The Role of NADPH Oxidase 2 in Leukocytes
Abstract
:1. Introduction
2. NADPH Oxidase Isoforms and Distribution
3. Role of NOX in Neutrophils
4. NOX in Lymphoid Cells
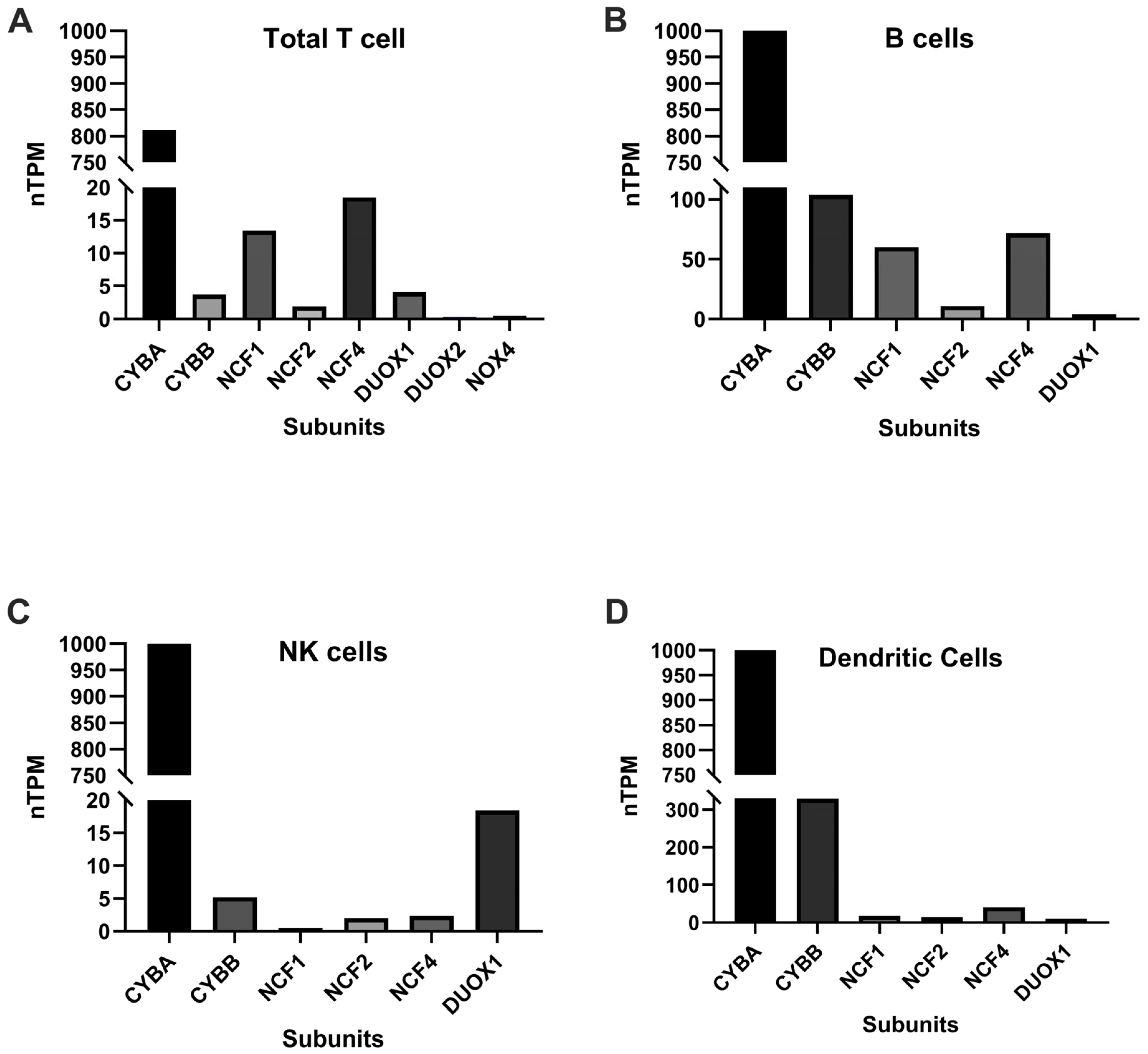
4.1. NOX and T Cells
4.2. NOX and B Cells
4.3. NOX and NK Cells
5. NOX and Dendritic Cells
6. Diseases Caused by NOX Deficiency
7. Myeloperoxidase Deficiency
8. Glucose-6-Phosphate Dehydrogenase Deficiency
9. NOX Deficiency and Autoimmunity
9.1. Rheumatoid Arthritis
9.2. Systemic Lupus Erythematosus (SLE)
9.3. Psoriasis
10. Treatment
11. Summary and Gaps in the Knowledge of the Field
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reeves, E.P.; Lu, H.; Jacobs, H.L.; Messina, C.G.; Bolsover, S.; Gabella, G.; Potma, E.O.; Warley, A.; Roes, J.; Segal, A.W. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 2002, 416, 291–297. [Google Scholar] [CrossRef]
- Cipriano, A.; Viviano, M.; Feoli, A.; Milite, C.; Sarno, G.; Castellano, S.; Sbardella, G. NADPH Oxidases: From Molecular Mechanisms to Current Inhibitors. J. Med. Chem. 2023, 66, 11632–11655. [Google Scholar] [CrossRef] [PubMed]
- Nocella, C.; D’Amico, A.; Cammisotto, V.; Bartimoccia, S.; Castellani, V.; Loffredo, L.; Marini, L.; Ferrara, G.; Testa, M.; Motta, G.; et al. Structure, Activation, and Regulation of NOX2: At the Crossroad between the Innate Immunity and Oxidative Stress-Mediated Pathologies. Antioxidants 2023, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Laurent, E.; McCoy, J.W., 3rd; Macina, R.A.; Liu, W.; Cheng, G.; Robine, S.; Papkoff, J.; Lambeth, J.D. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int. J. Cancer 2008, 123, 100–107. [Google Scholar] [CrossRef]
- Lim, S.D.; Sun, C.; Lambeth, J.D.; Marshall, F.; Amin, M.; Chung, L.; Petros, J.A.; Arnold, R.S. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 2005, 62, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.L.; Brockman, D.; Campos, B.; Myatt, L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: Involvement in preeclampsia. Placenta 2006, 27, 422–431. [Google Scholar] [CrossRef]
- Yin, W.; Voit, E.O. Function and design of the Nox1 system in vascular smooth muscle cells. BMC Syst. Biol. 2013, 7, 20. [Google Scholar] [CrossRef]
- Banfi, B.; Malgrange, B.; Knisz, J.; Steger, K.; Dubois-Dauphin, M.; Krause, K.H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004, 279, 46065–46072. [Google Scholar] [CrossRef]
- Weyemi, U.; Caillou, B.; Talbot, M.; Ameziane-El-Hassani, R.; Lacroix, L.; Lagent-Chevallier, O.; Al Ghuzlan, A.; Roos, D.; Bidart, J.M.; Virion, A.; et al. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr. Relat. Cancer 2010, 17, 27–37. [Google Scholar] [CrossRef]
- Chen, F.; Haigh, S.; Barman, S.; Fulton, D.J. From form to function: The role of Nox4 in the cardiovascular system. Front. Physiol. 2012, 3, 412. [Google Scholar] [CrossRef]
- Geiszt, M.; Kopp, J.B.; Varnai, P.; Leto, T.L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 8010–8014. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, S.; Deffert, C.; Donati, Y.; Basset, O.; Hinz, B.; Preynat-Seauve, O.; Guichard, C.; Arbiser, J.L.; Banfi, B.; Pache, J.C.; et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 2011, 15, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Grange, L.; Nguyen, M.V.; Lardy, B.; Derouazi, M.; Campion, Y.; Trocme, C.; Paclet, M.H.; Gaudin, P.; Morel, F. NAD(P)H oxidase activity of Nox4 in chondrocytes is both inducible and involved in collagenase expression. Antioxid. Redox Signal. 2006, 8, 1485–1496. [Google Scholar] [CrossRef]
- Meitzler, J.L.; Makhlouf, H.R.; Antony, S.; Wu, Y.; Butcher, D.; Jiang, G.; Juhasz, A.; Lu, J.; Dahan, I.; Jansen-Durr, P.; et al. Decoding NADPH oxidase 4 expression in human tumors. Redox Biol. 2017, 13, 182–195. [Google Scholar] [CrossRef]
- Cui, C.; Jiang, M.; Jain, N.; Das, S.; Lo, Y.H.; Kermani, A.A.; Pipatpolkai, T.; Sun, J. Structural basis of human NOX5 activation. Nat. Commun. 2024, 15, 3994. [Google Scholar] [CrossRef]
- Rigutto, S.; Hoste, C.; Grasberger, H.; Milenkovic, M.; Communi, D.; Dumont, J.E.; Corvilain, B.; Miot, F.; De Deken, X. Activation of dual oxidases Duox1 and Duox2: Differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J. Biol. Chem. 2009, 284, 6725–6734. [Google Scholar] [CrossRef] [PubMed]
- Ashtiwi, N.M.; Sarr, D.; Rada, B. DUOX1 in mammalian disease pathophysiology. J. Mol. Med. 2021, 99, 743–754. [Google Scholar] [CrossRef]
- Szanto, I.; Pusztaszeri, M.; Mavromati, M. H2O2 Metabolism in Normal Thyroid Cells and in Thyroid Tumorigenesis: Focus on NADPH Oxidases. Antioxidants 2019, 8, 126. [Google Scholar] [CrossRef]
- El Hassani, R.A.; Benfares, N.; Caillou, B.; Talbot, M.; Sabourin, J.C.; Belotte, V.; Morand, S.; Gnidehou, S.; Agnandji, D.; Ohayon, R.; et al. Dual oxidase2 is expressed all along the digestive tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G933–G942. [Google Scholar] [CrossRef]
- Gabig, T.G.; Babior, B.M. The O2−-forming oxidase responsible for the respiratory burst in human neutrophils. Properties of the solubilized enzyme. J. Biol. Chem. 1979, 254, 9070–9074. [Google Scholar] [CrossRef]
- Gabig, T.G.; Kipnes, R.S.; Babior, B.M. Solubilization of the O2−-forming activity responsible for the respiratory burst in human neutrophils. J. Biol. Chem. 1978, 253, 6663–6665. [Google Scholar] [CrossRef]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Investig. 2018, 48 (Suppl. S2), e12951. [Google Scholar] [CrossRef]
