Carbon Monoxide: A Pleiotropic Redox Regulator of Life and Death
Abstract
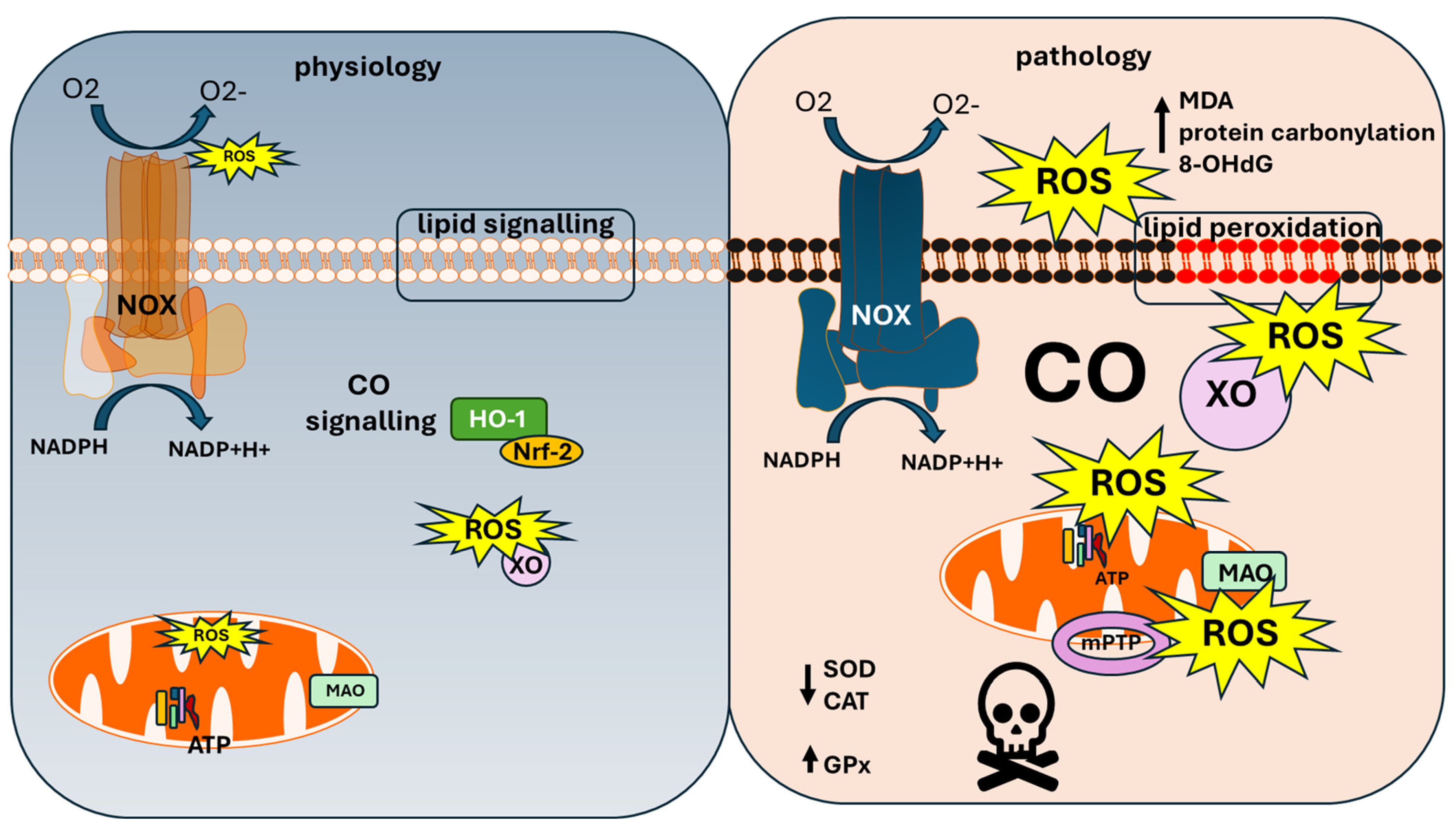
:1. Introduction
2. Physiological Role of CO
3. Carbon Monoxide and Mitochondria
4. NADPH Oxidase and Carbon Monoxide Toxicity
5. Xanthine Oxidase and CO Toxicity
6. Monoaminoxidase and Carbon Monoxide
7. CO Toxicity by Lipid Peroxidation-Driven Ferroptosis
8. CO Poisoning Oxidative Stress and Cellular Antioxidant Parameters
9. Sequence of Activation of ROS Production from Various Enzymatic Sources in the Mechanism of CO-Induced Neurotoxicity
10. Strategies to Overcome CO-Induced DNS
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ETC | electron transport chain |
| MAO | monoamine oxidase |
| ROS | reactive oxygen species |
| OXPHOS | oxidative phosphorylation |
| HO-1 | haem oxygenase 1 |
| COHb | carboxyhaemoglobin |
| CYP | cytochrome P450-dependent monooxygenases |
| Nrf-2 | nuclear factor erythroid 2-related factor 2 |
| NOX | NADPH oxidase |
| DUOX | dual oxidase |
| P2Y | purinoreceptor Y type |
| HBOT | hyperbaric oxygen therapy |
| NAC | nicotinamide cysteine |
| DNS | delayed neurological sequelae |
| XO | xanthine oxidase |
References
- GBD 2021 Carbon Monoxide Poisoning Collaborators. Global, regional, and national mortality due to unintentional carbon monoxide poisoning, 2000–2021: Results from the Global Burden of Disease Study 2021. Lancet Public Health 2023, 8, e839–e849. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J. Medicolegal contributions of historical interest. The action of carbonic oxide on man. Forensic Sci. 1972, 1, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Safari, C.; Ghosh, S.; Andersson, R.; Johannesson, J.; Bath, P.; Uwangue, O.; Dahl, P.; Zoric, D.; Sandelin, E.; Vallejos, A.; et al. Time-resolved serial crystallography to track the dynamics of carbon monoxide in the active site of cytochrome c oxidase. Sci. Adv. 2023, 9, eadh4179. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Groves, J.T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. [Google Scholar] [CrossRef]
- Bansal, S.; Liu, D.; Mao, Q.; Bauer, N.; Wang, B. Carbon Monoxide as a Potential Therapeutic Agent: A Molecular Analysis of Its Safety Profiles. J. Med. Chem. 2024, 67, 9789–9815. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, M.; Pan, Y.; Ni, Z.; Min, Q.; Wang, B.; Ke, H.; Ji, X. Reactive Oxygen Species-Activated Metal-Free Carbon Monoxide Prodrugs for Targeted Cancer Treatment. J. Med. Chem. 2023, 66, 14583–14596. [Google Scholar] [CrossRef]
- Weaver, L.K.; Hopkins, R.O.; Chan, K.J.; Churchill, S.; Elliott, C.G.; Clemmer, T.P.; Orme, J.F., Jr.; Thomas, F.O.; Morris, A.H. Hyperbaric oxygen for acute carbon monoxide poisoning. N. Engl. J. Med. 2002, 347, 1057–1067. [Google Scholar] [CrossRef]
- Delvau, N.; Elens, L.; Penaloza, A.; Liistro, G.; Thys, F.; Roy, P.M.; Gianello, P.; Hantson, P. Carboxyhemoglobin half-life toxicokinetic profiles during and after normobaric oxygen therapy: On a swine model. Toxicol. Rep. 2024, 12, 271–279. [Google Scholar] [CrossRef]
