Phenolic Acid Composition of Coffee Cascara in Connection with Antioxidant Capacity: A Geographic Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Proximate Composition
2.3. Water Extraction of Coffee Cascara
2.4. Methanol Extraction of Coffee Cascara
2.5. Analysis of Cascara Polyphenol Composition
2.6. ABTS Assay
2.7. ORAC Assay
2.8. Caco-2 Cell Culture
2.9. Cell Viability Assay
2.10. Intracellular Oxidative Assay
2.11. Statistics
3. Results
3.1. Proximate Analysis of Cascara Samples
3.2. Phenolic Profile of Coffee Cascara Water and Methanol Extracts
3.3. Antioxidant Activity of Coffee Cascara Water and Methanol Extract
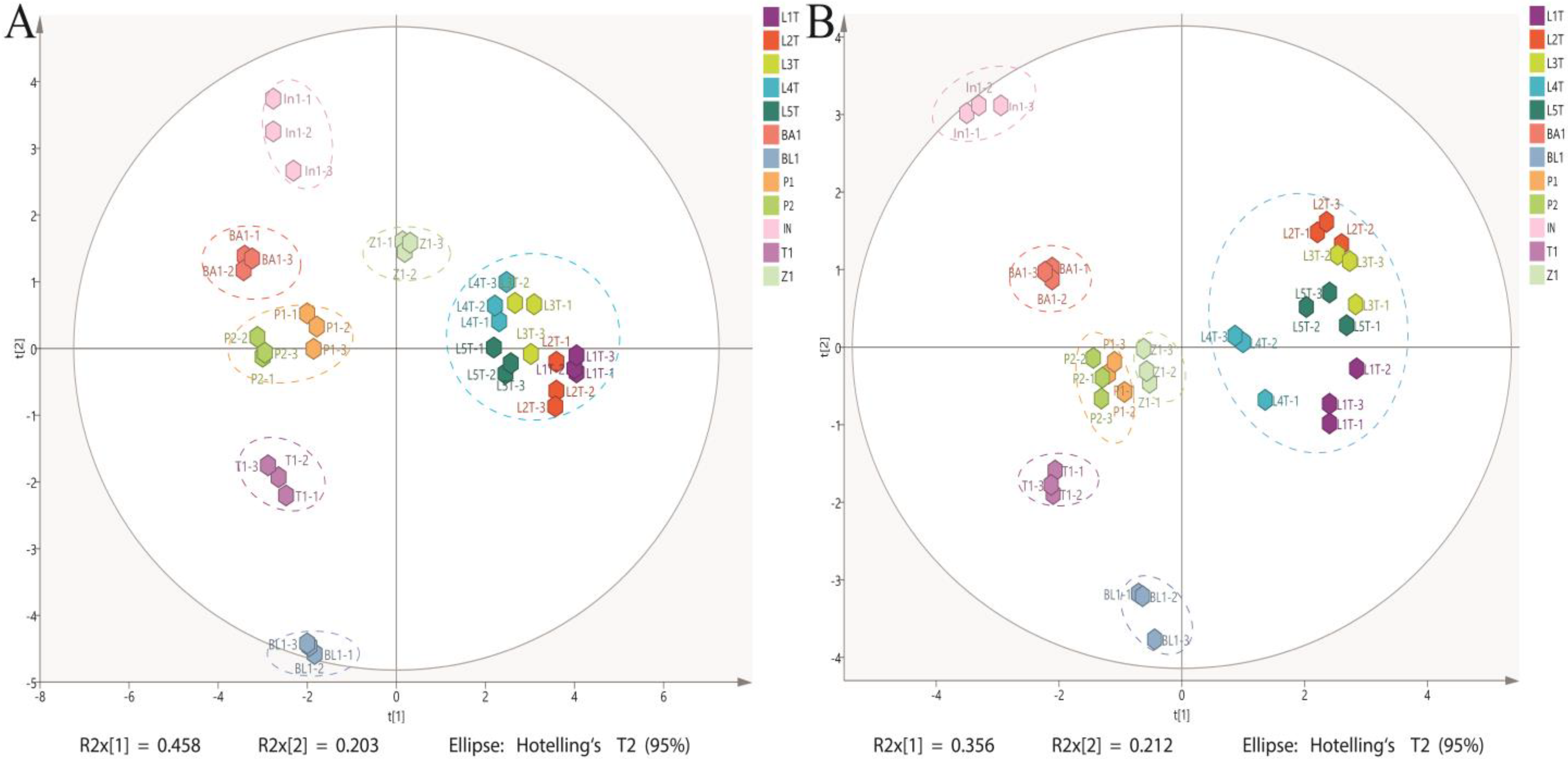
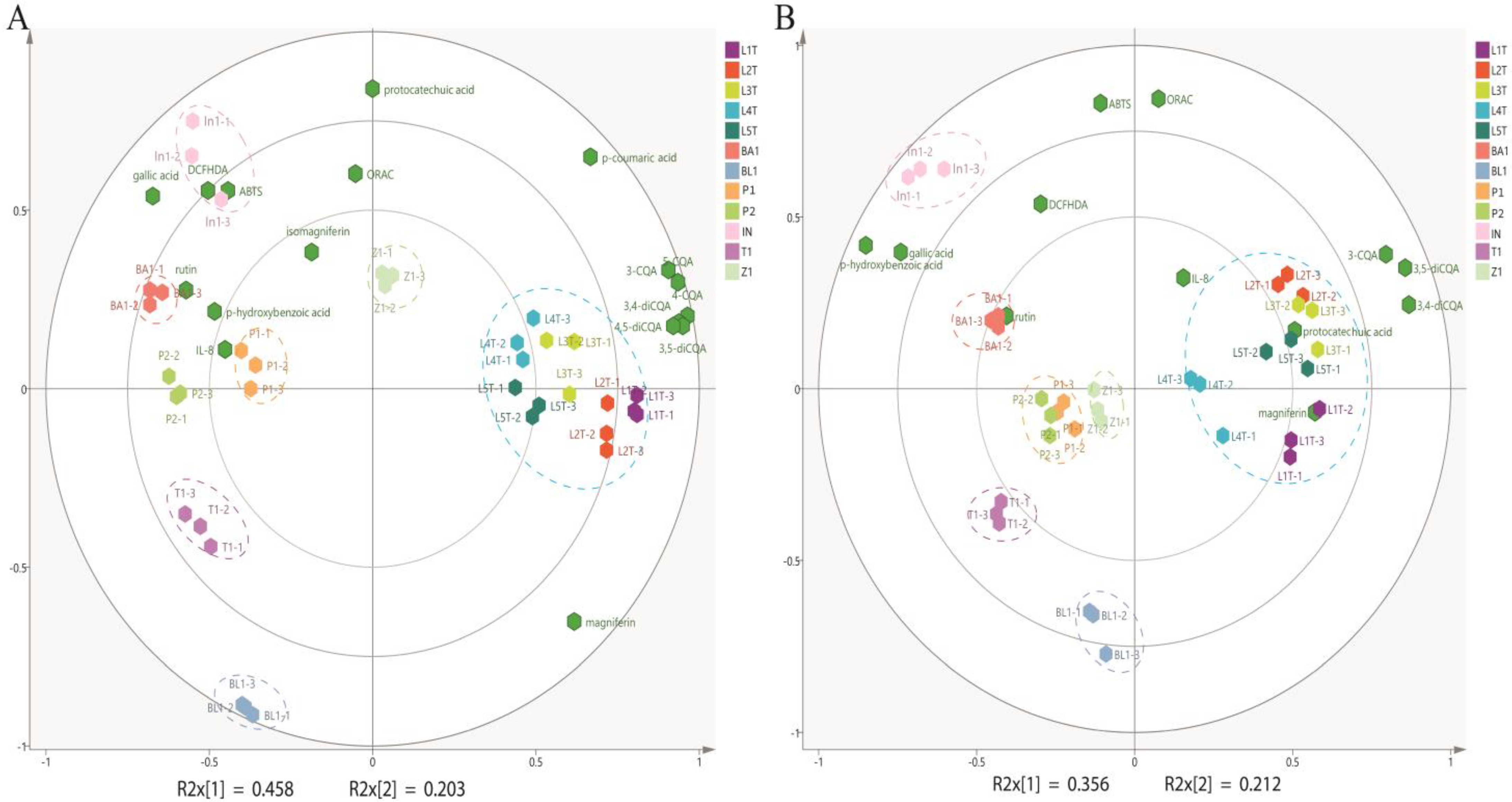
3.4. Principle Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| ACN | Acetonitrile |
| BA | Brazil |
| DCFH-DA | 2′,7′-Dichlorofluorescein diacetate |
| ET | Electron transfer |
| PCA | Principal component analysis |
| HPLC | High-performance liquid chromatography |
| Keap1 | Kelch ECH associating protein 1 |
| Nrf2 | Nuclear factor-erthyriod 2 -related factor 2 |
| ORAC | Oxygen radical absorbance capacity |
| HAT | Hydrogen atom transfer |
| TFA | Trifluoroacetic acid |
| P | Peru |
| In | Indonesia |
| Z | Zambia |
| L | Laos |
| BL | Boliva |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| CGA | Chlorogenic acid |
| 3-CQA | 3-caffeoylquinic acid |
| 4-CQA | 4-caffeoylquinic acid |
| 5-CQA | 5-caffeoylquinic acid |
| 3,4-diCQA | 3,4-dicaffeoylquinic acid |
| 3,5-diCQA | 3,5-dicaffeoylquinic acid |
| 4,5-diCQA | 4,5-dicaffeoylquinic acid |
References
- Manasa, V.; Padmanabhan, A.; Anu Appaiah, K.A. Utilization of Coffee Pulp Waste for Rapid Recovery of Pectin and Polyphenols for Sustainable Material Recycle. Waste Manag. 2021, 120, 762–771. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Mello, F.V.C.; Thode Filho, S.; Carpes, R.M.; Honório, J.G.; Marques, M.R.C.; Felzenszwalb, I.; Ferraz, E.R.A. Impacts of Discarded Coffee Waste on Human and Environmental Health. Ecotoxicol. Environ. Saf. 2017, 141, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Mata, A.; Delgado, G.; Fernández-Arteaga, A.; Fornasier, F.; Mondini, C. Spent Coffee Grounds By-Products and Their Influence on Soil C–N Dynamics. J. Environ. Manag. 2022, 302, 114075. [Google Scholar] [CrossRef] [PubMed]
