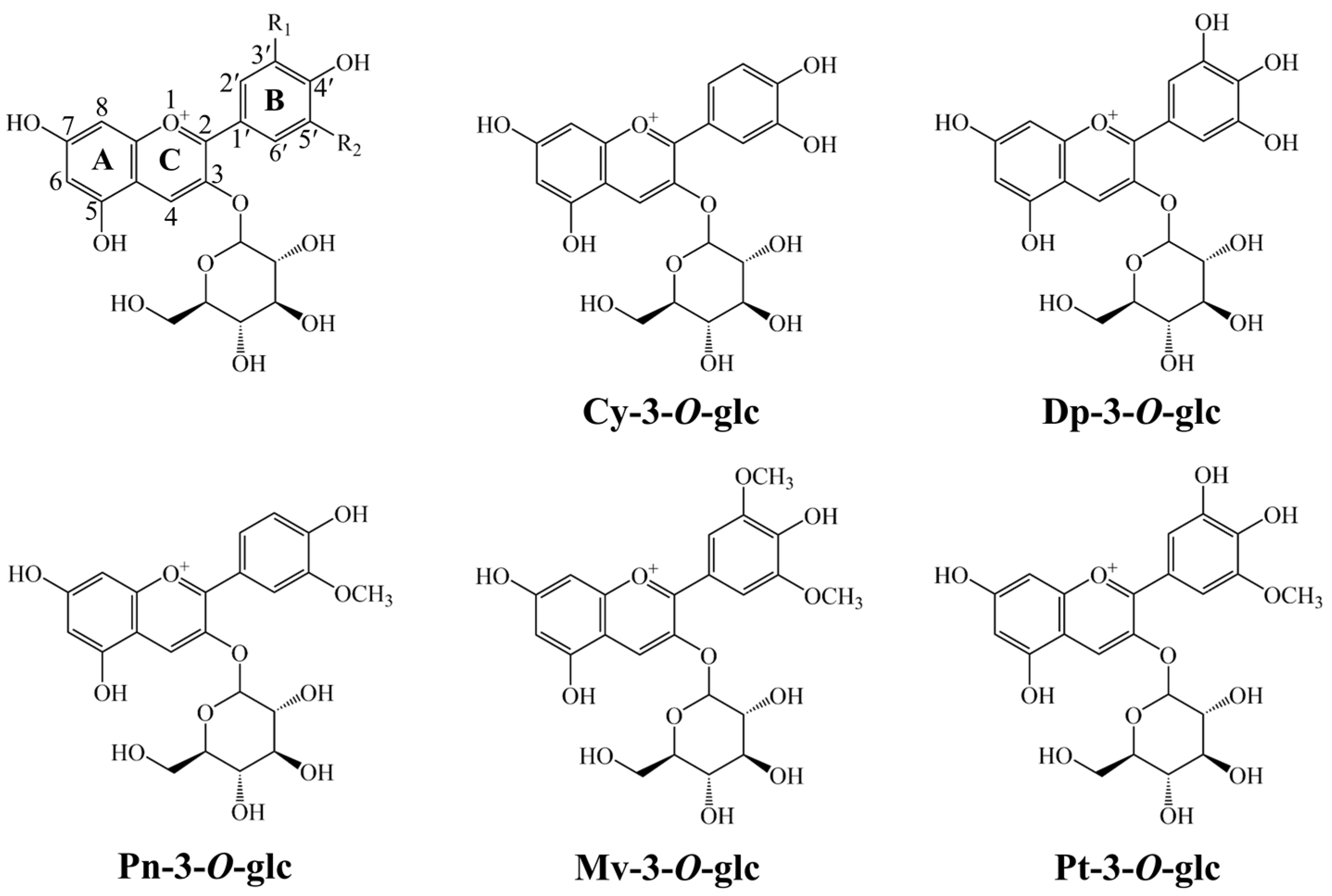
| Mechanism | HAT | SET-PT | SPLET | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE | IP | PDE | PA | ETE | |||||||||||
| Compounds | Gas | Water | Ethanol | Gas | Water | Ethanol | Gas | Water | Ethanol | Gas | Water | Ethanol | Gas | Water | Ethanol |
| Cy-3-O-glc | 244.5 | 139.7 | 135.9 | ||||||||||||
| 4′-OH | 94.0 | 88.9 | 88.2 | 162.8 | 3.1 | −0.7 | 255.7 | 25.8 | 25.0 | 151.6 | 117.0 | 110.3 | |||
| 5′-OH | 89.8 | 87.5 | 86.8 | 158.6 | 1.8 | −2.1 | 268.0 | 30.1 | 30.2 | 135.1 | 111.4 | 103.6 | |||
| 5-OH | 94.2 | 93.9 | 93.1 | 163.0 | 8.1 | 4.2 | 247.0 | 23.0 | 21.7 | 160.5 | 124.8 | 118.4 | |||
| 7-OH | 96.7 | 96.6 | 95.7 | 165.5 | 10.8 | 6.8 | 246.3 | 22.4 | 20.9 | 163.7 | 128.1 | 121.8 | |||
| Dp-3-O-glc | 243.2 | 139.4 | 135.6 | ||||||||||||
| 3′-OH | 94.7 | 89.6 | 89.2 | 164.8 | 4.2 | 0.7 | 276.7 | 31.6 | 32.6 | 131.3 | 112.0 | 103.6 | |||
| 4′-OH | 86.7 | 84.0 | 83.4 | 156.9 | −1.4 | −5.2 | 250.2 | 23.1 | 22.0 | 149.8 | 114.9 | 108.4 | |||
| 5′-OH | 90.6 | 88.5 | 87.6 | 160.7 | 3.1 | −0.9 | 268.0 | 29.8 | 29.9 | 135.9 | 112.7 | 104.8 | |||
| 5-OH | 94.1 | 93.9 | 93.2 | 164.2 | 8.4 | 4.7 | 246.9 | 22.9 | 21.6 | 160.5 | 124.9 | 118.7 | |||
| 7-OH | 96.9 | 96.6 | 96.1 | 167.0 | 11.2 | 7.5 | 246.3 | 22.5 | 20.9 | 163.9 | 128.1 | 122.2 | |||
| Pn-3-O-glc | 240.0 | 138.5 | 134.5 | ||||||||||||
| 4′-OH | 93.4 | 88.6 | 88.0 | 166.8 | 4.1 | 0.5 | 257.2 | 26.5 | 25.6 | 149.6 | 116.0 | 109.4 | |||
| 5-OH | 93.9 | 93.7 | 93.0 | 167.3 | 9.2 | 5.5 | 247.7 | 23.0 | 21.6 | 159.6 | 124.6 | 118.5 | |||
| 7-OH | 96.4 | 96.9 | 95.8 | 169.7 | 12.4 | 8.3 | 247.2 | 22.6 | 21.0 | 162.5 | 128.2 | 121.8 | |||
| Mv-3-O-glc | 234.8 | 138.9 | 134.0 | ||||||||||||
| 4′-OH | 87.4 | 86.3 | 85.0 | 165.9 | 1.4 | −2.0 | 255.5 | 24.9 | 23.7 | 145.3 | 115.4 | 108.3 | |||