- Angelova, P.R.; Myers, I.; Abramov, A.Y. Carbon monoxide neurotoxicity is triggered by oxidative stress induced by ROS production from three distinct cellular sources. Redox Biol. 2023, 60, 102598. [Google Scholar] [CrossRef]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef]
- Mancuso, C. Heme oxygenase and its products in the nervous system. Antioxid. Redox Signal 2004, 6, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C. The brain heme oxygenase/biliverdin reductase system as a target in drug research and development. Expert. Opin. Ther. Targets 2022, 26, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.W.; Bhat, G.K.; Lamar, C.A.; Mahesh, V.B. Gaseous transmitters and neuroendocrine regulation. Neuroendocrinology 1997, 65, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R. NO and CO as second messengers in oxygen sensing in the carotid body. Respir. Physiol. 1999, 115, 161–168. [Google Scholar] [CrossRef]
- Prabhakar, N.R. Carbon monoxide (CO) and hydrogen sulfide (H2S) in hypoxic sensing by the carotid body. Respir. Physiol. Neurobiol. 2012, 184, 165–169. [Google Scholar] [CrossRef]
- Mahan, V.L. Neuroprotective, neurotherapeutic, and neurometabolic effects of carbon monoxide. Med. Gas. Res. 2012, 2, 32. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef]
- Angelova, P.R. Sources and triggers of oxidative damage in neurodegeneration. Free Radic. Biol. Med. 2021, 173, 52–63. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016, 100, 81–85. [Google Scholar] [CrossRef]
- Novikova, I.N.; Manole, A.; Zherebtsov, E.A.; Stavtsev, D.D.; Vukolova, M.N.; Dunaev, A.V.; Angelova, P.R.; Abramov, A.Y. Adrenaline induces calcium signal in astrocytes and vasoconstriction via activation of monoamine oxidase. Free Radic. Biol. Med. 2020, 159, 15–22. [Google Scholar] [CrossRef]
- Cheng, X.; Vinokurov, A.Y.; Zherebtsov, E.A.; Stelmashchuk, O.A.; Angelova, P.R.; Esteras, N.; Abramov, A.Y. Variability of mitochondrial energy balance across brain regions. J. Neurochem. 2021, 157, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Vinokurov, A.Y.; Stelmashuk, O.A.; Ukolova, P.A.; Zherebtsov, E.A.; Abramov, A.Y. Brain region specificity in reactive oxygen species production and maintenance of redox balance. Free Radic. Biol. Med. 2021, 174, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, W.; Hopper, C.P.; Ke, B.; Wang, B. Nature’s marvels endowed in gaseous molecules I: Carbon monoxide and its physiological and therapeutic roles. Acta Pharm. Sin. B 2021, 11, 1434–1445. [Google Scholar] [CrossRef]
- Cardoso-Pires, C.; Vieira, H.L.A. Carbon monoxide and mitochondria: Cell energy and fate control. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167446. [Google Scholar] [CrossRef]
- Figueiredo-Pereira, C.; Dias-Pedroso, D.; Soares, N.L.; Vieira, H.L.A. CO-mediated cytoprotection is dependent on cell metabolism modulation. Redox Biol. 2020, 32, 101470. [Google Scholar] [CrossRef]
- Kaczara, P.; Proniewski, B.; Lovejoy, C.; Kus, K.; Motterlini, R.; Abramov, A.Y.; Chlopicki, S. CORM-401 induces calcium signalling, NO increase and activation of pentose phosphate pathway in endothelial cells. FEBS J. 2018, 285, 1346–1358. [Google Scholar] [CrossRef]
