Dimethyl Fumarate Strongly Ameliorates Gray and White Matter Brain Injury and Modulates Glial Activation after Severe Hypoxia–Ischemia in Neonatal Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hypoxia–Ischemia (HI)
- -
- Sham (n = 13): pups without ischemia or hypoxia.
- -
- Sham + DMF (n = 10): pups without ischemia or hypoxia receiving DMF.
- -
- HI (n = 24): hypoxic–ischemic non-treated pups.
- -
- HI + DMF (n = 13): hypoxic–ischemic pups receiving DMF.
2.2. DMF Administration
2.3. Obtention and Processing of Samples
2.4. Brain Injury Assessment
2.5. Immunohistochemistry
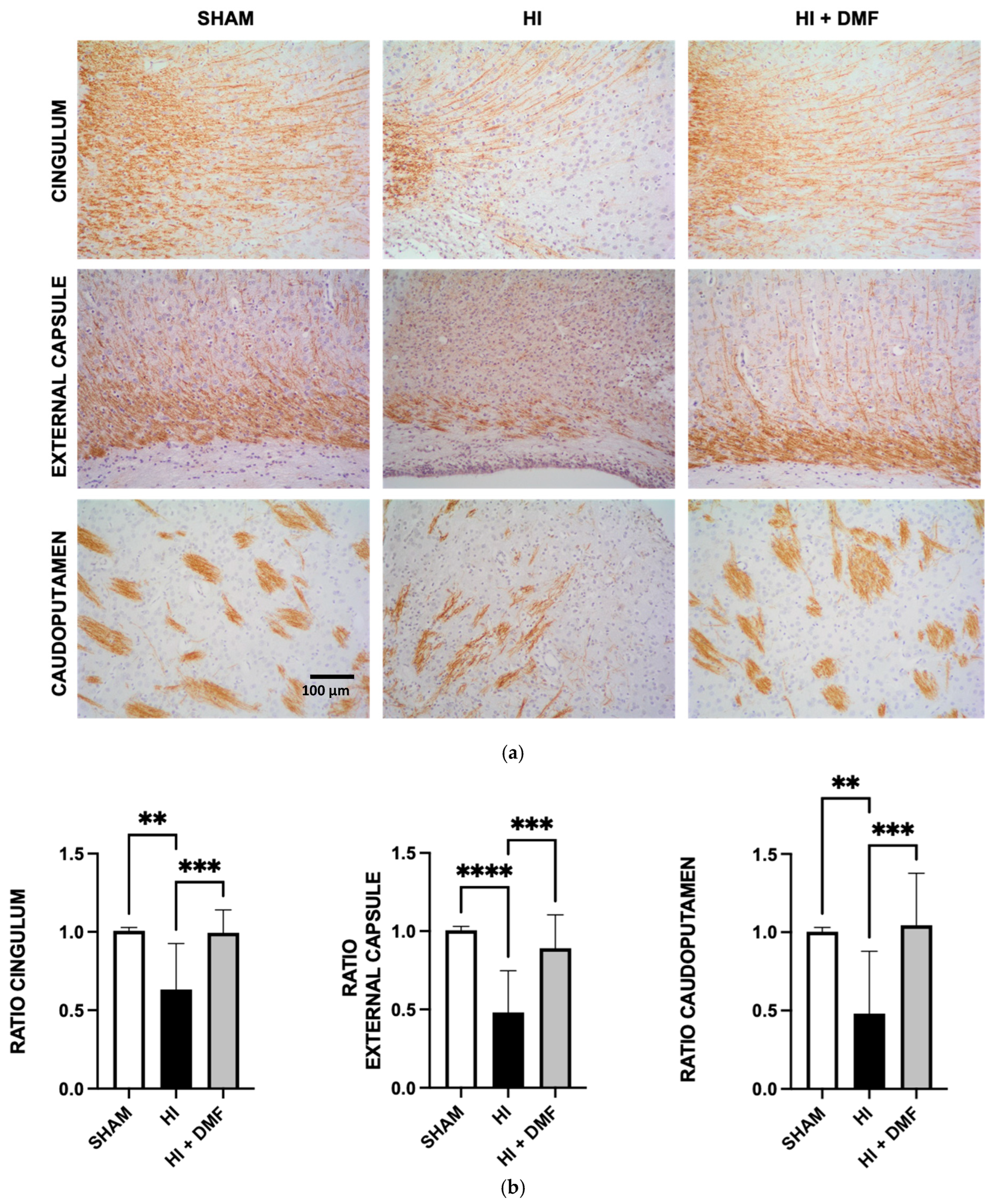
2.6. White Matter Injury Assessment
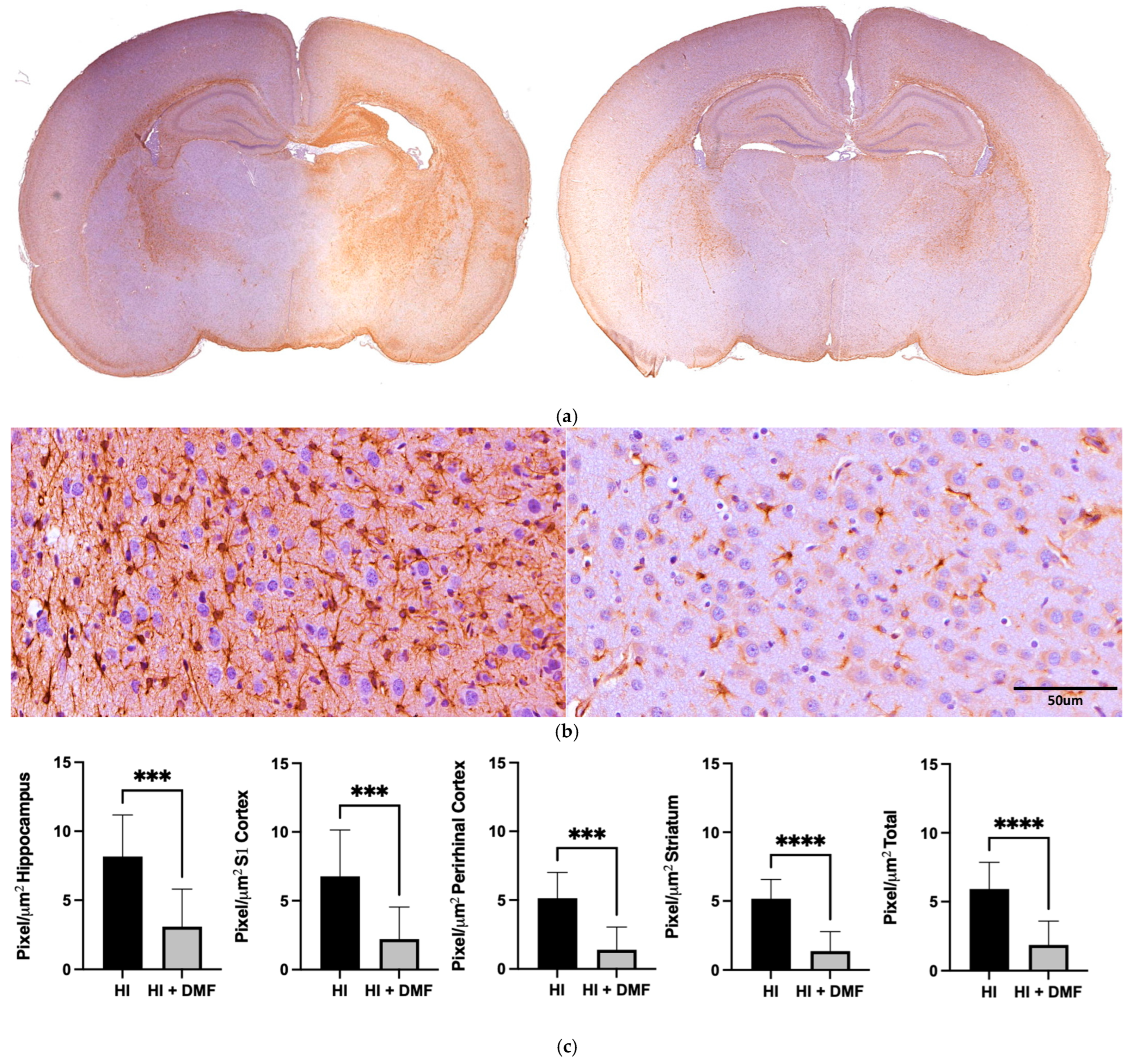
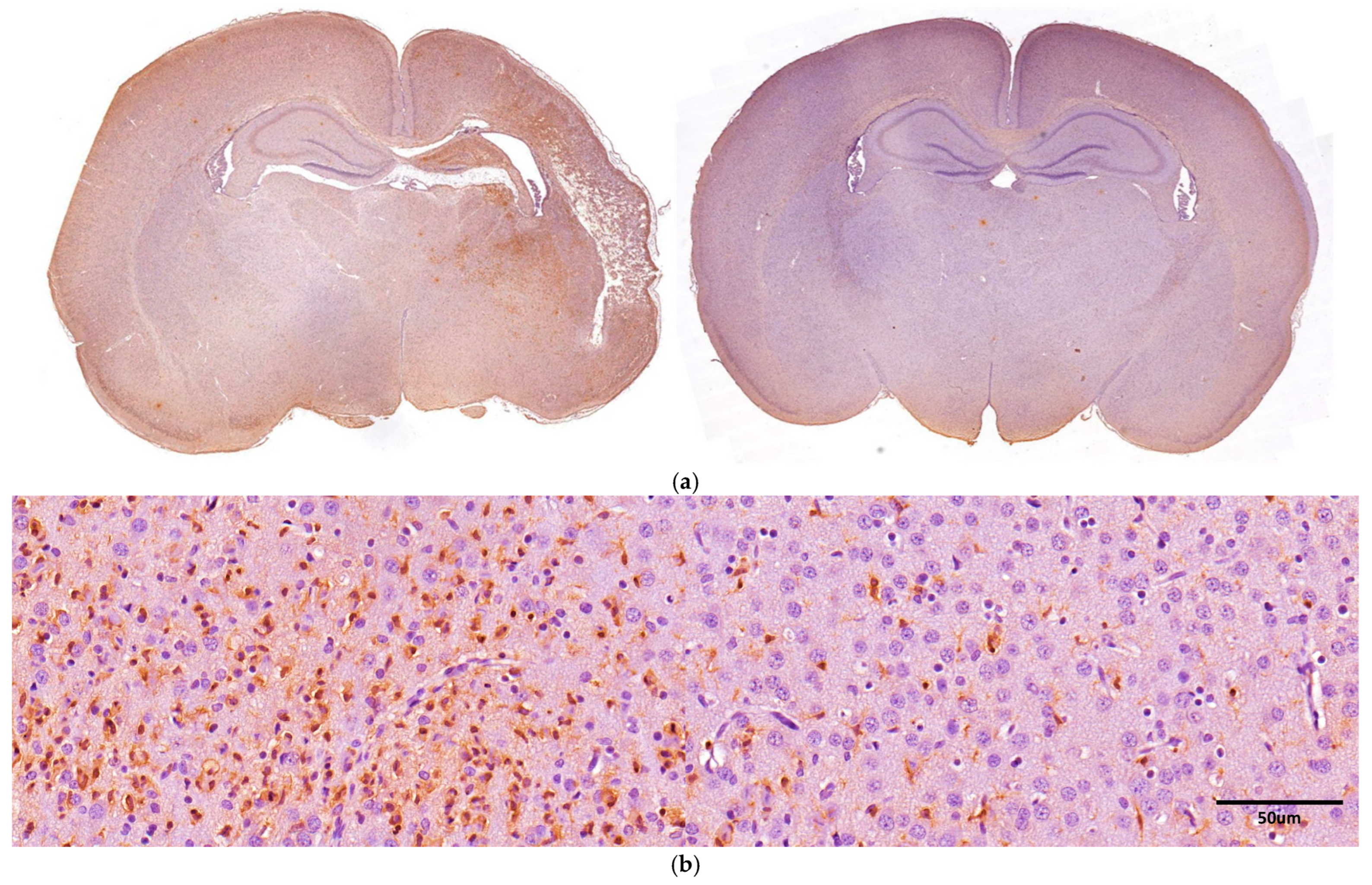
2.7. GFAP and Iba-1 Immunoreactivity
2.8. Statistical Analyses
3. Results
3.1. Neuropathological Score
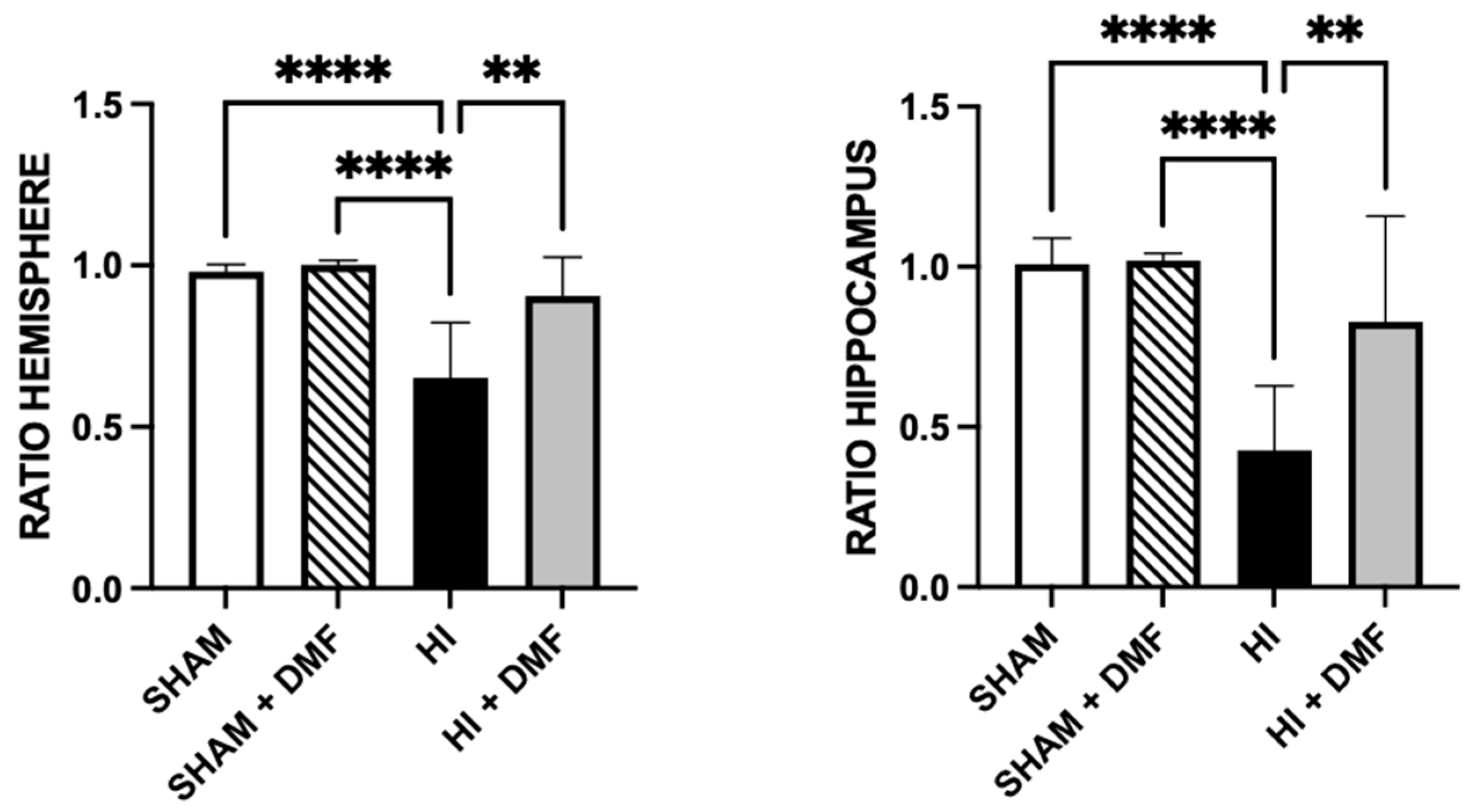
3.2. Brain Area Loss
3.3. Myelin Basic Protein Quantification
3.4. GFAP Immunoreactivity
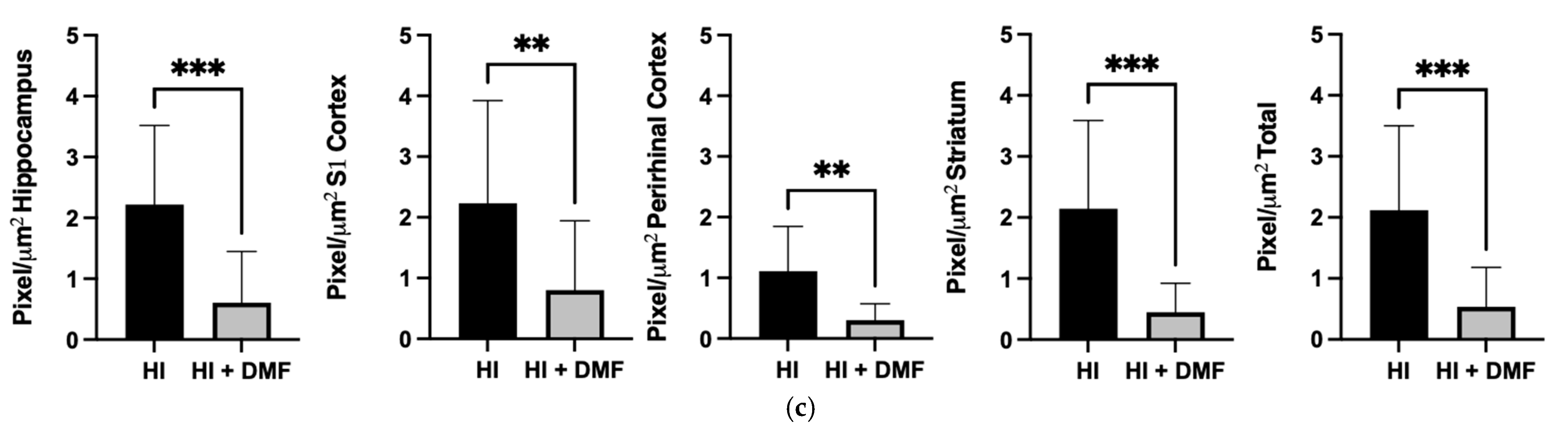
3.5. Iba-1 Immunoreactivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.W.; Comstock, B.A.; Gonzalez, F.F.; Mayock, D.E.; Goodman, A.M.; Maitre, N.L.; Chang, T.; Van Meurs, K.P.; Lampland, A.L.; Bendel-Stenzel, E.; et al. Trial of Erythropoietin for Hypoxic–Ischemic Encephalopathy in Newborns. N. Engl. J. Med. 2022, 387, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.D.; Brocklehurst, P.; Gunn, A.J.; Halliday, H.; Juszczak, E.; Levene, M.; Strohm, B.; Thoresen, M.; Whitelaw, A.; Azzopardi, D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ 2010, 340, c363. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.; Eide, S.; Sun, H.-S.; Feng, Z.-P. Animal models for neonatal brain injury induced by hypoxic ischemic conditions in rodents. Exp. Neurol. 2020, 334, 113457. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.; Osredkar, D.; Puchades, M.; Maes, E.; Falck, M.; Flatebø, T.; Walløe, L.; Sabir, H.; Thoresen, M. Treatment temperature and insult severity influence the neuroprotective effects of therapeutic hypothermia. Sci. Rep. 2016, 6, 23430. [Google Scholar] [CrossRef] [PubMed]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnár, Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef]
- Davidson, J.O.; Draghi, V.; Whitham, S.; Dhillon, S.K.; Wassink, G.; Bennet, L.; Gunn, A.J. How long is sufficient for optimal neuroprotection with cerebral cooling after ischemia in fetal sheep? J. Cereb. Blood Flow Metab. 2018, 38, 1047–1059. [Google Scholar] [CrossRef]
- Azzopardi, D.; Strohm, B.; Linsell, L.; Hobson, A.; Juszczak, E.; Kurinczuk, J.J.; Brocklehurst, P.; Edwards, A.D.; on behalf of the UK TOBY Cooling Register. Implementation and Conduct of Therapeutic Hypothermia for Perinatal Asphyxial Encephalopathy in the UK–Analysis of National Data. PLoS ONE 2012, 7, e38504. [Google Scholar] [CrossRef]
- Alonso-Alconada, D.; Broad, K.D.; Bainbridge, A.; Chandrasekaran, M.; Faulkner, S.D.; Kerenyi, Á.; Hassell, J.; Rocha-Ferreira, E.; Hristova, M.; Fleiss, B.; et al. Brain Cell Death Is Reduced With Cooling by 3.5 °C to 5 °C but Increased With Cooling by 8.5 °C in a Piglet Asphyxia Model. Stroke 2015, 46, 275–278. [Google Scholar] [CrossRef]
- Wassink, G.; Davidson, J.O.; Lear, C.A.; Juul, S.E.; Northington, F.; Bennet, L.; Gunn, A.J. A working model for hypothermic neuroprotection. J. Physiol. 2018, 596, 5641–5654. [Google Scholar] [CrossRef]
- Sabir, H.; Scull-Brown, E.; Liu, X.; Thoresen, M. Immediate Hypothermia Is Not Neuroprotective After Severe Hypoxia-Ischemia and Is Deleterious When Delayed by 12 Hours in Neonatal Rats. Stroke 2012, 43, 3364–3370. [Google Scholar] [CrossRef]