- Antoszewska-Smith, J.; Pawlowska, E.; Blasiak, J. Reactive oxygen species in BCR-ABL1-expressing cells—Relevance to chronic myeloid leukemia. Acta Biochim. Pol. 2017, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.; Chernatynskaya, A.V.; Newby, B.; Brusko, T.M.; Xu, Y.; Barra, J.M.; Morgan, N.; Santarlas, C.; Reeves, W.H.; et al. NADPH Oxidase 2-Derived Reactive Oxygen Species Promote CD8+ T Cell Effector Function. J. Immunol. 2024, 212, 258–270. [Google Scholar] [CrossRef]
- Tse, H.M.; Thayer, T.C.; Steele, C.; Cuda, C.M.; Morel, L.; Piganelli, J.D.; Mathews, C.E. NADPH oxidase deficiency regulates Th lineage commitment and modulates autoimmunity. J. Immunol. 2010, 185, 5247–5258. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.H.; Devadas, S.; Kwon, J.; Pinto, L.A.; Williams, M.S. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol. 2004, 5, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.J.; Oh, I.S.; Yoon, Y.H.; Kwon, B.I.; Kang, W.; Kim, H.J.; Nahm, S.H.; Choi, Y.H.; Lee, S.H.; Racanelli, V.; et al. Apocynin regulates cytokine production of CD8+ T cells. Clin. Exp. Med. 2014, 14, 261–268. [Google Scholar] [CrossRef]
- Bai, A.; Moss, A.; Rothweiler, S.; Serena Longhi, M.; Wu, Y.; Junger, W.G.; Robson, S.C. NADH oxidase-dependent CD39 expression by CD8+ T cells modulates interferon gamma responses via generation of adenosine. Nat. Commun. 2015, 6, 8819. [Google Scholar] [CrossRef]
- Kwon, J.; Shatynski, K.E.; Chen, H.; Morand, S.; de Deken, X.; Miot, F.; Leto, T.L.; Williams, M.S. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci. Signal. 2010, 3, ra59. [Google Scholar] [CrossRef]
- Wen, Z.; Shimojima, Y.; Shirai, T.; Li, Y.; Ju, J.; Yang, Z.; Tian, L.; Goronzy, J.J.; Weyand, C.M. NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs. J. Clin. Investig. 2016, 126, 1953–1967. [Google Scholar] [CrossRef]
- Thayer, T.C.; Delano, M.; Liu, C.; Chen, J.; Padgett, L.E.; Tse, H.M.; Annamali, M.; Piganelli, J.D.; Moldawer, L.L.; Mathews, C.E. Superoxide production by macrophages and T cells is critical for the induction of autoreactivity and type 1 diabetes. Diabetes 2011, 60, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Padgett, L.E.; Anderson, B.; Liu, C.; Ganini, D.; Mason, R.P.; Piganelli, J.D.; Mathews, C.E.; Tse, H.M. Loss of NOX-Derived Superoxide Exacerbates Diabetogenic CD4 T-Cell Effector Responses in Type 1 Diabetes. Diabetes 2015, 64, 4171–4183. [Google Scholar] [CrossRef] [PubMed]
- Burg, A.R.; Das, S.; Padgett, L.E.; Koenig, Z.E.; Tse, H.M. Superoxide Production by NADPH Oxidase Intensifies Macrophage Antiviral Responses during Diabetogenic Coxsackievirus Infection. J. Immunol. 2018, 200, 61–70. [Google Scholar] [CrossRef]
- Gabrion, A.; Hmitou, I.; Moshous, D.; Neven, B.; Lefevre-Utile, A.; Diana, J.S.; Suarez, F.; Picard, C.; Blanche, S.; Fischer, A.; et al. Mammalian target of rapamycin inhibition counterbalances the inflammatory status of immune cells in patients with chronic granulomatous disease. J. Allergy Clin. Immunol. 2017, 139, 1641–1649.e6. [Google Scholar] [CrossRef] [PubMed]
- Al, B.; Bruno, M.; Roring, R.J.; Moorlag, S.; Suen, T.K.; Kluck, V.; Liu, R.; Debisarun, P.A.; Gaal, O.; Bhat, J.; et al. Peripheral T Cell Populations are Differentially Affected in Familial Mediterranean Fever, Chronic Granulomatous Disease, and Gout. J. Clin. Immunol. 2023, 43, 2033–2048. [Google Scholar] [CrossRef]
- Albuquerque, A.S.; Fernandes, S.M.; Tendeiro, R.; Cheynier, R.; Lucas, M.; Silva, S.L.; Victorino, R.M.M.; Sousa, A.E. Major CD4 T-Cell Depletion and Immune Senescence in a Patient with Chronic Granulomatous Disease. Front. Immunol. 2017, 8, 543. [Google Scholar] [CrossRef]
- Kelkka, T.; Kienhofer, D.; Hoffmann, M.; Linja, M.; Wing, K.; Sareila, O.; Hultqvist, M.; Laajala, E.; Chen, Z.; Vasconcelos, J.; et al. Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature. Antioxid. Redox Signal. 2014, 21, 2231–2245. [Google Scholar] [CrossRef]
- Chiriaco, M.; Salfa, I.; Ursu, G.M.; Cifaldi, C.; Di Cesare, S.; Rossi, P.; Di Matteo, G.; Finocchi, A. Immunological Aspects of X-Linked Chronic Granulomatous Disease Female Carriers. Antioxidants 2021, 10, 891. [Google Scholar] [CrossRef]
- Zhi, L.; Ustyugova, I.V.; Chen, X.; Zhang, Q.; Wu, M.X. Enhanced Th17 differentiation and aggravated arthritis in IEX-1-deficient mice by mitochondrial reactive oxygen species-mediated signaling. J. Immunol. 2012, 189, 1639–1647. [Google Scholar] [CrossRef]
- van de Geer, A.; Cuadrado, E.; Slot, M.C.; van Bruggen, R.; Amsen, D.; Kuijpers, T.W. Regulatory T cell features in chronic granulomatous disease. Clin. Exp. Immunol. 2019, 197, 222–229. [Google Scholar] [CrossRef]
- Cemerski, S.; van Meerwijk, J.P.; Romagnoli, P. Oxidative-stress-induced T lymphocyte hyporesponsiveness is caused by structural modification rather than proteasomal degradation of crucial TCR signaling molecules. Eur. J. Immunol. 2003, 33, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Bevilacqua, D.; Caveggion, E.; Gasperini, S.; Zenaro, E.; Pettinella, F.; Donini, M.; Dusi, S.; Constantin, G.; Lonardi, S.; et al. Neutrophils inhibit gammadelta T cell functions in the imiquimod-induced mouse model of psoriasis. Front. Immunol. 2022, 13, 1049079. [Google Scholar] [CrossRef]
- Allan, E.R.; Tailor, P.; Balce, D.R.; Pirzadeh, P.; McKenna, N.T.; Renaux, B.; Warren, A.L.; Jirik, F.R.; Yates, R.M. NADPH oxidase modifies patterns of MHC class II-restricted epitopic repertoires through redox control of antigen processing. J. Immunol. 2014, 192, 4989–5001. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Batalla, R.; Acevedo, D.; Luo, Y.; Esteve-Sole, A.; Vlagea, A.; Correa-Rocha, R.; Seoane-Reula, M.E.; Alsina, L. Treg in inborn errors of immunity: Gaps, knowns and future perspectives. Front. Immunol. 2023, 14, 1278759. [Google Scholar] [CrossRef]
