Antioxidant Defenses and Poly(ADP-Ribose) Polymerase (PARP) Activity Provide “Radioresilience” Against Ionizing Radiation-Induced Stress in Dwarf Bean Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Irradiation Procedure
2.3. Chlorophyll a Fluorescence Emission Measurements and Total Chlorophyll Determination
2.4. Nuclei Isolation and Poly (ADP-Ribose) Polymerase (PARP) Activity
2.5. Hydro- and Lipo-Soluble Antioxidant Capacity Measurement
2.6. Total Polyphenol Determination and Catalase Activity Assay
2.7. Data Processing and Statistical Treatment
2.7.1. Hypothesis Testing
2.7.2. Dose–Response and Recovery Trend Modeling
3. Results
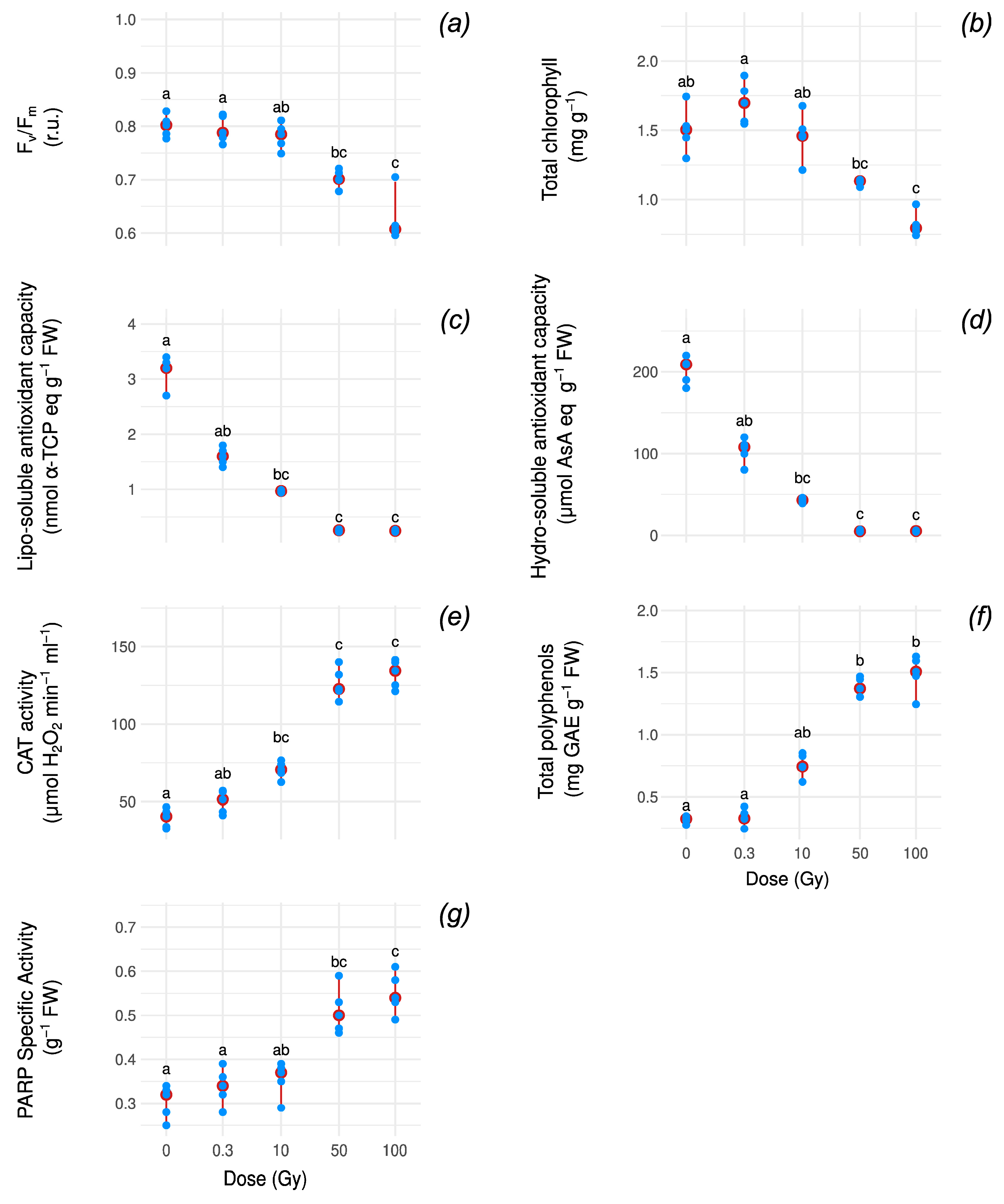
3.1. Oxidative Stress and Photosynthetic Markers Under Increasing Radiation Doses
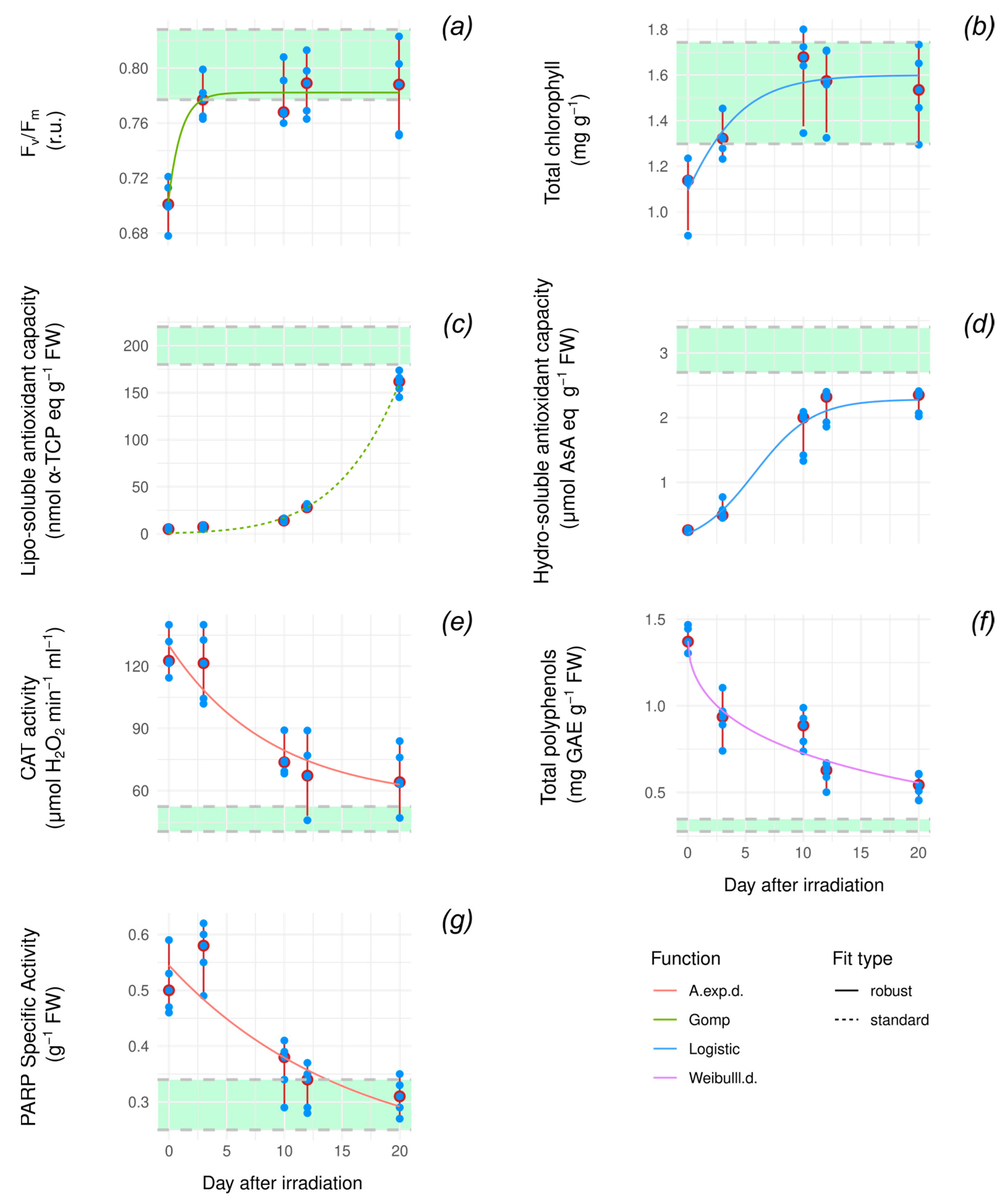
3.2. Recovery Dynamic After Exposure to High Doses of X-Ray Radiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Function | Fit Type | AIC | BIC | Delta_AIC |
|---|---|---|---|---|---|
| Fv/Fm | Linear | robust | −117.01 | −113.35 | 0.000 |
| Total chlorophylls | Exp. decay | robust | −51.49 | −47.83 | 0.000 |
| Hydro-AOX | A.exp.d | robust | −27.79 | −22.92 | 0.000 |
| Lipo-AOX | Weibull.d | robust | 188.61 | 193.49 | 0.000 |
| Catalase | Gompertz | standard | 180.10 | 184.97 | 0.115 |
| Total polyphenols | Gompertz | robust | −54.64 | −49.77 | 0.000 |
| PARP | Logistic | robust | −85.31 | −80.44 | 0.464 |
| Variable | Function | Fit Type | AIC | BIC | Delta_AIC |
|---|---|---|---|---|---|
| Fv/Fm | Gompertz | robust | −119.52 | −114.65 | 0 |
| Total chlorophylls | Logistic | robust | −21.99 | −17.11 | 0 |
| Hydro-AOX | Logistic | robust | −6.25 | −1.38 | 0 |
| Lipo-AOX | Gompertz | standard | 165.49 | 170.37 | 0 |
| Catalase | A.exp.d | robust | 205.02 | 209.89 | 0 |
| Total polyphenols | Weibull.d | robust | −38.53 | −33.66 | 0 |
| PARP | Gompertz | robust | −66.44 | −61.56 | 0 |
References
- Fenollosa, E.; Munné-Bosch, S. Increased chilling tolerance of the invasive species Carpobrotus edulis may explain its expansion across new territories. Conserv. Physiol. 2019, 7, coz075. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Duarte, G.T.; Horemans, N.; Ruytinx, J.; Gudkov, D.; Danchenko, M. Complexity of responses to ionizing Radiation in plants, and the impact on interacting biotic factors. Sci. Total Environ. 