- Figueiredo-Pereira, C.; Villarejo-Zori, B.; Cipriano, P.C.; Tavares, D.; Ramirez-Pardo, I.; Boya, P.; Vieira, H.L.A. Carbon Monoxide Stimulates Both Mitophagy and Mitochondrial Biogenesis to Mediate Protection against Oxidative Stress in Astrocytes. Mol. Neurobiol. 2023, 60, 851–863. [Google Scholar] [CrossRef]
- Lu, W.; Yang, X.; Wang, B. Carbon monoxide signaling and soluble guanylyl cyclase: Facts, myths, and intriguing possibilities. Biochem. Pharmacol. 2022, 200, 115041. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, P.; Alam, J.; Fu, X.Y.; Lee, P.J. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J. Biol. Chem. 2005, 280, 8714–8721. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Baird, L.; Holmstrom, K.M.; Meyer, C.J.; Abramov, A.Y. The spatiotemporal regulation of the Keap1-Nrf2 pathway and its importance in cellular bioenergetics. Biochem. Soc. Trans. 2015, 43, 602–610. [Google Scholar] [CrossRef]
- Kovac, S.; Angelova, P.R.; Holmstrom, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta 2015, 1850, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Esteras, N.; Blacker, T.S.; Zherebtsov, E.A.; Stelmashuk, O.A.; Zhang, Y.; Wigley, W.C.; Duchen, M.R.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates glucose uptake and metabolism in neurons and astrocytes. Redox Biol. 2023, 62, 102672. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; De La Cruz, L.K.; Yang, X.; Wang, B. Carbon Monoxide Signaling: Examining Its Engagement with Various Molecular Targets in the Context of Binding Affinity, Concentration, and Biologic Response. Pharmacol. Rev. 2022, 74, 823–873. [Google Scholar] [CrossRef]
- Kaczara, P.; Motterlini, R.; Kus, K.; Zakrzewska, A.; Abramov, A.Y.; Chlopicki, S. Carbon monoxide shifts energetic metabolism from glycolysis to oxidative phosphorylation in endothelial cells. FEBS Lett. 2016, 590, 3469–3480. [Google Scholar] [CrossRef]
- Ward, J.M.; Nickerson, W.J. Respiratory metabolism of normal and divisionless strains of Candida albicans. J. Gen. Physiol. 1958, 41, 703–724. [Google Scholar] [CrossRef]
- Miro, O.; Casademont, J.; Barrientos, A.; Urbano-Marquez, A.; Cardellach, F. Mitochondrial cytochrome c oxidase inhibition during acute carbon monoxide poisoning. Pharmacol. Toxicol. 1998, 82, 199–202. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Scorziello, A.; Duchen, M.R. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 2007, 27, 1129–1138. [Google Scholar] [CrossRef]
- Zuckerbraun, B.S.; Chin, B.Y.; Bilban, M.; d’Avila, J.C.; Rao, J.; Billiar, T.R.; Otterbein, L.E. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007, 21, 1099–1106. [Google Scholar] [CrossRef]
- Lo Iacono, L.; Boczkowski, J.; Zini, R.; Salouage, I.; Berdeaux, A.; Motterlini, R.; Morin, D. A carbon monoxide-releasing molecule (CORM-3) uncouples mitochondrial respiration and modulates the production of reactive oxygen species. Free Radic. Biol. Med. 2011, 50, 1556–1564. [Google Scholar] [CrossRef]
