Naringin Suppresses CoCl2-Induced Ferroptosis in ARPE-19 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Cell Cultures and Treatment
2.3. Cell Viability Assay and Morphology Examination
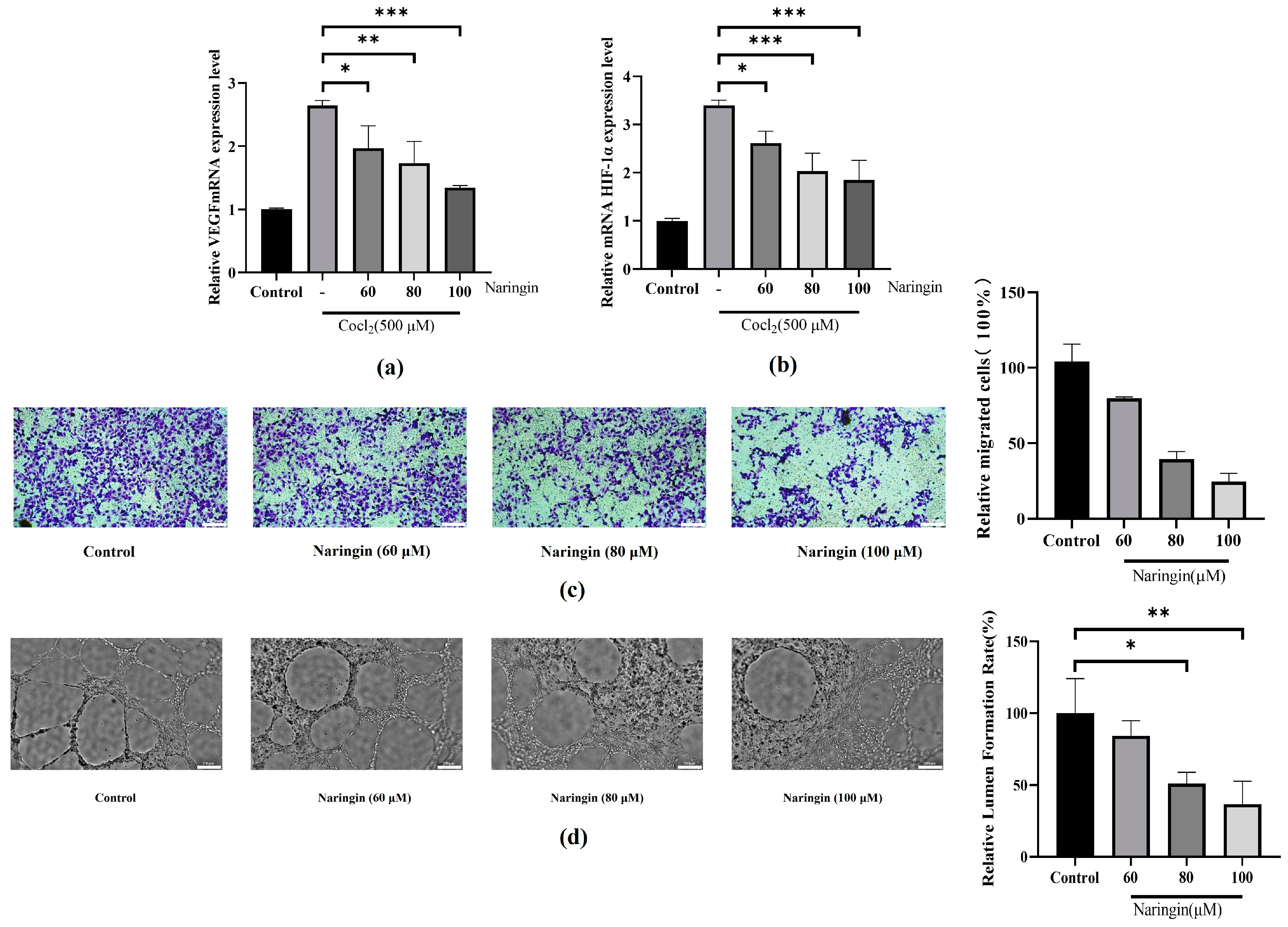
2.4. Transwell Assay
2.5. Tube Formation Assay
2.6. Measurement of ROS Levels
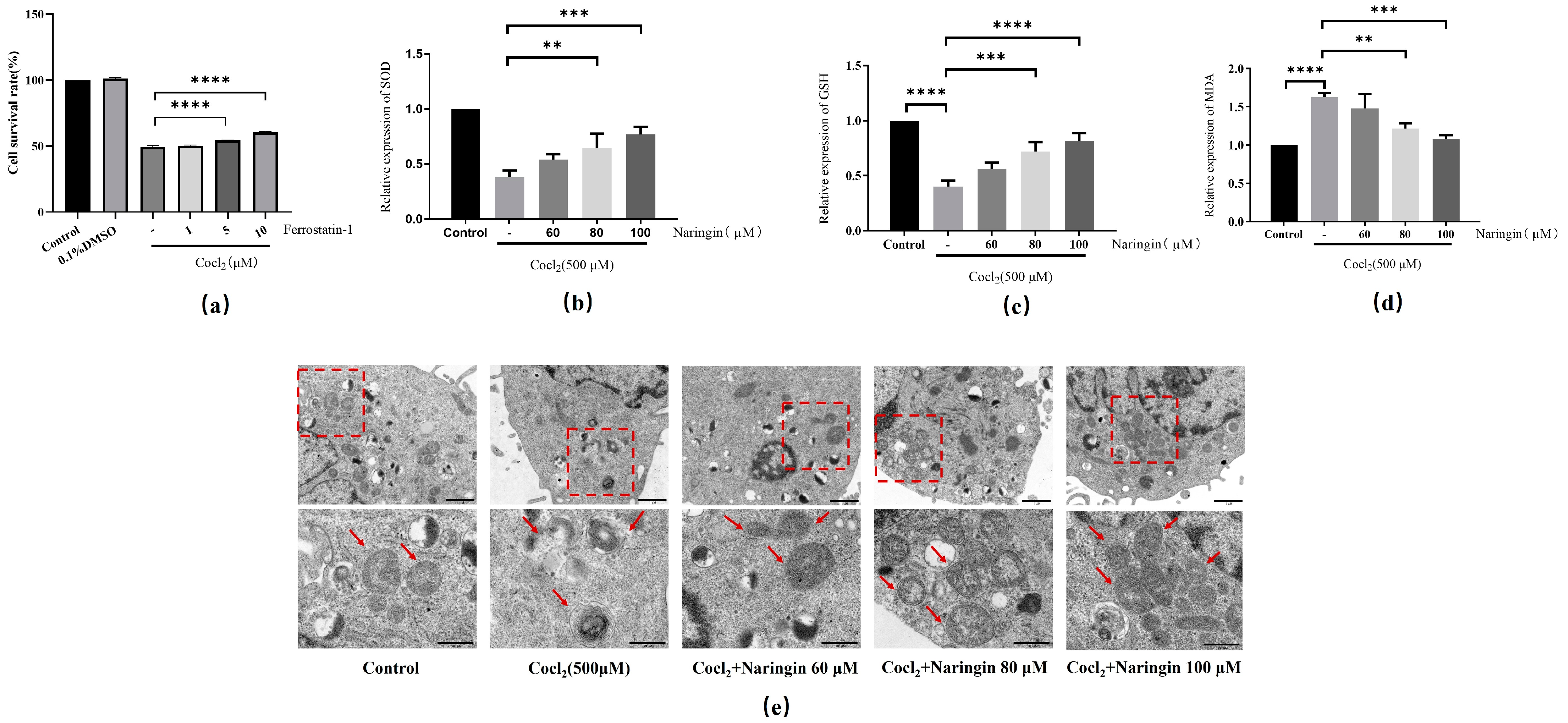
2.7. Measurement of GSH, MDA, and SOD Levels
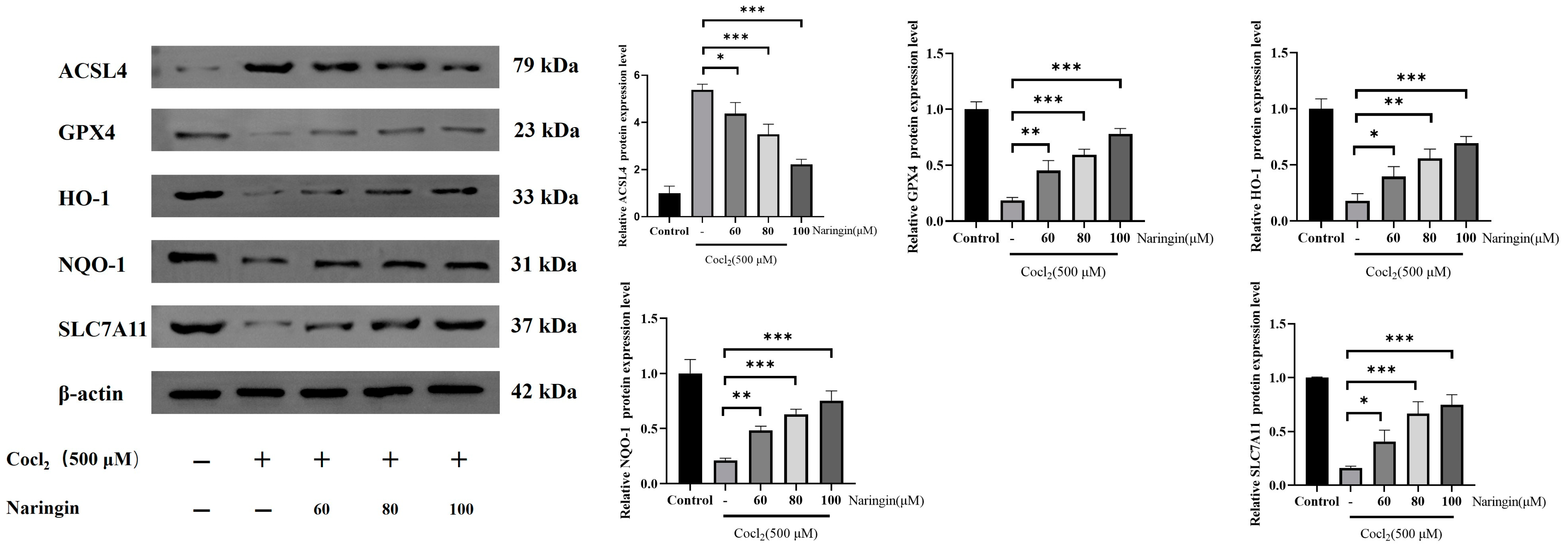
2.8. Western Blotting
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.10. Statistics
3. Results
3.1. Effects of CoCl2 and Naringin on Cell Viability
3.2. Naringin Pretreatment Attenuates Neovascularization in BRVO
3.3. Naringin Pretreatment Attenuates CoCl2-Induced Inflammation and Oxidative Stress in ARPE-19
3.4. Naringin Inhibited CoCl2-Induced Ferroptosis of ARPE-19
3.5. Naringin Adjusted HO-1/GPX4 Axis to Improve Ferroptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, I.U.; Campochiaro, P.A.; Newman, N.J.; Biousse, V. Retinal vascular occlusions. Lancet 2020, 396, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Lamanna, F.; Gabrielle, P.H.; Teo, K.Y.C.; Battaglia Parodi, M.; Iacono, P.; Fraser-Bell, S.; Cornish, E.E.; Nassisi, M.; Viola, F.; et al. Update on Retinal Vein Occlusion. Asia Pac. J. Ophthalmol. 2023, 12, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Yan, Z.Y.; Li, Y.L.; Wang, Y.H.; Zhang, S.J.; Jia, X.; Lu, L.; Shang, Y.X.; Wang, X.; Li, Y.H.; et al. Inhibition of Obtusifolin on retinal pigment epithelial cell growth under hypoxia. Int. J. Ophthalmol. 2019, 12, 1539–1547. [Google Scholar] [CrossRef]
- Barresi, C.; Chhablani, J.; Dolz-Marco, R.; Gallego-Pinazo, R.; Berni, A.; Bandello, F.; Borrelli, E. Retinal neurodegeneration in age-related macular degeneration. Eur. J. Ophthalmol. 2024, 34, 624–630. [Google Scholar] [CrossRef]
- Ghosh, S.; Sharma, R.; Bammidi, S.; Koontz, V.; Nemani, M.; Yazdankhah, M.; Kedziora, K.M.; Stolz, D.B.; Wallace, C.T.; Yu-Wei, C.; et al. The AKT2/SIRT5/TFEB pathway as a potential therapeutic target in non-neovascular AMD. Nat. Commun. 2024, 15, 6150. [Google Scholar] [CrossRef]