2024, 924, 171567. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y.; Sonne, C.; Lam, S.S.; Kirkham, M.B.; Bolan, N.; Rinklebe, J.; Chen, X.; Peng, W. A Strategy for Bioremediation of Nuclear Contaminants in the Environment. Environ. Pollut. 2023, 319, 120964. [Google Scholar] [CrossRef] [PubMed]
- Nuszkiewicz, J.; Woźniak, A.; Szewczyk-Golec, K. Ionizing Radiation as a Source of Oxidative Stress-The Protective Role of Melatonin and Vitamin D. Int. J. Mol. Sci. 2020, 21, 5804. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Mousseau, T.A. Strong Effects of Ionizing Radiation from Chernobyl on Mutation Rates. Sci. Rep. 2015, 5, 8363. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Møller, A.P. Plants in the Light of Ionizing Radiation: What Have We Learned From Chernobyl, Fukushima, and Other “Hot” Places? Front. Plant Sci. 2020, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; De Micco, V.; Macaeva, E.; Quintens, R. Space Radiation Effects on Plant and Mammalian Cells. Acta Astronaut. 2014, 104, 419–431. [Google Scholar] [CrossRef]
- Fesenko, S.; Spridonov, S.; Geras’kin, S. Radiation Effects in the Forest Ecosystems: Acute Irradiation. J. Environ. Radioact. 2022, 250, 106908. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; Vitale, E.; Hay Mele, B.; Cataletto, P.R.; Turano, M.; Simoniello, P.; De Micco, V. Suitability of Solanum lycopersicum L. ‘Microtom’ for Growth in Bioregenerative Life Support Systems: Exploring the Effect of High-LET Ionising Radiation on Photosynthesis, Leaf Structure and Fruit Traits. Plant Biol. 2019, 21, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Zaka, R. Effects of Low Chronic Doses of Ionizing Radiation on Antioxidant Enzymes and G6PDH Activities in Stipa capillata (Poaceae). J. Exp. Bot. 2002, 53, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.P.; Mishra, D.; Chakraborty, A.; Saha, A.; Santra, S.C.; Chanda, S. Modulation of Some Quantitative and Qualitative Characteristics in Rice (Oryza sativa L.) and Mung (Phaseolus mungo L.) by Ionizing Radiation. Radiat. Phys. Chem. 2005, 74, 391–394. [Google Scholar] [CrossRef]
