Agomelatine Alleviates Depressive-like Behaviors by Suppressing Hippocampal Oxidative Stress in the Chronic Social Defeat Stress Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Chronic Social Defeat Stress (CSDS) Model
2.3. Behavioral Tests
2.3.1. Social Interaction Test (SIT)

2.3.2. Three-Chambered Social Interaction Test (TBSIT)
2.3.3. Open Field Test (OFT)
2.3.4. Elevated Plus Maze (EPM)
2.3.5. Tail Suspension Test (TST)
2.3.6. Forced Swim Test (FST)
2.3.7. Sucrose Preference Test (SPT)
2.4. Measurement of MDA, SOD, GSH, and LDH
2.5. Western Blotting (WB)
2.6. DHE Staining
2.7. Immunofluorescence
2.8. Transmission Electron Microscopy (TEM)
2.9. Hippocampal Slice Preparation and Electrophysiological Recordings
2.10. LC–MS/MS Analysis
2.11. Statistical Analysis
3. Results
3.1. CSDS Modeling Induced Behavioral Disorders and Oxidative Stress
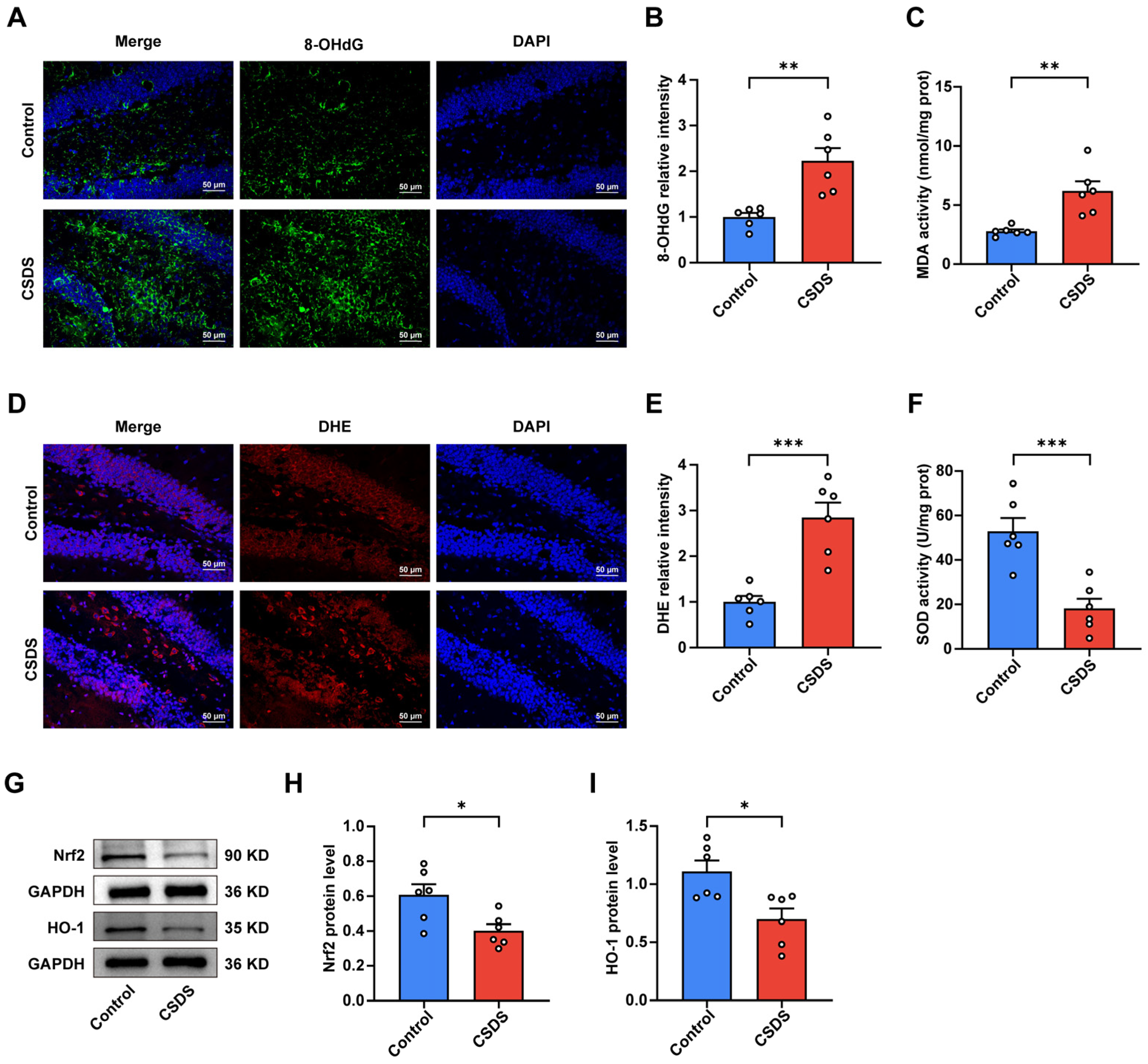
3.2. Oxidative Stress Was Observed in the Hippocampi of the CSDS-Induced Depression Group
3.3. Agomelatine Reverses Social Disorders After CSDS Exposure
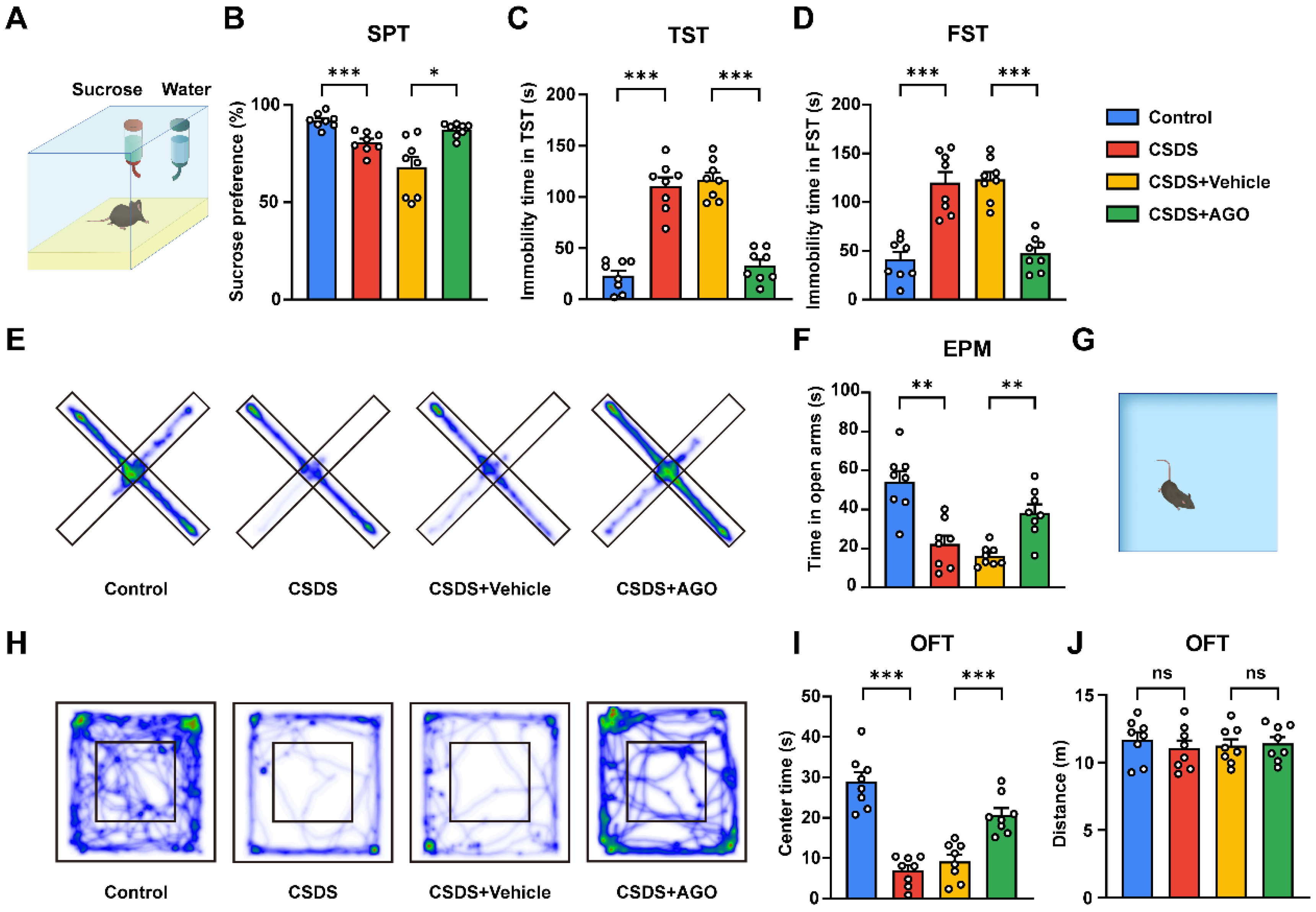
3.4. Agomelatine Ameliorates Depressive and Anxiety-like Behaviors Induced by CSDS Exposure
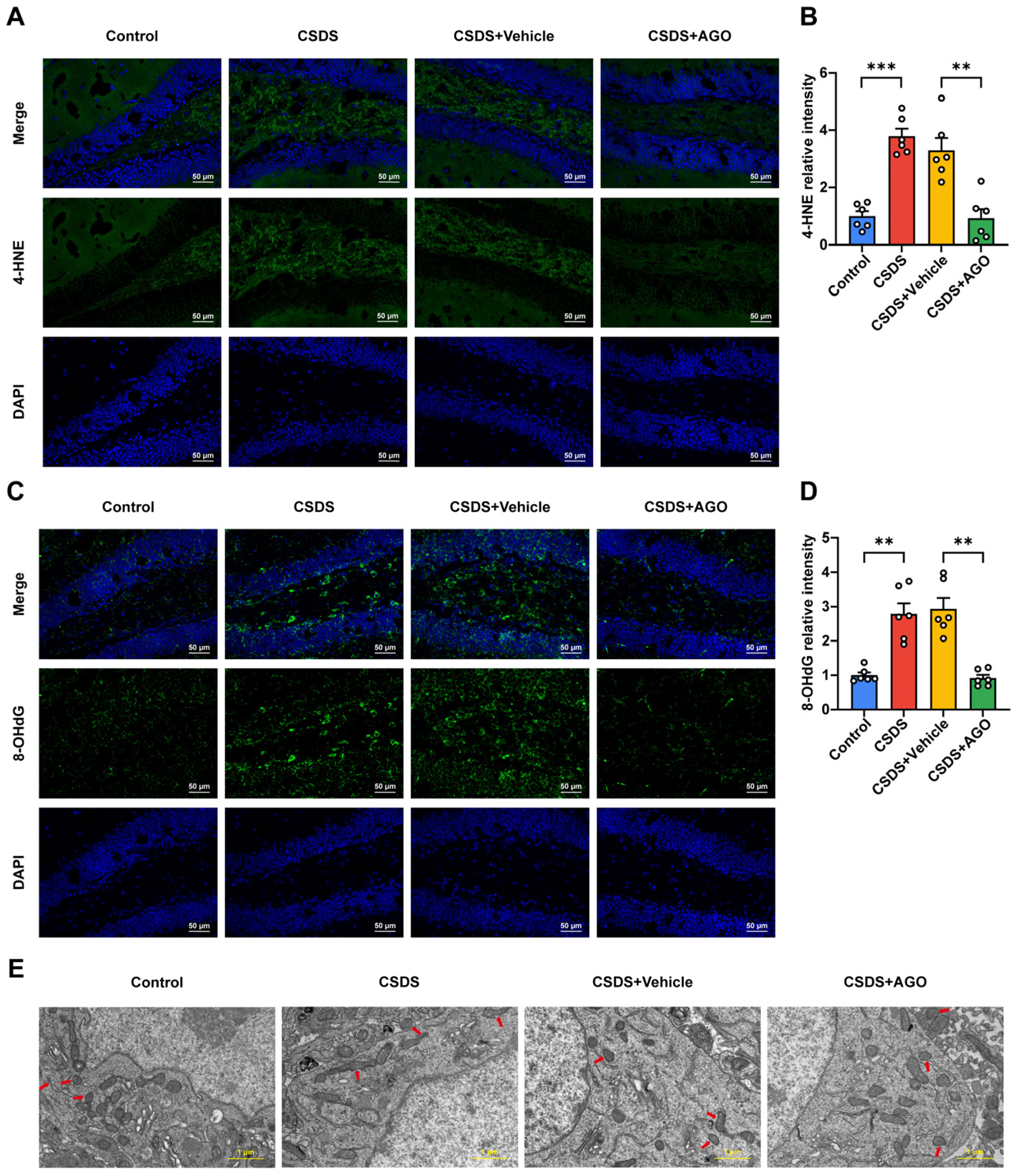
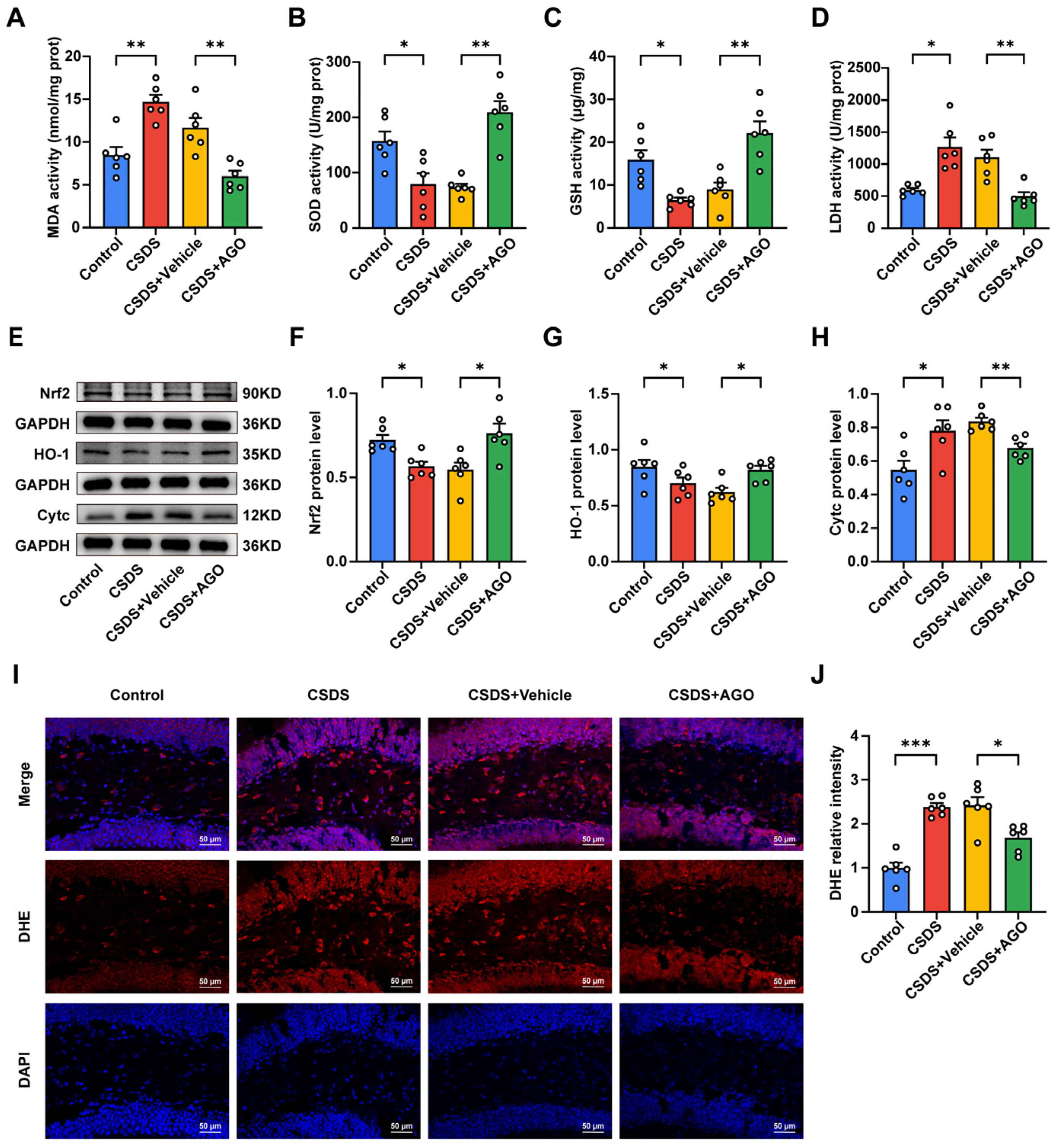
3.5. Agomelatine Suppressed Oxidative Stress in the Hippocampal of Mice CSDS Model
3.6. Agomelatine Ameliorates Mitochondrial Damage After CSDS Exposure
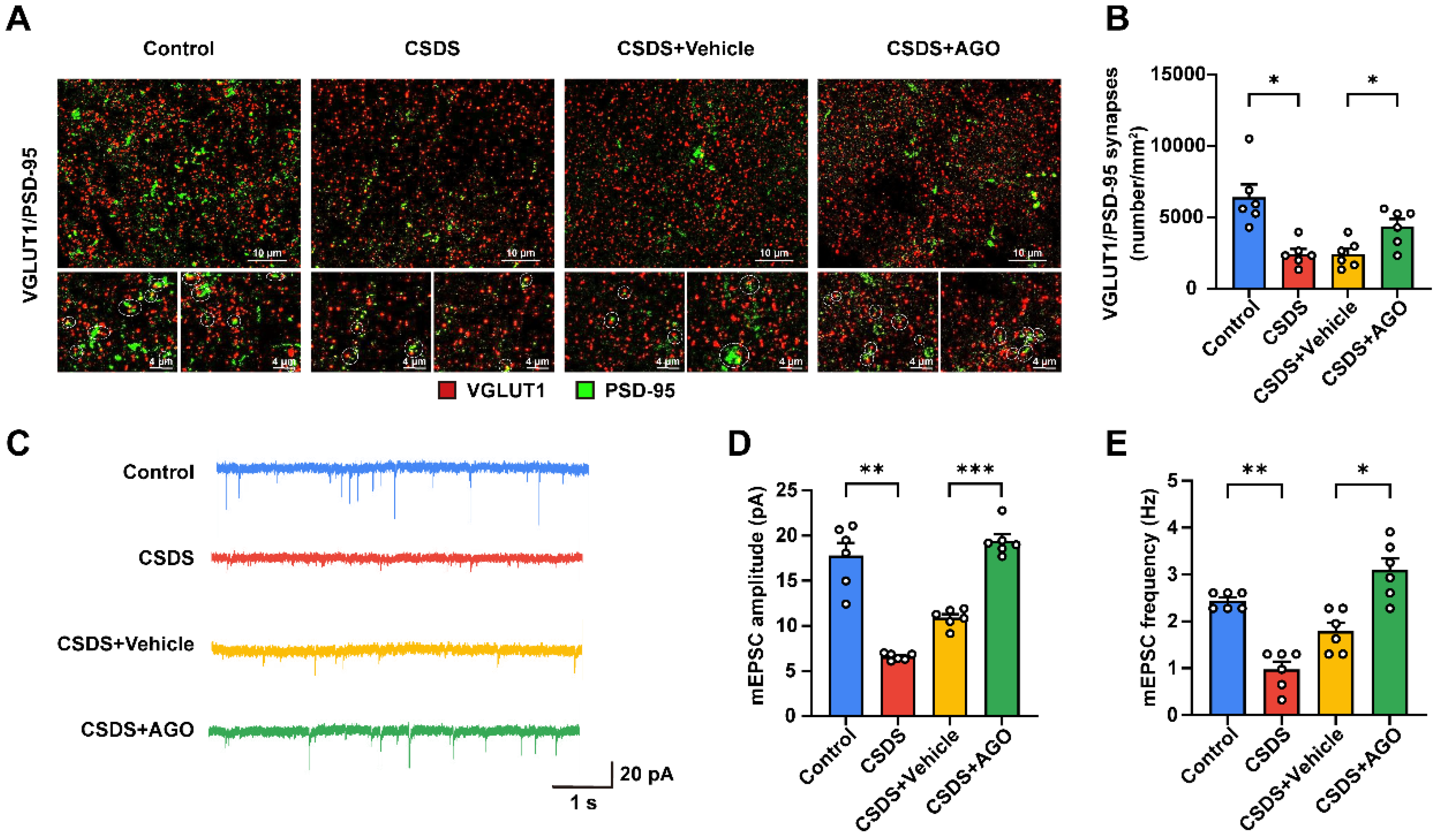
3.7. Agomelatine Ameliorates Synaptic Plasticity Impairment in the Hippocampi of CSDS-Exposed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDD | major depressive disorder |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| GSH | glutathione |
| SOD | superoxide dismutase |
| LDH | lactate dehydrogenase |
| BDNF | brain-derived neurotrophic factor |
| SSRIs | selective serotonin reuptake inhibitors |
| SNRIs | serotonin–norepinephrine reuptake inhibitors |
| TBSIT | three-chambered social interaction test |
| BSA | bovine serum albumin |
| TEM | transmission electron microscopy |
| ACSF | artificial cerebrospinal fluid |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| HO-1 | heme oxygenase-1 |
| DHE | dihydroethidium |