- Huemer, J.; Khalid, H.; Wagner, S.K.; Nicholson, L.; Fu, D.J.; Sim, D.A.; Patel, P.J.; Balaskas, K.; Rajendram, R.; Keane, P.A. Phenotyping of retinal neovascularization in ischemic retinal vein occlusion using wide field OCT angiography. Eye 2021, 35, 2812–2819. [Google Scholar] [CrossRef]
- Liu, H.; Stepicheva, N.A.; Ghosh, S.; Shang, P.; Chowdhury, O.; Daley, R.A.; Yazdankhah, M.; Gupta, U.; Hose, S.L.; Valapala, M.; et al. Reducing Akt2 in retinal pigment epithelial cells causes a compensatory increase in Akt1 and attenuates diabetic retinopathy. Nat. Commun. 2022, 13, 6045. [Google Scholar] [CrossRef]
- Dong, Y.; Qian, C.; Yan, P.; Wan, G. YTHDF1-regulated ALOX5 in retinal pigment epithelial cells under hypoxia enhances VEGF expression and promotes viability, migration, and angiogenesis of vascular endothelial cells. Sci. Rep. 2024, 14, 23226. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef]
- Qin, S.; Cao, G.; Tang, M.; Sun, S.; Dong, L. Baicalin alleviates the injury of human retinal pigment epithelium cells and improves branch retinal vein occlusion in rats by inhibiting the HIF-1α/VEGFA axis. Eur. J. Med. Res. 2024, 29, 564. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, J.; Chánez-Cárdenas, M.E. The use of cobalt chloride as a chemical hypoxia model. J. Appl. Toxicol. JAT 2019, 39, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Haley, M.J.; Bere, L.; Minshull, J.; Georgaka, S.; Garcia-Martin, N.; Howell, G.; Coope, D.J.; Roncaroli, F.; King, A.; Wedge, D.C.; et al. Hypoxia coordinates the spatial landscape of myeloid cells within glioblastoma to affect survival. Sci. Adv. 2024, 10, eadj3301. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, Y.; Jiang, S.; Xie, N.; Zhang, Y.; Meng, X.; Wang, X. Salidroside intensifies mitochondrial function of CoCl2-damaged HT22 cells by stimulating PI3K-AKT-MAPK signaling pathway. Phytomed. Int. J. Phytother. Phytopharm. 2023, 109, 154568. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.K.; Ahn, S.H.; Kim, G.; Kim, S.; Hong, J.; Park, K.S.; Park, I.C.; Jin, H.O. Inhibition of VDAC1 oligomerization blocks cysteine deprivation-induced ferroptosis via mitochondrial ROS suppression. Cell Death Dis. 2024, 15, 811. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Z.M.; Yi, X.; Wei, X.; Jiang, D.S. The interaction between ferroptosis and inflammatory signaling pathways. Cell Death Dis. 2023, 14, 205. [Google Scholar] [CrossRef]
