Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications
Abstract
1. Introduction
2. Pathophysiology of Oxidative Stress in Type 2 Diabetes Mellitus
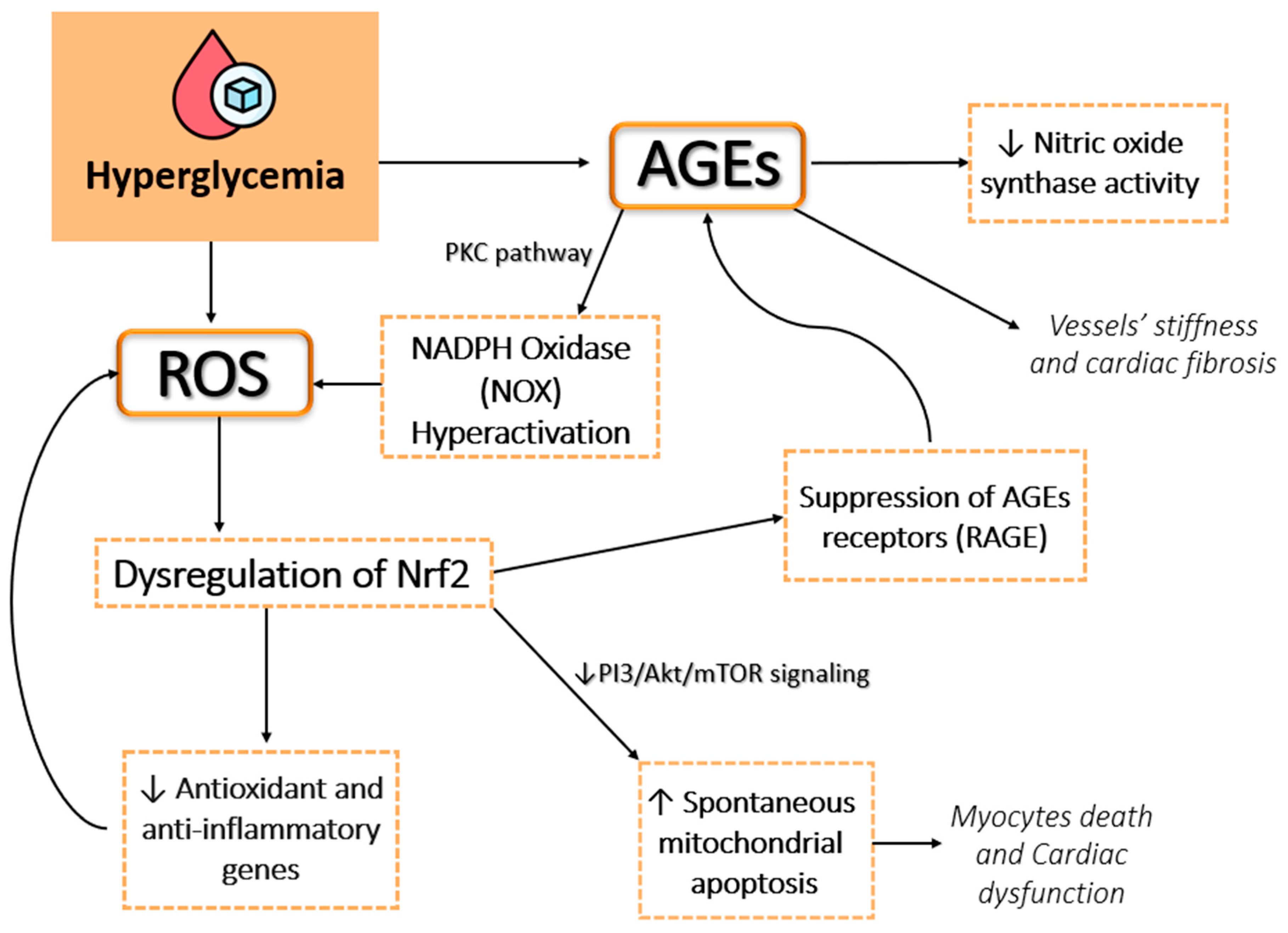
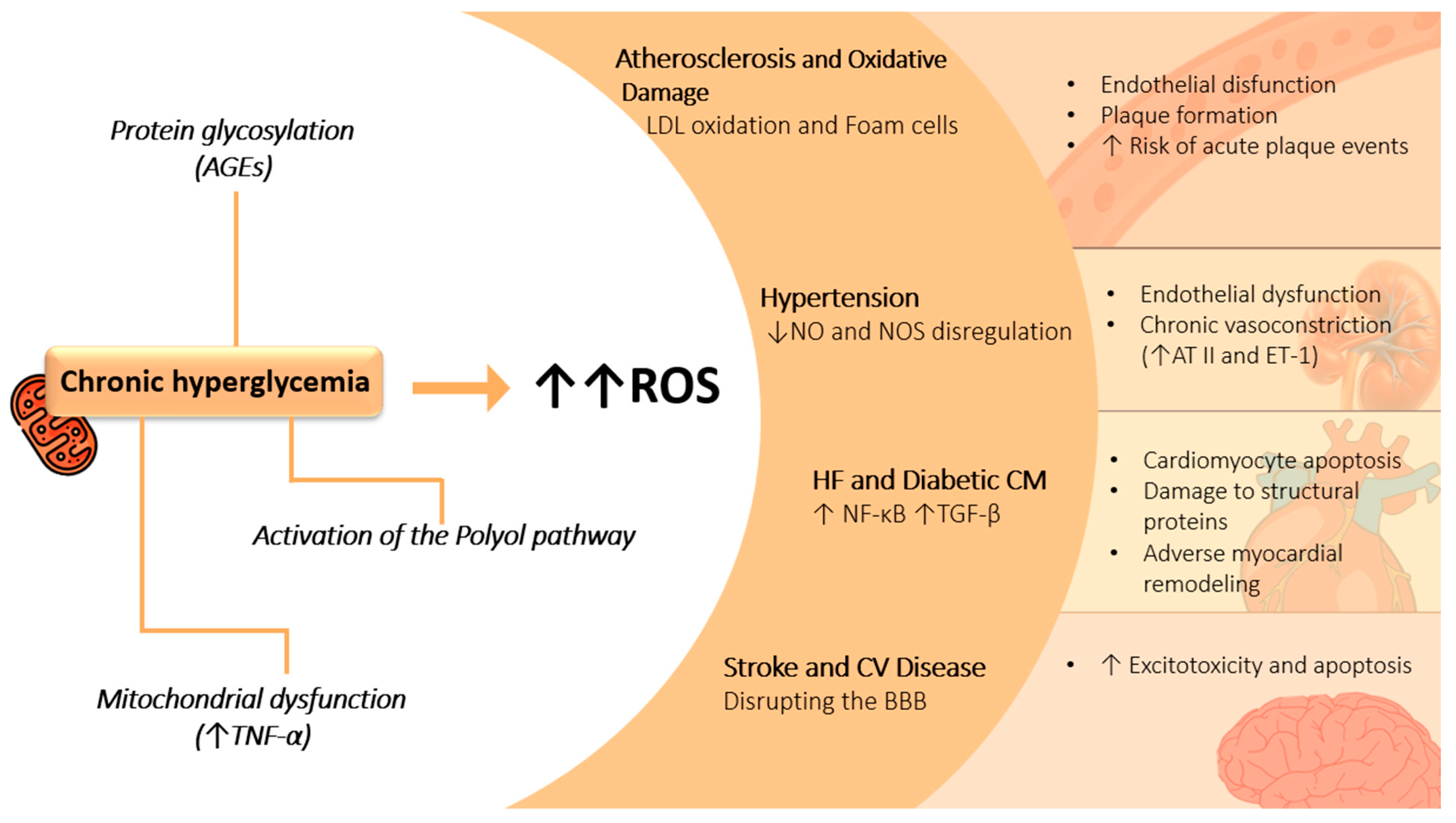
2.1. Hyperglycemia-Induced Oxidative Stress
2.2. Role of Advanced Glycation End Products and the Polyol Pathway
2.3. Mitochondrial Dysfunction
2.4. Implications for Vascular Damage and Cardiovascular Disease
2.5. Endothelial Dysfunction and Vascular Inflammation
2.6. Role of Lipids in Oxidative Stress and Cardiovascular Risk
2.7. Genetic Factors Modulating Oxidative Stress Pathways
3. Molecular Mechanisms Linking Oxidative Stress to Cardiovascular Disease in Type 2 Diabetes Mellitus
4. Oxidative Stress and Cardiovascular Complications in T2DM
4.1. Atherosclerosis and Oxidative Damage
4.2. Hypertension
4.3. Heart Failure and Diabetic Cardiomyopathy
4.4. Stroke and Cerebrovascular Disease
5. Lifestyle Modifications
5.1. Dietary Approaches
5.1.1. The Mediterranean Diet
5.1.2. Plant-Based Diets
5.1.3. Low-Glycemic Index Diets
5.1.4. DASH Diet
5.1.5. The Japanese Diet
5.1.6. Ketogenic Diet
5.1.7. Dietary Interventions and Gut Microbiota
5.2. Exercise
5.2.1. Exercise as a Modulator of Oxidative Stress and Vascular Health
5.2.2. Mechanisms of ROS Reduction and Enhanced Antioxidant Defenses
5.2.3. Exercise-Induced Mitochondrial Biogenesis
5.2.4. High-Intensity Interval Training
5.2.5. Exercise Synergy with Other Lifestyle Interventions
5.2.6. Impact of Duration and Intensity of Lifestyle, and Individual Characteristics
| Category | Key Points | References |
|---|---|---|
| Mediterranean Diet | Rich in antioxidants from vegetables, legumes, fruits, whole grains, EVOO, nuts, and seeds. | [149] |
| Polyphenols (e.g., hydroxycinnamic acids, quercetin, catechins, resveratrol, oleuropein, and hydroxytyrosol) are key antioxidants. | [149,150,151,152,153] | |
| Polyphenols enhance adiponectin secretion, AMPK activation, and inhibit NF-κB, iNOS, and macrophage infiltration. | [154,155,156] | |
| EVOO-derived compounds reduce LDL oxidation, NOX-mediated pathways, cytokines (e.g., IL-1β, COX-2), and increase PPARγ expression. | [157,158,161] | |
| Omega-3 and omega-9 fatty acids improve insulin sensitivity, beta-cell function, and endothelial health. | [162] | |
| Plant-Based Diets | Shown to reduce cardiovascular morbidity/mortality, BMI, cholesterol, HbA1C, and inflammation in some studies (e.g., AHS-2, ARIC, EPIC-Oxford, BROAD). | [164,165,166] |
| EVADE-CAD study found no significant advantages over AHA-recommended diets but noted reduced hsCRP in CAD patients. | [167] | |
| Low-Glycemic Index Diets | Stabilizes blood glucose levels, reducing postprandial oxidative stress. Foods like oats, legumes, and non-starchy vegetables enhance antioxidant enzyme activities and reduce ROS production. | [168,169] |
| DASH Diet | Rich in fruits, vegetables, low-fat dairy, and low in sodium; improves blood pressure, reduces oxidative stress, and enhances endothelial function in T2DM patients. | [173] |
| Increases antioxidant intake, reducing systemic inflammation and oxidative damage. | [173,174] | |
| Japanese Diet | High in fish, seaweed, soy, and green tea; rich in antioxidants like catechins, isoflavones, and omega-3 fatty acids, which reduce oxidative stress and inflammation. | [178] |
| Promotes improved endothelial function and reduced cardiovascular risk; omega-3 fatty acids support anti-inflammatory pathways. | [179] | |
| Fermented foods (e.g., miso, natto) may enhance gut microbiota and reduce systemic inflammation, contributing to oxidative stress modulation. | [193] | |
| Ketogenic Diet | Induces ketosis by restricting carbohydrates and increasing fat intake, improving insulin sensitivity and reducing blood glucose levels. | [186] |
| Lowers oxidative stress markers and inflammation, potentially improving endothelial function; effects on cardiovascular risk are still debated. | [188,189] | |
| May reduce mitochondrial ROS production but requires further long-term studies to assess safety and overall efficacy. | [188] | |
| Exercise Effects on Oxidative Stress | Reduces oxidative stress and inflammation, improving endothelial function and vascular health. | [204,205,206] |
| Aerobic Exercise | Enhances nitric oxide bioavailability, reduces peroxide levels, and promotes arterial remodeling (e.g., larger arteries, thinner walls, reduced stiffness). | [207,208] |
| Resistance vs. Aerobic | Insufficient activity reduces redox adaptability, emphasizing the need for consistent exercise. | [212] |
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 Diabetes Mellitus in Adults: Pathogenesis, Prevention and Therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the Obesity Epidemic: After the Sudden Rise, Is the Upward Trajectory Beginning to Flatten? Curr. Obes. Rep. 2023, 12, 514–527. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas 10th Edition Scientific Committee. In IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Caturano, A.; Vetrano, E.; Galiero, R.; Sardu, C.; Rinaldi, L.; Russo, V.; Monda, M.; Marfella, R.; Sasso, F.C. Advances in the Insulin–Heart Axis: Current Therapies and Future Directions. Int. J. Mol. Sci. 2024, 25, 10173. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The Burden and Risks of Emerging Complications of Diabetes Mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.D.; Sattar, N. Cardiovascular Risk in Diabetes Mellitus: Epidemiology, Assessment and Prevention. Nat. Rev. Cardiol. 2023, 20, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; Sardu, C.; et al. Efficacy and Durability of Multifactorial Intervention on Mortality and MACEs: A Randomized Clinical Trial in Type-2 Diabetic Kidney Disease. Cardiovasc. Diabetol. 2021, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.X.; Ma, X.N.; Guan, C.H.; Li, Y.D.; Mauricio, D.; Fu, S.B. Cardiovascular Disease in Type 2 Diabetes Mellitus: Progress Toward Personalized Management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef]
- Nakamura, K.; Miyoshi, T.; Yoshida, M.; Akagi, S.; Saito, Y.; Ejiri, K.; Matsuo, N.; Ichikawa, K.; Iwasaki, K.; Naito, T.; et al. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 3587. [Google Scholar] [CrossRef]
