Green Tea Pressurized Hot Water Extract in Atherosclerosis: A Multi-Approach Study on Cellular, Animal, and Molecular Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Determination of Total Phenolic Content
2.4. Determination of Total Flavonoid Content
2.5. Determination of Free Radical Scavenging Activity on DPPH
2.6. Determination of Free Radical Scavenging Activity on ABTS
2.7. Hydroxyl Radical Assay
2.8. Nitric Oxide Scavenging Assay
2.9. Cell Culture and Ox-LDL Induced RAW264.7 Macrophage Cell Toxicity
2.10. Ox-LDL Induced RAW264.7 Macrophage Cell Apoptosis
2.11. Animals and Study Design
- Group I received a normal chow diet plus distilled water (normal control group).
- Group II was given HFD plus distilled water (control group).
- Group III was treated with HFD plus GPHWE at 250 mg/kg BW.
- Group IV received HFD along with GPHWE at 500 mg/kg BW.
- Group V was administered HFD plus Simvastatin at 50 mg/kg BW.
2.12. Blood Analysis for Biochemical Parameters
2.13. Blood Analysis for Hematological Parameters
2.14. Determination of Lipid Peroxidation Marker
2.15. Preparation of Sample and LDL Oxidation
2.16. Inhibitory Effect of GHPWE on the Cu2+-Induced LDL Oxidation
2.17. Molecular Docking
2.18. Ultra-High-Performance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry (UHPLC-QTOF-MS) Analysis of GPHWE
2.19. Statistical Analysis
3. Results
3.1. Total Phenolic Content, Total Flavonoid Content, and Antioxidant Properties of GPHWE
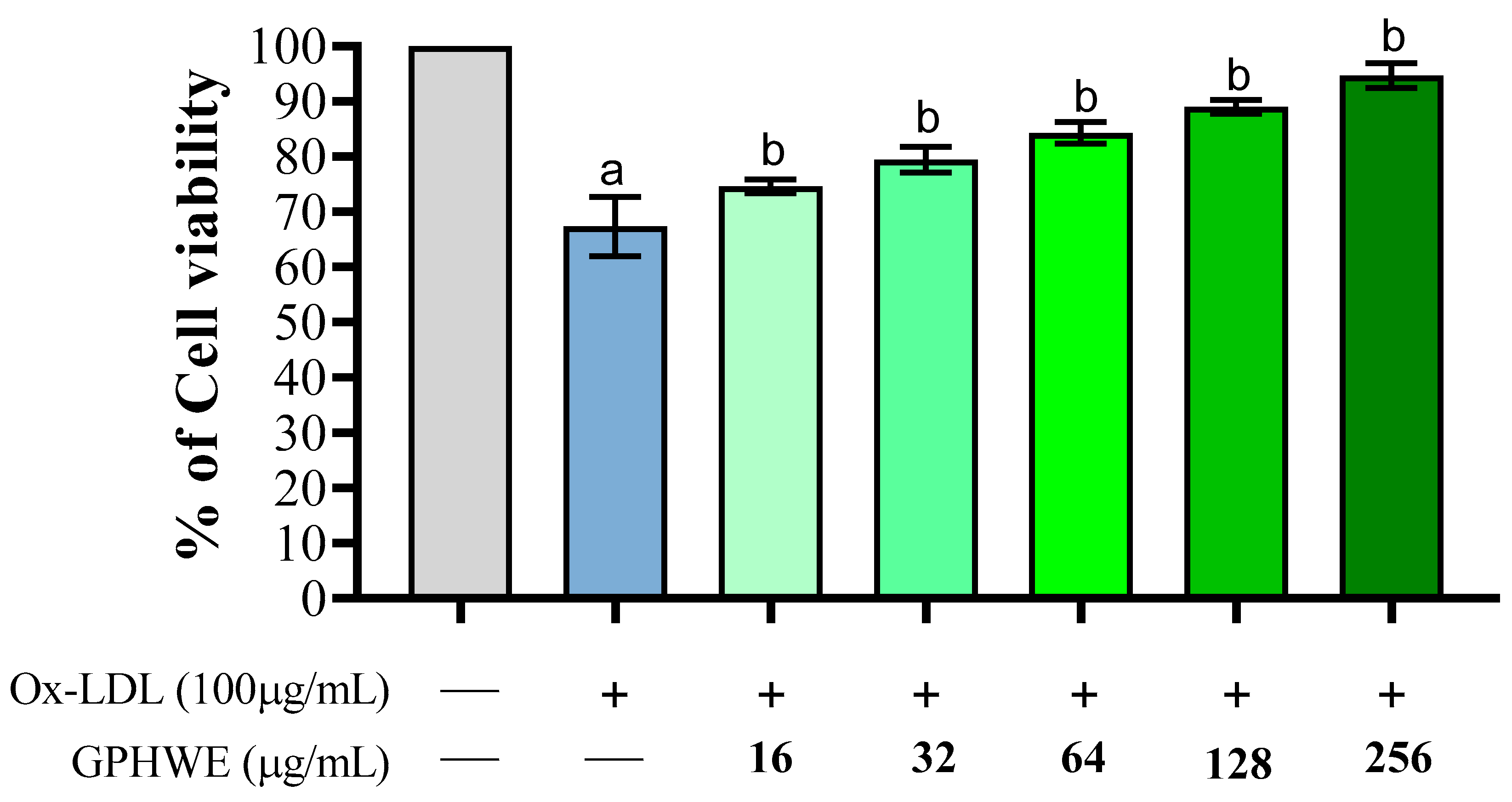
3.2. Ox-LDL Induced RAW264.7 Macrophage Cell Toxicity
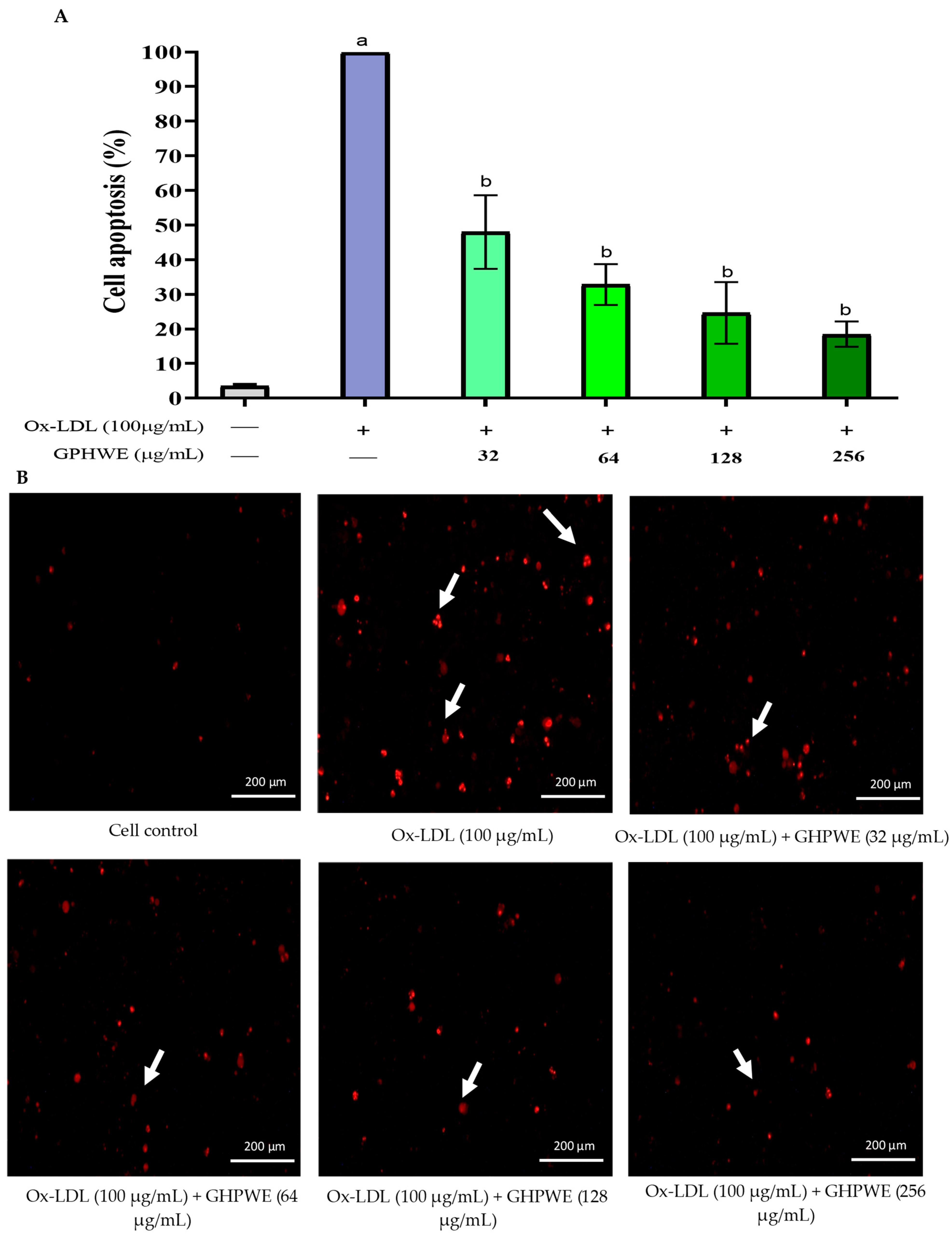
3.3. Ox-LDL-Induced RAW264.7 Macrophage Cell Apoptosis
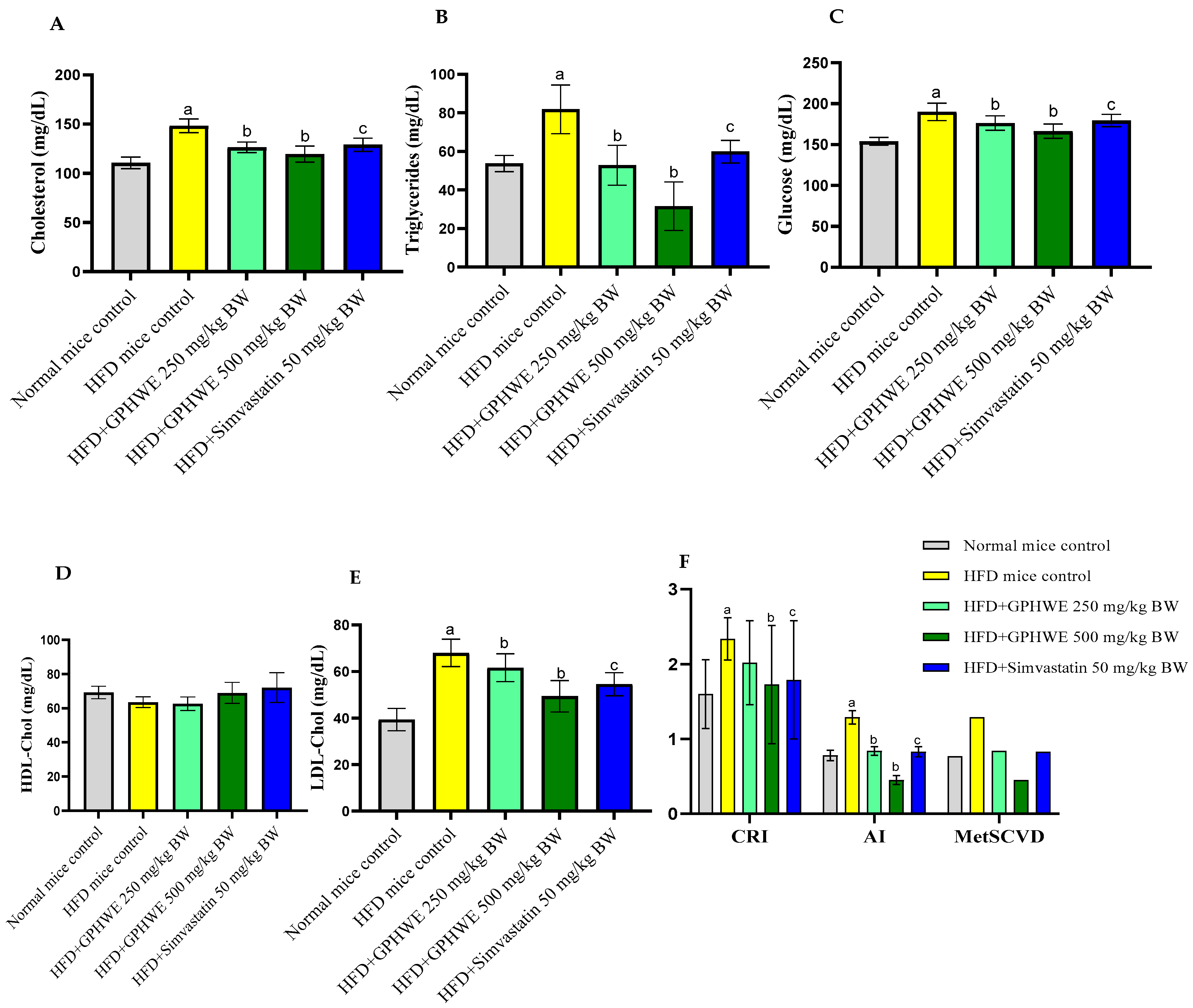
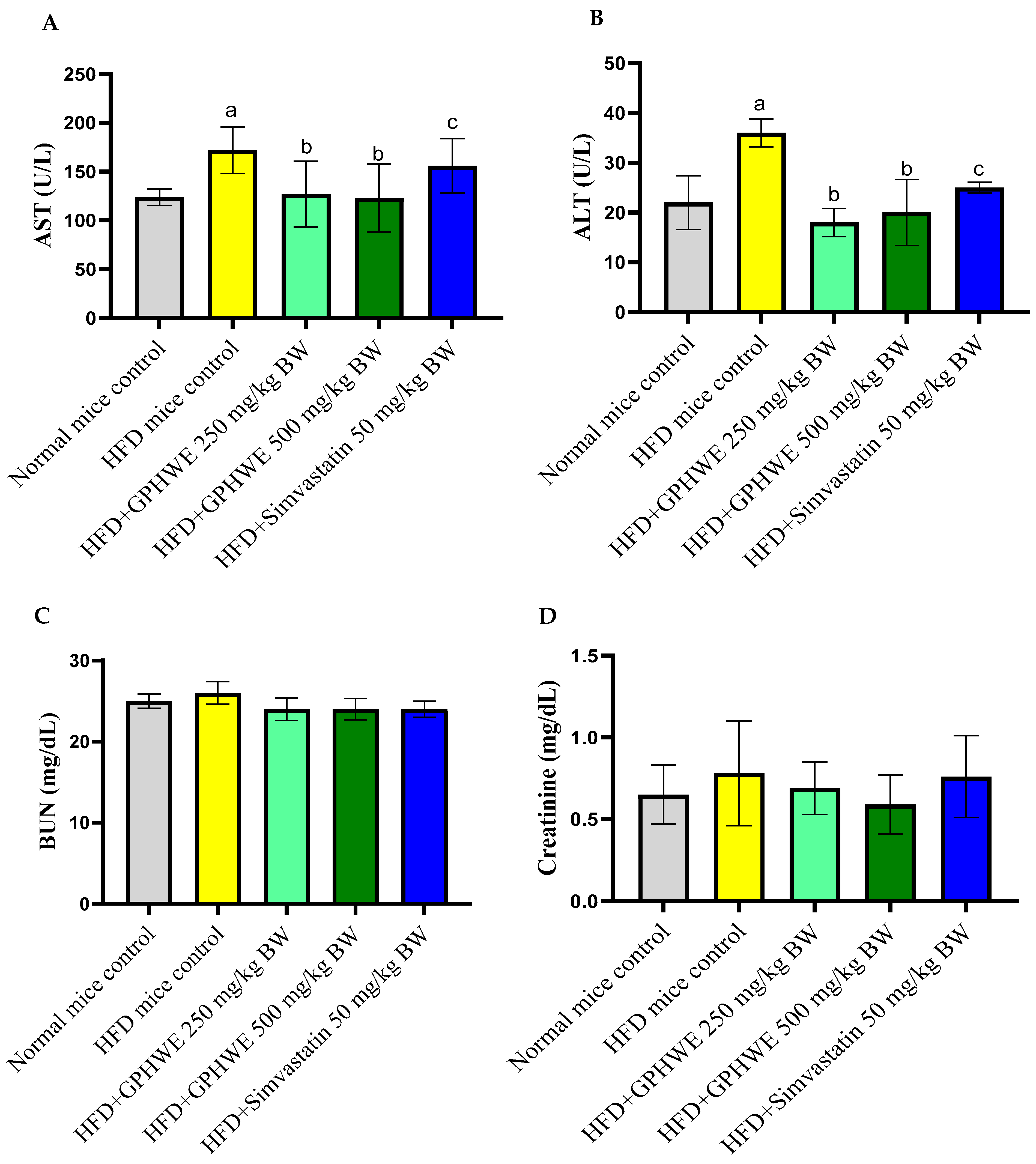
3.4. Animal Characteristics
3.5. Hematological Parameters
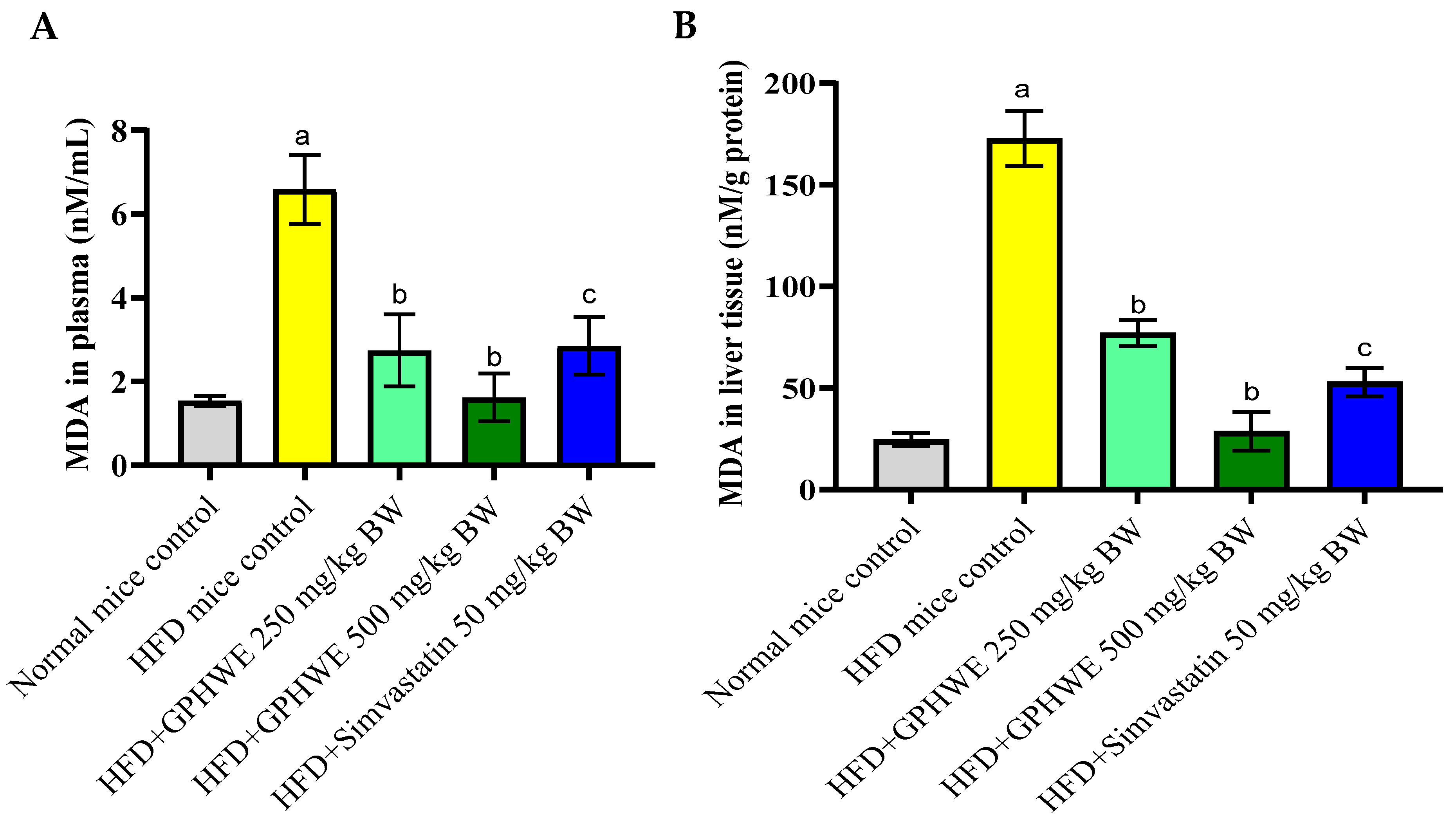
3.6. Lipid Peroxidation Product in HFD-Treated Mice
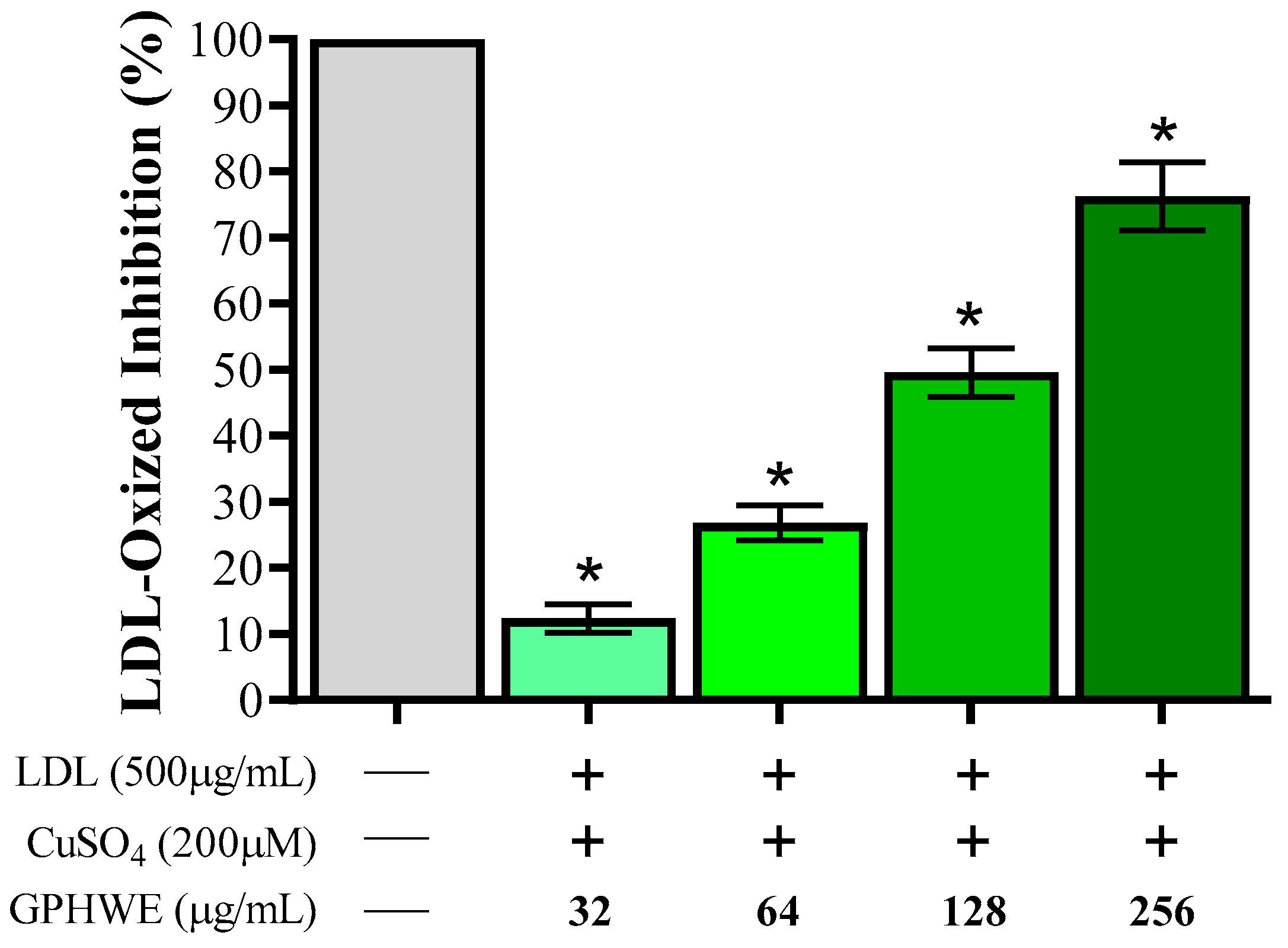
3.7. Inhibitory Effect of GPHWE on CuSO4-Induced Lipid Peroxidation
3.8. Molecular Docking Insights
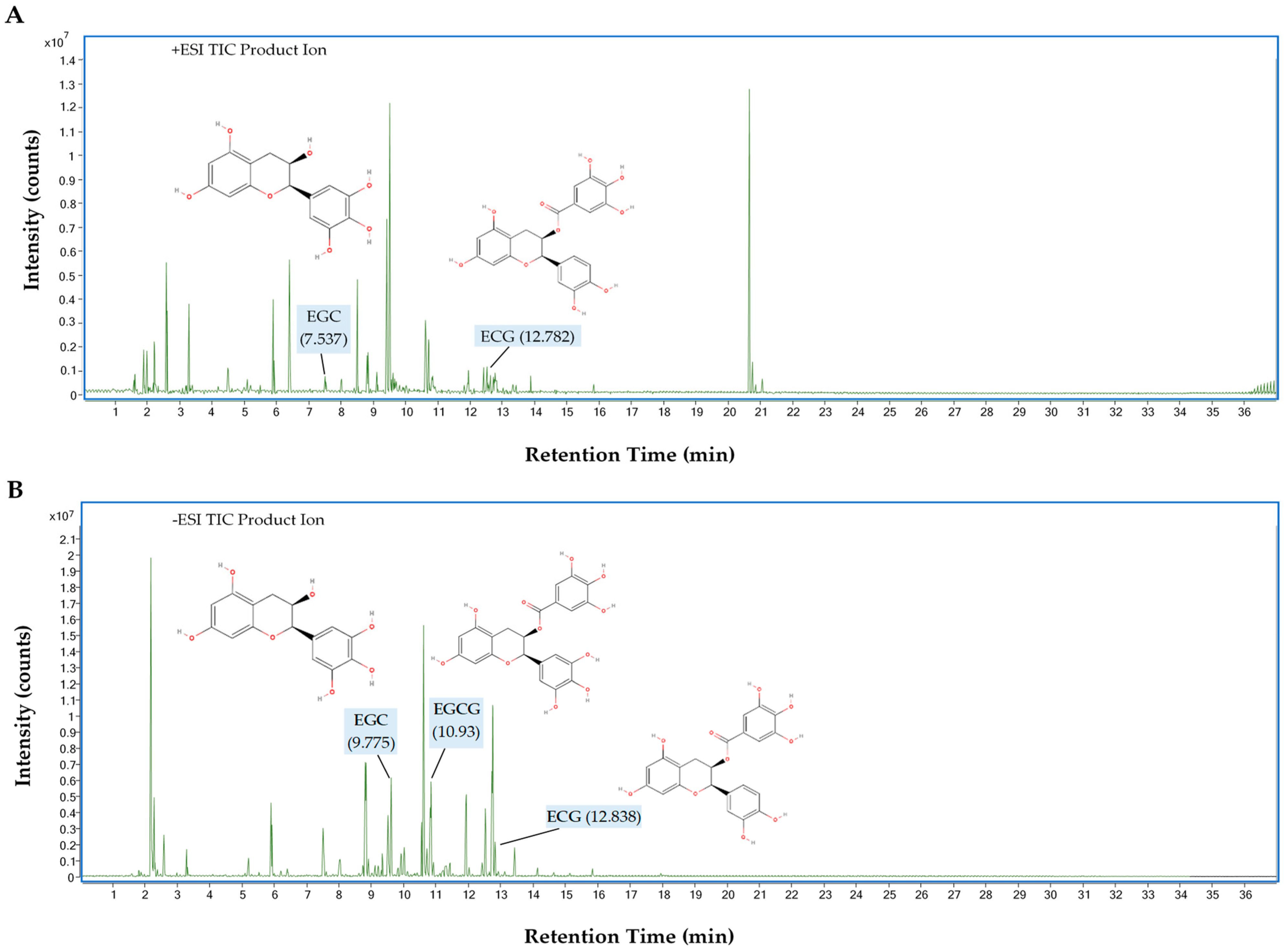
3.9. UHPLC-QTOF-MS Analysis of GPHWE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AI | Atherogenic Index |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| BUN | Blood Urea Nitrogen |
| CRI | Coronary Risk Index |
| DMSO | Dimethyl Sulfoxide |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ECG | Epicatechin Gallate |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin Gallate |
| FPG | Fasting Plasma Glucose |