- Rose, J.J.; Bocian, K.A.; Xu, Q.; Wang, L.; DeMartino, A.W.; Chen, X.; Corey, C.G.; Guimaraes, D.A.; Azarov, I.; Huang, X.N.; et al. A neuroglobin-based high-affinity ligand trap reverses carbon monoxide-induced mitochondrial poisoning. J. Biol. Chem. 2020, 295, 6357–6371. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.H.; Khatri, U.G.; Shortal, B.P.; Kelly, M.; Hardy, K.; Lambert, D.S.; Eckmann, D.M. Alterations in mitochondrial respiration and reactive oxygen species in patients poisoned with carbon monoxide treated with hyperbaric oxygen. Intensive Care Med. Exp. 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Kuriiwa, F.; Kobayashi, M.; Mizukami, H.; Hara, S. Mitochondrial toxins potentiate hydroxyl radical production in rat striatum during carbon monoxide poisoning. J. Pharmacol. Sci. 2021, 146, 29–32. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Jacobson, J.; Wientjes, F.; Hothersall, J.; Canevari, L.; Duchen, M.R. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J. Neurosci. 2005, 25, 9176–9184. [Google Scholar] [CrossRef]
- Hara, S.; Kobayashi, M.; Kuriiwa, F.; Ikematsu, K.; Mizukami, H. Hydroxyl radical production via NADPH oxidase in rat striatum due to carbon monoxide poisoning. Toxicology 2018, 394, 63–71. [Google Scholar] [CrossRef]
- Minnella, A.M.; Zhao, J.X.; Jiang, X.; Jakobsen, E.; Lu, F.; Wu, L.; El-Benna, J.; Gray, J.A.; Swanson, R.A. Excitotoxic superoxide production and neuronal death require both ionotropic and non-ionotropic NMDA receptor signaling. Sci. Rep. 2018, 8, 17522. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Duchen, M.R. Impaired mitochondrial bioenergetics determines glutamate-induced delayed calcium deregulation in neurons. Biochim. Biophys. Acta 2010, 1800, 297–304. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Interplay of mitochondrial calcium signalling and reactive oxygen species production in the brain. Biochem. Soc. Trans. 2024, 52, 1939–1946. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; Garcia-Montero, C.; Callejon-Pelaez, E.; Saez, M.A.; Alvarez-Mon, M.A.; Garcia-Honduvilla, N.; Monserrat, J.; Alvarez-Mon, M.; Bujan, J.; et al. A General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities. Medicina 2021, 57, 864. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, Z.; Meng, X.; Lv, Y.; Pan, S.; Guo, D. Effects of hyperbaric oxygen on NLRP3 inflammasome activation in the brain after carbon monoxide poisoning. Undersea Hyperb. Med. 2020, 47, 607–619. [Google Scholar] [CrossRef]
- Li, C.; Jackson, R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol. Cell Physiol. 2002, 282, C227–C241. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R. Dehydrogenase conversion to oxidase and lipid peroxidation in brain after carbon monoxide poisoning. J. Appl. Physiol. (1985) 1992, 73, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R. Leukocytes in carbon monoxide-mediated brain oxidative injury. Toxicol. Appl. Pharmacol. 1993, 123, 234–247. [Google Scholar] [CrossRef]
- Dong, G.; Ren, M.; Wang, X.; Jiang, H.; Yin, X.; Wang, S.; Wang, X.; Feng, H. Allopurinol reduces severity of delayed neurologic sequelae in experimental carbon monoxide toxicity in rats. Neurotoxicology 2015, 48, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Tatro, L.; Zhang, J. Hydroxyl radical production in the brain after CO hypoxia in rats. Free Radic. Biol. Med. 1995, 18, 603–609. [Google Scholar] [CrossRef]
