Chick Early Amniotic Fluid Alleviates Dextran-Sulfate-Sodium-Induced Colitis in Mice via T-Cell Receptor Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Experimental Animals
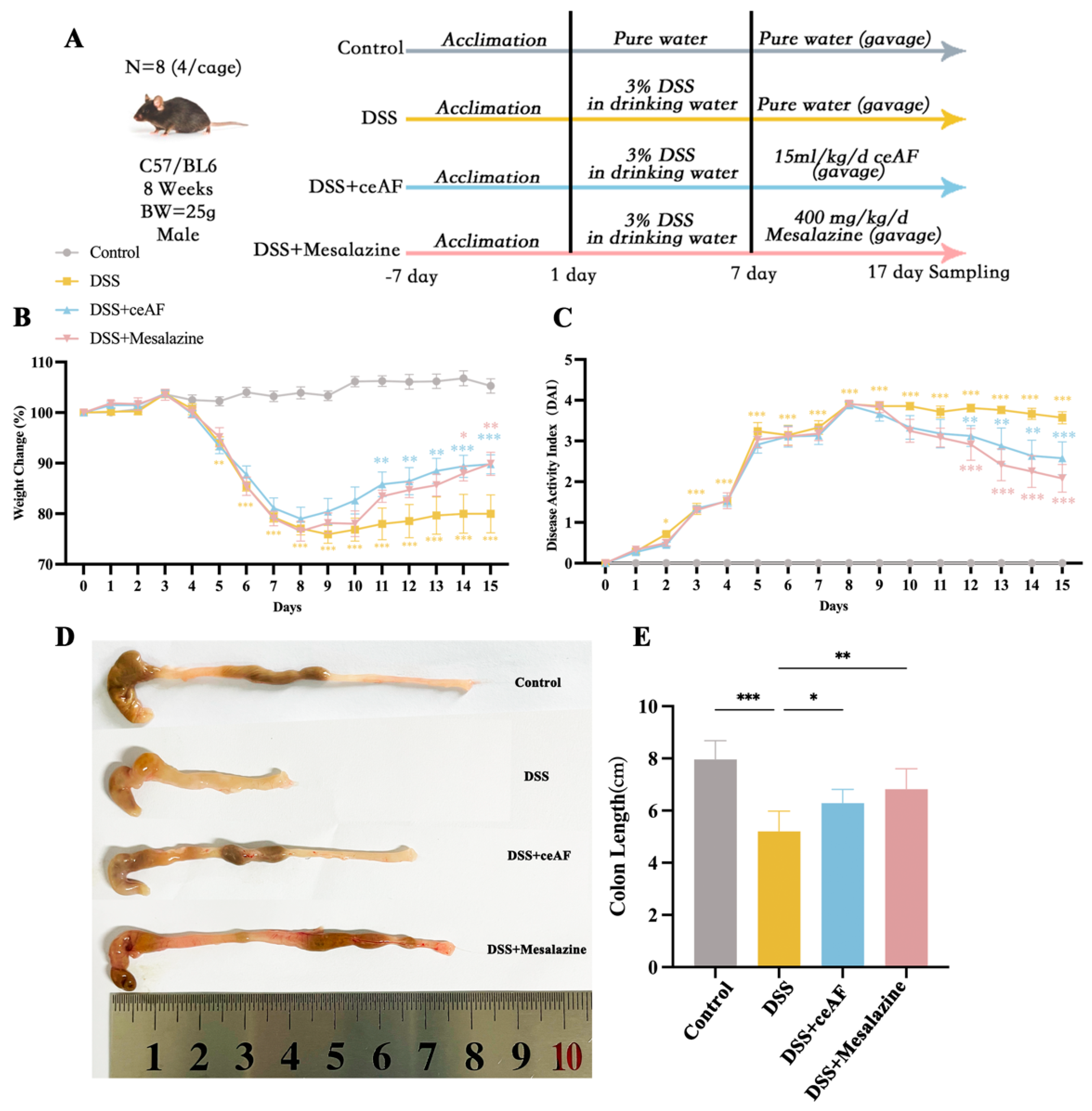
2.3. Experimental Design
2.4. Reagents and Antibodies
2.5. Chick Early Amniotic Fluid (ceAF) Preparation
2.6. Chemical Composition and Fatty Acid Content Analysis of ceAF
2.7. Observation of Signs and Disease Activity Index (DAI) in Mice
2.8. Histomorphopathologic Analysis
2.9. Immunohistochemical Analysis
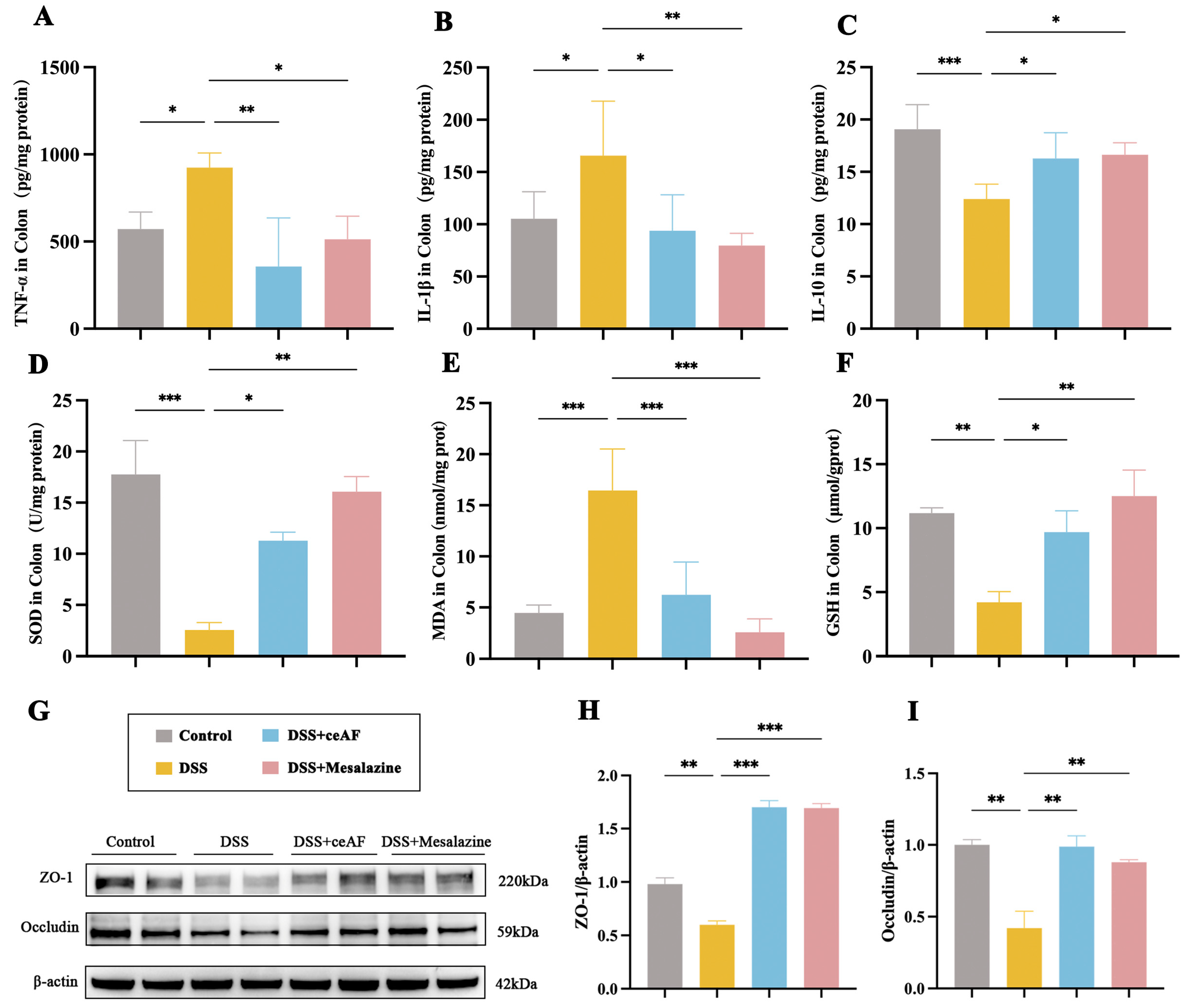
2.10. Determination of Inflammatory Factors and Antioxidant Indicators
2.11. Cell Viability Assay
2.12. Quantitative RT-PCR (qPCR)
2.13. RNA-Sequencing Analysis
2.14. Transfection with Lck Plasmid
2.15. Western Blotting Assays
2.16. Statistical Analysis
3. Results
3.1. Chemical Composition of ceAF
3.2. Anti-Inflammatory Effect of ceAF in DSS-Induced Caco-2 Cells
3.3. Effect of ceAF on DSS-Induced Pathological Parameters in UC Mice
3.4. ceAF Attenuates DSS-Induced Intestinal Inflammation and Oxidative Damage
3.5. General Characteristics of ceAF vs. DSS Transcriptomic Analysis
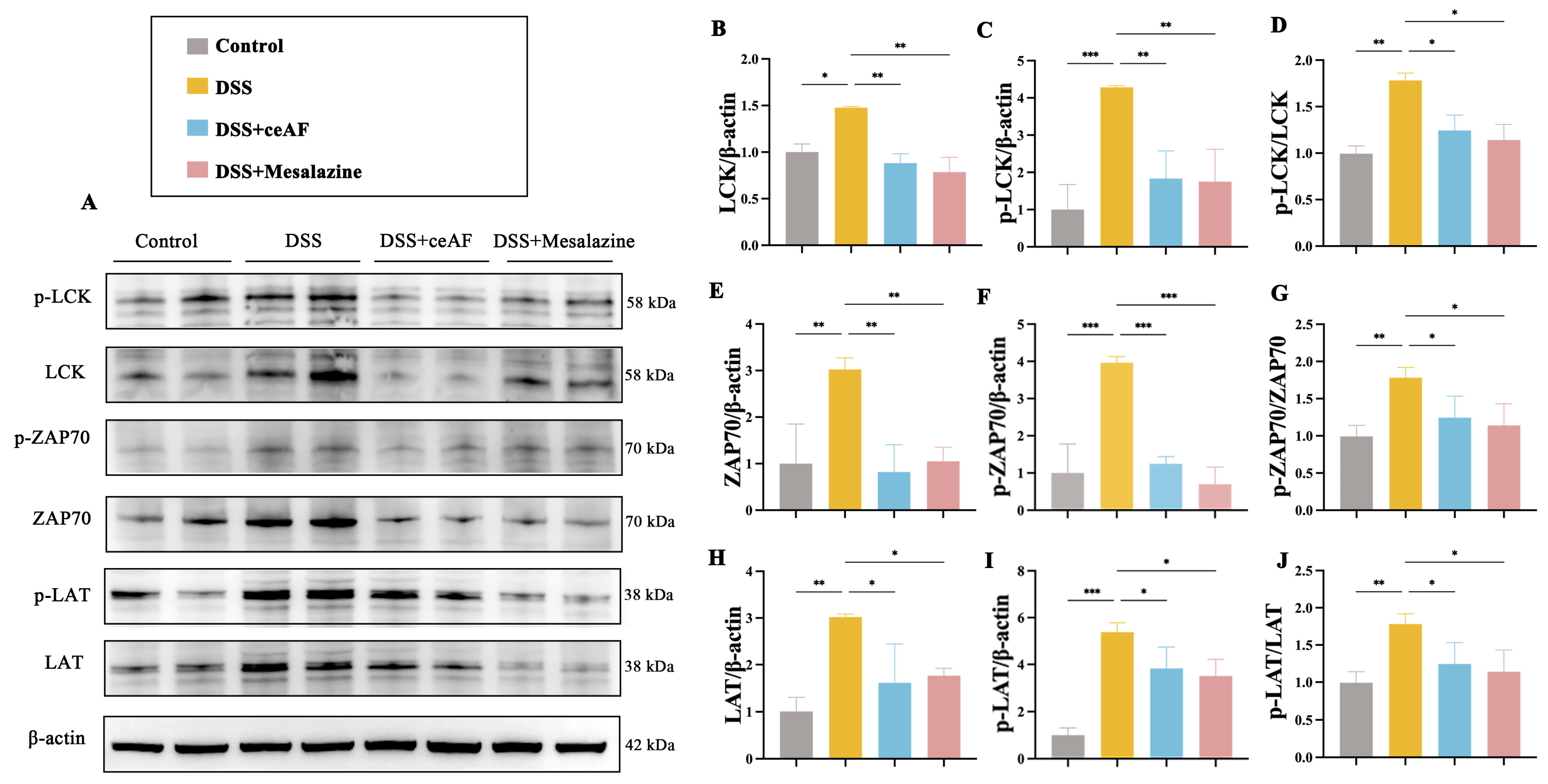
3.6. ceAF Alleviates DSS-Induced Hyperactivation of the TCR Signaling Pathway in Colon Tissue of UC Mice
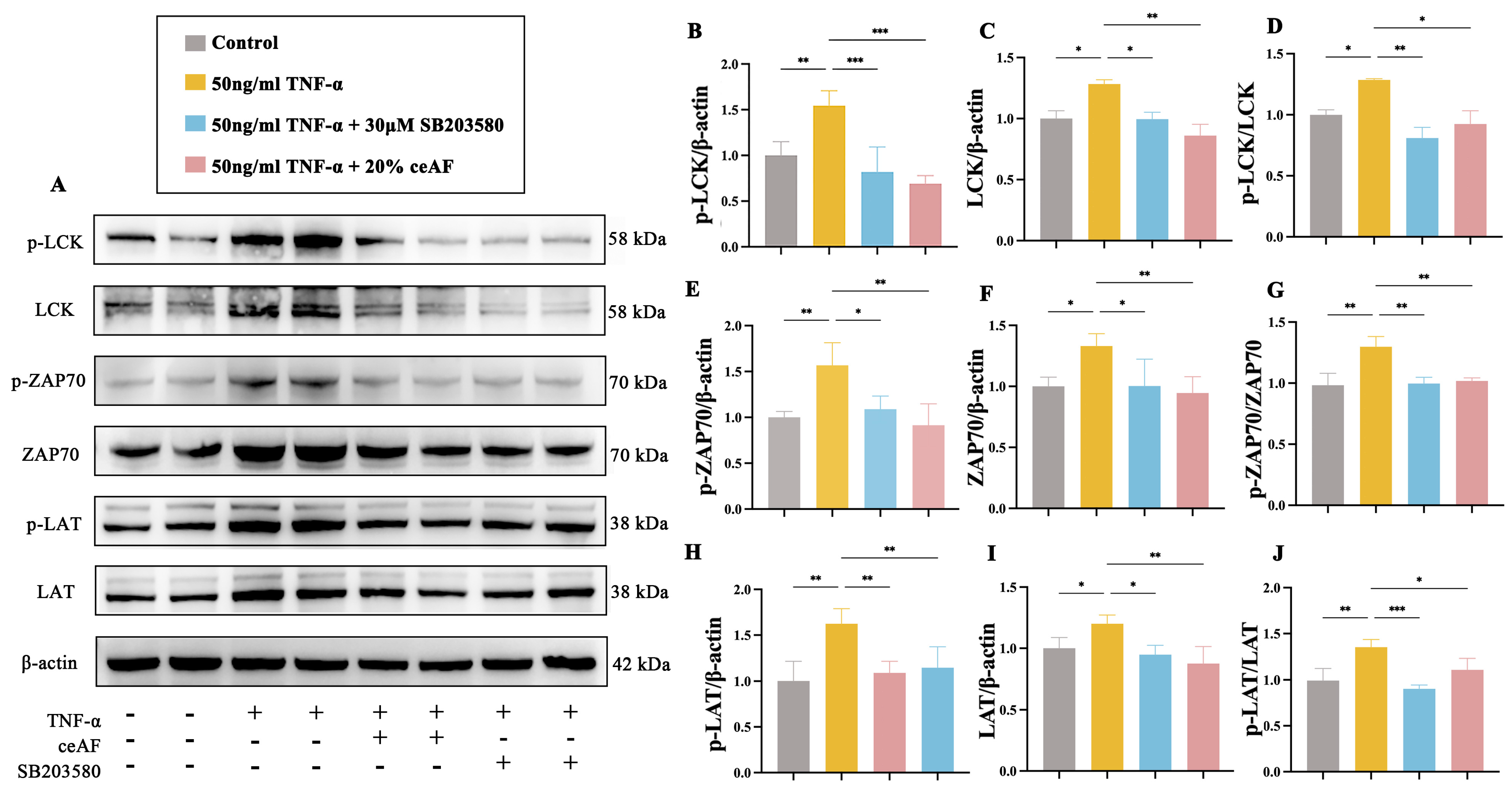
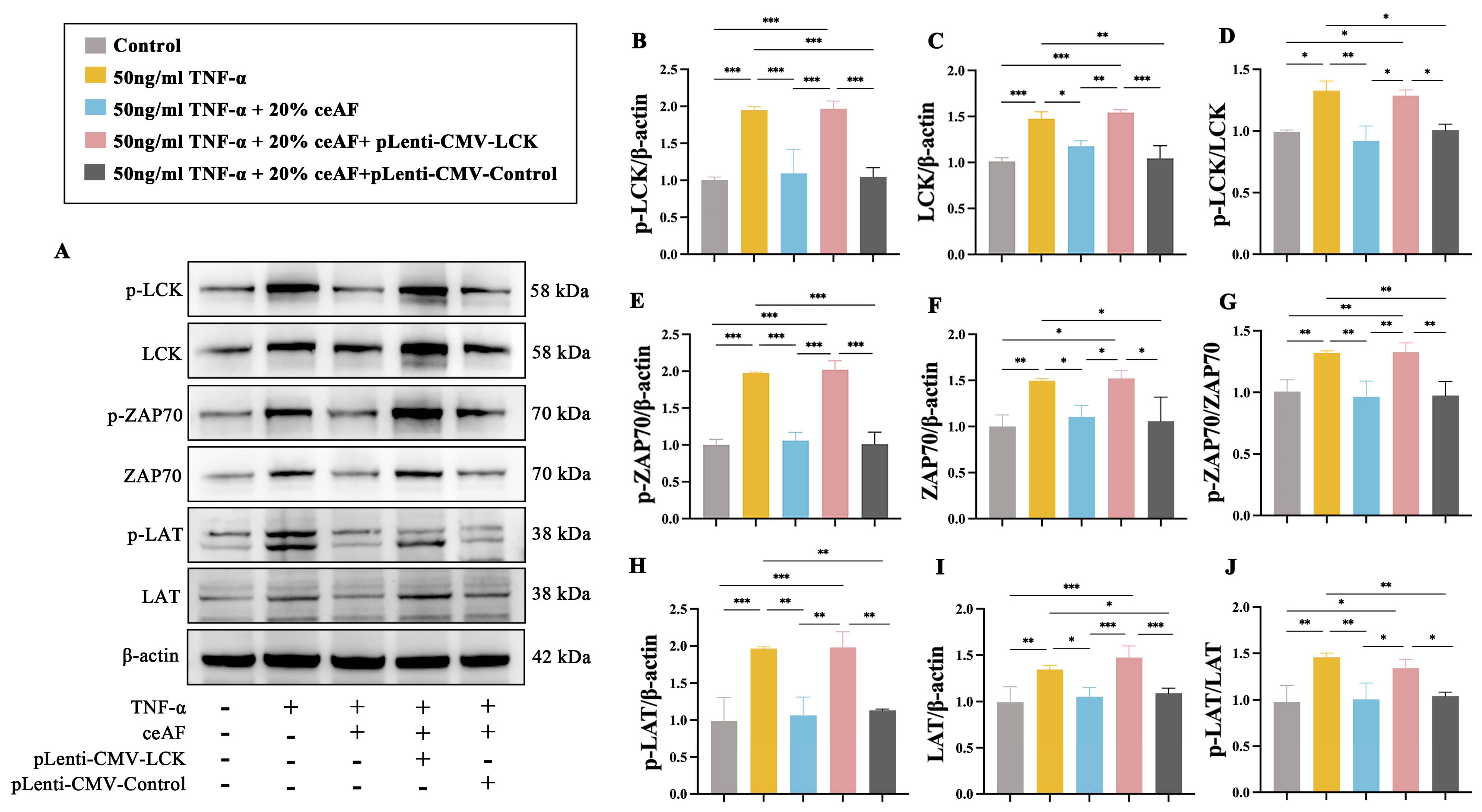
3.7. ceAF Inhibits the TCR Signaling Pathway in Jurkat Cells via the LCK/ZAP70/LAT Axis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Burri, E.; Maillard, M.H.; Schoepfer, A.M.; Seibold, F.; Van Assche, G.; Riviere, P.; Laharie, D.; Manz, M. Treatment Algorithm for Mild and Moderate-to-Severe Ulcerative Colitis: An Update. Digestion 2020, 101 (Suppl. 1), 2–15. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L.; Pogodziński, D. Nutrition and Supplementation in Ulcerative Colitis. Nutrients 2022, 14, 2469. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, F.; Dai, Z.; Feng, X.; Qiu, F. Combination of Coptis chinensis polysaccharides and berberine ameliorates ulcerative colitis by regulating gut microbiota and activating AhR/IL-22 pathway. J. Ethnopharmacol. 2024, 318, 117050. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Y.; Zhu, L.; Ying, Y.; Hao, W.; Wang, L.; He, L.; Zhao, D.; Chen, J.X.; Gao, Y.; et al. Liquiritin apioside alleviates colonic inflammation and accompanying depression-like symptoms in colitis by gut metabolites and the balance of Th17/Treg. Phytomedicine 2023, 120, 155039. [Google Scholar] [CrossRef]
- Zhao, M.; Xie, X.; Xu, B.; Chen, Y.; Cai, Y.; Chen, K.; Guan, X.; Ni, C.; Luo, X.; Zhou, L. Paeonol alleviates ulcerative colitis in mice by increasing short-chain fatty acids derived from Clostridium butyricum. Phytomedicine 2023, 120, 155056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, Y.; Huang, Y.; Zhang, S.; Wang, G.; Fang, X.; Bao, W. Aqueous M. oleifera leaf extract alleviates DSS-induced colitis in mice through suppression of inflammation. J. Ethnopharmacol. 2024, 318, 116929. [Google Scholar] [CrossRef] [PubMed]