| GPHWE | Green Tea Pressurized Hot Water Extract |
| Hb | Hemoglobin |
| Hct | Hematocrit |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HFD | High-Fat Diet |
| HMG-CoA | 3-Hydroxy-3-Methylglutaryl-Coenzyme A |
| IC50 | Inhibitory Concentration (50%) |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| LOX-1 | Lectin-Like Oxidized LDL Receptor 1 |
| MCH | Mean Corpuscular Hemoglobin |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| MDA | Malondialdehyde |
| MCV | Mean Corpuscular Volume |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| Ox-LDL | Oxidized Low-Density Lipoprotein |
| PBS | Phosphate-Buffered Saline |
| PHWE | Pressurized Hot Water Extraction |
| QC | Quality Control |
| QE | Quercetin Equivalent |
| RDW | Red Cell Distribution Width |
| SEM | Standard Error of the Mean |
| TC | Total Cholesterol |
| TFC | Total Flavonoid Content |
| TPC | Total Phenolic Content |
| TG | Triglycerides |
| UHPLC-QTOF-MS | Ultra-High-Performance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry |
| WBC | White Blood Cell |
References
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative stress-mediated atherosclerosis: Mechanisms and therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid. Med. Cell. Longev. 2020, 1, 5245308. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, M.; Sterchele, E.D.; Zaccarelli, M.; Mrakic-Sposta, S.; Welsby, I.J.; Balestra, C.; Taccone, F.S. Oxidative Stress and Cerebral Vascular Tone: The Role of Reactive Oxygen and Nitrogen Species. Int. J. Mol. Sci. 2024, 25, 3007. [Google Scholar] [CrossRef]
- Młynarska, E.; Czarnik, W.; Fularski, P.; Hajdys, J.; Majchrowicz, G.; Stabrawa, M.; Rysz, J.; Franczyk, B. From Atherosclerotic Plaque to Myocardial Infarction—The Leading Cause of Coronary Artery Occlusion. Int. J. Mol. Sci. 2024, 25, 7295. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Y.; Liang, Y. Cardioprotective effects of tea and its catechins. Health 2013, 5, 23–30. [Google Scholar] [CrossRef]
- Selvi, I.K.; Nagarajan, S. Separation of catechins from green tea (Camellia sinensis L.) by microwave assisted acetylation, evaluation of antioxidant potential of individual components and spectroscopic analysis. LWT 2018, 91, 391–397. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front. Pharmacol. 2023, 14, 1144878. [Google Scholar]
