Abstract
As a prevalent metabolic disorder, the increasing incidence of diabetes imposes a significant burden on global healthcare. Flavonoids in natural phytochemical products exhibit notable hypoglycemic properties, making them potential alternatives for diabetes treatment. This article summarizes the hypoglycemic properties of flavonoid subcategories studied in recent years, including flavones, isoflavones, flavonols, flavanols, and others. The relevant targets and signal pathways, such as α-amylase, α-glucosidase, insulin receptor substrate (IRS)/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), PKR-like endoplasmic reticulum kinase (PERK)/eukaryotic initiation factor 2α (eIF2α)/activation transcription factor 4 (ATF4)/C/EBP homologous protein (CHOP), etc., are also elaborated. Additionally, flavonoids have also been demonstrated to modulate the gut microbiota and its metabolites. Through the aforementioned mechanisms, flavonoids mainly suppress carbohydrate metabolism and gluconeogenesis; facilitate glucose uptake, glycogenesis, and insulin secretion; and mitigate insulin resistance, oxidative stress, inflammation, etc. Notably, several studies have indicated that certain flavonoids displayed synergistic hypoglycemic effects. In conclusion, this article provides a comprehensive review of the hypoglycemic effects of the flavonoids investigated in recent years, aiming to offer theoretical insights for their further exploration.
1. Introduction
Diabetes mellitus (DM) is a chronic disease associated with metabolic disorders of the endocrine system, characterized by high concentrations of blood glucose. In accordance with the etiology, type 1 diabetes (T1DM) and type 2 diabetes (T2DM) are the most common clinical types [1]. T1DM is significantly characterized by the absolute inadequacy of insulin secretion owing to pancreatic β-cell damage and destruction [2], whereas T2DM, accounting for more than 90% of all diabetes cases, is indicated by a relative inadequacy of insulin secretion caused by pancreatic β-cell dysfunction, as well as the depletion of insulin capacity to modulate glucose metabolism (insulin resistance, IR) [3].
Identified by the World Health Organization in 2019 as one of the top 10 causes of death worldwide from 2000 to 2019, diabetes is a severe threat to global health. The morbidity of diabetes has been growing at an alarming rate along with the improvement in living standards and alterations in lifestyle. It has been predicted that the prevalence of diabetes among adults aged 20–79 years could rise to 12.2% in 2045, meaning that there would be 783.2 million people with diabetes throughout the world. This represents staggering growth compared to the 10.5% (536.6 million people) living with diabetes in 2021. With the rising prevalence, the economic burden of diabetes is also enormous. The total expenditure for diabetes in China was projected to rise from USD 250.2 to USD 460.4 billion from 2020 to 2030, with an annual growth rate of 6.32% [4]. It was estimated that the global health expenditures related to diabetes would also rise to USD 1054 billion by 2045 [5]. Noteworthily, the explosion of diabetes mellitus will impose a huge burden on the global medical system and economy, resulting in an urgent need for strategies able to efficiently ameliorate and treat diabetes, along with its comorbidities.
Currently, a cure for DM is not available. Among all treatment options, lifestyle intervention, e.g., a reasonable diet, regular exercise, smoking cessation, and so on, remains paramount [6]. Medications for diabetes encompass oral and injectable preparations. The frequently used hypoglycemic oral drugs, including metformin, glinides, rosiglitazone, sulfonylureas, thiazolidinediones, glucagon-like peptide-1 agonists, α-glucosidase inhibitors (acarbose), and so on, may eventuate certain side effects when used permanently, such as lactic acidosis, excessive hypoglycemia, weight gain, gastrointestinal adverse reactions, and exacerbation of the hepatic and renal burden [7].
Natural products, including tannins, organic acids, polyphenols, terpenes, flavonoids, etc., have been validated to target the circulatory system, intestine, pancreas, hepatic tissue, muscular tissue, adipose tissue, etc., as potential therapeutic substitutes for diabetes treatment [8,9]. Natural products are a rich source of hypoglycemic medication discovery which, because of their mild effects, low risk of contraindications or harmful effects, and low cost, can replace traditional drugs that may cause many side effects when taken for a long period of time. Natural products can also promote the high-value utilization of agricultural resources [9]. Among these, flavonoids are important polyphenols naturally found in plants, vegetables, and fruits as a type of secondary metabolite, with anti-inflammatory, anti-cancer, pro-cardiovascular, anti-microbial, anti- severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and other biological activities. The chemical structures of flavonoids are diverse and modifiable, endowing them with significant potential for drug development. Moreover, flavonoids display abundant targets and pathways for lowering blood glucose, receiving significant attention in recent years [10].
This review gathers information from recent years on natural plant-derived flavonoids used in the treatment of conditions consistent with diabetes, summarizes the flavonoids mentioned and their molecular mechanisms, and emphasizes the synergistic hypoglycemic effects between the flavonoids. The literature search was conducted across several databases (Web of Science, PubMed, Scopus, Wiley, and Springer) from 2022 to date, using key search terms including diabetes, type 2 diabetes (T2DM), hypoglycemia, hyperglycemia, blood glucose, glycemic control, glycosylated hemoglobin (HbA1c), insulin resistance, flavonoids, flavone, isoflavone, flavonol, flavanone, flavanonol, chalcone, and anthocyanin.
2. Hypoglycemic Flavonoids Reported in In Vivo and In Vitro Studies
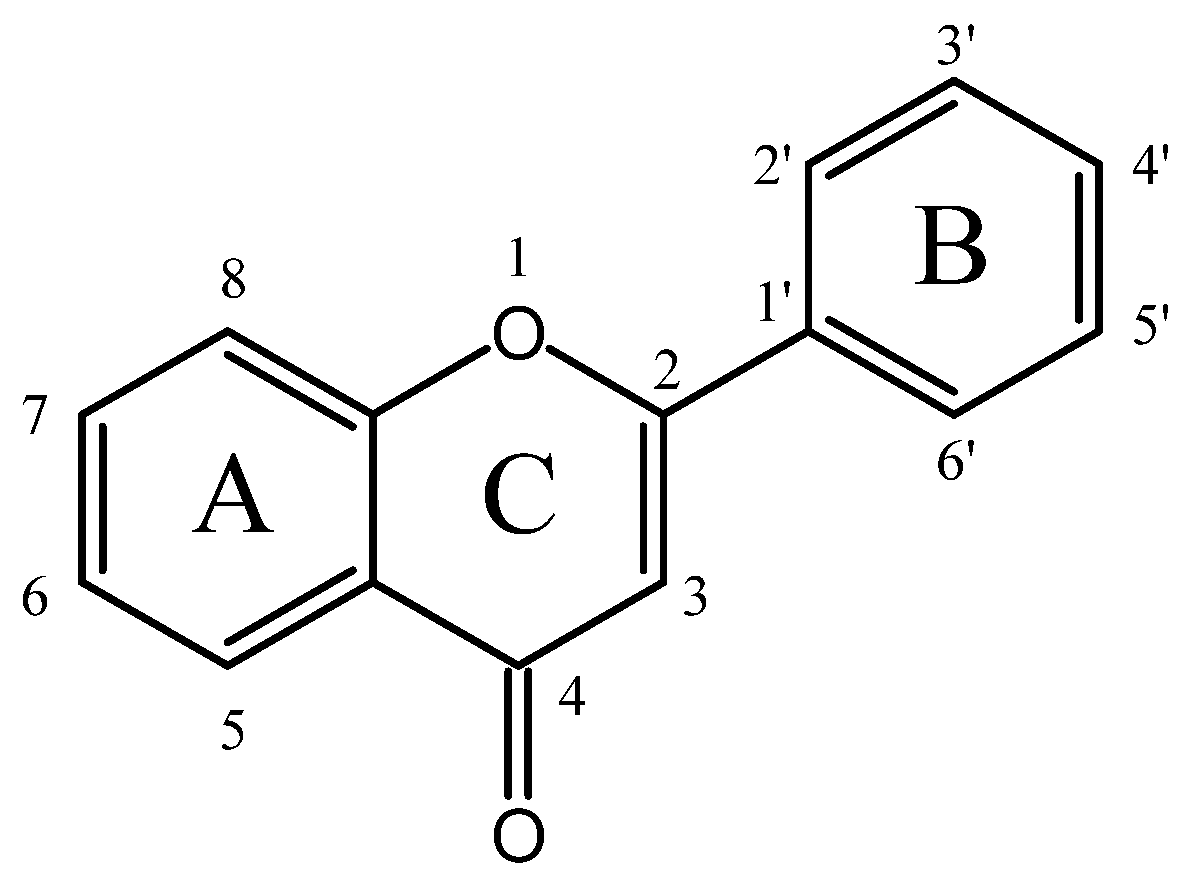
Flavonoids generally possess a benzo-c-pyran (C6-C3-C6) carbon skeletal configuration, which is composed of a three-carbon heterocyclic pyran ring C connecting two benzene rings A and B, as shown in Figure 1 [11]. According to the substitutional pattern and oxidative degree of ring C, flavonoids can be categorized into different isoforms, such as flavones, isoflavones, flavonols, flavanols, flavanones, flavanonols, anthocyanins, etc., as listed in Table S1. In plants, flavonoids are mostly present in the form of C-glycosides and O-glycosides. In recent years, numerous in vitro, in vivo, and in silico studies have substantiated that different subclasses of flavonoids display strikingly hypoglycemic effects through various targets and signaling pathways (as indicated in Table S2), some of which are even more effective than clinical hypoglycemic agents, providing new insights for the treatment of diabetes.
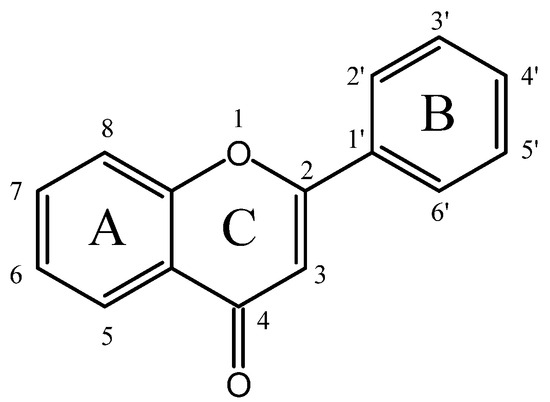
Figure 1.
The basic skeleton of flavonoids.
2.1. Flavones
Differing from other flavonoids, flavones display a double bond between C2 and C3, with oxidation at C4 and no substitution at C3 in ring C.
Acacetin, mainly extracted from Saussurea involucrate, Ziziphora clinopodioides Lam, and Robinia pseudoacacia, reduced blood glucose levels and increased body weight in a streptozocin (STZ)-induced zebrafish model [12]; it also diminished fasting blood glucose (FBG) and relieved pancreas and liver injuries in high-fat diet (HFD) and STZ-treated diabetic rats [13].
Baicalin, present in the water extract of Radix scutellariae, could ameliorate hyperglycemia and glucose tolerance in T2DM mice [14]. In the research to evaluate the hypoglycemic effects of baicalein, baicalin (baicalein 7-O-glucuronide), and the hypoglycemic agent metformin, it was confirmed that baicalein is the most effective in diminishing glycation, α-glucosidase, and free radicals, while baicalin exhibited similar activities [15].
Eupafolin, present in Artemisia princeps and Eremophila denticulate, was demonstrated to reduce FBG, serum insulin, IR, and liver oxidative stress; normalize the lipid profile; and elevate the hepatic glycogen level in nicotinamide-STZ-induced diabetic rat models [16].
Luteolin, which was widely present in Thespesia garckeana F. Hoffm., Rumex vescarius, Mentha arvensis, and Vernonia amygdalina, significantly decreased blood glucose [17] and FBG levels, improved glucose tolerance and metabolic markers in plasma, slightly increased the number and area of β cells in islets [18], and alleviated palmitic acid (PA)-induced β cell apoptosis and the impairment of glucose-stimulated insulin secretion (GSIS) [19]. In vitro, luteolin was proven to inhibit the catalytic activity of α-amylase [20], α-glucosidase [18,21], and cyclooxygenase [22]. Luteolin-7-O-rutinoside was found to enhance GSIS and glucose uptake [23]. Lutexin (luteolin-8-glucoside), present in Itea omeiensis, competitively inhibited α-glucosidase activity [24].
Nobiletin, a polymethoxylated flavone present in orange and lemon peels, was proven to exert anti-glucotoxicity properties, such as reducing blood glucose and islet injuries and elevating insulin levels [25]. Tectochrysin (5-hydroxy-7-methoxyflavone), one of the major flavonoids in propolis, showed an insulin-mimetic effect, as it could potentiate glucose uptake while repressing liver gluconeogenesis, which led to decreased glucose levels and the enhanced insulin sensitivity [26]. Furthermore, 3,3′,4′,5,6,7,8-heptamethoxyflavone [27], a morusin abundant in Morus alba L. roots [28], improved glucose metabolism in diabetic mice, and genkwanin (separated from Vietnamese Aquilaria crassna leaves) [29], eupafolin [30], oroxylin A (derived from Oroxylum indicum Bentham ex Kurz) [31], swertisin (isolated from Carex fraseriana) [32], and schaftoside (present in sugarcane leaves) [33] exhibited inhibitory capacity against α-glucosidase or α-amylase.
2.2. Isoflavones
Isoflavones, also known as phytoestrogens, are abundant in legumes such as chickpeas, soy, beans, peanuts, and red clover [34].
Biochanin A, an O-methylated isoflavone, was demonstrated to markedly restore the abnormal levels of plasma glucose and plasma insulin, attenuate insulin resistance, reduce liver and pancreatic injuries, enhance glucose tolerance, and modulate the activities of glucose and glycogen metabolizing enzymes in the diabetic group [35].
Daidzein, a soy isoflavone, demonstrated the ability to reduce plasma glucose and body weight loss in STZ-induced diabetic rats [36].
Formononetin, a methoxy isoflavone, is present in many medicinal plants, such as Astragalus membranaceus, Trifolium pratense L., and Pueraria lobata (Willd.), as well as legumes like Astragalus trimestris L., exhibiting the capacity to downregulate serum glucose, insulin resistance, and fasting glucose levels and to upregulate serum insulin [37], while relieving islet injuries [38].
Puerarin (8-β-D-grapes pyranose-4,7-dihydroxy isoflavone), the main active component extracted from kudzu root, enhanced glucose tolerance and insulin sensitivity, mitigated liver dysfunction and hyperlipidemia, and attenuated oxidative stress and inflammation in HFD/STZ-induced mice and PA-treated murine hepatocytes [39].
Isoflavones from the Apios americana Medik. tuber displayed outstanding antioxidant tendencies and α-glucosidase inhibitory activity [40]. A study on Crescentia cujete L. indicated that among all the compounds isolated from the plant, isoflavone was the most effective inhibitor of α-glucosidase [41]. Seven isoflavones with α-glucosidase inhibitory activity were identified from black soybeans, and there was a negative correlation between the degree of glycosylation of isoflavone and the α-glucosidase inhibitory activity [42].
2.3. Flavonols
Flavonols have been identified in almost all plants, including gymnosperms, angiosperms, mosses, ferns, and liverworts. Compared with flavones, the core carbon skeleton of flavonols contains an additional alkoxy group at the C3 position of ring C.
Quercetin, as a crucial active ingredient in Abelmoschus esculentus (L.) Moench (okra) and Euphorbia peplus, was shown to be capable of alleviating body weight loss; reducing blood glucose, FBG, IR, hepatic and pancreatic injuries, oxidant stress, and inflammation; and increasing insulin levels and oral glucose tolerance in diabetic animal models [43,44,45]. In a study to investigate the impacts of quercetin, isoquercitrin, and rutin (a mixture of quercetin mono-glycoside and oligo-glycosides) on Institute of Cancer Research mice, quercetin glycosides, particularly isoquercitrin (but not aglycone), were eventually confirmed to suppress acute hyperglycemia [46]. Quercetin could also reduce postprandial hyperglycemia through directly interacting with starch and suppressing the activity of α-glucosidase [47,48].
Astragalin, identified from Euphorbia peplus and Sauropus androgynus, could increase hexokinase in diabetic rats [49] and showed a higher α-glucosidase inhibitory activity (half maximal inhibitory concentration, IC50) = 55.03 ± 0.61 μg/mL) than that of acarbose (162.96 ± 0.59 μg/mL) [50].
Kaempferol, as the main flavonoid of the extract of Coreopsis tinctoria and Reseda lutea L., alleviated body weight gain, FBG, serum glucose, and HbAc1 levels; ameliorated glucose tolerance, insulin resistance, the lipid profile, and liver injury; promoted glucose uptake in hyperglycemia mice compared with that in the control group [51,52,53]; and suppressed the activity of α-amylase and α-glycosidase [54], while heightening the hexokinase activity in HFD/STZ-induced diabetic rats [49].
Myricetin, isolated from Annona cherimola Miller, impeded monosaccharide absorption [55], minimized the serum glucose and insulin levels, and ameliorated the apoptosis of pancreatic tissues in a HFD/STZ-treated rat models [56]. The research evaluated the prospective hypoglycemic properties of three flavonol glycosides present in Acacia mearnsii leaves, i.e., myricitrin, myricetin-3-O-glucoside, and myricetin-3-O-arabinoside, which displayed α-glucosidase and α-amylase inhibitory activity, and the effect of myricitrin was the most prominent [10].
In addition, epimedin C, derived from Epimedium [57]; fisetin, which is present in cucumbers, onions, apples, and strawberries [58]; morin, richly expressed in the Moraceae family [59]; silibinin, which is the main component of silymarin extracted from Cirsium japonicum [60]; and isorhamnetin [61] were found to ameliorate blood glucose, glucose intolerance, insulin resistance, oxidative stress, and inflammation, or to elevate hepatic glycogen, i.e., GSIS. Narcissoside (isorhamnetin 3-rutinoside), present in various herbs such as Anoectochilus roxburghii, Peucedanum aucheri Boiss., and Colicodendron scabridum, statically quenched α-glucosidase [62].
2.4. Flavanols
Unlike in the case of flavones, in flavanols, there are no double bonds between C2 and C3, nor is there oxidation at C4 of ring C; there is only an alkoxy group at the C3 position.
(-)-Epicatechin-6, isolated from Australian Acacia saligna, increased the uptake of glucose [63].
Epigallocatechin gallate (EGCG), an active substance obtained from Indian green tea extract, was proven to enhance the phosphorylation of insulin receptors and insulin sensitivity and promote glucose uptake in a dose-dependent manner [64]. EGCG also exhibited an inhibitory effect towards α-amylase, with an IC50 value of 0.548 ± 0.029 mg/mL [65].
Le et al. demonstrated that two galloylated flavanols, i.e., epi-catechin gallate and 3-O-galloyl-3,3′,5,5′,7-pentahydroxyflavan, extracted from the leaves of Acer ginnala Maxim., displayed inhibitory effects against α-glucosidase [66].
2.5. Flavanones
Flavanones have no double bond between the C2 and C3 of ring C, in contrast to the structure of flavones.
Bavachin isolated from the seeds and fruits of Psoralea corylifolia L. could ameliorate glucose homeostasis and insulin sensitivity [67]; pinocembrin (5,7-dihydroxyflavone) enhanced glucose consumption and improved insulin resistance [68].