- Caplin, N.; Willey, N. Ionizing Radiation, Higher Plants, and Radioprotection: From Acute High Doses to Chronic Low Doses. Front. Plant Sci. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Jeggo, P.A.; Lobrich, M. Artemis Links ATM to Double Strand Break Rejoining. Cell Cycle 2005, 4, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; Turano, M.; Hay Mele, B.; Cataletto, P.R.; Furia, M.; Pugliese, M.; De Micco, V. Anatomy, Photochemical Activity, and DNA Polymorphism in Leaves of Dwarf Tomato Irradiated with X-Rays. Biol. Plant. 2017, 61, 305–314. [Google Scholar] [CrossRef]
- Kim, J.-H.; Baek, M.-H.; Chung, B.Y.; Wi, S.G.; Kim, J.-S. Alterations in the Photosynthetic Pigments and Antioxidant Machineries of Red Pepper (Capsicum annuum L.) Seedlings from Gamma-Irradiated Seeds. J. Plant Biol. 2004, 47, 314–321. [Google Scholar] [CrossRef]
- Kurimoto, T.; Constable, J.V.H.; Huda, A. Effect of Ionizing Radiation Exposure on Arabidopsis thaliana. Health Phys. 2010, 99, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Marcu, D.; Damian, G.; Cosma, C.; Cristea, V. Gamma Radiation Effects on Seed Germination, Growth and Pigment Content, and ESR Study of Induced Free Radicals in Maize (Zea mays). J. Biol. Phys. 2013, 39, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Tyystjarvi, E. Photoinhibition of Photosystem II and Photodamage of the Oxygen Evolving Manganese Cluster. Coord. Chem. Rev. 2008, 252, 361–376. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; De Maio, A. Growth Alteration and Leaf Biochemical Responses in Phaseolus vulgaris Exposed to Different Doses of Ionising Radiation. Plant Biol. 2014, 16, 194–202. [Google Scholar] [CrossRef]
- Geras’kin, S. Plant Adaptation to Ionizing Radiation: Mechanisms and Patterns. Sci. Total Environ. 2024, 916, 170201. [Google Scholar] [CrossRef]
- Block, M.D.; Verduyn, C.; Brouwer, D.D.; Cornelissen, M. Poly(ADP-ribose) Polymerase in Plants Affects Energy Homeostasis, Cell Death and Stress Tolerance. Plant J. 2005, 41, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Doucet-Chabeaud, G.; Godon, C.; Brutesco, C.; De Murcia, G.; Kazmaier, M. Ionising Radiation Induces the Expression of PARP-1 and PARP-2 Genes in Arabidopsis. Mol. Gen. Genom. 