- Alsuwailem, N.S. Green Tea Facts Evidences. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2015. [Google Scholar]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Suksamran, N.; Anantawat, V.; Wattanaarsakit, P.; Wei, C.; Rahman, M.A.; Majima, H.J.; Tangpong, J. Mangosteen vinegar from Garcinia mangostana: Quality improvement and antioxidant properties. Heliyon 2022, 8, 12. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Romanet, R.; Coelho, C.; Liu, Y.; Bahut, F.; Ballester, J.; Nikolantonaki, M.; Gougeon, R.D. The antioxidant potential of white wines relies on the chemistry of sulfur-containing compounds: An optimized DPPH assay. Molecules 2019, 24, 1353. [Google Scholar] [CrossRef]
- Phyu, M.P.; Tangpong, J. Neuroprotective effects of xanthone derivative of Garcinia mangostana against lead-induced acetylcholinesterase dysfunction and cognitive impairment. Food Chem. Toxicol. 2014, 70, 151–156. [Google Scholar] [CrossRef]
- Ran, Y.; Moursy, M.; Hider, R.C.; Cilibrizzi, A. The colorimetric detection of the hydroxyl radical. Int. J. Mol. Sci. 2023, 24, 4162. [Google Scholar] [CrossRef]
- Rana, M.N.; Tangpong, J. In vitro free radical scavenging and anti-genotoxic activities of Thunbergia laurifolia aqueous leaf extract. J. Health Res. 2017, 31, 127–133. [Google Scholar]
- Baird, S.K.; Reid, L.; Hampton, M.B.; Gieseg, S.P. OxLDL induced cell death is inhibited by the macrophage synthesised pterin, 7, 8-dihydroneopterin, in U937 cells but not THP-1 cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2005, 1745, 361–369. [Google Scholar] [CrossRef]
- Arunkumar, E.; Forbes, C.C.; Noll, B.C.; Smith, B.D. Squaraine-derived rotaxanes: Sterically protected fluorescent near-IR dyes. J. Am. Chem. Soc. 2005, 127, 3288–3289. [Google Scholar] [CrossRef]
- Tian, C.; Ye, X.; Zhang, R.; Long, J.; Ren, W.; Ding, S.; Liao, D.; Jin, X.; Wu, H.; Xu, S.; et al. Green tea polyphenols reduced fat deposits in high fat-fed rats via erk1/2-PPARγ-adiponectin pathway. PLoS ONE 2013, 8, 53796. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Kazemi, T.; Hajihosseini, M.; Moossavi, M.; Hemmati, M.; Ziaee, M. Cardiovascular risk factors and atherogenic indices in an Iranian population: Birjand East of Iran. Clin. Med. Insights Cardiol. 2018, 12, 1179546818759286. [Google Scholar] [PubMed]
- Lovrić, J.; Mesić, M.; Macan, M.; Koprivanac, M.; Kelava, M.; Bradamante, V. Measurement of malondialdehyde (MDA) level in rat plasma after simvastatin treatment using two different analytical methods. Period. Biol. 2008, 110, 63–68. [Google Scholar]
- Sirichaiwetchakoon, K.; Lowe, G.M.; Eumkeb, G. The Free Radical Scavenging and Anti-Isolated Human LDL Oxidation Activities of Pluchea indica (L.) Less. Tea Compared to Green Tea (Camellia sinensis). BioMed Res. Int. 2020, 1, 4183643. [Google Scholar]