Supplementation with hesperetin at a dose of 20 mg/kg b.wt. for 6 weeks remarkably diminished serum glucose and hepatic glycogen, augmented glucose tolerance through increasing pancreatic islet cells, and thus promoted insulin secretion in T1DM rats [69]. Hesperetin was proven to be a mixed inhibitor for human salivary α-amylase [70]. Neoeriocitrin (hesperetin 7-O-neohesperidoside) could enhance GSIS and decrease intracellular reactive oxygen species (ROS) [71]. Hesperidin (hesperetin 7-rutinoside), mainly present in citrus fruits, could uncompetitively inhibit α-glucosidase activity, with an IC50 value of 18.52 μM [72].
Naringenin (4,5,7-trihydroxy-flavanone), a flavonoid phytoestrogen, was determined to exert antidiabetic activity in different organs of STZ-induced mouse models, such as alleviating the apoptosis of islet β-cells and hepatocytes and regulating glucose metabolism in skeletal muscles [73]. In STZ/Nicotinamide-treated diabetic rat models, naringenin considerably improved IR, glucose, and insulin levels, increased glucose tolerance [74], and appreciably restored pancreatic β cell mass and insulin secretion at a dosage of 50 mg/kg for 2 weeks [75]. In vitro, naringenin was found to protect HepG2 cells from PA-induced insulin resistance [76].
Nigragenon O, separated from the stems of Mours nigra, decreased the glucose level in IR 3T3-L1 cells [77].
2.6. Flavanonols
For flavanonols, there is an alkoxy group at C3 and a carbon–oxygen double bond at C4 of ring C.
Dihydromyricetin (DHM), the main flavonoid component in Ampelopsis grossedentata, ameliorated hepatic steatosis and insulin resistance in both HFD-treated rats and PA-induced HepG2 cells [78]. Chen et al. carried out an in vivo study that confirmed that dihydromyricetin (250 mg/kg), administrated once a day by gavage for 12 weeks, significantly decreased FBG, triglycerides (TG), and HbA1c and increased fasting insulin (FINS) levels in STZ-treated diabetic mice [79].
Sanggenon C, isolated from the root bark of Cortex Mori, could improve glucose uptake and oxidative stress, as well as lower lipid levels, which attenuated insulin resistance in palmitic acid-induced HepG2 cells [80].
2.7. Biflavonoids
Biflavonoids, typically isolated from Selaginella uncinate, are naturally present dimeric compounds consisting of two flavonoid parts.
In a study of the potential antidiabetic activity of extracts from Selaginella tamariscina (P.Beauv.) Spring, two biflavonoids were proven to enhance glucose consumption in both normal and insulin-resistant HepG2 cells [81].
Amentoflavone, a C–C type representative flavonoid isolated from Aletris spicata and Selaginella tamariscina, could improve cell viability, insulin-stimulated glucose uptake, and GLUT4 expression, suppressing cell apoptosis and oxidative stress [82].
The two biflavones, amentoflavone and bilobetin, which differ only in the C8 position, with the former being hydroxyl and the latter methoxy, were both able to improve insulin resistance and aberrant insulin secretion [83]. Moreover, amentoflavone and hinokiflavone displayed stronger α-glucosidase inhibitory tendency than did acarbose [84].
Sadeghi et al. evaluated the inhibitory activities of 18 biflavonoids against α-glucosidase, ultimately confirming that strychnobiflavone achieved the best score with a binding energy of −8.85 kcal/mol, and it acted as an α-glucosidase inhibitor in a mix-competitive pattern [85].
2.8. Chalcones
Chalcones exhibit a core structure of two aromatic rings connected by a three-carbon unsaturated carbonyl group and are easily cycled, which makes them one of the most structurally diverse flavonoids [86].
Licochalcone A, identified in licorice (the rhizomes and roots of Glycyrrhiza inflata Bat., Glycyrrhiza uralensis Fisch., and Glycyrrhiza glabra L.), could enhance hepatic glycogenesis and inhibit gluconeogenesis in HFD-induced diabetic mice [87].
Neohesperidin, a bicyclic dihydrochalcone, regulated glucolipid metabolism and inflammatory responses and improved insulin resistance in diabetic zebrafish. Moreover, neohesperidin dihydrochalcone markedly relieved diabetes-exacerbated lipid accumulation in liver tissues [88]. Nothofagin, a type of dihydrochalcone extracted from Aspalathus linearis (Burman f.) R. Dahlgren, was found to exhibit anti-glycation activity [89].
Phlorizin, a dihydrochalcone present in Lithocarpus litseifolius (Hance) Chun, promoted glucose consumption, glucose uptake, and glycogen synthesis, as well as inhibited gluconeogenesis, oxidative stress, and lipid accumulation in free fatty acids (FFA)-induced insulin-resistant HepG2 cells [90].
2.9. Anthocyanins
Anthocyanins, generally available in fruits, vegetables, cereals, and beans, are the predominant water-soluble pigments in plants and are commonly linked to a sugar molecule, such as glucose, rhamnose, galactose, arabinose, and rutinose.
Cyanidin-3-O-glucoside (C3G), always present in fruits and vegetables with dark color, including blueberries and red bayberries, enhanced glucose consumption, glycogen synthesis, and insulin sensitivity [91]; reduced blood glucose [92]; improved pancreatic β cell secretory dysfunction and apoptosis [93] in diabetic db/db mice and IR hepatic cells; as well as exerted a significant inhibitory effect on α-glucosidase [94]. Cyanidin-3-O-β-glucoside-rich haskap (Lonicera caerulea L.) berry extract could ameliorate glucose tolerance and insulin sensitivity [95].
Delphinidin could maintain glucokinase activity [96]. Delphinidin-3-O-glucoside prominently enhanced glucose uptake in high glucose (HG)oleic acid (OA)-treated HepG2 cells [97]. Delphinidin-3-O-galactoside and delphinidin-3-O-glucoside exhibited the prominent scavenging ability of radicals, along with inhibitory activity against α-glucosidase [97], and reduced advanced glycation end-product (AGE) accumulation [98].
Malvidin-3-arabinoside exhibited an inhibitory capacity against AGE accumulation and the secretions of nitric oxide and pro-inflammatory cytokines in RAW264.7 cells [98]. Malvidin-3-O-galactoside could remove 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid radical cation (ABTS+), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and oxygen free radicals; repress α-glucosidase activity; potentiate glucose uptake; and reduce total cholesterol (TC) and TG in HG-OA-evoked HepG2 cells [97].
Petunidin-3-O-galactoside and petunidin-3-O-glucoside enhanced glucose uptake in HepG2 cell models. It was confirmed that petunidin-type and malvidin-type anthocyanins induced more notable glucose uptake activity than did delphinidin-type anthocyanins. Moreover, the two flavonoids both significantly scavenged radicals and inhibited α-glucosidase activity [97].
2.10. Homoisoflavonoids
Homoisoflavonoids are a special subclass of flavonoids with rare occurrence in nature; they are uniquely characterized by having one more carbon (C9) than the regular flavonoids in a 16-carbon skeleton. They are classified into five groups based on their structures: sappanin-type, scillascillin-type, brazilin-type, caesalpin-type, and protosappanin-type.
(E)-5-hydroxy-7-methoxy-3-(2-hydroxybenzyl)-4-chromanone (HM-chromanone), isolated from Portulaca oleracea L., was able to reduce blood glucose and alleviate insulin resistance and inflammation in mice with endotoxin-induced insulin resistance [99].
HM-chromanone remarkedly restored impaired insulin pathways, reduced glucose production, and enhanced glycogen synthesis in ob/ob mice [100] and PA-induced HepG2 cells, respectively [101].
Brazilin-type homoisoflavonoids are commonly found in the heartwood of Caesalpinia sappan, Caesalpinia echinate, and Haematoxylon campechianum. Awotuya et al. examined nine brazilin-type homoisoflavonoids to determine their inhibition of protein tyrosine phosphatase 1B (PTP1B) [102].
3. Clinical Trials Regarding the Hypoglycemic Effect of Flavonoids
Although the hypoglycemic effect of flavonoids has been demonstrated in many animal models, in vitro experiments, and in silico modeling, the relevant clinical data are very limited. The trials to investigate individual flavonoids are as follows. Oral ingestion of hesperidin at a dose of 1 g/day for 12 weeks significantly lowered blood glucose (p < 0.05) in 129 T2DM patients with metabolic syndrome and neuropathy [103]. A 3-month double-blind, placebo-controlled trial involving 50 T2DM subjects showed that the administration of 500 mg/day of rutin significantly decreased FBG, insulin, HbA1c, homeostatic model assessment of insulin resistance (HOMA-IR) (p < 0.001), interleukin 6 (IL-6), malondialdehyde (MDA) (p < 0.05) levels and improved lipid profiles [104]. However, a randomized clinical trial involving 48 patients with T2DM validated that daily administration of 140 mg silymarin capsules for 12 weeks achieved no statistically significant difference in FBG (p = 0.789), HbA1c (p = 0.719), and lipid profiles compared to those of the control group. This may be due to the low dose, the short intervention period, the inclusion of fewer subjects, etc. [105].
Clinical trials of flavonoid mixtures include the following. A parallel, double-blind randomized controlled trial (RCT) (n = 45; age 63.4 ± 7.4 years; 64% male) revealed that 26 g (freeze-dried) of blueberries abundant in anthocyanins attenuated insulin (p < 0.01) and glucose levels (p < 0.001) induced by a high-fat/high-sugar meal after 24 h in individuals with metabolic syndrome [106]. A double-blind, randomized clinical trial (n = 30) showed that daily administration of 200 mg of Eriomin, containing 70% eriocitrin, 5% hesperidin, 4% naringin, 1% didymin, and 20% fiber material, for 12 weeks reduced blood glucose (−5%) by increasing glucagon-like peptide-1 (GLP-1) secretion (17%) and alleviated inflammation by decreasing IL-6 (−14%) and tumor necrosis factor-α (TNF-α) (−20%) in subjects with hyperglycemia (110–150 mg/dL) [107]. Another double-blind randomized controlled trial revealed that administration of 200 mg/day of Eriomin for 12 weeks decreased hyperglycemia (6%), increased GLP-1 blood levels (22%) (p < 0.05), and altered microbiota composition in prediabetic patients compared with the results for the placebo group [108]. A double blind, placebo-controlled crossover trial involving 25 healthy subjects showed that supplementation with the extract containing 320.4 mg of anthocyanins (mainly cyanidin and delphinidin) could mitigate high-fat diet-induced endotoxemia, reduce increases in plasma lipopolysaccharide (LPS) and LPS-binding protein, and lower blood glucose and triglycerides, but it had no significant effect on the increases in plasma insulin, GLP-1, GLP-2, or glucose-dependent insulinotropic polypeptide (GIP) [109]. A 5-week crossover study showed that dietary supplementation with elderberry juice containing pronounced anthocyanins significantly changed the gut microbiota and reduced blood glucose in 18 overweight or obese adults without chronic illnesses [110].
As can be seen from the above results, dietary supplementation with flavonoids exerts hypoglycemic actions by modulating blood glucose, insulin resistance, lipid profile, inflammation, oxidant stress, insulin signaling, endotoxemia, and gut microbiota. Additionally, comparative analysis shows that flavonoid mixtures are more effective than individual flavonoids at lower doses, and there are usually more glucose-lowering pathways involved due to the synergy of multiple flavonoids. However, it should be noted that in most of these clinical studies, the intervention period was 12 weeks, and the number of subjects was less than 50. Future clinical studies with longer intervention periods and larger numbers of participants are needed to verify the long-term hypoglycemic efficacy of flavonoids.
4. Molecular Mechanisms Underlying the Hypoglycemic Effects of Flavonoids
4.1. Targets
4.1.1. Carbohydrate-Metabolizing Enzymes
Blood glucose originates from the digestion and absorption of exogenous foods and is produced through two endogenous pathways, namely gluconeogenesis and glycogenolysis, while it is metabolized through anaerobic oxidation (glycolysis) and aerobic oxidation, as well as the pentose phosphate pathway, with glycogen synthesis serving as a sink for glucose. Therefore, regulating the activities of enzymes involved in the above reactions, and thus balancing blood glucose production and consumption, is essential for maintaining blood glucose homeostasis. Numerous studies have substantiated that flavonoids can regulate the catalytic activities of enzymes involved in blood glucose metabolism, such as α-amylase, α-glucosidase, glucokinase, etc., thereby inhibiting blood glucose absorption, promoting blood glucose consumption, and alleviating hyperglycemia.
- α-amylase
Carbohydrates serve as one of the primary energy sources for the human body. In comparison to fats and proteins, consuming foods high in carbohydrates can lead to a significant increase in postprandial blood glucose, particularly in diabetic patients who have a relative or absolute deficiency of insulin. Consequently, modulating carbohydrate metabolism emerges as a crucial strategy for managing blood glucose levels.
As one of the carbohydrates, starch is hydrolyzed into oligosaccharides by salivary and pancreatic enzymes, which is the first step in digestion and absorption. As illustrated in Table 1, numerous flavonoids are found to modulate the activity of α-amylase, thus suppressing starch absorption and lowering blood sugar levels. The IC50 values indicated that the order of inhibitory effect of flavones was nepetin > scutellarein > apigenin > hispidulin [111], and the order of flavonols was kaempferol > quercetin > rutin [112].

Table 1.
The molecular interactions of flavonoids with α-amylase.
Molecular docking results indicated that flavonoids spontaneously bind to α-amylase through non-covalent intermolecular interactions, including hydrogen bonds, hydrophobic interactions, and π-interactions [65,111,113], altering the structure and hydrophobic microenvironment of the enzyme and inhibiting α-amylase activity [112]. Specifically, the interaction between flavonoids and α-amylase diminished the α-helix content of the enzyme and modified its secondary structure, causing the enzyme to pronouncedly unfold, which hindered the substrate from binding to the active sites of the enzyme and influenced the microenvironment of Trp and Tyr residues in α-amylase, with a decreased polarity and an increased hydrophobicity around the residues [70,111]. Related studies have illustrated the structure–activity relationship between flavonoids and α-amylase, confirming the important role of hydroxyl groups. It was suggested that the number of OH groups in ring A and ring B and the inhibition of α-amylase presented a positive correlation; moreover, the OH groups at the A6, A7, B4’, B5’ position and the introduction of galloyl groups could enhance the inhibition of α-amylase [114,115]. That is because the hydroxyl groups located in these positions facilitate the formation of hydrogen bonds with the side chains of the amino acid residues of α-amylase. Moreover, ring C of flavones and flavonols is the 4-oxoflavonoid core, and the C2–C3 double bond is conjugated with the 4-ketone group on ring B, thus forming a highly conjugated π-system, resulting in better stability when interacting with the active site of the enzyme [116]. Noteworthily, the only difference between scutellarein, apigenin, and hispidulin was at the C6 position of ring A, where scutellarein was hydroxylated, and hispidulin was methoxylated compared to the results for apigenin [111]. However, the effects of rutin, quercetin, and kaempferol suggested that glycosylation at C3 on the C ring and hydroxylation at C3’ on the B ring attenuated the inhibitory effect [112].
Interestingly, Tonsic et al. discovered that although epicatechin, catechin, rutin, quercetin, and naringenin were in vitro inhibitors of α-amylase, only supplementation with 100 mg/kg of rutin inhibited starch absorption in mice, indicating that further in vivo studies are needed to verify the effect of α-amylase inhibitors on starch absorption in vivo [117].
- α-glucosidase
Normally, the oligosaccharides are further hydrolyzed into glucose by α-glucosidase in the small intestine. Therefore, restraining the activity of α-glucosidase also plays a pivotal role in restraining carbohydrate decomposition and controlling postprandial hyperglycemia [118]. A multitude of flavonoids have been proven to exert inhibitory effects on the catalytic activity of α-glucosidase, such as quercimeritrin [115], narcissoside [62], cyanidin-3-O-glucoside [94], etc. (Table 2). Some flavonoids are even more effective in vitro than are clinical α-glucosidase inhibitors (acarbose), such as scutellarein, nepetin, apigenin, hispidulin [118], and astragalin [50], and they could also synergistically inhibit α-glucosidase with acarbose [119]. According to the IC50 values, the order of inhibitory effect of flavonoids reported was scutellarein > nepetin > apigenin > hesperidin > hispidulin > luteolin.

Table 2.
The molecular interactions of flavonoids with α-glucosidase.
Molecular docking results indicated that flavonoids usually interacted with the amino acid residues in α-glucosidase via the van der Waals force, hydrogen bonds, hydrophobic interactions and π-interactions, and electrostatic force [24,31,115,118,119] which certainly altered the conformation, secondary structure, and microenvironment of α-glucosidase, and then influenced its functionality [118]. As an illustration, the combination with narcissoside loosened and expanded the protein skeleton of α-glucosidase, declined the α-helix, and disrupted the helical structure of the peptide chain and peptide chain elongation [62]. Likewise, cyanidin-3-O-glucoside decreased the α-helix and β-angle and increased β-folding and irregular helix formation [94]. In addition, flavonoids, such as narcissoside [62], luteolin [21], oroxylin A [31], hinokiflavone, amentoflavone [84], and quercimeritrin [115] generally altered the microenvironment around tyrosine and tryptophan of α-glucosidase, enhancing its hydrophobicity.
Numerous studies implied that the augmentation of hydroxyl groups could strengthen the inhibitory activity of flavonoids against α-glucosidase since they participate in the interaction with the enzyme [21,84,118]. Flavonoids with two catechol groups in rings A and B, together with an OH group in the C3 position, display high inhibitory activity. The hydroxyl group at the A5, A7, A8, B3’, B4’, and C6 position of the flavonoids increased the inhibitory activity of α-glucosidase, while the methoxylation of the C3, C6, B3’, and B4’corresponding sites decreased the inhibitory activity [62,120]. Moreover, it was revealed that the glycosylation at the A7 position was more effective at inhibiting α-glucosidase than the hydroxylation at the A7 position or the glycosylation at the C3 position, while the hydroxylation at C3 is more favorable than the glycosylation at this position. Galactoside in the C3 position was more favorable than the presence of rhamnose and arabinofuranoside. The OH groups at the B4’ and B5’ positions were beneficial to the inhibition of α-glucosidase [115].
- Gluconeogenic enzymes
Gluconeogenic enzymes catalyze the conversion of various non-glucose substances into glucose or glycogen, including glucose-6-phosphatase (G6Pase), which hydrolyzes glucose-6-phosphate and stimulates glucose release from the tissues; phosphoenolpyruvate carboxykinase (PEPCK), the key enzyme that catalyzes the first step of gluconeogenesis; and fructose-1,6-bisphosphatase, which maintains glucose homeostasis in the liver and kidney. In DM patients, the activity of gluconeogenic enzymes usually increases due to impaired insulin secretion or action. However, this phenomenon can be reversed by flavonoids.