- Silachev, D.N.; Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Balakireva, A.V.; Gulyaev, M.V.; Pirogov, Y.A.; Skulachev, V.P.; Zorov, D.B. Neuroprotective Effects of Mitochondria-Targeted Plastoquinone in a Rat Model of Neonatal Hypoxic–Ischemic Brain Injury. Molecules 2018, 23, 1871. [Google Scholar] [CrossRef] [PubMed]
- Kletkiewicz, H.; Klimiuk, M.; Woźniak, A.; Mila-Kierzenkowska, C.; Dokladny, K.; Rogalska, J. How to Improve the Antioxidant Defense in Asphyxiated Newborns—Lessons from Animal Models. Antioxidants 2020, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhu, P.; Fujino, M.; Zhuang, J.; Guo, H.; Sheikh, I.; Zhao, L.; Li, X.-K. Oxidative Stress in Hypoxic-Ischemic Encephalopathy: Molecular Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2016, 17, 2078. [Google Scholar] [CrossRef] [PubMed]
- Jellema, R.K.; Lima Passos, V.; Zwanenburg, A.; Ophelders, D.R.; De Munter, S.; Vanderlocht, J.; Germeraad, W.T.; Kuypers, E.; Collins, J.J.; Cleutjens, J.P.; et al. Cerebral inflammation and mobilization of the peripheral immune system following global hypoxia-ischemia in preterm sheep. J. Neuroinflamm. 2013, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Tataranno, M.L.; Perrone, S.; Longini, M.; Buonocore, G. New Antioxidant Drugs for Neonatal Brain Injury. Oxid. Med. Cell. Longev. 2015, 2015, 108251. [Google Scholar] [CrossRef] [PubMed]
- Majkutewicz, I. Dimethyl fumarate: A review of preclinical efficacy in models of neurodegenerative diseases. Eur. J. Pharmacol. 2022, 926, 175025. [Google Scholar] [CrossRef]