- Ye, Y.; Jiang, M.; Hong, X.; Fu, Y.; Chen, Y.; Wu, H.; Sun, Y.; Wang, X.; Zhou, E.; Wang, J.; et al. Quercetin Alleviates Deoxynivalenol-Induced Intestinal Damage by Suppressing Inflammation and Ferroptosis in Mice. J. Agric. Food Chem. 2023, 71, 10761–10772. [Google Scholar] [CrossRef]
- Wei, S.; Li, J.; Zhang, Y.; Li, Y.; Wang, Y. Ferroptosis in eye diseases: A systematic review. Eye 2024, 39, 18–27. [Google Scholar] [CrossRef]
- Henning, Y.; Blind, U.S.; Larafa, S.; Matschke, J.; Fandrey, J. Hypoxia aggravates ferroptosis in RPE cells by promoting the Fenton reaction. Cell Death Dis. 2022, 13, 662. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Shah, A.; Yu, M.; Liu, Y.; He, L.; Dang, J.; Yang, L.; Yan, M.; Ying, Y.; et al. Sodium iodate induces ferroptosis in human retinal pigment epithelium ARPE-19 cells. Cell Death Dis. 2021, 12, 230. [Google Scholar] [CrossRef]
- Zhan, S.; Liang, J.; Lin, H.; Cai, J.; Yang, X.; Wu, H.; Wei, J.; Wang, S.; Xian, M. SATB1/SLC7A11/HO-1 Axis Ameliorates Ferroptosis in Neuron Cells After Ischemic Stroke by Danhong Injection. Mol. Neurobiol. 2023, 60, 413–427. [Google Scholar] [CrossRef]
- Fantone, S.; Piani, F.; Olivieri, F.; Rippo, M.R.; Sirico, A.; Di Simone, N.; Marzioni, D.; Tossetta, G. Role of SLC7A11/xCT in Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 587. [Google Scholar] [CrossRef]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in cell death, autophagy, and disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef] [PubMed]
- Long, P.; Yan, W.; Liu, J.; Li, M.; Chen, T.; Zhang, Z.; An, J. Therapeutic Effect of Traditional Chinese Medicine on a Rat Model of Branch Retinal Vein Occlusion. J. Ophthalmol. 2019, 2019, 9521379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, Y. Quantitative Diagnosis of TCM Syndrome Types Based on Adaptive Resonant Neural Network. Comput. Intell. Neurosci. 2022, 2022, 2485089. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wei, W.B.; Yang, C.X.; Ding, N.; Liu, Y.; He, M.L.; Hou, Z.J.; Zhou, J.Q. Treatment of retinal vein occlusion in rabbits with traditional Chinese medicine Fufang XueShuan Tong. Chin. Med. J. 2010, 123, 3293–3298. [Google Scholar]