- Vrachnis, N.; Argyridis, S.; Vrachnis, D.; Antonakopoulos, N.; Valsamakis, G.; Iavazzo, C.; Zygouris, D.; Salakos, N.; Rodolakis, A.; Vlahos, N.; et al. Increased Fibroblast Growth Factor 21 (FGF21) Concentration in Early Second Trimester Amniotic Fluid and Its Association with Fetal Growth. Metabolites 2021, 11, 581. [Google Scholar] [CrossRef]
- Pressman, E.K.; Thornburg, L.L.; Glantz, J.C.; Earhart, A.; Wall, P.D.; Ashraf, M.; Pryhuber, G.S.; Woods, J.R., Jr. Inflammatory cytokines and antioxidants in midtrimester amniotic fluid: Correlation with pregnancy outcome. Am. J. Obstet. Gynecol. 2011, 204, 155.e1–155.e7. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, X.; Yan, X.; Qian, J.; Tan, Q. ceAF Ameliorates Diabetic Wound Healing by Alleviating Inflammation and Oxidative Stress via TLR4/NF-κB and Nrf2 Pathways. J. Diabetes Res. 2023, 2023, 2422303. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Zhang, L.; Zheng, Y.; Qian, J.; Sun, N.; Ding, X.; Cui, B. Chick early amniotic fluid component improves heart function and protects against inflammation after myocardial infarction in mice. Front. Cardiovasc. Med. 2022, 9, 1042852. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Serrano-Vega, R.; Pérez Gutiérrez, S.; Isiordia-Espinoza, M.A.; Solorio-Alvarado, C.R. Myristic acid reduces skin inflammation and nociception. J. Food Biochem. 2022, 46, e14013. [Google Scholar] [CrossRef]
- Gutiérrez-García, A.G.; Contreras, C.M.; Díaz-Marte, C. Myristic acid in amniotic fluid produces appetitive responses in human newborns. Early Hum. Dev. 2017, 115, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Shan, W.; Li, J.; Min, J.; Zeng, X.; Zuo, Z. Appropriate exercise level attenuates gut dysbiosis and valeric acid increase to improve neuroplasticity and cognitive function after surgery in mice. Mol. Psychiatry 2021, 26, 7167–7187. [Google Scholar] [CrossRef]
- Kim, C.R.; Kim, H.S.; Choi, S.J.; Kim, J.K.; Gim, M.C.; Kim, Y.J.; Shin, D.H. Erucamide from Radish Leaves Has an Inhibitory Effect Against Acetylcholinesterase and Prevents Memory Deficit Induced by Trimethyltin. J. Med. Food 2018, 21, 769–776. [Google Scholar] [CrossRef]
- Li, M.M.; Jiang, Z.E.; Song, L.Y.; Quan, Z.S.; Yu, H.L. Antidepressant and anxiolytic-like behavioral effects of erucamide, a bioactive fatty acid amide, involving the hypothalamus-pituitary-adrenal axis in mice. Neurosci. Lett. 2017, 640, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Nicotinamide as a Foundation for Treating Neurodegenerative Disease and Metabolic Disorders. Curr. Neurovasc Res. 2021, 18, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeong, E.J.; Hwang, B.; Lee, H.D.; Lee, S.; Jang, M.; Yeo, K.; Shin, Y.; Park, S.; Lim, W.T.; et al. Pharmacological effects of biologically synthesized ginsenoside CK-rich preparation (AceCK40) on the colitis symptoms in DSS-induced Caco-2 cells and C57BL mice. Phytomedicine 2024, 124, 155301. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, G.; Charrier-Hisamuddin, L.; Nguyen, H.T.; Yan, Y.; Sitaraman, S.; Merlin, D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology 2008, 134, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Kim, Y.S.; Lim, J.Y.; Min, S.J.; Shin, J.H.; Ko, H.C.; Kim, S.J.; Lim, Y.; Kim, Y. Sasa quelpaertensis leaf extract suppresses dextran sulfate sodium-induced colitis in mice by inhibiting the proinflammatory mediators and mitogen-activated protein kinase phosphorylation. Nutr. Res. 2014, 34, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Farid, M.S.; Shafique, B.; Xu, R.; Łopusiewicz, Ł.; Zhao, C. Potential interventions and interactions of bioactive polyphenols and functional polysaccharides to alleviate inflammatory bowel disease—A review. Food Chem. 2025, 462, 140951. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, P.; Su, D.; Zhao, Y.; Zhang, M. Therapeutic inhibition of the JAK-STAT pathway in the treatment of inflammatory bowel disease. Cytokine Growth Factor. Rev. 2024, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bamias, G.; Menghini, P.; Pizarro, T.T.; Cominelli, F. Targeting TL1A and DR3: The new frontier of anti-cytokine therapy in IBD. Gut 2024. ahead of print. [Google Scholar] [CrossRef]
- Massironi, S.; Furfaro, F.; Bencardino, S.; Allocca, M.; Danese, S. Immunity in digestive diseases: New drugs for inflammatory bowel disease treatment-insights from Phase II and III trials. J. Gastroenterol. 2024, 59, 761–787. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X.; Dong, H. Current advances in the anti-inflammatory effects and mechanisms of natural polysaccharides. Crit. Rev. Food Sci. Nutr. 2023, 63, 5890–5910. [Google Scholar] [CrossRef]
- Ahmad, M.; Yu, J.; Cheng, S.; Khan, Z.A.; Luo, Y.; Luo, H. Guanosine and Deoxyinosine Structural Analogs Extracted from Chick Early Amniotic Fluid Promote Cutaneous Wound Healing. Int. J. Mol. Sci. 2023, 24, 12817. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, Y.; Xu, Q.; Mao, L. Short Chain Fatty Acids: Essential Weapons of Traditional Medicine in Treating Inflammatory Bowel Disease. Molecules 2024, 29, 379. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, B.; Wiciński, M.; Sokołowska, M.M.; Hill, N.A.; Szambelan, M. The Rundown of Dietary Supplements and Their Effects on Inflammatory Bowel Disease-A Review. Nutrients 2020, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Robles, H.; Castro-Ochoa, K.F.; Citalan-Madrid, A.F.; Schnoor, M. Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models in vivo. World J. Gastroenterol. 2019, 25, 4181–4198. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Mizukure, T.; Park, S.B.; Kishino, S.; Kimura, I.; Hirano, K.; Bergamo, P.; Rossi, M.; Suzuki, T.; Arita, M.; et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J. Biol. Chem. 2015, 290, 2902–2918. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.D.; Lim, S.; Kim, D.H. Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-kappaB signaling pathway. Int. Immunopharmacol. 2015, 29, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, M.; Bijari, S.; Khazaei, A.H.; Shojaei-Ghahrizjani, F.; Rezakhani, L. The role of milk-derived exosomes in the treatment of diseases. Front. Genet. 2022, 13, 1009338. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zheng, H.; Xiong, Y.; Li, K. Extracellular vesicles affecting embryo development in vitro: A potential culture medium supplement. Front. Pharmacol. 2024, 15, 1366992. [Google Scholar] [CrossRef] [PubMed]
- Beretti, F.; Zavatti, M.; Casciaro, F.; Comitini, G.; Franchi, F.; Barbieri, V.; La Sala, G.B.; Maraldi, T. Amniotic fluid stem cell exosomes: Therapeutic perspective. Biofactors 2018, 44, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, R.; Liang, H.; An, J.; Yang, Y.; Huo, J.; Chen, Z.; Quan, W.; Jiang, L.; Li, C.; et al. Comparison and Investigation of Exosomes from Human Amniotic Fluid Stem Cells and Human Breast Milk in Alleviating Neonatal Necrotizing Enterocolitis. Stem Cell Rev. Rep. 2023, 19, 754–766. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, L.; Liu, Q.; Hou, J.; Qiu, X.; Chen, M.; Wu, Z.; Hu, D.; Cui, F.; Yan, T. Exosome-coated polydatin nanoparticles in the treatment of radiation-induced intestinal damage. Aging 2023, 15, 6905–6920. [Google Scholar] [CrossRef] [PubMed]
- Van Hese, I.; Goossens, K.; Vandaele, L.; Opsomer, G. Invited review: MicroRNAs in bovine colostrum-Focus on their origin and potential health benefits for the calf. J. Dairy Sci. 2020, 103, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xia, B.; Li, X.; Zhang, L.; Liu, X.; Shi, R.; Kou, R.; Liu, Z.; Liu, X. Sesamol Supplementation Attenuates DSS-Induced Colitis via Mediating Gut Barrier Integrity, Inflammatory Responses, and Reshaping Gut Microbiome. J. Agric. Food Chem. 2020, 68, 10697–10708. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, X.; Zhu, Y.; Ma, J.; Ma, H.; Zhang, H. Probiotic Mixture Protects Dextran Sulfate Sodium-Induced Colitis by Altering Tight Junction Protein Expressions and Increasing Tregs. Mediat. Inflamm. 2018, 2018, 9416391. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Antanaviciute, A.; Fawkner-Corbett, D.; Jagielowicz, M.; Aulicino, A.; Lagerholm, C.; Davis, S.; Kinchen, J.; Chen, H.H.; Alham, N.K.; et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019, 567, 49–55. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Honan, A.M.; Jacobsen, G.E.; Drum, H.; Vazquez, E.N.; Quintero, M.A.; Deshpande, A.R.; Sussman, D.A.; Kerman, D.H.; Damas, O.M.; Proksell, S.; et al. Stromal-Like Cells Are Found in Peripheral Blood of Patients With Inflammatory Bowel Disease and Correlate With Immune Activation State. Clin. Transl. Gastroenterol. 2024, 15, e1. [Google Scholar] [CrossRef]
