Abstract
Spinal cord injury (SCI) initiates a cascade of secondary damage driven by oxidative stress, characterized by the excessive production of reactive oxygen species and other reactive molecules, which exacerbate cellular and tissue damage through the activation of deleterious signaling pathways. This review provides a comprehensive and critical evaluation of recent advancements in antioxidant-based therapeutic strategies for SCI, including natural compounds, RNA-based therapies, stem cell interventions, and biomaterial applications. It emphasizes the limitations of single-regimen approaches, particularly their limited efficacy and suboptimal delivery to injured spinal cord tissue, while highlighting the synergistic potential of combination therapies that integrate multiple modalities to address the multifaceted pathophysiology of SCI. By analyzing emerging trends and current limitations, this review identifies key challenges and proposes future directions, including the refinement of antioxidant delivery systems, the development of multi-targeted approaches, and strategies to overcome the structural complexities of the spinal cord. This work underscores the pressing need for innovative and integrative therapeutic approaches to advance the clinical translation of antioxidant-based interventions and improve outcomes for SCI patients.
1. Introduction
Spinal cord injury (SCI) presents a significant challenge in medical science, often resulting in irreversible neurological deficits, including paralysis, sensory loss, autonomic dysfunction, and chronic neuralgia [1,2]. The complex pathophysiology of SCI involves both primary mechanical trauma and secondary injury mechanisms, such as inflammation, apoptosis, and oxidative stress, which collectively contribute to poor clinical outcomes. Among these, oxidative stress caused by the excessive production of reactive oxygen species (ROS) and free radicals plays a pivotal role in exacerbating tissue damage while simultaneously hindering recovery [3,4]. Under normal physiological conditions, ROS, including free radicals such as superoxide anions (O2•−) and hydroxyl radicals (•OH), as well as non-radical species like hydrogen peroxide (H2O2), function as critical signaling molecules necessary for maintaining cellular homeostasis. However, in SCI, the overproduction of ROS triggers extensive cellular damage, promoting lipid peroxidation, protein denaturation, and DNA double-strand breaks. These events lead to cell death, tissue degeneration, and worsened functional outcomes [5,6]. To counteract the damaging effects of ROS, both endogenous and exogenous antioxidants have been explored as therapeutic agents to alleviate oxidative stress in SCI. Antioxidants neutralize ROS, limit secondary injury, and support neuronal survival and regeneration. Nevertheless, single-agent antioxidant therapies have shown limited efficacy, as they fail to address the multifaceted pathophysiology of SCI. This limitation has prompted a shift toward combination strategies, which leverage the synergistic effects of multiple antioxidants. Despite promising preclinical evidence, no antioxidant therapies have been approved for clinical use in SCI. The main barriers to clinical translation include poor bioavailability and pharmacokinetics of antioxidants, limited efficacy of single agents, and insufficient delivery and targeting strategies.
This review aims to bridge the gap between promising preclinical findings and potential clinical applications by providing a comprehensive analysis of antioxidant-based strategies for SCI. It examines the mechanisms of antioxidant action, evaluates the efficacy of various experimental approaches, highlights challenges in delivery systems, and discusses recent advancements in combination strategies. By addressing the limitations and potential solutions, this review offers a framework for integrating antioxidant-based approaches into future therapeutic strategies for SCI.
2. Mechanisms of ROS-Induced Damage and Endogenous Antioxidant Responses in SCI
SCI initiates a cascade of pathological events that extend beyond the initial mechanical trauma, with the overproduction of ROS playing a central role in secondary injury. These highly reactive molecules significantly exacerbate neural damage following SCI. The trauma disrupts cellular membranes, induces mitochondrial dysfunction, and activates enzymatic pathways, leading to the rapid generation of ROS [3,7]. In addition, peroxynitrite (ONOO−), a reactive nitrogen species (RNS), is formed by the reaction of nitric oxide with superoxide anions, further amplifying oxidative damage [8,9]. Both ROS and RNS contribute to the pathophysiology of SCI by inducing mitochondrial dysfunction, lipid peroxidation, protein oxidation/nitration, and DNA damage (Figure 1).
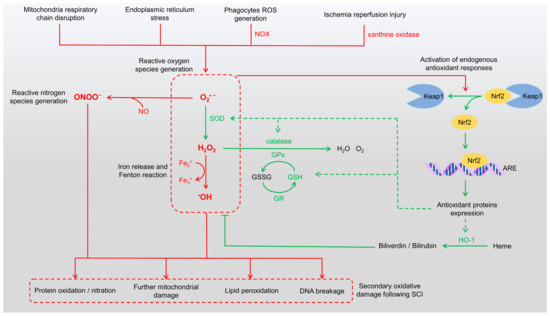
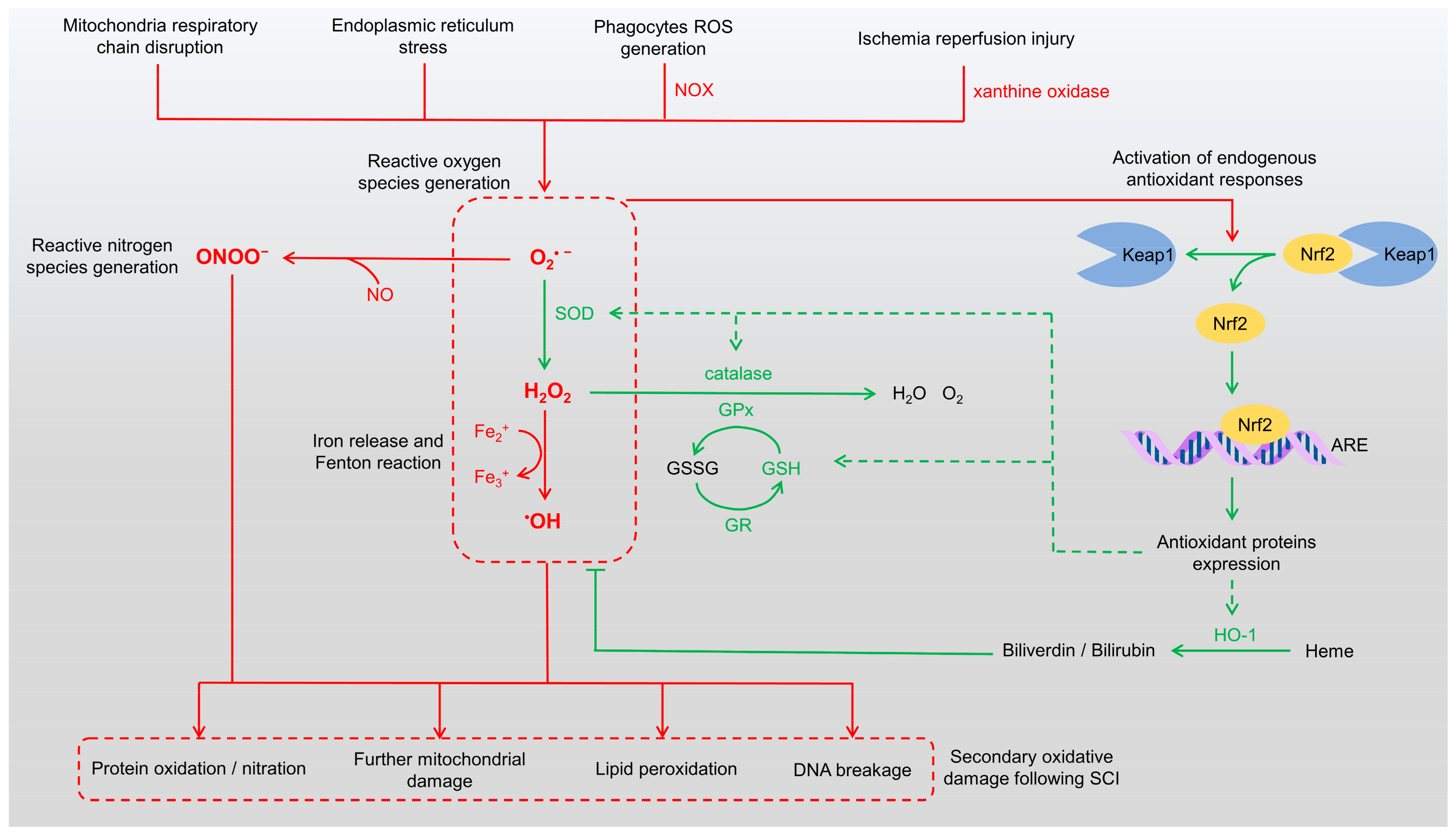
Figure 1.
Mechanisms of ROS generation and endogenous antioxidant responses. The schematic illustrates pathways contributing to oxidative stress and their pathological consequences in SCI (red pathways). ROS are generated from sources such as mitochondrial dysfunction, endoplasmic reticulum stress, and NADPH oxidase activity in phagocytes, leading to the production of superoxide anions (O2•−), hydroxyl radicals (•OH), and hydrogen peroxide (H2O2). Concurrently, RNS such as peroxynitrite (ONOO−) are formed through interactions between ROS and nitric oxide (NO). The Keap1/Nrf2/ARE pathway (green pathways) mediates endogenous antioxidant responses, upregulating antioxidant enzymes to neutralize ROS and RNS and attenuate oxidative damage. However, excessive oxidative stress results in protein oxidation/nitration, lipid peroxidation, DNA damage, and exacerbated mitochondrial dysfunction, contributing to secondary injury and hindering recovery in SCI. ARE: antioxidant response elements, GPx: glutathione peroxidase, GR: glutathione reductase, GSH: glutathione, GSSG: glutathione disulfide, HO-1: heme oxygenase-1, NOX: NADPH oxidase, RNS: reactive nitrogen species, ROS: reactive oxygen species, SOD: superoxide dismutase.
Mitochondria serve as a major source of ROS following SCI. The loss of cellular homeostasis impairs the electron transport chain, leading to electron leakage and the subsequent formation of superoxide anions [6,10,11]. Phagocytic cells such as neutrophils and macrophages also contribute to ROS production by generating extracellular superoxide via membrane-associated NADPH oxidase (NOX) [12,13]. Furthermore, ischemia/reperfusion injury, which occurs upon the restoration of blood flow to ischemic spinal cord tissue, exacerbates oxidative stress through the activation of ROS-producing enzymes like xanthine oxidase [14,15]. These processes not only damage cellular components but also amplify secondary injury by activating inflammatory pathways and inducing apoptosis in neurons and glial cells.
The excessive accumulation of ROS can overwhelm endogenous antioxidant defenses, leading to oxidative stress and widespread cellular damage. ROS directly attack macromolecules, including lipids, proteins, and nucleic acids, and indirectly disrupt cellular metabolic pathways and functions. Lipid peroxidation, a major mechanism of oxidative damage, involves the ROS-mediated oxidation of polyunsaturated fatty acids in membrane phospholipids. The spinal cord, enriched in polyunsaturated fatty acids, is particularly susceptible to this form of damage, which plays a critical role in the propagation of secondary injury after SCI [16,17]. ROS also inflict severe damage on DNA, particularly through hydroxyl radicals that interact with the DNA backbone, causing single- and double-strand breaks. Oxidation of nucleotide bases, especially guanine, forms 8-oxo-deoxyguanosine, a mutagenic lesion that interferes with replication fidelity [18,19]. Oxidative DNA damage activates repair mechanisms such as base excision repair and homologous recombination. However, when the damage exceeds the capacity of these repair systems, apoptosis or necrosis pathways are triggered, further exacerbating neuronal and glial cell loss and impeding spinal cord recovery.
In response to excessive ROS production, the body activates its endogenous antioxidant defense systems (Figure 1). Among these, nuclear factor erythroid 2-related factor 2 (Nrf2) serves as a central regulator of the cellular defense against oxidative stress [20,21,22]. Under basal conditions, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1), which facilitates its ubiquitination and proteasomal degradation. Upon exposure to elevated ROS levels, modifications to Keap1 result in the release of Nrf2, allowing it to translocate into the nucleus. Once in the nucleus, Nrf2 binds to antioxidant response elements (AREs), driving the transcription of numerous antioxidant and cytoprotective genes. These include key enzymes such as superoxide dismutase (SOD), catalase, heme oxygenase-1 (HO-1), glutathione peroxidase (GPx), and NADPH quinone oxidoreductase 1 (NQO1), which collectively counteract oxidative stress [23,24]. Following SCI, Nrf2 upregulation enhances antioxidant gene expression, reducing ROS levels, reducing lipid peroxidation, and protecting neurons and glial cells from apoptosis [25,26]. The Nrf2 pathway also promotes the synthesis of glutathione (GSH), bolstering cellular defenses and facilitating tissue repair and functional recovery in SCI [27]. Thus, Nrf2 is a critical modulator of oxidative stress and plays an essential role in mitigating secondary damage associated with SCI.
Enzymes activated by Nrf2 following SCI work collectively to neutralize ROS and maintain redox balance. SOD converts superoxide anions into hydrogen peroxide, which is subsequently decomposed by catalase and GPx into water and oxygen, thereby reducing the oxidative burden on injured spinal tissue [28,29]. Glutathione reductase (GR) regenerates reduced GSH from its oxidized form, glutathione disulfide (GSSG), maintaining high GSH levels necessary for GPx activity [30]. HO-1 degrades heme into biliverdin, which is further reduced to bilirubin, releasing free iron in the process. Both bilirubin and biliverdin possess antioxidant properties that neutralize ROS, while ferritin sequesters free iron to prevent oxidative damage [31]. NQO1 reduces quinone compounds, preventing the formation of semiquinone radicals, which can contribute to ROS generation [32]. Non-enzymatic antioxidants, including GSH, uric acid, melatonin, and vitamin E, also play a vital role by directly scavenging ROS, preventing excessive oxidative damage [33,34]. These endogenous antioxidants synergistically detoxify ROS and repair oxidative damage, aiding neuroprotection after SCI (Figure 1). However, excessive ROS production in SCI often overwhelms these defenses, highlighting the need for exogenous therapeutic approaches. This imbalance perpetuates oxidative stress, creating a self-sustaining cycle of inflammation, apoptosis, and further ROS production. Compounding this issue is the recruitment of immune cells such as neutrophils and macrophages, which generate additional ROS as part of the inflammatory response. The sustained oxidative stress exacerbates lipid peroxidation, protein oxidation, and DNA damage, further contributing to neuronal loss and worsening functional deficits.
3. Exogenous Antioxidants: Mechanisms and Roles in SCI Recovery
Antioxidant therapies for SCI are designed to alleviate oxidative stress and prevent secondary damage to the surrounding spinal cord tissue. A range of exogenous antioxidant strategies has been explored, including natural and pharmacological compounds, RNA-based therapies, stem cell therapies, and biomaterials, each exhibiting unique mechanisms of action and therapeutic potential. The following sections provide a detailed discussion of the antioxidative mechanisms, delivery methods, and therapeutic outcomes of these exogenous antioxidants in SCI models.
3.1. Natural Compounds as Antioxidant Therapeutics in SCI
Studies on pharmacological and natural compounds with antioxidant activities have been extensively reviewed in several articles, and we recommend that readers refer to these reviews for detailed information [35,36,37]. Here, we specifically highlight and discuss compounds that have been frequently tested in the context of SCI.
3.1.1. Curcumin
Curcumin, a natural polyphenolic compound derived from turmeric, has garnered significant attention for its therapeutic potential, particularly as an antioxidant, anti-inflammatory, and anti-tumor agent [38]. Curcumin exhibits robust antioxidant properties by directly scavenging free radicals [39]. In rat SCI models, intraperitoneal injection of curcumin has been shown to enhance the activity of antioxidant enzymes such as SOD, GPx, and catalase, leading to reduced lipid peroxidation and improved functional outcomes [40,41]. Additionally, curcumin activates the Nrf2 signaling pathway and its target genes, thereby amplifying the endogenous antioxidant response to diminish oxidative damage [42,43]. Curcumin also inhibits the nuclear factor-kappa B (NF-κB) pathway, which plays a critical role in inflammatory responses. By suppressing this pathway, curcumin reduces the production of inflammatory cytokines and limits the recruitment of immune cells that exacerbate ROS production [44,45]. A rat SCI model investigating the effects of curcumin demonstrated significant improvements in neurological function and reductions in oxidative damage, with therapeutic effects showing a dose-dependent relationship [46]. Curcumin’s potent antioxidant and anti-inflammatory properties, with only minor adverse events reported in human trials across various diseases [47,48], make it a promising therapeutic agent for SCI. However, its poor bioavailability limits clinical application. Advanced delivery systems, such as nanoparticles, have been developed to improve its stability and efficacy.
3.1.2. Tetramethylpyrazine (TMP)
TMP, a bioactive compound extracted from the traditional Chinese herb Ligusticum chuanxiong, has demonstrated a wide range of physiological effects, including antioxidant, anti-inflammatory, mitochondrial stabilization, and anti-apoptotic properties [49]. TMP is particularly noted for its neuroprotective effects in various neural injury models, such as cerebral ischemia, where it prevents ischemic brain damage and improves functional outcomes following middle cerebral artery occlusion in rats [50,51]. TMP also provides protection against spinal cord contusion and ischemia/reperfusion injuries [52,53,54]. The antioxidant effects of TMP include direct scavenging of free radicals [55], upregulating GSH levels via the Nrf2 signaling pathway and inhibiting HIF-1α/NOX2-mediated ROS generation. These mechanisms collectively alleviate oxidative stress and cellular apoptosis during neuronal hypoxia [56]. TMP also enhances the activities of key antioxidant enzymes, including catalase, SOD, and GPx [57,58], and reduces lipid peroxidation in spinal cord ischemia/reperfusion injury, thereby attenuating apoptotic signaling and improving neurological outcomes [58]. An animal SCI model study on TMP has demonstrated consistent reductions in oxidative stress markers and increases in antioxidant enzyme activity, contributing to improved neurological and motor recovery [59]. Clinical trials in cerebral infarction have shown that TMP generally has a high safety profile, with only a few mild adverse reactions reported, such as abdominal discomfort and dizziness [60]. However, further trials are necessary to evaluate the efficacy and safety of TMP specifically in spinal cord injury treatment. Additionally, the therapeutic efficacy of TMP as a standalone treatment is limited due to its low bioavailability and short half-life [61]. As a result, TMP is often combined with other antioxidants or incorporated into advanced delivery systems to achieve synergistic effects and enhance its potential for clinical applications.
3.1.3. Vitamins
Vitamins, particularly those with antioxidant properties such as vitamin C and vitamin E, play a crucial role in inhibiting oxidative stress under normal physiological conditions and have been widely explored for therapeutic use in various diseases [62]. Vitamin C, a well-characterized water-soluble antioxidant, reduces oxidative stress through direct ROS scavenging, protecting proteins, lipids, and DNA from oxidative damage [63,64]. Beyond its antioxidative properties, vitamin C has demonstrated neuroprotective and immunomodulatory effects in neurodegenerative diseases, further supporting its therapeutic potential [65,66,67]. Vitamin E, a lipid-soluble vitamin, primarily exerts its antioxidant ability by indirect interaction with ROS [68] and neutralizing lipid peroxyl radicals, thereby interrupting lipid peroxidation and maintaining the integrity of cell membrane lipid bilayers [69,70,71]. Additionally, vitamin E has biological activities beyond its antioxidative functions, including anti-inflammatory and anti-tumorigenic properties mediated through pathways such as NF-κB, MAPK, and mTOR [72]. It also demonstrates neuroprotective effects in various in vitro models [73]. These well-documented antioxidative and neuroprotective mechanisms have prompted extensive investigation into the therapeutic potential of vitamins C and E in SCI [74]. High-dose administration of vitamins C and E has shown beneficial outcomes in rat models of SCI, leading to improved motor recovery and reduced histopathological changes in the spinal cord [75,76]. In contrast, low-dose applications of these vitamins have primarily reduced inflammatory responses without significant improvements in neurological function [76]. Combined intraperitoneal administration of vitamins C and E following SCI has demonstrated synergistic effects, significantly decreasing oxidative stress by increasing SOD activity, reducing apoptosis, and enhancing autophagy, ultimately resulting in notable functional recovery [77]. Despite these promising findings, challenges remain in translating the therapeutic potential of vitamins to clinical practice. Excessive intake of vitamin C can lead to gastrointestinal discomfort [78] and an increased risk of kidney stone formation [79], while high doses of vitamin E may disrupt vitamin K-dependent clotting [80], raising the risks of hemorrhage and all-cause mortality [81]. Addressing these concerns, alongside determining optimal dosages, refining delivery mechanisms, and reducing variability in preclinical outcomes, will be essential for advancing the clinical application of vitamin-based therapies in SCI.
3.1.4. Quercetin
Quercetin, a natural flavonoid, has garnered significant attention recently for its therapeutic potential in SCI due to its potent antioxidant, anti-inflammatory, and anti-apoptotic properties [82]. Quercetin directly scavenges ROS and RNS [83,84] and enhances the expression and activity of endogenous antioxidant enzymes, including SOD1, SOD2, catalase, and GPx [85,86,87]. Furthermore, it activates the Nrf2 signaling pathway through the p38/MAPK pathway and promotes GSH synthesis [88], strengthening cellular defenses against oxidative damage. In addition to its antioxidant effects, quercetin provides mitochondrial protection by maintaining mitochondrial membrane potential, thereby reducing ROS production and lipid peroxidation [89,90]. It also inhibits microglia-mediated neuronal apoptosis by downregulating inflammatory cytokines such as TNF-α and interleukins [91] and protects other cell types, such as liver cells, from inflammatory damage through suppression of the PI3K/Akt/NF-κB signaling pathway [92]. In the context of SCI, quercetin’s therapeutic efficacy has been extensively studied in vivo, primarily using rat SCI models with intraperitoneal administration as the main delivery method [82]. In rat models, post-SCI quercetin treatment neutralizes excessive ROS and oxidative stress, reduces inflammatory cytokine levels [93], promotes the expression of antioxidant enzymes [94], and inhibits lipid peroxidation in spinal cord tissue [95]. These effects collectively facilitate improved behavioral and histopathological recovery in SCI models. Overall, quercetin’s multifunctional properties make it a highly promising therapeutic candidate for SCI. Adverse effects of quercetin are rarely reported in human applications; however, in vivo studies have demonstrated nephrotoxicity in rats subjected to long-term, high-dose administration of quercetin [96]. Ongoing research is focused on exploring its use in combination therapies and advanced drug delivery systems to enhance its efficacy and clinical applicability.