- Shilpa, V.S.; Shams, R.; Dash, K.K.; Pandey, V.K.; Dar, A.H.; Ayaz Mukarram, S.; Harsányi, E.; Kovács, B. Phytochemical Properties, Extraction, and Pharmacological Benefits of Naringin: A Review. Molecules 2023, 28, 5623. [Google Scholar] [CrossRef]
- Heidary Moghaddam, R.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef]
- Gu, L.; Wang, F.; Wang, Y.; Sun, D.; Sun, Y.; Tian, T.; Meng, Q.; Yin, L.; Xu, L.; Lu, X.; et al. Naringin protects against inflammation and apoptosis induced by intestinal ischemia-reperfusion injury through deactivation of cGAS-STING signaling pathway. Phytother. Res. 2023, 37, 3495–3507. [Google Scholar] [CrossRef]
- Guan, L.; Guo, L.; Zhang, H.; Liu, H.; Zhou, W.; Zhai, Y.; Yan, X.; Men, X.; Peng, L. Naringin Protects against Non-Alcoholic Fatty Liver Disease by Promoting Autophagic Flux and Lipophagy. Mol. Nutr. Food Res. 2024, 68, e2200812. [Google Scholar] [CrossRef]
- Benherlal, P.S.; Arumughan, C. Studies on modulation of DNA integrity in Fenton’s system by phytochemicals. Mutat. Res. 2008, 648, 1–8. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ke, X.; Wang, W.; Zhang, H.; Ma, N.; Fu, W.; Zhao, M.; Gao, X.; Hao, X.; Zhang, Z. Aloe-emodin suppresses hypoxia-induced retinal angiogenesis via inhibition of HIF-1α/VEGF pathway. Int. J. Biol. Sci. 2016, 12, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yi, C.; Pan, W.; Liu, J.; Qi, J.; Chen, J.; Zhou, Z.; Duan, Y.; Ning, X.; Li, J.; et al. Vascular Cell Adhesion Molecule-1 (VCAM-1) contributes to macular fibrosis in neovascular age-related macular degeneration through modulating macrophage functions. Immun. Ageing 2023, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Jiang, Y.; Zhang, J.; Shi, J.; Zheng, P.; Yang, C.; Chen, Y. Celastrol inhibits pathologic neovascularization in oxygen-induced retinopathy by targeting the miR-17-5p/HIF-1α/VEGF pathway. Cell Cycle 2022, 21, 2091–2108. [Google Scholar] [CrossRef]