- Mazzafera, P. Degradation of Caffeine by Microorganisms and Potential Use of Decaffeinated Coffee Husk and Pulp in Animal Feeding. Sci. Agric. Piracicaba Braz. 2002, 59, 815–821. [Google Scholar] [CrossRef]
- Oliveira, W.E.; Franca, A.S.; Oliveira, L.S.; Rocha, S.D. Untreated Coffee Husks as Biosorbents for the Removal of Heavy Metals from Aqueous Solutions. J. Hazard. Mater. 2008, 152, 1073–1081. [Google Scholar] [CrossRef]
- Son, J.; Kim, B.; Park, J.; Yang, J.; Lee, J.W. Wet in Situ Transesterification of Spent Coffee Grounds with Supercritical Methanol for the Production of Biodiesel. Bioresour. Technol. 2018, 259, 465–468. [Google Scholar] [CrossRef]
- Magoni, C.; Bruni, I.; Guzzetti, L.; Dell’Agli, M.; Sangiovanni, E.; Piazza, S.; Regonesi, M.E.; Maldini, M.; Spezzano, R.; Caruso, D.; et al. Valorizing Coffee Pulp By-Products as Anti-Inflammatory Ingredient of Food Supplements Acting on IL-8 Release. Food Res. Int. 2018, 112, 129–135. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Elizondo, A.S.; Iriondo-DeHond, M.; Ríos, M.B.; Mufari, R.; Mendiola, J.A.; Ibañez, E.; Del Castillo, M.D. Assessment of Healthy and Harmful Maillard Reaction Products in a Novel Coffee Cascara Beverage: Melanoidins and Acrylamide. Foods 2020, 9, 620. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Liu, P.; Zhang, T.; Jelderks, J.A.; Corke, H. Preliminary Characterization of Phytochemicals and Polysaccharides in Diverse Coffee Cascara Samples: Identification, Quantification and Discovery of Novel Compounds. Foods 2022, 11, 1710. [Google Scholar] [CrossRef]
- Sales, A.L.; Cunha, S.C.; Morgado, J.; Cruz, A.; Santos, T.F.; Ferreira, I.M.P.L.V.O.; Fernandes, J.O.; Miguel, M.A.L.; Farah, A. Volatile, Microbial, and Sensory Profiles and Consumer Acceptance of Coffee Cascara Kombuchas. Foods 2023, 12, 2710. [Google Scholar] [CrossRef]
- Sánchez-Martín, V.; López-Parra, M.B.; Iriondo-DeHond, A.; Haza, A.I.; Morales, P.; Del Castillo, M.D. Instant Cascara: A Potential Sustainable Promoter of Gastrointestinal Health. Proceedings 2023, 89, 21. [Google Scholar] [CrossRef]