3.2. Pharmacological Compounds as Antioxidant Therapeutics in SCI
3.2.1. Methylprednisolone and Methylprednisolone Sodium Succinate
Methylprednisolone, a widely utilized corticosteroid with potent anti-inflammatory and antioxidant properties, has been employed to treat various diseases, including SCI. It exerts its neuroprotective effects by suppressing ROS production and inflammatory cascades [97,98,99,100]. Methylprednisolone stabilizes cellular membranes by integrating into the phospholipid bilayer, thereby preventing lipid peroxidation [98,101]. Additionally, it enhances the activity of endogenous antioxidant enzymes such as SOD and GPx [102,103], while inhibiting pro-inflammatory cytokines, including TNF-α and interleukins, which are closely linked to oxidative stress and ROS generation [104,105,106]. A derivative of methylprednisolone, methylprednisolone sodium succinate, is chemically modified with a sodium succinate ester group to improve its pharmacological properties. This derivative has been extensively evaluated in various preclinical SCI models, demonstrating significant therapeutic potential in acute SCI [101,107]. Preclinical successes supported the use of methylprednisolone in clinical settings, further validated by the National Acute Spinal Cord Injury Study (NASCIS) trials [108,109]. Methylprednisolone was, at one point, the only treatment widely applied in clinical practice for acute SCI. However, subsequent clinical trials and studies yielded mixed results. While moderate neurological improvements were observed, these benefits were often accompanied by significant side effects, including increased risks of infection and gastrointestinal bleeding [110,111]. Consequently, the routine use of high-dose methylprednisolone for SCI patients is no longer recommended in contemporary clinical practice. These outcomes underscore both the therapeutic potential and the limitations of corticosteroids in SCI treatment, highlighting the need for more targeted and safer pharmacological approaches.
3.2.2. Minocycline
Minocycline, a clinically available tetracycline antibiotic, has demonstrated significant antioxidant, anti-inflammatory, and anti-apoptotic properties in addition to its well-known antimicrobial effects. These properties have been extensively studied in various neurodegenerative and neural injury models, including SCI [112,113,114]. Minocycline’s ability to target multiple secondary injury mechanisms—such as excessive oxidative stress, inflammation, glutamate excitotoxicity, cellular calcium influx, and mitochondrial dysfunction—positions it as a promising therapeutic agent for alleviating secondary injury processes following SCI [115,116]. Minocycline treatment in SCI models has been shown to enhance levels of GSH and increase the activity of antioxidant enzymes such as SOD and GPx, while significantly reducing lipid peroxidation [116,117]. Additionally, minocycline exerts neuroprotective effects by suppressing microglial activation, reducing oxidative stress, and reducing neuronal apoptosis [118,119,120]. It also stabilizes mitochondrial membranes, thereby decreasing ROS release from mitochondria and preserving mitochondrial function [121,122]. Furthermore, minocycline’s metal-chelating properties provide protection against iron-mediated neurotoxicity and membrane lipid peroxidation [123,124,125]. This capability is particularly relevant in SCI, where excess iron contributes to oxidative stress and neurodegeneration. Minocycline also inhibits NOX, a critical enzyme involved in ROS production by activated microglia and neutrophils [126,127]. While a phase II clinical trial found no significant difference in functional outcomes between placebo and minocycline-treated patients, the drug was deemed safe, with minimal adverse events reported even at high doses [128]. The multifaceted antioxidant and neuroprotective properties of minocycline make it a compelling candidate for addressing secondary injury after SCI. Its integration with other antioxidants or delivery systems is being increasingly explored to enhance its efficacy and therapeutic potential in preclinical and translational studies.
3.2.3. Metformin
Metformin, a widely used first-line treatment for diabetes mellitus, has demonstrated antioxidant properties that expand its therapeutic potential beyond glucose regulation. Metformin mitigates oxidative damage in various disease models by enhancing the activity of antioxidant enzymes such as SOD and GPx, reducing inflammatory cytokine levels [129,130], and increasing concentrations of antioxidant molecules like thiols and vitamin C [131]. Additionally, it protects against radiation-induced DNA damage through mechanisms such as ion chelation and oxidative DNA repair [132]. By activating the AMPK/Nrf2 signaling pathway, metformin amplifies endogenous antioxidant responses, providing neuroprotection in conditions such as cerebral ischemia and Alzheimer’s disease [133,134]. Metformin has been extensively studied in SCI animal models, particularly in rats, with intraperitoneal injection as the primary delivery route. Post-SCI metformin treatment has been shown to enhance functional recovery and neuronal survival [135]. It also promotes axonal regeneration by stabilizing microtubules through the PI3K/Akt signaling pathway, which preserves structural integrity and supports axonal elongation. Furthermore, metformin stabilizes mitochondrial membranes and bolsters antioxidant defenses by activating Nrf2 signaling and its downstream targets, including HO-1 and NQO1, reducing oxidative damage in injured spinal cord tissues [136]. In addition to these effects, metformin has been found to significantly reduce neuronal ferroptosis via HO-1 signaling in SCI models, further highlighting the importance of HO-1 in its antioxidant actions [137]. These properties—ranging from oxidative stress regulation to cellular repair promotion—position metformin as a promising therapeutic candidate for SCI. Approved by the Food and Drug Administration for managing type II diabetes mellitus, metformin is generally well-tolerated, with mild-to-moderate digestive disturbances being the most common side effects, and rarer occurrences of hypoglycemia, anemia, or lactic acidosis [138]. However, challenges remain, including its limited targeting efficiency within the spinal cord and the difficulty of translating in vivo results into clinical applications.
3.2.4. N-Acetylcysteine (NAC)
NAC, an acetylated derivative of L-cysteine, is commonly used as a mucolytic agent for respiratory diseases and as a detoxifying agent in acetaminophen overdose [139]. Its effectiveness in acetaminophen poisoning stems from its ability to replenish hepatic GSH levels [140]. As a precursor to GSH, NAC has attracted considerable interest for its potential to treat diseases associated with elevated oxidative stress, including various neurological disorders [141,142,143]. In rodent SCI models, NAC has been extensively studied, typically administered through intraperitoneal injection. NAC treatment significantly reduces lipid peroxidation and increases SOD activity, demonstrating its capacity to prevent oxidative damage after SCI. Its antioxidant effects have been shown to be comparable to those of methylprednisolone in a rat SCI model [144]. Additionally, NAC preserves mitochondrial function and mitigates associated oxidative stress, reducing neuronal loss, demyelination, and inflammation. These effects contribute to improved locomotor recovery following SCI [145,146]. Despite these promising findings, isolated use of NAC has shown limited therapeutic benefits in some studies. For instance, a recent investigation reported only slight improvements in locomotor activity, lesion size, and spinal cord morphology, with no significant effect on interlimb coordination [147]. In contrast, combining NAC with early decompression surgery in a rat SCI model significantly improved histological and functional outcomes by reducing inflammation and apoptosis [148]. This suggests that NAC’s therapeutic efficacy may be enhanced when used in combination with other interventions. Together, these findings highlight NAC’s potential as an antioxidant therapy for SCI.
3.3. RNA-Based Therapies for Antioxidant Treatment
3.3.1. MicroRNAs (miRNAs)
miRNAs are endogenous small non-coding RNAs that regulate gene expression by base-pairing with specific RNA sequences, resulting in the repression or degradation of their mRNA targets [149]. miRNAs play a crucial role in modulating the oxidative stress response across various disease models [150,151,152,153]. In SCI, several miRNAs have been identified as key regulators of the antioxidant response by targeting genes encoding antioxidant enzymes, highlighting their essential role in maintaining ROS homeostasis [154]. For instance, transcriptomic analysis has demonstrated that miR-137 exerts a protective role following SCI. Transfection of miR-137 into primary cultured spinal cord cells from mice reduced oxidative stress and inflammation, potentially via the degradation of NEUROD4 [155]. Following SCI, hyperactivation of Nrf2 in astrocytes has been shown to exert neuroprotective effects by upregulating miR-145-5p, which enhances the expression of antioxidant enzymes such as SOD2 and NQO1 [156]. In a rat SCI model, antisense miR-486 was directly injected into spinal cord tissue to knock down miR-486, resulting in significant motor function improvement. The decreased miR-486 levels induced NEUROD6 expression, which upregulated key antioxidative proteins, including thioredoxin-like 1 and GPX3, promoting ROS scavenging and neuroprotection [157]. Additionally, miR-99a agomir, a chemically modified RNA molecule mimicking endogenous miR-99a, was infused directly into the SCI lesion site via microinjection, improving allodynia and motor function recovery while reducing ROS levels in injured tissues [158]. Intrathecal administration of miR-7a mimics alleviated mitochondrial dysfunction, increased SOD-1 and HO-1 expression, reduced oxidative stress, and enhanced motor function recovery after SCI [159]. Infusion of miR-23b into injured mouse spinal cords targeted neuropathic pain by downregulating NOX4 expression, protecting motor neurons and astrocytes from ROS-mediated apoptosis, and improving neuropathic pain indices [160]. Mulberrin, a natural compound derived from the root of Ramulus Mori, diminishes oxidative stress and inflammation following rat SCI by downregulating miR-337 and modulating Nrf2 signaling [161]. These studies highlight miRNA as a promising therapeutic approach for modulating oxidative stress and promoting neuroprotection in SCI.
3.3.2. Small Interfering RNAs (siRNAs)
siRNAs can be either exogenous double-stranded RNAs introduced into cells to selectively silence specific genes or endogenous small RNAs processed from double-stranded RNA precursors within cells. Both forms function through the RNA interference (RNAi) pathway, binding to target mRNAs with perfect or near-perfect complementarity to induce mRNA cleavage and degradation [162,163]. Artificially synthesized or delivered siRNAs are particularly valuable for targeting genes implicated in oxidative stress and have been evaluated in various disease models [164,165,166]. In SCI, NOX4 siRNA injected into mouse spinal cords significantly increased thioredoxin-like 1 expression, reduced inflammatory markers, and improved neuropathic pain after SCI [160]. Knocking out TREM1, an innate immune receptor associated with inflammation, enhanced motor function, decreased oxidative stress markers, and increased antioxidant protein expression in injured spinal cord tissue. In vitro transfection of TREM1 siRNA into microglial and astrocytic cells further demonstrated that TREM1 silencing reduced oxidative stress via HO-1 signaling [167]. However, the application of siRNA as an antioxidant therapy in SCI remains limited, highlighting the need for further research to fully explore its therapeutic potential and efficacy.
3.3.3. Long Noncoding RNAs (lncRNAs)
LncRNAs, RNA molecules over 200 nucleotides long, regulate gene expression at transcriptional, translational, and epigenetic levels by interacting with nucleic acids and proteins [168]. They recruit chromatin-modifying complexes, form DNA hybrid structures to influence chromatin accessibility [169], act as miRNA sponges to regulate mRNA expression [170], and modulate mRNA splicing and protein turnover through lncRNA–protein complexes [171]. These numerous functions lead to their emergence as important therapeutic targets in various physiological and pathological contexts. In SCI, significant changes in lncRNA expression have been implicated in various processes, including the regulation of oxidative stress via miRNA modulation [172]. For instance, the lncRNA MALAT1 exhibits antioxidative and neuroprotective roles in multiple neurological disease models [173,174,175]. Delivered through viral vectors, MALAT1 exerts neuroprotective effects in SCI by sequestering miR-204 in a rat model of spinal cord ischemia/reperfusion injury [176] and inhibiting miR-125b-5p-induced microglial M1 polarization [173]. MALAT1 also promotes Nrf2 nuclear translocation and attenuates spinal cord tissue damage in SCI rats treated with low-dose lipopolysaccharide [177], highlighting its critical role in oxidative stress regulation and cell survival. Other lncRNAs also show altered expression following SCI. For example, lncRNA CASC9 is downregulated, while lncRNA H19 is upregulated in rat SCI models [178,179]. Overexpression of CASC9 in LPS-induced PC12 cells reduced apoptosis, lipid peroxidation, inflammatory cytokines, and lactic acid while increasing Nrf2 and HO-1 protein expression, highlighting its protective role against oxidative stress and inflammation [178]. In contrast, inhibition of lncRNA H19 with siRNA in lipopolysaccharide-stimulated spinal neurons reversed pathological changes, reducing ROS production and apoptosis while improving cell viability. Notably, the therapeutic effect of lncRNA H19 inhibition was counteracted by miR-370-3p upregulation, suggesting that the lncRNA H19/miR-370-3p pathway governs oxidative stress and neuronal apoptosis following SCI [179].
Despite their therapeutic potential, significant challenges remain in the development of RNA-based antioxidants for SCI. These include rapid degradation of single-stranded RNA molecules and off-target effects causing unexpected side effects. Effective delivery systems that prevent RNA degradation and enhance cellular selectivity are essential for unlocking the therapeutic potential of RNA therapies in SCI.
3.4. Antioxidant Properties of Stem Cell Therapy
Stem cell therapies have been extensively studied for SCI treatment due to their neuroprotective properties and ability to promote regeneration in damaged neural tissues [180]. Beyond their regenerative capacity, stem cells also exhibit significant antioxidant effects. They alleviate oxidative stress through multiple mechanisms: releasing antioxidant enzymes such as SOD, catalase, and GPx to neutralize ROS and reduce lipid peroxidation and DNA damage [181,182]; secreting neuroprotective factors like brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and vascular endothelial growth factor (VEGF) to attenuate oxidative stress and support cellular repair [183,184]; modulating microglia and astrocyte activation to shift the inflammatory response toward a neuroprotective, less oxidative profile [185,186]; and enhancing endogenous antioxidant defenses by upregulating antioxidant genes and activating intrinsic protective pathways, including Nrf2 signaling [184,187]. Collectively, these mechanisms reduce oxidative damage, preserve cellular integrity, and promote functional recovery after SCI.
3.4.1. Bone Marrow Mesenchymal Stem Cells (BM-MSCs)
BM-MSCs are multipotent stem cells found in adult bone marrow that support hematopoiesis and contribute to the regeneration of bone and connective tissue [188]. BM-MSCs have gained recognition for their potential in SCI treatment due to their robust paracrine signaling and antioxidant properties [189,190]. Their therapeutic effects are primarily attributed to paracrine actions rather than direct differentiation into neurons, enhancing neuroprotection by activating antioxidant and anti-inflammatory responses and secreting neurotrophic factors such as NGF and BDNF, which promote axon growth and synaptic connections [191,192,193]. In neural injury models, transplanted BM-MSCs reduce oxidative stress by secreting antioxidant enzymes such as SOD, catalase, and GPx, which neutralize ROS and RNS and prevent lipid peroxidation [194,195,196]. Studies in a bleomycin-induced pulmonary fibrosis model demonstrated that BM-MSCs exert antioxidant effects through Nrf2 signaling activation [197]. Additionally, BM-MSCs improve functional recovery following SCI by promoting tissue repair, reducing inflammation, and restoring motor function [198,199,200,201,202]. Combination therapies further enhance their efficacy. For example, BM-MSCs combined with plumbagin, a natural naphthoquinone compound, injected into injured rat spinal cords improved motor function, reduced edema, and exhibited antioxidant effects by increasing SOD levels and Nrf2 expression [203]. In another study, BM-MSCs overexpressing miR-200a activated Nrf2 signaling, reduced oxidative stress, and enhanced antioxidant enzyme activities, including SOD and catalase, while upregulating Nrf2 target proteins HO-1 and NQO1, resulting in improved locomotor recovery rat SCI model [204].
3.4.2. Adipose-Derived Mesenchymal Stem Cells (AD-MSCs)
AD-MSCs, isolated from adipose tissue through minimally invasive techniques, are another type of multipotent stem cells with high proliferation capacity and strong regenerative, immunomodulatory, and antioxidant properties [189,190]. Their accessibility and therapeutic potential make AD-MSCs a promising option for SCI treatment [205]. In SCI, AD-MSCs promote neural repair through various mechanisms, including replacing lost neurons after priming for neuronal differentiation and transplantation [206], secreting neurotrophic factors such as BDNF, VEGF, and ciliary neurotrophic factor (CTNF) [207,208], promoting angiogenesis [209], and modulating the immune response via Notch signaling [210]. To exert antioxidant effects in oxidative stress models, AD-MSCs enhance SOD activity and upregulate antioxidant enzymes such as NQO1 and GPx, reducing ROS, RNS, and lipid peroxidation levels [211,212,213]. Compared to BM-MSCs, AD-MSCs demonstrate similar antioxidant effects but with greater accessibility and a lower risk of immune rejection. In a canine SCI model, intravenously delivered AD-MSCs improved hindlimb function and neuronal survival, exhibiting superior antioxidative and anti-inflammatory effects compared to high-dose methylprednisolone, without adverse effects [214]. Additionally, AD-MSCs overexpressing HO-1, designed to boost antioxidant potential and resilience against oxidative stress, significantly improved motor function, reduced inflammation and astrogliosis, and enhanced neuroregeneration in SCI models [215,216]. Pre-conditioning AD-MSCs with melatonin, a hormone with versatile antioxidant properties, further enhanced engraftment and differentiation into neuronal lineages in a rat SCI model, resulting in better functional recovery [206]. These findings underscore the therapeutic promise of AD-MSCs in SCI treatment and highlight opportunities for further modifications to optimize their antioxidant effects.
In antioxidant research, MSCs excel over other stem cell types due to their exceptional immune modulation and robust paracrine functions. However, neuronal-lineage and pluripotent stem cells, despite less explored antioxidant properties, play a more direct role in neuronal replacement and integration into spinal cord neural circuits, which is essential for spinal cord regeneration. While their intrinsic antioxidant effects are limited, maintaining redox balance is critical for their survival and function. Reducing oxidative stress and enhancing endogenous antioxidant defenses could further improve their therapeutic efficacy in SCI. The following sections briefly discuss the antioxidant properties and therapeutic potential of stem cells other than MSCs, emphasizing how their modification can mitigate oxidative stress and support SCI recovery.
3.4.3. Neural Stem/Progenitor Cells (NSPCs)
NSPCs are lineage-specific stem/progenitor cells present in the developing embryonic central nervous system and, to a limited extent, in the adult CNS. In adults, NSCs have been identified in regions such as the subventricular zone of the lateral ventricles, the subgranular zone of the hippocampus, and the central canal of the spinal cord [217,218]. These cells can differentiate into primary neural lineages, including neurons [219,220,221], astrocytes, and oligodendrocytes [222,223,224], facilitating neurogenesis and remyelination following CNS injuries. Numerous studies on NSPC transplantation in animal models of SCI have shown improved motor function, reduced lesion size, and enhanced tissue preservation [225,226,227]. In neural injury and neurodegenerative models, NSPCs have been observed to upregulate SOD2 expression in neurons and reduce ROS production in response to neurotrophic factors [183,228]. The application of NSPC-derived secretomes in a rat SCI model demonstrated antioxidant and anti-inflammatory properties, leading to improved locomotor recovery and reduced neuropathic pain [229], highlighting their potential as antioxidant agents for SCI. Moreover, activation of Nrf2 signaling in NSPCs enhances the expression of antioxidant enzymes, protecting these cells from oxidative stress and promoting their survival while preserving self-renewal capabilities [230]. These findings emphasize the promise of NSPCs as a therapeutic option for SCI, particularly due to their dual role in regeneration and antioxidative defense.
3.4.4. Embryonic Stem Cells (ESCs)
ESCs are pluripotent stem cells derived from the inner cell mass of the blastocyst. They exhibit self-renewal capacity and can differentiate into all cell types of the three germ layers, making them a powerful tool in regenerative medicine [231]. In the context of SCI, undifferentiated ESCs are less commonly used due to their tumorigenic potential [232]. Instead, ESC-derived neural progenitors and oligodendrocyte progenitors are more frequently employed for SCI treatment [233]. In rodent SCI models, transplanted ESC-derived neural progenitors differentiate into neuronal and glial lineages, promoting axonal growth, remyelination, and angiogenesis [234,235,236]. ESC-derived oligodendrocyte progenitors have demonstrated substantial remyelination effects [237,238]. The role of intrinsic redox regulation and mitochondrial function in governing the characteristics of ESCs has been highlighted in several studies [239,240]. Expression of antioxidant enzymes such as SOD2 and catalase, which modulate redox status, is critical for determining whether ESCs maintain self-renewal or undergo neuronal differentiation [241].
3.4.5. Induced Pluripotent Stem Cells (iPSCs)
iPSCs function as pluripotent stem cells derived from reprogrammed adult somatic cells, such as skin or blood cells. This approach reduces the risk of immune rejection and avoids ethical concerns associated with ESCs [242,243]. In rodent SCI models, transplantation of human iPSC-derived NSPCs has led to their differentiation into neuronal and glial cells, resulting in the reconstruction of neuronal circuits with existing neurons, enhanced angiogenesis, axonal regeneration, and notable motor improvement [244,245]. Transplantation of iPSC-derived oligodendrocyte progenitors during the early stages of SCI has also been shown to promote remyelination of damaged axons and facilitate functional recovery [237,246]. Redox balance is critical for the survival and differentiation of iPSCs. The abundant expression of endogenous antioxidant enzymes in iPSCs reduces intracellular oxidative stress and maintains genomic integrity essential for stemness [247,248]. A combination therapy comprising human iPSC-derived NSPCs and a polyacetal-curcumin nanoconjugate has been developed, wherein curcumin protects iPSC-derived NSPC from H2O2-induced oxidative damage. This combination was injected directly into the lesion site following rat SCI, inhibiting glial scar formation while promoting the survival of motoneurons and axons [249].