- Cao, W.; Feng, S.J.; Kan, M.C. Naringin Targets NFKB1 to Alleviate Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury in PC12 Cells Via Modulating HIF-1α/AKT/mTOR-Signaling Pathway. J. Mol. Neurosci. 2021, 71, 101–111. [Google Scholar] [CrossRef]
- Li, S.; Jiang, J.; Fang, J.; Li, X.; Huang, C.; Liang, W.; Wu, K. Naringin protects H9C2 cardiomyocytes from chemical hypoxia-induced injury by promoting the autophagic flux via the activation of the HIF-1α/BNIP3 signaling pathway. Int. J. Mol. Med. 2021, 47, 102. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.C.; Ding, Y.X.; Bai, J.; Cao, F.; Li, F. The crosstalk between ferroptosis and mitochondrial dynamic regulatory networks. Int. J. Biol. Sci. 2023, 19, 2756–2771. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Zhao, T.; Chen, J.; Kang, L.; Wei, Y.; Han, L.; Shen, L.; Long, C.; Wu, S.; et al. Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1α/HO-1 signaling pathway in mouse testes. J. Hazard. Mater. 2022, 426, 127807. [Google Scholar] [CrossRef]
- Fu, C.; Wu, Y.; Liu, S.; Luo, C.; Lu, Y.; Liu, M.; Wang, L.; Zhang, Y.; Liu, X. Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J. Ethnopharmacol. 2022, 289, 115021. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Fan, Y.; Wang, X.; Qian, J.; Bian, Z. Shikonin induces ferroptosis in osteosarcomas through the mitochondrial ROS-regulated HIF-1α/HO-1 axis. Phytomed. Int. J. Phytother. Phytopharm. 2024, 135, 156139. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiang, Z.; Xing, Y.; Li, S.; Shi, S. Mitochondria bridge HIF signaling and ferroptosis blockage in acute kidney injury. Cell Death Dis. 2022, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; McIntosh, R.L.; Cheung, N.; Lim, L.; Wang, J.J.; Mitchell, P.; Kowalski, J.W.; Nguyen, H.; Wong, T.Y.; International Eye Disease Consortium. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010, 117, 313–319.e1. [Google Scholar] [CrossRef]
- Li, J.; Paulus, Y.M.; Shuai, Y.; Fang, W.; Liu, Q.; Yuan, S. New Developments in the Classification, Pathogenesis, Risk Factors, Natural History, and Treatment of Branch Retinal Vein Occlusion. J. Ophthalmol. 2017, 2017, 4936924. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Sophie, R.; Pearlman, J.; Brown, D.M.; Boyer, D.S.; Heier, J.S.; Marcus, D.M.; Feiner, L.; Patel, A.; RETAIN Study Group. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: The RETAIN study. Ophthalmology 2014, 121, 209–219. [Google Scholar] [CrossRef]