- Liddell, J. Are Astrocytes the Predominant Cell Type for Activation of Nrf2 in Aging and Neurodegeneration? Antioxidants 2017, 6, 65. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Zarbato, G.F.; De Souza Goldim, M.P.; Giustina, A.D.; Danielski, L.G.; Mathias, K.; Florentino, D.; De Oliveira Junior, A.N.; Da Rosa, N.; Laurentino, A.O.; Trombetta, T.; et al. Dimethyl Fumarate Limits Neuroinflammation and Oxidative Stress and Improves Cognitive Impairment After Polymicrobial Sepsis. Neurotox. Res. 2018, 34, 418–430. [Google Scholar] [CrossRef]
- Majkutewicz, I.; Kurowska, E.; Podlacha, M.; Myślińska, D.; Grembecka, B.; Ruciński, J.; Pierzynowska, K.; Wrona, D. Age-dependent effects of dimethyl fumarate on cognitive and neuropathological features in the streptozotocin-induced rat model of Alzheimer’s disease. Brain Res. 2018, 1686, 19–33. [Google Scholar] [CrossRef]
- Fowler, J.H.; McQueen, J.; Holland, P.R.; Manso, Y.; Marangoni, M.; Scott, F.; Chisholm, E.; Scannevin, R.H.; Hardingham, G.E.; Horsburgh, K. Dimethyl fumarate improves white matter function following severe hypoperfusion: Involvement of microglia/macrophages and inflammatory mediators. J. Cereb. Blood Flow Metab. 2018, 38, 1354–1370. [Google Scholar] [CrossRef] [PubMed]
- Krämer, T.; Grob, T.; Menzel, L.; Hirnet, T.; Griemert, E.; Radyushkin, K.; Thal, S.C.; Methner, A.; Schaefer, M.K.E. Dimethyl fumarate treatment after traumatic brain injury prevents depletion of antioxidative brain glutathione and confers neuroprotection. J. Neurochem. 2017, 143, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Miao, W.; Liu, Z.; Han, W.; Shi, K.; Shen, Y.; Li, H.; Liu, Q.; Fu, Y.; Huang, D.; et al. Dimethyl Fumarate and Monomethyl Fumarate Promote Post-Ischemic Recovery in Mice. Transl. Stroke Res. 2016, 7, 535–547. [Google Scholar] [CrossRef]