3.4.6. Extracellular Vesicles (EVs)
EVs have gained considerable attention due to their promising therapeutic potential. These small, lipid bilayer-enclosed vesicles are secreted by nearly all cell types and are present in various bodily fluids, including plasma, urine, and cerebrospinal fluid [250]. Stem cell-derived EVs, in particular, possess significant therapeutic potential [251,252]. EVs facilitate cell-cell communication by transporting bioactive molecules such as nucleic acids, proteins, and lipids [253]. Among EV subtypes, exosomes (50–200 nm in diameter) have garnered interest due to their low immunogenicity, high biocompatibility, and ability to preserve the biological activity of their cargo [254,255]. Additionally, exosomes exhibit strong chemotactic properties, enabling them to localize to injury sites and cross the blood-spinal cord barrier (BSCB), facilitating tissue repair [256,257]. In a rat SCI model, intravenously delivered NSPC-derived EVs reduced neuronal apoptosis, microglial activation, and neuroinflammation by enhancing autophagy and modulating apoptotic gene expression [258]. Systemically delivered MSC-derived exosomes facilitated SCI repair and functional recovery by downregulating inflammatory cytokines [259] and regulating apoptotic signaling via the miR-21-5p/FasL axis [260]. Beyond their anti-apoptotic and immunomodulatory effects, EVs are closely associated with oxidative stress [261]. EVs not only produce antioxidants and detoxify ROS but are also regulated by oxidative stress, which affects their production and cargo composition [262]. Pro-inflammatory or pro-oxidant conditions have been shown to induce EV release in vitro [263]. For example, exosomes derived from MSCs under hypoxic conditions reduced ROS accumulation, DNA damage, and apoptosis in a HIF-1α-dependent manner in a rat colitis model [264]. EVs also deliver molecules such as antioxidant enzymes, miRNAs, and proteins that scavenge ROS directly or activate antioxidative cascades in target cells [261]. The Nrf2/HO-1 axis has been identified as a key mediator of the antioxidative and anti-inflammatory effects of MSC-derived EVs [265,266]. In the context of SCI, AD-MSC-derived exosomes injected intravenously in a rat model promoted neuronal repair and angiogenesis by activating the Nrf2/GPX4 pathway and inhibiting endothelial ferroptosis [267]. Intrathecal delivery of MSC-derived exosomes significantly inhibited ferrous iron production, lipid peroxidation, ROS generation, and ferroptosis, improving neurological outcomes [268]. Hydrogel-loaded MSC-derived exosomes implanted directly into rat SCI lesions reduced inflammation and oxidative stress, resulting in improved motor function and preserved urinary function [269]. NSPC-derived exosomes were also shown to inhibit neuronal apoptosis post-SCI by activating autophagy via miR-374-5p and its target genes [270]. Despite these promising findings, the molecular mechanisms and signaling pathways underlying the therapeutic effects of EVs in SCI remain largely unexplored. Standardizing isolation protocols and characterizing active molecules within EVs are critical steps to ensure reproducibility and efficacy before translating EV-based therapies to clinical applications in SCI patients [271].
3.5. Biomaterials in Antioxidant-Based Therapeutics
Recent studies have increasingly focused on the application of biomaterials in SCI therapy, highlighting their potential as direct ROS scavengers or catalysts and their remarkable capacity for loading and controlled release of antioxidants. Leveraging their intrinsic enzyme-like antioxidant activities, carbon nanomaterials and metal-based nanoenzymes have been applied in various disease models, including SCI, to counteract excessive oxidative stress in injured tissues. Additionally, biocompatible polymers provide steady, continuous, and prolonged antioxidant release while serving as pro-survival platforms for hosting stem cells post-transplantation into injured spinal tissue. Nanoparticles and liposomes further enhance targeted antioxidant delivery through systemic circulation. The following sections discuss various biomaterials, focusing on their antioxidant mechanisms, therapeutic efficacy, delivery strategies, and associated challenges.
3.5.1. Biomaterials as Antioxidant Agents
Carbon nanomaterials have gained significant attention for their antioxidative potential, owing to unique physicochemical properties such as electrical conductivity, mechanical strength, and enzymatic activity [272]. Among these, fullerenes, spherical carbon molecules with a hollow structure, have demonstrated exceptional free radical scavenging abilities [273]. In a mouse model of chronic autoimmune encephalomyelitis, a fullerene derivative linked to an NMDA receptor antagonist effectively targeted oxidative stress and excitotoxic effects. This approach significantly reduced axonal loss, demyelination, oxidative injury, and immune cell infiltration into spinal cord tissues [274]. Carbon dots are nanoscale particles with unique photochemical properties and excellent biocompatibility that demonstrate strong ROS-scavenging capabilities [275]. Selenium-doped carbon dots have been shown to protect neuronal and astrocytic cell lines from H2O2-induced oxidative damage [276]. In a rat SCI model, these particles reduced inflammation, glial scar formation, and oxidative stress, enhancing locomotor recovery [277]. Carbon nanotubes, cylindrical graphene-based structures, are noted for their strength, conductivity, and ability to promote neural growth and circuit formation [278]. Their electron acceptability also makes them potent radical scavengers [279,280], demonstrating neuroprotection through antioxidant and immunomodulation properties [281,282]. In a rat SCI model, injecting multi-walled carbon nanotubes loaded with ivermectin reduced oxidative stress and inflammation, improving locomotor activity and neuropathic pain [283]. However, concerns about bioaccumulation, chronic toxicity, and immune activation remain challenges for their clinical translation, emphasizing the need for further research into safer, biodegradable nanomaterials.
Metal-based nanozymes are nanoparticles with intrinsic enzyme-like catalytic activities that mimic the functions of natural oxidoreductases or hydrolases and have shown substantial potential in scavenging ROS and reducing oxidative stress [284,285,286]. Cerium oxide (CeO2) nanoparticles are particularly well studied for their mimetic properties resembling SOD, catalase, and peroxidase. These nanoparticles alternate between Ce3+ and Ce4+ oxidation states to neutralize ROS and prevent oxidative damage [287]. In a rat model of contusion SCI, local injection of CeO2 nanoparticles into the lesion cavity inhibited the expression of genes involved in ROS generation, apoptosis, and inflammation while reducing lesion size and promoting motor function recovery [288]. Manganese (Mn) is a critical cofactor for several metalloenzymes, including SOD2 (Mn-SOD), glutamine synthetase, and arginase [289]. Mn-based nanoenzymes have been widely developed, exhibiting enzymatic activities that mimic those of GPx and SOD [290,291]. For instance, chitosan-modified manganese dioxide nanoparticles encapsulating resveratrol—a natural polyphenolic compound with antioxidant and anti-inflammatory properties—have been administered intravenously in SCI mice. This approach provided sustained antioxidant protection by reducing ROS levels and lipid peroxidation while enhancing antioxidant defenses through increased SOD and GPx activity in vivo [292]. Mn-porphyrins have also been extensively employed to alleviate oxidative stress following SCI. Intrathecal delivery of Mn-porphyrins significantly reduced oxidative damage while promoting the survival of neurons and glial cells [293]. Notably, this treatment demonstrated superior therapeutic outcomes compared to intravenous methylprednisolone administration [294,295]. Iron oxide (Fe2O3) nanoparticles, known for their peroxidase-like activity, catalyze the breakdown of H2O2, thereby reducing oxidative stress [296]. In a rat SCI model, Fe2O3 nanoparticles embedded in an agarose gel were implanted into the transection lesion and paired with an external electromagnetic field. This approach led to significant functional recovery and a reduction in lesion volume. In vitro experiments demonstrated effective cellular uptake of the nanoparticles and a marked decrease in H2O2-mediated oxidative stress [297]. Zinc oxide (ZnO), another metal-based nanozyme with peroxidase-like properties, has also shown promise in SCI treatment [298]. ZnO nanoparticles exhibited neuroprotective effects in an in vitro axotomy model by reducing oxidative stress and apoptosis through the activation of the PI3K-Akt signaling pathway [299]. When locally administered, ZnO nanoparticle-loaded hydrogel effectively reduced ROS production and the inflammatory response in the injured spinal cord of mice, providing neuroprotection and facilitating functional recovery [300]. Gold nanomaterials, with enzyme-mimicking activities resembling those of SOD, peroxidase, and catalase, have also demonstrated potential in SCI treatment [301]. In a mouse SCI model, intravenous injection of gold nanoclusters stabilized by zinc modification exhibited significant antioxidant properties. These nanoclusters reduced ROS-induced neuronal apoptosis and inflammation, improved ventral motor neuron survival, and caused minimal systemic toxicity [302]. Beyond these metallic nanoparticles, a hollow-structured single-atom cobalt nanozyme was developed with broad-spectrum capabilities to neutralize multiple ROS and RNS molecules. Its porous structure enabled the encapsulation and controlled release of minocycline, enhancing neuroprotection and motor function recovery in SCI rats [303].
Despite the well-recognized antioxidant properties of carbon nanomaterials and metal-based nanozymes, their application in SCI remains underexplored, with only a limited number of studies investigating their therapeutic potential. Challenges to their clinical translation persist, particularly due to the dual roles of metal-based nanozymes as both ROS scavengers and ROS producers [304,305,306]. Environmental factors such as pH variability [307], local ROS concentrations [308], and other conditions can influence the behavior of these nanozymes, sometimes resulting in unpredictable effects. In addition, carbon nanomaterials, while capable of scavenging ROS to alleviate oxidative stress, may, under specific stimuli, promote ROS generation [275,309,310,311], potentially causing unintended oxidative damage to non-target tissues. Furthermore, the lack of complete biodegradability of both metal-based nanozymes and carbon nanomaterials poses additional challenges. Their accumulation in organs during metabolism raises concerns about chronic toxicity and heightened immune responses. To address these limitations, further research is needed to optimize the design, functional modifications, and delivery methods for these nanomaterials, ensuring their efficacy and biocompatibility in clinical applications.
3.5.2. Biomaterials and Delivery Strategies for Antioxidant Therapy
Nanoparticles, ranging in size from 1 to 100 nanometers, possess unique features such as a high surface area, tunable properties, and small size, making them highly effective in the treatment of various diseases. Their applications include targeted tissue delivery, enhanced drug transport, and modulation of injury environments. Antioxidants such as curcumin and resveratrol face challenges of poor bioavailability due to limited absorption, rapid metabolism, and low systemic availability when administered orally or systemically [312]. Nanoparticles provide a promising solution by encapsulating these compounds, enabling enhanced penetration across the BSCB [313], shielding them from rapid metabolism, and improving their systemic bioavailability [314,315]. The following sections examine the antioxidant properties of various nanoparticles, along with their therapeutic cargo, delivery routes, and targeting mechanisms.
Intravenous administration remains the most common method for delivering nanoparticles in SCI therapy. For instance, double-coated nanozymes, where SOD1 was incorporated into a poly-ethylene glycol (PEG)–polylysine polycation complex and then coated with PEG–polyglutamic acid polyanion polymers, have been shown to prolong the circulation of active SOD1 in the bloodstream. These nanozymes reduced inflammation, oxidative stress, and edema, ultimately improving locomotor function recovery in a rat SCI model [316]. TMP-loaded nanoparticles conjugated with the HIV transactivator of transcription (TAT) peptide and human serum albumin facilitated neutrophil uptake and BSCB penetration. This system improved TMP solubility and bioavailability, resulting in reduced oxidative stress and inflammation, as well as enhanced locomotor recovery in SCI rats [317]. Nanoparticles co-loaded with curcumin and retinoic acid in a bovine serum albumin self-assembly system were applied to a mouse SCI model. These nanoparticles scavenged ROS effectively, induced macrophage polarization toward regenerative phenotypes, reduced inflammatory responses, promoted neuronal differentiation, and minimized scar tissue formation [318]. Another approach used zein-based nanoparticles encapsulating metformin and functionalized with the CAQK peptide, which targets chondroitin sulfate proteoglycans upregulated in SCI lesions. These inflammation-targeting nanoparticles exhibited potent anti-inflammatory, antioxidant, and neuroprotective effects, leading to increased neurofilament density, improved axonal myelination, reduced glial scarring, and enhanced motor function recovery [319]. Additionally, multi-functionalized selenium nanoparticles have been developed, incorporating a polysaccharide-protein complex for enhanced stability and the PG-6 peptide, which specifically targets matrix metalloproteinases-2 and -9, abundant in the SCI microenvironment. These nanoparticles were loaded with TMP and monosialotetrahexosylganglioside 1 to counteract excessive ROS and oxidative stress. This system demonstrated strong antioxidant and neuroprotective effects, reducing mitochondrial dysfunction, preventing apoptosis, and significantly improving functional recovery in a rat SCI model [320].
Local administration is an effective method for delivering nanoparticles, involving their direct injection into the injury site. This approach ensures a high concentration of nanoparticles at the target area, providing localized and sustained release of therapeutic agents while minimizing systemic exposure and reducing the risk of degradation and toxicity. For instance, polydopamine nanoparticles loaded with rapamycin, an mTOR inhibitor, were locally injected into spinal cord lesions in a rat SCI model. These nanoparticles exhibited antioxidant effects by inhibiting mTOR-mediated ROS overproduction and, in vivo, reduced cavity size, improved hind limb coordination, and enhanced neurogenesis and tissue regeneration [321]. Another example is a microcomposite system comprising a poly-lactic-co-glycolic acid (PLGA) polymer core containing methylprednisolone, coated with a polydopamine shell. This system targeted inflammatory cytokines, provided localized drug delivery, and protected spinal cord tissue in a rat SCI model [322]. Poly-caprolactone (PCL)-based nanoparticles loaded with minocycline were administered locally during the acute phase of SCI in mice, reducing pro-inflammatory microglial responses, sustaining a pro-regenerative environment, and supporting long-term functional recovery [323]. Localized delivery systems have also been employed for siRNA therapies, addressing challenges such as poor cellular uptake and rapid enzymatic degradation. For example, mesoporous silica nanoparticles were designed to deliver IRF5-targeted siRNA with ROS-responsive degradation and controlled release. This system promoted the transformation of macrophages into a pro-regenerative phenotype [324]. Chitosan nanoparticles loaded with siRNA targeting inducible nitric oxide synthase were used to reduce harmful nitric oxide production, minimizing secondary injury and promoting spinal cord recovery by limiting RNS release [325].
Oral administration of nanoparticles has also been explored as a promising alternative, offering flexible dosing schedules, improved patient compliance, and stable systemic concentrations. Selenium nanoparticles administered orally in a rat SCI model improved immune cell profiles and reduced histological markers of inflammation at the injury site [326]. In addition, oral gavage of selenium nanoparticles derived from selenium-enriched Proteus mirabilis cultures were delivered via oral gavage in a rat SCI model, demonstrating antioxidant and anti-inflammatory effects. The treatment reduced oxidative stress and lipid peroxidation levels, enhanced nerve regeneration, and improved hind limb motor function [327].
Liposomes, larger than nanoparticles and typically ranging from 50 to 500 nm in diameter, are vesicles composed of a phospholipid bilayer. Their stability, biocompatibility, ease of modification, high drug-loading capacity, and amphipathic properties—allowing encapsulation of both hydrophilic and hydrophobic drugs—have made them one of the most widely explored nanocarriers [328]. Similar to nanoparticles, liposomes are often conjugated with TAT peptides to facilitate penetration across cells and the BSCB. In a rat SCI model, TAT-modified liposomes demonstrated significantly higher accumulation at the lesion site [329]. Additionally, TAT- and PEG-conjugated magnetic polymeric liposomes containing superparamagnetic nanoparticles were found to target injury sites through magnetic force, enhancing BSCB penetration [330]. These findings suggest that TAT peptide modification and magnetic attraction offer feasible targeting strategies for liposome-based delivery in SCI. Further innovations have employed the natural targeting ability of macrophages toward injury sites. For example, macrophage membrane-camouflaged liposomes, isolated from macrophages and administered intravenously, selectively accumulated at the SCI site in a mouse model. When loaded with minocycline, these liposomes exhibited strong therapeutic effects, including anti-inflammatory and anti-pyroptotic actions, suppressed glial scar formation, reduced axonal necrosis [331], and decreased calcium-induced ROS generation and subsequent lipid peroxidation [332]. A complex delivery system was tested in a rat SCI model, combining oxidation resistance 1 (OXR1) plasmids condensed in vitamin E succinate-grafted polylysine nanoparticles encapsulated within liposomes. The OXR1 protein, which regulates genes involved in antioxidant defense, reduced oxidative damage. This system facilitated stable OXR1 plasmid transfection with minimal cytotoxicity, alleviating oxidative stress and promoting functional recovery by upregulating Nrf2, HO-1, catalase, and SOD1 [333].
Hydrogels are hydrophilic polymers capable of retaining substantial water while maintaining structural integrity, making them highly suitable for SCI applications due to their excellent biocompatibility, high water content, and similarity to the extracellular matrix. Studies have shown that alginate hydrogels support neurite outgrowth and alleviate oxidative stress in primary cultured neurons [334]. When implanted into a rat hemimyelonectomy SCI model, alginate hydrogels promoted functional recovery with reduced fibrous scar formation at the lesion site [335]. Innovative ROS-scavenging hydrogels have been developed by crosslinking thioketal-containing hyperbranched polymers with methacrylate hyaluronic acid. These hydrogels effectively neutralized hydrogen peroxide and superoxide anions in vitro. When loaded with BM-MSCs and growth factors and implanted into a rat SCI model, they attenuated oxidative damage and inflammatory cytokines, reduced fibrotic and glial scarring, and enhanced neurogenesis [336]. Antioxidant-loaded hydrogels, such as those containing curcumin or quercetin, have also been widely studied for their ability to optimize controlled release and localized delivery of antioxidants [337,338,339,340]. Several studies have evaluated the therapeutic efficacy of antioxidant-loaded hydrogels in SCI models. For instance, in a rat model of unilateral cervical contusion SCI, which impairs respiratory function, a minocycline-loaded agarose/chitosan hydrogel administered via subdural injection reduced spinal cord damage and preserved diaphragm motor neuron innervation, thereby maintaining respiratory function [341]. In another study, a rapamycin-loaded PVA hydrogel layered over polylactic acid electrospun fibers encapsulating BDNF was applied to a rat hemisection SCI model. The dynamic borate-ester crosslinked hydrogel exhibited responsive ROS-scavenging effects, while rapamycin facilitated autophagy by enhancing autophagosome formation. This system reduced exogenous ROS levels and stimulated endogenous antioxidant responses by upregulating Nrf2, HO-1, and related antioxidant enzymes. It simultaneously inhibited glial scar formation and neuronal apoptosis, while promoting autophagy, axonal growth, and neuroprotection [342]. Other examples include chitosan hydrogels loaded with curcumin and selenium nanoparticles, which were locally implanted at the SCI site. This combination synergized antioxidant effects, reducing edema and inflammation during the acute phase and decreasing necrotic neurons and inflammatory cell infiltration. However, it also induced reactive astrocytosis during the late phase of SCI [343]. TMP-loaded electroconductive hydrogels have also shown significant antioxidant properties and neuroprotection in a rat SCI model [344]. Stem cell-derived EVs are frequently incorporated into hydrogels to enhance therapeutic outcomes. A hydrogel composed of N-acryloyl glycinamide and gelatin methacrylate containing MSC-derived EVs and tannic acid demonstrated significant restoration of motor function and urinary function after implantation into the injured spinal cord. This system reduced oxidative DNA damage, lipid peroxidation, and inflammatory cytokines, facilitating tissue repair [269]. Moreover, a polylysine-based hydrogel designed for sustained release of AD-MSC-derived EVs and aminoguanidine reduced oxidative damage and scar tissue formation, and promoted remyelination and axonal regeneration at the lesion site [345].
Injectable hydrogels have been highlighted as a promising approach for SCI therapy, offering minimally invasive delivery and precise localization to the injury site. For example, a thermosensitive hydrogel containing MnO2/apoferritin nanoparticles encapsulating astragaloside, an antioxidant from Astragalus membranaceus, was directly injected into the lesion in a rat contusion SCI model. This treatment elevated GSH levels, enhanced SOD activity, and alleviated oxidative stress-induced ferroptosis, leading to improved neuronal survival, reduced tissue damage, and enhanced hindlimb motor function [346]. Furthermore, NSCs encapsulated in a ROS-scavenging gelatin hydrogel composed of CeO2 nanoparticles promoted neural differentiation and integration of transplanted cells, effectively reducing oxidative stress and inflammation in SCI tissue [347]. In another study, a hydrogel loaded with NSC-derived exosomes facilitated neurite regrowth and improved neurological function recovery in a mouse SCI model through the exosomal delivery of miR-34a-5p [348].