2001, 265, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Grinberg, M.A.; Sukhov, V.; Vodeneev, V. Effect of Ionizing Radiation on Physiological and Molecular Processes in Plants. J. Environ. Radioact. 2019, 202, 8–24. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Arena, C.; Aronne, G. Anatomical Alterations of Phaseolus vulgaris L. Mature Leaves Irradiated with X-rays. Plant Biol. 2014, 16, 187–193. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Aronne, G.; Caplin, N.; Carnero-Diaz, E.; Herranz, R.; Horemans, N.; Legué, V.; Medina, F.J.; Pereda-Loth, V.; Schiefloe, M.; et al. Perspectives for Plant Biology in Space and Analogue Environments. npj Microgravity 2023, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Arena, C.; Vitale, L.; Bianchi, A.; Mistretta, C.; Vitale, E.; Parisi, C.; Guerriero, G.; Magliulo, V.; De Maio, A. The Ageing Process Affects the Antioxidant Defences and the Poly (ADPribosyl)Ation Activity in Cistus incanus L. Leaves. Antioxidants 2019, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Spinelli, M.; Fontanarosa, C.; Paradiso, R.; Amoresano, A.; Arena, C. Modulation of Antioxidant Compounds in Fruits of Citrus reticulata Blanco Using Postharvest LED Irradiation. Biology 2023, 12, 1029. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2023. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 28 January 2025).
- Graves, S.; Piepho, H.-P.; Selzer, L.; Dorai-Raj, S. multcompView: Visualizations of Paired Comparisons. 2024. Available online: https://cran.r-project.org/web/packages/multcompView/index.html (accessed on 28 January 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- William, R.P. Procedures for Psychological, Psychometric, and Personality Research; Northwestern University: Evanston, IL, USA, 2024. [Google Scholar]
- Elzhov, T.V.; Mullen, K.M.; Spiess, A.-N.; Bolker, B. Minpack.Lm: R Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds. 2023. Available online: https://cran.r-project.org/web/packages/minpack.lm/index.html (accessed on 28 January 2025).
- Maechler, M.; Rousseeuw, P.; Croux, C.; Todorov, V.; Ruckstuhl, A.; Salibian-Barrera, M.; Verbeke, T.; Koller, M.; Conceicao, E.L.T.; di Palma, M.A. Robustbase: Basic Robust Statistics. 2024. Available online: https://cran.r-project.org/web/packages/robustbase/index.html (accessed on 28 January 2025).