- Ji, R.; Wang, A.; Shang, H.; Chen, L.; Bao, C.; Wu, L.; Wu, H.; Shi, Y. Herb-partitioned moxibustion upregulated the expression of colonic epithelial tight junction-related proteins in Crohn’s disease model rats. Chin. Med. 2016, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, A.; Mucida, D.; Bilate, A.M. Intraepithelial Lymphocytes of the Intestine. Annu. Rev. Immunol. 2024, 42, 289–316. [Google Scholar] [CrossRef] [PubMed]
- Schattgen, S.A.; Thomas, P.G. Bohemian T cell receptors: Sketching the repertoires of unconventional lymphocytes. Immunol. Rev. 2018, 284, 79–90. [Google Scholar] [CrossRef]
- McDonald, B.D.; Jabri, B.; Bendelac, A. Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 2018, 18, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L.; Olivares-Villagomez, D. Development, Homeostasis, and Functions of Intestinal Intraepithelial Lymphocytes. J. Immunol. 2018, 200, 2235–2244. [Google Scholar] [CrossRef]
- Turtle, C.J.; Delrow, J.; Joslyn, R.C.; Swanson, H.M.; Basom, R.; Tabellini, L.; Delaney, C.; Heimfeld, S.; Hansen, J.A.; Riddell, S.R. Innate signals overcome acquired TCR signaling pathway regulation and govern the fate of human CD161(hi) CD8alpha(+) semi-invariant T cells. Blood 2011, 118, 2752–2762. [Google Scholar] [CrossRef]
- Bilate, A.M.; London, M.; Castro, T.B.R.; Mesin, L.; Bortolatto, J.; Kongthong, S.; Harnagel, A.; Victora, G.D.; Mucida, D. T Cell Receptor Is Required for Differentiation, but Not Maintenance, of Intestinal CD4(+) Intraepithelial Lymphocytes. Immunity 2020, 53, 1001–1014.e1020. [Google Scholar] [CrossRef]
- Fan, Q.; Dai, W.; Li, M.; Wang, T.; Li, X.; Deng, Z.; Li, W.; Li, M. Inhibition of α2,6-sialyltransferase relieves symptoms of ulcerative colitis by regulating Th17 cells polarization. Int. Immunopharmacol. 2023, 125, 111130. [Google Scholar] [CrossRef]
- Yan, J.; Pandey, S.P.; Barnes, B.J.; Turner, J.R.; Abraham, C. T Cell-Intrinsic IRF5 Regulates T Cell Signaling, Migration, and Differentiation and Promotes Intestinal Inflammation. Cell Rep. 2020, 31, 107820. [Google Scholar] [CrossRef] [PubMed]
- Sarrabayrouse, G.; Bossard, C.; Chauvin, J.M.; Jarry, A.; Meurette, G.; Quevrain, E.; Bridonneau, C.; Preisser, L.; Asehnoune, K.; Labarriere, N.; et al. CD4CD8alphaalpha lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease. PLoS Biol. 2014, 12, e1001833. [Google Scholar] [CrossRef] [PubMed]
- Razvag, Y.; Neve-Oz, Y.; Sajman, J.; Yakovian, O.; Reches, M.; Sherman, E. T Cell Activation through Isolated Tight Contacts. Cell Rep. 2019, 29, 3506–3521.e3506. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Hu, J.; Xi, D.; Xiong, H.; He, W.; Liu, J.; Li, M.; Lu, H.; Zhao, J.; Lai, W.; et al. Lck Inhibits Heat Shock Protein 65-Mediated Reverse Cholesterol Transport in T Cells. J. Immunol. 2016, 197, 3861–3870. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, P.; Sundvold, V.; Chan, H.; Abrahamsen, G.; Kjelstrup, H.; Nyman, T.A.; Spurkland, A. Tyr(192) Regulates Lymphocyte-Specific Tyrosine Kinase Activity in T Cells. J. Immunol. 2021, 207, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, M.; Irving, B.A.; van Oers, N.S.; Chan, A.C.; Weiss, A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science 1994, 263, 1136–1139. [Google Scholar] [CrossRef]