| 4-HNE | 4-hydroxynonenal |
| Cytc | cytochrome C |
| VGLUT1 | vesicular glutamate transporter 1 |
| PSD-95 | postsynaptic density protein-95 |
| BCA | bicinchoninic acid |
| mEPSCs | miniature excitatory postsynaptic currents |
| CRS | chronic restraint stress |
| CMS | chronic mild stress |
| DAPI | 4′, 6-diamidino-2-phenylindole dihydrochloride |
| CUMS | chronic unpredictable mild stress |
| TST | tail suspension test |
| FST | forced swim test |
| EPM | elevated plus maze |
| OFT | open field test |
| SPT | sucrose preference test |
| i.p. | intraperitoneal |
| PBS | phosphate buffer saline |
| PFA | paraformaldehyde. |
| SIT | social interaction test |
| PPP | pentose phosphate pathway |
| CSDS | chronic social defeat stress |
| ANOVA | analysis of variance |
References
- Walker, E.R.; McGee, R.E.; Druss, B.G. Mortality in Mental Disorders and Global Disease Burden Implications. JAMA Psychiatry 2015, 72, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Yang, Y.; Yin, T.; Pan, M.; Xu, J.; Chen, R.; Zheng, W.; Gu, F. The Burden of Adolescent Depression and the Impact of COVID-19 across 204 Countries and Regions from 1990 to 2021: Results from the 2021 Global Burden of Disease Study. Sci. Rep. 2025, 15, 5658. [Google Scholar] [CrossRef]
- McCarron, R.M.; Shapiro, B.; Rawles, J.; Luo, J. Depression. Ann. Intern. Med. 2021, 174, ITC65–ITC80. [Google Scholar]
- Park, C.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Lee, Y.; Cao, B.; Zuckerman, H.; Kalantarova, A.; McIntyre, R.S. Stress, Epigenetics and Depression: A Systematic Review. Neurosci. Biobehav. Rev. 2019, 102, 139–152. [Google Scholar] [PubMed]
- Braithwaite, E.C.; O’Connor, R.M.; Degli-Esposti, M.; Luke, N.; Bowes, L. Modifiable Predictors of Depression Following Childhood Maltreatment: A Systematic Review and Meta-Analysis. Transl. Psychiatry 2017, 7, e1162. [Google Scholar]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major Depressive Disorder: Hypothesis, Mechanism, Prevention and Treatment. Signal Transduct. Target Ther. 2024, 9, 30. [Google Scholar]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular Pathways of Major Depressive Disorder Converge on the Synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef]