- Kraaij, M.D.; Savage, N.D.; van der Kooij, S.W.; Koekkoek, K.; Wang, J.; van den Berg, J.M.; Ottenhoff, T.H.; Kuijpers, T.W.; Holmdahl, R.; van Kooten, C.; et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2010, 107, 17686–17691. [Google Scholar] [CrossRef] [PubMed]
- Vaikunthanathan, T.; Landmann, E.; Correa, D.M.; Romano, M.; Trevelin, S.C.; Peng, Q.; Crespo, E.; Corrado, M.; Lozano, J.J.; Pearce, E.L.; et al. Dysregulated anti-oxidant signalling and compromised mitochondrial integrity negatively influence regulatory T cell function and viability in liver disease. EBioMedicine 2023, 95, 104778. [Google Scholar] [CrossRef]
- Cammarata, I.; Pinna, V.; Pacella, I.; Rotella, I.; Soresina, A.; Badolato, R.; Plebani, A.; Pignata, C.; Cirillo, E.; Zicari, A.M.; et al. In adult X-CGD patients, regulatory T cells are expanded while activated T cells display a NOX2-independent ROS increase. Immunol. Lett. 2024, 266, 106839. [Google Scholar] [CrossRef]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef]
- Sharma, A.K.; LaPar, D.J.; Stone, M.L.; Zhao, Y.; Mehta, C.K.; Kron, I.L.; Laubach, V.E. NOX2 Activation of Natural Killer T Cells Is Blocked by the Adenosine A2A Receptor to Inhibit Lung Ischemia-Reperfusion Injury. Am. J. Respir. Crit. Care Med. 2016, 193, 988–999. [Google Scholar] [CrossRef]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Defranco, A.L. Prolonged production of reactive oxygen species in response to B cell receptor stimulation promotes B cell activation and proliferation. J. Immunol. 2012, 189, 4405–4416. [Google Scholar] [CrossRef]
- Wienands, J.; Larbolette, O.; Reth, M. Evidence for a preformed transducer complex organized by the B cell antigen receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 7865–7870. [Google Scholar] [CrossRef]
- Taher, T.E.; Bystrom, J.; Ong, V.H.; Isenberg, D.A.; Renaudineau, Y.; Abraham, D.J.; Mageed, R.A. Intracellular B Lymphocyte Signalling and the Regulation of Humoral Immunity and Autoimmunity. Clin. Rev. Allergy Immunol. 2017, 53, 237–264. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Tang, M.; Suzuki, M.; Gunasekara, C.; Anbe, Y.; Hiraoka, Y.; Liu, J.; Grasberger, H.; Ohkita, M.; Matsumura, Y.; et al. Essential Role of NADPH Oxidase-Dependent Production of Reactive Oxygen Species in Maintenance of Sustained B Cell Receptor Signaling and B Cell Proliferation. J. Immunol. 2019, 202, 2546–2557. [Google Scholar] [CrossRef]
- Tsubata, T. Involvement of Reactive Oxygen Species (ROS) in BCR Signaling as a Second Messenger. Adv. Exp. Med. Biol. 2020, 1254, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, M.; Farinelli, G.; Galli, M.; Aiuti, A.; Sitia, R. AQP8 transports NOX2-generated H2O2 across the plasma membrane to promote signaling in B cells. J. Leukoc. Biol. 2016, 100, 1071–1079. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ono, Y. Involvement of reactive oxygen species produced via NADPH oxidase in tyrosine phosphorylation in human B- and T-lineage lymphoid cells. Biochem. Biophys. Res. Commun. 1999, 255, 262–267. [Google Scholar] [CrossRef]
- Richards, S.M.; Clark, E.A. BCR-induced superoxide negatively regulates B-cell proliferation and T-cell-independent type 2 Ab responses. Eur. J. Immunol. 2009, 39, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Kumar, D.; Siddiqui, Z.; Basu, S.K.; Kumar, V.; Rao, K.V. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell 2005, 121, 281–293. [Google Scholar] [CrossRef]
- Adler, L.N.; Jiang, W.; Bhamidipati, K.; Millican, M.; Macaubas, C.; Hung, S.C.; Mellins, E.D. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front. Immunol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Crotzer, V.L.; Matute, J.D.; Arias, A.A.; Zhao, H.; Quilliam, L.A.; Dinauer, M.C.; Blum, J.S. Cutting edge: NADPH oxidase modulates MHC class II antigen presentation by B cells. J. Immunol. 2012, 189, 3800–3804. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ma, X.; Gu, W.; Fu, M.; An, J.; Xing, Y.; Gao, T.; Li, W.; Liu, Y. Novel functions of murine B1 cells: Active phagocytic and microbicidal abilities. Eur. J. Immunol. 2012, 42, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Kinoshita, M.; Nakashima, H.; Habu, Y.; Miyazaki, H.; Shono, S.; Hiroi, S.; Shinomiya, N.; Nakanishi, K.; Seki, S. Pivotal advance: Characterization of mouse liver phagocytic B cells in innate immunity. J. Leukoc. Biol. 2012, 91, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Parra, D.; Rieger, A.M.; Li, J.; Zhang, Y.A.; Randall, L.M.; Hunter, C.A.; Barreda, D.R.; Sunyer, J.O. Pivotal advance: Peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J. Leukoc. Biol. 2012, 91, 525–536. [Google Scholar] [CrossRef]
- Choi, Y.S.; Dieter, J.A.; Rothaeusler, K.; Luo, Z.; Baumgarth, N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur. J. Immunol. 2012, 42, 120–129. [Google Scholar] [CrossRef]
- Mattos, M.S.; Vandendriessche, S.; Waisman, A.; Marques, P.E. The immunology of B-1 cells: From development to aging. Immun. Ageing 2024, 21, 54. [Google Scholar] [CrossRef]
- Muri, J.; Thut, H.; Bornkamm, G.W.; Kopf, M. B1 and Marginal Zone B Cells but Not Follicular B2 Cells Require Gpx4 to Prevent Lipid Peroxidation and Ferroptosis. Cell Rep. 2019, 29, 2731–2744.e4. [Google Scholar] [CrossRef]