- Zakir, M.; Ahuja, N.; Surksha, M.A.; Sachdev, R.; Kalariya, Y.; Nasir, M.; Kashif, M.; Shahzeen, F.; Tayyab, A.; Khan, M.S.M.; et al. Cardiovascular Complications of Diabetes: From Microvascular to Macrovascular Pathways. Cureus 2023, 15, e45835. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Colantuoni, S.; Medicamento, G.; et al. Dysregulated Epicardial Adipose Tissue as a Risk Factor and Potential Therapeutic Target of Heart Failure with Preserved Ejection Fraction in Diabetes. Biomolecules 2022, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Pafundi, P.C.; Gelso, A.; Bono, V.; Costagliola, C.; Marfella, R.; Sardu, C.; Rinaldi, L.; Galiero, R.; Acierno, C.; et al. High HDL Cholesterol: A Risk Factor for Diabetic Retinopathy? Findings from NO BLIND Study. Diabetes Res. Clin. Pract. 2019, 150, 236–244. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, E.A.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Caturano, A. Cardiovascular and Metabolic Disease: New Treatments and Future Directions 2.0. Biomedicines 2024, 12, 1356. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Understanding Mechanisms of Antioxidant Action in Health and Disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Ciotola, M.; Sasso, F.C.; Cozzolino, D.; Saccomanno, F.; Assaloni, R.; Ceriello, A.; Giugliano, D. Effect of a Single High-Fat Meal on Endothelial Function in Patients with the Metabolic Syndrome: Role of Tumor Necrosis Factor-Alpha. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 274–279. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Waldron, R.T.; Rey, O.; Zhukova, E.; Rozengurt, E. Oxidative stress induces protein kinase C-mediated activation loop phosphorylation and nuclear redistribution of protein kinase D. J. Biol. Chem. 2004, 279, 27482–27493. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic Hyperglycemia Mediated Physiological Alteration and Metabolic Distortion Leads to Organ Dysfunction, Infection, Cancer Progression and Other Pathophysiological Consequences: An Update on Glucose Toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef]
- Cosentino-Gomes, D.; Rocco-Machado, N.; Meyer-Fernandes, J.R. Cell Signaling Through Protein Kinase C Oxidation and Activation. Int. J. Mol. Sci. 2012, 13, 10697–10721. [Google Scholar] [CrossRef] [PubMed]
- Naruse, K.; Rask-Madsen, C.; Takahara, N.; Ha, S.W.; Suzuma, K.; Way, K.J.; Jacobs, J.R.; Clermont, A.C.; Ueki, K.; Ohshiro, Y.; et al. Activation of Vascular Protein Kinase C-Beta Inhibits Akt-Dependent Endothelial Nitric Oxide Synthase Function in Obesity-Associated Insulin Resistance. Diabetes 2006, 55, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.J.; Wang, X.; Xu, L.; Yang, X.C.; Zhao, W.S. PKC-Mediated Endothelin-1 Expression in Endothelial Cells Promotes Macrophage Activation in Atherogenesis. Am. J. Hypertens. 2019, 32, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Domingueti, C.P.; Dusse, L.M.; Carvalho, M.d.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes Mellitus: The Linkage Between Oxidative Stress, Inflammation, Hypercoagulability, and Vascular Complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.C. Lehninger. In Principles of Biochemistry, 4th ed.; W. H. Freeman & Co.: New York, NY, USA, 2004; ISBN 0-7167-4339-6. [Google Scholar]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging Mechanisms and Functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, J.; Rubio-Del-Campo, A.; Yebra, M.J. Regulatory Insights into the Production of UDP-N-Acetylglucosamine by Lactobacillus casei. Bioengineered 2012, 3, 339–342. [Google Scholar] [CrossRef]
- Olson, A.K.; Bouchard, B.; Zhu, W.Z.; Chatham, J.C.; Des Rosiers, C. First Characterization of Glucose Flux through the Hexosamine Biosynthesis Pathway (HBP) in Ex Vivo Mouse Heart. J. Biol. Chem. 2020, 295, 2018–2033. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; Shi, H.; Li, F.; Duan, Y.; Guo, Q. New Insights into the Role of Mitochondrial Dynamics in Oxidative Stress-Induced Diseases. Biomed. Pharmacother. 2024, 178, 117084. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- Wautier, J.L.; Schmidt, A.M. Protein glycation: A firm link to endothelial cell dysfunction. Circ. Res. 2004, 95, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Candido, R.; Forbes, J.M.; Thomas, M.C.; Thallas, V.; Dean, R.G.; Burns, W.C.; Tikellis, C.; Ritchie, R.H.; Twigg, S.M.; Cooper, M.E.; et al. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ. Res. 2003, 92, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Moore, J.E.; Sharkey, J.A.; Murphy, G.; Simpson, D.A.; Bucala, R.; Vlassara, H.; Archer, D.B. Advanced glycation end products in vitreous: Structural and functional implications for diabetic vitreopathy. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2517–2523. [Google Scholar]
- Niwa, T.; Katsuzaki, T.; Miyazaki, S.; Miyazaki, T.; Ishizaki, Y.; Hayase, F.; Tatemichi, N.; Takei, Y. Immunohistochemical detection of imidazolone, a novel advanced glycation end product, in kidneys and aortas of diabetic patients. J. Clin. Investig. 1997, 99, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Yang, M.-H.; Chyau, C.-C.; Chiu, C.-H.; Wang, H.-E.; Lin, Y.-C.; Chiu, W.-T.; Peng, R.Y. Kinetic analysis on the sensitivity of glucose- or glyoxal-induced LDL glycation to the inhibitory effect of Psidium guajava extract in a physiomimic system. Biosystems 2007, 88, 92–100. [Google Scholar] [CrossRef]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Watanabe, T.; Yamamoto, H. Roles of the receptor for advanced glycation endproducts in diabetes-induced vascular injury. J. Pharmacol. Sci. 2005, 97, 305–311. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef] [PubMed]
- El-Kabbani, O.; Darmanin, C.; Chung, R.P. Sorbitol Dehydrogenase: Structure, Function and Ligand Design. Curr. Med. Chem. 2004, 11, 465–476. [Google Scholar] [CrossRef]
- Kador, P.F.; Akagi, Y.; Kinoshita, J.H. The effect of aldose reductase and its inhibition on sugar cataract formation. Metabolism 1986, 35 (Suppl. S1), 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Araki, N.; Koh, N.; Nakamura, J.; Horiuchi, S.; Hotta, N. Rapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathway. Biochem. Biophys. Res. Commun. 1996, 228, 539–543. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2016, 26, 501–518. [Google Scholar] [CrossRef]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial Dysfunction in Type 2 Diabetes Mellitus: An Organ-Based Analysis. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef] [PubMed]
- Luengo, A.; Li, Z.; Gui, D.Y.; Sullivan, L.B.; Zagorulya, M.; Do, B.T.; Ferreira, R.; Naamati, A.; Ali, A.; Lewis, C.A.; et al. Increased Demand for NAD+ Relative to ATP Drives Aerobic Glycolysis. Mol. Cell 2021, 81, 691–707.e6. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation Between Mitochondrial Membrane Potential and ROS Formation. Methods Mol. Biol. 2012, 810, 183–205. [Google Scholar] [CrossRef]