- Vijayalaxmi, S.; Jayalakshmi, S.K.; Sreeramulu, K. Polyphenols from Different Agricultural Residues: Extraction, Identification and Their Antioxidant Properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, T.O.; Arowolo, R.; Olayemi, F.O. Phytochemical Screening and Toxicity Studies on the Methanol Extract of the Seeds of Moringa oleifera. J. Complement. Integr. Med. 2013, 10, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and Analysis of Terpenes/Terpenoids. Curr. Protoc. Plant Biol. 2016, 1, 345–358. [Google Scholar] [CrossRef]
- Pua, A.; Choo, W.X.D.; Goh, R.M.V.; Liu, S.Q.; Cornuz, M.; Ee, K.-H.; Sun, J.; Lassabliere, B.; Yu, B. A Systematic Study of Key Odourants, Non-Volatile Compounds, and Antioxidant Capacity of Cascara (Dried Coffea Arabica Pulp). LWT 2021, 138, 110630. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Nofitasari, D.; Rahmawati, A.; Eryanti, A.Y.; Rosmiati, M. Effects of Brewing Conditions on Total Phenolic Content, Antioxidant Activity and Sensory Properties of Cascara. Food Chem. Adv. 2023, 2, 100183. [Google Scholar] [CrossRef]
- Tores De La Cruz, S.; Iriondo-DeHond, A.; Herrera, T.; Lopez-Tofiño, Y.; Galvez-Robleño, C.; Prodanov, M.; Velazquez-Escobar, F.; Abalo, R.; Del Castillo, M.D. An Assessment of the Bioactivity of Coffee Silverskin Melanoidins. Foods 2019, 8, 68. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Relationship of the Phytochemicals from Coffee and Cocoa By-Products with Their Potential to Modulate Biomarkers of Metabolic Syndrome In Vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2006; pp. 38–46. [Google Scholar]
- Liang, N.; Xue, W.; Kennepohl, P.; Kitts, D.D. Interactions between Major Chlorogenic Acid Isomers and Chemical Changes in Coffee Brew That Affect Antioxidant Activities. Food Chem. 2016, 213, 251–259. [Google Scholar] [CrossRef]