Treatments with hesperetin increased the activity of glycogen phosphorylase and decreased glucose-6-phosphatase activity, resulting in a decrease in hepatic glucose production, which plays an important role in the control of blood glucose levels in diabetic rats [69]. Dihydromyricetin relieved hepatic insulin resistance through the inhibition of hepatic gluconeogenesis and the enhancement of glucose uptake and hepatic glycogen content via decreasing the expression of G6Pase and PEPCK [78]. Biochanin A restored the downregulation of the glycolytic enzyme (hexokinase) and the upregulation of gluconeogenic enzymes (glucose-6-phosphatase and fructose-1,6-bisphosphatase) in diabetic rats [35]. Myricetin significantly inhibited G6Pase and PEPCK [56].
- Other enzymes
Glycogen metabolic enzymes contain glycogen synthase (inactive when phosphorylated) and glycogen phosphorylase. Deficiency in insulin and resistance to insulin action cause the stimulation of glycogenolytic and gluconeogenic processes [69]. Biochanin A was able to increase glycogen in the liver and muscles and decrease glycogen in the kidneys via the regulation of glycogen synthase and glycogen phosphorylase activities [35].
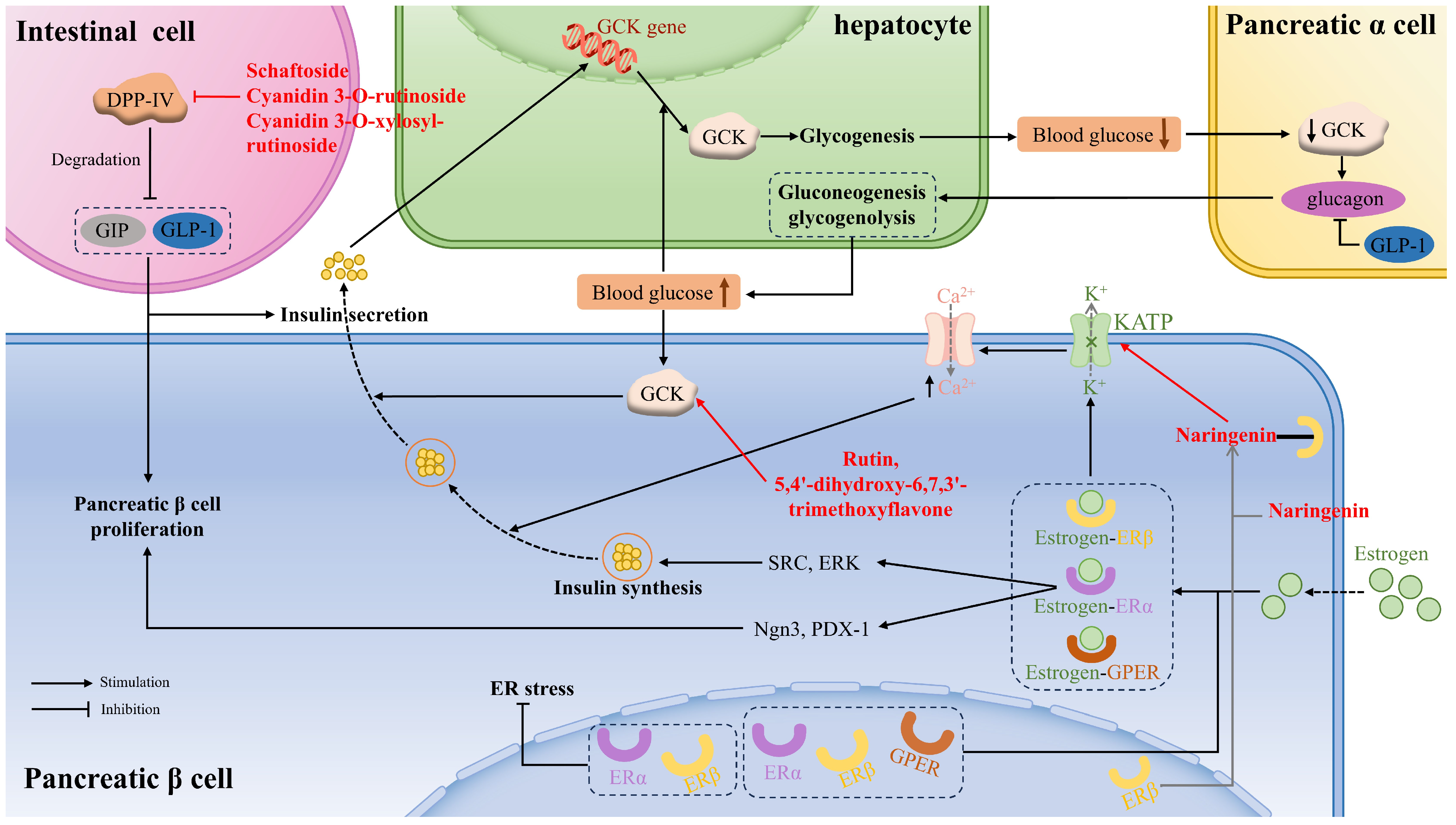
Glucokinase (GCK), also known as hexokinase, is often referred to as a glucose sensor, mainly occurring in liver cells and pancreatic cells, that plays a crucial role in whole-body glucose homeostasis. That means that when the blood glucose level rises to a certain threshold, GCK in pancreatic β cells is activated to stimulate insulin secretion. If blood glucose continues to rise, GCK in the liver cells will be activated to facilitate hepatic glycogen production. Moreover, GCK is a key determinant in glucose sensing within α-cells to regulate glucagon secretion, gluconeogenesis, and hepatic glycogenolysis. Flavonoids acting as small-molecule GCK activators not only reduce fasting and basal blood glucose levels but also improve glucose tolerance. Rutin and 5,4′-dihydroxy-6,7,3′-trimethoxyflavone showed appreciable binding interactions with the allosteric site residues of the human GCK, thus activating the enzyme [121] (Figure 2).
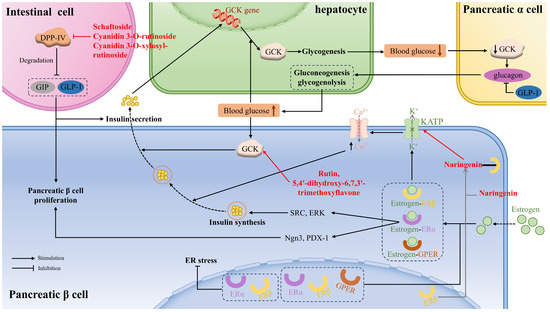
Figure 2.
The mechanism of flavonoids in regulating glucose metabolism, pancreatic β cell proliferation, and function via glucokinase (GCK), dipeptidyl peptidase IV (DPP-IV), estrogen receptors (ER), and GLP-1 between liver cells, pancreatic cells, and intestinal L cells. GPER: G protein-coupled ER; SRC: non-receptor tyrosine-protein kinase; ERK: extracellular signal-regulated kinase; Ngn3: neurogenin3; PDX-1: pancreatic and duodenal homeobox 1.
Moreover, glucokinase regulatory protein (GKRP) regulates GCK activity by establishing the GCK–GKRP complex, causing GCK to be inactive. It was demonstrated that some anthocyanidins showed lower binding energy to GKRP than did fructose-1-phosphate (F1P) (a well-known inhibitor of the GKRP–GCK complex), especially delphinidin, cyanidin, and pelargonidin, thus preventing the formation of GKRP–GCK and maintaining glucokinase activity [96].
4.1.2. Diabetic Kinome
The diabetic kinome is composed of protein kinases known to play a key role in modulating cell processes engaged in diabetes progression. For instance, the kinases responsible for the modulation of β cell proliferation and function, such as inhibiting dual specificity tyrosine phosphorylation-regulated kinase 1 A (DYRK1A), DPP-IV, and death-associated protein kinase-related apoptosis-inducing kinase-2 (Drak2). Additionally, the mechanistictarget of rapamycin (mTOR) and PTP1B plays a central role in insulin signaling.
DYRK1A has been shown to promote both insulin secretion and β cell proliferation. Five flavones and three aglycones (quercetin, baicalein, and herbacetin) were demonstrated to dose-dependently decrease DYRK1A activity in vitro, improve cell viability, and promote β-cells proliferation and insulin production. Mechanistically, the flavones were found to bind to the ATP-binding pocket of DYRK1A owing to the OH groups [122].
DPP-IV, a serine protease, is responsible for degrading GIP and GLP-1, which are peptide hormones secreted by intestinal cells to promote β-cell proliferation and insulin secretion. Some flavonoids can affirmatively inhibit DPP-IV activity and then ameliorate insulin-mediated glucose metabolism. The catalytic triad consisting of Ser630, Asn710, and His740 within the S1 pocket significantly affects DPP-IV activity. Schaftoside, a flavone that is present in sugarcane leaves, primarily repressed DPP-IV activity by forming hydrogen bonds, as well as carbon–hydrogen bonds, with the residues (Arg125, Glu205, Ser209, Ser630, Tyr666) [33]. Cyanidin 3-O-rutinoside, cyanidin 3-O-xylosyl-rutinoside, and malvin were affirmed to combine with amino acid residues in the active pocket of DPP-IV via salt bridge formation, π–π stackings, and hydrogen bonds [113] (Figure 2).
Drak2 plays a crucial role in modulating β cell survival. Luteolin potently inhibited Drak2 activity, with an IC50 of 346.7 ± 30.0 nM, as well as the expression of Drak2, thereby alleviating PA-induced β cell apoptosis and GSIS impairment owing to the promotion of autophagy and the mitigation of oxidative stress in INS-1E cells [19].
mTOR is a serine and threonine protein kinase with a specific role in insulin resistance. mTORC1 negatively regulates insulin signaling through a (p70S6) S6K-dependent feedback loop, which diminishes the interaction of insulin receptors and their downstream effectors. Licochalcone A enhanced glucose tolerance, insulin-induced glucose uptake, and insulin sensitivity through repressing mTORC1, which attenuated the negative impact of S6K on insulin signaling in diabetic mice [87].
PTP1B is a crucial tyrosine phosphatase involved in the regulation of insulin receptors [102] and is expressed in most cells in the body; however, it is overexpressed under diabetic conditions, which exacerbates insulin resistance. Consequently, PTB1B inhibitor has the potential to stimulate insulin-regulated glucose uptake, enhance insulin sensitivity, and mitigate insulin resistance, and it is regarded as a promising therapeutic agent for the treatment of diabetes [123]. Numerous flavonoids have been demonstrated to be potent PTB1B inhibitors. C3G was proven to suppress the expression of PTP1B, thereby strengthening the sensitivity of hepatic cells to insulin [91]. Among all identified O-methylated flavonoids from Eremophila clarkei, hispidulin showed the strongest inhibition against PTP1B, with an IC50 value of 24.8 ± 1.9 μM [124]. Cyanidin 3-(p-coumaroyl)-diglucoside-5-glucoside displayed a high binding affinity of −15.272 kcal/mol for PTP1B [113]. Epigallocatechin gallate (EGCG) and ECG could inhibit PTP1B and low molecular weight (LMW)-PTP. ECGG was proven to suppress PTP1B non-competitively by binding to multiple active sites of PTP1B via hydrogen bonds and hydrophobic interactions [64]. Baicalin, baicalein, chrysin, trihydroxy-dimethoxyflavone, salvigenin, and norwogonin displayed an outstanding binding affinity towards PTP1B through π–π and π–alkyl interactions [125].
4.1.3. Estrogen Receptors
Estrogen exerts its function by binding to the estrogen receptor (ER), including ERα, Erβ, and GPER, which can all be expressed in pancreatic β cells, important estrogen target organs. The activation of estrogen receptors is conducive to β cell proliferation and function, thereby improving systemic glucose metabolism. Moreover, the activation of ERs increases the secretion of GLP-1.
The activation of ERα in β cells promotes cell proliferation or regeneration through activating Ngn3 or PDX-1, increases proinsulin gene expression, followed by insulin synthesis, through extracellular ERα signaling to SRC and ERK. The activation of ERβ leads to the membrane atrial natriuretic peptide receptor-dependent reduction of KATP channel activity, leading to the opening of the voltage-dependent Ca2+ channel. The increased intracellular Ca2+ concentration promotes the fusion of insulin vesicles with the cell membrane and the subsequent release of insulin. GPER is also involved in GSIS in the β cells, which is related to the EGFR, ERK, and the AKT/mTOR/glucose transporter 2 (GLUT2) signaling pathways [75] (Figure 2).
Naringenin, a flavonoid phytoestrogen with potential estrogen-like effects, was found to specifically activate ERβ without affecting other ER isoforms, thus appreciably improving islet morphology, augmenting the percentage of β cell area in the pancreas, and elevating insulin secretion, which improved blood glucose and glucose tolerance in diabetic rat models. Further study revealed that naringenin directly bound to ERβ, activated the membrane atrial natriuretic peptide receptor, and subsequently decreased KATP channel activity, which enhanced calcium ion influx and promoted insulin release. Moreover, chronic administration of naringenin markedly upregulated the expression of genes related to β-cell function [75]. Silibinin was proven to reduce PA-caused GLUTag cell ER stress through the regulation of ERα and ERβ, improving GLP-1 secretion [126].
4.1.4. Glucose Transporter
Glucose transport proteins comprise sodium-dependent glucose transporters (SGLT) and facilitative GLUT. SGLT is a group of transmembrane proteins, including SGLT1 and SGLT2, whose main function is to mediate the transport of glucose and other monosaccharides on the cell membrane utilizing a gradient of sodium ions. SGLT plays an important role in the absorption and transport of glucose, especially in the intestine and kidneys: SGLT1, which is mainly expressed in the small intestine and kidneys, transports glucose from the intestinal lumen into intestinal epithelial cells, where it then enters the bloodstream through cytoplasmic transporters like GLUT, as well as prevents the loss of glucose in urine, while SGLT2 is predominantly expressed in the kidneys and acts as a main target responsible for transporting glucose from glomerular filtrate and glucose reabsorption. Thereupon, inhibiting SGLT is regarded as a promising strategy for controlling blood glucose levels, and many flavonoids have this effect.
For instance, molecular docking revealed that the ΔG value of myricetin and SGLT1 was −7.89 kcal·mol−1, while that of canagliflozin (a clinical inhibitor of SGLT1) was −10.08 kcal·mol−1, indicating a similar binding ability to SGLT1. Additionally, myricetin interacted with some aminoacidic residues (Asn78, His83, Glu102, Thr287, Tyr290, Trp291, Gln457) of SGLT1, playing a vital role in glucose transport and thus, impeding SGLT1-mediated glucose absorption [55]. Naringenin could occupy the glucose binding sites of proton-dependent glucose transporters via strong hydrophobic interactions, especially in the protonation state, limiting the glucose transport by SGLT [127].
GLUT transports monosaccharides through a diffusion gradient. As one of the GLUT isoforms, GLUT1 exists in a wide range of tissues to maintain the basal glucose supply. GLUT2, the most abundant GLUT isoform in hepatocytes, is accountable for most of the glucose uptake. GLU2 and GLUT3 participate in pancreatic insulin secretion. GLUT4 presents a pivotal role in regulating systemic glucose homeostasis and insulin-stimulated glucose transport. A number of documents elucidated that flavonoids can ameliorate glucose metabolism by enhancing the expression of GLUT. For example, liquiritigenin, naringenin, apigenin, kaempferol, and glabridin, isolated from the dichloromethane soluble fraction of the stem extract of Wendlandia tinctoria var. grandis (Roxb.) DC., exerted excellent hypoglycemic activities via combining with GLUT3 [128]. Myricetin significantly enhanced the expression of insulin receptors and GLUT4 [56]. Isoquercitrin, rutin [46], and isorhamnetin [129] promoted the translocation of GLUT4 to the plasma membrane.
4.1.5. The Human Islet Amyloid Polypeptide (hIAPP)
hIAPP, a peptide composed of 37 amino acids, is highly amyloidogenic and is synthesized in pancreatic β-cells, where it is co-secreted with insulin. The soluble and naturally occurring hIAPP functions in synergy with insulin to regulate glucose metabolism [130]. However, the misfolding and deposition of hIAPP generate toxic oligomeric intermediates, which give rise to membrane disruption and ion channel formation in β cells, resulting in β cell dysfunction, insulin resistance, and glucose metabolic disorders in T2DM, thereby worsening the progression of T2DM [131]. Accordingly, inhibitors that counteract the misfolding and aggregation of hIAPP have emerged as a therapeutic avenue for the treatment of type 2 diabetes.
Soluble monomeric hIAPP forms oligomerization and amyloid fibrils through a nucleation-dependent polymerization process. The canonical aggregation process involves a conformational shift of hIAPP from its native, primarily disordered state to an α-helical structure, subsequently adopting a predominant β-sheet fold, culminating with the precipitation of insoluble amyloid fibers [130]. Flavonoids, endowed with significant conformational advantages and spatial hindrance, effectively suppress hIAPP fibrillation, thus impeding the aggregation of hIAPP into oligomers and fibrils enriched in β-pleated sheets [131].
It was validated that IAPP aggregation is triggered by residues 20–29 (SNNFGAILSS), and flavonoids containing aromatic rings could interact with Phe23 of IAPP via π–π stacking, which prevented the binding between Phe23 and itself, thus reducing the β-strand content of IAPP (20–29), resulting in the inhibition of oligomer formation. Moreover, the carbonyl oxygen, hydroxyl, and vicinal hydroxyl group of flavonoids were proven to be beneficial to the suppression of IAPP aggregation [131]. Likewise, amentoflavone and bilobetin exhibited more efficacious inhibitory activity against the misfolding and aggregation of hIAPP than did some monoflavones (luteolin, apigenin), as they displayed a good steric configuration due to their globally symmetrical aromatic structures and hydroxyl groups. They were proven to suppress the self-assembly of hIAPP, depolymerize the aged aggregates into small oligomers and monomers, as well as reverse aberrant folding and mitigate hIAPP-induced cytotoxicity, insulin resistance, and aberrant insulin secretion. Further investigation revealed that the biflavones interfered with the microenvironment around Tyr37, as well as bound with the hydrophobic residues in hIAPP (22–28), especially Phe23, through hydrophobic interaction, hydrogen bonding, and π–π interaction [83]. Baicalein and baicalin maintain the secondary structure of hIAPP in an unfolded α-helix state and β-sheet conformation, respectively [130].
4.1.6. Other Targets
peroxisome proliferator-activated receptor γ (PPARγ): PPARγ can promote insulin sensitivity as a member of the nuclear hormone receptor superfamily [34]. Nigragenon O was found to interact with PPARγ, leading to a decrease in the blood glucose level and improvement in insulin sensitivity in IR 3T3-L1 cells [77]. Astragalin, quercitrin, and quercetin could interact with PPARγ active sites via polar bonding and hydrophobic interactions, as well as elevate liver PPARγ in HFD/STZ-induced diabetic rats [49]. Formononetin could interact with PPARγ through the π–alkyl bond and van der Waals interactions [34], while sulfuretin formed π–π stacking and hydrogen bond interactions [132], thereby boosting insulin sensitivity.
Inflammatory cytokines: T2DM is often accompanied by low inflammation, and there is a vicious cycle between inflammation and insulin resistance, which means insulin resistance-induced hyperglycemia can upregulate inflammation markers and ROS; likewise, increased oxidative stress and chronic low-grade inflammation give rise to insulin resistance and impaired insulin secretion. Consequently, the interventions against inflammation offer a therapeutic avenue for insulin resistance. Additionally, flavonoids, such as myricitrin, astragalin, quercitrin, quercetin [49], and puerarin [39], present anti-inflammation properties via suppressing some inflammatory cytokines involved in the inflammatory response through the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways, such as TNF-α, IL-6, and IL-1β, whose release can impede insulin signaling, causing impaired glucose metabolism and insulin resistance [133].