- Yang, J.Q.; Zhou, Q.X. Protective effect of nimodipine against cerebral injury induced by subacute carbon monoxide intoxication in mice. Acta Pharmacol. Sin. 2001, 22, 423–427. [Google Scholar]
- Hara, S.; Mukai, T.; Kurosaki, K.; Kuriiwa, F.; Endo, T. Modification of the striatal dopaminergic neuron system by carbon monoxide exposure in free-moving rats, as determined by in vivo brain microdialysis. Arch. Toxicol. 2002, 76, 596–605. [Google Scholar] [CrossRef]
- Park, E.J.; Min, Y.G.; Kim, G.W.; Cho, J.P.; Maeng, W.J.; Choi, S.C. Pathophysiology of brain injuries in acute carbon monoxide poisoning: A novel hypothesis. Med. Hypotheses 2014, 83, 186–189. [Google Scholar] [CrossRef]
- Weaver, L.K. Carbon monoxide poisoning. Crit. Care Clin. 1999, 15, 297–317. [Google Scholar] [CrossRef]
- Miro, O.; Alonso, J.R.; Casademont, J.; Jarreta, D.; Urbano-Marquez, A.; Cardellach, F. Oxidative damage on lymphocyte membranes is increased in patients suffering from acute carbon monoxide poisoning. Toxicol. Lett. 1999, 110, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Poon, H.F.; Calabrese, V.; Scapagnini, G.; Butterfield, D.A. Free radicals: Key to brain aging and heme oxygenase as a cellular response to oxidative stress. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Wang, P.; Zeng, T.; Zhang, C.L.; Gao, X.C.; Liu, Z.; Xie, K.Q.; Chi, Z.F. Lipid peroxidation was involved in the memory impairment of carbon monoxide-induced delayed neuron damage. Neurochem. Res. 2009, 34, 1293–1298. [Google Scholar] [CrossRef]
- Thom, S.R. Carbon monoxide-mediated brain lipid peroxidation in the rat. J. Appl. Physiol. (1985) 1990, 68, 997–1003. [Google Scholar] [CrossRef]
- Atalay, H.; Aybek, H.; Koseoglu, M.; Demir, S.; Erbay, H.; Bolaman, A.Z.; Avci, A. The effects of amifostine and dexamethasone on brain tissue lipid peroxidation during oxygen treatment of carbon monoxide-poisoned rats. Adv. Ther. 2006, 23, 332–341. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, B.; Tian, Y.; Hu, Q.; Jiang, X.; Zhang, J.; Chen, L.; Wang, R.; Li, M.; Zhou, X.; et al. Targeting Ferroptosis Promotes Functional Recovery by Mitigating White Matter Injury Following Acute Carbon Monoxide Poisoning. Mol. Neurobiol. 2024, 61, 1157–1174. [Google Scholar] [CrossRef]
- Teksam, O.; Sabuncuoglu, S.; Girgin, G.; Ozgunes, H. Evaluation of oxidative stress and antioxidant parameters in children with carbon monoxide poisoning. Hum. Exp. Toxicol. 2019, 38, 1235–1243. [Google Scholar] [CrossRef]
- Thom, S.R.; Kang, M.; Fisher, D.; Ischiropoulos, H. Release of glutathione from erythrocytes and other markers of oxidative stress in carbon monoxide poisoning. J. Appl. Physiol. (1985) 1997, 82, 1424–1432. [Google Scholar] [CrossRef]
- Roderique, J.D.; Josef, C.S.; Feldman, M.J.; Spiess, B.D. A modern literature review of carbon monoxide poisoning theories, therapies, and potential targets for therapy advancement. Toxicology 2015, 334, 45–58. [Google Scholar] [CrossRef]