- Driban, M.; Kedia, N.; Arora, S.; Chhablani, J. Novel pharmaceuticals for the management of retinal vein occlusion and linked disorders. Expert Rev. Clin. Pharmacol. 2023, 16, 1125–1139. [Google Scholar] [CrossRef]
- Hanada, N.; Iijima, H.; Sakurada, Y.; Imasawa, M. Recurrence of macular edema associated with branch retinal vein occlusion after intravitreal bevacizumab. Jpn. J. Ophthalmol. 2012, 56, 165–174. [Google Scholar] [CrossRef]
- Rivoira, M.A.; Rodriguez, V.; Talamoni, G.; Tolosa de Talamoni, N. New Perspectives in the Pharmacological Potential of Naringin in Medicine. Curr. Med. Chem. 2021, 28, 1987–2007. [Google Scholar] [CrossRef]
- Tang, G.; Pi, L.; Guo, H.; Hu, Z.; Zhou, C.; Hu, Q.; Peng, H.; Xiao, Z.; Zhang, Z.; Wang, M.; et al. Naringin Relieves Diabetic Cardiac Autonomic Neuropathy Mediated by P2Y14 Receptor in Superior Cervical Ganglion. Front. Pharmacol. 2022, 13, 873090. [Google Scholar] [CrossRef]
- Huang, Q.; Ru, Y.; Luo, Y.; Luo, X.; Liu, D.; Ma, Y.; Zhou, X.; Linghu, M.; Xu, W.; Gao, F.; et al. Identification of a targeted ACSL4 inhibitor to treat ferroptosis-related diseases. Sci. Adv. 2024, 10, eadk1200. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tsui, M.G.; Tsang, J.K.W.; Goit, R.K.; Yao, K.M.; So, K.F.; Lam, W.C.; Lo, A.C.Y. Involvement of FSP1-CoQ10-NADH and GSH-GPx-4 pathways in retinal pigment epithelium ferroptosis. Cell Death Dis. 2022, 13, 468. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Seong, H.; Ryu, J.; Jeong, J.Y.; Kang, T.S.; Nam, K.Y.; Seo, S.W.; Kim, S.J.; Kang, S.S.; Han, Y.S. Phosphorylation of STAT3 and ERBB2 mediates hypoxia-induced VEGF release in ARPE-19 cells. Mol. Med. Rep. 2020, 22, 2733–2740. [Google Scholar] [CrossRef]
- Yang, S.; Li, T.; Jia, H.; Gao, M.; Li, Y.; Wan, X.; Huang, Z.; Li, M.; Zhai, Y.; Li, X.; et al. Targeting C3b/C4b and VEGF with a bispecific fusion protein optimized for neovascular age-related macular degeneration therapy. Sci. Transl. Med. 2022, 14, eabj2177. [Google Scholar] [CrossRef] [PubMed]