- Kalpage, H.A.; Wan, J.; Morse, P.T.; Zurek, M.P.; Turner, A.A.; Khobeir, A.; Yazdi, N.; Hakim, L.; Liu, J.; Vaishnav, A.; et al. Cytochrome c Phosphorylation: Control of Mitochondrial Electron Transport Chain Flux and Apoptosis. Int. J. Biochem. Cell Biol. 2020, 121, 105704. [Google Scholar] [CrossRef] [PubMed]
- Kytövuori, L.; Lipponen, J.; Rusanen, H.; Komulainen, T.; Martikainen, M.H.; Majamaa, K. A novel mutation m.8561C>G in MT-ATP6/8 causing a mitochondrial syndrome with ataxia, peripheral neuropathy, diabetes mellitus, and hypergonadotropic hypogonadism. J. Neurol. 2016, 263, 2188–2195. [Google Scholar] [CrossRef]
- Diaz-Morales, N.; Rovira-Llopis, S.; Bañuls, C.; Escribano-Lopez, I.; de Marañon, A.M.; Lopez-Domenech, S.; Orden, S.; Roldan-Torres, I.; Alvarez, A.; Veses, S.; et al. Are Mitochondrial Fusion and Fission Impaired in Leukocytes of Type 2 Diabetic Patients? Antioxid. Redox Signal. 2016, 25, 108–115. [Google Scholar] [CrossRef]
- Hernandez-Mijares, A.; Rocha, M.; Apostolova, N.; Borras, C.; Jover, A.; Bañuls, C.; Sola, E.; Victor, V.M. Mitochondrial complex I impairment in leukocytes from type 2 diabetic patients. Free Radic. Biol. Med. 2011, 50, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Quirós, P.M.; Ramsay, A.J.; Sala, D.; Fernández-Vizarra, E.; Rodríguez, F.; Peinado, J.R.; Fernández-García, M.S.; Vega, J.A.; Enríquez, J.A.; Zorzano, A.; et al. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J. 2012, 31, 2117–2133. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, D.; Hernández-Alvarez, M.I.; Segalés, J.; Sorianello, E.; Muñoz, J.P.; Sala, D.; Waget, A.; Liesa, M.; Paz, J.C.; Gopalacharyulu, P.; et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 5523–5528. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Marechal, X.; Coisne, A.; Debry, N.; Modine, T.; Fayad, G.; Potelle, C.; El Arid, J.M.; Mouton, S.; Sebti, Y.; et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 2014, 130, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Kypson, A.P.; Rodriguez, E.; Anderson, C.A.; Lehr, E.J.; Neufer, P.D. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J. Am. Coll. Cardiol. 2009, 54, 1891–1898. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rodriguez, E.; Anderson, C.A.; Thayne, K.; Chitwood, W.R.; Kypson, A.P. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H118–H124. [Google Scholar] [CrossRef]
- Cho, Y.E.; Basu, A.; Dai, A.; Heldak, M.; Makino, A. Coronary endothelial dysfunction and mitochondrial reactive oxygen species in type 2 diabetic mice. Am. J. Physiol. Cell Physiol. 2013, 305, C1033–C1040. [Google Scholar] [CrossRef]
- Shenouda, S.M.; Widlansky, M.E.; Chen, K.; Xu, G.; Holbrook, M.; Tabit, C.E.; Hamburg, N.M.; Frame, A.A.; Caiano, T.L.; Kluge, M.A.; et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011, 124, 444–453. [Google Scholar] [CrossRef]
- Levine, T.B.; Levine, A.B. The endothelium and nitric oxide. In Metabolic Syndrome and Cardiovascular Disease 2006, 1st ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2006; pp. 173–210. [Google Scholar]
- Russo, G.; Leopold, J.A.; Loscalzo, J. Vasoactive substances: Nitric oxide and endothelial dysfunction in atherosclerosis. Vascul. Pharmacol. 2002, 38, 259–269. [Google Scholar] [CrossRef]
- Chew, G.T.; Watts, G.F. Coenzyme Q10 and diabetic endotheliopathy: Oxidative stress and the ‘recoupling hypothesis’. QJM 2004, 97, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Alp, N.J.; Channon, K.M. Regulation of Endothelial Nitric Oxide Synthase by Tetrahydrobiopterin in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Katusic, Z.S. Vascular endothelial dysfunction: Does tetrahydrobiopterin play a role? Am. J. Physiol.—Heart Circ. Physiol. 2001, 281, H981–H986. [Google Scholar] [CrossRef]
- Mitta, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction. A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Hirata, K.-I. Endothelial nitric oxide synthase uncoupling: Is it a physiological mechanism of endothelium-dependent relaxation in cerebral artery. Cardiovasc. Res. 2007, 73, 8–9. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Binjawhar, D.N.; Alhazmi, A.T.; Bin Jawhar, W.N.; MohammedSaeed, W.; Safi, S.Z. Hyperglycemia-Induced Oxidative Stress and Epigenetic Regulation of ET-1 Gene in Endothelial Cells. Front. Genet. 2023, 14, 1167773. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Gagliardi, S.; Nacci, C.; Carratu, M.R.; Montagnani, M. Endothelial dysfunction in diabetes: From mechanisms to therapeutic targets. Curr. Med. Chem. 2009, 16, 94–112. [Google Scholar] [CrossRef]
- Lu, S.; Kuang, M.; Qiu, J.; Li, W.; Zhang, M.; Sheng, G.; Zou, Y.; Peng, X. Lipids as the link between central obesity and diabetes: Perspectives from mediation analysis. BMC Endocr. Disord. 2024, 24, 229. [Google Scholar] [CrossRef]
- Soppert, J.; Lehrke, M.; Marx, N.; Jankowski, J.; Noels, H. Lipoproteins and lipids in cardiovascular disease: From mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 2020, 159, 4–33. [Google Scholar] [CrossRef]
- Trinh, M.D.; Plihalova, A.; Gojda, J.; Westlake, K.; Spicka, J.; Lattova, Z.; Pretl, M.; Polak, J. Obstructive sleep apnoea increases lipolysis and deteriorates glucose homeostasis in patients with type 2 diabetes mellitus. Sci. Rep. 2021, 11, 3567. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.P.; Valensi, P. Elevated triglycerides and low high-density lipoprotein cholesterol level as a marker of very high risk in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 118–129. [Google Scholar] [CrossRef]
- Caturano, A. New Advances in Cardiovascular Drugs: A Celebration of the 90th Birthday of Professor Akira Endo. Biomedicines 2024, 12, 2716. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Yan, K. Recent advances in the effect of adipose tissue inflammation on insulin resistance. Cell Signal. 2024, 120, 111229. [Google Scholar] [CrossRef]
- Chueire, V.B.; Muscelli, E. Effect of Free Fatty Acids on Insulin Secretion, Insulin Sensitivity and Incretin Effect—A Narrative Review. Arch. Endocrinol. Metab. 2021, 65, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, H.K.; Rajavelu, I.; Pereira, M.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Inside the Genome: Understanding Genetic Influences on Oxidative Stress. Front. Genet. 2024, 15, 1397352. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Vanita, V. Association of Aldose Reductase Gene (AKR1B1) Polymorphism with Diabetic Retinopathy. Diabetes Res. Clin. Pract. 2016, 121, 41–48. [Google Scholar] [CrossRef]
- Ramana, K.V. Aldose Reductase: New Insights for an Old Enzyme. Biomol. Concepts 2011, 2, 103–114. [Google Scholar] [CrossRef]
- Araki, S.; Haneda, M.; Sugimoto, T.; Isono, M.; Isshiki, K.; Kashiwagi, A.; Koya, D. Polymorphisms of the Protein Kinase C-Beta Gene (PRKCB1) Accelerate Kidney Disease in Type 2 Diabetes Without Overt Proteinuria. Diabetes Care 2006, 29, 864–868. [Google Scholar] [CrossRef]
