Antioxidant, Antithrombotic and Anti-Inflammatory Properties of Amphiphilic Bioactives from Water Kefir Grains and Its Apple Pomace-Based Fermented Beverage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Instrumentation
2.2. Preparation of WKGs and WKB Samples
2.3. Extraction of Lipid Bioactives from WKGs and WKB and Separation of Their Total Amphiphilic (TAC) and Total Lipophilic Content (TLC)
2.4. Total Carotenoid Content (TCC) Analysis
2.5. Total Phenolic Content (TPC) Analysis
2.6. Assessment of Antioxidant Activities of Extracts
2.7. Assessment of Antiplatelet and Anti-Inflammatory Properties of Extracts with Light Transmittance Aggregometry
2.8. ATR-FTIR Analysis
2.9. LC–MS Analysis
2.10. StatisticalAnalysis
3. Results and Discussion
3.1. Yield Extraction of Lipids from WKGs and WKB
3.2. Total Carotenoid Content (TCC) of Extracts from WKGs and WKB
3.3. Total Phenolic Content (TPC) of Extracts from WKGs and WKB
3.4. Antioxidant Activity of Extracts from WKGs and WKB
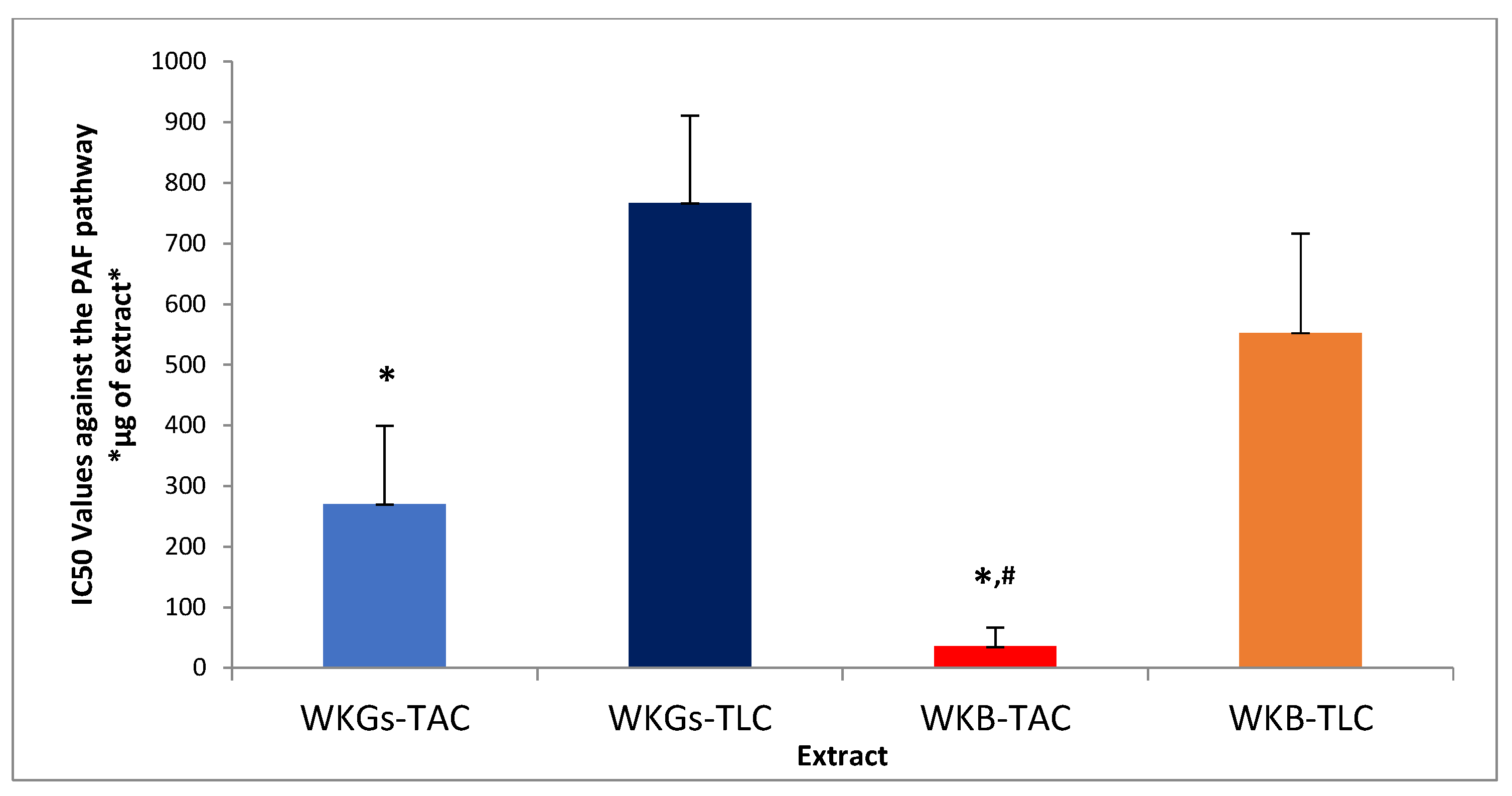
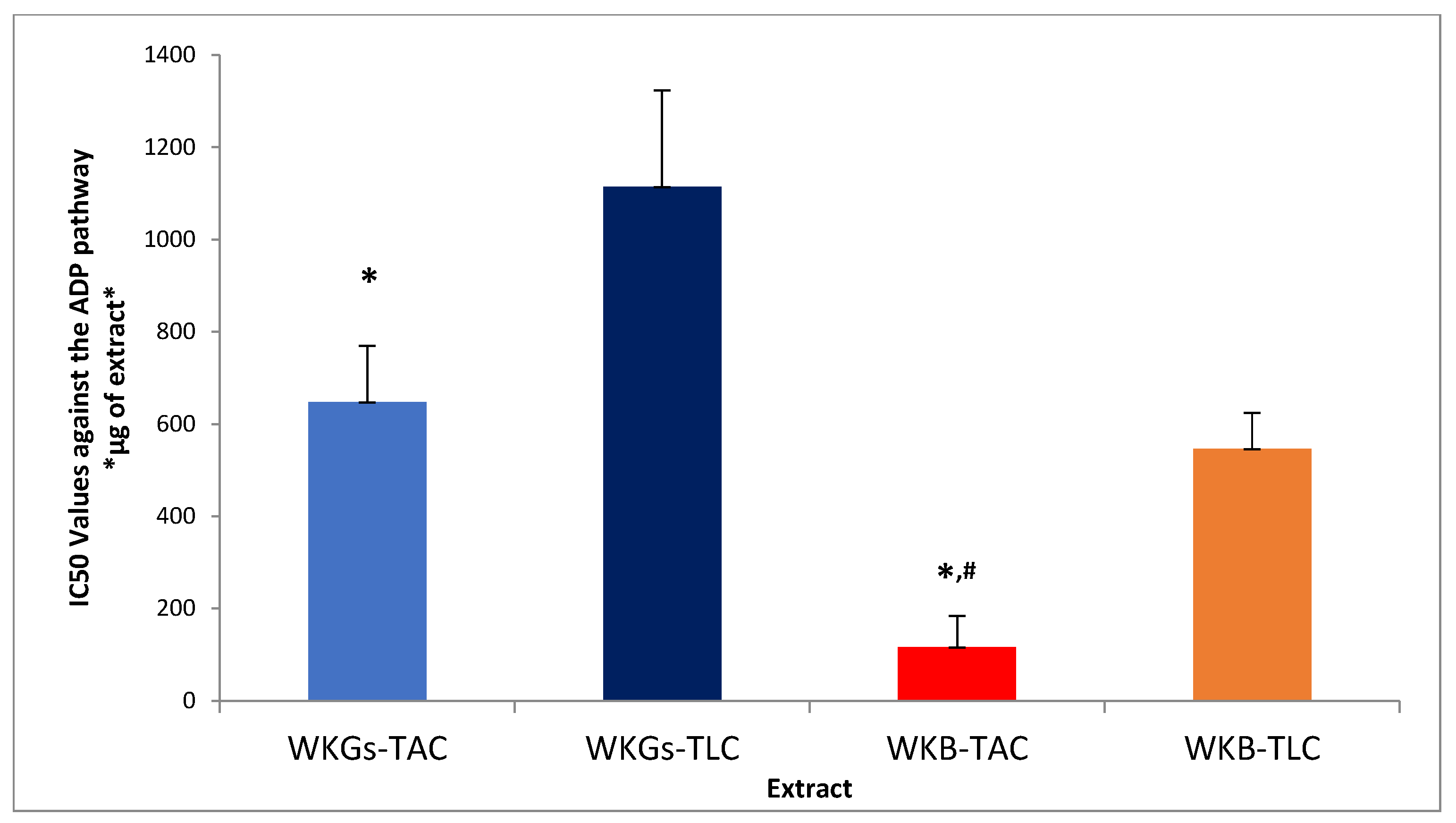
3.5. Anti-Inflammatory and Antiplatelet Properties of Extracts from WKGs and WKB
3.6. ATR-FTIR Analysis of Amphiphilic Extracts from WKGs and WKB
3.7. Fatty Acid Composition of the Amphiphilic Extracts from WKGs and WKB
3.8. Structural Elucidation of the PL Biocatives Present in the Amphiphilic Extracts from WKGs and WKB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 8 January 2025).
- Tsoupras, A.; Adamantidi, T.; Finos, M.A.; Philippopoulos, A.; Detopoulou, P.; Tsopoki, I.; Kynatidou, M.; Demopoulos, C.A. Re-Assessing the Role of Platelet Activating Factor and Its Inflammatory Signaling and Inhibitors in Cancer and Anti-Cancer Strategies. Front. Biosci.-Landmark 2024, 29, 345. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A. The Anti-Inflammatory and Antithrombotic Properties of Bioactives from Orange, Sanguine and Clementine Juices and from Their Remaining By-Products. Beverages 2022, 8, 39. [Google Scholar] [CrossRef]
- Tsoupras, A.; Moran, D.; Pleskach, H.; Durkin, M.; Traas, C.; Zabetakis, I. Beneficial Anti-Platelet and Anti-Inflammatory Properties of Irish Apple Juice and Cider Bioactives. Foods 2021, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Moran, D.; Shiels, K.; Saha, S.K.; Abu-Reidah, I.M.; Thomas, R.H.; Redfern, S. Enrichment of Whole-Grain Breads with Food-Grade Extracted Apple Pomace Bioactives Enhanced Their Anti-Inflammatory, Antithrombotic and Anti-Oxidant Functional Properties. Antioxidants 2024, 13, 225. [Google Scholar] [CrossRef]