In addition to hydrogels, various polymeric formulations have been explored for SCI repair, including scaffolds, nanofibers, and conduits, many of which have been specifically designed to target oxidative stress [349,350]. For instance, a hybrid nanofiber scaffold composed of PCL and polysialic acid encapsulating methylprednisolone was directly transplanted into the lesion area following rat SCI. This system effectively reduced the release of inflammatory cytokines and the expression of apoptotic proteins, while also preventing axon demyelination and reactive gliosis [351]. In a rat hemisection SCI model, insulin-like growth factor-1 (IGF-1) and BDNF were incorporated into biodegradable PLGA/graphene oxide electrospun nanofibers. This nanofiber membrane protected NSCs from H2O2-induced oxidative stress and promoted neuronal differentiation in vitro. When applied to the surface of the injured spinal cord, the membrane reduced cavity formation and increased neuron density at the injury site, resulting in improved locomotor recovery [352]. Chitosan/polyvinyl alcohol (PVA) nanofibers encapsulating genistein, a natural phytoestrogen with antioxidant and anti-inflammatory properties, were used to cover spinal cord lesions in another hemisection SCI model. This treatment enhanced SOD activity while reducing nitric oxide levels, lipid peroxidation, and inflammatory markers, ultimately mitigating oxidative stress and inflammation within the injured tissue [353]. To overcome the harsh environment with excessive ROS SCI, which threatens post-transplanted MSC survival, a composite scaffold system featured an outer ROS filter constructed from PVA crosslinking with a ROS-responsive linker tetramethylpropane-1,3-diaminium and the inner porous hydrogel housing MSC spheroids was implanted into transected rat spinal cord. The ROS filter rapidly scavenged excess ROS, shielding MSCs from oxidative damage and upregulating Nrf2 and HO-1 expression during the acute phase. The enhanced paracrine effect of MSCs promoted neuronal repair and significantly improved electrophysiological and motor function recovery in the chronic phase [354].
These polymer-based systems offer multifunctional capabilities, including structural support for axon guidance, creating a conducive environment for stem cells, intrinsic antioxidative properties, or efficient antioxidant delivery. Collectively, they represent promising candidates for integrating various therapeutic approaches to enhance SCI repair and recovery.
4. Challenges for Clinical Translation and Future Directions
4.1. Administration Routes and Drug Delivery Issues
Despite the promising efficacy of antioxidant therapies, demonstrated in preclinical studies, several challenges remain in translating these approaches into clinical practice. A significant hurdle lies in the delivery of antioxidants, as their therapeutic potential is most effective during the acute phase of injury when access to spinal cord tissue is inherently difficult. Systemic delivery, including nanoparticle-based formulations and liposomal encapsulations as discussed previously, represents an innovative approach for enhancing bioavailability and targeting. However, the efficiency of targeting and the controlled release of antioxidants through these systems remain limited due to physical and physiological barriers. To address these challenges, direct delivery of antioxidants into the spinal cord has been extensively explored. Intrathecal delivery, which administers therapeutic agents directly into the cerebrospinal fluid, bypasses the BSCB and enables more efficient access to spinal cord tissue [249,268,293]. This technique offers a significant advantage in achieving localized and effective drug concentrations. Future advancements may include the use of intrathecal catheters, which allow for the steady and repeated infusion of antioxidants to the injury site, potentially enhancing controlled and targeted interventions [355,356]. Additionally, innovative delivery methods such as convection-enhanced delivery have shown potential for SCI treatment. Convection-enhanced delivery employs pressure-driven bulk flow generated through specialized catheters, facilitating more extensive and uniform distribution of therapeutic agents within spinal cord tissue [357,358]. Another promising strategy involves injectable hydrogels, which have garnered considerable interest for their ability to encapsulate therapeutic agents. These hydrogels can be administered directly into the lesion site without requiring invasive surgery, offering a minimally invasive alternative to traditional implants [346,347].
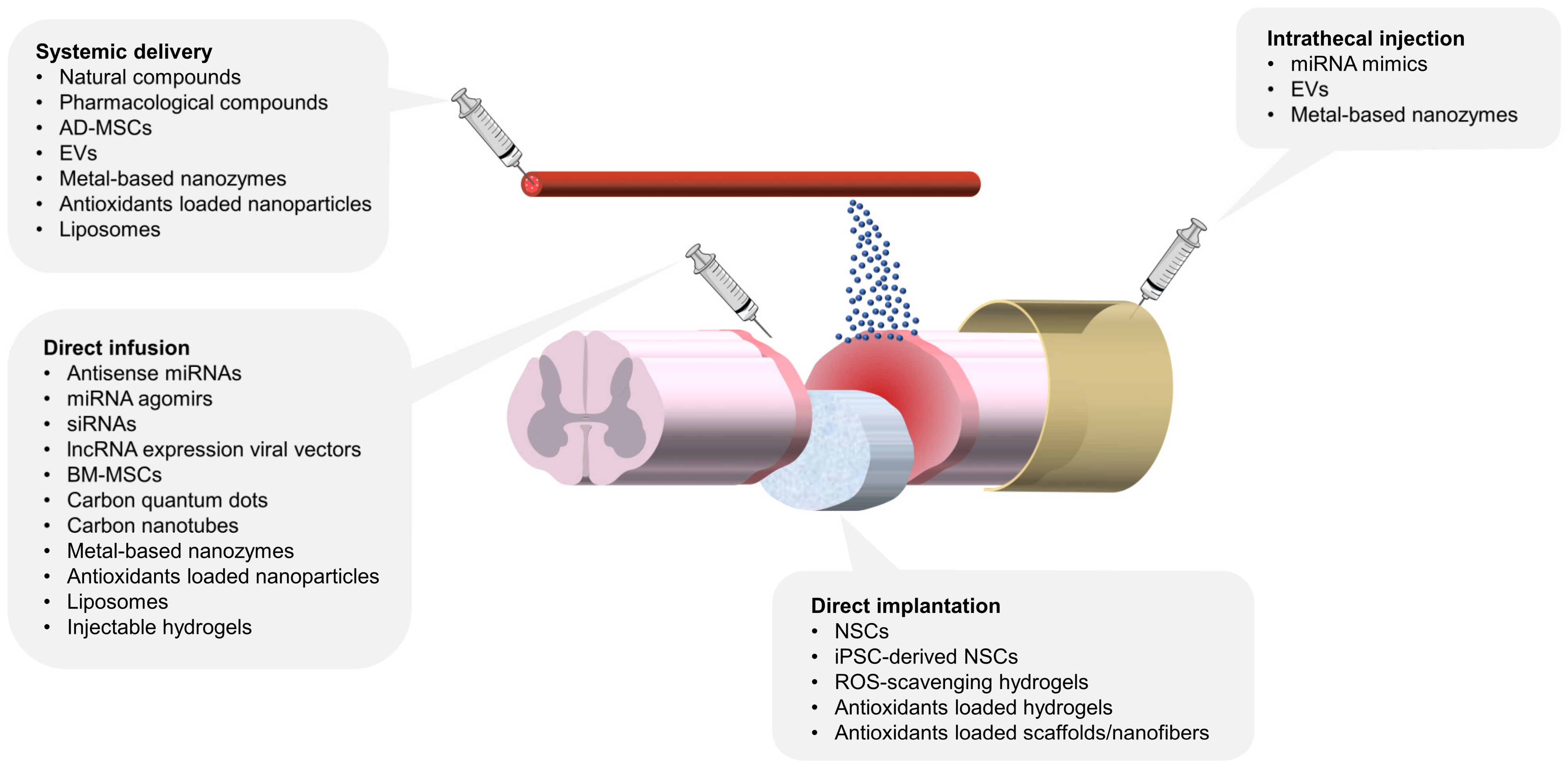
Figure 2 provides an overview of various antioxidant therapies under investigation, highlighting their respective delivery routes, including systemic administration, direct infusion, implantation, and intrathecal injection. Each method offers distinct advantages in addressing the challenges of targeting SCI lesions and optimizing therapeutic efficacy.
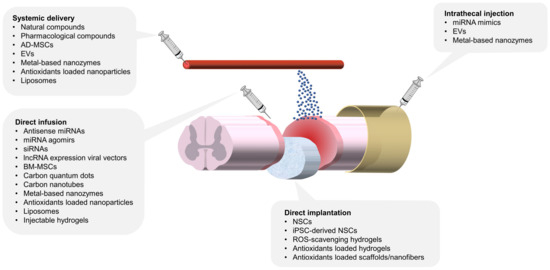
Figure 2.
Multiple antioxidant therapies for SCI and their delivery routes. This schematic illustrates various delivery methods for antioxidant strategies targeting spinal cord injuries. The delivery methods include direct infusion, direct implantation, systemic delivery, and intrathecal injection. Direct infusion and implantation focus on localized therapeutic action, while systemic delivery emphasizes immunomodulation and targeting SCI lesions. Intrathecal injection, though less explored in experimental models, holds significant clinical potential due to well-established techniques for spinal tapping and drug administration. AD-MSC: adipose-derived mesenchymal stem cell, BM-MSC: bone marrow mesenchymal stem cell, NSC: neural stem cell, EVs: extracellular vesicles.
4.2. The Complexity of Spinal Cord Tissue
The spinal cord’s intricate anatomical structure, diverse cellular composition, and highly organized neural circuits present significant challenges for effective SCI treatment. Following injury, this complexity is further exacerbated by the formation of necrotic cavities, glial scars, and cystic spaces [359,360]. These structural irregularities hinder the uniform distribution of therapeutic agents, making it difficult for systemically administered antioxidants to penetrate and reach all damaged areas. The diversity of cell types within the spinal cord also complicates antioxidant treatments. Neurons, oligodendrocytes, astrocytes, microglia, and infiltrating immune cells exhibit distinct responses to oxidative stress and injury. Neurons and oligodendrocytes are particularly vulnerable to ROS-induced damage, which leads to neural death and demyelination [361,362,363]. Concurrently, oxidative stress stimulates M1 polarization of macrophages, enhancing phagocytic function and debris clearance. However, a controlled level of ROS can promote the polarization of microglia toward the M2 phenotype, which supports tissue repair and neuroprotection [364,365]. Therefore, antioxidant therapies must be carefully designed to achieve both optimal distribution within the spinal cord and a delicate balance of ROS levels across different cell types, creating an environment conducive to tissue regeneration and functional recovery.
To address the complexity of spinal cord injury, advanced stereoscopic biostructures have been developed. Organoids, which mimic the tissue architecture and cellular diversity of an organ, represent a significant advancement in this area [366]. Spinal cord organoids derived from iPSCs provide a physiologically relevant in vitro model to study the neural networks and cellular interactions within the spinal cord, as well as to screen experimental therapies [367,368]. These organoids can be utilized to model oxidative damage after SCI, enabling high-throughput testing of antioxidant treatments. In therapeutic applications, organoids derived from reprogrammed human astrocytes have been transplanted into mice SCI lesions, where they demonstrated neuronal differentiation and integration into host neural circuits [369]. This highlights the potential of organoids as a therapeutic strategy for SCI repair. Another promising approach is 3D bioprinting, which offers precision in creating complex structures and combines cells with biomaterials in a spatially controlled manner [370]. For example, a dual-network hydrogel was 3D printed with a temperature-responsive outer layer and an N-cadherin-modified inner layer, targeting temporal regulation of oxidative stress and neuroregeneration in SCI. The hydrogel facilitated the release of ROS scavengers to protect endogenous neural stem/progenitor cells during the acute phase of injury, while supporting their migration and neuronal differentiation during the later phase [371]. These advanced bioengineering technologies offer innovative solutions to address the challenges posed by the spinal cord’s complexity. By combining antioxidant therapies with organoid and 3D bioprinting technologies, researchers can overcome the limitations of current treatment strategies, paving the way for more effective and precise SCI interventions.
5. Conclusions
The spinal cord is particularly susceptible to oxidative stress and free radical-mediated damage due to its high lipid content and active oxygen metabolism. Despite extensive basic and clinical research into the pathophysiology of secondary injury after SCI, the complexity of these mechanisms continues to hinder the development of effective therapies. This review has highlighted several antioxidants with potential therapeutic roles in SCI treatment, encompassing a wide range of approaches, including antioxidative molecules, stem cells, and biomaterials. Each therapeutic approach demonstrates significant antioxidative activity but faces distinct challenges in SCI treatment, such as adverse effects and insufficient efficacy as standalone regimens, limited bioavailability and biocompatibility, poor targeting of the injured spinal cord, and inadequate penetration across the BSCB. These limitations underscore the necessity of combining multiple antioxidant strategies to achieve synergistic and complementary effects, with particular emphasis on the role of biomaterials as a platform for integrating diverse antioxidant therapies.
While numerous antioxidant therapies have demonstrated promising results in experimental SCI models, no effective antioxidant-based treatments are currently available for clinical use. The translation of these findings into clinical practice faces significant challenges, including issues related to the timing and delivery of antioxidants and the complex structural and cellular composition of the spinal cord. This review has proposed potential strategies to address these obstacles, such as innovative delivery systems and advanced bioengineering technologies. Nevertheless, further research and development are required to refine multiplex antioxidant therapies and facilitate the translation of preclinical success into clinical applications, ultimately improving outcomes for SCI patients.
Author Contributions
Conceptualization: Y.-J.S., Y.-C.H. and Y.-C.C.; data curation: Y.-J.S., Y.-C.H. and Y.-C.C.; writing—original draft preparation: Y.-J.S. and Y.-C.C.; writing—review and editing: Y.-J.S., Y.-C.H. and Y.-C.C.; supervision: Y.-C.C.; project administration: Y.-C.C.; funding acquisition: Y.-C.H. and Y.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from Chang Gung Medical Foundation and Chang Gung Memorial Hospital (CMRPD1M0092, CMRPD1M0431, CMRPD1M0432, CMRPD1P0041, and BMRP857) and the National Science and Technology Council, Taiwan (113-2320-B-182-029-MY3).
Data Availability Statement
No new data were created in this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| AD-MSC | adipose-derived mesenchymal stem cell. |
| ARE | antioxidant response elements. |
| BDNF | brain-derived neurotrophic factor. |
| BM-MSC | bone marrow mesenchymal stem cell. |
| BSCB | blood-spinal cord barrier. |
| CeO2 | cerium oxide. |
| CTNF | ciliary neurotrophic factor. |
| ESC | embryonic stem cell. |
| EVs | extracellular vesicles. |
| Fe2O3 | iron oxide. |
| GPx | glutathione peroxidase. |
| GR | glutathione reductase. |
| GSH | glutathione. |
| GSSG | glutathione disulfide. |
| H2O2 | hydrogen peroxide. |
| HO-1 | heme oxygenase-1. |
| iPSC | induced pluripotent stem cell. |
| Keap1 | Kelch-like ECH-associated protein 1. |
| lncRNA | long noncoding RNA. |
| miRNA | microRNA. |
| Mn | manganese. |
| Mn-SOD | manganese superoxide dismutase. |
| NAC | N-acetylcysteine. |
| NF-κB | nuclear factor-kappa B. |
| NGF | nerve growth factor. |
| NOX | NADPH oxidase. |
| NQO1 | NADPH quinone oxidoreductase 1. |
| Nrf2 | nuclear factor erythroid 2-related factor 2. |
| NSC | neural stem cell. |
| O2•− | superoxide anions. |
| •OH | hydroxyl radicals. |
| ONOO− | peroxynitrite. |
| PCL | poly-caprolactone. |
| PEG | poly-ethylene glycol. |
| PLGA | poly-lactic-co-glycolic acid. |
| PVA | poly-vinyl alcohol. |
| RNS | reactive nitrogen species. |
| ROS | reactive oxygen species. |
| SCI | spinal cord injury. |
| siRNA | small interfering RNA. |
| SOD | superoxide dismutase. |
| TAT | transactivator of transcription. |
| TMP | Tetramethylpyrazine. |
| TNF-α | tumor necrosis factor-α. |
| VEGF | vascular endothelial growth factor. |
| ZnO | zinc oxide. |
References
- Yezierski, R.P. Pain following spinal cord injury: Pathophysiology and central mechanisms. Prog. Brain Res. 2000, 129, 429–449. [Google Scholar] [PubMed]
- Sekhon, L.H.; Fehlings, M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 2001, 26, S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chi, L.; Xu, R.; Ke, Y.; Luo, C.; Cai, J.; Qiu, M.; Gozal, D.; Liu, R. Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord. 2005, 43, 204–213. [Google Scholar] [CrossRef]
- Juurlink, B.H.; Paterson, P.G. Review of oxidative stress in brain and spinal cord injury: Suggestions for pharmacological and nutritional management strategies. J. Spinal Cord. Med. 1998, 21, 309–334. [Google Scholar] [CrossRef]
- He, Z.; Zhang, C.; Liang, J.X.; Zheng, F.F.; Qi, X.Y.; Gao, F. Targeting Mitochondrial Oxidative Stress: Potential Neuroprotective Therapy for Spinal Cord Injury. J. Integr. Neurosci. 2023, 22, 153. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, T.; Zhu, J.; Wei, D.; Ren, S.; Yang, Y.; Gao, F.; Zhao, L. Research Progress of Antioxidants in Oxidative Stress Therapy after Spinal Cord Injury. Neurochem. Res. 2023, 48, 3473–3484. [Google Scholar] [CrossRef]
- Liu, D.; Ling, X.; Wen, J.; Liu, J. The role of reactive nitrogen species in secondary spinal cord injury: Formation of nitric oxide, peroxynitrite, and nitrated protein. J. Neurochem. 2000, 75, 2144–2154. [Google Scholar] [CrossRef]
- Xiong, Y.; Rabchevsky, A.G.; Hall, E.D. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J. Neurochem. 2007, 100, 639–649. [Google Scholar] [CrossRef]
- Kim, H.Y.; Chung, J.M.; Chung, K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: The effect of mitochondrial electron transport complex inhibitors. Neurosci. Lett. 2008, 447, 87–91. [Google Scholar] [CrossRef]
- Azbill, R.D.; Mu, X.; Bruce-Keller, A.J.; Mattson, M.P.; Springer, J.E. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997, 765, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Bailey, W.M.; McVicar, A.L.; Stewart, A.N.; Veldhorst, A.K.; Gensel, J.C. Reducing age-dependent monocyte-derived macrophage activation contributes to the therapeutic efficacy of NADPH oxidase inhibition in spinal cord injury. Brain Behav. Immun. 2019, 76, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Bailey, C.S.; Gurr, K.R.; Bailey, S.I.; Rosas-Arellano, M.P.; Dekaban, G.A.; Weaver, L.C. Increased oxidative activity in human blood neutrophils and monocytes after spinal cord injury. Exp. Neurol. 2009, 215, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Sun, J.; Su, B.; Yang, R.; Dong, H.; Xiong, L. Ischemic postconditioning protects the spinal cord from ischemia-reperfusion injury via modulation of redox signaling. J. Thorac. Cardiovasc. Surg. 2013, 146, 688–695. [Google Scholar] [CrossRef]
- Granger, D.N. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am. J. Physiol. 1988, 255, H1269–H1275. [Google Scholar] [CrossRef]
- Michael-Titus, A.T. Omega-3 fatty acids and neurological injury. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 295–300. [Google Scholar] [CrossRef]
- Paterniti, I.; Impellizzeri, D.; Di Paola, R.; Esposito, E.; Gladman, S.; Yip, P.; Priestley, J.V.; Michael-Titus, A.T.; Cuzzocrea, S. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: In-vivo and in-vitro studies. J. Neuroinflammation 2014, 11, 6. [Google Scholar] [CrossRef]
- Bolin, C.; Stedeford, T.; Cardozo-Pelaez, F. Single extraction protocol for the analysis of 8-hydroxy-2’-deoxyguanosine (oxo8dG) and the associated activity of 8-oxoguanine DNA glycosylase. J. Neurosci. Methods 2004, 136, 69–76. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Gasparutto, D.; Ravanat, J.L. Oxidative damage to DNA: Formation, measurement and biochemical features. Mutat. Res. 2003, 531, 5–23. [Google Scholar] [CrossRef]
- Chen, X.L.; Kunsch, C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: A new therapeutic approach for the treatment of inflammatory diseases. Curr. Pharm. Des. 2004, 10, 879–891. [Google Scholar] [CrossRef]
- Mansouri, A.; Reiner, Ž.; Ruscica, M.; Tedeschi-Reiner, E.; Radbakhsh, S.; Bagheri Ekta, M.; Sahebkar, A. Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases. J. Clin. Med. 2022, 11, 1313. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Xiao, C.L.; Lai, H.T.; Zhou, J.J.; Liu, W.Y.; Zhao, M.; Zhao, K. Nrf2 Signaling Pathway: Focus on Oxidative Stress in Spinal Cord Injury. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef]
- Wang, X.; de Rivero Vaccari, J.P.; Wang, H.; Diaz, P.; German, R.; Marcillo, A.E.; Keane, R.W. Activation of the nuclear factor E2-related factor 2/antioxidant response element pathway is neuroprotective after spinal cord injury. J. Neurotrauma 2012, 29, 936–945. [Google Scholar] [CrossRef]
- Ryoo, I.G.; Kwak, M.K. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol. Appl. Pharmacol. 2018, 359, 24–33. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Lee, Y.S.; Lin, C.Y.; Lin, V.W.; Sindhu, R.K. NAD(P)H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Res. 2004, 995, 76–83. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Patel, S.P.; VanRooyen, J.L.; Sullivan, P.G.; Rabchevsky, A.G. Cellular and subcellular oxidative stress parameters following severe spinal cord injury. Redox Biol. 2016, 8, 59–67. [Google Scholar] [CrossRef]
- Yang, M.S.; Chan, H.W.; Yu, L.C. Glutathione peroxidase and glutathione reductase activities are partially responsible for determining the susceptibility of cells to oxidative stress. Toxicology 2006, 226, 126–130. [Google Scholar] [CrossRef]