- Han, X.; Hu, Z.; Chen, J.; Huang, J.; Huang, C.; Liu, F.; Gu, C.; Yang, X.; Hixson, J.E.; Lu, X.; et al. Associations Between Genetic Variants of NADPH Oxidase-Related Genes and Blood Pressure Responses to Dietary Sodium Intervention: The GenSalt Study. Am. J. Hypertens. 2017, 30, 427–434. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T.A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat. Inflamm. 2010, 2010, 453892. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Galiero, R.; Pafundi, P.C.; Cesaro, A.; Vetrano, E.; Palmiero, G.; Rinaldi, L.; Salvatore, T.; Marfella, R.; Sardu, C.; et al. Does a strict glycemic control during acute coronary syndrome play a cardioprotective effect? Pathophysiology and clinical evidence. Diabetes Res. Clin. Pract. 2021, 178, 108959. [Google Scholar] [CrossRef]
- Russo, V.; Falco, L.; Tessitore, V.; Mauriello, A.; Catapano, D.; Napolitano, N.; Tariq, M.; Caturano, A.; Ciccarelli, G.; D’Andrea, A.; et al. Anti-Inflammatory and Anticancer Effects of Anticoagulant Therapy in Patients with Malignancy. Life 2023, 13, 1888. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Khotina, V.; Ivanova, E.A.; Orekhov, A.N. NADPH Oxidases and Their Role in Atherosclerosis. Biomedicines 2020, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Sun, W.; Tan, Y.; Liu, Y.; Zheng, Y.; Liu, Q.; Cai, L.; Sun, J. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid. Med. Cell. Longev. 2014, 2014, 123963. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Khan, S.U.; Suleman, M.; Khan, M.U.; Khan, M.S.; Arbi, F.M.; Hussain, T.; Mohammed Alsuhaibani, A.; S Refat, M. Natural Allies for Heart Health: Nrf2 Activation and Cardiovascular Disease Management. Curr. Probl. Cardiol. 2024, 49 Pt B, 102084. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, J.H. Recent Advances in Understanding Nrf2 Agonism and Its Potential Clinical Application to Metabolic and Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 2846. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Galiero, R.; Caturano, A.; Vetrano, E.; Beccia, D.; Brin, C.; Alfano, M.; Di Salvo, J.; Epifani, R.; Piacevole, A.; Tagliaferri, G.; et al. Peripheral Neuropathy in Diabetes Mellitus: Pathogenetic Mechanisms and Diagnostic Options. Int. J. Mol. Sci. 2023, 24, 3554. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Cai, L. Diabetic cardiomyopathy and its mechanisms: Role of oxidative stress and damage. J. Diabetes Investig. 2014, 5, 623–634. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Marfella, R.; Sardu, C.; et al. Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence. Biomolecules 2021, 11, 1834. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Kma, L.; Baruah, T.J. The Interplay of ROS and the PI3K/Akt Pathway in Autophagy Regulation. Biotechnol. Appl. Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.; Tsao, P. Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Beckman, J.; Creager, M.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Jha, J.; Ho, F.; Dan, C.; Jandeleit-Dahm, K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. 2018, 132, 1811–1836. [Google Scholar] [CrossRef]
- Sasso, F.C.; Simeon, V.; Galiero, R.; Caturano, A.; De Nicola, L.; Chiodini, P.; Rinaldi, L.; Salvatore, T.; Lettieri, M.; Nevola, R.; et al. The Number of Risk Factors Not at Target Is Associated with Cardiovascular Risk in a Type 2 Diabetic Population with Albuminuria in Primary Cardiovascular Prevention: Post-Hoc Analysis of the NID-2 Trial. Cardiovasc. Diabetol. 2022, 21, 235. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Sardu, C.; Marfella, R.; et al. Sex-Difference of Multifactorial Intervention on Cardiovascular and Mortality Risk in DKD: Post-Hoc Analysis of a Randomised Clinical Trial. Cardiovasc. Diabetol. 2024, 23, 285. [Google Scholar] [CrossRef]
- Cesaro, A.; Acerbo, V.; Vetrano, E.; Signore, G.; Scherillo, G.; Rotolo, F.P.; De Michele, G.; Scialla, F.; Raucci, G.; Panico, D.; et al. Sodium–Glucose Cotransporter 2 Inhibitors in Patients with Diabetes and Coronary Artery Disease: Translating the Benefits of the Molecular Mechanisms of Gliflozins into Clinical Practice. Int. J. Mol. Sci. 2023, 24, 8099. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Nevola, R.; Alfano, M.; Pafundi, P.C.; Brin, C.; Gragnano, F.; Calabrò, P.; Adinolfi, L.E.; Rinaldi, L.; Sasso, F.C.; Caturano, A. Cardiorenal Impact of SGLT-2 Inhibitors: A Conceptual Revolution in the Management of Type 2 Diabetes, Heart Failure and Chronic Kidney Disease. Rev. Cardiovasc. Med. 2022, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2018, 20, 247–260. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, M.; Singh, J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: Molecular insights and therapeutic strategies. Cardiovasc. Diabetol. 2018, 17, 121. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.; Aldasoro, M.; Víctor, V.; Mauricio, M. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Sinning, C.; Post, F.; Warnholtz, A.; Schulz, E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008, 40, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Pafundi, P.C.; Garofalo, C.; Galiero, R.; Borrelli, S.; Caturano, A.; Rinaldi, L.; Provenzano, M.; Salvatore, T.; De Nicola, L.; Minutolo, R.; et al. Role of Albuminuria in Detecting Cardio-Renal Risk and Outcome in Diabetic Subjects. Diagnostics 2021, 11, 290. [Google Scholar] [CrossRef]
- Förstermann, U. Nitric oxide and oxidative stress in vascular disease. Pflügers Arch.-Eur. J. Physiol. 2010, 459, 923–939. [Google Scholar] [CrossRef]
- Schulz, E.; Jansen, T.; Wenzel, P.; Daiber, A.; Münzel, T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signal. 2008, 10, 1115–1126. [Google Scholar] [CrossRef]
- Inoguchi, T.; Sonta, T.; Tsubouchi, H.; Etoh, T.; Kakimoto, M.; Sonoda, N.; Sato, N.; Sekiguchi, N.; Kobayashi, K.; Sumimoto, H.; et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: Role of vascular NAD(P)H oxidase. J. Am. Soc. Nephrol. 2003, 14, S227–S232. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Albanese, G.; Di Martino, A.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Sasso, F.C. The Diabetic Cardiomyopathy: The Contributing Pathophysiological Mechanisms. Front. Med. 2021, 8, 695792. [Google Scholar] [CrossRef] [PubMed]