- Courtney, A.H.; Lo, W.L.; Weiss, A. TCR Signaling: Mechanisms of Initiation and Propagation. Trends Biochem. Sci. 2018, 43, 108–123. [Google Scholar] [CrossRef] [PubMed]
- van Oers, N.S.; Lowin-Kropf, B.; Finlay, D.; Connolly, K.; Weiss, A. alpha beta T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity 1996, 5, 429–436. [Google Scholar] [CrossRef]
- Sabat, M.; Vanrens, J.C.; Brugel, T.A.; Maier, J.; Laufersweiler, M.J.; Golebiowski, A.; De, B.; Easwaran, V.; Hsieh, L.C.; Rosegen, J.; et al. The development of novel 1,2-dihydro-pyrimido[4,5-c]pyridazine based inhibitors of lymphocyte specific kinase (Lck). Bioorg. Med. Chem. Lett. 2006, 16, 4257–4261. [Google Scholar] [CrossRef]
- Takayama, T.; Umemiya, H.; Amada, H.; Yabuuchi, T.; Koami, T.; Shiozawa, F.; Oka, Y.; Takaoka, A.; Yamaguchi, A.; Endo, M.; et al. Ring-fused pyrazole derivatives as potent inhibitors of lymphocyte-specific kinase (Lck): Structure, synthesis, and SAR. Bioorg. Med. Chem. Lett. 2010, 20, 112–116. [Google Scholar] [CrossRef]
- Kumar Singh, P.; Kashyap, A.; Silakari, O. Exploration of the therapeutic aspects of Lck: A kinase target in inflammatory mediated pathological conditions. Biomed. Pharmacother. 2018, 108, 1565–1571. [Google Scholar] [CrossRef]
- Bottini, N.; Musumeci, L.; Alonso, A.; Rahmouni, S.; Nika, K.; Rostamkhani, M.; MacMurray, J.; Meloni, G.F.; Lucarelli, P.; Pellecchia, M.; et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Genet. 2004, 36, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Gurzov, E.N.; Stanley, W.J.; Brodnicki, T.C.; Thomas, H.E. Protein tyrosine phosphatases: Molecular switches in metabolism and diabetes. Trends Endocrinol. Metab. 2015, 26, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Patry, M.; Teinturier, R.; Goehrig, D.; Zetu, C.; Ripoche, D.; Kim, I.S.; Bertolino, P.; Hennino, A. βig-h3 Represses T-Cell Activation in Type 1 Diabetes. Diabetes 2015, 64, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Pain, E.; Snowden, S.; Oddy, J.; Shinhmar, S.; Alhammad, Y.M.A.; King, J.S.; Muller-Taubenberger, A.; Williams, R.S.B. Pharmacological inhibition of ENT1 enhances the impact of specific dietary fats on energy metabolism gene expression. Proc. Natl. Acad. Sci. USA 2024, 121, e2321874121. [Google Scholar] [CrossRef]
- Wei, X.; Luo, D.; Yan, Y.; Yu, H.; Sun, L.; Wang, C.; Song, F.; Ge, H.; Qian, H.; Li, X.; et al. Kojic acid inhibits senescence of human corneal endothelial cells via NF-kappaB and p21 signaling pathways. Exp. Eye Res. 2019, 180, 174–183. [Google Scholar] [CrossRef]