- Vandorou, M.; Plakidis, C.; Tsompanidou, I.M.; Adamantidi, T.; Panagopoulou, E.A.; Tsoupras, A. A Review on Apple Pomace Bioactives for Natural Functional Food and Cosmetic Products with Therapeutic Health-Promoting Properties. IJMS 2024, 25, 10856. [Google Scholar] [CrossRef]
- Tsoupras, A.; Panagopoulou, E.A.; Kyzas, G.Z. Anti-Inflammatory, Antithrombotic and Anti-Oxidant Bioactives of Beer and Brewery by-Products, as Ingredients of Bio-Functional Foods, Nutraceuticals, Cosmetics, Cosmeceuticals and Pharmaceuticals with Health Promoting Properties. AIMS Agric. Food 2024, 9, 568–606. [Google Scholar] [CrossRef]
- Tsoupras, A.; Ni, V.L.J.; O’Mahony, É.; Karali, M. Winemaking: “With One Stone, Two Birds”? A Holistic Review of the Bio-Functional Compounds, Applications and Health Benefits of Wine and Wineries’ By-Products. Fermentation 2023, 9, 838. [Google Scholar] [CrossRef]
- Moran, D.; Fleming, M.; Daly, E.; Gaughan, N.; Zabetakis, I.; Traas, C.; Tsoupras, A. Anti-Platelet Properties of Apple Must/Skin Yeasts and of Their Fermented Apple Cider Products. Beverages 2021, 7, 54. [Google Scholar] [CrossRef]
- Tsoupras, A.; Davi, K.G. Bioactive Metabolites from Fungi with Anti-Inflammatory and Antithrombotic Properties: Current Status and Future Perspectives for Drug Development. In Fungi Bioactive Metabolites; Deshmukh, S.K., Takahashi, J.A., Saxena, S., Eds.; Springer Nature: Singapore, 2024; pp. 427–494. ISBN 978-981-9956-95-1. [Google Scholar]
- Moretti, A.F.; Moure, M.C.; Quiñoy, F.; Esposito, F.; Simonelli, N.; Medrano, M.; León-Peláez, Á. Water Kefir, a Fermented Beverage Containing Probiotic Microorganisms: From Ancient and Artisanal Manufacture to Industrialized and Regulated Commercialization. Future Foods 2022, 5, 100123. [Google Scholar] [CrossRef]
- Papadopoulou, D.; Chrysikopoulou, V.; Rampaouni, A.; Tsoupras, A. Antioxidant and Anti-Inflammatory Properties of Water Kefir Microbiota and Its Bioactive Metabolites for Health Promoting Bio-Functional Products and Applications. Aimsmicro 2024, 10, 756–811. [Google Scholar] [CrossRef]
- Bueno, R.S.; Ressutte, J.B.; Hata, N.N.Y.; Henrique-Bana, F.C.; Guergoletto, K.B.; De Oliveira, A.G.; Spinosa, W.A. Quality and Shelf Life Assessment of a New Beverage Produced from Water Kefir Grains and Red Pitaya. LWT 2021, 140, 110770. [Google Scholar] [CrossRef]
- How to Make Water Kefir with Bio-Ferment Water Kefir Grains; The Ferment Company. Available online: https://www.thefermentcompany.nl/hoe-maak-je-waterkefir/ (accessed on 8 January 2025).