- Hu, C.; Kwok, B.H.L.; Kitts, D.D. Saskatoon Berries (Amelanchier alnifolia Nutt.) Scavenge Free Radicals and Inhibit Intracellular Oxidation. Food Res. Int. 2005, 38, 1079–1085. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of Coffee Cherry Pulp and Its Utilisation for Production of Cascara Beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Plata-Oviedo, M.S.V.; de Mattos, G.; Carpes, S.T.; Branco, I.G. Extraction and Quantification of Phenolic Acids and Flavonols from Eugenia Pyriformis Using Different Solvents. J. Food Sci. Technol. 2014, 51, 2862–2866. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.M.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M.; Khan, S.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alfagham, A.; Alkahtani, J. Optimization Method for Phenolic Compounds Extraction from Medicinal Plant (Juniperus procera) and Phytochemicals Screening. Molecules 2021, 26, 7454. [Google Scholar] [CrossRef]
- Tran, T.M.K.; Akanbi, T.; Kirkman, T.; Nguyen, M.H.; Vuong, Q.V. Optimal Aqueous Extraction Conditions as A Green Technique for Recovery of Phenolic Antioxidants from Robusta Dried Coffee Pulp. Eur. J. Eng. Technol. Res. 2020, 5, 1069–1074. [Google Scholar] [CrossRef]
- Mu, K.; Yao, Y.; Wang, D.; Kitts, D.D. Prooxidant Capacity of Phenolic Acids Defines Antioxidant Potential. Biochim. Biophys. Acta (BBA) Gen. Subj. 2023, 1867, 130371. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and Limitations of Common Testing Methods for Antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC Assays Comparison to Measure the Antioxidant Capacity of Food Products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Carvalho, J.R.B.; Meireles, A.N.; Marques, S.S.; Gregório, B.J.R.; Ramos, I.I.; Silva, E.M.P.; Barreiros, L.; Segundo, M.A. Exploiting Kinetic Features of ORAC Assay for Evaluation of Radical Scavenging Capacity. Antioxidants 2023, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Olszowy-Tomczyk, M. Synergistic, Antagonistic and Additive Antioxidant Effects in the Binary Mixtures. Phytochem. Rev. 2020, 19, 63–103. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The Synergistic and Antagonistic Antioxidant Interactions of Dietary Phytochemical Combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef] [PubMed]