- Vlaminck, B.; Calay, D.; Genin, M.; Sauvage, A.; Ninane, N.; Zouaoui Boudjeltia, K.; Raes, M.; Michiels, C. Effects of copper sulfate-or myeloperoxidase-modified LDL on lipid loading and programmed cell death in macrophages under hypoxia. Hypoxia 2014, 2, 153–169. [Google Scholar]
- Yagi, K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976, 15, 212–216. [Google Scholar]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The single global macromolecular structure archive. Protein Crystallogr. Methods Protoc. 2017, 1067, 627–641. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of oxidized LDL-mediated endothelial dysfunction and its consequences for the development of atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef]
- Wang, H. Prediction of protein–ligand binding affinity via deep learning models. Brief. Bioinform. 2024, 25, bbae081. [Google Scholar]
- Arouri, A.; Garidel, P.; Kliche, W.; Blume, A. Hydrophobic interactions are the driving force for the binding of peptide mimotopes and Staphylococcal protein A to recombinant human IgG1. Eur. Biophys. J. 2007, 36, 647–660. [Google Scholar] [PubMed]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and its impact on cardiovascular health: Focus on atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Ballester, M.; Herrero-Cervera, A.; Vinué, Á.; Martínez-Hervás, S.; González-Navarro, H. Impact of cholesterol metabolism in immune cell function and atherosclerosis. Nutrients 2020, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam cells in atherosclerosis: Novel insights into its origins, consequences, and molecular mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar]
- Young, I.S.; McEneny, J. Lipoprotein oxidation and atherosclerosis. Biochem. Soc. Trans. 2001, 29, 358–362. [Google Scholar]
- Zhu, Q.Y.; Huang, Y.; Tsang, D.; Chen, Z.Y. Regeneration of α-tocopherol in human low-density lipoprotein by green tea catechin. J. Agric. Food Chem. 1999, 47, 2020–2025. [Google Scholar]
- Suzuki-Sugihara, N.; Kishimoto, Y.; Saita, E.; Taguchi, C.; Kobayashi, M.; Ichitani, M.; Ukawa, Y.; Sagesaka, Y.M.; Suzuki, E.; Kondo, K. Green tea catechins prevent low-density lipoprotein oxidation via their accumulation in low-density lipoprotein particles in humans. Nutr. Res. 2016, 36, 16–23. [Google Scholar]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar]