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Galanos, D.S.; Kapoulas, V.M. Isolation of Polar Lipids from Triglyceride Mixtures. J. Lipid Res. 1962, 3, 134–136. [Google Scholar] [CrossRef]
- Tsoupras, A.; Moran, D.; Byrne, T.; Ryan, J.; Barrett, L.; Traas, C.; Zabetakis, I. Anti-Inflammatory and Anti-Platelet Properties of Lipid Bioactives from Apple Cider By-Products. Molecules 2021, 26, 2869. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Tsoupras, A.; Cholidis, P.; Kranas, D.; Galouni, E.A.; Ofrydopoulou, A.; Efthymiopoulos, P.; Shiels, K.; Saha, S.K.; Kyzas, G.Z.; Anastasiadou, C. Anti-Inflammatory, Antithrombotic, and Antioxidant Properties of Amphiphilic Lipid Bioactives from Shrimp. Pharmaceuticals 2024, 18, 25. [Google Scholar] [CrossRef]
- Vordos, N.; Giannakopoulos, S.; Vansant, E.F.; Kalaitzis, C.; Nolan, J.W.; Bandekas, D.V.; Karavasilis, I.; Mitropoulos, A.C.; Touloupidis, S. Small-Angle X-Ray Scattering (SAXS) and Nitrogen Porosimetry (NP): Two Novel Techniques for the Evaluation of Urinary Stone Hardness. Int. Urol. Nephrol. 2018, 50, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Lordan, R.; Harrington, J.; Pienaar, R.; Devaney, K.; Heaney, S.; Koidis, A.; Zabetakis, I. The Effects of Oxidation on the Antithrombotic Properties of Tea Lipids against PAF, Thrombin, Collagen, and ADP. Foods 2020, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative Study on Total Lipid Determination Using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and Modified Bligh & Dyer Extraction Methods. J. Food Compos. Anal. 2001, 14, 93–100. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Matsakas, L. An Overview of Current Pretreatment Methods Used to Improve Lipid Extraction from Oleaginous Microorganisms. Molecules 2018, 23, 1562. [Google Scholar] [CrossRef]
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A.; Sahin, S. Comparison of Antioxidant Capacity of Cow and Ewe Milk Kefirs. J. Dairy. Sci. 2018, 101, 3788–3798. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, G.; Chen, W. Beneficial Effects of Tomato Juice Fermented by Lactobacillus Plantarum and Lactobacillus Casei: Antioxidation, Antimicrobial Effect, and Volatile Profiles. Molecules 2018, 23, 2366. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.Y.; Lim, X.X.; Khor, B.H.; Rasti, B.; Tan, T.C.; Kobun, R.; Uthumporn, U. Production and Optimisation of Fermented Pumpkin-Based Mature Coconut Water Kefir Beverage Using Response Surface Methodology. Beverages 2024, 10, 34. [Google Scholar] [CrossRef]
- Santos, D.C.D.; Pimentel, T.C.; Sousa, T.L.D.; Almeida, A.B.D.; Oliveira, M.S.; Oliveira Filho, J.G.D.; Silva, F.G.; Egea, M.B. Cerrado Cagaita (Eugenia Dysenterica) Cloudy and Clarified Beverages: Effect of Kefir Fermentation and Inulin Addition. Food Biosci. 2024, 61, 104767. [Google Scholar] [CrossRef]
- Silva, J.C.D.M.; Santana, R.V.; Almeida, A.B.D.; Takeuchi, K.P.; Egea, M.B. Changes in the Chemical, Technological, and Microbiological Properties of Kefir-Fermented Soymilk after Supplementation with Inulin and Acrocomia Aculeata Pulp. Appl. Sci. 2021, 11, 5575. [Google Scholar] [CrossRef]
- Priyadarshini Pradhan, S.; Padhi, S.; Dash, M.; Heena, M.B.; Behera, A. Chapter 7—Carotenoids. Nutraceuticals Health Care 2022, 7, 135–157. [Google Scholar] [CrossRef]
- Salari, S.; Ferreira, J.; Lima, A.; Sousa, I. Effects of Particle Size on Physicochemical and Nutritional Properties and Antioxidant Activity of Apple and Carrot Pomaces. Foods 2024, 13, 710. [Google Scholar] [CrossRef]
- Popescu, L.; Ceșco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods 2022, 11, 3565. [Google Scholar] [CrossRef] [PubMed]
- Darvishzadeh, P.; Orsat, V.; Martinez, J.L. Process Optimization for Development of a Novel Water Kefir Drink with High Antioxidant Activity and Potential Probiotic Properties from Russian Olive Fruit (Elaeagnus Angustifolia). Food Bioprocess. Technol. 2021, 14, 248–260. [Google Scholar] [CrossRef]
- Azi, F.; Tu, C.; Rasheed, H.A.; Dong, M. Comparative Study of the Phenolics, Antioxidant and Metagenomic Composition of Novel Soy Whey-based Beverages Produced Using Three Different Water Kefir Microbiota. Int. J. Food Sci. Tech. 2020, 55, 1689–1697. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.-C.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H.; Kubow, S.; Almajwal, A.M.; Razzak Mahmood, A.A.; Al-Qaisi, A.; Bawadi, H. Enhancing the Quality of Lentil Proteins via Combination with Whey Proteins Based on a Dual Process: A Novel Strategy through the Incorporation of Complexation and Fermentation. Food Sci. Biotechnol. 2024, 34, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Queiroz Santos, V.A.; Nascimento, C.G.; Schmidt, C.A.P.; Mantovani, D.; Dekker, R.F.H.; Da Cunha, M.A.A. Solid-State Fermentation of Soybean Okara: Isoflavones Biotransformation, Antioxidant Activity and Enhancement of Nutritional Quality. LWT 2018, 92, 509–515. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Solid-State Fermentation of Apple Pomace Using Phanerocheate Chrysosporium—Liberation and Extraction of Phenolic Antioxidants. Food Chem. 2011, 126, 1071–1080. [Google Scholar] [CrossRef]