- Lip, P.L.; Kolli, H.; Trivedi, D. Ultra-Widefield Fluorescein Angiographic Patterns, Retinal Microvascular Anomalies and Retinal Ischemic Index in Branch Retinal Vein Occlusions with Established Retinal Neovascularization. Clin. Ophthalmol. 2020, 14, 2965–2974. [Google Scholar] [CrossRef]
- Arrigo, A.; Aragona, E.; Bandello, F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann. Med. 2022, 54, 1089–1111. [Google Scholar] [CrossRef]
- Yang, Z.; Ni, B.; Zhou, T.; Huang, Z.; Zhou, H.; Zhou, Y.; Lin, S.; He, C.; Liu, X. HIF-1α Reduction by Lowering Intraocular Pressure Alleviated Retinal Neovascularization. Biomolecules 2023, 13, 1532. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Y.; Martinez, M.; Flores-Bellver, M.; Rodrigues, M.; Dinabandhu, A.; Cao, X.; Deshpande, M.; Qin, Y.; Aparicio-Domingo, S.; et al. HIF-1α and HIF-2α redundantly promote retinal neovascularization in patients with ischemic retinal disease. J. Clin. Investig. 2021, 131, e139202. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, L.; Jin, J.; Zhou, Y.; Zhang, H.; Lv, C.; Lu, D.; Wu, Y.; Zhang, H.; Liu, S.; et al. Bruceine D inhibits HIF-1α-mediated glucose metabolism in hepatocellular carcinoma by blocking ICAT/β-catenin interaction. Acta Pharm. Sin. B 2021, 11, 3481–3492. [Google Scholar] [CrossRef]
- Qiu, B.; Yuan, P.; Du, X.; Jin, H.; Du, J.; Huang, Y. Hypoxia inducible factor-1α is an important regulator of macrophage biology. Heliyon 2023, 9, e17167. [Google Scholar] [CrossRef]
- Li, Z.L.; Ding, L.; Ma, R.X.; Zhang, Y.; Zhang, Y.L.; Ni, W.J.; Tang, T.T.; Wang, G.H.; Wang, B.; Lv, L.L.; et al. Activation of HIF-1α C-terminal transactivation domain protects against hypoxia-induced kidney injury through hexokinase 2-mediated mitophagy. Cell Death Dis. 2023, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, H.; Mei, C.; Cui, M.; He, Q.; Wang, Q.; Li, D.; Song, Y.; Li, J.; Chen, S.; et al. Sirtuin4 alleviates severe acute pancreatitis by regulating HIF-1α/HO-1 mediated ferroptosis. Cell Death Dis. 2023, 14, 694. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, D.; Zhang, Q.; Yang, F.; Wong, Y.K.; Xia, F.; Zhang, J.; Chen, J.; Tian, Y.; Yang, C.; et al. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1. Acta Pharm. Sin. B 2022, 12, 2300–2314. [Google Scholar] [CrossRef]
