Optimization of Supercritical Fluid Extraction for the Recovery of γ-Oryzanol-Rich Extracts with Improved Bioactivity from Rice Bran
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Raw Material
2.3. Extraction Procedures
2.3.1. Supercritical CO2 Extractions
Experimental Design Analysis/Statistical Analysis
2.3.2. Soxhlet Extraction
2.4. Chemical Characterization
2.4.1. Analysis of γ-Oryzanol by LC-MS
2.4.2. Analysis of Fatty Acids by GC
2.5. Antioxidant Activity—Oxygen Radical Absorbance Capacity (ORAC) Assay
2.6. Cell-Based Assays
2.6.1. Cell Culture
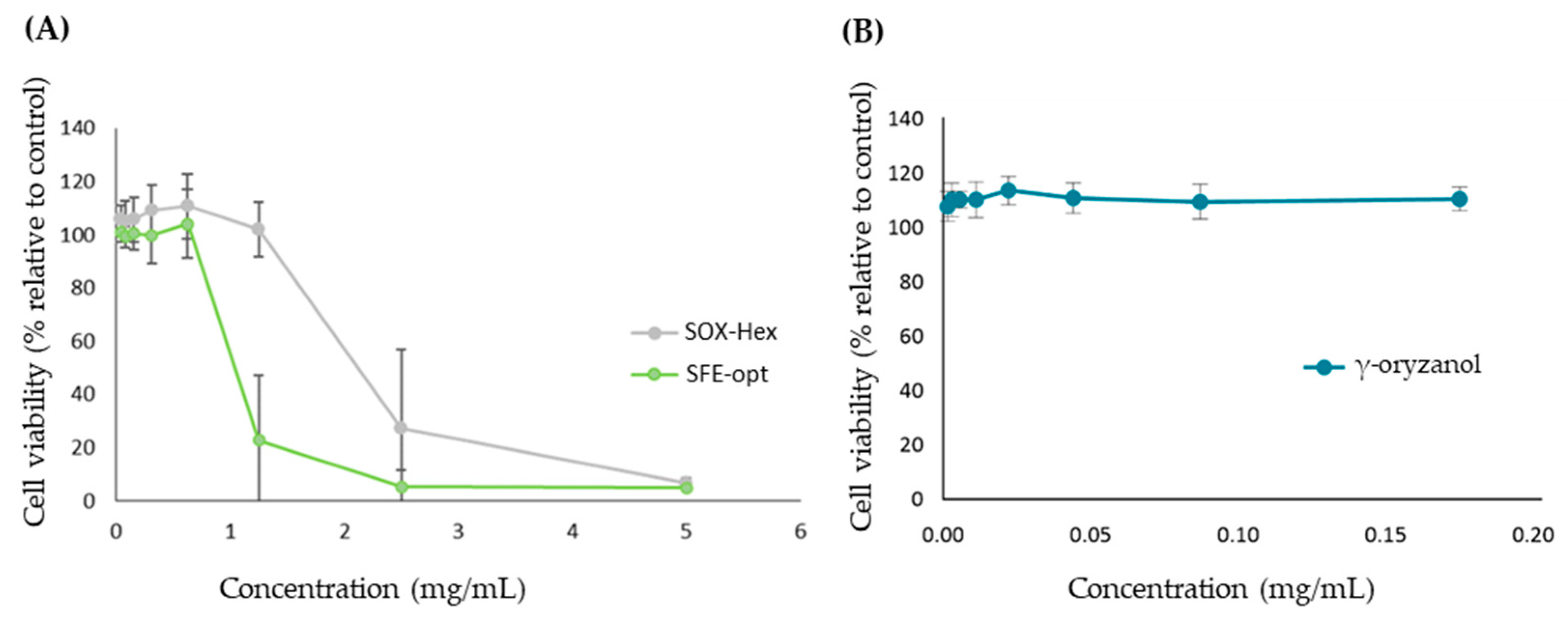
2.6.2. Cytotoxicity Assays
2.6.3. Antiproliferative Activity
2.6.4. Cellular Antioxidant Activity
2.6.5. Statistical Analysis
3. Results and Discussion
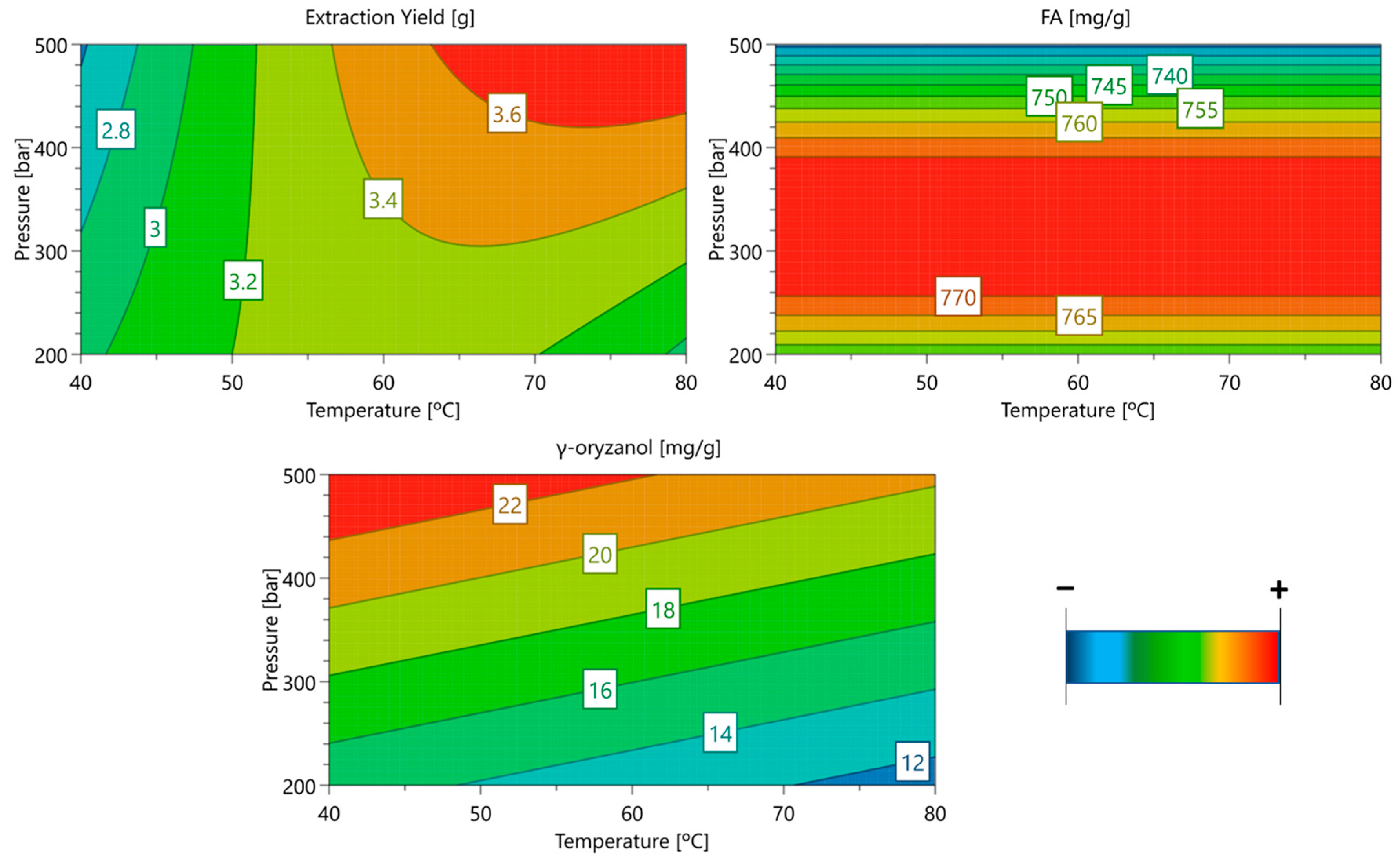
3.1. Optimization of Bioactive Extraction from RB Using Supercritical CO2
3.2. Bioactivity Evaluation of the RBO
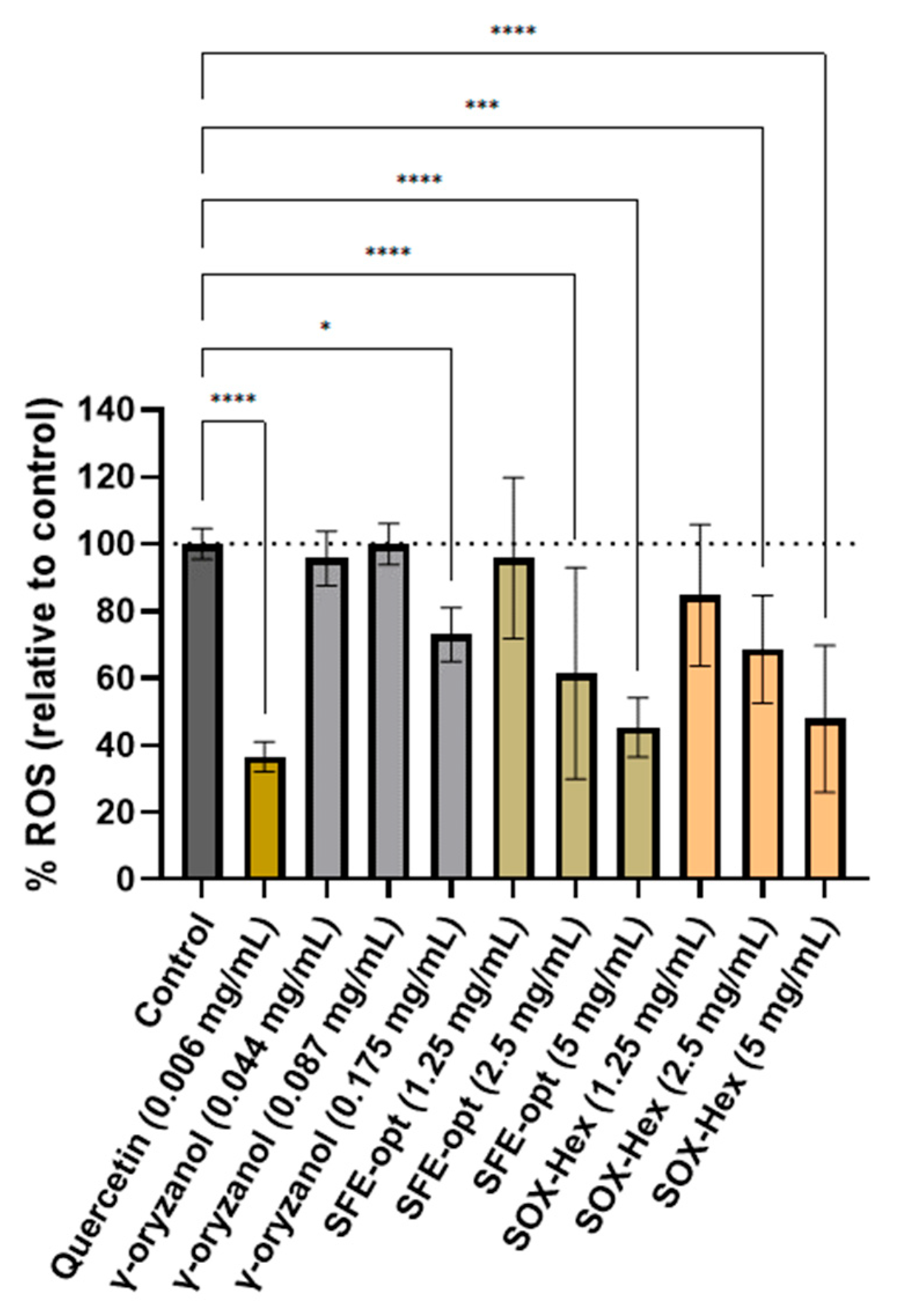
3.2.1. Antioxidant Activity
3.2.2. Antiproliferative Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saji, N.; Francis, N.; Schwarz, L.J.; Blanchard, C.L.; Santhakumar, A.B. The Antioxidant and Anti-Inflammatory Properties of Rice Bran Phenolic