- Khazipov, R.; Zaynutdinova, D.; Ogievetsky, E.; Valeeva, G.; Mitrukhina, O.; Manent, J.-B.; Represa, A. Atlas of the Postnatal Rat Brain in Stereotaxic Coordinates. Front. Neuroanat. 2015, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Beldarrain, G.; Chillida, M.; Hilario, E.; Herrero de la Parte, B.; Álvarez, A.; Alonso-Alconada, D. URB447 Is Neuroprotective in Both Male and Female Rats after Neonatal Hypoxia–Ischemia and Enhances Neurogenesis in Females. Int. J. Mol. Sci. 2024, 25, 1607. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Wyatt, J.S.; Azzopardi, D.; Ballard, R.; Edwards, A.D.; Ferriero, D.M.; Polin, R.A.; Robertson, C.M.; Thoresen, M.; Whitelaw, A.; et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet Lond. Engl. 2005, 365, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Ehlting, A.; Zweyer, M.; Maes, E.; Schleehuber, Y.; Doshi, H.; Sabir, H.; Bernis, M.E. Impact of Hypoxia-Ischemia on Neurogenesis and Structural and Functional Outcomes in a Mild–Moderate Neonatal Hypoxia-Ischemia Brain Injury Model. Life 2022, 12, 1164. [Google Scholar] [CrossRef]
- Owjfard, M.; Bigdeli, M.R.; Safari, A.; Haghani, M.; Namavar, M.R. Effect of Dimethyl Fumarate on the Motor Function and Spatial Arrangement of Primary Motor Cortical Neurons in the Sub-Acute Phase of Stroke in a Rat Model. J. Stroke Cerebrovasc. Dis. 2021, 30, 105630. [Google Scholar] [CrossRef]
- Safari, A.; Fazeli, M.; Namavar, M.R.; Tanideh, N.; Jafari, P.; Borhani-Haghighi, A. Therapeutic effects of oral dimethyl fumarate on stroke induced by middle cerebral artery occlusion: An animal experimental study. Restor. Neurol. Neurosci. 2017, 35, 265–274. [Google Scholar] [CrossRef]
- Meng, S.; Qiao, M.; Scobie, K.; Tomanek, B.; Tuor, U.I. Evolution of magnetic resonance imaging changes associated with cerebral hypoxia-ischemia and a relatively selective white matter injury in neonatal rats. Pediatr. Res. 2006, 59, 554–559. [Google Scholar] [CrossRef]