- Casoni, D.; Sârbu, C. Comprehensive Evaluation of Antioxidant Activity: A Chemometric Approach Using Principal Component Analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 118, 343–348. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E.; Karpowicz, D.; Leśniewska, B. Antioxidant Properties of Coffee Substitutes Rich in Polyphenols and Minerals. Food Chem. 2019, 278, 101–109. [Google Scholar] [CrossRef]
- Trinh, N.T.N.; Tuan, N.N.; Thang, T.D.; Kuo, P.-C.; Thanh, N.B.; Tam, L.N.; Tuoi, L.H.; Nguyen, T.H.D.; Vu, D.C.; Ho, T.L.; et al. Chemical Composition Analysis and Antioxidant Activity of Coffea Robusta Monofloral Honeys from Vietnam. Foods 2022, 11, 388. [Google Scholar] [CrossRef]
- Hossain, M.B.; Patras, A.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Application of Principal Component and Hierarchical Cluster Analysis to Classify Different Spices Based on In Vitro Antioxidant Activity and Individual Polyphenolic Antioxidant Compounds. J. Funct. Foods 2011, 3, 179–189. [Google Scholar] [CrossRef]
- Chen, X.; Kitts, D.D.; Ji, D.; Ding, J. Free Radical Scavenging Activities of Phytochemical Mixtures and Aqueous Methanolic Extracts Recovered from Processed Coffee Leaves. Int. J. Food Sci. Technol. 2019, 54, 2872–2879. [Google Scholar] [CrossRef]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Phytochemical Profile and Antioxidant Capacity of Coffee Plant Organs Compared to Green and Roasted Coffee Beans. Antioxidants 2020, 9, 93. [Google Scholar] [CrossRef]
- Guan, Y.; Lin, H.; Ma, L.; Yang, Y.; Hu, X. Nitric Oxide and Hydrogen Peroxide Are Important Signals Mediating the Allelopathic Response of Arabidopsis to p-hydroxybenzoic Acid. Physiol. Plant. 2014, 152, 275–285. [Google Scholar] [CrossRef]
- Kimura, M.; Yamamoto, T.; Zhang, J.; Itoh, K.; Kyo, M.; Kamiya, T.; Aburatani, H.; Katskoka, F.; Karokawa, H.; Tanaka, T.; et al. Molecular Basis Distinguishing the DNA Binding Profile of Nrf2-Maf Heterodimer From that of Maf Ho-modimer. J. Biol. Chem. 2015, 290, 10644. [Google Scholar] [CrossRef] [PubMed]
| Parameter | L | BA | BL | P | In | T | Z |
|---|---|---|---|---|---|---|---|
| Moisture (%) | 20.1 ± 0.4 | 13.4 ± 0.3 | 16.2 ± 0.4 | 17.6 ± 0.7 | 15.3 ± 0.7 | 15.1 ± 0.8 | 13.3 ± 0.7 |