Adipocytokines: Adipocytokines, containing leptin and adiponectin, are secreted by adipocytes and are involved in maintaining glucose homeostasis owing to the regulation of food and energy consumption, glucose uptake, and insulin sensitivity [134]. The total flavonoids from Mela stoma dodecandrum Lour. were found to upregulate adiponectin levels, while downregulating leptin to improve insulin resistance in high-fat-diet-induced rat models [135]. Nigragenon O was found to promote adiponectin secretion in insulin-resistant 3T3-L1 cells, resulting in decreased blood glucose levels and improved insulin resistance [77]. Vitexin and luteolin were considered to interact with adiponectin and its receptor [136].
4.2. Signal Pathways
4.2.1. IRS/PI3K/AKT
The insulin-mediated IRS/PI3K/AKT biochemical pathway plays a pivotal role in glucose metabolism through downregulating gluconeogenesis and heightening glycogen synthesis, along with improving glucose utilization.
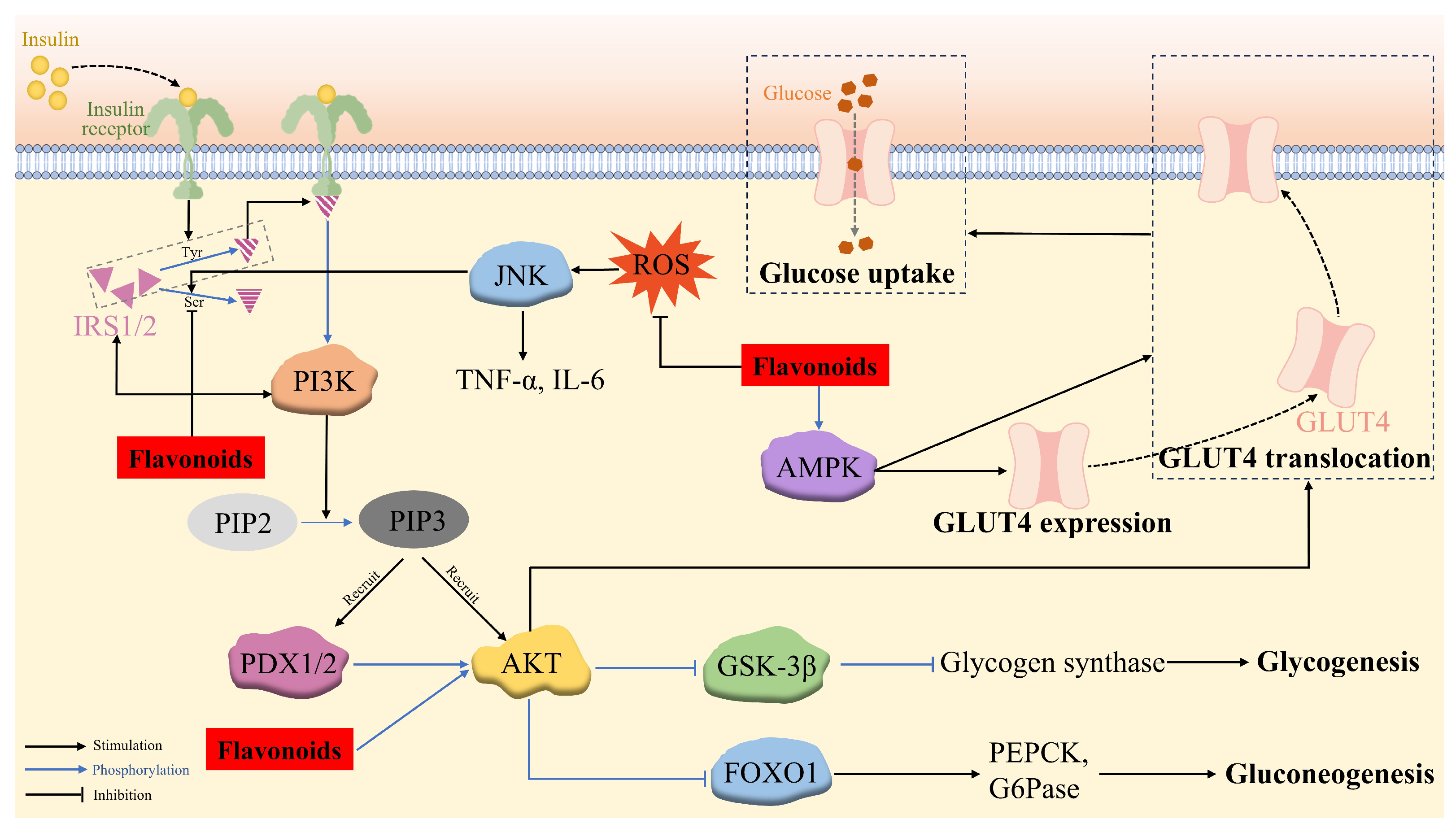
The binding of insulin to its receptors results in the autophosphorylation and activation of the receptors, which recruits tyrosine to phosphorylate IRS-1/2, leading to tyrosine phosphorylation and the activation of PI3K. This catalyzes the phosphorylation of the 3-hydroxy group of phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 then acts as a second messenger, which simultaneously recruits 3-phosphoinositide-dependent protein kinase-1 and -2 (PDK1 and PDX2) and AKT to the plasma membrane, where PDK1 and PDX2 phosphorylate threonine at position 308 and serine at position 473 of AKT, respectively. However, the phosphorylation of IRS at serine prevents IRS from binding to the insulin receptors, resulting in the suppression of the PI3K/Akt pathway [137].
The phosphorylated AKT triggers downstream signaling proteins, including forkhead box transcription factor O1 (FOXO1), glycogen synthase kinase-3β (GSK-3β), and GLUT4. FOXO1 is a key transcription factor in regulating glucose production through insulin signaling in the liver, which is localized to the nucleus and enhances hepatic gluconeogenesis by binding to the promoters of the gluconeogenic enzymes (PEPCK and G6Pase). However, P-AKT phosphorylates FOXO1, leading to its dissociation from the nucleus and its decomposition and thereby, the suppression of gluconeogenesis. GSK-3β, a serine protein kinase with an inhibitory effect on glycogen synthesis via phosphorylating glycogen synthase, is deactivated by p-Akt-induced phosphorylation, which inhibits the phosphorylation of glycogen synthase and enhances glycogen synthesis in the hepatocytes [138]. P-AKT also stimulates the translocation of insulin-responsive GLUT4 to the cell surface, thus potentiating glucose uptake in muscle and adipose cells and maintaining blood glucose homeostasis [129,139] (Figure 3).
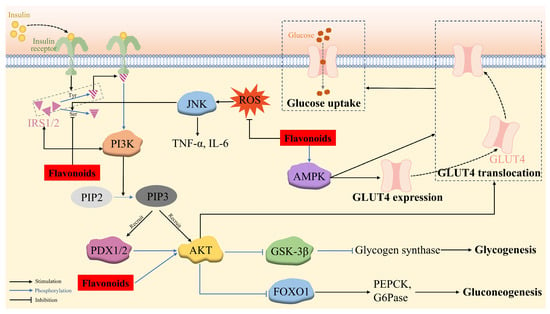
Figure 3.
The mechanism of flavonoid regulation of insulin-mediated glucose metabolism via the IRS/PI3K/AKT signaling pathway and insulin-independent glucose uptake via the AMPK pathway, together with ROS/c-Jun N-terminal kinase (JNK) pathway.
Several flavonoids have been validated to upregulate the IRS/PI3K/AKT pathway, consequently exerting anti-hyperglycemia and anti-insulin-resistance activity. Quercetin-3-O-α-L-arabinopyranosyl-(1→2)-β-D-glucopyranoside (QAG) stimulated the IRS-1/PI3K/Akt/GSK-3β pathway via suppressing the phosphorylation of IRS-1 at Ser612, resulting in the activation of insulin signaling. Furthermore, the molecular docking result revealed that QAG achieved the above activities by combining with the residues in the active pocket of the insulin receptor [140]. HM-chromanone was able to activate the IRS/PI3K/AKT channel to reduce blood glucose levels and alleviate insulin resistance contributed by the decreased phosphorylation of IRS-1ser307 and elevated GLUT4, p-FOXO1, and p-GSK-3β levels in PA-treated IR HepG2 cells and ob/ob mice [99,100,101]. Naringenin efficiently restored the decreased phosphorylation of Akt at the Ser473 residue site [76]. Astragalin, quercetin-3-arabinoside, and quercetin derived from Hypericum attenuatum exhibited appreciable hypoglycemic activity manifested by declined glucose production and accelerated glycogen synthesis in IR HepG-2 cells owing to the decreased expression of PEPCK and G6Pase and the increased expression of glycogen synthase, respectively, via the PI3K/AKT/FOXO1 and PI3K/AKT/GSK3β pathways [139].
4.2.2. AMPK/GLUT4
Skeletal muscle is the principal target organ for insulin-mediated glucose uptake, which is achieved through GLUT4 on its cell membrane. In addition to the insulin-dependent IRS/PI3K/AKT pathway, the translocation of GLUT4 can also be enhanced through an insulin-independent mechanism, namely the AMPK pathway. During this signaling, the AMP-activated protein kinase (AMPK) phosphorylates and deactivates the Akt substrate, detectable at a molecular weight of 160 (AS160), which is a functional Rab-GTPase-activating protein (rab GAP) [141], thus promoting the transcription of the GLUT4 gene and the transposition of GLUT4 to the cell surface, facilitating glucose uptake [61,142]. Therefore, the activation of AMPK/GLUT4 is an effective way to improve insulin sensitivity in T2DM [37]. Licochalcone A alleviated abnormal liver glucose metabolism, partly owing to the activation of AMPK [87]. Formononetin [37] and isorhamnetin [61] were capable of upregulating p-AMPK-α levels, potentiating the expression and translocation of GLUT4. Kaempferol [52] and phlorizin [90] promoted glucose uptake in lipotoxicity-impaired human skeletal muscle cells and mitigated insulin resistance in FFA-treated IR HepG2 cells, respectively, through upregulating p-AMPK and PI3K/Akt/GLUT4 (Figure 3).
4.2.3. ROS/JNK
Insulin resistance is caused by a variety of factors, including ROS-induced oxidative stress. Long-term exposure to FFA, such as palmitic acid, is one of the factors causing mitochondrial dysfunction that leads to excess ROS formation. Increased ROS levels cause oxidative stress and promote the activation of JNK, a stress kinase belonging to the mitogen-activated protein kinase family, which has been reported to be involved in the mechanism of FFA-induced hepatic insulin resistance. The activated JNK pathway facilitates the production of the inflammatory response factors TNF-α and IL-6, as well as IRS-1 phosphorylation at S307, thus inhibiting the PI3K/AKT pathway and providing a mechanism for insulin resistance. HM-chromanone was proven to inhibit ROS production and ROS-induced JNK activation, leading to a significant dose-dependent reduction in TNF-α and IL-6 levels, along with the decreased phosphorylation of IRS-1 at Ser307 and increased AKT/FOXO1 and AKT/GSK-3β levels compared to those of the insulin-resistant HepG2 cells [101] (Figure 3).
4.2.4. PERK/eIF2α/ATF4/CHOP
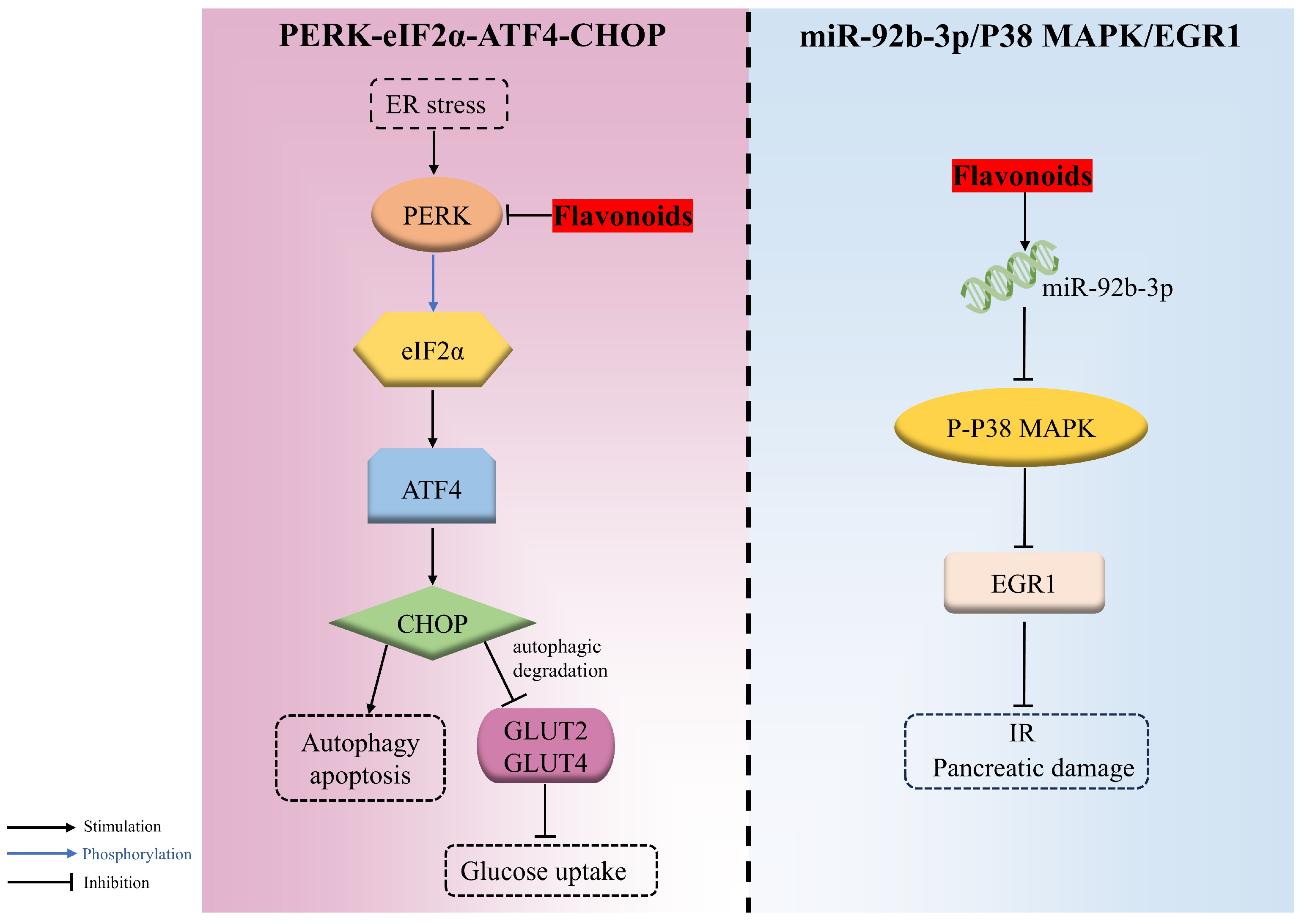
The endoplasmic reticulum, the vital organelle responsible for the synthesis, proper folding, and secretion of insulin, is highly sensitive to various stress factors that induce misfolded and unfolded proteins to accumulate there, triggering the ER stress and activating the unfolded protein response (UPR) response. However, when the adaptive responses evoked by UPR are inadequate to resume normal cellular function, the apoptosis and autophagy signaling pathways are then triggered. PERK-eIF2α-ATF4-CHOP is an important signaling pathway involved in endoplasmic reticulum stress-induced apoptosis. PERK, an endoplasmic reticulum transmembrane protein acting as a UPR sensor, can phosphorylate eIF2α at ser51, causing the activation of ATF4, followed by CHOP, which gives rise to autophagy and apoptosis during endoplasmic reticulum stress.
Cyanidin-3-O-glucoside remarkably alleviated PA-evoked pancreatic β cell malfunction and apoptosis in a dose-dependent manner through downregulating the ER-stress-related PERK/eIF2α/ATF4/CHOP pathway. It was confirmed that C3G decreased the expression of genes, including eif2αk3 (PERK) and ddit3 (CHOP), as well as the expression of PERK, CHOP, glucose regulated protein 78 (GRP78), and GLUT2 and the phosphorylation of PERK and eif2α [93].
Additionally, the autophagic degradation of two hepatic glucose transporters, namely GLUT2 (insulin-independent) and GLUT4 (insulin-dependent), during hyperglycemia was motivated via PERK/eIF2α/ATF4/CHOP, thereupon decreasing glucose uptake. Nevertheless, morin was proven to ameliorate the hyperglycemia and insulin sensitivity attributed to the repression of PERK-adjusted transporter degradation and the boosted expression of glucose transporter proteins in both HepG2 cells and diabetic rats [143] (Figure 4).
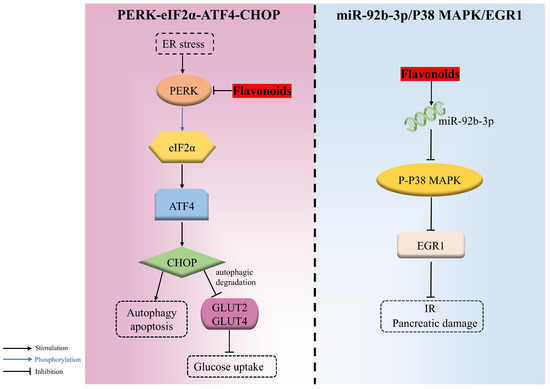
Figure 4.
The regulation mechanisms of flavonoids in PERK-eIF2α-ATF4-CHOP and miR-92b-3p/ P38 mitogen-activated protein kinase (P38 MAPK)/ early growth response 1 (EGR1) signaling pathways.
4.2.5. miR-92b-3p/P38 MAPK/EGR1
miR-92b-3p is the dominant form during miRNA formation, which inhibits the phosphorylation of P38 MAPK and then suppresses EGR1 expression, which is associated with β-cell proliferation and transactivation of the insulin gene. EGR1, a stress-responsive gene highly expressed during diabetes, is responsible for the release of inflammatory factors that can cause IR. It was demonstrated that quercetin was capable of upregulating miR-92b-3p, thus decreasing the activation of P38 MAPK and further repressing EGR1 expression, which resulted in decreased blood glucose and the mitigation of IR and pancreatic damage in HFD/STZ-induced T2DM mice [45] (Figure 4).