- Muri, J.; Corak, B.; Matsushita, M.; Baes, M.; Kopf, M. Peroxisomes Are Critical for the Development and Maintenance of B1 and Marginal Zone B Cells but Dispensable for Follicular B Cells and T Cells. J. Immunol. 2022, 208, 839–850. [Google Scholar] [CrossRef]
- Kovacs, I.; Horvath, M.; Lanyi, A.; Petheo, G.L.; Geiszt, M. Reactive oxygen species-mediated bacterial killing by B lymphocytes. J. Leukoc. Biol. 2015, 97, 1133–1137. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Catalan, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillon, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Thimmulappa, R.; Kombairaju, P.; Biswal, S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J. Immunol. 2010, 185, 569–577. [Google Scholar] [CrossRef]
- Liu, S.; Lagos, J.; Shumlak, N.M.; Largent, A.D.; Lewis, S.T.E.; Holder, U.; Du, S.W.; Liu, Y.; Hou, B.; Acharya, M.; et al. NADPH oxidase exerts a B cell-intrinsic contribution to lupus risk by modulating endosomal TLR signals. J. Exp. Med. 2024, 221, e20230774. [Google Scholar] [CrossRef]
- Moon, E.Y.; Lee, J.H.; Oh, S.Y.; Ryu, S.K.; Kim, H.M.; Kwak, H.S.; Yoon, W.K. Reactive oxygen species augment B-cell-activating factor expression. Free. Radic. Biol. Med. 2006, 40, 2103–2111. [Google Scholar] [CrossRef]
- Gordon, R.A.; Cosgrove, H.A.; Marinov, A.; Gingras, S.; Tilstra, J.S.; Campbell, A.M.; Bastacky, S.I.; Kashgarian, M.; Perl, A.; Nickerson, K.M.; et al. NADPH oxidase in B cells and macrophages protects against murine lupus by regulation of TLR7. JCI Insight 2024, 9, e178563. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Luo, H.; Coelho, A.; Liu, M.; Li, Q.; Xu, J.; Kramer, A.; Malin, S.; Yuan, Z.; Holmdahl, R. NCF4 dependent intracellular reactive oxygen species regulate plasma cell formation. Redox Biol. 2022, 56, 102422. [Google Scholar] [CrossRef] [PubMed]
- Hultqvist, M.; Olofsson, P.; Holmberg, J.; Backstrom, B.T.; Tordsson, J.; Holmdahl, R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc. Natl. Acad. Sci. USA 2004, 101, 12646–12651. [Google Scholar] [CrossRef]
- Yu, J.E.; De Ravin, S.S.; Uzel, G.; Landers, C.; Targan, S.; Malech, H.L.; Holland, S.M.; Cao, W.; Harpaz, N.; Mayer, L.; et al. High levels of Crohn’s disease-associated anti-microbial antibodies are present and independent of colitis in chronic granulomatous disease. Clin. Immunol. 2011, 138, 14–22. [Google Scholar] [CrossRef]
- Cachat, J.; Deffert, C.; Alessandrini, M.; Roux-Lombard, P.; Le Gouellec, A.; Stasia, M.J.; Hugues, S.; Krause, K.H. Altered Humoral Immune Responses and IgG Subtypes in NOX2-Deficient Mice and Patients: A Key Role for NOX2 in Antigen-Presenting Cells. Front. Immunol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Mace, E.M. Human natural killer cells: Form, function, and development. J. Allergy Clin. Immunol. 2023, 151, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kay, H.D.; Smith, D.L.; Sullivan, G.; Mandell, G.L.; Donowitz, G.R. Evidence for a nonoxidative mechanism of human natural killer (NK) cell cytotoxicity by using mononuclear effector cells from healthy donors and from patients with chronic granulomatous disease. J. Immunol. 1983, 131, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Duwe, A.K.; Werkmeister, J.; Roder, J.C.; Lauzon, R.; Payne, U. Natural killer cell-mediated lysis involves an hydroxyl radical-dependent step. J. Immunol. 1985, 134, 2637–2644. [Google Scholar] [CrossRef]
- Aydin, E.; Johansson, J.; Nazir, F.H.; Hellstrand, K.; Martner, A. Role of NOX2-Derived Reactive Oxygen Species in NK Cell-Mediated Control of Murine Melanoma Metastasis. Cancer Immunol. Res. 2017, 5, 804–811. [Google Scholar] [CrossRef]
- Hansson, M.; Asea, A.; Ersson, U.; Hermodsson, S.; Hellstrand, K. Induction of apoptosis in NK cells by monocyte-derived reactive oxygen metabolites. J. Immunol. 1996, 156, 42–47. [Google Scholar] [CrossRef]
- Kotsafti, A.; Scarpa, M.; Castagliuolo, I.; Scarpa, M. Reactive Oxygen Species and Antitumor Immunity-From Surveillance to Evasion. Cancers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Nakamura, K.; Matsunaga, K. Susceptibility of natural killer (NK) cells to reactive oxygen species (ROS) and their restoration by the mimics of superoxide dismutase (SOD). Cancer Biother. Radiopharm. 1998, 13, 275–290. [Google Scholar] [CrossRef]
- Aurelius, J.; Thoren, F.B.; Akhiani, A.A.; Brune, M.; Palmqvist, L.; Hansson, M.; Hellstrand, K.; Martner, A. Monocytic AML cells inactivate antileukemic lymphocytes: Role of NADPH oxidase/gp91(phox) expression and the PARP-1/PAR pathway of apoptosis. Blood 2012, 119, 5832–5837. [Google Scholar] [CrossRef]
- Pierson, B.A.; Miller, J.S. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood 1996, 88, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Mellqvist, U.H.; Hansson, M.; Brune, M.; Dahlgren, C.; Hermodsson, S.; Hellstrand, K. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: Role of reactive oxygen species and regulation by histamine. Blood 2000, 96, 1961–1968. [Google Scholar] [CrossRef]
- Akhiani, A.A.; Hallner, A.; Kiffin, R.; Aydin, E.; Werlenius, O.; Aurelius, J.; Martner, A.; Thoren, F.B.; Hellstrand, K. Idelalisib Rescues Natural Killer Cells from Monocyte-Induced Immunosuppression by Inhibiting NOX2-Derived Reactive Oxygen Species. Cancer Immunol. Res. 2020, 8, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xu, S.; Zhang, M.; Liu, S.; Siu, S.P.; Peng, H.; Ng, J.C.; Tsao, G.S.; Chan, A.W.; Chow, V.L.; et al. NADPH oxidase 5alpha promotes the formation of CD271 tumor-initiating cells in oral cancer. Am. J. Cancer Res. 2020, 10, 1710–1727. [Google Scholar] [PubMed]