| Protein (%) | 10.2 ± 0.2 | 7.3 ± 0.1 | 10.7 ± 0.1 | 7.1 ± 0.1 | 8.8 ± 0.2 | 7.2 ± 0.1 | 9.1 ± 0.1 |
| Fat (%) | 3.0 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.8 ± 0.1 | 2.0 ± 0.1 | 3.0 ± 0.1 | 1.9 ± 0.1 |
| Carbohydrate (%) | 61 ± 5 | 72 ± 7 | 65 ± 5 | 68 ± 3 | 67 ± 4 | 67 ± 6 | 70 ± 4 |
| Crude Fiber (%) | 9.0 ± 0.7 | 10.7 ± 0.7 | 11.8 ± 0.8 | 9.3 ± 0.6 | 12.9 ± 0.6 | 8.0 ± 0.4 | 9.9 ± 0.5 |
| Ash (%) | 5.8 ± 0.1 | 6.1 ± 0.1 | 6.8 ± 0.1 | 7.4 ± 0.1 | 6.6 ± 0.1 | 7.7 ± 0.1 | 6.3 ± 0.1 |
| Energy (calories/100 g) | 315 | 330 | 317 | 326 | 322 | 325 | 327 |
| Code | Protocatechuic Acid | Gallic Acid | p-Coumaric Acid 2 | p-Hydroxybenzoic Acid | CGA 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Water | Methanol | Water | Methanol | Water | Methanol | Water | Methanol | Water | Methanol | |
| L | 2952 ± 47 a,x | 2313 ± 84 a,x | 1035 ± 31 b,x | 56 ± 9 c,y | 121 ± 13 c | ND | 15 ± 3 a,y | 24 ± 4 a,x | 8665 ± 62 c,x | 7867 ± 242 d,x |
| BA | 3050 ± 38 a,x | 3186 ± 49 a,x | 7958 ± 111 c,x | 884 ± 99 a,y | 73 ± 17 b | ND | 91 ± 4 b,x | 101 ± 13 b,x | 3861 ± 1899 e,x | 3725 ± 58 c,x |
| BL | 2409 ± 66 a,x | 2563 ± 27 a,x | 1226 ± 342 a,x | 124 ± 14 c,y | 17 ± 15 a | ND | 125 ± 1 b,x | 174 ± 14 b,x | 1195 ± 431 a,x | 1058 ± 20 s,x |
| P | 2757 ± 18 a,x | 2715 ± 25 a,x | 4895 ± 120 b,x | 101 ± 7 c,y | 65 ± 35 b | ND | 101 ± 12 b,x | 213 ± 8 b,c,y | 3597 ± 18 b,x | 3529 ± 15 b,x |
| In | 2862 ± 22 a,x | 2410 ± 50 a,x | 7553 ± 131 c,x | 882 ± 128 a,y | 121 ± 23 c | ND | 231 ± 18 c,x | 312 ± 80 c,y | 3837 ± 18 a,x | 2787 ± 26 g,y |
| T | 2671 ± 14 a,x | 2486 ± 30 a,x | 849 ± 32 d,x | 408 ± 13 b,y | 64 ± 3 d | ND | 51 ± 13 b,x | 49. ± 10 a,x | 2047 ± 28 a,y | 1394 ± 13 s,x |
| Z | 2983 ± 22 a,x | 3160 ± 64 a,x | 1173 ± 40 b,x | 395 ± 66 b,x | 122 ± 19 a | ND | 133 ± 11 b,x | 233 ± 4 b,c,y | 6886 ± 63 c,x | 6371 ± 98 d,x |
| Code 2 | Mangiferin | Rutin | Isomangiferin | |||
|---|---|---|---|---|---|---|
| Water | Methanol | Water | Methanol | Water | Methanol | |
| BA | 2039 ± 25 b,x | 2554 ± 27 b,y | 250 ± 106 c,x | 116 ± 15 a,y | 40 ± 7 | ND |
| BL | 3107 ± 86 c,x | 4294 ± 18 a,y | 83 ± 45 a,x | 80 ± 2 a,x | 21 ± 7 | ND |