4.3. Gut Microbiota
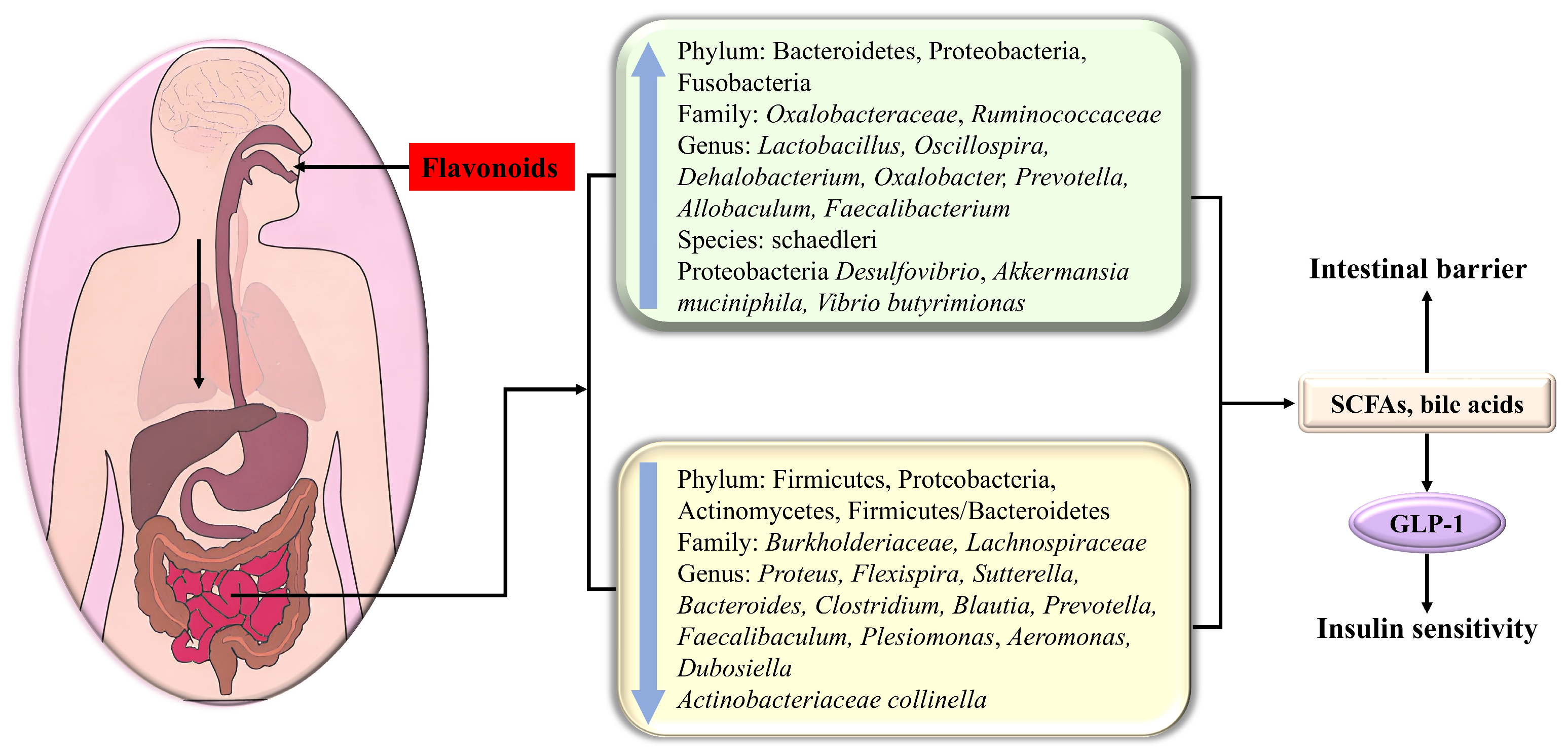
The human gut contains trillions of microorganisms, encompassing over 1000 species of bacteria [144]. The intestinal flora is implicated in the release of intestinal hormones, bile acid metabolism, insulin sensitivity regulation, and lipid metabolism disorder, and its metabolites are linked to the functionality of remote organs and systems via the circulatory system. In healthy individuals, the gut microbiota maintains a dynamic equilibrium. Abnormalities in the abundance and proportions of bacteria can lead to an imbalance of the gut microbiota, resulting in intestinal disorders, as well as various metabolic disorders such as diabetes, obesity, cardiovascular diseases, and non-alcoholic fatty liver disease. Numerous studies indicate that diabetes is accompanied by alterations in gut microbiota composition [51]. Accordingly, restoring disrupted gut microbiota and promoting the growth of hypoglycemic probiotics are effective strategies for treating diabetes.
A significant number of studies have declared that the low bioavailability of flavonoids affords ample time for interaction with the intestinal tract, which effectively modulates the composition of gut microbiota and its metabolites and protects the intestinal barrier [145] (Figure 5). Therefore, flavonoids represent potentially efficacious agents for regulating disruptions in the gut microbiota.
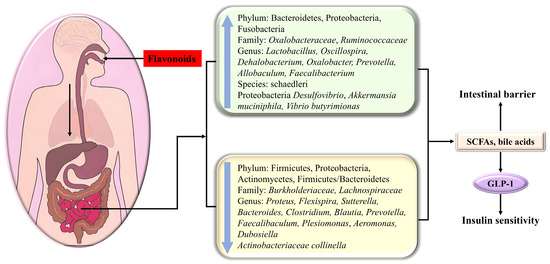
Figure 5.
The flavonoid modulation of glucose metabolism via adjusting the composition of intestinal flora and its metabolites.
Diabetes usually leads to the rarefication of the dominant flora Bacteroidetes, which participate in some vital metabolism roles, including carbohydrate fermentation, i.e., the increment of Firmicutes, Proteobacteria (containing various pathogens), Actinomycetes, and Firmicutes/Bacteroidetes. Watanabe et al. indicated that the addition of soy isoflavone could decrease the ratio of Firmicutes/Bacteroidetes [146], which was beneficial for glycemic control and GLP-1 secretion.
Kaempferol had the potential to significantly decrease fasting blood glucose and ameliorate glucose tolerance and insulin sensitivity as a consequence of reversing the abnormal alterations of the gut microbiota in HFD-treated mice compared with that of the control group, including the reduction in the Firmicute Dehalobacterium, the Proteobacteria Desulfovibrio, as well as in Proteus flexispira and Sutterella; the increment of Actinobacteriaceae collinella, Oscillospira, Dehalobacterium, and schaedleri species; and the increase in the Firmicute Vibrio butyricimonas. In the microbiotas studied, Collinsella is related to insulin, and the increase in Desulfovibrio can improve glucose metabolism [51]. Nobiletin enhanced the abundance of Allobaculum, which was indicated to be an active glucose assimilator and could improve energy homeostasis in HFD-fed mice [25]. Neohesperidin dihydrochalcone had the potential to bolster glycogenesis and restrain gluconeogenesis in the liver of diabetic zebrafish as a consequence of upregulating Prevotella [147].
Apigenin increases Akkermansia and decreases Faecalibaculum and Dubosiella at the genus level [148]. Cyanidin-3-O-glucoside (C3G) demonstrated efficacy in treating hyperglycemia through adjusting the intestinal flora distribution, manifested by the decline in Firmicutes/Bacteroidetes, Burkholderiaceae, Lactobacillus, and Bacteroides and the increase in Oxalobacteraceae, Oxalobacter, Prevotellaceae, and Helicobacter [92]. HMF (3,3′,4′,5,6,7,8-heptamethoxyflavone) prominently changed the composition, function, and metabolism of the gut microbiota, especially Faecalibaculum rodentium, Collinsella aerofaciens and Lactobacillus fermentum, in HFD-fed mice, thus displaying a beneficial effect on metabolic syndrome [27]. Isoxanthohumol, a prenylated flavanone found in beer hops, markedly influenced the gut microbiota composition, specifically promoting the growth and abundance of Akkermansia muciniphila, while inhibiting some Bacteroides and Clostridiums [149]. Quercetin decreased the abundance of Proteobacteria, Bacteroides, Escherichia-Shigella, and Escherichia coli in db/db mice, thereby reducing glucose and alleviating insulin resistance [150].
In addition to causing compositional changes, metabolites produced by the gut microbiota also have a striking impact on T2DM. For instance, short-chain fatty acids (SCFAs) derived from polysaccharide fermentation heighten insulin sensitivity and deplete blood sugar levels by modulating the secretion of the gastrointestinal hormone GLP-1. Additionally, bile acids, involved in food digestion, serve as crucial signaling molecules impacting various metabolic disorders, including cancer, obesity, and type 2 diabetes. Secondary bile acids can engage with the G protein-coupled receptor 5 on intestinal epithelial L cells, thereby stimulating the release of GLP-1 to modulate glucose metabolism [92]. Dietary flavonoids can modulate microbial metabolites, subsequently impacting host metabolism.
Neohesperidin dihydrochalcone significantly alleviated insulin resistance due to the promotion of SCFAs and the suppression of pro-inflammatory cytokines contributed by the decrease in harmful bacteria, including Proteobacteria, Plesiomonas, and Aeromonas, together with the increase in Faecalibacterium and Fusobacteria. Among the aforementioned bacteria, Faecalibacterium has the potential to ferment glucose to produce SCFAs, including butyric acid, formic acid, and D-lactic acid, meanwhile boosting glycogenesis [147]. Kaempferol upregulated Vibrio butyrimionas, which can generate short-chain fatty acids targeting the intestines, liver, and other organs to improve intestinal health, blood glucose, and insulin resistance [51]. The addition of soy isoflavone could enhance the abundance of short-chain fatty acid-producing bacteria in intestinal bacteria [146]. HMF (3,3′,4′,5,6,7,8-heptamethoxyflavone) enhanced short-chain fatty acid- and bile acid-producing bacteria and inhibited harmful bacteria, thereby promoting bile acid metabolism and reducing fatty acid metabolism and inflammatory response [27]. C3G enriched the abundance of the microbiomes involved in SCFA biosynthesis and bile acid metabolism in diabetic db/db mice, giving rise to the expansion of short-chain fatty acids (propanoic acid, isobutyric acid, butyric acid) and bile acids, followed by the secretion of GLP-1, which in turn bolstered insulin release, lowering blood glucose [92].
Noteworthily, isoxanthohumol enhanced the intestinal barrier protective function manifested by increased mucin layer thickness and claudin-1 (a tight-junction-related protein in the colon), which might be contributed by the A. muciniphila-induced enhanced turnover of epithelial cells or the production of SCFAs (the main nutrient for intestinal cells) [149]. Neohesperidin dihydrochalcone adjusted the composition of intestinal flora and thus improved the functionality of the intestinal barrier in diabetic zebrafish [147].
5. Synergistic Hypoglycemic Effects of Flavonoids
Quercetin could also be useful in combination with other drugs to potentially enhance the effects or synergistically interact with them in order to reduce their side effects and related toxicity.
Several studies have suggested that the combination of different flavonoids can synergistically alleviate diabetes, showing surprising effects. It was demonstrated that the addition of curcumin and baicalein (75 mg/kg each) remarkedly diminished FBG (13.30 ± 2.97 mmol/L) in mice fed with a high-fat diet for 4 weeks compared with the results for model diabetic group (25.49 ± 9.28 mmol/L). Additionally, the ability of the combination to decrease FBG was superior to that of individual 150 mg/kg curcumin (21.91 ± 9.08 mmol/L) or 150 mg/kg baicalein (25.85 ± 9.41 mmol/L) treatments. The common gene targets and the kyoto encyclopedia of genes and genomes (KEGG) pathway might be decisive in elucidating the mechanisms of the synergistic effect of curcumin and baicalein [151].
Koh et al. revealed that the combination of myricetin (40 μM) and luteolin (100 μM) required to produce a 50% inhibition of the activity of α-glucosidase was 6.41 ± 0.17 μM myricetin and 16.02 ± 0.43 μM luteolin, suggesting a synergistic inhibition [152]. The combinations of apigenin (5, 10, 15 μM) and nepetin/scutellarein/hispidulin (5, 10, 15 μM) were able to synergistically repress the activity of α-amylase apart from the conjunction of apigenin (5 μM) and hispidulin (5 μM), which exerted additional effect. Since apigenin was a non-competitive inhibitor of α-amylase, while the other three flavonoids were competitive inhibitors, they could interact with different sites of α-amylase, displaying a synergistical inhibitory effect [111].
Scarpa et al. elucidated that naringenin and hesperetin were able to synergistically increase insulin receptor (INSR) mRNA expression in insulin-resistant HepG2 and Hep3B cells, synergistically reduce GLUT3 mRNA levels in IR Hep3B cells, and synergistically elevate sirtuin 1 (SIRT1) expression in IR HepG2 cells. Furthermore, naringenin, hesperetin, curcumin, polydatin, and quercetin presented a synergistic inhibition against SEMA3E mRNA expression in insulin-evoked IR HepG2 cells and DPP-IV catalytic activity in IR Hep3B cells, suggesting an effective novel strategy for the treatment of T2DM [153]. The combined treatment with 25 mg/kg kaempferol and 25 mg/kg myricetin decreased body weight, glycogen level, α-amylase activity, pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α), and elevated insulin level more significantly than the addition with 50 mg/kg kaempferol or 50 mg/kg myricetin alone in STZ-induced diabetic rats, suggesting that co-treatment with kaempferol and myricetin exerted a better hypoglycemic effect than did treatment with one of the compounds independently [154].
6. Limitations in the Application of Flavonoids
6.1. Absorption and Bioavailability of Flavonoids
Despite the objective hypoglycemic activities, flavonoids generally exhibit low oral bioavailability due to their poor water solubility. The bioavailability of quercetin is often relatively low (<10%) and is influenced by the type of sugar moiety [155], by which its hypoglycemic activity is also affected. In addition, because of massive free hydroxyl groups, flavonoids are easily glucuronidated and sulfated in the intestinal cells, and then expelled by efflux transporters, resulting in poor and unstable oral absorption. Moreover, flavonoids are also sensitive to various environmental stresses (heat, light, pH) due to the presence of hydroxyl, ketone, and unsaturated double bonds. This may lead to degradation or biotransformation during storage and systemic circulation, severely limiting their efficacy through oral absorption [156].
In conclusion, the clinical application of flavonoids is greatly limited. To address these issues, a number of promising strategies have been developed and applied, such as the use of absorption enhancers (e.g., apple pectin, chitosan), structural transformations (e.g., prodrugs, glycosylation), the glycosylation of flavonoids (e.g., chemical synthesis, enzyme pathway manipulation), and pharmaceutical techniques (e.g., carrier complexes, nanotechnology, cocrystals) [157] to deliver poorly water-soluble flavonoid. Using these strategies alone or in combination effectively improves the oral bioavailability of flavonoids, prevents its degradation or metabolism in the gastrointestinal tract, or delivers it directly to the targets. In the future, we should pay more attention to the toxicity and safety of these modified oral preparations, accelerate the translation of results and clinical research, and promote the clinical application of flavonoids for lowering blood glucose.
6.2. Toxicity and Safety of Flavonoids
Although flavonoids have shown significant potential for improving glucose metabolism, recent research indicates a growing concern about the endocrine disruption, developmental toxicity, and mutagenicity of many flavonoids.
Anthocyanins are widely found in plants, fruits, and vegetables and are generally safe to consume via a normal dietary intake. According to early toxicological studies, anthocyanin-rich extracts exhibit very low mutagenicity, reproductive toxicity, teratogenicity, and acute and short-term toxicity. However, data on the long-term toxicity and carcinogenic effects of anthocyanins are still lacking [158].
Luteolin, apigenin (flavones), quercetin (flavonol), and genistein (isoflavone) showed specific estrogenic activity, developmental toxicity, and mutagenicity. Luteolin displayed higher developmental toxicity and genotoxicity than did apigenin, and quercetin exhibited lower developmental toxicity and estrogenic activity among the flavonoids. Luteolin and genistein showed high developmental toxicity (45–477 μg/kg), with up to 50% mortality in the chicken embryo test [159]. A toxicity study of two isoflavones, genistein and daidzein, showed that the hatching rate of zebrafish larvae was significantly reduced at 72–96 h post fertilization (hpf) at the doses of 20 mg of daidzein and 10 mg of genistein. Genistein showed higher toxicity than did daidzein, often inducing greater transcriptional responses, as the LC50 of genistein estimated at 96 hpf was 4.4 mg/L, which is significantly lower than that of daidzein (over 65.15 mg/L) [160].
Supplementation with 800 mg of EGCG daily for 5 days did not cause liver injury or abnormal serum folate levels in 39 women aged 18 to 40 years [161]. However, high doses of dietary EGCG supplements may be associated with certain health risks. An acute toxicity study of EGCG showed that EGCG doses exceeding 30 mg/L resulted in 100% mortality and organ-specific toxicity. Continuous exposure to high concentrations of EGCG may cause severe tissue changes in experimental animals [162]. EGCG showed a weak degradation effect on 5-methenyltetrahydrofolic acid (5-MTHF), the active form of folate, which can be used directly by the body. In serum, 2 μm EGCG had no significant effect on 5-MTHF within 48 h, but 0.1 mM and 1 mM EGCG showed a pro-degradation effect on 5-MTHF from 6 h, and 20 μm EGCG decreased the concentration of 5-MTHF for 48 h [163].
Quercetin treatment for 24 h did not affect the cellular metabolic activity of MCF7 cells but showed cytotoxic effects after 48 h and 72 h of treatment, with IC50 values of 43.18 μg/mL and 18.49 μg/mL, respectively. The results of live cell imaging showed that no apoptosis was found after 24 h, but prolonged exposure reduced the apoptosis-promoting effect of quercetin [164].
A toxicological study on biflavonoids from Ginkgo biloba leaves (amentoflavone, sciadopitysin, ginkgetin, isoginkgetin, and bilobetin) showed that all five flavonoids reduced cell survival of human renal tubular epithelial cells and human normal hepatocytes in a dose-dependent manner, suggesting potential hepatorenal toxicity. After intragastrical administration at a dose of 20 mg/kg per day for 7 days, the activity of alkaline phosphatase was significantly increased, and all five biflavonoids induced acute kidney injury in treated mice. More attention should be paid to ensure the safety of Ginkgo biloba preparations [165].
Given the above facts, the supplementation of flavonoids should be scientific and cautious to maximize their health benefits and avoid potential risks. A large number of studies are still needed in the future to fully elucidate the potential toxicity of flavonoids, especially their safety under long-term or low-dose conditions, which still requires careful evaluation. The relationship between toxicity and the structure of flavonoids should also be additionally studied to guide its further modification and application.
7. Conclusions and Future Perspectives
As the incidence of diabetes continues to rise around the world, there is increasing interest in natural plant-derived flavonoids exhibiting low side effects and considerable anti-diabetes activity. The subclasses of hypoglycemic flavonoids discussed in this review comprise flavones, isoflavones, flavonols, flavanols, flavanones, flavanonols, biflavonoids, chalcones, anthocyanins, and homoisoflavonoids, which exhibit therapeutic properties against diabetes through a variety of targets and pathways, e.g., α-amylase, α-glucosidase, gluconeogenic enzymes, DPP-IV, estrogen receptors, GLUT, IRS/PI3K/AKT, AMPK/GLUT4, PERK/eIF2α/ATF4, etc. In addition, flavonoids also modulate abnormal gut microbiota to alleviate the symptoms of diabetes. Moreover, with further research regarding the hypoglycemic mechanism of flavonoids, the synergistic effects between flavonoids have also received widespread attention; however, the exploration of this aspect is relatively fractional and lacks further investigation and validation regarding the mechanism of the synergistic action between related targets. Overall, flavonoids offer a new insight into hypoglycemic therapy.