- Jansen, T.; Hortmann, M.; Oelze, M.; Opitz, B.; Steven, S.; Schell, R.; Knorr, M.; Karbach, S.; Schuhmacher, S.; Wenzel, P.; et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell Cardiol. 2010, 49, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Mustacich, D.J.; Bruno, R.S.; Traber, M.G. Vitamin E. Vitam. Horm. 2007, 76, 1–21. [Google Scholar]
- Barreiro-Sisto, U.; Fernández-Fariña, S.; González-Noya, A.M.; Pedrido, R.; Maneiro, M. Enemies or Allies? Hormetic and Apparent Non-Dose-Dependent Effects of Natural Bioactive Antioxidants in the Treatment of Inflammation. Int. J. Mol. Sci. 2024, 25, 1892. [Google Scholar] [CrossRef]
- Banaszak, M.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. The Impact of Curcumin, Resveratrol, and Cinnamon on Modulating Oxidative Stress and Antioxidant Activity in Type 2 Diabetes: Moving beyond an Anti-Hyperglycaemic Evaluation. Antioxid 2024, 13, 510. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2019, 7, 447. [Google Scholar] [CrossRef]
- Lin, M.S.; Lee, Y.H.; Chiu, W.T.; Hung, K.S. Curcumin provides neuroprotection after spinal cord injury. J. Surg. Res. 2011, 166, 280–289. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar]
- Cemil, B.; Topuz, K.; Demircan, M.N.; Kurt, G.; Tun, K.; Kutlay, M.; Ipcioglu, O.; Kucukodaci, Z. Curcumin improves early functional results after experimental spinal cord injury. Acta Neurochir. 2010, 152, 1583–1590, discussion 1590. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Bahroudi, Z.; Hussen, B.M.; Talebi, S.F.; Taheri, M.; Ayatollahi, S.A. Nrf2-Related Therapeutic Effects of Curcumin in Different Disorders. Biomolecules 2022, 12, 82. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Alsaab, H.O.; Golmohammadi, M.; Yumashev, A.; Jabba, A.M.; Abid, M.K.; Joshi, A.; Alawadi, A.H.; Jafer, N.S.; Kianifar, F.; et al. NF-κB pathway as a molecular target for curcumin in diabetes mellitus treatment: Focusing on oxidative stress and inflammation. Cell Biochem. Funct. 2024, 42, e4030. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Yao, M.; Yang, L.; Wang, J.; Sun, Y.L.; Dun, R.L.; Wang, Y.J.; Cui, X.J. Neurological recovery and antioxidant effects of curcumin for spinal cord injury in the rat: A network meta-analysis and systematic review. J. Neurotrauma 2015, 32, 381–391. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and Safety of Curcumin and Curcuma longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Q.; Zhou, S.; Xu, S.; Yao, K. Tetramethylpyrazine: A review on its mechanisms and functions. Biomed. Pharmacother. 2022, 150, 113005. [Google Scholar] [CrossRef]
- Lin, J.; Hao, C.; Gong, Y.; Zhang, Y.; Li, Y.; Feng, Z.; Xu, X.; Huang, H.; Liao, W. Effect of Tetramethylpyrazine on Neuroplasticity after Transient Focal Cerebral Ischemia Reperfusion in Rats. Evid. Based Complement. Altern. Med. 2021, 2021, 1587241. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, Y.; Qi, C.; Qiu, F.; Chen, X.; Zhang, J.; Yang, P. Neuroprotection and enhanced neurogenesis by tetramethylpyrazine in adult rat brain after focal ischemia. Neurol. Res. 2010, 32, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, K.; Shi, Z.; Die, J.; Wang, C.; Dang, X. Tetramethylpyrazine protects spinal cord and reduces inflammation in a rat model of spinal cord ischemia-reperfusion injury. J. Vasc. Surg. 2011, 54, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.X.; Lü, H.B.; Li, X.M.; Xu, D.Q.; Hu, J.Z.; Wang, X.Y. Tetramethylpyrazine accelerated spinal cord repair through regulation of caspase-3 and neurofilament protein expression: An acute spinal cord injury model in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008, 33, 693–699. [Google Scholar] [PubMed]
- Huang, J.H.; Cao, Y.; Zeng, L.; Wang, G.; Cao, M.; Lu, H.B.; Hu, J.Z. Tetramethylpyrazine enhances functional recovery after contusion spinal cord injury by modulation of MicroRNA-21, FasL, PDCD4 and PTEN expression. Brain Res. 2016, 1648, 35–45. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yu, S.Z.; Wang, Z.T.; Zhao, B.L.; Hou, J.W.; Yang, F.J.; Xin, W.J. Scavenging effects of tetramethylpyrazine on active oxygen free radicals. Zhongguo Yao Li Xue Bao 1994, 15, 229–231. [Google Scholar]
- Guan, D.; Su, Y.; Li, Y.; Wu, C.; Meng, Y.; Peng, X.; Cui, Y. Tetramethylpyrazine inhibits CoCl2-induced neurotoxicity through enhancement of Nrf2/GCLc/GSH and suppression of HIF1α/NOX2/ROS pathways. J. Neurochem. 2015, 134, 551–565. [Google Scholar] [CrossRef]
- Guo, L.; Wang, A.; Sun, Y.; Xu, C. Evaluation of antioxidant and immunity function of tetramethylpyrazine phosphate tablets in vivo. Molecules 2012, 17, 5412–5421. [Google Scholar] [CrossRef]
- Fan, L.H.; Wang, K.Z.; Cheng, B.; Wang, C.S.; Dang, X.Q. Anti-apoptotic and neuroprotective effects of Tetramethylpyrazine following spinal cord ischemia in rabbits. BMC Neurosci. 2006, 7, 48. [Google Scholar] [CrossRef]
- Li, G.; Sng, K.S.; Shu, B.; Wang, Y.J.; Yao, M.; Cui, X.J. Effects of tetramethylpyrazine treatment in a rat model of spinal cord injury: A systematic review and meta-analysis. Eur. J. Pharmacol. 2023, 945, 175524. [Google Scholar] [CrossRef]
- Yu, T.; Guo, X.; Zhang, Z.; Liu, R.; Zou, L.; Fu, J.; Shi, Z. Meta-Analysis of the Clinical Effectiveness and Safety of Ligustrazine in Cerebral Infarction. Evid. Based Complement. Altern. Med. 2016, 2016, 3595946. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Cui, W.; Zhang, Y.; Liang, X. Regulatory mechanisms of tetramethylpyrazine on central nervous system diseases: A review. Front. Pharmacol. 2022, 13, 948600. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Hiremath, P.; John, J.; Ranadive, N.; Nandakumar, K.; Mudgal, J. Modulatory role of vitamins A, B3, C, D, and E on skin health, immunity, microbiome, and diseases. Pharmacol. Rep. 2023, 75, 1096–1114. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Moseley, P.L. EPR spin trapping of free radicals produced by bleomycin and ascorbate. Free Radic. Res. Commun. 1993, 19 (Suppl. 1), S89–S93. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Naseer, M.I.; Ullah, N.; Ullah, I.; Koh, P.O.; Lee, H.Y.; Park, M.S.; Kim, M.O. Vitamin C protects against ethanol and PTZ-induced apoptotic neurodegeneration in prenatal rat hippocampal neurons. Synapse 2011, 65, 562–571. [Google Scholar] [CrossRef]
- Ahmad, A.; Shah, S.A.; Badshah, H.; Kim, M.J.; Ali, T.; Yoon, G.H.; Kim, T.H.; Abid, N.B.; Rehman, S.U.; Khan, S.; et al. Neuroprotection by Vitamin C Against Ethanol-Induced Neuroinflammation Associated Neurodegeneration in the Developing Rat Brain. CNS Neurol. Disord. Drug Targets 2016, 15, 360–370. [Google Scholar] [CrossRef]
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, Aging and Alzheimer’s Disease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Gebicki, J.M. Oxidation of alpha-tocopherol in micelles and liposomes by the hydroxyl, perhydroxyl, and superoxide free radicals. Arch. Biochem. Biophys. 1983, 226, 242–251. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Neuzil, J.; Thomas, S.R.; Stocker, R. Requirement for, promotion, or inhibition by alpha-tocopherol of radical-induced initiation of plasma lipoprotein lipid peroxidation. Free Radic. Biol. Med. 1997, 22, 57–71. [Google Scholar] [CrossRef]
- Babbs, C.F.; Steiner, M.G. Simulation of free radical reactions in biology and medicine: A new two-compartment kinetic model of intracellular lipid peroxidation. Free Radic. Biol. Med. 1990, 8, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Kolnik, S.; Wood, T.R. Role of Vitamin E in Neonatal Neuroprotection: A Comprehensive Narrative Review. Life 2022, 12, 1083. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Sarveazad, A.; Babahajian, A.; Baikpour, M.; Vaccaro, A.R.; Chapman, J.R.; Yousefifard, M.; Rahimi-Movaghar, V. Effect of vitamins C and E on recovery of motor function after spinal cord injury: Systematic review and meta-analysis of animal studies. Nutr. Rev. 2020, 78, 465–473. [Google Scholar] [CrossRef]
- Zadeh-Ardabili, P.M.; Rad, S.K.; Rad, S.K.; Khazaài, H.; Sanusi, J.; Zadeh, M.H. Palm vitamin E reduces locomotor dysfunction and morphological changes induced by spinal cord injury and protects against oxidative damage. Sci. Rep. 2017, 7, 14365. [Google Scholar] [CrossRef]
- Yan, M.; Yang, M.; Shao, W.; Mao, X.G.; Yuan, B.; Chen, Y.F.; Ye, Z.X.; Liang, W.; Luo, Z.J. High-dose ascorbic acid administration improves functional recovery in rats with spinal cord contusion injury. Spinal Cord. 2014, 52, 803–808. [Google Scholar] [CrossRef]
- Chen, H.C.; Hsu, P.W.; Tzaan, W.C.; Lee, A.W. Effects of the combined administration of vitamins C and E on the oxidative stress status and programmed cell death pathways after experimental spinal cord injury. Spinal Cord. 2014, 52, 24–28. [Google Scholar] [CrossRef]
- Han, P.L.; Fu, Q.L.; Dong, J.F.; Zhang, J.; Qin, Y.X.; Cui, Y.; Li, Q. Effects of open heart surgery under normothermic and hypothermic cardiopulmonary bypass on cytokines and complements. Di Yi Jun. Yi Da Xue Xue Bao 2003, 23, 1317–1318, 1322. [Google Scholar]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Total, Dietary, and Supplemental Vitamin C Intake and Risk of Incident Kidney Stones. Am. J. Kidney Dis. 2016, 67, 400–407. [Google Scholar] [CrossRef]
- Dowd, P.; Zheng, Z.B. On the mechanism of the anticlotting action of vitamin E quinone. Proc. Natl. Acad. Sci. USA 1995, 92, 8171–8175. [Google Scholar] [CrossRef]
- Miller, E.R., 3rd; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Gravandi, M.M.; Abdian, S.; Moradi, S.Z.; Echeverría, J. Quercetin Derivatives in Combating Spinal Cord Injury: A Mechanistic and Systematic Review. Life 2022, 12, 1960. [Google Scholar] [CrossRef] [PubMed]
- Rezaei-Sadabady, R.; Eidi, A.; Zarghami, N.; Barzegar, A. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 128–134. [Google Scholar] [CrossRef]
- Kang, S.G.; Lee, G.B.; Vinayagam, R.; Do, G.S.; Oh, S.Y.; Yang, S.J.; Kwon, J.B.; Singh, M. Anti-Inflammatory, Antioxidative, and Nitric Oxide-Scavenging Activities of a Quercetin Nanosuspension with Polyethylene Glycol in LPS-Induced RAW 264.7 Macrophages. Molecules 2022, 27, 7432. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, C.; Liu, H.; Wang, M.; Zhao, H.; Yan, Y.; Han, L. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Dixit, S. Quercetin attenuates the development of 7, 12-dimethyl benz (a) anthracene (DMBA) and croton oil-induced skin cancer in mice. J. Biomed. Res. 2015, 29, 139–144. [Google Scholar]
- Chen, B.H.; Park, J.H.; Ahn, J.H.; Cho, J.H.; Kim, I.H.; Lee, J.C.; Won, M.H.; Lee, C.H.; Hwang, I.K.; Kim, J.D.; et al. Pretreated quercetin protects gerbil hippocampal CA1 pyramidal neurons from transient cerebral ischemic injury by increasing the expression of antioxidant enzymes. Neural Regen. Res. 2017, 12, 220–227. [Google Scholar]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem. Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, C.; Xing, M.; Li, Y.; Zhu, L.; Wang, D.; Yang, X.; Liu, L.; Yao, P. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem. Toxicol. 2012, 50, 1194–1200. [Google Scholar] [CrossRef]
- Chakraborty, S.; Stalin, S.; Das, N.; Thakur Choudhury, S.; Ghosh, S.; Swarnakar, S. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials 2012, 33, 2991–3001. [Google Scholar] [CrossRef]
- Bureau, G.; Longpré, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, X.; Zhao, J. Quercetin prevents alcohol-induced liver injury through targeting of PI3K/Akt/nuclear factor-κB and STAT3 signaling pathway. Exp. Ther. Med. 2017, 14, 6169–6175. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, Y.; Han, N.; He, F.; Li, M.; Bian, Z.; Liu, J.; Sun, T.; Zhu, L. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord. 2016, 54, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Çiftçi, U.; Delen, E.; Vural, M.; Uysal, O.; Turgut Coşan, D.; Baydemir, C.; Doğaner, F. Efficiacy of resveratrol and quercetin after experimental spinal cord injury. Ulus. Travma Acil Cerrahi Derg. 2016, 22, 423–431. [Google Scholar]
- Ocal, O.; Borcek, A.O.; Pasaoglu, O.; Gundogdu, A.C.; Kaplanoglu, G.T.; Baykaner, M.K. Can Quercetin be an Option for Treatment of Spinal Cord Injury? An Experimental Study. Turk. Neurosurg. 2019, 29, 247–253. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Hall, E.D.; Braughler, J.M. Glucocorticoid mechanisms in acute spinal cord injury: A review and therapeutic rationale. Surg. Neurol. 1982, 18, 320–327. [Google Scholar] [CrossRef]
- Hall, E.D. The neuroprotective pharmacology of methylprednisolone. J. Neurosurg. 1992, 76, 13–22. [Google Scholar] [CrossRef]
- Lee, H.J.; Suh, J.K.; Song, H.H.; Jeong, M.A.; Yeom, J.H.; Kim, D.W. Antioxidant effects of methylprednisolone and hydrocortisone on the impairment of endothelium dependent relaxation induced by reactive oxygen species in rabbit abdominal aorta. Korean J. Anesthesiol. 2013, 64, 54–60. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Fan, G.; Chen, S.; Wu, Y.; Xu, X.M.; Hsu, C.Y. Methylprednisolone inhibition of TNF-α expression and NF-kB activation after spinal cord injury in rats. Mol. Brain Res. 1998, 59, 135–142. [Google Scholar] [CrossRef]
- Hall, E.D. Antioxidant therapies for acute spinal cord injury. Neurotherapeutics 2011, 8, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhu, W.; Tang, M.; Zhang, M.; Liu, Y.; Li, Z.; Rao, Z.; He, X.; Ma, R.; Xue, X. Protective effects of methylprednisolone-cyclophosphamide treatment on bleomycin-induced pulmonary fibrosis. Cytokine 2023, 166, 156188. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.L.; Torres, I.L.; Laste, G.; Ferreira, M.B.; Cardoso, P.F.; Belló-Klein, A. Effects of acute and chronic administration of methylprednisolone on oxidative stress in rat lungs. J. Bras. Pneumol. 2014, 40, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Van Zanden, J.E.; ’T Hart, N.A.; Ottens, P.J.; Liu, B.; Rebolledo, R.A.; Erasmus, M.E.; Leuvenink, H.G.D. Methylprednisolone Treatment in Brain Death-Induced Lung Inflammation-A Dose Comparative Study in Rats. Front. Pharmacol. 2021, 12, 587003. [Google Scholar] [CrossRef]
- Wang, X.; Luo, F.; Zhao, H. Paraquat-induced reactive oxygen species inhibit neutrophil apoptosis via a p38 MAPK/NF-κB-IL-6/TNF-α positive-feedback circuit. PLoS ONE 2014, 9, e93837. [Google Scholar] [CrossRef]
- Mathy-Hartert, M.; Hogge, L.; Sanchez, C.; Deby-Dupont, G.; Crielaard, J.M.; Henrotin, Y. Interleukin-1β and interleukin-6 disturb the antioxidant enzyme system in bovine chondrocytes: A possible explanation for oxidative stress generation. Osteoarthr. Cartil. 2008, 16, 756–763. [Google Scholar] [CrossRef]
- Braughler, J.M.; Hall, E.D. Correlation of methylprednisolone levels in cat spinal cord with its effects on (Na+ + K+)-ATPase, lipid peroxidation, and alpha motor neuron function. J. Neurosurg. 1982, 56, 838–844. [Google Scholar] [CrossRef]
- Bracken, M.B.; Shepard, M.J.; Collins, W.F.; Holford, T.R.; Young, W.; Baskin, D.S.; Eisenberg, H.M.; Flamm, E.; Leo-Summers, L.; Maroon, J. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: Results of the Second National Acute Spinal Cord Injury Study. New Engl. J. Med. 1990, 322, 1405–1411. [Google Scholar] [CrossRef]
- Bracken, M.B.; Shepard, M.J.; Holford, T.R.; Leo-Summers, L.; Aldrich, E.F.; Fazl, M.; Fehlings, M.; Herr, D.L.; Hitchon, P.W.; Marshall, L.F. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: Results of the third national acute spinal cord injury randomized controlled trial. Jama 1997, 277, 1597–1604. [Google Scholar] [CrossRef]
- Bracken, M.B.; Shepard, M.J.; Collins, W.F., Jr.; Holford, T.R.; Baskin, D.S.; Eisenberg, H.M.; Flamm, E.; Leo-Summers, L.; Maroon, J.C.; Marshall, L.F.; et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J. Neurosurg. 1992, 76, 23–31. [Google Scholar] [CrossRef]
- Cheung, V.; Hoshide, R.; Bansal, V.; Kasper, E.; Chen, C.C. Methylprednisolone in the management of spinal cord injuries: Lessons from randomized, controlled trials. Surg. Neurol. Int. 2015, 6, 142. [Google Scholar] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. What is behind the non-antibiotic properties of minocycline? Pharmacol. Res. 2013, 67, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Yin, J.H.; Hwang, C.S.; Tang, C.M.; Yang, D.I. Anti-apoptotic and anti-oxidative mechanisms of minocycline against sphingomyelinase/ceramide neurotoxicity: Implication in Alzheimer’s disease and cerebral ischemia. Free Radic. Res. 2012, 46, 940–950. [Google Scholar] [CrossRef]
- Kraus, R.L.; Pasieczny, R.; Lariosa-Willingham, K.; Turner, M.S.; Jiang, A.; Trauger, J.W. Antioxidant properties of minocycline: Neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J. Neurochem. 2005, 94, 819–827. [Google Scholar] [CrossRef]
- Shultz, R.B.; Zhong, Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen. Res. 2017, 12, 702–713. [Google Scholar]
- Aras, M.; Altas, M.; Motor, S.; Dokuyucu, R.; Yilmaz, A.; Ozgiray, E.; Seraslan, Y.; Yilmaz, N. Protective effects of minocycline on experimental spinal cord injury in rats. Injury 2015, 46, 1471–1474. [Google Scholar] [CrossRef]
- Sonmez, E.; Kabatas, S.; Ozen, O.; Karabay, G.; Turkoglu, S.; Ogus, E.; Yilmaz, C.; Caner, H.; Altinors, N. Minocycline treatment inhibits lipid peroxidation, preserves spinal cord ultrastructure, and improves functional outcome after traumatic spinal cord injury in the rat. Spine 2013, 38, 1253–1259. [Google Scholar] [CrossRef]
- Möller, T.; Bard, F.; Bhattacharya, A.; Biber, K.; Campbell, B.; Dale, E.; Eder, C.; Gan, L.; Garden, G.A.; Hughes, Z.A.; et al. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia 2016, 64, 1788–1794. [Google Scholar] [CrossRef]
- Abdo Qaid, E.Y.; Abdullah, Z.; Zakaria, R.; Long, I. Minocycline protects against lipopolysaccharide-induced glial cells activation and oxidative stress damage in the medial prefrontal cortex (mPFC) of the rat. Int. J. Neurosci. 2024, 134, 56–65. [Google Scholar] [CrossRef]
- Verma, D.K.; Singh, D.K.; Gupta, S.; Gupta, P.; Singh, A.; Biswas, J.; Singh, S. Minocycline diminishes the rotenone induced neurotoxicity and glial activation via suppression of apoptosis, nitrite levels and oxidative stress. NeuroToxicology 2018, 65, 9–21. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Wang, Y.; Tang, S.; Xiao, X.; Velkov, T. Minocycline attenuates colistin-induced neurotoxicity via suppression of apoptosis, mitochondrial dysfunction and oxidative stress. J. Antimicrob. Chemother. 2017, 72, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Karachitos, A.; García Del Pozo, J.S.; de Groot, P.W.; Kmita, H.; Jordán, J. Minocycline mediated mitochondrial cytoprotection: Premises for therapy of cerebrovascular and neurodegenerative diseases. Curr. Drug Targets 2013, 14, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Grenier, D.; Huot, M.P.; Mayrand, D. Iron-chelating activity of tetracyclines and its impact on the susceptibility of Actinobacillus actinomycetemcomitans to these antibiotics. Antimicrob. Agents Chemother. 2000, 44, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, H.; Yu, X.; Deng, Y. Minocycline attenuates neurological impairment and regulates iron metabolism in a rat model of traumatic brain injury. Arch. Biochem. Biophys. 2020, 682, 108302. [Google Scholar] [CrossRef]