- Aiello, F.; Restuccia, D.; Spizzirri, U.G.; Carullo, G.; Leporini, M.; Loizzo, M.R. Improving Kefir Bioactive Properties by Functional Enrichment with Plant and Agro-Food Waste Extracts. Fermentation 2020, 6, 83. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.-C.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H.; Aleid, G.M.; Alhamad, M.N.; Maghaydah, S. Evaluation of Quality and Protein Structure of Natural Water Kefir-Fermented Quinoa Protein Concentrates. Food Chem. 2023, 404, 134614. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaisi, A.; Alrosan, M.; Almajwal, A.M.; Gammoh, S.; Alu’datt, M.H.; Kubow, S.; Tan, T.; Mahmood, A.A.R.; Qudsi, F.R.A. Evaluation of Structure, Quality, Physicochemical Properties, and Phenolics Content of Pea Proteins: A Novel Strategy through the Incorporation of Fermentation. J. Food Sci. 2024, 89, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Şafak, H.; Gün, İ.; Tudor Kalit, M.; Kalit, S. Physico-Chemical, Microbiological and Sensory Properties of Water Kefir Drinks Produced from Demineralized Whey and Dimrit and Shiraz Grape Varieties. Foods 2023, 12, 1851. [Google Scholar] [CrossRef]
- Koh, W.Y.; Lim, X.X.; Uthumporn, U.; Tan, T.C.; Kobun, R.; Rasti, B. Characterisation of Physicochemical Properties, Anti-Hyperglycaemic Effects, and Probiotic Potentials in Fermented Pumpkin Drink Utilising Lactobacillus Mali Isolated from Water Kefir. IFRJ 2024, 31, 398–416. [Google Scholar] [CrossRef]
- Agirman, B.; Yildiz, I.; Polat, S.; Erten, H. The Evaluation of Black Carrot, Green Cabbage, Grape, and Apple Juices as Substrates for the Production of Functional Water Kefir-like Beverages. Food Sci. Nutr. 2024, 12, 6595–6611. [Google Scholar] [CrossRef]
- Aziz, N.S.; Chin, Z.K.; Mohd Razali, N.S.; Sofian-Seng, N.S.; Kasim, K.F. Development of Mature Coconut (Cocos Nucifera L.) Probiotic Beverage: Physicochemical Characteristics, Microbial Count, Antioxidant Activity, and Sensory Acceptance. IFRJ 2023, 30, 119–129. [Google Scholar] [CrossRef]
- Kumar, M.; Yeap, S.; Lee, H.; Mohamad, N.; Nazirul Mubin Aziz, M.; Khalid, M.; Masarudin, M.; Leow, A.; Abdullah, J.; Alitheen, N. Selected Kefir Water from Malaysia Attenuates Hydrogen Peroxide-Induced Oxidative Stress by Upregulating Endogenous Antioxidant Levels in SH-SY5Y Neuroblastoma Cells. Antioxidants 2021, 10, 940. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Sereti, V.; Kotsiou, K.; Ciurlă, L.; Patras, A.; Irakli, M.; Lazaridou, A. Valorizing Apple Pomace as Stabilizer of Olive Oil-Water Emulsion Used for Reduction of Saturated Fat in Biscuits. Food Hydrocoll. 2024, 151, 109746. [Google Scholar] [CrossRef]
- Lin, L.; Peng, A.; Yang, K.; Zou, Y. Monomeric Phenolics in Different Parts of High-acid Apple (Malus Sieversii f Niedzwetzkyana (Dieck) Langenf): A Promising Source of Antioxidants for Application in Nutraceuticals. Int. J. Food Sci. Tech. 2018, 53, 1503–1509. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Application of the Pulsed Electric Field to Release Bound Phenolics in Sorghum Flour and Apple Pomace. Innov. Food Sci. Emerg. Technol. 2016, 35, 29–35. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, T.; Wang, X.; Lü, X. Apple Pomace as a Potential Valuable Resource for Full-Components Utilization: A Review. J. Clean. Prod. 2021, 329, 129676. [Google Scholar] [CrossRef]
- Sudha, M.L. Apple Pomace (By-Product of Fruit Juice Industry) as a Flour Fortification Strategy. In Flour and Breads and their Fortification in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 395–405. ISBN 978-0-12-380886-8. [Google Scholar]
- Luang-In, V.; Saengha, W.; Yotchaisarn, M.; Halaslova, M.; Udomwong, P.; Deeseenthum, S. Microbial Strains and Bioactive Exopolysaccharide Producers from Thai Water Kefir. Microbiol. Biotechnol. Lett. 2018, 46, 403–415. [Google Scholar] [CrossRef]
- Tu, C.; Azi, F.; Huang, J.; Xu, X.; Xing, G.; Dong, M. Quality and Metagenomic Evaluation of a Novel Functional Beverage Produced from Soy Whey Using Water Kefir Grains. LWT 2019, 113, 108258. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P. Red Beetroot Juice Fermented by Water Kefir Grains: Physicochemical, Antioxidant Profile and Anticancer Activity. Eur. Food Res. Technol. 2023, 249, 939–950. [Google Scholar] [CrossRef]
- Tiwari, S.; Jha, N.K. Kalaivany Nutraceuticals from Apple Waste. In Nutraceuticals from Fruit and Vegetable Waste; Tomer, V., Chhikara, N., Kumar, A., Panghal, A., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 97–119. ISBN 978-1-119-80350-8. [Google Scholar]
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.M.G.C.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Revisiting the Chemistry of Apple Pomace Polyphenols. Food Chem. 2019, 294, 9–18. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Zhang, H.; Qu, G.; Li, C.; Liu, L. Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus Rhamnosus L08. Foods 2021, 10, 1343. [Google Scholar] [CrossRef] [PubMed]
- Aligita, W.; Tarigan, P.N.; Susilawati, E. Anti inflammatory and antioxidant activity of kefir water. IJBPAS 2020, 9, 2454–2464. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Araújo, T.H.; Schneedorf, J.M.; de Souza Ferreira, C.; Moraes, G. de O.I.; Coimbra, R.S.; Rodrigues, M.R. A Novel Beer Fermented by Kefir Enhances Anti-Inflammatory and Anti-Ulcerogenic Activities Found Isolated in Its Constituents. J. Funct. Foods 2016, 21, 58–69. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Demopoulos, C.A.; Pappas, K.M. Platelet-activating Factor Detection, Metabolism, and Inhibitors in the Ethanologenic Bacterium Zymomonas mobilis. Euro J. Lipid Sci. Tech. 2012, 114, 123–133. [Google Scholar] [CrossRef]