Of particular note, a substantial number of studies have focused only on the hypoglycemic activity of total flavonoids derived from the plant extract, without further identifying and validating the exact physiological activity of the flavonoid contained therein, which is not conducive to the symptomatic use and clinical application of flavonoids. Moreover, numerous studies on the hypoglycemic effects of flavonoids remain confined to the in silico phase, without subsequent in vivo validation. Previous studies have detected that flavonoids displaying carbohydrate metabolism enzyme inhibitory activity in silico were unable to significantly suppress carbohydrate absorption in vivo [117]. Therefore, complementing the in vitro characterization with in vivo experiments is certainly necessary. For in vivo experiments, while many animal models of diabetes exist, more convincing human experimental data are still lacking. However, most studies are conducted in vitro, and further in vivo and clinical studies should determine the efficacy and safety of flavonoids. With the development of bioavailability research, increasing attention has been paid to the physiological function of flavonoid metabolites. Flavonoids may exhibit different positive effects at different stages. In the future, we should further study the cellular sites and various signaling pathways that its decomposition products may reach, as well as the effects on the structure and function of gut microbiota.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14040378/s1, Table S1: The subclasses of flavonoids and their core carbon skeleton; representative flavonoids; Table S2: The hypoglycemic effects of flavonoids studied in recent years. Refs. [166,167,168,169,170] are cited in the Supplementary Table S2.
Author Contributions
Conceptualization, M.L., C.L. and Z.X.; validation, C.L. and Z.X.; writing—original draft preparation, M.L. and C.L.; writing—review and editing, Z.X., P.Z., X.K. and Y.L.; visualization, M.L., C.L., P.Z., X.K. and Y.L.; supervision, Z.X., P.Z., X.K. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31571825), the 2024 Graduate International Teaching System Construction Project (ENT24004), the Tianjin University Independent Innovation Foundation (2024XC-0001), and the Natural Science Foundation of the Xizang Autonomous Region (XZ202501ZR0016, XZ202501ZR0015).
Conflicts of Interest
The authors declare no conflicts of interest. Author Chunlong Liu is employed by Tianjin Longsheng Biotechnology Co., Ltd., and the company had no role in the conceptualization of the study, the writing of the manuscript, or the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| DM | diabetes mellitus |
| T1DM | type 1 diabetes |
| T2DM | type 2 diabetes |
| IR | insulin resistance |
| FBG | fasting blood glucose |
| STZ | streptozocin |
| HFD | high-fat diet |
| PA | palmitic acid |
| GSIS | glucose-stimulated insulin secretion |
| EGCG | epigallocatechin gallate |
| ROS | reactive oxygen species |
| FINS | fasting insulin |
| C3G | cyanidin-3-O-glucoside |
| HbA1c | glycosylated hemoglobin |
| AGE | advanced glycation end-product |
| G6Pase | glucose-6-phosphatase |
| PEPCK | phosphoenolpyruvate carboxykinase |
| GCK | glucokinase |
| GKRP | glucokinase regulatory protein |
| DYRK1A | dual specificity tyrosine phosphorylation-regulated kinase 1 A |
| DPP-IV | dipeptidyl peptidase IV |
| Drak2 | death-associated protein kinase-related apoptosis-inducing kinase-2 |
| mTOR | mammalian target of rapamycin |
| PTP1B | protein tyrosine phosphatase 1B |
| GIP | glucose-dependent insulinotropic polypeptide |
| GLP-1 | glucagon-like peptide-1 |
| ER | estrogen receptor |
| SGLT | sodium-dependent glucose transporter |
| GLUT | facilitative glucose transporter |
| hIAPP | human islet amyloid polypeptide |
| TNF-α | tumor necrosis factor-α |
| IL-6 | interleukin 6 |
| FFA | free fatty acids |
| SCFAs | short-chain fatty acids |
References
- Care, D. Classification and Diagnosis of Diabetes. Diabetes Care 2015, 38, S8–S16. [Google Scholar]
- Katsarou, A.; Gudbjornsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, A. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Liu, J.L.; Liu, M.; Chai, Z.L.; Li, C.; Wang, Y.A.; Shen, M.W.; Zhuang, G.H.; Zhang, L. Projected rapid growth in diabetes disease burden and economic burden in China: A spatio-temporal study from 2020 to 2030. Lancet Reg. Health West. Pac. 2023, 33, 100700. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Diabetes Care in the Hospital: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S267–S278. [Google Scholar] [CrossRef]
- Kumar, R.; Kerins, D.M.; Walther, T. Cardiovascular safety of anti-diabetic drugs. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.A.; Serafini, M.; Alves, I.A.; Lang, K.L.; Silva, F.; Aragon, D.M. Natural Products as Outstanding Alternatives in Diabetes Mellitus: A Patent Review. Pharmaceutics 2023, 15, 85. [Google Scholar] [CrossRef]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef]
- Wu, C.; He, L.; Zhang, Y.; You, C.; Li, X.; Jiang, P.; Wang, F. Separation of flavonoids with significant biological activity from Acacia mearnsii leaves. RSC Adv. 2023, 13, 9119–9127. [Google Scholar]
- Wang, T.-y.; Li, Q.; Bi, K.-s. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [PubMed]
- Priya, P.S.; Pavithra, V.; Vaishnavi, S.; Pachaiappan, R.; Kumar, T.T.A.; Rady, A.; Darwish, N.M.; Arokiyaraj, S.; Namasivayam, S.K.R.; Arockiaraj, J. Understanding the mechanisms and implications of acacetin in mitigating diabetic osteoporosis: Insights from a zebrafish model. Process Biochem. 2023, 134, 63–74. [Google Scholar]
- Wang, Y.; Liu, L.; Ge, M.; Cui, J.; Dong, X.; Shao, Y. Acacetin attenuates the pancreatic and hepatorenal dysfunction in type 2 diabetic rats induced by high-fat diet combined with streptozotocin. J. Nat. Med. 2023, 77, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, Y.; Peng, Y.; Li, X. The water extract of Radix scutellariae, its total flavonoids and baicalin inhibited CYP7A1 expression, improved bile acid, and glycolipid metabolism in T2DM mice. J. Ethnopharmacol. 2022, 293, 115238. [Google Scholar]
- Froldi, G.; Djeujo, F.M.; Bulf, N.; Caparelli, E.; Ragazzi, E. Comparative Evaluation of the Antiglycation and Anti-α-Glucosidase Activities of Baicalein, Baicalin (Baicalein 7-O-Glucuronide) and the Antidiabetic Drug Metformin. Pharmaceutics 2022, 14, 2141. [Google Scholar] [CrossRef]
- Wu, S.; Ai, W.; Nie, L.; Lu, X. Antidiabetic activity of eupafolin through peroxisome proliferator-activated receptor-gamma and PI3K/Akt signaling in Type 2 diabetic rats. J. Biochem. Mol. Toxicol. 2023, 37, e23463. [Google Scholar]
- Al-Masri, A.A.; Ameen, F.; Davella, R.; Mamidala, E. Antidiabetic effect of flavonoid from Rumex vesicarius on alloxan induced diabetes in Male Albino Wistar rats and its validation through in silico molecular docking and dynamic simulation studies. Biotechnol. Genet. Eng. Rev. 2023, 40, 4479–4494. [Google Scholar]
- Djeujo, F.M.; Stablum, V.; Pangrazzi, E.; Ragazzi, E.; Froldi, G. Luteolin and Vernodalol as Bioactive Compounds of Leaf and Root Vernonia amygdalina Extracts: Effects on α-Glucosidase, Glycation, ROS, Cell Viability, and In Silico ADMET Parameters. Pharmaceutics 2023, 15, 1541. [Google Scholar] [CrossRef]
- Han, M.; Lu, Y.; Tao, Y.; Zhang, X.; Dai, C.; Zhang, B.; Xu, H.; Li, J. Luteolin Protects Pancreatic β Cells against Apoptosis through Regulation of Autophagy and ROS Clearance. Pharmaceuticals 2023, 16, 975. [Google Scholar] [CrossRef]
- Faisal, S.; Tariq, M.H.; Ullah, R.; Zafar, S.; Rizwan, M.; Bibi, N.; Khattak, A.; Amir, N.; Abdullah. Exploring the antibacterial, antidiabetic, and anticancer potential of Mentha arvensis extract through in-silico and in-vitro analysis. BMC Complement. Med. Ther. 2023, 23, 267. [Google Scholar]
- Djeujo, F.M.; Ragazzi, E.; Urettini, M.; Sauro, B.; Cichero, E.; Tonelli, M.; Froldi, G. Magnolol and luteolin inhibition of α-glucosidase activity: Kinetics and type of interaction detected by in vitro and in silico studies. Pharmaceuticals 2022, 15, 205. [Google Scholar] [CrossRef]
- Alozieuwa, U.B.; Lawal, B.; Sani, S.; Onikanni, A.S.; Osuji, O.; Ibrahim, Y.O.; Babalola, S.B.; Mostafa-Hedeab, G.; Alsayegh, A.A.; Albogami, S.; et al. Luteolin-Rich Extract of Thespesia garckeana F. Hoffm. (Snot Apple) Contains Potential. Drug-Like Candidates and Modulates Glycemic and Oxidoinflammatory Aberrations in Experimental Animals. Oxidative Med. Cell. Longev. 2022, 2022, 1215097. [Google Scholar]
- Subash-Babu, P.; Abdulaziz AlSedairy, S.; Abdulaziz Binobead, M.; Alshatwi, A.A. Luteolin-7-O-rutinoside Protects RIN-5F Cells from High-Glucose-Induced Toxicity, Improves Glucose Homeostasis in L6 Myotubes, and Prevents Onset of Type 2 Diabetes. Metabolites 2023, 13, 269. [Google Scholar] [CrossRef]
- Sadeghi, M.; Shakouri Khomartash, M.; Taslimi, P. The Potential of C-Glycosylflavonoids as α-Glucosidase Inhibitors Determined by Virtual Screening, Molecular Docking, Molecular Dynamics, and IC50 Studies. ChemistrySelect 2023, 8, e202300847. [Google Scholar]
- Yuan, S.; Ye, Z.; Li, Y.; Zou, J.; Wu, M.; Wang, K.; Liao, W.; Shen, J. Hypoglycemic Effect of Nobiletin via Regulation of Islet beta-Cell Mitophagy and Gut Microbiota Homeostasis in Streptozocin-Challenged Mice. J. Agric. Food Chem. 2022, 70, 5805–5818. [Google Scholar]
- Zhang, J.; Wu, J.; Shi, X.; Li, D.; Yang, S.; Zhang, R.; Xia, B.; Yang, G. A Propolis-Derived Small Molecule Tectochrysin Ameliorates Type 2 Diabetes in Mice by Activating Insulin Receptor β. Mol. Nutr. Food Res. 2024, 68, e2300283. [Google Scholar]
- Feng, K.; Zhang, H.; Chen, C.; Ho, C.-T.; Kang, M.; Zhu, S.; Xu, J.; Deng, X.; Huang, Q.; Cao, Y. Heptamethoxyflavone Alleviates Metabolic Syndrome in High-Fat Diet-Fed Mice by Regulating the Composition, Function, and Metabolism of Gut Microbiota. J. Agric. Food Chem. 2023, 71, 10050–10064. [Google Scholar]
- Zhou, Q.Y.-J.; Liao, X.; Kuang, H.-M.; Li, J.-Y.; Zhang, S.-H. LC-MS Metabolite Profiling and the Hypoglycemic Activity of Morus alba L. Extracts. Molecules 2022, 27, 5360. [Google Scholar]
- Thi Ngoc Tram, N.; Thanh Duy, L.; Phuoc Long, N.; Duc Hanh, N.; Huynh Van Thi, N.; Tan Khanh, N.; Manh Hung, T.; Thi Hong Van, L. α-Glucosidase Inhibitory Activity and Quantitative Contribution of Phenolic Compounds from Vietnamese Aquilaria crassna Leaves. Nat. Product. Commun. 2022, 17, 1934578X221080326. [Google Scholar]
- Zhao, Y.; Li, T.; Kjaerulff, L.; Venter, H.; Coriani, S.; Moller, B.L.; Semple, S.; Staerk, D. Orthogonal Reversed-Phase C18 and Pentafluorophenyl HPLC Separation for Phytochemical Profiling of Serrulatanes in Eremophila denticulata. J. Nat. Prod. 2023, 86, 2638–2650. [Google Scholar]
- Wang, S.; Fan, Y.; Wang, M.; Tao, Y.; Lian, D.; Cui, J.; Li, L. Comparative analysis of the interaction of oroxylin A with two sources of α glucosidase and α amylase. J. Mol. Struct. 2023, 1292, 136176. [Google Scholar] [CrossRef]
- Sadeghi, M.; Miroliaei, M.; Rahimmalek, M.; Taslimi, P.; Szumny, A.; Sadeghian, N. Comparison of Flavonoid and Flavonoid Glycoside in the Inhibition of the Starch Hydrolyzing Enzymes and AGEs; A Virtual Approaches. J. Oleo Sci. 2023, 72, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Kan, R.; Ren, P.; Wu, A.; Tang, Q.; Kong, B.; Xue, C. Identification and molecular docking study of sugarcane leaf-derived compounds as potent dipeptidyl peptidase IV, α-glucosidase, and α-amylase inhibitors. J. Sci. Food Agric. 2023, 103, 5388–5400. [Google Scholar] [CrossRef]
- Yu, S.; Caruso, F.; Belli, S.; Rossi, M. Scavenging of Superoxide in Aprotic Solvents of Four Isoflavones That Mimic Superoxide Dismutase. Int. J. Mol. Sci. 2023, 24, 3815. [Google Scholar] [CrossRef]
- Pudhupalayam, S.P.; Uddandrao, V.V.S.; Ponnusamy, C.; Singaravel, S.; Periyasamy, T.; Ponnusamy, P.; Sasikumar, V.; Begum, M.S.; Ganapathy, S. Biochanin A Attenuates Hyperglycemia in High-Fat Diet-Streptozotocin-Induced Diabetic Rats by Modulating the Activities of Carbohydrate-Metabolizing Enzymes in Vital Organs. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2022, 32, 608–617. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. Daidzein attenuates urinary bladder dysfunction in streptozotocin-induced diabetes in rats by NOX-4 and RAC-1 inhibition. Naunyn-Schmiedebergs Arch. Pharmacol. 2022, 395, 975–986. [Google Scholar] [CrossRef]
- Qnais, E.; Alqudah, A.; Wedyan, M.; Gammoh, O.; Alkhateeb, H.; Al-Noaimi, M. Formononetin suppresses hyperglycaemia through activation of GLUT4-AMPK pathway. Pharmacia 2023, 70, 527–536. [Google Scholar] [CrossRef]
- Chen, H.; Lou, Y.; Lin, S.; Tan, X.; Zheng, Y.; Yu, H.; Jiang, R.; Wei, Y.; Huang, H.; Qi, X.; et al. Formononetin, a bioactive isoflavonoid constituent from Astragalus membranaceus (Fisch.) Bunge, ameliorates type 1 diabetes mellitus via activation of Keap1/Nrf2 signaling pathway: An integrated study supported by network pharmacology and experimental validation. J. Ethnopharmacol. 2024, 322, 117576. [Google Scholar]
- Yang, M.; Xia, L.; Song, J.; Hu, H.; Zang, N.; Yang, J.; Zou, Y.; Wang, L.; Zheng, X.; He, Q.; et al. Puerarin ameliorates metabolic dysfunction-associated fatty liver disease by inhibiting ferroptosis and inflammation. Lipids Health Dis. 2023, 22, 1–16. [Google Scholar] [CrossRef]
- Deng, K.; Chen, L.; Yang, L.; Hu, Y.; Xu, G.; Zhang, Y.; Wang, Y.; Ni, Q. Preparation of Isoflavones from the Tuber of Apios americana Medik. and Their Protective Effects on RIN-m5F Cells from Oxidative Damage. Food Sci. 2022, 43, 200–207. [Google Scholar]
- Balogun, F.O.; Singh, K.; Rampadarath, A.; Akoonjee, A.; Naidoo, K.; Sabiu, S. Cheminformatics identification of modulators of key carbohydrate-metabolizing enzymes from C. cujete for type-2 diabetes mellitus intervention. J. Diabetes Metab. Disord. 2023, 22, 1299–1317. [Google Scholar]
- Zheng, Y.; Zhang, R.; Huang, F.; Cheng, L.-H.; Xu, L.; Jia, X. α-Glucosidase inhibitors derived from black soybean and their inhibitory mechanisms. Lwt-Food Sci. Technol. 2023, 189, 115502. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.A.; Khan, A.A.; Almatroodi, S.A.A. Quercetin, a Plant Flavonol Attenuates Diabetic Complications, Renal Tissue Damage, Renal Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats. Metabolites 2023, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, Z.; ShahaniPour, K.; Monajemi, R.; Ahadi, A.M. Abelmoschus esculentus (L.) Moench improved blood glucose, lipid, and down-regulated PPAR-alpha, PTP1B genes expression in diabetic rats. J. Food Biochem. 2022, 46, e14097. [Google Scholar]
- Ke, R.Q.; Wang, Y.; Hong, S.H.; Xiao, L.X. Anti-diabetic effect of quercetin in type 2 diabetes mellitus by regulating the microRNA-92B-3P/EGR1 axis. J. Physiol. Pharmacol. 2023, 74, 149–158. [Google Scholar]
- Yamashita, Y.; Jiang, H.; Okada, F.; Kitakaze, T.; Yoshioka, Y.; Ashida, H. Single oral administration of quercetin glycosides prevented acute hyperglycemia by promoting GLUT4 translocation in skeletal muscles through the activation of AMPK in mice. J. Clin. Biochem. Nutr. 2023, 74, 37. [Google Scholar]
- Zhou, J.-F.; Xu, H.-X.; Yin, Z.-P.; Chen, J.-G.; Zhang, Q.-F. The combination effects of quercetin on starch and digestive enzymes reduce postprandial blood glucose in rats. Eur. Food Res. Technol. 2024, 250, 1189–1199. [Google Scholar]
- Gozcu, S.; Ugan, R.A.; Ozbek, H.; Gundogdu, B.; Guvenalp, Z. Evaluation of antidiabetic and antioxidant effects of Polygonum cognatum Meisn. and phytochemical analysis of effective extracts. Biomed. Chromatogr. 2023, 38, e5809. [Google Scholar]