- Orčić, D.Z.; Mimica-Dukić, N.M.; Francišković, M.M.; Petrović, S.S.; Jovin, E.Đ. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem. Cent. J. 2011, 5, 34. [Google Scholar]
- Fuhrman, B.; Aviram, M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 41–48. [Google Scholar] [PubMed]
- Hartonen, K.; Parshintsev, J.; Sandberg, K.; Bergelin, E.; Nisula, L.; Riekkola, M.L. Isolation of flavonoids from aspen knotwood by pressurized hot water extraction and comparison with other extraction techniques. Talanta 2007, 74, 32–38. [Google Scholar] [PubMed]
- Bokuchava, M.A.; Skobeleva, N.I.; Sanderson, G.W. The biochemistry and technology of tea manufacture. Crit. Rev. Food Sci. Nutr. 1980, 12, 303–370. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar]
- Conforti, F.; Menichini, F. Phenolic compounds from plants as nitric oxide production inhibitors. Curr. Med. Chem. 2011, 18, 1137–1145. [Google Scholar]
- Atale, N.; Gupta, S.; Yadav, U.C.S.; Rani, V. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014, 255, 7–19. [Google Scholar]
- Carresi, C.; Mollace, R.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Ruga, S.; Zito, M.C.; et al. Oxidative stress triggers defective autophagy in endothelial cells: Role in atherothrombosis development. Antioxidants 2021, 10, 387. [Google Scholar] [CrossRef]
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar]
- Lankin, V.Z.; Tikhaze, A.K.; Melkumyants, A.M. Malondialdehyde as an important key factor of molecular mechanisms of vascular wall damage under heart diseases development. Int. J. Mol. Sci. 2022, 24, 128. [Google Scholar] [CrossRef]
- Ismail, B.S.; Mahmoud, B.; Abdel-Reheim, E.S.; Soliman, H.A.; Ali, T.M.; Elesawy, B.H.; Zaky, M.Y. Cinnamaldehyde Mitigates Atherosclerosis Induced by High-Fat Diet via Modulation of Hyperlipidemia, Oxidative Stress, and Inflammation. Oxid. Med. Cell. Longev. 2022, 2022, 4464180. [Google Scholar]
- Wang, L.; Geng, C.; Jiang, L.; Gong, D.; Liu, D.; Yoshimura, H.; Zhong, L. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur. J. Nutr. 2008, 47, 235–243. [Google Scholar] [PubMed]
- Puttananjaiah, M.K.H.; Dhale, M.A.; Gaonkar, V.; Keni, S. Statins: 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors demonstrate anti-atherosclerotic character due to their antioxidant capacity. Appl. Biochem. Biotechnol. 2011, 163, 215–222. [Google Scholar] [PubMed]
- Baskaran, G.; Salvamani, S.; Ahmad, S.A.; Shaharuddin, N.A.; Pattiram, P.D.; Shukor, M.Y. HMG-CoA reductase inhibitory activity and phytocomponent investigation of Basella alba leaf extract as a treatment for hypercholesterolemia. Drug Des. Dev. Ther. 2015, 9, 509–517. [Google Scholar]