- Won, E.; Na, K.-S.; Kim, Y.-K. Associations between Melatonin, Neuroinflammation, and Brain Alterations in Depression. Int. J. Mol. Sci. 2021, 23, 305. [Google Scholar] [CrossRef]
- Cathomas, F.; Lin, H.-Y.; Chan, K.L.; Li, L.; Parise, L.F.; Alvarez, J.; Durand-de Cuttoli, R.; Aubry, A.V.; Muhareb, S.; Desland, F.; et al. Circulating Myeloid-Derived MMP8 in Stress Susceptibility and Depression. Nature 2024, 626, 1108–1115. [Google Scholar]
- Hammen, C. Stress and Depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar]
- Li, H.-H.; Liu, Y.; Chen, H.-S.; Wang, J.; Li, Y.-K.; Zhao, Y.; Sun, R.; He, J.-G.; Wang, F.; Chen, J.-G. PDGF-BB-Dependent Neurogenesis Buffers Depressive-Like Behaviors by Inhibition of GABAergic Projection from Medial Septum to Dentate Gyrus. Adv. Sci. 2023, 10, e2301110. [Google Scholar]
- Zhu, Y.-J.; Huang, J.; Chen, R.; Zhang, Y.; He, X.; Duan, W.-X.; Zou, Y.-L.; Sun, M.-M.; Sun, H.-L.; Cheng, S.-M.; et al. Autophagy Dysfunction Contributes to NLRP1 Inflammasome-Linked Depressive-like Behaviors in Mice. J. Neuroinflamm. 2024, 21, 6. [Google Scholar]
- Kuang, X.-J.; Zhang, C.-Y.; Yan, B.-Y.; Cai, W.-Z.; Lu, C.-L.; Xie, L.-J.; Li, S.-J.; Kong, P.-L.; Fan, J.; Pan, S.-M.; et al. P2X2 Receptors in Pyramidal Neurons Are Critical for Regulating Vulnerability to Chronic Stress. Theranostics 2022, 12, 3703–3718. [Google Scholar]
- Wang, Y.-J.; Zan, G.-Y.; Xu, C.; Li, X.-P.; Shu, X.; Yao, S.-Y.; Xu, X.-S.; Qiu, X.; Chen, Y.; Jin, K.; et al. The Claustrum-Prelimbic Cortex Circuit through Dynorphin/κ-Opioid Receptor Signaling Underlies Depression-like Behaviors Associated with Social Stress Etiology. Nat. Commun. 2023, 14, 7903. [Google Scholar] [PubMed]
- Naß, J.; Abdelfatah, S.; Efferth, T. The Triterpenoid Ursolic Acid Ameliorates Stress in Caenorhabditis Elegans by Affecting the Depression-Associated Genes Skn-1 and Prdx2. Phytomedicine 2021, 88, 153598. [Google Scholar]