- Wang, S.; Wu, E.X.; Tam, C.N.; Lau, H.-F.; Cheung, P.-T.; Khong, P.-L. Characterization of white matter injury in a hypoxic-ischemic neonatal rat model by diffusion tensor MRI. Stroke 2008, 39, 2348–2353. [Google Scholar] [CrossRef] [PubMed]
- Silbereis, J.C.; Huang, E.J.; Back, S.A.; Rowitch, D.H. Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Dis. Model. Mech. 2010, 3, 678–688. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Cheung, P.-Y.; Chen, C. Long-term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic-ischemic brain injury. Brain Res. 2009, 1301, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hagberg, H.; Zhu, C.; Jacobsson, B.; Mallard, C. Effects of intrauterine inflammation on the developing mouse brain. Brain Res. 2007, 1144, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Inder, T.E.; Wells, S.J.; Mogridge, N.B.; Spencer, C.; Volpe, J.J. Defining the nature of the cerebral abnormalities in the premature infant: A qualitative magnetic resonance imaging study. J. Pediatr. 2003, 143, 171–179. [Google Scholar] [CrossRef]
- Yadav, S.K.; Ito, N.; Soin, D.; Ito, K.; Dhib-Jalbut, S. Dimethyl Fumarate Suppresses Demyelination and Axonal Loss through Reduction in Pro-Inflammatory Macrophage-Induced Reactive Astrocytes and Complement C3 Deposition. J. Clin. Med. 2021, 10, 857. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Raimondo, D.; di Sciacca, R.; Pinto, A.; Licata, G. Inflammatory Cytokines in Acute Ischemic Stroke. Curr. Pharm. Des. 2008, 14, 3574–3589. [Google Scholar] [CrossRef]
- Volpe, J.J.; Kinney, H.C.; Jensen, F.E.; Rosenberg, P.A. The developing oligodendrocyte: Key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 2011, 29, 423–440. [Google Scholar] [CrossRef]