- Akyol, S.; Yuksel, S.; Pehlivan, S.; Erdemli, H.K.; Gulec, M.A.; Adam, B.; Akyol, O. Possible role of antioxidants and nitric oxide inhibitors against carbon monoxide poisoning: Having a clear conscience because of their potential benefits. Med. Hypotheses 2016, 92, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Akyol, S.; Erdogan, S.; Idiz, N.; Celik, S.; Kaya, M.; Ucar, F.; Dane, S.; Akyol, O. The role of reactive oxygen species and oxidative stress in carbon monoxide toxicity: An in-depth analysis. Redox Rep. 2014, 19, 180–189. [Google Scholar] [CrossRef]
- Howard, R.J.; Blake, D.R.; Pall, H.; Williams, A.; Green, I.D. Allopurinol/N-acetylcysteine for carbon monoxide poisoning. Lancet 1987, 2, 628–629. [Google Scholar] [CrossRef]
- Dogan, G.; Kayir, S.; Ayaz, E.; Ozcan, O.; Akdagli Ekici, A. Curcumin as a Potential Therapeutic Agent for Mitigating Carbon Monoxide Poisoning: Evidence from an Experimental Rat Study. Med. Sci. Monit. 2024, 30, e943739. [Google Scholar] [CrossRef]
- Spina, V.; Tomaiuolo, F.; Celli, L.; Bonfiglio, L.; Cecchetti, L.; Carboncini, M.C. A Case of Carbon Monoxide-Induced Delayed Neurological Sequelae Successfully Treated with Hyperbaric Oxygen Therapy, N-Acetylcysteine, and Glucocorticoids: Clinical and Neuroimaging Follow-Up. Case Rep. Neurol. Med. 2019, 2019, 9360542. [Google Scholar] [CrossRef]
- Akyol, S.; Gulec, M.A.; Erdemli, H.K.; Akyol, O. A new therapeutic approach for carbon monoxide poisoning: Antioxidants. Toxicology 2015, 336, 34–35. [Google Scholar] [CrossRef]
- Nesterowicz, M.; Zendzian-Piotrowska, M.; Ladny, J.R.; Zalewska, A.; Maciejczyk, M. Antiglycoxidative properties of amantadine—A systematic review and comprehensive in vitro study. J. Enzyme Inhib. Med. Chem. 2023, 38, 138–155. [Google Scholar] [CrossRef]
- Tabrizian, K.; Shahraki, J.; Bazzi, M.; Rezaee, R.; Jahantigh, H.; Hashemzaei, M. Neuro-Protective Effects of Resveratrol on Carbon Monoxide-Induced Toxicity in Male Rats. Phytother. Res. 2017, 31, 1310–1315. [Google Scholar] [CrossRef]
- Zhao, N.; Liang, P.; Zhuo, X.; Su, C.; Zong, X.; Guo, B.; Han, D.; Yan, X.; Hu, S.; Zhang, Q.; et al. After Treatment with Methylene Blue is Effective against Delayed Encephalopathy after Acute Carbon Monoxide Poisoning. Basic. Clin. Pharmacol. Toxicol. 2018, 122, 470–480. [Google Scholar] [CrossRef]
- Teerapuncharoen, K.; Sharma, N.S.; Barker, A.B.; Wille, K.M.; Diaz-Guzman, E. Successful Treatment of Severe Carbon Monoxide Poisoning and Refractory Shock Using Extracorporeal Membrane Oxygenation. Respir. Care 2015, 60, e155–e160. [Google Scholar] [CrossRef]
- Schauer, R.J.; Kalmuk, S.; Gerbes, A.L.; Leiderer, R.; Meissner, H.; Schildberg, F.W.; Messmer, K.; Bilzer, M. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J. Gastroenterol. 2004, 10, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Ishkaeva, R.A.; Zoughaib, M.; Laikov, A.V.; Angelova, P.R.; Abdullin, T.I. Probing Cell Redox State and Glutathione-Modulating Factors Using a Monochlorobimane-Based Microplate Assay. Antioxidants 2022, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.D.; Wang, J.L.; Guo, D.D.; Jiang, W.W.; Li, Z.K.; Wang, L.; Zou, Y.; Bi, M.J.; Li, Q. Neuroprotective effect of targeted regulatory Nrf2 gene on rats with acute brain injury induced by carbon monoxide poisoning. Environ. Toxicol. 2021, 36, 1742–1757. [Google Scholar] [CrossRef]