- Ashraf, M.; Nookala, V. Biochemistry of Platelet Activating Factor. In Statpearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Keating, F.K.; Schneider, D.J. The Influence of Platelet Activating Factor on the Effects of Platelet Agonists and Antiplatelet Agents In Vitro. J. Thromb. Thrombolysis 2009, 28, 38–45. [Google Scholar] [CrossRef]
- Klein, D.R. Organic Chemistry II John Wiley and Sons; John Wiley and Sons: Hoboken, NJ, USA, 2015; ISBN 10:1118452283. [Google Scholar]
- Wongsa, P.; Phatikulrungsun, P.; Prathumthong, S. FT-IR Characteristics, Phenolic Profiles and Inhibitory Potential against Digestive Enzymes of 25 Herbal Infusions. Sci. Rep. 2022, 12, 6631. [Google Scholar] [CrossRef] [PubMed]
- Alrosan, M.; Tan, T.-C.; Mat Easa, A.; Gammoh, S.; Alu’datt, M.H.; Kubow, S.; Madi Almajwal, A.; Maghaydah, S.; Razzak Mahmood, A.A.; Al-Qaisi, A.; et al. Characterisation of the Protein Quality and Composition of Water Kefir-Fermented Casein. Food Chem. 2024, 443, 138574. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Gabriela Medina-Meza, I. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Güzel-Seydim, Z.B.; Şatır, G.; Gökırmaklı, Ç. Use of Mandarin and Persimmon Fruits in Water Kefir Fermentation. Food Sci. Nutr. 2023, 11, 5890–5897. [Google Scholar] [CrossRef]
- Tornabene, T.G.; Holzer, G.; Bittner, A.S.; Grohmann, K. Characterization of the Total Extractable Lipids of Zymomonas Mobilis Var. Mobilis. Can. J. Microbiol. 1982, 28, 1107–1118. [Google Scholar] [CrossRef]
- Drucker, D.B.; Megson, G.; Harty, D.W.; Riba, I.; Gaskell, S.J. Phospholipids of Lactobacillus Spp. J. Bacteriol. 1995, 177, 6304–6308. [Google Scholar] [CrossRef]
- Shah, N.; Cynkar, W.; Smith, P.; Cozzolino, D. Use of Attenuated Total Reflectance Midinfrared for Rapid and Real-Time Analysis of Compositional Parameters in Commercial White Grape Juice. J. Agric. Food Chem. 2010, 58, 3279–3283. [Google Scholar] [CrossRef]
- Vinogradov, E.; Sadovskaya, I.; Grard, T.; Chapot-Chartier, M.-P. Structural Studies of the Rhamnose-Rich Cell Wall Polysaccharide of Lactobacillus Casei BL23. Carbohydr. Res. 2016, 435, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H.; Manners, D.J. Isolation and Composition of an Alkali-Soluble Glucan from the Cell Walls of Saccharomyces Cerevisiae. J. Gen. Microbiol. 1976, 94, 180–192. [Google Scholar] [CrossRef]
- Da Silva Araújo, C.; Macedo, L.L.; Teixeira, L.J.Q. Evaluation of Mid-Infrared Spectra Associated with Chemometrics for the Determination of Physicochemical Properties during Fermentation of a New Strawberry-Based Beverage with Water Kefir Grains. J. Food Compos. Anal. 2023, 123, 105490. [Google Scholar] [CrossRef]
- Araújo, C.D.S.; Macedo, L.L.; Teixeira, L.J.Q. Use of Mid-Infrared Spectroscopy to Predict the Content of Bioactive Compounds of a New Non-Dairy Beverage Fermented with Water Kefir. LWT 2023, 176, 114514. [Google Scholar] [CrossRef]
- Chand, P.; Pakade, Y.B. Removal of Pb from Water by Adsorption on Apple Pomace: Equilibrium, Kinetics, and Thermodynamics Studies. J. Chem. 2013, 2013, 164575. [Google Scholar] [CrossRef]
- Zlatanović, S.; Ostojić, S.; Micić, D.; Rankov, S.; Dodevska, M.; Vukosavljević, P.; Gorjanović, S. Thermal Behaviour and Degradation Kinetics of Apple Pomace Flours. Thermochim. Acta 2019, 673, 17–25. [Google Scholar] [CrossRef]
- Coma, M.E.; Peltzer, M.A.; Delgado, J.F.; Salvay, A.G. Water Kefir Grains as an Innovative Source of Materials: Study of Plasticiser Content on Film Properties. Eur. Polym. J. 2019, 120, 109234. [Google Scholar] [CrossRef]
- Lago, A.; Delgado, J.F.; Rezzani, G.D.; Cottet, C.; Ramírez Tapias, Y.A.; Peltzer, M.A.; Salvay, A.G. Multi-Component Biodegradable Materials Based on Water Kefir Grains and Yeast Biomasses: Effect of the Mixing Ratio on the Properties of the Films. Polymers 2023, 15, 2594. [Google Scholar] [CrossRef] [PubMed]
- Marinho, C.O.; Vianna, T.C.; Cecci, R.R.R.; Marangoni Júnior, L.; Alves, R.M.V.; Vieira, R.P. Effect of Water Kefir Grain Biomass on Chitosan Film Properties. Mater. Today Commun. 2022, 32, 103902. [Google Scholar] [CrossRef]
- Carey, V.C.; Ingram, L.O. Lipid Composition of Zymomonas Mobilis: Effects of Ethanol and Glucose. J. Bacteriol. 1983, 154, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Eckel, V.P.L.; Ziegler, L.-M.; Vogel, R.F.; Ehrmann, M. Bifidobacterium Tibiigranuli Sp. Nov. Isolated from Homemade Water Kefir. Int. J. Syst. Evol. Microbiol. 2020, 70, 1562–1570. [Google Scholar] [CrossRef]
- Gientka, I.; Kieliszek, M.; Jermacz, K.; Błażejak, S. Identification and Characterization of Oleaginous Yeast Isolated from Kefir and Its Ability to Accumulate Intracellular Fats in Deproteinated Potato Wastewater with Different Carbon Sources. BioMed Res. Int. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Bechtner, J.; Behr, J.; Eisenbach, L.; Geißler, A.J.; Vogel, R.F. Lifestyle of Lactobacillus Hordei Isolated from Water Kefir Based on Genomic, Proteomic and Physiological Characterization. Int. J. Food Microbiol. 2019, 290, 141–149. [Google Scholar] [CrossRef]