- Alonso-Alconada, D.; Alvarez, A.; Lacalle, J.; Hilario, E. Histological study of the protective effect of melatonin on neural cells after neonatal hypoxia-ischemia. Histol. Histopathol. 2012, 27, 771–783. [Google Scholar] [CrossRef]
- Carloni, S.; Crinelli, R.; Palma, L.; Álvarez, F.J.; Piomelli, D.; Duranti, A.; Balduini, W.; Alonso-Alconada, D. The Synthetic Cannabinoid URB447 Reduces Brain Injury and the Associated White Matter Demyelination after Hypoxia-Ischemia in Neonatal Rats. ACS Chem. Neurosci. 2020, 11, 1291–1299. [Google Scholar] [CrossRef]
- Kaur, C.; Rathnasamy, G.; Ling, E.-A. Roles of Activated Microglia in Hypoxia Induced Neuroinflammation in the Developing Brain and the Retina. J. Neuroimmune Pharmacol. 2013, 8, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Bains, S.K.; Pitchumony, T.S.; de Castro Brás, L.E.; Drago, F.; Dubois-Randé, J.-L.; Bucolo, C.; Motterlini, R. Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol. Res. 2013, 76, 132–148. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, H.; Sheehy, A.; Cullen, P.; Allaire, N.; Scannevin, R.H. Dimethyl fumarate alters microglia phenotype and protects neurons against proinflammatory toxic microenvironments. J. Neuroimmunol. 2016, 299, 35–44. [Google Scholar] [CrossRef]
- Lin, R.; Cai, J.; Kostuk, E.W.; Rosenwasser, R.; Iacovitti, L. Fumarate modulates the immune/inflammatory response and rescues nerve cells and neurological function after stroke in rats. J. Neuroinflamm. 2016, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Kunze, R.; Urrutia, A.; Hoffmann, A.; Liu, H.; Helluy, X.; Pham, M.; Reischl, S.; Korff, T.; Marti, H.H. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood–brain barrier integrity. Exp. Neurol. 