- Sánchez-León, M.E.; Loaeza-Reyes, K.J.; Matias-Cervantes, C.A.; Mayoral-Andrade, G.; Pérez-Campos, E.L.; Pérez-Campos-Mayoral, L.; Hernández-Huerta, M.T.; Zenteno, E.; Pérez-Cervera, Y.; Pina-Canseco, S. LOX-1 in Cardiovascular Disease: A Comprehensive Molecular and Clinical Review. Int. J. Mol. Sci. 2024, 25, 5276. [Google Scholar] [CrossRef]
- Bustos, C.; Hernández-Presa, M.A.; Ortego, M.; Tuñón, J.; Ortega, L.; Pérez, F.; Díaz, C.; Hernández, G.; Egido, J. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. J. Am. Coll. Cardiol. 1998, 32, 2057–2064. [Google Scholar]
- Grootaert, M.O.; Schrijvers, D.M.; Hermans, M.; Van Hoof, V.O.; De Meyer, G.R.; Martinet, W. Caspase-3 deletion promotes necrosis in atherosclerotic plaques of ApoE knockout mice. Oxidative Med. Cell. Longev. 2016, 2016, 3087469. [Google Scholar]
- Mimura, J.; Itoh, K. Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic. Biol. Med. 2015, 88, 221–232. [Google Scholar]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar]
- Jo, Y.; DeBose-Boyd, R.A. Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 185–198. [Google Scholar]
- Hassanein, E.H.; Shalkami, A.G.S.; Khalaf, M.M.; Mohamed, W.R.; Hemeida, R.A. The impact of Keap1/Nrf2, P38MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. [Google Scholar]
- Nain, C.W.; Mignolet, E.; Herent, M.-F.; Quetin-Leclercq, J.; Debier, C.; Page, M.M.; Larondelle, Y. The Catechins Profile of Green Tea Extracts Affects the Antioxidant Activity and Degradation of Catechins in DHA-Rich Oil. Antioxidants 2022, 11, 1844. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [PubMed]
- Huang, B.; Ong, A.; Wang, W.; He, Y.; Xiao, Y. Application of microdialysis combined with UHPLC-QTOF/MS to screen for endogenous metabolites in aquatic organisms as biomarkers of exposure to an emerging contaminant, triclosan. Anal. Bioanal. Chem. 2023, 415, 1571–1581. [Google Scholar] [PubMed]
- Naldi, M.; Fiori, J.; Gotti, R.; Périat, A.; Veuthey, J.L.; Guillarme, D.; Andrisano, V. UHPLC determination of catechins for the quality control of green tea. J. Pharm. Biomed. Anal. 2014, 88, 307–314. [Google Scholar] [CrossRef]
- Chen, Y.; Lai, L.; You, Y.; Gao, R.; Xiang, J.; Wang, G.; Yu, W. Quantitative Analysis of Bioactive Compounds in Commercial Teas: Profiling Catechin Alkaloids, Phenolic Acids, and Flavonols Using Targeted Statistical Approaches. Foods 2023, 12, 3098. [Google Scholar] [CrossRef]