- Kono, K.; Salazar-Onfray, F.; Petersson, M.; Hansson, J.; Masucci, G.; Wasserman, K.; Nakazawa, T.; Anderson, P.; Kiessling, R. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur. J. Immunol. 1996, 26, 1308–1313. [Google Scholar] [CrossRef]
- Gardiner, G.J.; Deffit, S.N.; McLetchie, S.; Perez, L.; Walline, C.C.; Blum, J.S. A role for NADPH oxidase in antigen presentation. Front. Immunol. 2013, 4, 295. [Google Scholar] [CrossRef]
- Mantegazza, A.R.; Savina, A.; Vermeulen, M.; Perez, L.; Geffner, J.; Hermine, O.; Rosenzweig, S.D.; Faure, F.; Amigorena, S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood 2008, 112, 4712–4722. [Google Scholar] [CrossRef]
- Savina, A.; Jancic, C.; Hugues, S.; Guermonprez, P.; Vargas, P.; Moura, I.C.; Lennon-Dumenil, A.M.; Seabra, M.C.; Raposo, G.; Amigorena, S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 2006, 126, 205–218. [Google Scholar] [CrossRef]
- Rybicka, J.M.; Balce, D.R.; Chaudhuri, S.; Allan, E.R.; Yates, R.M. Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner. EMBO J. 2012, 31, 932–944. [Google Scholar] [CrossRef]
- Zhang, S.; Chopin, M.; Nutt, S.L. Type 1 conventional dendritic cells: Ontogeny, function, and emerging roles in cancer immunotherapy. Trends Immunol. 2021, 42, 1113–1127. [Google Scholar] [CrossRef]
- Sancho, D.; Joffre, O.P.; Keller, A.M.; Rogers, N.C.; Martinez, D.; Hernanz-Falcon, P.; Rosewell, I.; Reis e Sousa, C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 2009, 458, 899–903. [Google Scholar] [CrossRef]
- Canton, J.; Blees, H.; Henry, C.M.; Buck, M.D.; Schulz, O.; Rogers, N.C.; Childs, E.; Zelenay, S.; Rhys, H.; Domart, M.C.; et al. The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens. Nat. Immunol. 2021, 22, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.W.; Kotur, M.B.; Mundt, S.; Dokalis, N.; Ligeon, L.A.; Shah, A.M.; Prinz, M.; Becher, B.; Munz, C.; Lunemann, J.D. CYBB/NOX2 in conventional DCs controls T cell encephalitogenicity during neuroinflammation. Autophagy 2021, 17, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Whitener, R.L.; Lin, A.; Xu, Y.; Chen, J.; Savinov, A.; Leiding, J.W.; Wallet, M.A.; Mathews, C.E. Neutrophil Cytosolic Factor 1 in Dendritic Cells Promotes Autoreactive CD8+ T Cell Activation via Cross-Presentation in Type 1 Diabetes. Front. Immunol. 2019, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Vasilevsky, S.; Liu, Q.; Koontz, S.M.; Kastenmayer, R.; Shea, K.; Jackson, S.H. Role of p47phox in antigen-presenting cell-mediated regulation of humoral immunity in mice. Am. J. Pathol. 2011, 178, 2774–2782. [Google Scholar] [CrossRef]
- Roos, D.; van Leeuwen, K.; Hsu, A.P.; Priel, D.L.; Begtrup, A.; Brandon, R.; Rawat, A.; Vignesh, P.; Madkaikar, M.; Stasia, M.J.; et al. Hematologically important mutations: The autosomal forms of chronic granulomatous disease (third update). Blood Cells Mol. Dis. 2021, 92, 102596. [Google Scholar] [CrossRef] [PubMed]
- Roos, D.; van Leeuwen, K.; Hsu, A.P.; Priel, D.L.; Begtrup, A.; Brandon, R.; Stasia, M.J.; Bakri, F.G.; Koker, N.; Koker, M.Y.; et al. Hematologically important mutations: X-linked chronic granulomatous disease (fourth update). Blood Cells Mol. Dis. 2021, 90, 102587. [Google Scholar] [CrossRef]
- Fattahi, F.; Badalzadeh, M.; Sedighipour, L.; Movahedi, M.; Fazlollahi, M.R.; Mansouri, S.D.; Khotaei, G.T.; Bemanian, M.H.; Behmanesh, F.; Hamidieh, A.A.; et al. Inheritance pattern and clinical aspects of 93 Iranian patients with chronic granulomatous disease. J. Clin. Immunol. 2011, 31, 792–801. [Google Scholar] [CrossRef]
- Winkelstein, J.A.; Marino, M.C.; Johnston, R.B., Jr.; Boyle, J.; Curnutte, J.; Gallin, J.I.; Malech, H.L.; Holland, S.M.; Ochs, H.; Quie, P.; et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine 2000, 79, 155–169. [Google Scholar] [CrossRef]
- Babior, B.M.; Lambeth, J.D.; Nauseef, W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002, 397, 342–344. [Google Scholar] [CrossRef]
- Segal, B.H.; Leto, T.L.; Gallin, J.I.; Malech, H.L.; Holland, S.M. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine 2000, 79, 170–200. [Google Scholar] [CrossRef] [PubMed]
- Arnadottir, G.A.; Norddahl, G.L.; Gudmundsdottir, S.; Agustsdottir, A.B.; Sigurdsson, S.; Jensson, B.O.; Bjarnadottir, K.; Theodors, F.; Benonisdottir, S.; Ivarsdottir, E.V.; et al. A homozygous loss-of-function mutation leading to CYBC1 deficiency causes chronic granulomatous disease. Nat. Commun. 2018, 9, 4447. [Google Scholar] [CrossRef]
- Kuhns, D.B.; Alvord, W.G.; Heller, T.; Feld, J.J.; Pike, K.M.; Marciano, B.E.; Uzel, G.; DeRavin, S.S.; Priel, D.A.; Soule, B.P.; et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N. Engl. J. Med. 2010, 363, 2600–2610. [Google Scholar] [CrossRef]
- Peng, J.; Redman, C.M.; Wu, X.; Song, X.; Walker, R.H.; Westhoff, C.M.; Lee, S. Insights into extensive deletions around the XK locus associated with McLeod phenotype and characterization of two novel cases. Gene 2007, 392, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Coman, D.; Yaplito-Lee, J.; La, P.; Nasioulas, S.; Bruno, D.; Slater, H.R.; Stock-Myer, S.E.; Lynch, E.L.; Gardner, R.J. Three Mendelian disorders (chronic granulomatous disease, retinitis pigmentosa, ornithine transcarbamylase deficiency) in a young woman with an X chromosome deletion, del(X)(p11.4p21.1). Mol. Genet. Metab. 2010, 99, 329. [Google Scholar] [CrossRef]
- Hwang, S.W.; Kirst, M.J.; Chiu, S.; Tolomiczenko, G.; Kiss, A.; Cowan, L.; Levinson, W. Multidimensional social support and the health of homeless individuals. J. Urban Health 2009, 86, 791–803. [Google Scholar] [CrossRef] [PubMed]