| 5-OH | 93.9 | 93.3 | 93.0 | 172.4 | 8.3 | 6.0 | 248.2 | 22.8 | 21.2 | 159.0 | 124.4 | 118.9 | |||
| 7-OH | 96.4 | 97.0 | 95.5 | 174.9 | 12.1 | 8.5 | 247.8 | 22.3 | 20.6 | 162.0 | 128.6 | 122.0 | |||
| Pt-3-O-glc | 239.6 | 138.1 | 134.3 | ||||||||||||
| 3′-OH | 94.6 | 89.6 | 89.1 | 168.4 | 5.4 | 1.8 | 278.0 | 32.7 | 33.2 | 130.0 | 110.9 | 102.9 | |||
| 4′-OH | 86.2 | 83.7 | 83.0 | 160.0 | −0.4 | −4.3 | 251.7 | 23.8 | 22.5 | 147.8 | 113.9 | 107.5 | |||
| 5-OH | 94.0 | 93.8 | 93.2 | 167.7 | 9.7 | 5.9 | 247.7 | 22.8 | 21.5 | 159.6 | 125.0 | 118.8 | |||
| 7-OH | 96.7 | 96.8 | 96.0 | 170.4 | 12.7 | 8.7 | 247.3 | 22.2 | 20.8 | 162.7 | 128.6 | 122.3 qstat | |||
| Reactions | ΔG (kcal/mol) | ΔG≠ (kcal/mol) | k (M−1 s−1) |
|---|---|---|---|
| Pt-3-O-glc-3′-OH + •OH | −22.7 | 9.8 | 7.45 × 107 |
| Pt-3-O-glc-4′-OH + •OH | −31.1 | 7.6 | 5.72 × 109 |
| Pt-3-O-glc-5-OH + •OH | −24.0 | 8.1 | 1.33 × 109 |
| Pt-3-O-glc-7-OH + •OH | −21.2 | 8.3 | 9.31 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.-Q.; Li, J.; Wang, B.; Qin, S. Computational Insights into the Radical Scavenging Activity and Xanthine Oxidase Inhibition of the Five Anthocyanins Derived from Grape Skin. Antioxidants 2024, 13, 1117. https://doi.org/10.3390/antiox13091117
Lu X-Q, Li J, Wang B, Qin S. Computational Insights into the Radical Scavenging Activity and Xanthine Oxidase Inhibition of the Five Anthocyanins Derived from Grape Skin. Antioxidants. 2024; 13(9):1117. https://doi.org/10.3390/antiox13091117
Chicago/Turabian StyleLu, Xiao-Qin, Jindong Li, Bin Wang, and Shu Qin. 2024. "Computational Insights into the Radical Scavenging Activity and Xanthine Oxidase Inhibition of the Five Anthocyanins Derived from Grape Skin" Antioxidants 13, no. 9: 1117. https://doi.org/10.3390/antiox13091117