Extracts. Foods 2020, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice Bran: A Novel Functional Ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Kumar, M.; Sandhu, K.S.; Whiteside, W.S. Rice-bran oil: An emerging source of functional oil. J. Food Process. Preserv. 2021, 45, e15318. [Google Scholar] [CrossRef]
- Sapwarobol, S.; Saphyakhajorn, W.; Astina, J. Biological Functions and Activities of Rice Bran as a Functional Ingredient: A Review. Nutr. Metab. Insights 2021, 14, 11786388211058559. [Google Scholar] [CrossRef]
- Lemus, C.; Angelis, A.; Halabalaki, M.; Skaltsounis, A.L. γ-Oryzanol: An Attractive Bioactive Component from Rice Bran. In Wheat and Rice in Disease Prevention and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 409–430. [Google Scholar] [CrossRef]
- Patel, M.; Naik, S.N. Gamma-Oryzanol from Rice Bran Oil—A Review. J. Sci. Ind. Res. 2004, 63, 569–578. [Google Scholar]
- Mastinu, A.; Bonini, S.A.; Rungratanawanich, W.; Aria, F.; Marziano, M.; Maccarinelli, G.; Abate, G.; Premoli, M.; Memo, M.; Uberti, D. Gamma-oryzanol prevents LPS-induced brain inflammation and cognitive impairment in adult mice. Nutrients 2019, 11, 728. [Google Scholar] [CrossRef]