- van de Geer, A.; Nieto-Patlan, A.; Kuhns, D.B.; Tool, A.T.; Arias, A.A.; Bouaziz, M.; de Boer, M.; Franco, J.L.; Gazendam, R.P.; van Hamme, J.L.; et al. Inherited p40phox deficiency differs from classic chronic granulomatous disease. J. Clin. Invest. 2018, 128, 3957–3975. [Google Scholar] [CrossRef]
- Vowells, S.J.; Fleisher, T.A.; Sekhsaria, S.; Alling, D.W.; Maguire, T.E.; Malech, H.L. Genotype-dependent variability in flow cytometric evaluation of reduced nicotinamide adenine dinucleotide phosphate oxidase function in patients with chronic granulomatous disease. J. Pediatr. 1996, 128, 104–107. [Google Scholar] [CrossRef]
- Leiding, J.W.; Holland, S.M. Chronic Granulomatous Disease. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Marciano, B.E.; Spalding, C.; Fitzgerald, A.; Mann, D.; Brown, T.; Osgood, S.; Yockey, L.; Darnell, D.N.; Barnhart, L.; Daub, J.; et al. Common severe infections in chronic granulomatous disease. Clin. Infect. Dis. 2015, 60, 1176–1183. [Google Scholar] [CrossRef]
- van den Berg, J.M.; van Koppen, E.; Ahlin, A.; Belohradsky, B.H.; Bernatowska, E.; Corbeel, L.; Espanol, T.; Fischer, A.; Kurenko-Deptuch, M.; Mouy, R.; et al. Chronic granulomatous disease: The European experience. PLoS ONE 2009, 4, e5234. [Google Scholar] [CrossRef]
- Grammatikos, A.; Gennery, A.R. Inflammatory Complications in Chronic Granulomatous Disease. J. Clin. Med. 2024, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Falcone, E.L.; Holland, S.M. Gastrointestinal Complications in Chronic Granulomatous Disease. Methods Mol. Biol. 2019, 1982, 573–586. [Google Scholar] [CrossRef] [PubMed]
- LaBere, B.; Gutierrez, M.J.; Wright, H.; Garabedian, E.; Ochs, H.D.; Fuleihan, R.L.; Secord, E.; Marsh, R.; Sullivan, K.E.; Cunningham-Rundles, C.; et al. Chronic Granulomatous Disease With Inflammatory Bowel Disease: Clinical Presentation, Treatment, and Outcomes From the USIDNET Registry. J. Allergy Clin. Immunol. Pract. 2022, 10, 1325–1333.e1325. [Google Scholar] [CrossRef]
- Marciano, B.E.; Rosenzweig, S.D.; Kleiner, D.E.; Anderson, V.L.; Darnell, D.N.; Anaya-O’Brien, S.; Hilligoss, D.M.; Malech, H.L.; Gallin, J.I.; Holland, S.M. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics 2004, 114, 462–468. [Google Scholar] [CrossRef]
- Marks, D.J.; Miyagi, K.; Rahman, F.Z.; Novelli, M.; Bloom, S.L.; Segal, A.W. Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn’s disease. Am. J. Gastroenterol. 2009, 104, 117–124. [Google Scholar] [CrossRef]
- Conrad, A.; Neven, B.; Mahlaoui, N.; Suarez, F.; Sokol, H.; Ruemmele, F.M.; Rouzaud, C.; Moshous, D.; Lortholary, O.; Blanche, S.; et al. Infections in Patients with Chronic Granulomatous Disease Treated with Tumor Necrosis Factor Alpha Blockers for Inflammatory Complications. J. Clin. Immunol. 2021, 41, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Uzel, G.; Orange, J.S.; Poliak, N.; Marciano, B.E.; Heller, T.; Holland, S.M. Complications of tumor necrosis factor-alpha blockade in chronic granulomatous disease-related colitis. Clin. Infect. Dis. 2010, 51, 1429–1434. [Google Scholar] [CrossRef]
- Feld, J.J.; Hussain, N.; Wright, E.C.; Kleiner, D.E.; Hoofnagle, J.H.; Ahlawat, S.; Anderson, V.; Hilligoss, D.; Gallin, J.I.; Liang, T.J.; et al. Hepatic involvement and portal hypertension predict mortality in chronic granulomatous disease. Gastroenterology 2008, 134, 1917–1926. [Google Scholar] [CrossRef]
- Hussain, N.; Feld, J.J.; Kleiner, D.E.; Hoofnagle, J.H.; Garcia-Eulate, R.; Ahlawat, S.; Koziel, D.E.; Anderson, V.; Hilligoss, D.; Choyke, P.; et al. Hepatic abnormalities in patients with chronic granulomatous disease. Hepatology 2007, 45, 675–683. [Google Scholar] [CrossRef]
- Salvator, H.; Mahlaoui, N.; Catherinot, E.; Rivaud, E.; Pilmis, B.; Borie, R.; Crestani, B.; Tcherakian, C.; Suarez, F.; Dunogue, B.; et al. Pulmonary manifestations in adult patients with chronic granulomatous disease. Eur. Respir. J. 2015, 45, 1613–1623. [Google Scholar] [CrossRef]
- De Ravin, S.S.; Naumann, N.; Cowen, E.W.; Friend, J.; Hilligoss, D.; Marquesen, M.; Balow, J.E.; Barron, K.S.; Turner, M.L.; Gallin, J.I.; et al. Chronic granulomatous disease as a risk factor for autoimmune disease. J. Allergy Clin. Immunol. 2008, 122, 1097–1103. [Google Scholar] [CrossRef]
- Freeman, A.F.; Marciano, B.E.; Anderson, V.L.; Uzel, G.; Costas, C.; Holland, S.M. Corticosteroids in the treatment of severe nocardia pneumonia in chronic granulomatous disease. Pediatr. Infect. Dis. J. 2011, 30, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Leiding, J.W.; Freeman, A.F.; Marciano, B.E.; Anderson, V.L.; Uzel, G.; Malech, H.L.; DeRavin, S.; Wilks, D.; Venkatesan, A.M.; Zerbe, C.S.; et al. Corticosteroid therapy for liver abscess in chronic granulomatous disease. Clin. Infect. Dis. 2012, 54, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Straughan, D.M.; McLoughlin, K.C.; Mullinax, J.E.; Marciano, B.E.; Freeman, A.F.; Anderson, V.L.; Uzel, G.; Azoury, S.C.; Sorber, R.; Quadri, H.S.; et al. The Changing Paradigm of Management of Liver Abscesses in Chronic Granulomatous Disease. Clin. Infect. Dis. 2018, 66, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Bode, S.F.; Ammann, S.; Al-Herz, W.; Bataneant, M.; Dvorak, C.C.; Gehring, S.; Gennery, A.; Gilmour, K.C.; Gonzalez-Granado, L.I.; Gross-Wieltsch, U.; et al. The syndrome of hemophagocytic lymphohistiocytosis in primary immunodeficiencies: Implications for differential diagnosis and pathogenesis. Haematologica 2015, 100, 978–988. [Google Scholar] [CrossRef]