- Palmiero, G.; Cesaro, A.; Galiero, R.; Loffredo, G.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Salvatore, T.; Ruggiero, R.; Di Palo, M.R.; et al. Impact of Gliflozins on Cardiac Remodeling in Patients with Type 2 Diabetes Mellitus & Reduced Ejection Fraction Heart Failure: A Pilot Prospective Study. GLISCAR Study. Diabetes Res. Clin. Pract. 2023, 200, 110686. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc. Res. 2008, 81, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Trincado, C.; García-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.; Rothermel, B.; Lavandero, S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef]
- Russo, V.; Malvezzi Caracciolo D’Aquino, M.; Caturano, A.; Scognamiglio, G.; Pezzullo, E.; Fabiani, D.; Del Giudice, C.; Carbone, A.; Bottino, R.; Caso, V.; et al. Improvement of Global Longitudinal Strain and Myocardial Work in Type 2 Diabetes Patients on Sodium-Glucose Cotransporter 2 Inhibitors Therapy. J. Cardiovasc. Pharmacol. 2023, 82, 196–200. [Google Scholar] [CrossRef]
- Palomer, X.; Salvadó, L.; Barroso, E.; Vázquez-Carrera, M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int. J. Cardiol. 2013, 168, 3160–3172. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Loffredo, G.; Rinaldi, L.; Catalini, C.; Gjeloshi, K.; Albanese, G.; Di Martino, A.; et al. Coronary Microvascular Dysfunction in Diabetes Mellitus: Pathogenetic Mechanisms and Potential Therapeutic Options. Biomedicines 2022, 10, 2274. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, M.; Cheema, Y.; Shahbaz, A.U.; Bhattacharya, S.K.; Weber, K.T. Intracellular Calcium Overloading and Oxidative Stress in Cardiomyocyte Necrosis via a Mitochriocentric Signal-Transducer-Effector Pathway. Exp. Clin. Cardiol. 2011, 16, 109–115. [Google Scholar]
- Kamada, H.; Yu, F.; Nito, C.; Chan, P. Influence of Hyperglycemia on Oxidative Stress and Matrix Metalloproteinase-9 Activation After Focal Cerebral Ischemia/Reperfusion in Rats: Relation to Blood-Brain Barrier Dysfunction. Stroke 2007, 38, 1044–1049. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic Mechanisms Following Ischemic Stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Di Francia, R.; Rinaldi, L.; Cillo, M.; Varriale, E.; Facchini, G.; D’Aniello, C.; Marotta, G.; Berretta, M. Antioxidant Diet and Genotyping as Tools for the Prevention of Liver Disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 5155–5163. [Google Scholar] [PubMed]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Criscuolo, L.; Di Martino, A.; Albanese, G.; Vetrano, E.; Catalini, C.; Sardu, C.; et al. Current Knowledge on the Pathophysiology of Lean/Normal-Weight Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 658. [Google Scholar] [CrossRef]
- Sandri, E.; Sguanci, M.; Cantín Larumbe, E.; Cerdá Olmedo, G.; Werner, L.U.; Piredda, M.; Mancin, S. Plant-Based Diets versus the Mediterranean Dietary Pattern and Their Socio-Demographic Determinants in the Spanish Population: Influence on Health and Lifestyle Habits. Nutrients 2024, 16, 1278. [Google Scholar] [CrossRef]
- Selinger, E.; Neuenschwander, M.; Koller, A.; Gojda, J.; Kühn, T.; Schwingshackl, L.; Barbaresko, J.; Schlesinger, S. Evidence of a Vegan Diet for Health Benefits and Risks—An Umbrella Review of Meta-Analyses of Observational and Clinical Studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 9926–9936. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Nardini, M.; Baldi, A.; Scaccini, C. Antioxidant Activity of Different Phenolic Fractions Separated from an Italian Red Wine. J. Agric. Food Chem. 1998, 46, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Sinatra, D.; Blanco, I.; Mulè, S.; La Verde, M.; Marranzano, M. Association between Dietary Phenolic Acids and Hypertension in a Mediterranean Cohort. Nutrients 2017, 9, 1069. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Kopp, C.; Singh, S.P.; Regenhard, P.; Müller, U.; Sauerwein, H.; Mielenz, M. Trans-Cinnamic Acid Increases Adiponectin and the Phosphorylation of AMP-Activated Protein Kinase through G-Protein-Coupled Receptor Signaling in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2014, 15, 2906–2915. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.; Loarca-Piña, G.; Gonzalez de Mejia, E. Maize extract rich in ferulic acid and anthocyanins prevents high-fat-induced obesity in mice by modulating SIRT1, AMPK and IL-6 associated metabolic and inflammatory pathways. J. Nutr. Biochem. 2020, 79, 108343. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef]
- Sebai, H.; Sani, M.; Yacoubi, M.T.; Aouani, E.; Ghanem-Boughanmi, N.; Ben-Attia, M. Resveratrol, a red wine polyphenol, attenuates lipopolysaccharide-induced oxidative stress in rat liver. Ecotoxicol. Environ. Saf. 2010, 73, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- De Groote, D.; Van Belleghem, K.; Devière, J.; Van Brussel, W.; Mukaneza, A.; Amininejad, L. Effect of the intake of resveratrol, resveratrol phosphate, and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann. Nutr. Metab. 2012, 61, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Carpi, S.; Scoditti, E.; Massaro, M.; Polini, B.; Manera, C.; Digiacomo, M.; Esposito Salsano, J.; Poli, G.; Tuccinardi, T.; Doccini, S.; et al. The Extra-Virgin Olive Oil Polyphenols Oleocanthal and Oleacein Counteract Inflammation-Related Gene and miRNA Expression in Adipocytes by Attenuating NF-κB Activation. Nutrients 2019, 11, 2855. [Google Scholar] [CrossRef] [PubMed]
- Dayi, T.; Ozgoren, M. Effects of the Mediterranean diet on the components of metabolic syndrome. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E56–E64. [Google Scholar] [CrossRef]
- Salehin, S.; Rasmussen, P.; Mai, S.; Mushtaq, M.; Agarwal, M.; Hasan, S.M.; Salehin, S.; Raja, M.; Gilani, S.; Khalife, W.I. Plant Based Diet and Its Effect on Cardiovascular Disease. Int. J. Environ. Res. Public Health 2023, 20, 3337. [Google Scholar] [CrossRef] [PubMed]
- Giosuè, A.; Calabrese, I.; Vitale, M.; Riccardi, G.; Vaccaro, O. Consumption of Dairy Foods and Cardiovascular Disease: A Systematic Review. Nutrients 2022, 14, 831. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional Status and the Influence of the Vegan Diet on the Gut Microbiota and Human Health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Papier, K.; Tong, T.Y.N. Plant-Based Diets and Long-Term Health: Findings from the EPIC-Oxford Study. Proc. Nutr. Soc. 2022, 81, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association-Recommended Diet in Coronary Artery Disease Trial. J. Am. Heart Assoc. 2018, 7, e011367. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.L. Low-Glycemic Index Diets as an Intervention for Diabetes: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kaur, K.; Singh, B.; Kaur, H.; Thakur, A.; Dhaliwal, S.S.; Singh, S. Insights into the latest advances in low glycemic foods, their mechanism of action and health benefits. Food Meas. 2022, 16, 533–546. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Kraakman, M.J.; Flynn, M.C.; Nagareddy, P.R.; Schalkwijk, C.G.; Murphy, A.J. Postprandial Glucose Spikes, an Important Contributor to Cardiovascular Disease in Diabetes? Front. Cardiovasc. Med. 2020, 7, 570553. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Ojo, O.; Ojo, O.O.; Wang, X.-H.; Adegboye, A.R.A. The Effects of a Low GI Diet on Cardiometabolic and Inflammatory Parameters in Patients with Type 2 and Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2019, 11, 1584. [Google Scholar] [CrossRef]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Ghodeshwar, G.K.; Dube, A.; Khobragade, D. Impact of Lifestyle Modifications on Cardiovascular Health: A Narrative Review. Cureus 2023, 15, e42616. [Google Scholar] [CrossRef]