- Chen-Roetling, J.; Chen, L.; Regan, R.F. Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem. Biophys. Res. Commun. 2009, 386, 322–326. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, D.Y.; Chung, E.S.; Hong, Y.B.; Kim, S.U.; Jin, B.K. Inhibition of thrombin-induced microglial activation and NADPH oxidase by minocycline protects dopaminergic neurons in the substantia nigra in vivo. J. Neurochem. 2005, 95, 1755–1765. [Google Scholar] [CrossRef]
- Hernandes, M.S.; Santos, G.D.; Café-Mendes, C.C.; Lima, L.S.; Scavone, C.; Munhoz, C.D.; Britto, L.R. Microglial cells are involved in the susceptibility of NADPH oxidase knockout mice to 6-hydroxy-dopamine-induced neurodegeneration. PLoS ONE 2013, 8, e75532. [Google Scholar] [CrossRef]
- Casha, S.; Zygun, D.; McGowan, M.D.; Bains, I.; Yong, V.W.; Hurlbert, R.J. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012, 135, 1224–1236. [Google Scholar] [CrossRef]
- Sabzali, M.; Eidi, A.; Khaksari, M.; Khastar, H. Anti-inflammatory, Antioxidant, and Antiapoptotic Action of Metformin Attenuates Ethanol Neurotoxicity in the Animal Model of Fetal Alcohol Spectrum Disorders. Neurotox. Res. 2022, 40, 605–613. [Google Scholar] [CrossRef]
- Yang, X.; Ding, H.; Qin, Z.; Zhang, C.; Qi, S.; Zhang, H.; Yang, T.; He, Z.; Yang, K.; Du, E.; et al. Metformin Prevents Renal Stone Formation through an Antioxidant Mechanism In Vitro and In Vivo. Oxid. Med. Cell Longev. 2016, 2016, 4156075. [Google Scholar] [CrossRef]
- Manica, D.; Sandri, G.; da Silva, G.B.; Manica, A.; da Silva Rosa Bonadiman, B.; Dos Santos, D.; Flores É, M.M.; Bolzan, R.C.; Barcelos, R.C.S.; Tomazoni, F.; et al. Evaluation of the effects of metformin on antioxidant biomarkers and mineral levels in patients with type II diabetes mellitus: A cross-sectional study. J. Diabetes Complicat. 2023, 37, 108497. [Google Scholar] [CrossRef] [PubMed]
- Karmanova, E.E.; Chernikov, A.V.; Popova, N.R.; Sharapov, M.G.; Ivanov, V.E.; Bruskov, V.I. Metformin mitigates radiation toxicity exerting antioxidant and genoprotective properties. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Ashabi, G.; Khalaj, L.; Khodagholi, F.; Goudarzvand, M.; Sarkaki, A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2015, 30, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Niloufar, D.; Samira, M.; Hamid Reza, M.; Matin, R. Comparing the acute and chronic effects of metformin and antioxidant protective effects of N-acetyl cysteine on memory retrieval and oxidative stress in rats with Alzheimer’s disease. Pak. J. Pharm. Sci. 2023, 36, 731–739. [Google Scholar]
- Zhou, L.Y.; Chen, X.Q.; Yu, B.B.; Pan, M.X.; Fang, L.; Li, J.; Cui, X.J.; Yao, M.; Lu, X. The effect of metformin on ameliorating neurological function deficits and tissue damage in rats following spinal cord injury: A systematic review and network meta-analysis. Front. Neurosci. 2022, 16, 946879. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Z.; Han, W.; Yuan, Y.; Li, Y.; Zhou, K.; Wang, Q.; Xie, L.; Xu, K.; Zhang, H.; et al. Metformin Promotes Axon Regeneration after Spinal Cord Injury through Inhibiting Oxidative Stress and Stabilizing Microtubule. Oxid. Med. Cell Longev. 2020, 2020, 9741369. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, W.; Zhang, Z.; Zhang, L.; Li, M. Metformin alleviates spinal cord injury by inhibiting nerve cell ferroptosis through upregulation of heme oxygenase-1 expression. Neural Regen. Res. 2024, 19, 2041–2049. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef]
- Hazelton, G.A.; Hjelle, J.J.; Klaassen, C.D. Effects of cysteine pro-drugs on acetaminophen-induced hepatotoxicity. J. Pharmacol. Exp. Ther. 1986, 237, 341–349. [Google Scholar]
- Kyyriäinen, J.; Kajevu, N.; Bañuelos, I.; Lara, L.; Lipponen, A.; Balosso, S.; Hämäläinen, E.; Das Gupta, S.; Puhakka, N.; Natunen, T.; et al. Targeting Oxidative Stress with Antioxidant Duotherapy after Experimental Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 10555. [Google Scholar] [CrossRef] [PubMed]
- Pauletti, A.; Terrone, G.; Shekh-Ahmad, T.; Salamone, A.; Ravizza, T.; Rizzi, M.; Pastore, A.; Pascente, R.; Liang, L.P.; Villa, B.R.; et al. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain 2019, 142, e39. [Google Scholar] [CrossRef] [PubMed]
- Sabetghadam, M.; Mazdeh, M.; Abolfathi, P.; Mohammadi, Y.; Mehrpooya, M. Evidence for a Beneficial Effect of Oral N-acetylcysteine on Functional Outcomes and Inflammatory Biomarkers in Patients with Acute Ischemic Stroke. Neuropsychiatr. Dis. Treat. 2020, 16, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Hanci, V.; Kerimoğlu, A.; Koca, K.; Başkesen, A.; Kiliç, K.; Taştekin, D. The biochemical effectiveness of N-acetylcysteine in experimental spinal cord injury in rats. Ulus. Travma Acil Cerrahi Derg. 2010, 16, 15–21. [Google Scholar] [PubMed]
- Patel, S.P.; Sullivan, P.G.; Pandya, J.D.; Goldstein, G.A.; VanRooyen, J.L.; Yonutas, H.M.; Eldahan, K.C.; Morehouse, J.; Magnuson, D.S.; Rabchevsky, A.G. N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp. Neurol. 2014, 257, 95–105. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Chen, Z.; He, Z.; Zhang, B.; Li, Y.; Hu, J.; Han, M.; Xu, Y.; Li, Y. N-acetylcysteine treatment following spinal cord trauma reduces neural tissue damage and improves locomotor function in mice. Mol. Med. Rep. 2015, 12, 37–44. [Google Scholar] [CrossRef]
- Olakowska, E.; Marcol, W.; Właszczuk, A.; Woszczycka-Korczyńska, I.; Lewin-Kowalik, J. The neuroprotective effect of N-acetylcysteine in spinal cord-injured rats. Adv. Clin. Exp. Med. 2017, 26, 1329–1334. [Google Scholar] [CrossRef]
- Guo, X.; He, J.; Zhang, R.; Wang, T.; Chen, J.; Wang, J.; Wang, Z.; Chang, G.; Niu, Y.; Niu, Z.; et al. N-Acetylcysteine alleviates spinal cord injury in rats after early decompression surgery by regulating inflammation and apoptosis. Neurol. Res. 2022, 44, 605–613. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Młynarska, E.; Hajdys, J.; Czarnik, W.; Fularski, P.; Leszto, K.; Majchrowicz, G.; Lisińska, W.; Rysz, J.; Franczyk, B. The Role of Antioxidants in the Therapy of Cardiovascular Diseases-A Literature Review. Nutrients 2024, 16, 2587. [Google Scholar] [CrossRef]
- Wei, Z.; Hang, S.; Wiredu Ocansey, D.K.; Zhang, Z.; Wang, B.; Zhang, X.; Mao, F. Human umbilical cord mesenchymal stem cells derived exosome shuttling mir-129-5p attenuates inflammatory bowel disease by inhibiting ferroptosis. J. Nanobiotechnology 2023, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Bardallo, R.G.; Panisello-Roselló, A.; Sanchez-Nuno, S.; Alva, N.; Roselló-Catafau, J.; Carbonell, T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. Febs J. 2022, 289, 5463–5479. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, Q.; Chen, L.; He, Y.J.; Wang, X.; Qu, S. miR-100a-5p-enriched exosomes derived from mesenchymal stem cells enhance the anti-oxidant effect in a Parkinson’s disease model via regulation of Nox4/ROS/Nrf2 signaling. J. Transl. Med. 2023, 21, 747. [Google Scholar] [CrossRef]
- Liu, N.K.; Wang, X.F.; Lu, Q.B.; Xu, X.M. Altered microRNA expression following traumatic spinal cord injury. Exp. Neurol. 2009, 219, 424–429. [Google Scholar] [CrossRef]
- Dai, J.; Xu, L.J.; Han, G.D.; Sun, H.L.; Zhu, G.T.; Jiang, H.T.; Yu, G.Y.; Tang, X.M. MiR-137 attenuates spinal cord injury by modulating NEUROD4 through reducing inflammation and oxidative stress. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1884–1890. [Google Scholar]
- Ebrahimy, N.; Gasterich, N.; Behrens, V.; Amini, J.; Fragoulis, A.; Beyer, C.; Zhao, W.; Sanadgol, N.; Zendedel, A. Neuroprotective effect of the Nrf2/ARE/miRNA145-5p signaling pathway in the early phase of spinal cord injury. Life Sci. 2022, 304, 120726. [Google Scholar] [CrossRef]
- Jee, M.K.; Jung, J.S.; Choi, J.I.; Jang, J.A.; Kang, K.S.; Im, Y.B.; Kang, S.K. MicroRNA 486 is a potentially novel target for the treatment of spinal cord injury. Brain 2012, 135, 1237–1252. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Jing, L. MiRNA-99a alleviates inflammation and oxidative stress in lipopolysaccharide-stimulated PC-12 cells and rats post spinal cord injury. Bioengineered 2022, 13, 4248–4259. [Google Scholar] [CrossRef]
- Ding, L.Z.; Xu, J.; Yuan, C.; Teng, X.; Wu, Q.M. MiR-7a ameliorates spinal cord injury by inhibiting neuronal apoptosis and oxidative stress. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11–17. [Google Scholar]
- Im, Y.B.; Jee, M.K.; Choi, J.I.; Cho, H.T.; Kwon, O.H.; Kang, S.K. Molecular targeting of NOX4 for neuropathic pain after traumatic injury of the spinal cord. Cell Death Dis. 2012, 3, e426. [Google Scholar] [CrossRef]
- Xia, P.; Gao, X.; Duan, L.; Zhang, W.; Sun, Y.F. Mulberrin (Mul) reduces spinal cord injury (SCI)-induced apoptosis, inflammation and oxidative stress in rats via miroRNA-337 by targeting Nrf-2. Biomed. Pharmacother. 2018, 107, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Behlke, M.A. Progress towards in vivo use of siRNAs. Mol. Ther. 2006, 13, 644–670. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.; Chow, M.Y.; Zhang, Y.; Leung, S.W. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, Y. NADPH oxidase 4 contributes to oxidative stress in a mouse model of myocardial infarction. Physiol. Res. 2023, 72, 177–186. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Shen, X.; Xu, T.; Yin, Y.; Zhang, H.; Ding, S.; Zhao, Y.; Zhang, Y.; Guan, Y.; et al. Inhibition of NOX2-NLRP1 signaling pathway protects against chronic glucocorticoids exposure-induced hippocampal neuronal damage. Int. Immunopharmacol. 2019, 74, 105721. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Lu, L.Q.; Jiang, Y.Q.; Li, N.S.; Luo, X.J.; Peng, J.W.; Peng, J. Allopurinol attenuates oxidative injury in rat hearts suffered ischemia/reperfusion via suppressing the xanthine oxidase/vascular peroxidase 1 pathway. Eur. J. Pharmacol. 2021, 908, 174368. [Google Scholar] [CrossRef]
- Li, Z.; Wu, F.; Xu, D.; Zhi, Z.; Xu, G. Inhibition of TREM1 reduces inflammation and oxidative stress after spinal cord injury (SCI) associated with HO-1 expressions. Biomed. Pharmacother. 2019, 109, 2014–2021. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef]
- Khalil, A.M.; Guttman, M.; Huarte, M.; Garber, M.; Raj, A.; Rivea Morales, D.; Thomas, K.; Presser, A.; Bernstein, B.E.; van Oudenaarden, A.; et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 11667–11672. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Analyzing MiRNA-LncRNA Interactions. Methods Mol. Biol. 2016, 1402, 271–286. [Google Scholar]
- Dykes, I.M.; Emanueli, C. Transcriptional and Post-Transcriptional Gene Regulation by Long Non-Coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.; Wang, X.; Chen, J.; Kong, Q.; Ye, B.; Li, Z. The Emerging Role of lncRNAs in Spinal Cord Injury. Biomed. Res. Int. 2019, 2019, 3467121. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, H.; Han, X.; Sun, J.; Ni, M.; Hou, X.; Fang, F.; Zhang, W.; Ma, P. Long Non-Coding RNA MALAT1 Protects Against Spinal Cord Injury via Suppressing microRNA-125b-5p Mediated Microglial M1 Polarization, Neuroinflammation, and Neural Apoptosis. Mol. Neurobiol. 2024, 61, 2136–2150. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Li, Y.; Zhang, W.; Fang, F.; Sun, J.; Liu, M.; Li, K.; Dong, L. Long Non-coding RNA MALAT1 Inhibits Neuron Apoptosis and Neuroinflammation While Stimulates Neurite Outgrowth and Its Correlation With MiR-125b Mediates PTGS2, CDK5 and FOXQ1 in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 596–612. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, G.; Wang, G.; Zhang, F. Oxidative Stress and Neuroinflammation Potentiate Each Other to Promote Progression of Dopamine Neurodegeneration. Oxid. Med. Cell Longev. 2020, 2020, 6137521. [Google Scholar] [CrossRef]
- Qiao, Y.; Peng, C.; Li, J.; Wu, D.; Wang, X. LncRNA MALAT1 is Neuroprotective in a Rat Model of Spinal Cord Ischemia-Reperfusion Injury Through miR-204 Regulation. Curr. Neurovasc Res. 2018, 15, 211–219. [Google Scholar] [CrossRef]
- Hu, J.; Huang, K.; Bao, F.; Zhong, S.; Fan, Q.; Li, W. Low-dose lipopolysaccharide inhibits spinal cord injury-induced neuronal apoptosis by regulating autophagy through the lncRNA MALAT1/Nrf2 axis. PeerJ 2023, 11, e15919. [Google Scholar] [CrossRef]
- Guan, C.; Wang, Y. LncRNA CASC9 attenuates lactate dehydrogenase-mediated oxidative stress and inflammation in spinal cord injury via sponging miR-383-5p. Inflammation 2021, 44, 923–933. [Google Scholar] [CrossRef]
- Li, X.; Qian, Y.; Tang, K.; Li, Y.; Tao, R.; Gong, C.; Huang, L.; Zou, K.; Liu, L. Inhibition of lncRNA H19/miR-370-3p pathway mitigates neuronal apoptosis in an in vitro model of spinal cord injury (SCI). Transl. Neurosci. 2021, 12, 103–113. [Google Scholar] [CrossRef]
- Sahni, V.; Kessler, J.A. Stem cell therapies for spinal cord injury. Nat. Rev. Neurol. 2010, 6, 363–372. [Google Scholar] [CrossRef]
- Valle-Prieto, A.; Conget, P.A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010, 19, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Konyalioglu, S.; Armagan, G.; Yalcin, A.; Atalayin, C.; Dagci, T. Effects of resveratrol on hydrogen peroxide-induced oxidative stress in embryonic neural stem cells. Neural Regen. Res. 2013, 8, 485–495. [Google Scholar] [PubMed]
- Madhavan, L.; Ourednik, V.; Ourednik, J. Neural stem/progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. Stem Cells 2008, 26, 254–265. [Google Scholar] [CrossRef]
- Santos, M.F.D.; Roxo, C.; Solá, S. Oxidative-Signaling in Neural Stem Cell-Mediated Plasticity: Implications for Neurodegenerative Diseases. Antioxidants 2021, 10, 1088. [Google Scholar] [CrossRef]
- Fu, S.P.; Chen, S.Y.; Pang, Q.M.; Zhang, M.; Wu, X.C.; Wan, X.; Wan, W.H.; Ao, J.; Zhang, T. Advances in the research of the role of macrophage/microglia polarization-mediated inflammatory response in spinal cord injury. Front. Immunol. 2022, 13, 1014013. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhu, W.; Cao, K.; Wu, F.; Li, J.; Wang, G.; Li, H.; Lu, M.; Ren, Y.; He, X. Anti-Inflammatory Mechanism of Neural Stem Cell Transplantation in Spinal Cord Injury. Int. J. Mol. Sci. 2016, 17, 1380. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Yu, X.; Tian, N.; Liu, Y.; Yu, D. Identifying key antioxidative stress factors regulating Nrf2 in the genioglossus with human umbilical cord mesenchymal stem-cell therapy. Sci. Rep. 2024, 14, 5838. [Google Scholar] [CrossRef]
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef]
- Kim, G.U.; Sung, S.E.; Kang, K.K.; Choi, J.H.; Lee, S.; Sung, M.; Yang, S.Y.; Kim, S.K.; Kim, Y.I.; Lim, J.H.; et al. Therapeutic Potential of Mesenchymal Stem Cells (MSCs) and MSC-Derived Extracellular Vesicles for the Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 13672. [Google Scholar] [CrossRef]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef]
- Hernández, R.; Jiménez-Luna, C.; Perales-Adán, J.; Perazzoli, G.; Melguizo, C.; Prados, J. Differentiation of Human Mesenchymal Stem Cells towards Neuronal Lineage: Clinical Trials in Nervous System Disorders. Biomol. Ther. 2020, 28, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Cízková, D.; Rosocha, J.; Vanický, I.; Jergová, S.; Cízek, M. Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol. Neurobiol. 2006, 26, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xuan, A.; Chen, Y.; Zhang, J.; Xu, L.; Yan, Q.; Long, D. Combined effect of nerve growth factor and brain-derived neurotrophic factor on neuronal differentiation of neural stem cells and the potential molecular mechanisms. Mol. Med. Rep. 2014, 10, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Estirado, A.; Redondo, C.; Pacheco-Torres, J.; Sirerol-Piquer, M.S.; Garcia-Verdugo, J.M.; Martinez, S. Mesenchymal stem cells improve motor functions and decrease neurodegeneration in ataxic mice. Mol. Ther. 2015, 23, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.A.; El-Shamarka, M.; Khadrawy, Y.; El-Shebiney, S. New prospects of mesenchymal stem cells for ameliorating temporal lobe epilepsy. Inflammopharmacology 2018, 26, 963–972. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Zhang, X.; He, X.; Lai, L.; Liu, Y.; Zhu, G.; Li, W.; Li, H.; Fang, Q.; et al. The inhibitory effect of mesenchymal stem cell on blood-brain barrier disruption following intracerebral hemorrhage in rats: Contribution of TSG-6. J. Neuroinflammation 2015, 12, 61. [Google Scholar] [CrossRef]
- Ni, S.; Wang, D.; Qiu, X.; Pang, L.; Song, Z.; Guo, K. Bone marrow mesenchymal stem cells protect against bleomycin-induced pulmonary fibrosis in rat by activating Nrf2 signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 7752–7761. [Google Scholar]
- Deng, Y.B.; Liu, X.G.; Liu, Z.G.; Liu, X.L.; Liu, Y.; Zhou, G.Q. Implantation of BM mesenchymal stem cells into injured spinal cord elicits de novo neurogenesis and functional recovery: Evidence from a study in rhesus monkeys. Cytotherapy 2006, 8, 210–214. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Schwarz, E.J.; Hess, D.; Widenfalk, J.; El Manira, A.; Prockop, D.J.; Olson, L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA 2002, 99, 2199–2204. [Google Scholar] [CrossRef]
- Quertainmont, R.; Cantinieaux, D.; Botman, O.; Sid, S.; Schoenen, J.; Franzen, R. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS ONE 2012, 7, e39500. [Google Scholar] [CrossRef]
- Matyas, J.J.; Stewart, A.N.; Goldsmith, A.; Nan, Z.; Skeel, R.L.; Rossignol, J.; Dunbar, G.L. Effects of Bone-Marrow-Derived MSC Transplantation on Functional Recovery in a Rat Model of Spinal Cord Injury: Comparisons of Transplant Locations and Cell Concentrations. Cell Transpl. 2017, 26, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Gopinath, C.; Rao, N.M.; Banerjee, P.; Krishnamoorthy, V.; Venkataramana, N.K.; Totey, S. Functional recovery after transplantation of bone marrow-derived human mesenchymal stromal cells in a rat model of spinal cord injury. Cytotherapy 2010, 12, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, Y.; Yang, J.Y.; Liang, M.; Song, J. Treatment with bone marrow mesenchymal stem cells combined with plumbagin alleviates spinal cord injury by affecting oxidative stress, inflammation, apoptotis and the activation of the Nrf2 pathway. Int. J. Mol. Med. 2016, 37, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, L.; Zhang, K.; Gao, L.; Xiao, J.; Zhang, Y. Upregulation of microRNA-200a in bone marrow mesenchymal stem cells enhances the repair of spinal cord injury in rats by reducing oxidative stress and regulating Keap1/Nrf2 pathway. Artif. Organs 2020, 44, 744–752. [Google Scholar] [CrossRef]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef]