- Fujiwara, M.; Sato, N.; Okamoto, K. Hypoxanthine Reduces Radiation Damage in Vascular Endothelial Cells and Mouse Skin by Enhancing ATP Production via the Salvage Pathway. Radiat. Res. 2022, 197, 583–593. [Google Scholar] [CrossRef]
- Ogura, J.; Yamanoi, K.; Ishida, K.; Nakamura, E.; Ito, S.; Aoyama, N.; Nakanishi, Y.; Menju, T.; Kawaguchi, K.; Hosoe, Y.; et al. A stearate-rich diet and oleate restriction directly inhibit tumor growth via the unfolded protein response. Exp. Mol. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Nutritional importance of choline for brain development. J. Am. Coll. Nutr. 2004, 23, 621S–626S. [Google Scholar] [CrossRef] [PubMed]
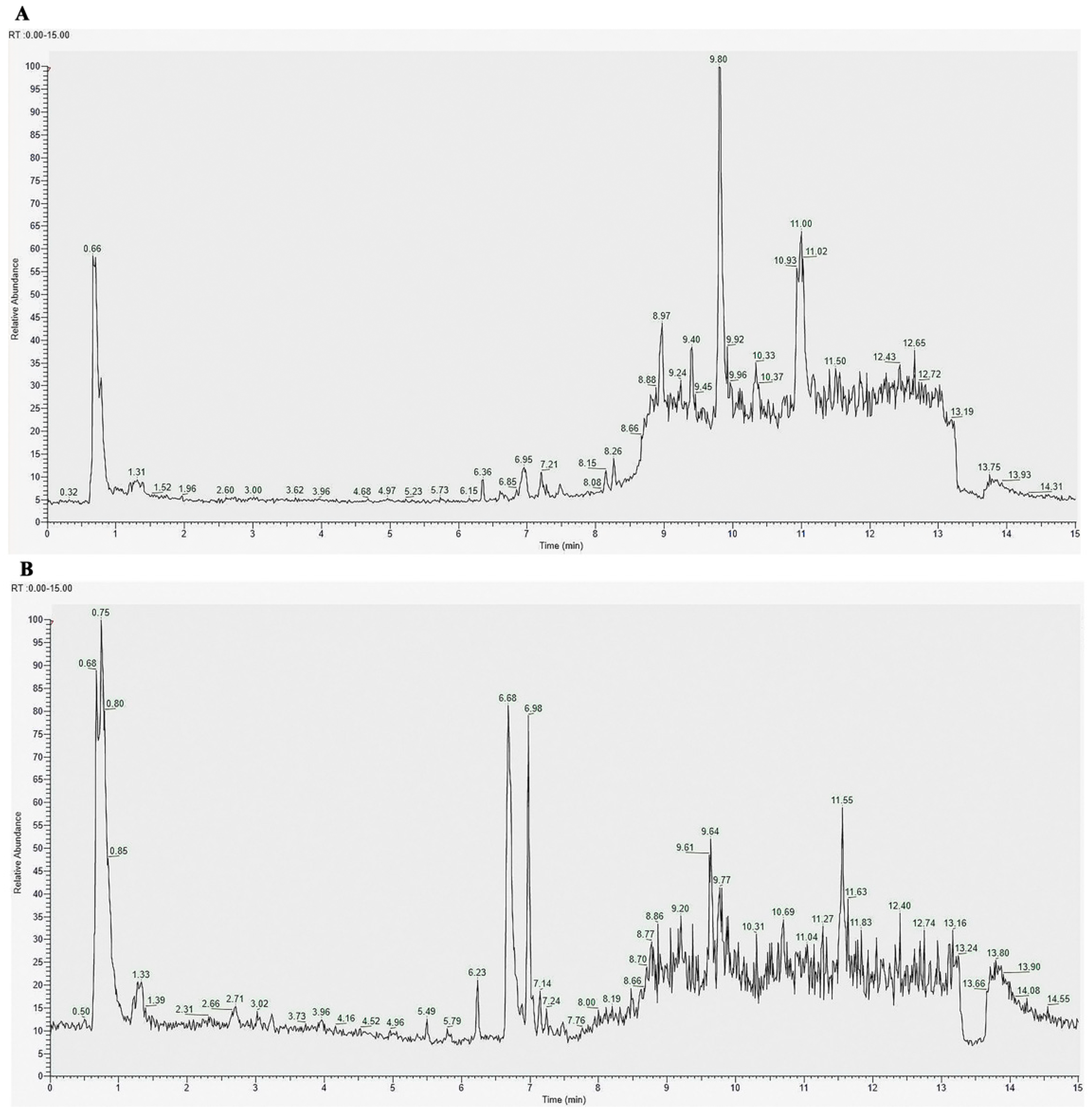






| Negative Ion Mode | |||||
| No. | Compound Name | Formula | m/z | RT [min] | Group Area |
| 1 | Palmitic acid | C16 H32 O2 | 255.23287 | 9.813 | 4,357,510,384 |
| 2 | Stearic acid | C18 H36 O2 | 283.26453 | 10.958 | 4,061,435,490 |
| 3 | Valeric acid | C5 H10 O2 | 101.06094 | 13.288 | 2,520,589,319 |
| 4 | Myristic acid | C14 H28 O2 | 227.20191 | 8.96 | 870,899,862.9 |
| 5 | dl-Lactic acid | C3 H6 O3 | 89.02454 | 0.791 | 358,035,153.6 |
| 6 | Kojic acid | C6 H6 O4 | 141.01717 | 0.664 | 348,432,111.8 |
| 7 | Caprylic acid | C8 H16 O2 | 143.10794 | 13.207 | 244,523,673.5 |
| 8 | 2,6-di-tert-Butylphenol | C14 H22 O | 205.15992 | 8.259 | 177,305,015.2 |
| 9 | Oleic acid | C18 H34 O2 | 281.2489 | 9.978 | 155,150,822.5 |
| 10 | Pentadecanoic acid | C15 H30 O2 | 241.21766 | 9.384 | 149,309,887.4 |
| 11 | Lauric acid | C12 H24 O2 | 199.17056 | 8.143 | 148,878,532.1 |
| 12 | Uric acid | C5 H4 N4 O3 | 167.02128 | 1.196 | 130,110,907.9 |
| 13 | Palmitoleic acid | C16 H30 O2 | 253.21766 | 9.193 | 100,995,495.3 |
| 14 | 4-Dodecylbenzenesulfonic acid | C18 H30 O3 S | 325.18482 | 10.975 | 94,350,125.41 |
| 15 | 3-Hydroxybutyric acid | C4 H8 O3 | 103.04017 | 1.078 | 78,136,007.21 |
| Positive Ion Mode | |||||
| No. | Compound Name | Formula | m/z | RT [min] | Group Area |
| 1 | 2-Amino-1,3,4-octadecanetriol | C18 H39 N O3 | 318.30018 | 6.677 | 1,609,178,255 |
| 2 | Erucamide | C22 H43 N O | 338.34161 | 11.545 | 1,603,181,304 |
| 3 | Choline | C5 H13 N O | 104.10702 | 0.754 | 1,184,765,704 |
| 4 | Hexamethylenetetramine | C6 H12 N4 | 141.1135 | 13.246 | 851,641,374 |
| 5 | Granisetron | C18 H24 N4 O | 313.19831 | 8.857 | 782,405,096.8 |
| 6 | Bis(4-ethylbenzylidene)sorbitol | C24 H30 O6 | 415.21155 | 6.97 | 593,893,594.6 |
| 7 | Nicotinamide | C6 H6 N2 O | 123.05532 | 2.374 | 444,547,455.8 |
| 8 | l-Phenylalanine | C9 H11 N O2 | 166.08634 | 2.687 | 295,842,507.5 |
| 9 | δ-Valerolactam | C5 H9 N O | 100.07577 | 2.922 | 288,263,436.4 |
| 10 | N-cyclohexyl-1-methyl-5-(1H-pyrrol-1-yl)-1H-pyrazole-4-carboxamide | C15 H20 N4 O | 273.16737 | 13.23 | 251,908,905 |
| 11 | Indan-1-one 1-(4,5-dihydro-1H-imidazol-2-yl)hydrazone | C12 H14 N4 | 215.12525 | 8.84 | 219,406,038 |