- Caturano, A.; Vetrano, E.; Galiero, R.; Salvatore, T.; Docimo, G.; Epifani, R.; Alfano, M.; Sardu, C.; Marfella, R.; Rinaldi, L.; et al. Cardiac Hypertrophy: From Pathophysiological Mechanisms to Heart Failure Development. Rev. Cardiovasc. Med. 2022, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Shen, X.; Li, C.; Li, Y.; Li, T.; Chen, B. Adherence to the Dietary Approaches to Stop Hypertension Diet Reduces the Risk of Diabetes Mellitus: A Systematic Review and Dose-Response Meta-Analysis. Endocrine 2024, 86, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural Antioxidants from Some Fruits, Seeds, Foods, Natural Products, and Associated Health Benefits: An Update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Gabriel, A.S.; Ninomiya, K.; Uneyama, H. The Role of the Japanese Traditional Diet in Healthy and Sustainable Dietary Patterns around the World. Nutrients 2018, 10, 173. [Google Scholar] [CrossRef]
- Levitan, E.B.; Wolk, A.; Mittleman, M.A. Fatty Fish, Marine Omega-3 Fatty Acids and Incidence of Heart Failure. Eur. J. Clin. Nutr. 2010, 64, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jung, Y.S.; Jang, D.; Cho, C.H.; Lee, S.H.; Han, N.S.; Kim, D.O. Antioxidant Capacity of 12 Major Soybean Isoflavones and Their Bioavailability Under Simulated Digestion and in Human Intestinal Caco-2 Cells. Food Chem. 2022, 374, 131493. [Google Scholar] [CrossRef]
- Salido, M.; Soto, M.; Seoane, S. Seaweed: Nutritional and Gastronomic Perspective. A Review. Algal Res. 2024, 77, 103357. [Google Scholar] [CrossRef]
- Maruyama, C.; Nakano, R.; Shima, M.; Mae, A.; Shijo, Y.; Nakamura, E.; Okabe, Y.; Park, S.; Kameyama, N.; Hirai, S.; et al. Effects of a Japan Diet Intake Program on Metabolic Parameters in Middle-Aged Men. J. Atheroscler. Thromb. 2017, 24, 393–401. [Google Scholar] [CrossRef]
- Shirota, M.; Watanabe, N.; Suzuki, M.; Kobori, M. Japanese-Style Diet and Cardiovascular Disease Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutrients 2022, 14, 2008. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative Stress in the Pathophysiology of Type 2 Diabetes and Related Complications: Current Therapeutic Strategies and Future Perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic Diet for Human Diseases: The Underlying Mechanisms and Potential for Clinical Implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, D.; Żendzian-Piotrowska, M. Ketogenic Diet: A Review of Composition Diversity, Mechanism of Action and Clinical Application. J. Nutr. Metab. 2024, 2024, 6666171. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., III; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Greco, T.; Glenn, T.C.; Hovda, D.A.; Prins, M.L. Ketogenic Diet Decreases Oxidative Stress and Improves Mitochondrial Respiratory Complex Activity. J. Cereb. Blood Flow Metab. 2016, 36, 1603–1613. [Google Scholar] [CrossRef]
- Batch, J.T.; Lamsal, S.P.; Adkins, M.; Sultan, S.; Ramirez, M.N. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus 2020, 12, e9639. [Google Scholar] [CrossRef] [PubMed]
- Pirola, L.; Górecka, K.; Gois Leandro, C.; Balcerczyk, A. Nutritional Studies Evaluating Ketogenic Diets as a Treatment for Obesity and Obesity-Associated Morbidities: Underlying Mechanisms and Potential for Clinical Implementation. Endocrines 2024, 5, 585–599. [Google Scholar] [CrossRef]
- Dowis, K.; Banga, S. The Potential Health Benefits of the Ketogenic Diet: A Narrative Review. Nutrients 2021, 13, 1654. [Google Scholar] [CrossRef]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The Role of Diet in Shaping Human Gut Microbiota. Best Pract. Res. Clin. Gastroenterol. 2023, 62–63, 101828. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the Role of Diet in Maintaining Gut Health to Reduce the Risk of Obesity, Cardiovascular and Other Age-Related Inflammatory Diseases: Recent Challenges and Future Recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, M.; Budinska, E.; Kuzma, M.; Pelantova, H.; Hradecky, J.; Heczkova, M.; Daskova, N.; Bratova, M.; Modos, I.; Videnska, P.; et al. Vegan Diet Is Associated with Favorable Effects on the Metabolic Performance of Intestinal Microbiota: A Cross-Sectional Multi-Omics Study. Front. Nutr. 2022, 8, 783302. [Google Scholar] [CrossRef] [PubMed]
- Daskova, N.; Heczkova, M.; Modos, I.; Hradecky, J.; Hudcovic, T.; Kuzma, M.; Pelantova, H.; Buskova, I.; Sticova, E.; Funda, D.; et al. Protective Effect of Vegan Microbiota on Liver Steatosis Is Conveyed by Dietary Fiber: Implications for Fecal Microbiota Transfer Therapy. Nutrients 2023, 15, 454. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Gojda, J.; Rossmeislová, L.; Straková, R.; Tůmová, J.; Elkalaf, M.; Jaček, M.; Tůma, P.; Potočková, J.; Krauzová, E.; Waldauf, P.; et al. Chronic dietary exposure to branched chain amino acids impairs glucose disposal in vegans but not in omnivores. Eur. J. Clin. Nutr. 2017, 71, 594–601. [Google Scholar] [CrossRef]
- Jamar, G.; Ribeiro, D.A.; Pisani, L.P. High-Fat or High-Sugar Diets as Triggers of Inflammation in the Microbiota-Gut-Brain Axis. Crit. Rev. Food Sci. Nutr. 2021, 61, 836–854. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2017, 54, 4432–4451. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Di Murro, E.; Di Giuseppe, G.; Soldovieri, L.; Moffa, S.; Improta, I.; Capece, U.; Nista, E.C.; Cinti, F.; Ciccarelli, G.; Brunetti, M.; et al. Physical Activity and Type 2 Diabetes: In Search of a Personalized Approach to Improving β-Cell Function. Nutrients 2023, 15, 4202. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Su, C.-H. The Impact of Physical Exercise on Oxidative and Nitrosative Stress: Balancing the Benefits and Risks. Antioxidants 2024, 13, 573. [Google Scholar] [CrossRef]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. Biomed. Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef]