- Ismail, M.; Al-Naqeeb, G.; bin Mamat, W.A.A.; Ahmad, Z. Gamma-oryzanol rich fraction regulates the expression of antioxidant and oxidative stress related genes in stressed rat’s liver. Nutr. Metab. 2010, 7, 23. [Google Scholar] [CrossRef]
- Klongpityapong, P.; Supabphol, R.; Supabphol, A. Antioxidant effects of gamma-oryzanol on human prostate cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 5421–5425. [Google Scholar] [CrossRef]
- Singanusong, R.; Jacoby, J.J. Nutrition and applications of rice bran oil: A mini-overview. Nutrition 2021, 29, 47–53. [Google Scholar] [CrossRef]
- Jesus, S.P.; Grimaldi, R.; Hense, H. Recovery of γ-oryzanol from rice bran oil byproduct using supercritical fluid extraction. J. Supercrit. Fluids 2010, 55, 149–155. [Google Scholar] [CrossRef]
- Nunes, A.N.; do Carmo, C.; Duarte, C.M.M. Production of a natural red pigment derived from Opuntia spp. using a novel high pressure CO2 assisted-process. RSC Adv. 2015, 5, 83106–83114. [Google Scholar] [CrossRef]
- Benito-Román, O.; Varona, S.; Sanz, M.T.; Beltrán, S. Valorization of rice bran: Modified supercritical CO2 extraction of bioactive compounds. J. Ind. Eng. Chem. 2019, 80, 273–282. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Filho, W.A.R.; Paula, J.T.; Garmus, T.T.; Cabral, F.A. Solubility of γ-oryzanol in supercritical carbon dioxide and extraction from rice bran. J. Supercrit. Fluids 2016, 107, 196–200. [Google Scholar] [CrossRef]
- Tomita, K.; Machmudah, S.; Wahyudiono; Fukuzato, R.; Kanda, H.; Quitain, A.T.; Sasaki, M.; Goto, M. Extraction of rice bran oil by supercritical carbon dioxide and solubility consideration. Sep. Purif. Technol. 2014, 125, 319–325. [Google Scholar] [CrossRef]
- Sookwong, P.; Suttiarporn, P.; Boontakham, P.; Seekhow, P.; Wangtueai, S.; Mahatheeranont, S. Simultaneous quantification of Vitamin E, γ-oryzanols and xanthophylls from rice bran essences extracted by supercritical CO2. Food Chem. 2016, 211, 140–147. [Google Scholar] [CrossRef]
- Kayathi, A.; Chakrabarti, P.P.; Bonfim-Rocha, L.; Cardozo-Filho, L.; Bollampalli, A.; Jegatheesan, V. Extraction of γ-Oryzanol from defatted rice bran using supercritical carbon dioxide (SC-CO2): Process optimisation of extract yield, scale-up and economic analysis. Process Saf. Environ. Prot. 2021, 148, 179–188. [Google Scholar] [CrossRef]
- ISO 5509:2000; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2000.