| P | 1913 ± 22 b,x | 2246 ± 10 a,x | 164 ± 10 b,x | 173 ± 9 b,x | 35 ± 7 | ND |
| In | 818 ± 12 c,x | 2874 ± 70 a,y | 147 ± 19 b,x | 150 ± 21 b,x | 31 ± 4 | ND |
| T | 2051 ± 34 b,x | 2155 ± 15 b,x | 133 ± 51 b,x | 187 ± 41 b,y | 32 ± 4 | ND |
| Z | 2462 ± 44 a,b,x | 1555 ± 28 c,x | 207 ± 12 c,x | 184 ± 21 b,x | 30 ± 8 | ND |
| ORAC | ABTS | DCFH-DA | ||||
|---|---|---|---|---|---|---|
| Code 2 | Water | Methanol 3 | Water | Methanol 3 | Water | Methanol 3 |
| L | 252 ± 13 b,x | 418 ± 16 c,y | 72 ± 9 a,x | 126 ± 7 b,y | 20 ± 7 a,x | 26 ± 8 a,x |
| BA | 339 ± 5 c,x | 364 ± 4 b,y | 86 ± 6a,x | 171 ± 5 b,y | 16 ± 10 a,y | 36 ± 6 b,y |
| BL | 160 ± 7 a,x | 216 ± 12 a,y | 61 ± 4 a,x | 73 ± 12 c,x | 12 ± 11 a,x | 16 ± 7 a,x |
| P | 273 ± 19 b,x | 403 ± 18 c,y | 123 ± 21b,x | 165 ± 5.0 b,x | 14 ± 5 a,x | 31 ± 14 b,y |
| I | 252 ± 11 b,x | 614 ± 26 a,y | 183 ± 9 c,x | 293 ± 15 a,y | 35 ± 4 b,x | 39 ± 9 b,x |
| T | 133 ± 14 a,x | 341 ± 24 b,y | 105 ± 4 b,x | 66 ± 28 c,y | 36 ± 7 b,x | 19 ± 4 a,y |
| Z | 244 ± 5 b,x | 391 ± 33 b,y | 208 ± N/A c,x | 119 ±14 b,y | 26 ±3 a,x | 16 ± 6 a,y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, N.; Kitts, D.D.; Wang, X.; Hu, Z.; Sabier, M. Phenolic Acid Composition of Coffee Cascara in Connection with Antioxidant Capacity: A Geographic Assessment. Antioxidants 2025, 14, 502. https://doi.org/10.3390/antiox14050502
Liang N, Kitts DD, Wang X, Hu Z, Sabier M. Phenolic Acid Composition of Coffee Cascara in Connection with Antioxidant Capacity: A Geographic Assessment. Antioxidants. 2025; 14(5):502. https://doi.org/10.3390/antiox14050502
Chicago/Turabian StyleLiang, Ningjian, David D. Kitts, Xiwen Wang, Ziying Hu, and Maidinai Sabier. 2025. "Phenolic Acid Composition of Coffee Cascara in Connection with Antioxidant Capacity: A Geographic Assessment" Antioxidants 14, no. 5: 502. https://doi.org/10.3390/antiox14050502
APA StyleLiang, N., Kitts, D. D., Wang, X., Hu, Z., & Sabier, M. (2025). Phenolic Acid Composition of Coffee Cascara in Connection with Antioxidant Capacity: A Geographic Assessment. Antioxidants, 14(5), 502. https://doi.org/10.3390/antiox14050502