- Vina, J.; Sanchis-Gomar, F.; Martinez-Bello, V.; Gomez-Cabrera, M.C. Exercise acts as a drug; the pharmacological benefits of exercise. Br. J. Pharmacol. 2012, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise Effects on Physiological Function During Aging. Free Radic. Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef]
- El Assar, M.; Álvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodríguez-Mañas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef] [PubMed]
- Korsager Larsen, M.; Matchkov, V.V. Hypertension and Physical Exercise: The Role of Oxidative Stress. Medicina 2016, 52, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.J.; Fegers-Wustrow, I.; Halle, M.; Haykowsky, M.J.; Chung, E.H.; Kovacic, J.C. Exercise for Primary and Secondary Prevention of Cardiovascular Disease: JACC Focus Seminar 1/4. J. Am. Coll. Cardiol. 2022, 80, 1091–1106. [Google Scholar] [CrossRef]
- Königstein, K.; Dipla, K.; Zafeiridis, A. Training the Vessels: Molecular and Clinical Effects of Exercise on Vascular Health—A Narrative Review. Cells 2023, 12, 2544. [Google Scholar] [CrossRef]
- Versic, S.; Idrizovic, K.; Ahmeti, G.B.; Sekulic, D.; Majeric, M. Differential Effects of Resistance- and Endurance-Based Exercise Programs on Muscular Fitness, Body Composition, and Cardiovascular Variables in Young Adult Women: Contextualizing the Efficacy of Self-Selected Exercise Modalities. Medicina 2021, 57, 654. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine Supplementation, Physical Exercise and Oxidative Stress Markers: A Review of the Mechanisms and Effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Exercise Metabolism: Fuels for the Fire. Cold Spring Harb. Perspect. Med. 2018, 8, a029744. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Yan, J.; Zhao, L.; Wu, G.; Wang, Y.L. Regulation of Mitochondrial Dynamics by Aerobic Exercise in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 8, 788505. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.G.R.; Hawley, J.A. Molecular Basis of Exercise-Induced Skeletal Muscle Mitochondrial Biogenesis: Historical Advances, Current Knowledge, and Future Challenges. Cold Spring Harb. Perspect. Med. 2018, 8, a029686. [Google Scholar] [CrossRef]
- Jung, S.; Kim, K. Exercise-induced PGC-1α Transcriptional Factors in Skeletal Muscle. Integr. Med. Res. 2014, 3, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step Towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.X. Interplay of mitochondria and diabetes: Unveiling novel therapeutic strategies. Mitochondrion 2024, 75, 101850. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef]
- Gillen, J.B.; Gibala, M.J. Is High-Intensity Interval Training a Time-Efficient Exercise Strategy to Improve Health and Fitness? Appl. Physiol. Nutr. Metab. 2014, 39, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Shishira, K.B.; Vaishali, K.; Kadavigere, R.; Sukumar, S.; Shivashankara, K.N.; Pullinger, S.A.; Bommasamudram, T. Effects of High-Intensity Interval Training versus Moderate-Intensity Continuous Training on Vascular Function Among Individuals with Overweight and Obesity—A Systematic Review. Int. J. Obes. 2024, 48, 1517–1533. [Google Scholar] [CrossRef]
- Srivastava, S.; Tamrakar, S.; Nallathambi, N.; Vrindavanam, S.A.; Prasad, R.; Kothari, R. Assessment of Maximal Oxygen Uptake (VO2 Max) in Athletes and Nonathletes Assessed in Sports Physiology Laboratory. Cureus 2024, 16, e61124. [Google Scholar] [CrossRef]
- Atakan, M.M.; Li, Y.; Koşar, Ş.N.; Turnagöl, H.H.; Yan, X. Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. Int. J. Environ. Res. Public Health 2021, 18, 7201. [Google Scholar] [CrossRef]
- Ito, S. High-Intensity Interval Training for Health Benefits and Care of Cardiac Diseases—The Key to an Efficient Exercise Protocol. World J. Cardiol. 2019, 11, 171–188. [Google Scholar] [CrossRef]
- Caprara, G. Mediterranean-Type Dietary Pattern and Physical Activity: The Winning Combination to Counteract the Rising Burden of Non-Communicable Diseases (NCDs). Nutrients 2021, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Erbasan, H.; Riso, P.; Perna, S. Impact of the Mediterranean Diet on Athletic Performance, Muscle Strength, Body Composition, and Antioxidant Markers in Both Athletes and Non-Professional Athletes: A Systematic Review of Intervention Trials. Nutrients 2024, 16, 3454. [Google Scholar] [CrossRef]
- Hershey, M.S.; Martínez-González, M.Á.; Álvarez-Álvarez, I.; Martínez Hernández, J.A.; Ruiz-Canela, M. The Mediterranean Diet and Physical Activity: Better Together than Apart for the Prevention of Premature Mortality. Br. J. Nutr. 2022, 128, 1413–1424. [Google Scholar] [CrossRef]
- Myburgh, K.H. Polyphenol Supplementation: Benefits for Exercise Performance or Oxidative Stress? Sports Med. 2014, 44 (Suppl. S1), S57–S70. [Google Scholar] [CrossRef]
- O’Donoghue, G.; O’Sullivan, C.; Corridan, I.; Daly, J.; Finn, R.; Melvin, K.; Peiris, C. Lifestyle Interventions to Improve Glycemic Control in Adults with Type 2 Diabetes Living in Low-and-Middle Income Countries: A Systematic Review and Meta-Analysis of Randomized Controlled Trials (RCTs). Int. J. Environ. Res. Public Health 2021, 18, 6273. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Dyer, K.A.; Hyde, B.; Davis, C.R.; Bracci, E.L.; Woodman, R.J.; Hodgson, J.M. Long-Term Adherence to a Mediterranean Diet 1-Year after Completion of the MedLey Study. Nutrients 2022, 14, 3098. [Google Scholar] [CrossRef]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Huang, Q.; Zhang, Q.; Li, M.; Wu, Y. The Effectiveness of Lifestyle Interventions for Diabetes Remission on Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Worldviews Evid. Based Nurs. 2023, 20, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; You, W.; Almeida, F.; Estabrooks, P.; Davy, B. The Effectiveness and Cost of Lifestyle Interventions Including Nutrition Education for Diabetes Prevention: A Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2017, 117, 404–421.e36. [Google Scholar] [CrossRef]
- Langhammer, B.; Bergland, A.; Rydwik, E. The Importance of Physical Activity Exercise among Older People. Biomed. Res. Int. 2018, 2018, 7856823. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Lu, Y.; Zhang, H.; Zhu, C.; Tian, L.; Chen, J.; He, E.; Li, Y. Optimal Strategies for Exercise Intervention in Older People Diabetic Patients: The Impacts of Intensity, Form, and Frequency on Glycemic Control: An Exercise Prescription for Older People with Diabetes. Arch. Gerontol. Geriatr. 2025, 128, 105621. [Google Scholar] [CrossRef]