- Bechtner, J.; Xu, D.; Behr, J.; Ludwig, C.; Vogel, R.F. Proteomic Analysis of Lactobacillus Nagelii in the Presence of Saccharomyces Cerevisiae Isolated From Water Kefir and Comparison With Lactobacillus Hordei. Front. Microbiol. 2019, 10, 325. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Xie, Y.; Zhang, L.; Li, J.; Liu, H. Effects of Fermentation with Kefir Grains on Nutrient Composition, Flavor Volatiles, and Product Physical Stability of a Hemp Seed (Cannabis Sativa L.) Beverage. LWT 2023, 183, 114934. [Google Scholar] [CrossRef]
- Nascimento, R.Q.; Deamici, K.M.; Tavares, P.P.L.G.; De Andrade, R.B.; Guimarães, L.C.; Costa, J.A.V.; Magalhães-Guedes, K.T.; Druzian, J.I.; De Souza, C.O. Improving Water Kefir Nutritional Quality via Addition of Viable Spirulina Biomass. Bioresour. Technol. Rep. 2022, 17, 100914. [Google Scholar] [CrossRef]
- Wulansari, P.D.; Widodo; Sunarti; Nurliyani. Incorporation of Oat Milk with Probiotic Lacticaseibacillus Casei AP Improves the Quality of Kefir Produced from Goat Milk. Food Sci. Technol. 2022, 42, e10322. [Google Scholar] [CrossRef]
- Setyawardani, T.; Sumarmono, J.; Arief, I.I.; Rahardjo, A.H.D.; Widayaka, K.; Santosa, S.S. Improving Composition and Microbiological Characteristics of Milk Kefir Using Colostrum. Food Sci. Technol. 2020, 40, 699–707. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Chiș, M.S.; Dulf, F.V.; Podea, P.; Tofană, M. Analysis of Fatty Acids, Amino Acids and Volatile Profile of Apple By-Products by Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 1987. [Google Scholar] [CrossRef]
- Tavakkoli-Kakhki, M.; Motavasselian, M.; Mosaddegh, M.; Esfahani, M.M.; Kamalinejad, M.; Nematy, M.; Eslami, S. Omega-3 and Omega-6 Content of Medicinal Foods for Depressed Patients: Implications from the Iranian Traditional Medicine. Avicenna J. Phytomedicine 2014, 4, 225. [Google Scholar]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp Biol Med 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, C.; Li, G.; Yu, C.; Yin, M. Stearic Acid Protects Primary Cultured Cortical Neurons against Oxidative Stress. Acta Pharmacol. Sin. 2007, 28, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.-H.; Lin, S.-Y.; Ou, Y.-C.; Chen, W.-Y.; Chuang, Y.-H.; Yen, Y.-J.; Liao, S.-L.; Raung, S.-L.; Chen, C.-J. Stearic Acid Attenuates Cholestasis-Induced Liver Injury. Biochem. Biophys. Res. Commun. 2010, 391, 1537–1542. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de La Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Zadeh Hashem, E.; Khodadadi, M.; Asadi, F.; Koohi, M.K.; Eslami, M.; Hasani-Dizaj, S.; Taleb Zadeh, R. The Antioxidant Activity of Palmitoleic Acid on the Oxidative Stress Parameters of Palmitic Acid in Adult Rat Cardiomyocytes. Ann. Mil. Health Sci. Res. 2016, 14, e11467. [Google Scholar] [CrossRef]
- Tsai, Y.-W.; Lu, C.-H.; Chang, R.C.-A.; Hsu, Y.-P.; Ho, L.-T.; Shih, K.-C. Palmitoleic Acid Ameliorates Palmitic Acid-Induced Proinflammation in J774A.1 Macrophages via TLR4-Dependent and TNF-α-Independent Signallings. Prostaglandins Leukot. Essent. Fat. Acids 2021, 169, 102270. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated Fatty Acids as Antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Azi, F.; Tu, C.; Meng, L.; Zhiyu, L.; Cherinet, M.T.; Ahmadullah, Z.; Dong, M. Metabolite Dynamics and Phytochemistry of a Soy Whey-Based Beverage Bio-Transformed by Water Kefir Consortium. Food Chem. 2021, 342, 128225. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, X.; Yue, Y.; Du, G.; Chen, H.; Ning, M.; Yuan, Y.; Yue, T. Metagenomic Features of Tibetan Kefir Grains and Its Metabolomics Analysis during Fermentation. LWT 2023, 175, 114502. [Google Scholar] [CrossRef]
- Rombaut, R.; Dewettinck, K.; Van Camp, J. Phospho- and Sphingolipid Content of Selected Dairy Products as Determined by HPLC Coupled to an Evaporative Light Scattering Detector (HPLC–ELSD). J. Food Compos. Anal. 2007, 20, 308–312. [Google Scholar] [CrossRef]
- Li, C.; Huang, R.; Guo, M.; Ge, Y. Lipidomics Reveals the Regulatory Mechanism of Exogenous Caffeic Acid on Glycerophospholipid Metabolism in Apple Fruit. Sci. Hortic. 2024, 329, 112990. [Google Scholar] [CrossRef]
- Wang, S.Y.; Faust, M. Variation in Lipid Composition of Apples in Relation to Watercore. J. Amer. Soc. Hort. Sci. 1992, 117, 829–833. [Google Scholar] [CrossRef]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef]
- Velissaridou, A.; Panoutsopoulou, E.; Prokopiou, V.; Tsoupras, A. Cardio-Protective-Promoting Properties of Functional Foods Inducing HDL-Cholesterol Levels and Functionality. Nutraceuticals 2024, 4, 469–502. [Google Scholar] [CrossRef]
- Maeda, N.; Hada, T.; Yoshida, H.; Mizushina, Y. Inhibitory Effect on Replicative DNA Polymerases, Human Cancer Cell Proliferation, and In Vivo Anti-Tumor Activity by Glycolipids from Spinach. CMC 2007, 14, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Quasney, M.E.; Carter, L.C.; Oxford, C.; Watkins, S.M.; Gershwin, M.E.; German, J.B. Inhibition of Proliferation and Induction of Apoptosis in SNU-1 Human Gastric Cancer Cells by the Plant Sulfolipid, Sulfoquinovosyldiacylglycerol. J. Nutr. Biochem. 2001, 12, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Kokai, Y.; Ohtani, S.; Sahara, H.; Kumamoto-Yonezawa, Y.; Kuriyama, I.; Hada, T.; Sato, N.; Yoshida, H.; Mizushina, Y. Anti-Tumor Effect of Orally Administered Spinach Glycolipid Fraction on Implanted Cancer Cells, Colon-26, in Mice. Lipids 2008, 43, 741. [Google Scholar] [CrossRef]