- Eren, I.; Naziroğlu, M.; Demirdaş, A. Protective Effects of Lamotrigine, Aripiprazole and Escitalopram on Depression-Induced Oxidative Stress in Rat Brain. Neurochem. Res. 2007, 32, 1188–1195. [Google Scholar]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is Depression Associated with Increased Oxidative Stress? A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A Review of Peripheral Biomarkers in Major Depression: The Potential of Inflammatory and Oxidative Stress Biomarkers. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar]
- Kotan, V.O.; Sarandol, E.; Kirhan, E.; Ozkaya, G.; Kirli, S. Effects of Long-Term Antidepressant Treatment on Oxidative Status in Major Depressive Disorder: A 24-Week Follow-up Study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1284–1290. [Google Scholar]
- Rossetti, A.C.; Paladini, M.S.; Riva, M.A.; Molteni, R. Oxidation-Reduction Mechanisms in Psychiatric Disorders: A Novel Target for Pharmacological Intervention. Pharmacol. Ther. 2020, 210, 107520. [Google Scholar]
- Michel, T.M.; Pülschen, D.; Thome, J. The Role of Oxidative Stress in Depressive Disorders. Curr. Pharm. Des. 2012, 18, 5890–5899. [Google Scholar] [PubMed]
- Fridovich, I. Biological Effects of the Superoxide Radical. Arch. Biochem. Biophys. 1986, 247, 1–11. [Google Scholar] [PubMed]
- Gawryluk, J.W.; Wang, J.-F.; Andreazza, A.C.; Shao, L.; Young, L.T. Decreased Levels of Glutathione, the Major Brain Antioxidant, in Post-Mortem Prefrontal Cortex from Patients with Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2011, 14, 123–130. [Google Scholar] [PubMed]
- Chung, C.P.; Schmidt, D.; Stein, C.M.; Morrow, J.D.; Salomon, R.M. Increased Oxidative Stress in Patients with Depression and Its Relationship to Treatment. Psychiatry Res. 2013, 206, 213–216. [Google Scholar]
- Pomara, N.; Bruno, D.; Sarreal, A.S.; Hernando, R.T.; Nierenberg, J.; Petkova, E.; Sidtis, J.J.; Wisniewski, T.M.; Mehta, P.D.; Pratico, D.; et al. Lower CSF Amyloid Beta Peptides and Higher F2-Isoprostanes in Cognitively Intact Elderly Individuals with Major Depressive Disorder. Am. J. Psychiatry 2012, 169, 523–530. [Google Scholar]
- Romano, A.; Serviddio, G.; Calcagnini, S.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Linking Lipid Peroxidation and Neuropsychiatric Disorders: Focus on 4-Hydroxy-2-Nonenal. Free Radic. Biol. Med. 2017, 111, 281–293. [Google Scholar]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and Oxidative Stress: Results from a Meta-Analysis of Observational Studies. Psychosom. Med. 2014, 76, 12–19. [Google Scholar]