| 12 | Betaine | C5 H11 N O2 | 118.08628 | 0.735 | 210,445,968.9 |
| 13 | Hypoxanthine | C5 H4 N4 O | 137.04589 | 2.071 | 186,643,512.7 |
| 14 | l-Norleucine | C6 H13 N O2 | 132.10202 | 1.328 | 178,446,405.3 |
| 15 | 2-[(3S)-1-Isopropyl-3-pyrrolidinyl]-1H-benzimidazole-5-carbonitrile | C15 H18 N4 | 255.15664 | 8.865 | 157,960,783 |
| No. | Fatty Acid | Content (μg/mL) | No. | Fatty Acid | Content (μg/mL) |
|---|---|---|---|---|---|
| 1 | Methyl Oleate | 43.491 | 20 | Methyl Elaidate | 0.388 |
| 2 | Methyl Palmitate | 32.613 | 21 | Methyl 10-Transnonadecenoate | 0.323 |
| 3 | Methyl Linoelaidate | 11.532 | 22 | Methyl 11-Eicosenoate | 0.323 |
| 4 | Methyl Stearate | 11.113 | 23 | Methyl Petroselaidate | 0.32 |
| 5 | Methyl Palmitoleate | 3.74 | 24 | Methyl Transvaccenate | 0.314 |
| 6 | Methyl Transvaccenate | 2.551 | 25 | Methyl Nervonoate | 0.3 |
| 7 | Methyl Petroselaidate | 2.249 | 26 | Methyl Erucate | 0.287 |
| 8 | Methyl 10-Heptadecenoate | 1.495 | 27 | Methyl Heptadecanoate | 0.197 |
| 9 | Methyl Arachidonate | 1.461 | 28 | Methyl Brassidate | 0.177 |
| 10 | Methyl Myristoleate | 1.178 | 29 | Methyl Alpha Linolenate | 0.15 |
| 11 | Methyl Myristelaidate | 1.061 | 30 | Methyl Pentadecanoate | 0.098 |
| 12 | Methyl 10-Transpentadecenoate | 0.804 | 31 | Methyl Lignocerate | 0.035 |
| 13 | Methyl 10-Pentadecenoate | 0.556 | 32 | Methyl Linoelaidate | 0.032 |
| 14 | Methyl 10-Transsheptadecenoate | 0.556 | 33 | Methyl Laurate | 0.02 |
| 15 | Methyl Trans 11-Eicosenoate | 0.45 | 34 | Methyl Docosadienoate | 0.014 |
| 16 | Methyl Docosahexaenoate | 0.448 | 35 | Methyl Undecanoate | 0.005 |
| 17 | Methyl Docosapentaenoate | 0.416 | 36 | Methyl Caprate | 0.002 |
| 18 | Methyl Palmitelaidate | 0.412 | 37 | Methyl Docosatetraenoate | 0.002 |
| 19 | Methyl Myristate | 0.404 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Zhao, Y.; Dai, Y.; Sun, N.; Gao, X.; Yin, J.; Zhou, Z.; Wu, K.-j. Chick Early Amniotic Fluid Alleviates Dextran-Sulfate-Sodium-Induced Colitis in Mice via T-Cell Receptor Pathway. Antioxidants 2025, 14, 51. https://doi.org/10.3390/antiox14010051
Chen F, Zhao Y, Dai Y, Sun N, Gao X, Yin J, Zhou Z, Wu K-j. Chick Early Amniotic Fluid Alleviates Dextran-Sulfate-Sodium-Induced Colitis in Mice via T-Cell Receptor Pathway. Antioxidants. 2025; 14(1):51. https://doi.org/10.3390/antiox14010051
Chicago/Turabian StyleChen, Fan, Yining Zhao, Yanfa Dai, Ning Sun, Xuezheng Gao, Jiajun Yin, Zhenhe Zhou, and Ke-jia Wu. 2025. "Chick Early Amniotic Fluid Alleviates Dextran-Sulfate-Sodium-Induced Colitis in Mice via T-Cell Receptor Pathway" Antioxidants 14, no. 1: 51. https://doi.org/10.3390/antiox14010051
APA StyleChen, F., Zhao, Y., Dai, Y., Sun, N., Gao, X., Yin, J., Zhou, Z., & Wu, K.-j. (2025). Chick Early Amniotic Fluid Alleviates Dextran-Sulfate-Sodium-Induced Colitis in Mice via T-Cell Receptor Pathway. Antioxidants, 14(1), 51. https://doi.org/10.3390/antiox14010051