- Beermann, C.; Möbius, M.; Winterling, N.; Schmitt, J.J.; Boehm, G. Sn-Position Determination of Phospholipid-linked Fatty Acids Derived from Erythrocytes by Liquid Chromatography Electrospray Ionization Ion-trap Mass Spectrometry. Lipids 2005, 40, 211–218. [Google Scholar] [CrossRef]
| Yield of Extraction | |||
|---|---|---|---|
| g/100 g Grains or 100 mL Beverage | |||
| Extract | Minimum | Median | Maximum |
| WKGs-TAC | 0.160 | 0.410 | 0.560 |
| WKGs-TLC | 0.064 | 0.088 | 0.187 |
| WKB-TAC | 0.002 | 0.020 | 0.048 |
| WKB-TLC | 0.002 | 0.003 | 0.004 |
| Total Carotenoid Content (TCC) | |||
|---|---|---|---|
| mg CE/g Extract | |||
| Extract | Minimum | Median | Maximum |
| WKGs-TAC | 10.14 | 11.71 | 28.50 |
| WKGs-TLC | 15.61 | 19.09 | 50.63 |
| WKB-TAC | 19.37 | 114.00 | 192.00 |
| WKB-TLC | 108.57 | 122.50 | 315.56 |
| Total Phenolic Content (TPC) | |||
|---|---|---|---|
| mg GAE/g Extract | |||
| Extract | Minimum | Median | Maximum |
| WKGs-TAC | 92.26 | 101.63 | 245.54 |
| WKGs-TLC | 3.72 | 129.87 | 160.43 |
| WKB-TAC | 47.62 | 351.19 | 1404.76 |
| WKB-TLC | 370.37 | 386.90 | 442.18 |
| ABTS | TEAC | FRAP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| μmol TE/g Extract | |||||||||
| Extract | Minimum | Median | Maximum | Minimum | Median | Maximum | Minimum | Median | Maximum |
| WKGs-TAC | 5.12 | 8.62 | 10.06 | 0.0005 | 0.0023 | 0.0040 | 21.65 | 21.91 | 53.69 |
| WKGs-TLC | ND | ND | ND | ND | ND | ND | 0.89 | 4.12 | 6.16 |
| WKB-TAC | 5.84 | 44.62 | 199.76 | 0.0028 | 0.0083 | 0.0507 | 8.26 | 46.02 | 351.01 |
| WKB-TLC | ND | ND | ND | ND | ND | ND | 14.27 | 14.27 | 15.85 |
| Peak (cm−1) | Extract | Bond/Functional Group Correlation | |
|---|---|---|---|
| WKGs-TAC | WKB-TAC | ||
| Broad peak at about 3330 cm−1 | + | + | O-H (hydroxyl) bonds, characteristic of this functional group in phenolic compounds (e.g., gallic acid, catechin, and quercetin) |
| Peaks at about 3000 cm−1 | + | + | Vibrations of C-H, both for the double bonds in alkenes and/or aromatic rings but also in single -C-H (alkyl) bonds, also present in β-carotene and polar lipids |
| Peak at about 1660 cm−1 | + | + | C=C bonds, also present in polar lipids containing unsaturated fatty acids and in carotenoids with double bonds) |
| Three peaks in the 1580–1300 cm−1 region | + | + | Stretching vibrations of C-H and C=C-C bonds of the aromatic ring of phenolic compounds |
| Peak at about 1120 cm−1 | + | + | C-O-C (ether) bond, also present in catechin and quercetin |
| Peak at about 950 cm−1 | + | + | Hydrogen atoms bonded to sp2 hybridized carbon atoms (=C-H2 bond), also found in β-carotene |
| Peak at about 810 cm−1 | + | + | Out-of-plane C-H bending vibrations (900–690 cm−1), characteristic of aromatic rings that are also found in β-carotene, gallic acid, catechin, and quercetin |
| Fatty Acid Emperical Name | Lipid Number | WKGs-TAC | WKB-TAC |
|---|---|---|---|
| Caprylic | C8:0 | 0.063 ± 0.002 | 0.134 ± 0.016 |
| Pelargonic | C9:0 | 0.229 ± 0.009 | 0.465 ± 0.010 |
| Capric | C10:0 | ND | ND |
| Lauric | C12:0 | 0.139 ± 0.013 | 0.424 ± 0.035 |
| Tridecylic | C13:0 | ND | ND |
| Myristic | C14:0 | 0.558 ± 0.063 | 0.826 ± 0.036 |
| Pentadecylic | C15:0 | ND | ND |
| Palmitic | C16:0 | 24.876 ± 1.601 | 25.293 ± 4.178 |
| Palmitoleic | C16:1 c9 (n7 MUFA) | 9.305 ± 0.272 | 0.789 ± 0.118 |
| Margaric | C17:0 | 1.156 ± 0.134 | 2.388 ± 0.598 |
| Stearic | C18:0 | 49.801 ± 1.731 | 65.423 ± 4.952 |
| Oleic | C18:1 c9 (n9 MUFA) | 11.517 ± 0.255 | 3.135 ± 0.361 |
| Linoleic | C18:2 c9,12 (n6 PUFA) | 0.523 ± 0.063 | 0.870 ± 0.048 |
| Linolenic (α + γ) | C18:3 c9,12,15 (n3 PUFA) | 0.099 ± 0.010 | 0.133 ± 0.008 |
| Stearidonic | C18:4 c6,9,12,15 (n3 PUFA) | ND | ND |
| Nonadecylic | C19:0 | ND | ND |
| Arachidic | C20:0 | ND | ND |
| Gadoleic | C20:1 c9 (n11 MUFA) | 0.614 ± 0.012 | ND |
| DihomoLinoleic | C18:2 c10,12 (n6 PUFA) | 0.116 ± 0.013 | ND |
| Dihomolinolenic | C20:3 c8,11,14 (n6 PUFA) | ND | ND |
| Arachidonic | C20:4 c5,8,11,14 (n6 PUFA) | 0.140 ± 0.004 | ND |
| EPA | C20:5 c5,8,11,14,17 (n3 PUFA) | 0.316 ± 0.066 | 0.121 ± 0.013 |
| Docosadienoic | C22:2 c13,16 (n6 PUFA) | ND | ND |
| Eranthic | C22:3 c5,13,16 (n6 PUFA) | ND | ND |
| Ardenic | C22:4 c7,10,13,16 (n6 PUFA) | ND | ND |
| DPA | C22:5 c7,10,13,16,19 (n3 PUFA) | ND | ND |
| DHA | C22:6 c4,7,10,13,16,19 (n3 PUFA) | 0.548 ± 0.095 | ND |
| SFA | 76.822 * ± 0.328 | 94.953 * ± 0.498 | |
| UFA | 23.178 ± 0.328 | 5.047 ± 0.498 | |
| MUFA | 21.436 ** ± 0.333 | 3.924 ** ± 0.448 | |
| PUFA | 1.742 ± 0.239 | 1.123 ± 0.053 | |
| n3PUFA | 0.963 ± 0.172 | 0.253 ± 0.021 | |
| n6PUFA | 0.779 ± 0.073 | 0.870 ± 0.048 | |
| n6/n3 | 0.820 ± 0.095 | 3.451 ± 0.347 |
| Fatty Acid Emperical Name | Lipid Number | WKGs-TAC | WKB-TAC |
|---|---|---|---|
| Caprylic | C8:0 | ND | ND |
| Pelargonic | C9:0 | 0.468 ± 0.025 | 0.458 ± 0.028 |
| Lauric | C12:0 | ND | ND |
| Tridecylic | C13:0 | ND | ND |
| Myristic | C14:0 | 0.714 ± 0.055 | ND |