- Alruhaimi, R.S.; Mostafa-Hedeab, G.; Abduh, M.S.; Bin-Ammar, A.; Hassanein, E.H.M.; Kamel, E.M.; Mahmoud, A.M. DataSheet1_A flavonoid-rich fraction of Euphorbia peplus attenuates hyperglycemia, insulin resistance, and oxidative stress in a type 2 diabetes rat model. Front. Pharmacol. 2023, 14, 1204641. [Google Scholar]
- Putra, N.; Garmana, A.N.; Qomaladewi, N.P.; Amrianto; Al Muqarrabun, L.M.R.; Rosandy, A.R.; Chahyadi, A.; Insanu, M.; Elfahmi. Bioactivity-guided isolation of a bioactive compound with aglucosidase inhibitory activity from the leaves extract of Sauropus androgynus. Sustain. Chem. Pharm. 2023, 31, 100907. [Google Scholar]
- Zhang, F.; Yang, M.; Xu, J.; Hu, Y.; Gao, R.; Huang, K.; He, X. Coreopsis tinctoria and Its Flavonoids Ameliorate Hyperglycemia in Obese Mice Induced by High-Fat Diet. Nutrients 2022, 14, 1160. [Google Scholar] [CrossRef]
- Moore, W.T.; Luo, J.; Liu, D. Kaempferol improves glucose uptake in skeletal muscle via an AMPK-dependent mechanism. Food Sci. Human. Wellness 2023, 12, 2087–2094. [Google Scholar] [CrossRef]
- Li, N.; Yin, L.; Shang, J.; Liang, M.; Liu, Z.; Yang, H.; Qiang, G.; Du, G.; Yang, X. Kaempferol attenuates nonalcoholic fatty liver disease in type 2 diabetic mice via the Sirt1/AMPK signaling pathway. Biomed. Pharmacother. 2023, 165, 115113. [Google Scholar] [CrossRef]
- Kiziltas, H. Comprehensive evaluation of Reseda lutea L. (Wild Mignonette) and 7 isolated flavonol glycosides: Determination of antioxidant activity, anti-Alzheimer, antidiabetic and cytotoxic effects with in vitro and in silico methods. Turk. J. Chem. 2022, 46, 1185–1198. [Google Scholar]
- Calzada, F.; Valdes, M.; Martinez-Solis, J.; Velazquez, C.; Barbosa, E. Annona cherimola Miller and Its Flavonoids, an Important Source of Products for the Treatment of Diabetes Mellitus: In Vivo and In Silico Evaluations. Pharmaceuticals 2023, 16, 724. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, J.; Chen, Y.; Dai, C.; Fan, J.; Guo, H. Hypoglycemic activity and mechanisms of myricetin. Nat. Prod. Res. 2022, 36, 6177–6180. [Google Scholar]
- Zhou, X.; Liu, Z.; Yang, X.; Feng, J.; Gins, M.S.; Yan, T.; Han, L.; Zhang, H. The Mechanism Underlying the Hypoglycemic Effect of Epimedin C on Mice with Type 2 Diabetes Mellitus Based on Proteomic Analysis. Nutrients 2024, 16, 25. [Google Scholar] [CrossRef]
- Zou, T.-F.; Liu, Z.-G.; Cao, P.-C.; Zheng, S.-H.; Guo, W.-T.; Wang, T.-X.; Chen, Y.-L.; Duan, Y.-J.; Li, Q.-S.; Liao, C.-Z.; et al. Fisetin treatment alleviates kidney injury in mice with diabetes-exacerbated atherosclerosis through inhibiting CD36/fibrosis pathway. Acta Pharmacol. Sin. 2023, 44, 2065–2074. [Google Scholar] [CrossRef]
- Bahramzadeh, A.; Samavarchi Tehrani, S.; Goodarzi, G.; Seyyedebrahimi, S.; Meshkani, R. Combination therapy of metformin and morin attenuates insulin resistance, inflammation, and oxidative stress in skeletal muscle of high-fat diet-fed mice. Phytother. Res. 2024, 38, 912–924. [Google Scholar] [CrossRef]
- Mazraesefidi, M.; Mahmoodi, M.; Hajizadeh, M. Effects of silibinin on apoptosis and insulin secretion in rat RINm5F pancreatic beta-cells. Biotech. Histochem. 2023, 98, 201–209. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Altaber, S.; Bseiso, Y.; Oqal, M.; AbuDalo, R.; Alrosan, K.; Alrosan, A.Z.; Melhim, S.B.; et al. Isorhamnetin Reduces Glucose Level, Inflammation, and Oxidative Stress in High-Fat Diet/Streptozotocin Diabetic Mice Model. Molecules 2023, 28, 502. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Tao, Y.; Wang, S.; Wang, M.; Li, L. Inhibitory interaction of narcissoside on alpha-glucosidase from Aspergillus niger and Saccharomyces cerevisiae by spectral analysis and molecular docking. J. Mol. Struct. 2022, 1263, 133262. [Google Scholar] [CrossRef]
- Asmara, A.P.; Prasansuklab, A.; Chiabchalard, A.; Chen, H.; Ung, A.T. Antihyperglycemic Properties of Extracts and Isolated Compounds from Australian Acacia saligna on 3T3-L1 Adipocytes. Molecules 2023, 28, 4054. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.; Luti, S.; Pardella, E.; Vivoli-Vega, M.; Pazzagli, L.; Parri, M.; Caselli, A.; Cirri, P.; Paoli, P. Differential impact of cold and hot tea extracts on tyrosine phosphatases regulating insulin receptor activity: A focus on PTP1B and LMW-PTP. Eur. J. Nutr. 2022, 61, 1905–1918. [Google Scholar] [CrossRef]
- Ni, J.; Wen, X.; Wang, S.; Zhou, X.; Wang, H. Investigation of the inhibitory combined effect and mechanism of (-)-epigallocatechin gallate and chlorogenic acid on amylase: Evidence of synergistic interaction. Food Biosci. 2023, 56, 103406. [Google Scholar] [CrossRef]
- Le, T.T.; Ha, M.T.; Cao, T.Q.; Kim, J.A.; Choi, J.S.; Min, B.S. 1,5-Anhydro-D-glucitol derivative and galloylated flavonoids isolated from the leaves of Acer ginnala Maxim. as dual inhibitors of PTP1B and α-glucosidase enzymes: In vitro and in silico studies. Phytochemistry 2023, 213, 113769. [Google Scholar] [CrossRef]
- Wei, X.; Lin, L.; Yuan, Q.-q.; Wang, X.-y.; Zhang, Q.; Zhang, X.-m.; Tang, K.-c.; Guo, M.-y.; Dong, T.-y.; Han, W.; et al. Bavachin protects against diet-induced hepatic steatosis and obesity in mice. Acta Pharmacol. Sin. 2023, 44, 1416–1428. [Google Scholar] [CrossRef]
- Hu, K.; Sun, Y.; Wang, J.; Wu, S.; Ren, J.; Su, D.; Tang, L.; Gong, J.; Fang, H.; Xu, S.; et al. Integrating network analysis and experimental validation to reveal the mechanism of pinocembrin in alleviating high glucose and free fatty acid-induced lipid accumulation in HepG2 cells. J. Funct. Food. 2023, 110, 105879. [Google Scholar]
- Ahmed, O.M.; Saleh, A.S.; Ahmed, E.A.; Ghoneim, M.M.; Ebrahim, H.A.; Abdelgawad, M.A.; Abdel-Gabbar, M. Efficiency of Bone Marrow-Derived Mesenchymal Stem Cells and Hesperetin in the Treatment of Streptozotocin-Induced Type 1 Diabetes in Wistar Rats. Pharmaceuticals 2023, 16, 859. [Google Scholar] [CrossRef]
- Adebayo, M.A.; Kolawole, A.N.; Falese, B.A.; Kolawole, A.O. Spectroscopic and in silico evaluation of hesperetin, aglycone flavanone, as a prospective regulatory ligand for human salivary a-amylase. J. Biomol. Struct. Dyn. 2023, 42, 3177–3192. [Google Scholar]
- Aldemir, E.E.; Selmanoglu, G.; Karacaoglu, E. The Potential Protective Role of Neoeriocitrin in a Streptozotocin-Induced Diabetic Model in INS-1E Pancreatic ß-Cells. Braz. Arch. Biol. Technol. 2023, 66, e23220138. [Google Scholar]
- Kaliaperumal, K.; Zhang, L.; Gao, L.; Xiong, Q.; Liang, Y.; Jiang, Y.; Zhang, J. Insight into the Inhibitory Mechanisms of Hesperidin on α-Glucosidase through Kinetics, Fluorescence Quenching, and Molecular Docking Studies. Foods 2023, 12, 4142. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Sim, K.-S.; Hwangbo, Y.; Park, S.-J.; Kim, Y.-J.; Kim, J.-H. Naringenin and Phytoestrogen 8-Prenylnaringenin Protect against Islet Dysfunction and Inhibit Apoptotic Signaling in Insulin-Deficient Diabetic Mice. Molecules 2022, 27, 4227. [Google Scholar] [CrossRef]
- Khan, M.F.; Mathur, A.; Pandey, V.K.; Kakkar, P. Endoplasmic reticulum stress-dependent activation of TRB3-FoxO1 signaling pathway exacerbates hyperglycemic nephrotoxicity: Protection accorded by Naringenin. Eur. J. Pharmacol. 2022, 917, 174745. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, X.; Zhu, B.; Gao, J.; Yin, D.; Zeng, J.; Kang, Z. Naringenin protects pancreatic β cells in diabetic rat through activation of estrogen receptor β. Eur. J. Pharmacol. 2023, 960, 176115. [Google Scholar]
- Maity, S.; Chakrabarti, P.; Chakraborti, A.S. Naringenin ameliorates palmitic acid-induced fatty acid stress in hepatocytes. Nat. Prod. J. 2022, 12, 2–8. [Google Scholar] [CrossRef]
- Jia, A.; Wang, L.; Jin, J.; Huang, C.; Hu, X. A new sanggenon-type flavanone from Morus nigra and its insulin-sensitizing activity. Chin. Tradit. Herbal. Drugs 2023, 54, 45–50. [Google Scholar]
- Yang, Y.; Qiu, W.; Xiao, J.; Sun, J.; Ren, X.; Jiang, L. Dihydromyricetin ameliorates hepatic steatosis and insulin resistance via AMPK/PGC-1alpha and PPARalpha-mediated autophagy pathway. J. Transl. Med. 2024, 22, 309. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Chen, R.; Shen, J.; Zhang, S.; Gu, Y.; Shi, J.; Meng, G. Dihydromyricetin attenuates diabetic cardiomyopathy by inhibiting oxidative stress, inflammation and necroptosis via sirtuin 3 activation. Antioxidants 2023, 12, 200. [Google Scholar] [CrossRef]
- Shou, L.; Zhou, L.; Hu, J.; Zhu, Q.; Luo, H. Sanggenon C alleviates palmitic acid-induced insulin resistance in HepG2 cells via AMPK pathway. Trop. J. Pharm. Res. 2023, 22, 1361–1366. [Google Scholar] [CrossRef]
- Long, H.P.; Liu, J.; Xu, P.S.; Xu, K.P.; Li, J.; Tan, G.S. Hypoglycemic flavonoids from Selaginella tamariscina (P.Beauv.) Spring. Phytochemistry 2022, 195, 113073. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wang, B.; Sun, M.; Li, S. Attenuation of glucolipotoxicity-evoked dysfunction and insulin resistance by amentoflavone involves in AMPK/Nrf2 signaling in cardiomyocytes. J. Biol. Regul. Homeost. Agents 2023, 37, 3333–3342. [Google Scholar]
- Xu, J.; Wang, Y.; Zheng, T.; Huo, Y.; Du, W. Biflavones inhibit the fibrillation and cytotoxicity of the human islet amyloid polypeptide. J. Mater. Chem. B 2022, 10, 4650–4661. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Wang, M.; Ma, X.; Peng, X. Studies on the inhibition of a-glucosidase by biflavonoids and their interaction mechanisms. Food Chem. 2023, 420, 136113. [Google Scholar] [CrossRef]
- Sadeghi, M.; Miroliaei, M.; Ghanadian, M.; Szumny, A.; Rahimmalek, M. Exploring the inhibitory properties of biflavonoids on α-glucosidase; computational and experimental approaches. Int. J. Biol. Macromol. 2023, 253, 127380. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Wang, D.; Yang, L.; Ding, W.; Chen, Z.; Yang, X.; Jiang, Y.; Liu, Y. Licochalcone A alleviates abnormal glucolipid metabolism and restores energy homeostasis in diet-induced diabetic mice. Phytother. Res. 2024, 38, 196–213. [Google Scholar] [CrossRef]
- Tao, F.; Wang, P.; Dai, Z.; Zhang, C.; Li, D.; Fan, H.; Qin, M.; Qi, C.; Hao, J. Neohesperidin dihydrochalcone improves hyperglycemia and insulin resistance in diabetic zebrafish. ACS Food Sci. Technol. 2023, 3, 1548–1558. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I. Aspalathin and other rooibos flavonoids trapped α-dicarbonyls and inhibited formation of advanced glycation end products in vitro. Int. J. Mol. Sci. 2022, 23, 14738. [Google Scholar] [CrossRef]
- Zhao, H.; Zhai, B.-W.; Zhang, M.Y.; Huang, H.; Zhu, H.L.; Yang, H.; Ni, H.-Y.; Fu, Y.-J. Phlorizin from Lithocarpus litseifolius Hance Chun ameliorates FFA-induced insulin resistance by regulating AMPK/PI3K/AKT signaling pathway. Phytomedicine 2024, 130, 155743. [Google Scholar] [CrossRef]
- Ye, X.; Chen, W.; Huang, X.-F.; Yan, F.-J.; Deng, S.-G.; Zheng, X.-D.; Shan, P.-F. Anti-diabetic effect of anthocyanin cyanidin-3-O-glucoside: Data from insulin resistant hepatocyte and diabetic mouse. Nutr. Diabetes 2024, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, W.; Yan, F.-J.; Zheng, X.-D.; Tu, P.-C.; Shan, P.-F. Exploring the effects of cyanidin-3-O-glucoside on type 2 diabetes mellitus: Insights into gut microbiome modulation and potential antidiabetic benefits. J. Agric. Food Chem. 2023, 71, 20047–20061. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Su, L.; Hu, Q.; Li, W.; He, J.; Zhao, L. Cyanidin-3-O-glucoside ameliorates palmitic-acid-induced pancreatic β cell dysfunction by modulating CHOP-mediated endoplasmic reticulum stress pathways. Nutrients 2022, 14, 1835. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tian, Y.; Wang, L.; Tan, J. Activity guided-assisted high-speed counter-current chromatography separation of active compound from blueberry and the interaction between the active compound and α-glucosidase. J. Chin. Inst. Food Sci. Technol. 2023, 23, 41–53. [Google Scholar]
- Biswas, D.; De Silva, A.B.K.H.; Mercer, A.; Sarkar, S.; Kienesberger, P.; Langille, M.; Rupasinghe, H.P.V.; Pulinilkunnil, T. Supplementation of cyanidin-3-O-β-glucoside-rich haskap (Lonicera caerulea L.) berry extract attenuates hepatic lipid dysregulation in diet-induced obese mice. J. Funct. Food. 2023, 108, 105635. [Google Scholar] [CrossRef]
- Kenneth, C.; Anugrah, D.S.B.; Julianus, J.; Junedi, S. Molecular insights into the inhibitory potential of anthocyanidins on glucokinase regulatory protein. PLoS ONE 2023, 18, e0288810. [Google Scholar] [CrossRef]
- Zhu, C.-w.; Lu, H.; Du, L.-l.; Li, J.; Chen, H.; Zhao, H.-f.; Wu, W.-l.; Chen, J.; Li, W.-l. Five blueberry anthocyanins and their antioxidant, hypoglycemic, and hypolipidemic effects in vitro. Front. Nutr. 2023, 10, 1172982. [Google Scholar] [CrossRef]
- Peng, J.; Liang, G.; Wen, W.; Huang, W.; Qiu, Y.; Xiao, G.; Wang, Q. Blueberry anthocyanins extract inhibits advanced glycation end-products (AGEs) production and AGEs-stimulated inflammation in RAW264.7 cells. J. Sci. Food Agric. 2024, 104, 75–82. [Google Scholar] [CrossRef]
- Lim, H.J.; Park, J.E.; Han, J.S. HM-chromanone alleviates hyperglycemia and inflammation in mice with endotoxin-induced insulin resistance. Toxicol. Res. 2023, 12, 665–674. [Google Scholar] [CrossRef]
- Yoo, J.; Park, J.E.; Han, J.S. HMC Ameliorates Hyperglycemia via Acting PI3K/AKT Pathway and Improving FOXO1 Pathway in ob/ob Mice. Nutrients 2023, 15, 2023. [Google Scholar] [CrossRef]
- Park, J.E.; Han, J.S. HM-chromanone isolated from Portulaca oleracea L. alleviates insulin resistance and inhibits gluconeogenesis by regulating palmitate-induced activation of ROS/JNK in HepG2 cells. Toxicol. Res. 2023, 12, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Awotuya, I.O.; Fakola, E.G.; Olusola, A.J.; Olanudun, E.A.; Bello, O.I.; Ogunremi, B.I.; Gboyero, F.O.; Adesida, S.A.; Faloye, K.O. Exploring the Protein Tyrosine Phosphatase 1B Inhibitory Potentials of Naturally Occurring Brazilin-Type Homoisoflavonoids: A Computational Approach. Chem. Afr. 2022, 5, 1493–1502. [Google Scholar]
- Osama, H.; Hamed, E.O.; Mahmoud, M.A.; Abdelrahim, M.E.A. The Effect of Hesperidin and Diosmin Individually or in Combination on Metabolic Profile and Neuropathy among Diabetic Patients with Metabolic Syndrome: A Randomized Controlled Trial. J. Diet. Suppl. 2023, 20, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Moradi, L.; Zaman, F.; Zare Javid, A. The effects of rutin flavonoid supplement on glycemic status, lipid profile, atherogenic index of plasma, brain-derived neurotrophic factor (BDNF), some serum inflammatory, and oxidative stress factors in patients with type 2 diabetes mellitus: A double-blind, placebo-controlled trial. Phytother. Res. 2023, 37, 271–284. [Google Scholar]
- Ferdowsi, S.; Shidfar, F.; Heidari, I.; Kashi, M.; Sohouli, M.H.; Sarrafi Zadeh, S. Effect of silymarin on lipid profile and glycemic control in patients with type 2 diabetes mellitus. Phytother. Res. 2024, 38, 4667–4674. [Google Scholar] [CrossRef]
- Curtis, P.J.; Berends, L.; van der Velpen, V.; Jennings, A.; Haag, L.; Chandra, P.; Kay, C.D.; Rimm, E.B.; Cassidy, A. Blueberry anthocyanin intake attenuates the postprandial cardiometabolic effect of an energy-dense food challenge: Results from a double blind, randomized controlled trial in metabolic syndrome participants. Clin. Nutr. 2022, 41, 165–176. [Google Scholar]
- Cesar, T.B.; Ramos, F.M.M.; Ribeiro, C.B. Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial. J. Med. Food 2022, 25, 1050–1058. [Google Scholar]
- Ramos, F.M.M.; Ribeiro, C.B.; Cesar, T.B.; Milenkovic, D.; Cabral, L.; Noronha, M.F.; Sivieri, K. Lemon flavonoids nutraceutical (Eriomin®) attenuates prediabetes intestinal dysbiosis: A double-blind randomized controlled trial. Food Sci. Nutr. 2023, 11, 7283–7295. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Iglesias, D.E.; Kang, J.; Wang, Z.; Gray, R.; Mastaloudis, A.; Kay, C.D.; Hester, S.N.; Wood, S.M.; et al. A randomized placebo-controlled cross-over study on the effects of anthocyanins on inflammatory and metabolic responses to a high-fat meal in healthy subjects. Redox Biol. 2022, 51, 102273. [Google Scholar]