- Gao, S.; Guo, X.; Zhao, S.; Jin, Y.; Zhou, F.; Yuan, P.; Cao, L.; Wang, J.; Qiu, Y.; Sun, C.; et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. 2019, 10, 597. [Google Scholar] [CrossRef]
- Aras, Y.; Sabanci, P.A.; Kabatas, S.; Duruksu, G.; Subasi, C.; Erguven, M.; Karaoz, E. The Effects of Adipose Tissue-Derived Mesenchymal Stem Cell Transplantation During the Acute and Subacute Phases Following Spinal Cord Injury. Turk. Neurosurg. 2016, 26, 127–139. [Google Scholar] [CrossRef]
- Kolar, M.K.; Kingham, P.J.; Novikova, L.N.; Wiberg, M.; Novikov, L.N. The therapeutic effects of human adipose-derived stem cells in a rat cervical spinal cord injury model. Stem Cells Dev. 2014, 23, 1659–1674. [Google Scholar] [CrossRef]
- Ohta, Y.; Hamaguchi, A.; Ootaki, M.; Watanabe, M.; Takeba, Y.; Iiri, T.; Matsumoto, N.; Takenaga, M. Intravenous infusion of adipose-derived stem/stromal cells improves functional recovery of rats with spinal cord injury. Cytotherapy 2017, 19, 839–848. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, X.; Mo, B.; Xu, H.; Zhang, L.; Huang, L.; Yao, S.; Huang, Z.; Wang, Y.; Xie, H.; et al. Adipose mesenchymal stem cell transplantation alleviates spinal cord injury-induced neuroinflammation partly by suppressing the Jagged1/Notch pathway. Stem Cell Res. Ther. 2020, 11, 212. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Zang, G.; Wang, T.; Yu, Z.; Wang, S.; Tang, Z.; Liu, J. Adipose-derived stem cells improve erectile function partially through the secretion of IGF-1, bFGF, and VEGF in aged rats. Andrology 2018, 6, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Sung, P.H.; Sun, C.K.; Chen, C.H.; Chiang, H.J.; Huang, T.H.; Chen, Y.L.; Zhen, Y.Y.; Chai, H.T.; Chung, S.Y.; et al. Protective effect of melatonin-supported adipose-derived mesenchymal stem cells against small bowel ischemia-reperfusion injury in rat. J. Pineal Res. 2015, 59, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Shojafar, E.; Soleimani Mehranjani, M.; Shariatzadeh, S.M.A. Adipose-derived mesenchymal stromal cell transplantation at the graft site improves the structure and function of autografted mice ovaries: A stereological and biochemical analysis. Cytotherapy 2018, 20, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jo, S.H.; Kim, W.H.; Kweon, O.K. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res. Ther. 2015, 6, 229. [Google Scholar] [CrossRef]
- Khan, I.U.; Yoon, Y.; Kim, A.; Jo, K.R.; Choi, K.U.; Jung, T.; Kim, N.; Son, Y.; Kim, W.H.; Kweon, O.K. Improved Healing after the Co-Transplantation of HO-1 and BDNF Overexpressed Mesenchymal Stem Cells in the Subacute Spinal Cord Injury of Dogs. Cell Transpl. 2018, 27, 1140–1153. [Google Scholar] [CrossRef]
- Khan, I.U.; Yoon, Y.; Choi, K.U.; Jo, K.R.; Kim, N.; Lee, E.; Kim, W.H.; Kweon, O.K. Therapeutic Effects of Intravenous Injection of Fresh and Frozen Thawed HO-1-Overexpressed Ad-MSCs in Dogs with Acute Spinal Cord Injury. Stem Cells Int. 2019, 2019, 8537541. [Google Scholar] [CrossRef]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [CrossRef]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef]
- Ogawa, Y.; Sawamoto, K.; Miyata, T.; Miyao, S.; Watanabe, M.; Nakamura, M.; Bregman, B.S.; Koike, M.; Uchiyama, Y.; Toyama, Y.; et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. Res. 2002, 69, 925–933. [Google Scholar] [CrossRef]
- Tarasenko, Y.I.; Gao, J.; Nie, L.; Johnson, K.M.; Grady, J.J.; Hulsebosch, C.E.; McAdoo, D.J.; Wu, P. Human fetal neural stem cells grafted into contusion-injured rat spinal cords improve behavior. J. Neurosci. Res. 2007, 85, 47–57. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, Y.N.; Kim, Y.H.; Kim, Y.J.; Park, J.P.; Kwon, D.K.; Kwon, O.S.; Heo, J.H.; Kim, Y.H.; Ryu, S.; et al. Effects of human neural stem cell transplantation in canine spinal cord hemisection. Neurol. Res. 2009, 31, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.L.; Zhang, Y.P.; Howard, R.M.; Walters, W.M.; Tsoulfas, P.; Whittemore, S.R. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp. Neurol. 2001, 167, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Tator, C.H. Transplanted neural stem/progenitor cells generate myelinating oligodendrocytes and Schwann cells in spinal cord demyelination and dysmyelination. Exp. Neurol. 2008, 213, 176–190. [Google Scholar] [CrossRef]
- Eftekharpour, E.; Karimi-Abdolrezaee, S.; Wang, J.; El Beheiry, H.; Morshead, C.; Fehlings, M.G. Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction. J. Neurosci. 2007, 27, 3416–3428. [Google Scholar] [CrossRef]
- Nemati, S.N.; Jabbari, R.; Hajinasrollah, M.; Zare Mehrjerdi, N.; Azizi, H.; Hemmesi, K.; Moghiminasr, R.; Azhdari, Z.; Talebi, A.; Mohitmafi, S.; et al. Transplantation of adult monkey neural stem cells into a contusion spinal cord injury model in rhesus macaque monkeys. Cell J. 2014, 16, 117–130. [Google Scholar]
- Sankavaram, S.R.; Hakim, R.; Covacu, R.; Frostell, A.; Neumann, S.; Svensson, M.; Brundin, L. Adult Neural Progenitor Cells Transplanted into Spinal Cord Injury Differentiate into Oligodendrocytes, Enhance Myelination, and Contribute to Recovery. Stem Cell Rep. 2019, 12, 950–966. [Google Scholar] [CrossRef]
- Yuan, T.; Liu, Q.; Kang, J.; Gao, H.; Gui, S. High-Dose Neural Stem/Progenitor Cell Transplantation Increases Engraftment and Neuronal Distribution and Promotes Functional Recovery in Rats after Acutely Severe Spinal Cord Injury. Stem Cells Int. 2019, 2019, 9807978. [Google Scholar] [CrossRef]
- Willis, C.M.; Nicaise, A.M.; Peruzzotti-Jametti, L.; Pluchino, S. The neural stem cell secretome and its role in brain repair. Brain Res. 2020, 1729, 146615. [Google Scholar] [CrossRef]
- Semita, I.N.; Utomo, D.N.; Suroto, H.; Sudiana, I.K.; Gandi, P. The mechanism of human neural stem cell secretomes improves neuropathic pain and locomotor function in spinal cord injury rat models: Through antioxidant, anti-inflammatory, anti-matrix degradation, and neurotrophic activities. Korean J. Pain. 2023, 36, 72–83. [Google Scholar] [CrossRef]
- Kahroba, H.; Ramezani, B.; Maadi, H.; Sadeghi, M.R.; Jaberie, H.; Ramezani, F. The role of Nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res. Rev. 2021, 65, 101211. [Google Scholar] [CrossRef]
- Damdimopoulou, P.; Rodin, S.; Stenfelt, S.; Antonsson, L.; Tryggvason, K.; Hovatta, O. Human embryonic stem cells. Best. Pr. Res. Clin. Obs. Gynaecol. 2016, 31, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lopez, M.; Fernandez-Muñoz, B.; Canovas, S. Pluripotent Stem Cells for Spinal Cord Injury Repair. Cells 2021, 10, 3334. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, G.; Okada, Y.; Yamane, J.; Nagoshi, N.; Kitamura, K.; Mukaino, M.; Tsuji, O.; Fujiyoshi, K.; Katoh, H.; Okada, S.; et al. Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PLoS ONE 2009, 4, e7706. [Google Scholar] [CrossRef]
- Kimura, H.; Yoshikawa, M.; Matsuda, R.; Toriumi, H.; Nishimura, F.; Hirabayashi, H.; Nakase, H.; Kawaguchi, S.; Ishizaka, S.; Sakaki, T. Transplantation of embryonic stem cell-derived neural stem cells for spinal cord injury in adult mice. Neurol. Res. 2005, 27, 812–819. [Google Scholar] [CrossRef]
- McDonald, J.W.; Liu, X.Z.; Qu, Y.; Liu, S.; Mickey, S.K.; Turetsky, D.; Gottlieb, D.I.; Choi, D.W. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 1999, 5, 1410–1412. [Google Scholar] [CrossRef]
- Keirstead, H.S.; Nistor, G.; Bernal, G.; Totoiu, M.; Cloutier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005, 25, 4694–4705. [Google Scholar] [CrossRef]
- Sharp, J.; Frame, J.; Siegenthaler, M.; Nistor, G.; Keirstead, H.S. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 2010, 28, 152–163. [Google Scholar] [CrossRef]
- Pereira, S.L.; Grãos, M.; Rodrigues, A.S.; Anjo, S.I.; Carvalho, R.A.; Oliveira, P.J.; Arenas, E.; Ramalho-Santos, J. Inhibition of mitochondrial complex III blocks neuronal differentiation and maintains embryonic stem cell pluripotency. PLoS ONE 2013, 8, e82095. [Google Scholar]
- Chung, S.; Arrell, D.K.; Faustino, R.S.; Terzic, A.; Dzeja, P.P. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J. Mol. Cell Cardiol. 2010, 48, 725–734. [Google Scholar] [CrossRef]
- Trouillas, M.; Saucourt, C.; Guillotin, B.; Gauthereau, X.; Ding, L.; Buchholz, F.; Doss, M.X.; Sachinidis, A.; Hescheler, J.; Hummel, O.; et al. Three LIF-dependent signatures and gene clusters with atypical expression profiles, identified by transcriptome studies in mouse ES cells and early derivatives. BMC Genom. 2009, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Zhao, R.; West, J.A.; Yabuuchi, A.; Huo, H.; Ince, T.A.; Lerou, P.H.; Lensch, M.W.; Daley, G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008, 451, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Romanyuk, N.; Amemori, T.; Turnovcova, K.; Prochazka, P.; Onteniente, B.; Sykova, E.; Jendelova, P. Beneficial Effect of Human Induced Pluripotent Stem Cell-Derived Neural Precursors in Spinal Cord Injury Repair. Cell Transpl. 2015, 24, 1781–1797. [Google Scholar] [CrossRef]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E.; et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 16825–16830. [Google Scholar] [CrossRef]
- Kawabata, S.; Takano, M.; Numasawa-Kuroiwa, Y.; Itakura, G.; Kobayashi, Y.; Nishiyama, Y.; Sugai, K.; Nishimura, S.; Iwai, H.; Isoda, M.; et al. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ye, Z.; Jang, Y.Y. Roles of reactive oxygen species in the fate of stem cells. Antioxid. Redox Signal 2014, 20, 1881–1890. [Google Scholar] [CrossRef]
- Dannenmann, B.; Lehle, S.; Hildebrand, D.G.; Kübler, A.; Grondona, P.; Schmid, V.; Holzer, K.; Fröschl, M.; Essmann, F.; Rothfuss, O.; et al. High glutathione and glutathione peroxidase-2 levels mediate cell-type-specific DNA damage protection in human induced pluripotent stem cells. Stem Cell Rep. 2015, 4, 886–898. [Google Scholar] [CrossRef]
- Bonilla, P.; Hernandez, J.; Giraldo, E.; González-Pérez, M.A.; Alastrue-Agudo, A.; Elkhenany, H.; Vicent, M.J.; Navarro, X.; Edel, M.; Moreno-Manzano, V. Human-Induced Neural and Mesenchymal Stem Cell Therapy Combined with a Curcumin Nanoconjugate as a Spinal Cord Injury Treatment. Int. J. Mol. Sci. 2021, 22, 5966. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [PubMed]
- Familtseva, A.; Jeremic, N.; Tyagi, S.C. Exosomes: Cell-created drug delivery systems. Mol. Cell Biochem. 2019, 459, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Botchway, B.O.A.; Zhang, Y.; Yuan, J.; Liu, X. Combinational Treatment of Bioscaffolds and Extracellular Vesicles in Spinal Cord Injury. Front. Mol. Neurosci. 2019, 12, 81. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Romanelli, P.; Bieler, L.; Scharler, C.; Pachler, K.; Kreutzer, C.; Zaunmair, P.; Jakubecova, D.; Mrowetz, H.; Benedetti, B.; Rivera, F.J.; et al. Extracellular Vesicles Can Deliver Anti-inflammatory and Anti-scarring Activities of Mesenchymal Stromal Cells After Spinal Cord Injury. Front. Neurol. 2019, 10, 1225. [Google Scholar] [CrossRef]
- Lankford, K.L.; Arroyo, E.J.; Nazimek, K.; Bryniarski, K.; Askenase, P.W.; Kocsis, J.D. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS ONE 2018, 13, e0190358. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019, 10, 340. [Google Scholar] [CrossRef]
- Sun, G.; Li, G.; Li, D.; Huang, W.; Zhang, R.; Zhang, H.; Duan, Y.; Wang, B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 194–204. [Google Scholar] [CrossRef]
- Zhou, X.; Chu, X.; Yuan, H.; Qiu, J.; Zhao, C.; Xin, D.; Li, T.; Ma, W.; Wang, H.; Wang, Z.; et al. Mesenchymal stem cell derived EVs mediate neuroprotection after spinal cord injury in rats via the microRNA-21-5p/FasL gene axis. Biomed. Pharmacother. 2019, 115, 108818. [Google Scholar] [CrossRef]
- Bodega, G.; Alique, M.; Puebla, L.; Carracedo, J.; Ramírez, R.M. Microvesicles: ROS scavengers and ROS producers. J. Extracell. Vesicles 2019, 8, 1626654. [Google Scholar] [CrossRef] [PubMed]
- Yarana, C.; St Clair, D.K. Chemotherapy-Induced Tissue Injury: An Insight into the Role of Extracellular Vesicles-Mediated Oxidative Stress Responses. Antioxidants 2017, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Benedikter, B.J.; Weseler, A.R.; Wouters, E.F.M.; Savelkoul, P.H.M.; Rohde, G.G.U.; Stassen, F.R.M. Redox-dependent thiol modifications: Implications for the release of extracellular vesicles. Cell Mol. Life Sci. 2018, 75, 2321–2337. [Google Scholar] [CrossRef]
- Zhu, F.; Wei, C.; Wu, H.; Shuai, B.; Yu, T.; Gao, F.; Yuan, Y.; Zuo, D.; Liu, X.; Zhang, L.; et al. Hypoxic mesenchymal stem cell-derived exosomes alleviate ulcerative colitis injury by limiting intestinal epithelial cells reactive oxygen species accumulation and DNA damage through HIF-1α. Int. Immunopharmacol. 2022, 113, 109426. [Google Scholar] [CrossRef]
- Su, H.; Wang, Z.; Zhou, L.; Liu, D.; Zhang, N. Regulation of the Nrf2/HO-1 axis by mesenchymal stem cells-derived extracellular vesicles: Implications for disease treatment. Front. Cell Dev. Biol. 2024, 12, 1397954. [Google Scholar] [CrossRef]
- Wang, C.-C.; Hu, X.-M.; Long, Y.-F.; Huang, H.-R.; He, Y.; Xu, Z.-R.; Qi, Z.-Q. Treatment of Parkinson’s disease model with human umbilical cord mesenchymal stem cell-derived exosomes loaded with BDNF. Life Sci. 2024, 356, 123014. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Z.; Wu, Y.; Shi, Q.; Yang, E.; Zhang, B.; Qian, Y.; Lian, X.; Xu, J. ADSC-Exos enhance functional recovery after spinal cord injury by inhibiting ferroptosis and promoting the survival and function of endothelial cells through the NRF2/SLC7A11/GPX4 pathway. Biomed. Pharmacother. 2024, 172, 116225. [Google Scholar] [CrossRef]
- Chen, Y.; Li, B.; Quan, J.; Li, Z.; Li, Y.; Tang, Y. Inhibition of Ferroptosis by Mesenchymal Stem Cell-Derived Exosomes in Acute Spinal Cord Injury: Role of Nrf2/GCH1/BH4 Axis. Neurospine 2024, 21, 642–655. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, S.; Dong, L.; Wu, P.; Li, K.; Li, X.; Li, X.; Qian, H.; Fu, Q. A tannic acid doped hydrogel with small extracellular vesicles derived from mesenchymal stem cells promotes spinal cord repair by regulating reactive oxygen species microenvironment. Mater. Today Bio 2022, 16, 100425. [Google Scholar] [CrossRef]
- Zhang, L.; Han, P. Neural stem cell-derived exosomes suppress neuronal cell apoptosis by activating autophagy via miR-374-5p/STK-4 axis in spinal cord injury. J. Musculoskelet. Neuronal Interact. 2022, 22, 411–421. [Google Scholar]
- Patel, D.B.; Santoro, M.; Born, L.J.; Fisher, J.P.; Jay, S.M. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: Impact of the bioproduction microenvironment. Biotechnol. Adv. 2018, 36, 2051–2059. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chem. Int. Ed. Engl. 2018, 57, 9224–9237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Ma, H.; Zhen, M.; Guo, J.; Wang, L.; Jiang, L.; Shu, C.; Wang, C. Biocompatible [60]/[70] Fullerenols: Potent Defense against Oxidative Injury Induced by Reduplicative Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 35539–35547. [Google Scholar] [CrossRef]
- Basso, A.S.; Frenkel, D.; Quintana, F.J.; Costa-Pinto, F.A.; Petrovic-Stojkovic, S.; Puckett, L.; Monsonego, A.; Bar-Shir, A.; Engel, Y.; Gozin, M.; et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J. Clin. Invest. 2008, 118, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shen, X.; Xing, D. Carbon quantum dots as ROS-generator and -scavenger: A comprehensive review. Dye. Pigment. 2023, 208, 110784. [Google Scholar] [CrossRef]
- Tang, N.; Ding, Z.; Zhang, J.; Cai, Y.; Bao, X. Recent advances of antioxidant low-dimensional carbon materials for biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1121477. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Lin, F.; Liu, Y.; Gu, R.; Liu, W.; Xiao, C. Selenium-Doped Carbon Quantum Dots Efficiently Ameliorate Secondary Spinal Cord Injury via Scavenging Reactive Oxygen Species. Int. J. Nanomed. 2020, 15, 10113–10125. [Google Scholar] [CrossRef]
- Mattson, M.P.; Haddon, R.C.; Rao, A.M. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J. Mol. Neurosci. 2000, 14, 175–182. [Google Scholar] [CrossRef]
- Galano, A. Influence of diameter, length, and chirality of single-walled carbon nanotubes on their free radical scavenging capability. J. Phys. Chem. C 2009, 113, 18487–18491. [Google Scholar] [CrossRef]
- Lucente-Schultz, R.M.; Moore, V.C.; Leonard, A.D.; Price, B.K.; Kosynkin, D.V.; Lu, M.; Partha, R.; Conyers, J.L.; Tour, J.M. Antioxidant single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 3934–3941. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.; Yoon, O.J.; Kim, H.W.; Lee, D.Y.; Kim, D.H.; Lee, W.B.; Lee, N.E.; Bonventre, J.V.; Kim, S.S. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat. Nanotechnol. 2011, 6, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.H.; Park, J.H.; Son, J.I.; Kim, K.Y.; Lee, H.J. Both Intracranial and Intravenous Administration of Functionalized Carbon Nanotubes Protect Dopaminergic Neuronal Death from 6-Hydroxydopamine. Int. J. Nanomed. 2020, 15, 7615–7626. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.; Shakyba, S.; Ghaderi, M.; Kazemi, K.; Fagheh, A.F.; Farsinejad, P.; Khosravi, A.; Louyeh, P.A.; Mirzaeyian, E.; Chamanara, M.; et al. Ivermectin-functionalized multiwall carbon nanotube enhanced the locomotor activity and neuropathic pain by modulating M1/M2 macrophage and decrease oxidative stress in rat model of spinal cord injury. Heliyon 2021, 7, e07311. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Dong, Z.; Liang, C.; Chen, M.; Tao, D.; Cheng, L.; Yang, K.; Liu, Z. Iridium nanocrystals encapsulated liposomes as near-infrared light controllable nanozymes for enhanced cancer radiotherapy. Biomaterials 2018, 181, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A Review on Metal- and Metal Oxide-Based Nanozymes: Properties, Mechanisms, and Applications. Nanomicro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Wang, J.; Sun, K.; Si, Y. Metal and metal-oxide nanozymes: Bioenzymatic characteristics, catalytic mechanism, and eco-environmental applications. Nanoscale 2019, 11, 15783–15793. [Google Scholar] [CrossRef]
- Saifi, M.A.; Seal, S.; Godugu, C. Nanoceria, the versatile nanoparticles: Promising biomedical applications. J. Control. Release 2021, 338, 164–189. [Google Scholar] [CrossRef]
- Kim, J.W.; Mahapatra, C.; Hong, J.Y.; Kim, M.S.; Leong, K.W.; Kim, H.W.; Hyun, J.K. Functional Recovery of Contused Spinal Cord in Rat with the Injection of Optimal-Dosed Cerium Oxide Nanoparticles. Adv. Sci. 2017, 4, 1700034. [Google Scholar] [CrossRef]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Luo, S.; Gao, J.; Yuan, H.; Yang, J.; Fan, Y.; Wang, L.; Ouyang, H.; Fu, Z. Mn Single-Atom Nanozymes with Superior Loading Capability and Superb Superoxide Dismutase-like Activity for Bioassay. Anal. Chem. 2023, 95, 9366–9372. [Google Scholar] [CrossRef]