| Pentadecylic | C15:0 | 0.680 ± 0.191 | ND |
| Palmitic | C16:0 | 24.943 ± 1.361 | 25.544 ± 1.781 |
| Palmitoleic | C16:1 c9 (n7 MUFA) | 5.056 ± 0.091 | ND |
| Margaric | C17:0 | 2.862 ± 0.059 | ND |
| Stearic | C18:0 | 53.091 ± 1.242 | 66.794 ± 1.831 |
| Oleic | C18:1 c9 (n9 MUFA) | 9.569 ± 1.289 | 7.051 ± 0.156 |
| Linoleic | C18:2 c9,12 (n6 PUFA) | 1.673 ± 0.079 | ND |
| Linolenic (α + γ) | C18:3 c9,12,15 (n3 PUFA) | 0.200 ± 0.023 | ND |
| Stearidonic | C18:4 c6,9,12,15 (n3 PUFA) | ND | ND |
| Nonadecylic | C19:0 | ND | ND |
| Gadoleic | C20:1 c9 (n11 MUFA) | ND | ND |
| DihomoLinoleic | C18:2 c10,12 (n6 PUFA) | ND | ND |
| Dihomolinolenic | C20:3 c8,11,14 (n6 PUFA) | ND | ND |
| Arachidonic | C20:4 c5,8,11,14 (n6 PUFA) | ND | ND |
| EPA | C20:5 c5,8,11,14,17 (n3 PUFA) | 0.345 ± 0.036 | 0.153 ± 0.023 |
| Docosadienoic | C22:2 c13,16 (n6 PUFA) | ND | ND |
| Eranthic | C22:3 c5,13,16 (n6 PUFA) | ND | ND |
| Ardenic | C22:4 c7,10,13,16 (n6 PUFA) | ND | ND |
| DPA | C22:5 c7,10,13,16,19 (n3 PUFA) | ND | ND |
| DHA | C22:6 c4,7,10,13,16,19 (n3 PUFA) | 0.399 ± 0.015 | ND |
| SFA | 82.758 * ± 1.328 | 92.796 * ± 0.155 | |
| UFA | 17.242 ± 1.328 | 7.204 ± 0.155 | |
| MUFA | 14.625 ** ± 1.353 | 7.051 ** ± 0.156 | |
| PUFA | 2.617 ± 0.146 | 0.153 ± 0.023 | |
| n3PUFA | 0.944 ± 0.071 | 0.153 ± 0.023 | |
| n6PUFA | 1.673 ± 0.079 | - | |
| n6/n3 | 1.775 ± 0.068 | - |
| TAC Extracts from WKG | TAC Extracts from WKB | |||||||
|---|---|---|---|---|---|---|---|---|
| Main Classes of PLs | Elution Time (min) | Mr | Representative Molecular Species | Proposed Structures | Elution Time (min) | Mr | Representative Molecular Species | Proposed Structures |
| PC | 10.2–10.3 | 792.9 | PC O-38:6;O | (i.e PC O-16:0/22:6 or PC O-18:1/20:5) | 10.2–10.3 | 734.4 | PC 32:0;O | (i.e PC 18:0/14:0;O or 16:0/16:0;O) |
| 11.7–11.8 | 770.9 | PC 36:2 | (i.e PC 18:0/18:2) | 11.7–11.8 | 742.6 | PC 34:2 | (i.e PC 16:0/18:2) | |
| 10.2–10.3 | 744.4 | PC 34:1 | (i.e PC 18:0/16:1 or PC 16:0/18:1) | |||||
| PE | 9.0–9.2 | 792.9 | PE O-40:6;O | (i.e PE O-18:0/22:6;O) | 10.2–10.3 | 714.4 | PE 34:2 | (i.e PE 16:0/18:2) |
| 10.2–10.3 | 714.8 | PE 34:2 | (i.e PE 16:0/18:2) | 10.2–10.3 | 698.4 | PE O-34:3 | (i.e PE 16:0/18:3) | |
| 10.2–10.3 | 744.4 | PE 36:1 | (i.e PE 18:0/18:1) | |||||
| 10.2–10.3 | 734.4 | PE 34:0;O | (i.e., PE 18:0/16:0;O) | |||||
| 11.7–11.8 | 742.6 | PE 36:2 | (i.e PE 18:0/18:2) | |||||
| PI | 9.9–10.1 | 835.5 | PI 34:1 | (i.e PI 18:0/16:1 or PI 16:0/18:1) | 12.6–12.8 | 821.5 | PI O-34:1 | (i.ePI O-16:0/18:1) |
| 12.0–12.5 | 835.5 | PI O-34:2;O | (i.e PI O-16:0/18:2) | |||||
| 12.6–12.8 | 821.5 | PI O-34:1 | (i.ePI O-18:0/16:1 or PI O-16:0/18:1) | |||||
| PS | 9.9–10.1 | 792.9 | PS O-36:0;O | (i.e., PS O-18:0/18:0;O) | 10.2–10.3 | 734.4 | PS 32:0 | (i.e PS 18:0/14:0 or 16:0/16:0) |
| 9.9–10.1 | 808.8 | PS O-38:6;O | (i.e PS O-16:0/22:6;O or PS O-18:1/20:5;O) | 11.7–11.8 | 742.6 | PS O-34:3 | (i.e PS 16:0/18:3) | |
| 10.2–10.3 | 744.4 | PS O-34:2 | (i.e PS 16:0/18:2) | |||||
| Cer | 12.1–12.5 | 520.9 | Cer 34:1;O | (i.e., Cer18:0/16:1 or Cer 16:0/18:1) | 12.1–12.5 | 520.9 | Cer 34:1;O | (i.eCer18:0/16:1 or Cer 16:0/18:1) |
| 2.6–2.7 | 612.3 | Cer 36:1;O5 | (i.e., Cer 18:0/18:1;O5) | 12.1–12.5 | 626.4 | Cer 36:2;O6 | (i.eCer 18:0/18:2;O6) | |
| 12.6–12.8 | 626.4 | Cer 36:2;O6 | (i.eCer 18:0/18:2;O6) | |||||
| HexCer | 10.2–10.3 | 792.9 | HexCer 36:0;O6 | (i.e., HexCer 18:0/18:0;O6) | 10.2–10.3 | 792.9 | HexCer 36:0;O6 | (i.e., HexCer 18:0/18:0;O6) |
| 9.9–10.1 | 808.8 | HexCer 38:6;O6 | (i.eHexCer 16:0/22:6;O6 or HexCer 18:1/20:5;O6) | 11.7–11.8 | 742.6 | HexCer 36:1;O3 | (i.eHexCer 18:0/18:1;O3) | |
| SQDG | 12.6–12.8 | 821.5 | SQDG 34:0 | (i.e., SQDG 18:0/16:0) | ||||
| DGDG | 13.0–13.1 | 915.6 | DGDG 34:2 | (i.e DGDG 16:0/18:2) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, D.; Chrysikopoulou, V.; Rampaouni, A.; Plakidis, C.; Ofrydopoulou, A.; Shiels, K.; Saha, S.K.; Tsoupras, A. Antioxidant, Antithrombotic and Anti-Inflammatory Properties of Amphiphilic Bioactives from Water Kefir Grains and Its Apple Pomace-Based Fermented Beverage. Antioxidants 2025, 14, 164. https://doi.org/10.3390/antiox14020164
Papadopoulou D, Chrysikopoulou V, Rampaouni A, Plakidis C, Ofrydopoulou A, Shiels K, Saha SK, Tsoupras A. Antioxidant, Antithrombotic and Anti-Inflammatory Properties of Amphiphilic Bioactives from Water Kefir Grains and Its Apple Pomace-Based Fermented Beverage. Antioxidants. 2025; 14(2):164. https://doi.org/10.3390/antiox14020164
Chicago/Turabian StylePapadopoulou, Dimitra, Vasiliki Chrysikopoulou, Aikaterini Rampaouni, Christos Plakidis, Anna Ofrydopoulou, Katie Shiels, Sushanta Kumar Saha, and Alexandros Tsoupras. 2025. "Antioxidant, Antithrombotic and Anti-Inflammatory Properties of Amphiphilic Bioactives from Water Kefir Grains and Its Apple Pomace-Based Fermented Beverage" Antioxidants 14, no. 2: 164. https://doi.org/10.3390/antiox14020164
APA StylePapadopoulou, D., Chrysikopoulou, V., Rampaouni, A., Plakidis, C., Ofrydopoulou, A., Shiels, K., Saha, S. K., & Tsoupras, A. (2025). Antioxidant, Antithrombotic and Anti-Inflammatory Properties of Amphiphilic Bioactives from Water Kefir Grains and Its Apple Pomace-Based Fermented Beverage. Antioxidants, 14(2), 164. https://doi.org/10.3390/antiox14020164