- Zalachoras, I.; Hollis, F.; Ramos-Fernández, E.; Trovo, L.; Sonnay, S.; Geiser, E.; Preitner, N.; Steiner, P.; Sandi, C.; Morató, L. Therapeutic Potential of Glutathione-Enhancers in Stress-Related Psychopathologies. Neurosci. Biobehav. Rev. 2020, 114, 134–155. [Google Scholar]
- Götz, M.E.; Künig, G.; Riederer, P.; Youdim, M.B. Oxidative Stress: Free Radical Production in Neural Degeneration. Pharmacol. Ther. 1994, 63, 37–122. [Google Scholar]
- Koutsilieri, E.; Scheller, C.; Grünblatt, E.; Nara, K.; Li, J.; Riederer, P. Free Radicals in Parkinson’s Disease. J. Neurol. 2002, 249 (Suppl. S2), II1–II5. [Google Scholar]
- Baek, S.-E.; Lee, G.-J.; Rhee, C.-K.; Rho, D.-Y.; Kim, D.-H.; Huh, S.; Lee, S.-K. Decreased Total Antioxidant Activity in Major Depressive Disorder Patients Non-Responsive to Antidepressant Treatment. Psychiatry Investig. 2016, 13, 222–226. [Google Scholar] [PubMed]
- Moylan, S.; Berk, M.; Dean, O.M.; Samuni, Y.; Williams, L.J.; O’Neil, A.; Hayley, A.C.; Pasco, J.A.; Anderson, G.; Jacka, F.N.; et al. Oxidative & Nitrosative Stress in Depression: Why so Much Stress? Neurosci. Biobehav. Rev. 2014, 45, 46–62. [Google Scholar]
- Demirdaş, A.; Nazıroğlu, M.; Ünal, G.Ö. Agomelatine Reduces Brain, Kidney and Liver Oxidative Stress but Increases Plasma Cytokine Production in the Rats with Chronic Mild Stress-Induced Depression. Metab. Brain. Dis. 2016, 31, 1445–1453. [Google Scholar] [PubMed]
- de Bodinat, C.; Guardiola-Lemaitre, B.; Mocaër, E.; Renard, P.; Muñoz, C.; Millan, M.J. Agomelatine, the First Melatonergic Antidepressant: Discovery, Characterization and Development. Nat. Rev. Drug Discov. 2010, 9, 628–642. [Google Scholar] [PubMed]
- Dagyte, G.; Trentani, A.; Postema, F.; Luiten, P.G.; Den Boer, J.A.; Gabriel, C.; Mocaër, E.; Meerlo, P.; Van der Zee, E.A. The Novel Antidepressant Agomelatine Normalizes Hippocampal Neuronal Activity and Promotes Neurogenesis in Chronically Stressed Rats. CNS Neurosci. Ther. 2010, 16, 195–207. [Google Scholar]
- Kalkman, H.O.; Feuerbach, D. Antidepressant Therapies Inhibit Inflammation and Microglial M1-Polarization. Pharmacol. Ther. 2016, 163, 82–93. [Google Scholar]