- Sivanesan, I.; Muthu, M.; Kannan, A.; Pushparaj, S.S.C.; Oh, J.-W.; Gopal, J. Identification of Epigallocatechin-3-Gallate (EGCG) from Green Tea Using Mass Spectrometry. Separations 2022, 9, 209. [Google Scholar] [CrossRef]


| Total Phenolic Content (mg GAE/g Dry Weight) | Total Flavonoid Content (mg QE/g Dry Weight) | DPPH Radical Scavenging IC50 (μg/mL) | OH- Radical Scavenging IC50 (μg/mL) | NO Radical Scavenging IC50 (μg/mL) |
|---|---|---|---|---|
| 258.61 ± 32.48 | 98.91 ± 22.19 | 72.84 ± 4.90 | 94.87 ± 8.27 | 77.82 ± 8.04 |
| Group | WBC (cells/µL) | RBC (cells/µL) | Hb (g/dL) | Hct (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | RDW (%) | Platelet (103/µL) |
|---|---|---|---|---|---|---|---|---|---|
| Normal control | 7.42 ± 0.98 | 8.64 ± 0.67 | 13.42 ± 0.89 | 39.68 ± 1.78 | 54.55 ± 0.98 | 18.63 ± 0.34 | 32.08 ± 0.86 | 15.62 ± 0.47 | 742.50 ± 167.41 |
| HFD control | 8.30 ± 0.59 | 8.07 ± 0.94 | 13.08 ± 0.86 | 39.24 ± 0.85 | 54.02 ± 0.82 | 18.23 ± 0.38 | 31.98 ± 0.98 | 15.56 ± 0.85 | 865.41 ± 125.16 |
| HFD + GPHWE250 mg/kg BW | 8.02 ± 0.66 | 8.42 ± 0.74 | 13.36 ± 0.75 | 40.06 ± 0.64 | 54.65 ± 0.58 | 18.45 ± 0.84 | 32.06 ± 0.82 | 15.58 ± 0.67 | 765.64 ± 132.14 |
| HFD + GPHWE500 mg/kg BW | 7.45 ± 0.86 | 8.74 ± 0.76 | 13.58 ± 0.59 | 40.64 ± 0.85 | 54.83 ± 0.86 | 18.68 ± 0.96 | 32.65 ± 0.87 | 15.82 ± 0.45 | 763.25 ± 112.35 |
| HFD + Simvastatin50 mg/kg BW | 8.12 ± 0.68 | 8.32 ± 0.65 | 13.21 ± 0.75 | 39.62 ± 0.94 | 54.32 ± 0.87 | 18.36 ± 0.62 | 31.88 ± 0.94 | 15.48 ± 0.84 | 786.98 ± 154.36 |
| Target | Compound | Binding Energy (Kcal/mol) | Hydrogen Bond (Length Å) |
|---|---|---|---|
| LOX-1 (3R6Y) | EGCG | −1.69 | ILE-36 (5.67) |
| ECG | −2.60 | GLY-180 (4.42), ASN-394 (5.78), MET-184 (4.46), LEU-306 (5.13, 6.02) | |
| EGC | −3.08 | LEU-58 (4.62), LYS-54 (5.25), ASP-79 (4.38, 4.68) | |
| HMG-CoA (1PKM) | EGCG | −1.40 | LYS-392 (5.82), PHE-25 (5.04), GLU-395 (4.97, 4.25) |
| ECG | −3.21 | LYS-134 (5.63), THR-138 (3.63), ASN-154 (4.32), GLU-130 (4.18, 4.57) | |
| EGC | −3.30 | LYS-172 (3.33), ILE-298 (5.42) | |
| Caspase-3 (3I9I) | EGCG | −6.50 | TRP-41 (5.72, 5.24), LYS-70 (5.85, 6.29), LYS-35 (5.24), GLU-92 (5.27, 6.03), LYS-70 (4.74), GLU-92 (5.59, 5.71, 6.06) |
| ECG | −5.23 | VAL-122 (2.90), TYR-114 (5.96) | |
| EGC | −6.95 | ASN-27 (3.59), ASP-74 (3.88), ILE-26 (5.34) | |
| Nrf2 (2FLU) | EGCG | −5.09 | GLY-81 (4.23), PRO-384 (3.52), SER-383 (4.47, 4.77) |
| ECG | −6.40 | VAL-561 (3.52), VAL-418 (4.09), VAL-512 (4.79, 4.88), ILE-559 (4.82), VAL-465 (5.12), ARG-326 (6.51) | |