- Bartosz, G. Oxidative Stress in Plants. Acta Physiol. Plant. 1997, 19, 47–64. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of Oxidative Stress in Plants: From Classical Chemistry to Cell Biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; Aronne, G.; Pugliese, M.; Virzo De Santo, A.; De Maio, A. Response of Phaseolus vulgaris L. Plants to Low-Let Ionizing Radiation: Growth and Oxidative Stress. Acta Astron. 2013, 91, 107–114. [Google Scholar] [CrossRef]
- Challapalli, S.; Kumar, A.; Rai, R.; Kini, J.; Kumarchandra, R. Standardization of Mean Lethal Dose (LD50/30) of X-Rays Using Linear Accelerator (LINIAC) in Albino Wistar Rat Model Based on Survival Analysis Studies and Hematological Parameters. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1215–1219. [Google Scholar]
- Von Zallinger, C.; Tempel, K. The Physiologic Response of Domestic Animals to Ionizing Radiation: A Review. Vet. Radiol. Ultrasound 1998, 39, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ludovici, G.M.; Oliveira De Souza, S.; Chierici, A.; Cascone, M.G.; d’Errico, F.; Malizia, A. Adaptation to Ionizing Radiation of Higher Plants: From Environmental Radioactivity to Chernobyl Disaster. J. Environ. Rad. 2020, 222, 106375. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and Antioxidant Signalling in Plants: A Re-evaluation of the Concept of Oxidative Stress in a Physiological Context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.I.; Van Breusegem, F. Improving Oxidative Stress Resilience in Plants. Plant J. 2022, 109, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, C.J.; Davies, M.I.; Goodwin, P.M.; Halldorsson, H.; Lewis, P.J.; Shall, S.; Zia’Ee, A. The Involvement of Poly(ADP-ribose) Polymerase in the Degradation of NAD Caused by γ-Radiation and N-Methyl-N-Nitrosourea. Eur. J. Biochem. 1979, 101, 135–142. [Google Scholar] [CrossRef]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ Consumption by PARP1 in Response to DNA Damage Triggers Metabolic Shift Critical for Damaged Cell Survival. Mol. Biol. Cell 2019, 30, 2584–2597. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, S.; Piccolella, S.; Manti, L.; Pacifico, S. Could Polyphenols Really Be a Good Radioprotective Strategy? Molecules 2021, 26, 4969. [Google Scholar] [CrossRef] [PubMed]
- Rissel, D.; Peiter, E. Poly(ADP-Ribose) Polymerases in Plants and Their Human Counterparts: Parallels and Peculiarities. Int. J. Mol. Sci. 2019, 20, 1638. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-Induced Reactive Oxygen Species Compartmentalization, Perception and Signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hay Mele, B.; Bianchi, A.R.; Guerretti, V.; Pugliese, M.; De Maio, A.; Arena, C. Antioxidant Defenses and Poly(ADP-Ribose) Polymerase (PARP) Activity Provide “Radioresilience” Against Ionizing Radiation-Induced Stress in Dwarf Bean Plants. Antioxidants 2025, 14, 261. https://doi.org/10.3390/antiox14030261
Hay Mele B, Bianchi AR, Guerretti V, Pugliese M, De Maio A, Arena C. Antioxidant Defenses and Poly(ADP-Ribose) Polymerase (PARP) Activity Provide “Radioresilience” Against Ionizing Radiation-Induced Stress in Dwarf Bean Plants. Antioxidants. 2025; 14(3):261. https://doi.org/10.3390/antiox14030261
Chicago/Turabian StyleHay Mele, Bruno, Anna Rita Bianchi, Valeria Guerretti, Mariagabriella Pugliese, Anna De Maio, and Carmen Arena. 2025. "Antioxidant Defenses and Poly(ADP-Ribose) Polymerase (PARP) Activity Provide “Radioresilience” Against Ionizing Radiation-Induced Stress in Dwarf Bean Plants" Antioxidants 14, no. 3: 261. https://doi.org/10.3390/antiox14030261
APA StyleHay Mele, B., Bianchi, A. R., Guerretti, V., Pugliese, M., De Maio, A., & Arena, C. (2025). Antioxidant Defenses and Poly(ADP-Ribose) Polymerase (PARP) Activity Provide “Radioresilience” Against Ionizing Radiation-Induced Stress in Dwarf Bean Plants. Antioxidants, 14(3), 261. https://doi.org/10.3390/antiox14030261