- D’Agata, V.; D’Amico, A.G.; Maugeri, G.; Bucolo, C.; Rossi, S.; Giunta, S. Carnosol attenuates high glucose damage in human retinal endothelial cells through regulation of ERK/Nrf2/HO-1 pathway. J. Asian Nat. Prod. Res. 2023, 25, 783–795. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Tie, H.; Tian, W.; Zhao, Y.; Qin, L.; Guo, S.; Li, Q.; Bao, C. Eriodictyol regulated ferroptosis, mitochondrial dysfunction, and cell viability via Nrf2/HO-1/NQO1 signaling pathway in ovarian cancer cells. J. Biochem. Mol. Toxicol. 2023, 37, e23368. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Liang, D.; Feng, Y.; Zandkarimi, F.; Wang, H.; Zhang, Z.; Kim, J.; Cai, Y.; Gu, W.; Stockwell, B.R.; Jiang, X. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 2023, 186, 2748–2764. [Google Scholar] [CrossRef]
- Miao, Z.; Tian, W.; Ye, Y.; Gu, W.; Bao, Z.; Xu, L.; Sun, G.; Li, C.; Tu, Y.; Chao, H.; et al. Hsp90 induces Acsl4-dependent glioma ferroptosis via dephosphorylating Ser637 at Drp1. Cell Death Dis. 2022, 13, 548. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Liu, M.; Dong, X.; Bai, J.; Shi, W.; Zhu, Q.; Liu, J.; Wang, Z.; Yi, L.; Yin, X.; et al. Naringin Suppresses CoCl2-Induced Ferroptosis in ARPE-19 Cells. Antioxidants 2025, 14, 236. https://doi.org/10.3390/antiox14020236
Yang Y, Liu M, Dong X, Bai J, Shi W, Zhu Q, Liu J, Wang Z, Yi L, Yin X, et al. Naringin Suppresses CoCl2-Induced Ferroptosis in ARPE-19 Cells. Antioxidants. 2025; 14(2):236. https://doi.org/10.3390/antiox14020236
Chicago/Turabian StyleYang, Yuchang, Manting Liu, Xiaoxv Dong, Jie Bai, Wenjuan Shi, Qian Zhu, Juan Liu, Ziheng Wang, Lisa Yi, Xingbin Yin, and et al. 2025. "Naringin Suppresses CoCl2-Induced Ferroptosis in ARPE-19 Cells" Antioxidants 14, no. 2: 236. https://doi.org/10.3390/antiox14020236
APA StyleYang, Y., Liu, M., Dong, X., Bai, J., Shi, W., Zhu, Q., Liu, J., Wang, Z., Yi, L., Yin, X., Ni, J., & Qu, C. (2025). Naringin Suppresses CoCl2-Induced Ferroptosis in ARPE-19 Cells. Antioxidants, 14(2), 236. https://doi.org/10.3390/antiox14020236