- Squire, J.D.; Vazquez, S.N.; Chan, A.; Smith, M.E.; Chellapandian, D.; Vose, L.; Teppa, B.; Hanson, I.C.; Chinn, I.K.; Forbes-Satter, L.; et al. Case Report: Secondary Hemophagocytic Lymphohistiocytosis with Disseminated Infection in Chronic Granulomatous Disease-A Serious Cause of Mortality. Front. Immunol. 2020, 11, 581475. [Google Scholar] [CrossRef]
- Marciano, B.E.; Zerbe, C.S.; Falcone, E.L.; Ding, L.; DeRavin, S.S.; Daub, J.; Kreuzburg, S.; Yockey, L.; Hunsberger, S.; Foruraghi, L.; et al. X-linked carriers of chronic granulomatous disease: Illness, lyonization, and stability. J. Allergy Clin. Immunol. 2018, 141, 365–371. [Google Scholar] [CrossRef]
- Miranda, M.A.; Tsalatsanis, A.; Trotter, J.R.; Arnold, D.E.; Squire, J.D.; Kidd, S.; Parikh, S.; Marsh, R.A.; Griffith, L.M.; Mallhi, K.; et al. High symptom burden in female X-linked chronic granulomatous disease carriers. Clin. Immunol. 2024, 268, 110364. [Google Scholar] [CrossRef]
- Patel, N.C.; Younger, M.E.M.; Williams, K.; Matias Lopes, J.P.; Kuhns, D.B.; Patel, M.N.; Miranda, M.A.; Marciano, B.E.; Deal, C.L.; Leiding, J.W. Severe clinical phenotypes of heterozygous females with X-linked chronic granulomatous disease. J. Allergy Clin. Immunol. Pract. 2024, 12, 3452–3456.e3451. [Google Scholar] [CrossRef]
- Gallin, J.I.; Alling, D.W.; Malech, H.L.; Wesley, R.; Koziol, D.; Marciano, B.; Eisenstein, E.M.; Turner, M.L.; DeCarlo, E.S.; Starling, J.M.; et al. Itraconazole to prevent fungal infections in chronic granulomatous disease. N. Engl. J. Med. 2003, 348, 2416–2422. [Google Scholar] [CrossRef]
- The International Chronic Granulomatous Disease Cooperative Study Group. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N. Engl. J. Med. 1991, 324, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, R.; Wang, J.; Blok, H.J.; Hazelaar, S.; Neven, B.; Moshous, D.; Schulz, A.; Hoenig, M.; Hauck, F.; Al Seraihy, A.; et al. Hematopoietic cell transplantation in chronic granulomatous disease: A study of 712 children and adults. Blood 2020, 136, 1201–1211. [Google Scholar] [CrossRef]
- Leiding, J.W.; Arnold, D.E.; Parikh, S.; Logan, B.; Marsh, R.A.; Griffith, L.M.; Wu, R.; Kidd, S.; Mallhi, K.; Chellapandian, D.; et al. Genotype, oxidase status, and preceding infection or autoinflammation do not affect allogeneic HCT outcomes for CGD. Blood 2023, 142, 2105–2118. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.B.; Booth, C.; Kang, E.M.; Pai, S.Y.; Shaw, K.L.; Santilli, G.; Armant, M.; Buckland, K.F.; Choi, U.; De Ravin, S.S.; et al. Lentiviral gene therapy for X-linked chronic granulomatous disease. Nat. Med. 2020, 26, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.L.; Sackey, S.; Brown, D.; Senadheera, S.; Masiuk, K.; Quintos, J.P.; Colindres, N.; Riggan, L.; Morgan, R.A.; Malech, H.L.; et al. Lentiviral gene therapy for X-linked chronic granulomatous disease recapitulates endogenous CYBB regulation and expression. Blood 2023, 141, 1007–1022. [Google Scholar] [CrossRef]
- Chiang, A.K.; Chan, G.C.; Ma, S.K.; Ng, Y.K.; Ha, S.Y.; Lau, Y.L. Disseminated fungal infection associated with myeloperoxidase deficiency in a premature neonate. Pediatr. Infect. Dis. J. 2000, 19, 1027–1029. [Google Scholar] [CrossRef]
- Hoyos, S.; Agudelo, C.C.; Lozano, D.M.; Buitrago, L.E. Systemic paracoccidioidomycosis in a patient with enzymatic myeloperoxidase deficiency and acute lymphoblastic leukemia: Case report. Bull. Natl. Res. Cent. 2022, 46, 286. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 371, 64–74. [Google Scholar] [CrossRef]
- Sun, B.; Li, Q.; Dong, X.; Hou, J.; Wang, W.; Ying, W.; Hui, X.; Zhou, Q.; Yao, H.; Sun, J.; et al. Severe G6PD deficiency leads to recurrent infections and defects in ROS production: Case report and literature review. Front. Genet. 2022, 13, 1035673. [Google Scholar] [CrossRef]
- van Bruggen, R.; Bautista, J.M.; Petropoulou, T.; de Boer, M.; van Zwieten, R.; Gomez-Gallego, F.; Belohradsky, B.H.; Hartwig, N.G.; Stevens, D.; Mason, P.J.; et al. Deletion of leucine 61 in glucose-6-phosphate dehydrogenase leads to chronic nonspherocytic anemia, granulocyte dysfunction, and increased susceptibility to infections. Blood 2002, 100, 1026–1030. [Google Scholar] [CrossRef]
- Rees, M.D.; Kennett, E.C.; Whitelock, J.M.; Davies, M.J. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic. Biol. Med. 2008, 44, 1973–2001. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Hirshberg, J.; Lyle, D.; Freij, J.B.; Caturegli, P. Reactive oxygen species in organ-specific autoimmunity. Autoimmun. Highlights 2016, 7, 11. [Google Scholar] [CrossRef]
- Hultqvist, M.; Olsson, L.M.; Gelderman, K.A.; Holmdahl, R. The protective role of ROS in autoimmune disease. Trends Immunol. 2009, 30, 201–208. [Google Scholar] [CrossRef]
- Olofsson, P.; Holmberg, J.; Tordsson, J.; Lu, S.; Akerstrom, B.; Holmdahl, R. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat. Genet. 2003, 33, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.M.; Johansson, A.C.; Gullstrand, B.; Jonsen, A.; Saevarsdottir, S.; Ronnblom, L.; Leonard, D.; Wettero, J.; Sjowall, C.; Svenungsson, E.; et al. A single nucleotide polymorphism in the NCF1 gene leading to reduced oxidative burst is associated with systemic lupus erythematosus. Ann. Rheum. Dis. 2017, 76, 1607–1613. [Google Scholar] [CrossRef]