| EGC | −7.19 | VAL-467 (4.40), VAL-418 (3.66, 4.09, 4.38), ALA-510 (4.32), LEU-557 (4.61, 4.91), VAL-465 (3.64) |
| Retention Time (RT, min) | M/Z | Observed Mass (g/mol) | Possible Compound | Molecular Formula | Response | Score | Base Peak | Ionization Mode |
|---|---|---|---|---|---|---|---|---|
| 12.782 | 443.0974 | 442.0901 | Epicatechin gallate (ECG) | C22H18O10 | 916,981 | 49.83 | 123.0442 | Positive (+ESI) |
| 7.537 | 307.0812 | 306.0739 | Epigallocatechin (EGC) | C15H14O7 | 536,828 | 49.48 | 139.0387 | Positive (+ESI) |
| 10.93 | 457.0773 | 458.0846 | Epigallocatechin Gallate (EGCG) | C22H18O11 | 4,336,442 | 98.27 | 169.0144 | Negative (−ESI) |
| 12.838 | 441.0825 | 442.0898 | Epicatechin gallate (ECG) | C22H18O10 | 6,574,501 | 49.19 | 169.0142 | Negative (−ESI) |
| 9.775 | 305.0663 | 306.0737 | Epigallocatechin (EGC) | C15H14O7 | 76,723 | 49.38 | 196.9156 | Negative (−ESI) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, R.; Kongchain, A.; Chatatikun, M.; Klangbud, W.K.; Yupanqui, C.T.; Majima, H.J.; Indo, H.P.; Sompol, P.; Sekeroglu, N.; Phongphithakchai, A.; et al. Green Tea Pressurized Hot Water Extract in Atherosclerosis: A Multi-Approach Study on Cellular, Animal, and Molecular Mechanisms. Antioxidants 2025, 14, 404. https://doi.org/10.3390/antiox14040404
Hossain R, Kongchain A, Chatatikun M, Klangbud WK, Yupanqui CT, Majima HJ, Indo HP, Sompol P, Sekeroglu N, Phongphithakchai A, et al. Green Tea Pressurized Hot Water Extract in Atherosclerosis: A Multi-Approach Study on Cellular, Animal, and Molecular Mechanisms. Antioxidants. 2025; 14(4):404. https://doi.org/10.3390/antiox14040404
Chicago/Turabian StyleHossain, Rahni, Anawat Kongchain, Moragot Chatatikun, Wiyada Kwanhian Klangbud, Chutha Takahashi Yupanqui, Hideyuki J. Majima, Hiroko P. Indo, Pradoldej Sompol, Nazim Sekeroglu, Atthaphong Phongphithakchai, and et al. 2025. "Green Tea Pressurized Hot Water Extract in Atherosclerosis: A Multi-Approach Study on Cellular, Animal, and Molecular Mechanisms" Antioxidants 14, no. 4: 404. https://doi.org/10.3390/antiox14040404
APA StyleHossain, R., Kongchain, A., Chatatikun, M., Klangbud, W. K., Yupanqui, C. T., Majima, H. J., Indo, H. P., Sompol, P., Sekeroglu, N., Phongphithakchai, A., & Tangpong, J. (2025). Green Tea Pressurized Hot Water Extract in Atherosclerosis: A Multi-Approach Study on Cellular, Animal, and Molecular Mechanisms. Antioxidants, 14(4), 404. https://doi.org/10.3390/antiox14040404