- Batsis, J.A.; Roderka, M.N.; Rauch, V.K.; Seo, L.M.; Li, X.; DiMilia, P.R.; Gooding, T.; Gilbert-Diamond, D.; McClure, A.C.; Roth, R.M. Impact of Diet and Exercise on Weight and Cognition in Older Adults: A Rapid Review. Am. J. Health Promot. 2021, 35, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Morros, A.; Franch-Nadal, J.; Real, J.; Gratacòs, M.; Mauricio, D. Sex Differences in Cardiovascular Prevention in Type 2: Diabetes in a Real-World Practice Database. J. Clin. Med. 2022, 11, 2196. [Google Scholar] [CrossRef] [PubMed]
- Koceva, A.; Herman, R.; Janez, A.; Rakusa, M.; Jensterle, M. Sex- and Gender-Related Differences in Obesity: From Pathophysiological Mechanisms to Clinical Implications. Int. J. Mol. Sci. 2024, 25, 7342. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, G.L.; da Silva Dias, D.; Malfitano, C.; Sanches, I.C.; Llesuy, S.; Ulloa, L.; Irigoyen, M.C.; De Angelis, K. Combined Aerobic and Resistance Exercise Training Improve Hypertension Associated with Menopause. Front. Physiol. 2018, 9, 1471. [Google Scholar] [CrossRef] [PubMed]
- Mazza, E.; Troiano, E.; Ferro, Y.; Lisso, F.; Tosi, M.; Turco, E.; Pujia, R.; Montalcini, T. Obesity, Dietary Patterns, and Hormonal Balance Modulation: Gender-Specific Impacts. Nutrients 2024, 16, 1629. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.T.; Li, Y.; Whitbourne, S.B.; Djousse, L.; Wang, D.D.; Ivey, K.; Willett, W.C.; Gaziano, J.M.; Cho, K.; Hu, F.B.; et al. Racial and Ethnic Disparities in Dietary Intake and Quality Among United States Veterans. Curr. Dev. Nutr. 2024, 8, 104461. [Google Scholar] [CrossRef]
- Haw, J.S.; Shah, M.; Turbow, S.; Egeolu, M.; Umpierrez, G. Diabetes Complications in Racial and Ethnic Minority Populations in the USA. Curr. Diab. Rep. 2021, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Feairheller, D.L.; Park, J.Y.; Sturgeon, K.M.; Williamson, S.T.; Diaz, K.M.; Veerabhadrappa, P.; Brown, M.D. Racial Differences in Oxidative Stress and Inflammation: In Vitro and In Vivo. Clin. Transl. Sci. 2011, 4, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.A.; Zhao, L.; Patel, R.S.; Jones, D.P.; Ahmed, Y.; Stoyanova, N.; Gibbons, G.H.; Vaccarino, V.; Din-Dzietham, R.; Quyyumi, A.A. Differences in Systemic Oxidative Stress Based on Race and the Metabolic Syndrome: The Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) Study. Metab. Syndr. Relat. Disord. 2012, 10, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Monterrosa, E.C.; Frongillo, E.A.; Drewnowski, A.; de Pee, S.; Vandevijvere, S. Sociocultural Influences on Food Choices and Implications for Sustainable Healthy Diets. Food Nutr. Bull. 2020, 41 (Suppl. S2), 59S–73S. [Google Scholar] [CrossRef] [PubMed]
- Yang, A. Cultural Cuisines and Cardiovascular Care: Tailoring Dietary Interventions for Effective Prevention. Adv. Nutr. 2024, 15, 100331. [Google Scholar] [CrossRef]
- Brinkmann, C. Road Map for Personalized Exercise Medicine in T2DM. Trends Endocrinol. Metab. 2023, 34, 789–798. [Google Scholar] [CrossRef]
- Yahaya, J.J.; Doya, I.F.; Morgan, E.D.; Ngaiza, A.I.; Bintabara, D. Poor Glycemic Control and Associated Factors Among Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study. Sci. Rep. 2023, 13, 9673. [Google Scholar] [CrossRef] [PubMed]
- Galiero, R.; Caturano, A.; Vetrano, E.; Monda, M.; Marfella, R.; Sardu, C.; Salvatore, T.; Rinaldi, L.; Sasso, F.C. Precision Medicine in Type 2 Diabetes Mellitus: Utility and Limitations. Diabetes Metab. Syndr. Obes. 2023, 16, 3669–3689. [Google Scholar] [CrossRef] [PubMed]
- Tebani, A.; Bekri, S. Paving the Way to Precision Nutrition Through Metabolomics. Front. Nutr. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Shea, L.; Pesa, J.; Geonnotti, G.; Powell, V.; Kahn, C.; Peters, W. Improving Diversity in Study Participation: Patient Perspectives on Barriers, Racial Differences and the Role of Communities. Health Expect. 2022, 25, 1979–1987. [Google Scholar] [CrossRef]
- Caturano, A.; Galiero, R.; Loffredo, G.; Vetrano, E.; Medicamento, G.; Acierno, C.; Rinaldi, L.; Marrone, A.; Salvatore, T.; Monda, M.; et al. Effects of a Combination of Empagliflozin Plus Metformin vs. Metformin Monotherapy on NAFLD Progression in Type 2 Diabetes: The IMAGIN Pilot Study. Biomedicines 2023, 11, 322. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Gelso, A.; Bono, V.; Costagliola, C.; Marfella, R.; Sardu, C.; Rinaldi, L.; Galiero, R.; Acierno, C.; et al. Relationship between Albuminuric CKD and Diabetic Retinopathy in a Real-World Setting of Type 2 Diabetes: Findings from the No Blind Study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Trenfield, S.J.; Pollard, T.D.; Ong, J.J.; Elbadawi, M.; McCoubrey, L.E.; Goyanes, A.; Gaisford, S.; Basit, A.W. Connected healthcare: Improving patient care using digital health technologies. Adv. Drug Deliv. Rev. 2021, 178, 113958. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Pafundi, P.C.; Gelso, A.; Bono, V.; Costagliola, C.; Marfella, R.; Sardu, C.; Rinaldi, L.; Galiero, R.; Acierno, C.; et al. Telemedicine for Screening Diabetic Retinopathy: The NO BLIND Italian Multicenter Study. Diabetes Metab. Res. Rev. 2019, 35, e3113. [Google Scholar] [CrossRef]
- Galiero, R.; Pafundi, P.C.; Nevola, R.; Rinaldi, L.; Acierno, C.; Caturano, A.; Salvatore, T.; Adinolfi, L.E.; Costagliola, C.; Sasso, F.C. The importance of telemedicine during COVID-19 pandemic: A focus on diabetic retinopathy. J. Diabetes Res. 2020, 2020, 9036847. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V. The Next Generation of Evidence-Based Medicine. Nat. Med. 2023, 29, 49–58. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caturano, A.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Iadicicco, I.; Donnarumma, M.; Galiero, R.; Acierno, C.; et al. Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications. Antioxidants 2025, 14, 72. https://doi.org/10.3390/antiox14010072
Caturano A, Rocco M, Tagliaferri G, Piacevole A, Nilo D, Di Lorenzo G, Iadicicco I, Donnarumma M, Galiero R, Acierno C, et al. Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications. Antioxidants. 2025; 14(1):72. https://doi.org/10.3390/antiox14010072
Chicago/Turabian StyleCaturano, Alfredo, Maria Rocco, Giuseppina Tagliaferri, Alessia Piacevole, Davide Nilo, Giovanni Di Lorenzo, Ilaria Iadicicco, Mariarosaria Donnarumma, Raffaele Galiero, Carlo Acierno, and et al. 2025. "Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications" Antioxidants 14, no. 1: 72. https://doi.org/10.3390/antiox14010072
APA StyleCaturano, A., Rocco, M., Tagliaferri, G., Piacevole, A., Nilo, D., Di Lorenzo, G., Iadicicco, I., Donnarumma, M., Galiero, R., Acierno, C., Sardu, C., Russo, V., Vetrano, E., Conte, C., Marfella, R., Rinaldi, L., & Sasso, F. C. (2025). Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications. Antioxidants, 14(1), 72. https://doi.org/10.3390/antiox14010072