- Guardiola-Lemaitre, B.; De Bodinat, C.; Delagrange, P.; Millan, M.J.; Munoz, C.; Mocaër, E. Agomelatine: Mechanism of Action and Pharmacological Profile in Relation to Antidepressant Properties. Br. J. Pharmacol. 2014, 171, 3604–3619. [Google Scholar]
- Papp, M.; Gruca, P.; Boyer, P.-A.; Mocaër, E. Effect of Agomelatine in the Chronic Mild Stress Model of Depression in the Rat. Neuropsychopharmacology 2003, 28, 694–703. [Google Scholar]
- Bourin, M.; Mocaër, E.; Porsolt, R. Antidepressant-like Activity of S 20098 (Agomelatine) in the Forced Swimming Test in Rodents: Involvement of Melatonin and Serotonin Receptors. J. Psychiatry Neurosci. 2004, 29, 126–133. [Google Scholar]
- Lan, T.; Wu, Y.; Zhang, Y.; Li, S.; Zhu, Z.; Wang, L.; Mao, X.; Li, Y.; Fan, C.; Wang, W.; et al. Agomelatine Rescues Lipopolysaccharide-Induced Neural Injury and Depression-like Behaviors via Suppression of the Gαi-2-PKA-ASK1 Signaling Pathway. J. Neuroinflamm. 2022, 19, 117. [Google Scholar]
- Cardinali, D.P.; Srinivasan, V.; Brzezinski, A.; Brown, G.M. Melatonin and Its Analogs in Insomnia and Depression. J. Pineal Res. 2012, 52, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Boulle, F.; Massart, R.; Stragier, E.; Païzanis, E.; Zaidan, L.; Marday, S.; Gabriel, C.; Mocaer, E.; Mongeau, R.; Lanfumey, L. Hippocampal and Behavioral Dysfunctions in a Mouse Model of Environmental Stress: Normalization by Agomelatine. Transl. Psychiatry 2014, 4, e485. [Google Scholar] [CrossRef]
- Dastgheib, M.; Moezi, L. Acute and Chronic Effects of Agomelatine on Intravenous Penthylenetetrazol-Induced Seizure in Mice and the Probable Role of Nitric Oxide. Eur. J. Pharmacol. 2014, 736, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.A.; Covington, H.E.; Berton, O.; Russo, S.J. A Standardized Protocol for Repeated Social Defeat Stress in Mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; et al. Edaravone Ameliorates Depressive and Anxiety-like Behaviors via Sirt1/Nrf2/HO-1/Gpx4 Pathway. J. Neuroinflamm. 2022, 19, 41. [Google Scholar] [CrossRef]