- Wang, X.; Ren, X.; Yang, J.; Zhao, Z.; Zhang, X.; Yang, F.; Zhang, Z.; Chen, P.; Li, L.; Zhang, R. Mn-single-atom nano-multizyme enabled NIR-II photoacoustically monitored, photothermally enhanced ROS storm for combined cancer therapy. Biomater. Res. 2023, 27, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, Z.; An, J.; Wu, Q.; Tong, L.; Mei, X.; Tian, H.; Wu, C. Chitosan-modified hollow manganese dioxide nanoparticles loaded with resveratrol for the treatment of spinal cord injury. Drug Deliv. 2022, 29, 2498–2512. [Google Scholar] [CrossRef] [PubMed]
- Valluru, L.; Diao, Y.; Hachmeister, J.E.; Liu, D. Mn (III) tetrakis (4-benzoic acid) porphyrin protects against neuronal and glial oxidative stress and death after spinal cord injury. CNS Neurol. Disord. Drug Targets 2012, 11, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Spasojevic, I.; Warner, D.S.; Batinic-Haberle, I. Mouse spinal cord compression injury is ameliorated by intrathecal cationic manganese(III) porphyrin catalytic antioxidant therapy. Neurosci. Lett. 2004, 366, 220–225. [Google Scholar] [CrossRef]
- Hachmeister, J.E.; Valluru, L.; Bao, F.; Liu, D. Mn (III) tetrakis (4-benzoic acid) porphyrin administered into the intrathecal space reduces oxidative damage and neuron death after spinal cord injury: A comparison with methylprednisolone. J. Neurotrauma 2006, 23, 1766–1778. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Pal, A.; Singh, A.; Nag, T.C.; Chattopadhyay, P.; Mathur, R.; Jain, S. Iron oxide nanoparticles and magnetic field exposure promote functional recovery by attenuating free radical-induced damage in rats with spinal cord transection. Int. J. Nanomed. 2013, 8, 2259–2272. [Google Scholar]
- Tripathi, R.M.; Ahn, D.; Kim, Y.M.; Chung, S.J. Enzyme Mimetic Activity of ZnO-Pd Nanosheets Synthesized via a Green Route. Molecules 2020, 25, 2585. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Yin, S.; Jiang, Y.; Shao, L. Protective effect of zinc oxide nanoparticles on spinal cord injury. Front. Pharmacol. 2022, 13, 990586. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, H.S.; Xu, C.; Zhou, Z.P.; Wang, D.H.; Chen, S.R.; Mei, X.F. Bioengineered Zinc Oxide Nanoparticle-Loaded Hydrogel for Combinative Treatment of Spinal Cord Transection. Front. Bioeng. Biotechnol. 2021, 9, 796361. [Google Scholar] [CrossRef]
- Lou-Franco, J.; Das, B.; Elliott, C.; Cao, C. Gold Nanozymes: From Concept to Biomedical Applications. Nanomicro Lett. 2020, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, D.; Zhou, Z.; Xu, C.; Mei, X.; Tian, H. Therapy of spinal cord injury by zinc modified gold nanoclusters via immune-suppressing strategies. J. Nanobiotechnology 2021, 19, 281. [Google Scholar] [CrossRef]
- Jiang, Y.; Rong, H.; Wang, Y.; Liu, S.; Xu, P.; Luo, Z.; Guo, L.; Zhu, T.; Rong, H.; Wang, D.; et al. Single-atom cobalt nanozymes promote spinal cord injury recovery by anti-oxidation and neuroprotection. Nano Res. 2023, 16, 9752–9759. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, B.; Lu, M.; Li, S.; Guo, J.; Chen, F.; Xiong, X.; Yin, Z.; Liu, H.; Zhou, D. Ultrasmall Fe-doped carbon dots nanozymes for photoenhanced antibacterial therapy and wound healing. Bioact. Mater. 2022, 12, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Savi, M.; Bocchi, L.; Cacciani, F.; Vilella, R.; Buschini, A.; Perotti, A.; Galati, S.; Montalbano, S.; Pinelli, S.; Frati, C.; et al. Cobalt oxide nanoparticles induce oxidative stress and alter electromechanical function in rat ventricular myocytes. Part. Fibre Toxicol. 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, H.; Phua, S.Z.F.; Yang, G.; Qi Lim, W.; Gu, L.; Qian, C.; Wang, H.; Guo, Z.; Chen, H.; et al. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat. Commun. 2020, 11, 357. [Google Scholar] [CrossRef]
- Feng, K.; Wang, G.; Wang, S.; Ma, J.; Wu, H.; Ma, M.; Zhang, Y. Breaking the pH Limitation of Nanozymes: Mechanisms, Methods, and Applications. Adv. Mater. 2024, 36, e2401619. [Google Scholar] [CrossRef]
- Bu, J.-W.; Wang, Z.-G.; Liu, H.-Y.; Liu, S.-L. Metal nanozymes modulation of reactive oxygen species as promising strategies for cancer therapy. Int. J. Pharm. 2024, 662, 124453. [Google Scholar] [CrossRef]
- Getachew, G.; Korupalli, C.; Rasal, A.S.; Chang, J.-Y. ROS generation/scavenging modulation of carbon dots as phototherapeutic candidates and peroxidase mimetics to integrate with polydopamine nanoparticles/GOx towards cooperative cancer therapy. Compos. Part. B Eng. 2021, 226, 109364. [Google Scholar] [CrossRef]
- Hsieh, H.-S.; Wu, R.; Jafvert, C.T. Light-Independent Reactive Oxygen Species (ROS) Formation through Electron Transfer from Carboxylated Single-Walled Carbon Nanotubes in Water. Environ. Sci. Technol. 2014, 48, 11330–11336. [Google Scholar] [CrossRef]
- Du, Z.; Gao, N.; Wang, X.; Ren, J.; Qu, X. Near-Infrared Switchable Fullerene-Based Synergy Therapy for Alzheimer’s Disease. Small 2018, 14, e1801852. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ciciriello, A.J.; Dumont, C.M.; Pearson, R.M. Nanoparticle-Based Delivery to Treat Spinal Cord Injury-a Mini-review. AAPS PharmSciTech 2021, 22, 101. [Google Scholar] [CrossRef]
- Nukolova, N.V.; Aleksashkin, A.D.; Abakumova, T.O.; Morozova, A.Y.; Gubskiy, I.L.; Kirzhanova, E.; Abakumov, M.A.; Chekhonin, V.P.; Klyachko, N.L.; Kabanov, A.V. Multilayer polyion complex nanoformulations of superoxide dismutase 1 for acute spinal cord injury. J. Control Release 2018, 270, 226–236. [Google Scholar] [CrossRef]
- Li, J.; Wei, J.; Wan, Y.; Du, X.; Bai, X.; Li, C.; Lin, Y.; Liu, Z.; Zhou, M.; Zhong, Z. TAT-modified tetramethylpyrazine-loaded nanoparticles for targeted treatment of spinal cord injury. J. Control Release 2021, 335, 103–116. [Google Scholar] [CrossRef]
- Gao, X.; Han, Z.; Huang, C.; Lei, H.; Li, G.; Chen, L.; Feng, D.; Zhou, Z.; Shi, Q.; Cheng, L.; et al. An anti-inflammatory and neuroprotective biomimetic nanoplatform for repairing spinal cord injury. Bioact. Mater. 2022, 18, 569–582. [Google Scholar] [CrossRef]
- Li, T.; Liu, Z.; Wang, J.; Ye, H.; Wan, Y.; Du, X.; Sun, X.; Zhou, M.; Lin, Y.; Jing, P.; et al. Nanoformulated metformin enhanced the treatment of spinal cord injury. Chem. Eng. J. 2022, 446, 137227. [Google Scholar] [CrossRef]
- Rao, S.; Lin, Y.; Du, Y.; He, L.; Huang, G.; Chen, B.; Chen, T. Designing multifunctionalized selenium nanoparticles to reverse oxidative stress-induced spinal cord injury by attenuating ROS overproduction and mitochondria dysfunction. J. Mater. Chem. B 2019, 7, 2648–2656. [Google Scholar] [CrossRef]
- Shi, H.; Jin, L.; Li, J.; Liang, K.; Li, X.; Ye, Z.; Zhu, X.; Oliveira, J.M.; Reis, R.L.; Mao, Z.; et al. Mesoporous polydopamine nanoparticles for sustained release of rapamycin and reactive oxygen species scavenging to synergistically accelerate neurogenesis after spinal cord injury. J. Mater. Chem. B 2022, 10, 6351–6359. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Jiang, D.; Hu, S.; Yang, Z.; Zhang, Z.; Li, W.; Cai, W.; Liu, D. Polydopamine-Decorated Microcomposites Promote Functional Recovery of an Injured Spinal Cord by Inhibiting Neuroinflammation. ACS Appl. Mater. Interfaces 2021, 13, 47341–47353. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Caron, I.; Erba, E.; Panini, N.; De Paola, M.; Mariani, A.; Colombo, C.; Ferrari, R.; Pozzer, D.; Zanier, E.R.; et al. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials 2016, 75, 13–24. [Google Scholar] [CrossRef]
- Ma, D.; Shen, H.; Chen, F.; Liu, W.; Zhao, Y.; Xiao, Z.; Wu, X.; Chen, B.; Lu, J.; Shao, D.; et al. Inflammatory Microenvironment-Responsive Nanomaterials Promote Spinal Cord Injury Repair by Targeting IRF5. Adv. Heal. Mater. 2022, 11, e2201319. [Google Scholar] [CrossRef]
- Gao, W.; Li, J. Targeted siRNA delivery reduces nitric oxide mediated cell death after spinal cord injury. J. Nanobiotechnology 2017, 15, 38. [Google Scholar] [CrossRef]
- Javdani, M.; Habibi, A.; Shirian, S.; Kojouri, G.A.; Hosseini, F. Effect of Selenium Nanoparticle Supplementation on Tissue Inflammation, Blood Cell Count, and IGF-1 Levels in Spinal Cord Injury-Induced Rats. Biol. Trace Elem. Res. 2019, 187, 202–211. [Google Scholar] [CrossRef]
- Liu, X.; Mao, Y.; Huang, S.; Li, W.; Zhang, W.; An, J.; Jin, Y.; Guan, J.; Wu, L.; Zhou, P. Selenium nanoparticles derived from Proteus mirabilis YC801 alleviate oxidative stress and inflammatory response to promote nerve repair in rats with spinal cord injury. Regen. Biomater. 2022, 9, rbac042. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: The problems and the possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Liao, Z.; Wang, C.; Liu, Y.; Feng, S.; Jiang, X.; Chang, J. PEGlated magnetic polymeric liposome anchored with TAT for delivery of drugs across the blood-spinal cord barrier. Biomaterials 2010, 31, 6589–6596. [Google Scholar] [CrossRef]
- Tang, W.; Yang, Y.; Yang, L.; Tang, M.; Chen, Y.; Li, C. Macrophage membrane-mediated targeted drug delivery for treatment of spinal cord injury regardless of the macrophage polarization states. Asian J. Pharm. Sci. 2021, 16, 459–470. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Jiang, X.; Wang, Z.; Li, Y.; Zou, Z.; Wu, Q.; Tong, L.; Mei, X.; Tian, H.; Wu, C. Codelivery of minocycline hydrochloride and dextran sulfate via bionic liposomes for the treatment of spinal cord injury. Int. J. Pharm. 2022, 628, 122285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Xiong, J.; Xu, H.; Xiang, G.; Fan, M.; Zhou, K.; Lin, Y.; Chen, X.; Xie, L.; et al. Delivery of pOXR1 through an injectable liposomal nanoparticle enhances spinal cord injury regeneration by alleviating oxidative stress. Bioact. Mater. 2021, 6, 3177–3191. [Google Scholar] [CrossRef] [PubMed]
- Matyash, M.; Despang, F.; Mandal, R.; Fiore, D.; Gelinsky, M.; Ikonomidou, C. Novel soft alginate hydrogel strongly supports neurite growth and protects neurons against oxidative stress. Tissue Eng. Part. A 2012, 18, 55–66. [Google Scholar] [CrossRef]
- Sitoci-Ficici, K.H.; Matyash, M.; Uckermann, O.; Galli, R.; Leipnitz, E.; Later, R.; Ikonomidou, C.; Gelinsky, M.; Schackert, G.; Kirsch, M. Non-functionalized soft alginate hydrogel promotes locomotor recovery after spinal cord injury in a rat hemimyelonectomy model. Acta Neurochir. 2018, 160, 449–457. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, T.; Ding, J.; Gu, H.; Wang, Q.; Wang, Y.; Zhang, D.; Gao, C. A reactive oxygen species-responsive hydrogel encapsulated with bone marrow derived stem cells promotes repair and regeneration of spinal cord injury. Bioact. Mater. 2023, 19, 550–568. [Google Scholar] [CrossRef]
- Gupta, P.; Jordan, C.T.; Mitov, M.I.; Butterfield, D.A.; Hilt, J.Z.; Dziubla, T.D. Controlled curcumin release via conjugation into PBAE nanogels enhances mitochondrial protection against oxidative stress. Int. J. Pharm. 2016, 511, 1012–1021. [Google Scholar] [CrossRef]
- Wattamwar, P.P.; Biswal, D.; Cochran, D.B.; Lyvers, A.C.; Eitel, R.E.; Anderson, K.W.; Hilt, J.Z.; Dziubla, T.D. Synthesis and characterization of poly(antioxidant β-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomater. 2012, 8, 2529–2537. [Google Scholar] [CrossRef]
- Gupta, P.; Authimoolam, S.P.; Hilt, J.Z.; Dziubla, T.D. Quercetin conjugated poly(β-amino esters) nanogels for the treatment of cellular oxidative stress. Acta Biomater. 2015, 27, 194–204. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hwang, C.H.; Kim, H.E.; Jeong, S.H. Enhancement of bio-stability and mechanical properties of hyaluronic acid hydrogels by tannic acid treatment. Carbohydr. Polym. 2018, 186, 290–298. [Google Scholar] [CrossRef]
- Ghosh, B.; Nong, J.; Wang, Z.; Urban, M.W.; Heinsinger, N.M.; Trovillion, V.A.; Wright, M.C.; Lepore, A.C.; Zhong, Y. A hydrogel engineered to deliver minocycline locally to the injured cervical spinal cord protects respiratory neural circuitry and preserves diaphragm function. Neurobiol. Dis. 2019, 127, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhou, L.; Tang, J.; Xu, Y.; Wang, W.; Shi, W.; Li, Z.; Zhang, L.; Ding, Z.; Xi, K.; et al. Reactive Oxygen Species-Responsive Composite Fibers Regulate Oxidative Metabolism through Internal and External Factors to Promote the Recovery of Nerve Function. Small 2024, 20, e2401241. [Google Scholar] [CrossRef] [PubMed]
- Lajmiri, E.; Javdani, M.; Khosravian, P.; Hashemnia, M.; Kazemi Mehrjerdi, H. Preparation and evaluation of controlled released implant containing mesoporous selenium nanoparticles loaded with curcumin in rats with spinal cord injury. Vet. Res. Forum 2024, 15, 357–367. [Google Scholar] [PubMed]
- Deng, B.; Jiang, S.; Liu, G.; Li, X.; Zhao, Y.; Fan, X.; Ren, J.; Ning, C.; Xu, L.; Ji, L.; et al. Tetramethylpyrazine-loaded electroconductive hydrogels promote tissue repair after spinal cord injury by protecting the blood-spinal cord barrier and neurons. J. Mater. Chem. B 2024, 12, 4409–4426. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Chen, J.; Yang, B.; Shu, J.; Cheng, F.; Tao, Y.; Shi, K.; Wang, C.; Wang, J.; et al. Controlled extracellular vesicles release from aminoguanidine nanoparticle-loaded polylysine hydrogel for synergistic treatment of spinal cord injury. J. Control Release 2023, 363, 27–42. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Gu, Y.; Liu, T.; Chen, L. Biomineralized MnO2 Nanoparticle-Constituted Hydrogels Promote Spinal Cord Injury Repair by Modulating Redox Microenvironment and Inhibiting Ferroptosis. Pharmaceutics 2024, 16, 1057. [Google Scholar] [CrossRef]
- Liu, D.; Lu, G.; Shi, B.; Ni, H.; Wang, J.; Qiu, Y.; Yang, L.; Zhu, Z.; Yi, X.; Du, X.; et al. ROS-Scavenging Hydrogels Synergize with Neural Stem Cells to Enhance Spinal Cord Injury Repair via Regulating Microenvironment and Facilitating Nerve Regeneration. Adv. Heal. Mater. 2023, 12, e2300123. [Google Scholar] [CrossRef]
- Qin, T.; Li, C.; Xu, Y.; Qin, Y.; Jin, Y.; He, R.; Luo, Z.; Zhao, J.; Duan, C.; Lu, H.; et al. Local delivery of EGFR+NSCs-derived exosomes promotes neural regeneration post spinal cord injury via miR-34a-5p/HDAC6 pathway. Bioact. Mater. 2024, 33, 424–443. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, B.; Ding, J.; Yan, L.; Thawani, J.P.; Fu, C.; Chen, X. Polymer scaffolds facilitate spinal cord injury repair. Acta Biomater. 2019, 88, 57–77. [Google Scholar] [CrossRef]
- Zafar, M.S.; Quarta, A.; Marradi, M.; Ragusa, A. Recent Developments in the Reduction of Oxidative Stress through Antioxidant Polymeric Formulations. Pharmaceutics 2019, 11, 505. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.-J.; Li, W.-S.; Xu, X.-L.; Hu, J.-B.; Kang, X.-Q.; Qi, J.; Ying, X.-Y.; You, J.; Du, Y.-Z. Polycaprolactone/polysialic acid hybrid, multifunctional nanofiber scaffolds for treatment of spinal cord injury. Acta Biomater. 2018, 77, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Qi, Z.; Li, Q.; Ma, Y.; Fu, C.; Zheng, S.; Kong, W.; Liu, Q.; Yang, X. Graphene oxide-PLGA hybrid nanofibres for the local delivery of IGF-1 and BDNF in spinal cord repair. Artif. Cells Nanomed. Biotechnol. 2019, 47, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Ibrahim, S.; El-Amir, A.; El-Rafei, A.M.; Allam, N.K.; Abdellatif, A. Genistein Loaded Nanofibers Protect Spinal Cord Tissue Following Experimental Injury in Rats. Biomedicines 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wu, J.; Mu, J.; Lin, L.; Zhang, X.; Huang, T.; Ma, T.; Zhu, M.; Dai, X.; Wang, X.; et al. ROS filter coating scaffold protects 3D mesenchymal stem cell spheroids for dual-phase treatment of spinal cord injury. Chem. Eng. J. 2023, 462, 142192. [Google Scholar] [CrossRef]
- Cizkova, D.; Novotna, I.; Slovinska, L.; Vanicky, I.; Jergova, S.; Rosocha, J.; Radonak, J. Repetitive intrathecal catheter delivery of bone marrow mesenchymal stromal cells improves functional recovery in a rat model of contusive spinal cord injury. J. Neurotrauma 2011, 28, 1951–1961. [Google Scholar] [CrossRef]
- Cizkova, D.; Cubinkova, V.; Smolek, T.; Murgoci, A.-N.; Danko, J.; Vdoviakova, K.; Humenik, F.; Cizek, M.; Quanico, J.; Fournier, I. Localized intrathecal delivery of mesenchymal stromal cells conditioned medium improves functional recovery in a rat model of spinal cord injury. Int. J. Mol. Sci. 2018, 19, 870. [Google Scholar] [CrossRef]
- Martin Bauknight, W.; Chakrabarty, S.; Hwang, B.Y.; Malone, H.R.; Joshi, S.; Bruce, J.N.; Sander Connolly, E.; Winfree, C.J.; Cunningham, M.G.; Martin, J.H.; et al. Convection enhanced drug delivery of BDNF through a microcannula in a rodent model to strengthen connectivity of a peripheral motor nerve bridge model to bypass spinal cord injury. J. Clin. Neurosci. 2012, 19, 563–569. [Google Scholar] [CrossRef]
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706. [Google Scholar] [CrossRef]
- Fitch, M.T.; Doller, C.; Combs, C.K.; Landreth, G.E.; Silver, J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: In vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 1999, 19, 8182–8198. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Connor, J.R.; Menzies, S.L. Relationship of iron to oligodendrocytes and myelination. Glia 1996, 17, 83–93. [Google Scholar] [CrossRef]
- Smith, K.J.; Kapoor, R.; Felts, P.A. Demyelination: The role of reactive oxygen and nitrogen species. Brain Pathol. 1999, 9, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Guha, L.; Singh, N.; Kumar, H. Different Ways to Die: Cell Death Pathways and Their Association With Spinal Cord Injury. Neurospine 2023, 20, 430–448. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; López, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal 2014, 21, 1766–1801. [Google Scholar] [CrossRef]
- Arlotta, P. Organoids required! A new path to understanding human brain development and disease. Nat. Methods 2018, 15, 27–29. [Google Scholar] [CrossRef]
- Xue, W.; Li, B.; Liu, H.; Xiao, Y.; Li, B.; Ren, L.; Li, H.; Shao, Z. Generation of dorsoventral human spinal cord organoids via functionalizing composite scaffold for drug testing. iScience 2023, 26, 105898. [Google Scholar] [CrossRef]
- Andersen, J.; Revah, O.; Miura, Y.; Thom, N.; Amin, N.D.; Kelley, K.W.; Singh, M.; Chen, X.; Thete, M.V.; Walczak, E.M.; et al. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell 2020, 183, 1913–1929.e1926. [Google Scholar] [CrossRef]
- Xu, J.; Fang, S.; Deng, S.; Li, H.; Lin, X.; Huang, Y.; Chung, S.; Shu, Y.; Shao, Z. Generation of neural organoids for spinal-cord regeneration via the direct reprogramming of human astrocytes. Nat. Biomed. Eng. 2023, 7, 253–269. [Google Scholar] [CrossRef]
- Yuan, T.Y.; Zhang, J.; Yu, T.; Wu, J.P.; Liu, Q.Y. 3D Bioprinting for Spinal Cord Injury Repair. Front. Bioeng. Biotechnol. 2022, 10, 847344. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, S.; Wen, H.; Xiao, L.; Deng, Z.; Huang, J.; Zhang, Z. Coaxial 3D printing of hierarchical structured hydrogel scaffolds for on-demand repair of spinal cord injury. Acta Biomater. 2023, 168, 400–415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).