- Teets, C.; Ghanem, N.; Ma, G.; Minj, J.; Perkins-Veazie, P.; Johnson, S.A.; Etter, A.J.; Carbonero, F.G.; Solverson, P.M. A One-Week Elderberry Juice Intervention Augments the Fecal Microbiota and Suggests Improvement in Glucose Tolerance and Fat Oxidation in a Randomized Controlled Trial. Nutrients 2024, 16, 3555. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Li, H.; Shi, S.; Peng, X. Mechanistic study and synergistic effect on inhibition of α-amylase by structurally similar flavonoids. J. Mol. Liq. 2022, 360, 119485. [Google Scholar]
- Qin, Y.; Wang, P.; Chen, X.; Xu, F.; Zhu, K.; Zhang, Y.; Tan, L. Inhibition Mechanism of Three Flavonoids on alpha-Amylase. J. Food Sci. Technol. 2023, 41, 110–122. [Google Scholar]
- Akinnusi, P.A.; Olubode, S.O.; Alade, A.A.; Ashimi, A.A.; Onawola, O.L.; Agbolade, A.O.; Emeka, A.P.; Shodehinde, S.A.; Adeniran, O.Y. Potential Inhibitory Biomolecular Interactions of Natural Compounds with Different Molecular Targets of Diabetes. Bioinform. Biol. Insights 2023, 17, 11779322231167970. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.A.; Omar, A.M.M.; El-Araby, M.E.E.; Mass, S.; Ibrahim, S.R.M. Assessments of Alpha-Amylase Inhibitory Potential of Tagetes Flavonoids through In Vitro, Molecular Docking, and Molecular Dynamics Simulation Studies. Int. J. Mol. Sci. 2023, 24, 10195. [Google Scholar] [CrossRef]
- Guo, F.; An, J.; Wang, M.; Zhang, W.; Chen, C.; Mao, X.; Liu, S.; Wang, P.; Ren, F. Inhibitory Mechanism of Quercimeritrin as a Novel α-Glucosidase Selective Inhibitor. Foods 2023, 12, 3415. [Google Scholar] [CrossRef]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human α-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef]
- Tonsic, B.R.; Correa, V.G.; Garcia-Manieri, J.A.A.; Bracht, A.; Peralta, R.M. An in vivo approach to the reported effects of phenolic acids and flavonoids on the pancreatic α-amylase activity. Food Biosci. 2023, 51, 102357. [Google Scholar] [CrossRef]
- Yang, J.C.; Wang, X.L.; Zhang, C.Y.; Ma, L.; Wei, T.; Zhao, Y.J.; Peng, X. Comparative study of inhibition mechanisms of structurally different flavonoid compounds on α-glucosidase and synergistic effect with acarbose. Food Chem. 2021, 347, 129056. [Google Scholar]
- Zhao, L.; Pei, Y.; Zhang, G.; Li, J.; Zhu, Y.; Xia, M.; Yan, K.; Mu, W.; Han, J.; Zhang, S.; et al. Efficient Synthesis and In Vitro Hypoglycemic Activity of Rare Apigenin Glycosylation Derivatives. Molecules 2023, 28, 533. [Google Scholar] [CrossRef]
- Proenca, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tome, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar]
- Rani, J.; Jagta, N.; Deswal, G.; Chopra, B.; Dhingra, A.K.; Guarve, K.; Grewal, A.S. Molecular docking-guided screening of phytoconstituents from Artemisia princeps as Allosteric Glucokinase Activators. Chem. Biol. Lett. 2023, 10, 547. [Google Scholar]
- Pustelny, K.; Grygier, P.; Barzowska, A.; Pucelik, B.; Matsuda, A.; Mrowiec, K.; Slugocka, E.; Popowicz, G.M.; Dubin, G.; Czarna, A. Binding mechanism and biological effects of flavone DYRK1A inhibitors for the design of new antidiabetics. Sci. Rep. 2023, 13, 18114. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Zhi, Y.; Tian, F.; Huang, F.; Cheng, X.; Wu, A.; Tao, L.; Guo, Z.; Shen, X. Ameliorative effect of black raspberry anthocyanins on diabetes retinopathy by inhibiting axis protein tyrosine phosphatase 1B-endoplasmic reticulum stress. J. Funct. Food. 2023, 107, 105696. [Google Scholar] [CrossRef]
- Liang, C.; Zang, J.; Ndi, C.; Semple, S.J.; Buirchell, B.; Coriani, S.; Moller, B.L.; Staerk, D. Identification of new PTP1B-inhibiting decipiene diterpenoid esters from Eremophila clarkei by high-resolution PTP1B inhibition profiling, enzyme kinetics analysis, and molecular docking. Bioorg. Chem. 2023, 139, 106744. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, H.; Liu, K.; Yang, X.; Xing, S.; Li, W. Unveiling Anti-Diabetic Potential of Baicalin and Baicalein from Baikal Skullcap: LC-MS, In Silico, and In Vitro Studies. Int. J. Mol. Sci. 2024, 25, 3654. [Google Scholar] [CrossRef]
- Shi, X.Y.; Chu, C.; Li, X.; Zhang, L.X.; Zhang, X.R.; Chen, N.; Liu, W.W.; Jiao, Z.X.; Ikejima, T.; Xu, F.X. Silibinin protects GLUTag cells from PA-induced injury via suppressing endoplasmic reticulum stress. Cyta J. Food 2023, 21, 442–450. [Google Scholar] [CrossRef]
- Zeng, X.; Na, R.; Yang, L.; Zhao, X.; Huang, X. Inhibition mechanism understanding from molecular dynamics simulation of the interactions between several flavonoids and proton-dependent glucose transporter. J. Biomol. Struct. Dyn. 2023, 43, 1278–1289. [Google Scholar] [CrossRef]
- Farzana, M.; Hossain, M.J.; El-Shehawi, A.M.; Sikder, M.A.A.; Rahman, M.S.; Al-Mansur, M.A.; Albogami, S.; Elseehy, M.M.; Roy, A.; Uddin, M.A.; et al. Phenolic Constituents from Wendlandia tinctoria var. grandis (Roxb.) DC. Stem Deciphering Pharmacological Potentials against Oxidation, Hyperglycemia, and Diarrhea: Phyto-Pharmacological and Computational Approaches. Molecules 2022, 27, 5957. [Google Scholar] [CrossRef]
- Ollinger, N.; Neuhauser, C.; Schwarzinger, B.; Wallner, M.; Schwarzinger, C.; Blank-Landeshammer, B.; Hager, R.; Sadova, N.; Drotarova, I.; Mathmann, K.; et al. Anti-Hyperglycemic Effects of Oils and Extracts Derived from Sea Buckthorn—A Comprehensive Analysis Utilizing In Vitro and In Vivo Models. Mol. Nutr. Food Res. 2022, 66, e2101133. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, T.; Wei, J.; Yin, X.; Gao, Z.; Li, H. Inhibitory activities of flavonoids from Scutellaria baicalensis Georgi on amyloid aggregation related to type 2 diabetes and the possible structural requirements for polyphenol in inhibiting the nucleation phase of hIAPP aggregation. Int. J. Biol. Macromol. 2022, 215, 531–540. [Google Scholar] [CrossRef]
- King, K.M.; Bevan, D.R.; Brown, A.M. Molecular Dynamics Simulations Indicate Aromaticity as a Key Factor in the Inhibition of IAPP((20–29)) Aggregation. Acs Chem. Neurosci. 2022, 13, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Wu, X.; Guan, Y.; Lin, J.; Sun, Y.; Hu, M.; Xiao, P.; He, C.; Jiang, B. Integration of network pharmacology, transcriptomics and molecular docking reveals two novel hypoglycemic components in snow chrysanthemum. Biomed. Pharmacother. 2023, 163, 114818. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-L.; Tseng, Y.-H.; Cheng, P.-W.; Hao, C.-L.; Tu, Y.-C.; Yeh, B.-C.; Shen, K.-P. Content Analysis of Chenopodium formosanum (Djulis) Collected from Two Production Areas and Evaluation of Their Ability to Improve Insulin Resistance. Nat. Product. Commun. 2023, 18, 1934578X231179416. [Google Scholar] [CrossRef]
- Yu, W.; Zhong, J.Q.; Zhang, Z.Y.; Ge, S.X. Leptin-adiponectin ratio: A reliable indicator in metabolic health. Chin. Bull. Life Sci. 2024, 36, 658–668. [Google Scholar]
- Tang, Y.; Zhou, J.; Mo, Y.; Zhu, P.; Yang, Q.; Li, X.; Li, L. Effect of Total Flavonoids from Melastoma dodecandrum L. on Lipid Metabolism of Nonalcoholic Fatty Liver Rats. induced by High-fat Diet. Chin. Anim. Husb. Vet. Med. 2023, 50, 3438–3447. [Google Scholar]
- Dwivedi, P.S.R.; Rasal, V.P.; Chavan, R.S.; Khanal, P.; Gaonkar, V.P. Insulin sensitization by Feronia elephantum in fructose-induced hyperinsulinemic rats: Insights from computational and experimental pharmacology. J. Ethnopharmacol. 2023, 316, 116686. [Google Scholar] [CrossRef]
- Guo, S.D. Insulin signaling, resistance, and metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, H.; Cheong, M.S.; Zhong, R.; Garcia-oliveira, P.; Prieto, M.A.; Cheng, K.-w.; Wang, M.; Cao, H.; Nie, S.; et al. Anti-diabetic potential of apigenin, luteolin, and baicalein via partially activating PI3K/Akt/GLUT-4 signaling pathways in insulin-resistant HepG2 cells. Food Sci. Human. Wellness 2023, 12, 1991–2000. [Google Scholar] [CrossRef]
- Jin, D.-X.; He, J.-F. PI3K/AKT Signaling Pathway-Mediated Three Flavonoids’ Modulation on Glucose Metabolism. Rev. Bras. De Farmacogn. Braz. J. Pharmacogn. 2022, 32, 834–839. [Google Scholar] [CrossRef]
- Tang, P.; Tang, Y.; Liu, Y.; He, B.; Shen, X.; Zhang, Z.-J.; Qin, D.-L.; Tian, J. Quercetin-3-O-α-L-arabinopyranosyl-(1→2)-β-D-glucopyranoside Isolated from Eucommia ulmoides Leaf Relieves Insulin Resistance in HepG2 Cells via the IRS-1/PI3K/Akt/GSK-3β Pathway. Biol. Pharm. Bull. 2023, 46, 219–229. [Google Scholar] [CrossRef]
- Taylor, E.B.; An, D.; Kramer, H.F.; Yu, H.; Fujii, N.L.; Roeckl, K.S.C.; Bowles, N.; Hirshman, M.F.; Xie, J.; Feener, E.P.; et al. Discovery of TBC1D1 as an Insulin-, AICAR-, and Contraction-stimulated Signaling Nexus in Mouse Skeletal Muscle. J. Biol. Chem. 2008, 283, 9787–9796. [Google Scholar] [PubMed]
- Long, Y.C.; Cheng, Z.; Copps, K.D.; White, M.F. Insulin Receptor Substrates Irs1 and Irs2 Coordinate Skeletal Muscle Growth and Metabolism via the Akt and AMPK Pathways. Mol. Cell. Biol. 2023, 31, 430–441. [Google Scholar]
- Pandey, V.K.; Mathur, A.; Khan, M.F.; Kakkar, P. Endoplasmic reticulum stress induces degradation of glucose transporter proteins during hyperglycemic hepatotoxicity: Role of PERK-eIF2 alpha-ATF4 axis. Eur. J. Pharmacol. 2022, 926, 175012. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.-H.; Lin, S.-Y.; Chen, L.-L.; Ouyang, K.-H.; Wang, W.-J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Z.; Zhao, L.; Chen, L.; Yu, Y.; Wang, D.; Mao, X.; Cao, G.; Zhao, Z.; Yang, H. Unique roles in health promotion of dietary flavonoids through gut microbiota regulation: Current understanding and future perspectives. Food Chem. 2023, 399, 133959. [Google Scholar]
- Watanabe, S.; Namba, F.; Suzuki, T.; Kosaka, H.; Toda, T. Agent or Additive Used for e.g., Improving Intestinal Flora for Improving Decrease in Diversity of Intestinal Flora, and Reducing Secondary Bile Acid Production, and Increasing Primary Bile Acid Production, Comprises Soy Isoflavone. JP2023080372-A, 27 April 2023. [Google Scholar]
- Wang, P.; Tao, F.; Dai, Z.; Wang, T.; Zhang, C.; Fan, H.; Qin, M.; Qi, C.; Li, Y.; Hao, J. Neohesperidin dihydrochalcone can improve intestinal structure and microflora composition of diabetic zebrafish. J. Funct. Food. 2024, 115, 106118. [Google Scholar]
- Qiao, Y.; Zhang, Z.; Zhai, Y.; Yan, X.; Zhou, W.; Liu, H.; Guan, L.; Peng, L. Apigenin Alleviates Obesity-Associated Metabolic Syndrome by Regulating the Composition of the Gut Microbiome. Front. Microbiol. 2022, 12, 805827. [Google Scholar]
- Watanabe, Y.; Fujisaka, S.; Morinaga, Y.; Watanabe, S.; Nawaz, A.; Hatta, H.; Kado, T.; Nishimura, A.; Bilal, M.; Aslam, M.R.; et al. Isoxanthohumol improves obesity and glucose metabolism via inhibiting intestinal lipid absorption with a bloom of Akkermansia muciniphila in mice. Mol. Metab. 2023, 77, 101797. [Google Scholar] [CrossRef]
- Yuan, M.; Sun, T.; Zhang, Y.; Guo, C.; Wang, F.; Yao, Z.; Yu, L. Quercetin Alleviates Insulin Resistance and Repairs Intestinal Barrier in db/db Mice by Modulating Gut Microbiota. Nutrients 2024, 16, 1870. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Liu, W.; Liu, Y.; Afzal, S.; Grover, J.; Chang, D.; Munch, G.; Li, C.G.; Lin, S.; et al. Protective effect of the curcumin-baicalein combination against macrovascular changes in diabetic angiopathy. Front. Endocrinol. 2022, 13, 953305. [Google Scholar] [CrossRef]
- Koh, Y.Q.; Sin, Y.A.D.; Rong, H.J.; Chua, T.H.S.; Ho, S.-H.S.; Ho, H.K. Evaluation of anthoxanthins and their actions on digestive enzyme inhibition when used independently and in combination. Heliyon 2022, 8, e10131. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.-S.; Giordani, C.; Antonelli, A.; Petrelli, M.; Balercia, G.; Silvetti, F.; Pieroni, A.; Sabbatinelli, J.; Rippo, M.R.; Olivieri, F.; et al. The Combination of Natural Molecules Naringenin, Hesperetin, Curcumin, Polydatin and Quercetin Synergistically Decreases SEMA3E Expression Levels and DPPIV Activity in In Vitro Models of Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 8071. [Google Scholar] [CrossRef] [PubMed]
- Al-Abbasi, F.A.; Kazmi, I. Therapeutic role of kaempferol and myricetin in streptozotocin-induced diabetes synergistically via modulation in pancreatic amylase, glycogen storage and insulin secretion. Mol. Cell. Biochem. 2023, 478, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; McGillivray, D.J. Recent advances to improve curcumin oral bioavailability. Trends Food Sci. Technol. 2021, 110, 253–266. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, C. In Silico, In Vitro, and In Vivo Evaluation of the Developmental Toxicity, Estrogenic Activity, and Mutagenicity of Four Natural Phenolic Flavonoids at Low Exposure Levels. ACS Omega 2022, 7, 4757–4768. [Google Scholar] [CrossRef]
- Sarasquete, C.; Ubeda-Manzanaro, M.; Ortiz-Delgado, J.B. Toxicity and non-harmful effects of the soya isoflavones, genistein and daidzein, in embryos of the zebrafish, Danio rerio. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 211, 57–67. [Google Scholar] [CrossRef]
- Siblini, H.; Al-Hendy, A.; Segars, J.; Gonzalez, F.; Taylor, H.S.; Singh, B.; Flaminia, A.; Flores, V.A.; Christman, G.M.; Huang, H.; et al. Assessing the Hepatic Safety of Epigallocatechin Gallate (EGCG) in Reproductive-Aged Women. Nutrients 2023, 15, 320. [Google Scholar] [CrossRef]
- Jamir, A.; Longkumer, S.; Punj, P.P. Acute Toxicity of Epigallocatechin Gallate and C-Phycocyanin in Zebrafish (Danio rerio): Behavioral and Histopathological Studies. India J. Pharm. Sci. 2023, 86, 546–555. [Google Scholar]
- Zhou, G.; Zhang, M.; Sun, X.; Huang, T.; Hou, K.; Zhou, S.; Yin, J.; Guan, L. EGCG induces degradation of active folate in serum via H2O2 generation, while L-ascorbic acid effectively reverses this effect. Biochem. Biophys. Rep. 2024, 38, 101719. [Google Scholar]
- Ramli, S.; Eshak, Z.; Ayub, N.; Halim, H. Morphological changes of MCF7 cells incubated with quercetin. Res. J. Biotechnol. 2022, 17, 58–62. [Google Scholar]
- Li, Y.-Y.; Lu, X.-Y.; Sun, J.-L.; Wang, Q.-Q.; Zhang, Y.-D.; Zhang, J.-B.; Fan, X.-H. Potential hepatic and renal toxicity induced by the biflavonoids from Ginkgo biloba. Chin. J. Nat. Med. 2019, 17, 672–681. [Google Scholar] [CrossRef]
- Pavasutti, V.; Sinthuvanich, C.; Tayana, N.; Kongkiatpaiboon, S.; Sae-tan, S. Mung bean seed coat water extract restores insulin sensitivity via upregulation of antioxidant defense system and downregulation of inflammation in insulin-resistant HepG2 cells. NFS J. 2023, 32, 100145. [Google Scholar]
- Madubuike, K.G.; Nnadi, C.O.; Anaga, A.O.; Asuzu, I.U. Bioactivity-guided isolation of the antidiabetic principle in Pterocarpus Santalinoides leaf extract. Braz. J. Pharm. Sci. 2023, 59, e21726. [Google Scholar]
- Nguyen Minh, T.; Le Ba, V.; Nguyen Van, T.; Nguyen Viet, P. Inhibition of PTP1B by isosinensetin, a polymethoxylated flavone isolated from trifoliate orange peel: Kinetic studies, molecular docking, and molecular dynamics simulation. Chem. Pap. 2022, 77, 1751–1757. [Google Scholar]
- Maradesha, T.; Patil, S.M.; Phanindra, B.; Achar, R.R.; Silina, E.; Stupin, V.; Ramu, R. Multiprotein Inhibitory Effect of Dietary Polyphenol Rutin from Whole Green Jackfruit Flour Targeting Different Stages of Diabetes Mellitus: Defining a Bio-Computational Stratagem. Separations 2022, 9, 262. [Google Scholar] [CrossRef]
- Bazyar, H.; Javid, A.Z.; Ahangarpour, A.; Zaman, F.; Hosseini, S.A.; Zohoori, V.; Aghamohammadi, V.; Yazdanfar, S.; Cheshmeh, M.G.D. The effects of rutin supplement on blood pressure markers, some serum antioxidant enzymes, and quality of life in patients with type 2 diabetes mellitus compared with placebo. Front. Nutr. 2023, 10, 1214420. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).