- Li, Y.; Fan, C.; Wang, C.; Wang, L.; Yi, Y.; Mao, X.; Chen, X.; Lan, T.; Wang, W.; Yu, S.Y. Stress-Induced Reduction of Na+/H+ Exchanger Isoform 1 Promotes Maladaptation of Neuroplasticity and Exacerbates Depressive Behaviors. Sci. Adv. 2022, 8, eadd7063. [Google Scholar] [CrossRef]
- Pan, L.; Zheng, L.; Wu, X.; Zhu, Z.; Wang, S.; Lu, Y.; He, Y.; Yang, Q.; Ma, X.; Wang, X.; et al. A Short Period of Early Life Oxytocin Treatment Rescues Social Behavior Dysfunction via Suppression of Hippocampal Hyperactivity in Male Mice. Mol. Psychiatry 2022, 27, 4157–4171. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.A.; Villumsen, K.R.; Ernst, M.; Hansen, M.; Forberg, T.; Gopalakrishnan, S.; Gilbert, M.T.P.; Bojesen, A.M.; Kristiansen, K.; Limborg, M.T. A Multi-Omics Approach Unravels Metagenomic and Metabolic Alterations of a Probiotic and Synbiotic Additive in Rainbow Trout (Oncorhynchus Mykiss). Microbiome 2022, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; HUSERMET Consortium; et al. Development of a Robust and Repeatable UPLC-MS Method for the Long-Term Metabolomic Study of Human Serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef]
- Navarro-Reig, M.; Jaumot, J.; García-Reiriz, A.; Tauler, R. Evaluation of Changes Induced in Rice Metabolome by Cd and Cu Exposure Using LC-MS with XCMS and MCR-ALS Data Analysis Strategies. Anal. Bioanal. Chem. 2015, 407, 8835–8847. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global Metabolic Profiling of Animal and Human Tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Demurtas, A.; Pescina, S.; Nicoli, S.; Santi, P.; Ribeiro de Araujo, D.; Padula, C. Validation of a HPLC-UV Method for the Quantification of Budesonide in Skin Layers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1164, 122512. [Google Scholar]
- Lan, T.; Li, Y.; Chen, X.; Wang, W.; Wang, C.; Lou, H.; Chen, S.; Yu, S. Exercise-Activated mPFC Tri-Synaptic Pathway Ameliorates Depression-Like Behaviors in Mouse. Adv. Sci. 2025, 12, 2408618. [Google Scholar]
- Torres-Berrío, A.; Estill, M.; Patel, V.; Ramakrishnan, A.; Kronman, H.; Minier-Toribio, A.; Issler, O.; Browne, C.J.; Parise, E.M.; van der Zee, Y.Y.; et al. Mono-Methylation of Lysine 27 at Histone 3 Confers Lifelong Susceptibility to Stress. Neuron 2024, 112, 2973–2989.e10. [Google Scholar]