- Olsson, L.M.; Nerstedt, A.; Lindqvist, A.K.; Johansson, S.C.; Medstrand, P.; Olofsson, P.; Holmdahl, R. Copy number variation of the gene NCF1 is associated with rheumatoid arthritis. Antioxid. Redox Signal. 2012, 16, 71–78. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, J.; Deng, Y.; Kelly, J.A.; Kim, K.; Bang, S.Y.; Lee, H.S.; Li, Q.Z.; Wakeland, E.K.; Qiu, R.; et al. A missense variant in NCF1 is associated with susceptibility to multiple autoimmune diseases. Nat. Genet. 2017, 49, 433–437. [Google Scholar] [CrossRef]
- Hahn, J.; Euler, M.; Kilgus, E.; Kienhofer, D.; Stoof, J.; Knopf, J.; Hahn, M.; Harrer, T.; Hultqvist, M.; Olofsson, P.; et al. NOX2 mediates quiescent handling of dead cell remnants in phagocytes. Redox Biol. 2019, 26, 101279. [Google Scholar] [CrossRef] [PubMed]
- Hultqvist, M.; Backlund, J.; Bauer, K.; Gelderman, K.A.; Holmdahl, R. Lack of reactive oxygen species breaks T cell tolerance to collagen type II and allows development of arthritis in mice. J. Immunol. 2007, 179, 1431–1437. [Google Scholar] [CrossRef]
- Kienhofer, D.; Hahn, J.; Stoof, J.; Csepregi, J.Z.; Reinwald, C.; Urbonaviciute, V.; Johnsson, C.; Maueroder, C.; Podolska, M.J.; Biermann, M.H.; et al. Experimental lupus is aggravated in mouse strains with impaired induction of neutrophil extracellular traps. JCI Insight 2017, 2, e92920. [Google Scholar] [CrossRef]
- Smith, M.H.; Berman, J.R. What Is Rheumatoid Arthritis? JAMA 2022, 327, 1194. [Google Scholar] [CrossRef]
- Cedergren, J.; Forslund, T.; Sundqvist, T.; Skogh, T. Intracellular oxidative activation in synovial fluid neutrophils from patients with rheumatoid arthritis but not from other arthritis patients. J. Rheumatol. 2007, 34, 2162–2170. [Google Scholar] [PubMed]
- Eggleton, P.; Wang, L.; Penhallow, J.; Crawford, N.; Brown, K.A. Differences in oxidative response of subpopulations of neutrophils from healthy subjects and patients with rheumatoid arthritis. Ann. Rheum. Dis. 1995, 54, 916–923. [Google Scholar] [CrossRef]
- Tiku, M.L.; Gupta, S.; Deshmukh, D.R. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free Radic. Res. 1999, 30, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Bohanec Grabar, P.; Logar, D.; Tomsic, M.; Rozman, B.; Dolzan, V. Genetic polymorphisms modifying oxidative stress are associated with disease activity in rheumatoid arthritis patients. Dis. Markers 2009, 26, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.P.; Girija, A.S.S. Exploring the ROS reduction strategies in chronic lupus management. Front. Immunol. 2024, 15, 1346656. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef]
- Campbell, A.M.; Kashgarian, M.; Shlomchik, M.J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 2012, 4, 157ra141. [Google Scholar] [CrossRef]
- Jacob, C.O.; Eisenstein, M.; Dinauer, M.C.; Ming, W.; Liu, Q.; John, S.; Quismorio, F.P., Jr.; Reiff, A.; Myones, B.L.; Kaufman, K.M.; et al. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc. Natl. Acad. Sci. USA 2012, 109, E59–E67. [Google Scholar] [CrossRef]
- Gladman, D.D.; Brockbank, J. Psoriatic arthritis. Expert Opin. Investig. Drugs 2000, 9, 1511–1522. [Google Scholar] [CrossRef]
- Blagov, A.; Sukhorukov, V.; Guo, S.; Zhang, D.; Eremin, I.; Orekhov, A. The Role of Oxidative Stress in the Induction and Development of Psoriasis. Front. Biosci. 2023, 28, 118. [Google Scholar] [CrossRef] [PubMed]
- Cannavo, S.P.; Riso, G.; Casciaro, M.; Di Salvo, E.; Gangemi, S. Oxidative stress involvement in psoriasis: A systematic review. Free Radic. Res. 2019, 53, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Medovic, M.V.; Jakovljevic, V.L.; Zivkovic, V.I.; Jeremic, N.S.; Jeremic, J.N.; Bolevich, S.B.; Ravic Nikolic, A.B.; Milicic, V.M.; Srejovic, I.M. Psoriasis between Autoimmunity and Oxidative Stress: Changes Induced by Different Therapeutic Approaches. Oxid. Med. Cell Longev. 2022, 2022, 2249834. [Google Scholar] [CrossRef] [PubMed]
- Bilski, R.; Kupczyk, D.; Wozniak, A. Oxidative Imbalance in Psoriasis with an Emphasis on Psoriatic Arthritis: Therapeutic Antioxidant Targets. Molecules 2024, 29, 5460. [Google Scholar] [CrossRef]
- Khmaladze, I.; Kelkka, T.; Guerard, S.; Wing, K.; Pizzolla, A.; Saxena, A.; Lundqvist, K.; Holmdahl, M.; Nandakumar, K.S.; Holmdahl, R. Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E3669–E3678. [Google Scholar] [CrossRef]
- Hultqvist, M.; Olofsson, P.; Wallner, F.K.; Holmdahl, R. Pharmacological Potential of NOX2 Agonists in Inflammatory Conditions. Antioxid. Redox Signal. 2015, 23, 446–459. [Google Scholar] [CrossRef]
- Kajaste-Rudnitsk, A.; Aiuti, A. Towards improved yet regulated gene therapy for X-CGD. Blood 2023, 142, 2035. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Yang, S.C.; Hwang, T.L. Formyl peptide receptor modulators: A patent review and potential applications for inflammatory diseases (2012–2015). Expert Opin. Ther. Pat. 2016, 26, 1139–1156. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiding, J.W.; Mathews, C.E.; Arnold, D.E.; Chen, J. The Role of NADPH Oxidase 2 in Leukocytes. Antioxidants 2025, 14, 309. https://doi.org/10.3390/antiox14030309
Leiding JW, Mathews CE, Arnold DE, Chen J. The Role of NADPH Oxidase 2 in Leukocytes. Antioxidants. 2025; 14(3):309. https://doi.org/10.3390/antiox14030309
Chicago/Turabian StyleLeiding, Jennifer W., Clayton E. Mathews, Danielle E. Arnold, and Jing Chen. 2025. "The Role of NADPH Oxidase 2 in Leukocytes" Antioxidants 14, no. 3: 309. https://doi.org/10.3390/antiox14030309
APA StyleLeiding, J. W., Mathews, C. E., Arnold, D. E., & Chen, J. (2025). The Role of NADPH Oxidase 2 in Leukocytes. Antioxidants, 14(3), 309. https://doi.org/10.3390/antiox14030309