2015, 266, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Liu, L.; Vollmer, M.K.; Kelly, M.G.; Fernandez, V.M.; Fernandez, T.G.; Kim, H.; Doré, S. Reactive Gliosis Contributes to Nrf2-dependent Neuroprotection by Pretreatment with Dimethyl Fumarate or Korean Red Ginseng against Hypoxic-Ischemia: Focus on Hippocampal Injury. Mol. Neurobiol. 2020, 57, 105–117. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Bona, E.; Hagberg, H.; Løberg, E.M.; Bågenholm, R.; Thoresen, M. Protective Effects of Moderate Hypothermia after Neonatal Hypoxia-Ischemia: Short- and Long-Term Outcome. Pediatr. Res. 1998, 43, 738–745. [Google Scholar] [CrossRef]
- Wang, Q.; Chuikov, S.; Taitano, S.; Wu, Q.; Rastogi, A.; Tuck, S.; Corey, J.; Lundy, S.; Mao-Draayer, Y. Dimethyl Fumarate Protects Neural Stem/Progenitor Cells and Neurons from Oxidative Damage through Nrf2-ERK1/2 MAPK Pathway. Int. J. Mol. Sci. 2015, 16, 13885–13907. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alart, J.A.; Álvarez, A.; Catalan, A.; Herrero de la Parte, B.; Alonso-Alconada, D. Dimethyl Fumarate Strongly Ameliorates Gray and White Matter Brain Injury and Modulates Glial Activation after Severe Hypoxia–Ischemia in Neonatal Rats. Antioxidants 2024, 13, 1122. https://doi.org/10.3390/antiox13091122
Alart JA, Álvarez A, Catalan A, Herrero de la Parte B, Alonso-Alconada D. Dimethyl Fumarate Strongly Ameliorates Gray and White Matter Brain Injury and Modulates Glial Activation after Severe Hypoxia–Ischemia in Neonatal Rats. Antioxidants. 2024; 13(9):1122. https://doi.org/10.3390/antiox13091122
Chicago/Turabian StyleAlart, Jon Ander, Antonia Álvarez, Ana Catalan, Borja Herrero de la Parte, and Daniel Alonso-Alconada. 2024. "Dimethyl Fumarate Strongly Ameliorates Gray and White Matter Brain Injury and Modulates Glial Activation after Severe Hypoxia–Ischemia in Neonatal Rats" Antioxidants 13, no. 9: 1122. https://doi.org/10.3390/antiox13091122