- Serra, A.T.; Duarte, R.O.; Bronze, M.R.; Duarte, C.M.M. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011, 125, 318–325. [Google Scholar] [CrossRef]
- Rodrigues, L.; Silva, I.; Poejo, J.; Serra, A.T.; Matias, A.A.; Simplício, A.L.; Bronze, M.R.; Duarte, C.M.M. Recovery of antioxidant and antiproliferative compounds from watercress using pressurized fluid extraction. RSC Adv. 2016, 6, 30905–30918. [Google Scholar] [CrossRef]
- Melgosa, R.; Trigueros, E.; Sanz, M.T.; Cardeira, M.; Rodrigues, L.; Fernández, N.; Matias, A.A.; Bronze, M.R.; Marques, M.; Paiva, A.; et al. Supercritical CO2 and subcritical water technologies for the production of bioactive extracts from sardine (Sardina pilchardus) waste. J. Supercrit. Fluids 2020, 164, 104943. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Original Contribution quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free. Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Matias, A.A.; Frade, R.F.M.; Duarte, R.O.; Feliciano, R.P.; Bronze, M.R.; Figueira, M.E.; de Carvalho, A.; Duarte, C.M.M. Characterization of traditional and exotic apple varieties from Portugal. Part 2—Antioxidant and antiproliferative activities. J. Funct. Foods 2010, 2, 46–53. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Zhimin, X.; Samuel, G. Comparison of Supercritical Fluid and Solvent Extraction Methods in Extracting γ-Oryzanol from Rice Bran. J. Am. Oil Chem. Soc. 2000, 77, 547–551. [Google Scholar]
- Perretti, G.; Miniati, E.; Montanari, L.; Fantozzi, P. Improving the value of rice by-products by SFE. J. Supercrit. Fluids 2003, 26, 63–71. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Rui, H.L. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Valério, R.; Serra, A.T.; Baixinho, J.; Cardeira, M.; Fernández, N.; Bronze, M.R.; Duarte, L.C.; Tavares, M.L.; Crespo, J.G.; Brazinha, C. Combined hydrothermal pre-treatment and enzymatic hydrolysis of corn fibre: Production of ferulic acid extracts and assessment of their antioxidant and antiproliferative properties. Ind. Crops Prod. 2021, 170, 113731. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Tyagi, A.; Shabbir, U.; Chen, X.; Chelliah, R.; Elahi, F.; Ham, H.J.; Oh, D.-H. Phytochemical profiling and cellular antioxidant efficacy of different rice varieties in colorectal adenocarcinoma cells exposed to oxidative stress. PLoS ONE 2022, 17, e0269403. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Karbalaii, M.T.; Jaafar, H.Z.E.; Rahmat, A. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem. Cent. J. 2018, 12, 17. [Google Scholar] [CrossRef]

| Exp No. | Temperature (°C) | Pressure (bar) | Temperature Coded | Pressure Coded |