- Li, Q.; Bi, M.J.; Bi, W.K.; Kang, H.; Yan, L.J.; Guo, Y.L. Edaravone attenuates brain damage in rats after acute CO poisoning through inhibiting apoptosis and oxidative stress. Environ. Toxicol. 2016, 31, 372–379. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Y.; Bi, M.; Lin, H.; Chen, Y.; Zou, Y.; Liu, Y.; Kang, H.; Guo, Y. Effects of N-butylphthalide on the activation of Keap1/Nrf-2 signal pathway in rats after carbon monoxide poisoning. Environ. Toxicol. Pharmacol. 2015, 40, 22–29. [Google Scholar] [CrossRef]
- Bi, M.; Li, Q.; Guo, D.; Ding, X.; Bi, W.; Zhang, Y.; Zou, Y. Sulphoraphane Improves Neuronal Mitochondrial Function in Brain Tissue in Acute Carbon Monoxide Poisoning Rats. Basic. Clin. Pharmacol. Toxicol. 2017, 120, 541–549. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Bai, Y.; Xiao, J.; Jiao, H.; He, R. Ginaton improves neurological function in ischemic stroke rats via inducing autophagy and maintaining mitochondrial homeostasis. Neuropsychiatr. Dis. Treat. 2019, 15, 1813–1822. [Google Scholar] [CrossRef]
- Wang, W.Z.; Qi, H.N.; Xiao, Q.M.; Gao, X.; Zhu, B.Y.; Li, J.; Liu, Y.J.; Li, W.; Ma, G.Y.; Wang, P. [Effects of Ginaton on nitric oxide and nitric oxide synthase in patients with delayed encephalopathy after carbon monoxide poisoning]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2017, 35, 30–33. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.; Ko, Y.; Lee, C.A.; Kim, G.W.; Moon, J.E.; Nah, S.; Han, S. Dexamethasone therapy prevents delayed neuropsychiatric sequelae after carbon monoxide poisoning: A prospective registry-based study. Clin. Toxicol. 2023, 61, 98–103. [Google Scholar] [CrossRef]
- Hayashi, M.; Otsuki, K.; Miura, S.; Mihara, Y.; Abe, S.; Inagaki, M. Delayed encephalopathy after carbon monoxide poisoning treated with corticosteroid monotherapy: Case report. Psychiatry Clin. Neurosci. 2022, 76, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Ikeda, K.; Mizumura, S.; Tachiki, K.; Yanagihashi, M.; Iwasaki, Y. Combined treatment of methylprednisolone pulse and memantine hydrochloride prompts recovery from neurological dysfunction and cerebral hypoperfusion in carbon monoxide poisoning: A case report. J. Stroke Cerebrovasc. Dis. 2014, 23, 592–595. [Google Scholar] [CrossRef]
- Maurice, T.; Phan, V.; Sandillon, F.; Urani, A. Differential effect of dehydroepiandrosterone and its steroid precursor pregnenolone against the behavioural deficits in CO-exposed mice. Eur. J. Pharmacol. 2000, 390, 145–155. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramov, A.Y.; Myers, I.; Angelova, P.R. Carbon Monoxide: A Pleiotropic Redox Regulator of Life and Death. Antioxidants 2024, 13, 1121. https://doi.org/10.3390/antiox13091121
Abramov AY, Myers I, Angelova PR. Carbon Monoxide: A Pleiotropic Redox Regulator of Life and Death. Antioxidants. 2024; 13(9):1121. https://doi.org/10.3390/antiox13091121
Chicago/Turabian StyleAbramov, Andrey Y., Isabella Myers, and Plamena R. Angelova. 2024. "Carbon Monoxide: A Pleiotropic Redox Regulator of Life and Death" Antioxidants 13, no. 9: 1121. https://doi.org/10.3390/antiox13091121