|---|---|---|---|---|
| 1 | 40 | 200 | −1 | −1 |
| 2 | 80 | 200 | +1 | −1 |
| 3 | 40 | 500 | −1 | +1 |
| 4 | 80 | 500 | +1 | +1 |
| 5 | 40 | 350 | −1 | 0 |
| 6 | 80 | 350 | +1 | 0 |
| 7 | 60 | 200 | 0 | −1 |
| 8 | 60 | 500 | 0 | +1 |
| 9 | 60 | 350 | 0 | 0 |
| 10 | 60 | 350 | 0 | 0 |
| 11 | 60 | 350 | 0 | 0 |
| Exp No. | Temperature (°C) | Pressure (Bar) | Extraction Yield (%) | Total FA | γ-Oryzanol |
|---|---|---|---|---|---|
| (mg/g Extract) | |||||
| 1 | 40 | 200 | 15.1% | 757.64 | 14.49 |
| 2 | 80 | 200 | 14.7% | 772.31 | 8.94 |
| 3 | 40 | 500 | 12.6% | 716.35 | 24.89 |
| 4 | 80 | 500 | 18.2% | 707.41 | 22.72 |
| 5 | 40 | 350 | 13.8% | 752.64 | 20.62 |
| 6 | 80 | 350 | 17.6% | 787.22 | 17.56 |
| 7 | 60 | 200 | 15.9% | 723.50 | 15.4 |
| 8 | 60 | 500 | 18.3% | 746.41 | 18.78 |
| 9 | 60 | 350 | 16.8% | 784.49 | 15.38 |
| 10 | 60 | 350 | 15.8% | 782.52 | 16.75 |
| 11 | 60 | 350 | 16.7% | 745.21 | 19.41 |
| Extraction Yield (%) | Total FA (mg/g Extract) | Total FA (mg FA/g RB) | γ-Oryzanol (mg/g Extract) | γ-Oryzanol (mg/g RB) |
|---|---|---|---|---|
| 17.3 ± 0.5 | 784.4 ± 18.4 | 135.7 ± 3.9 | 36.4 ± 3.5 | 6.3 ± 0.7 |
| Extract | Antiproliferative Effect—EC50 (mg/mL) |
|---|---|
| SFE-opt | 0.9 ± 0.04 a |
| SOX-Hex | 1.5 ± 0.19 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baixinho, J.P.; Cardeira, M.; Bento-Silva, A.; Partidário, A.M.C.; Serra, A.T.; Bronze, M.d.R.; Fernández, N. Optimization of Supercritical Fluid Extraction for the Recovery of γ-Oryzanol-Rich Extracts with Improved Bioactivity from Rice Bran. Antioxidants 2025, 14, 206. https://doi.org/10.3390/antiox14020206
Baixinho JP, Cardeira M, Bento-Silva A, Partidário AMC, Serra AT, Bronze MdR, Fernández N. Optimization of Supercritical Fluid Extraction for the Recovery of γ-Oryzanol-Rich Extracts with Improved Bioactivity from Rice Bran. Antioxidants. 2025; 14(2):206. https://doi.org/10.3390/antiox14020206
Chicago/Turabian StyleBaixinho, João P., Martim Cardeira, Andreia Bento-Silva, Ana Maria Carvalho Partidário, Ana Teresa Serra, Maria do Rosário Bronze, and Naiara Fernández. 2025. "Optimization of Supercritical Fluid Extraction for the Recovery of γ-Oryzanol-Rich Extracts with Improved Bioactivity from Rice Bran" Antioxidants 14, no. 2: 206. https://doi.org/10.3390/antiox14020206
APA StyleBaixinho, J. P., Cardeira, M., Bento-Silva, A., Partidário, A. M. C., Serra, A. T., Bronze, M. d. R., & Fernández, N. (2025). Optimization of Supercritical Fluid Extraction for the Recovery of γ-Oryzanol-Rich Extracts with Improved Bioactivity from Rice Bran. Antioxidants, 14(2), 206. https://doi.org/10.3390/antiox14020206