- Huang, J.; Huang, W.; Yi, J.; Deng, Y.; Li, R.; Chen, J.; Shi, J.; Qiu, Y.; Wang, T.; Chen, X.; et al. Mesenchymal Stromal Cells Alleviate Depressive and Anxiety-like Behaviors via a Lung Vagal-to-Brain Axis in Male Mice. Nat. Commun. 2023, 14, 7406. [Google Scholar] [PubMed]
- Fox, M.E.; Lobo, M.K. The Molecular and Cellular Mechanisms of Depression: A Focus on Reward Circuitry. Mol. Psychiatry 2019, 24, 1798–1815. [Google Scholar]
- Holtzheimer, P.E.; Mayberg, H.S. Stuck in a Rut: Rethinking Depression and Its Treatment. Trends Neurosci. 2011, 34, 1–9. [Google Scholar] [PubMed]
- Pillinger, T.; Howes, O.D.; Correll, C.U.; Leucht, S.; Huhn, M.; Schneider-Thoma, J.; Gaughran, F.; Jauhar, S.; McGuire, P.K.; Taylor, D.M.; et al. Antidepressant and Antipsychotic Side-Effects and Personalised Prescribing: A Systematic Review and Digital Tool Development. Lancet Psychiatry 2023, 10, 860–876. [Google Scholar]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [PubMed]
- Protti, M.; Mandrioli, R.; Marasca, C.; Cavalli, A.; Serretti, A.; Mercolini, L. New-Generation, Non-SSRI Antidepressants: Drug-Drug Interactions and Therapeutic Drug Monitoring. Part 2: NaSSAs, NRIs, SNDRIs, MASSAs, NDRIs, and Others. Med. Res. Rev. 2020, 40, 1794–1832. [Google Scholar] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major Depressive Disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [PubMed]
- Berger, T.; Lee, H.; Young, A.H.; Aarsland, D.; Thuret, S. Adult Hippocampal Neurogenesis in Major Depressive Disorder and Alzheimer’s Disease. Trends Mol. Med. 2020, 26, 803–818. [Google Scholar]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol. Psychiatry 2019, 85, 443–453. [Google Scholar]
- Lu, J.; Gong, X.; Yao, X.; Guang, Y.; Yang, H.; Ji, R.; He, Y.; Zhou, W.; Wang, H.; Wang, W.; et al. Prolonged Chronic Social Defeat Stress Promotes Less Resilience and Higher Uniformity in Depression-like Behaviors in Adult Male Mice. Biochem. Biophys. Res. Commun. 2021, 553, 107–113. [Google Scholar]
- Bullich, S.; Delcourte, S.; Haddjeri, N.; Guiard, B.P. Learned Immobility Produces Enduring Impairment of the HPA Axis Reactivity in Mice without Replicating the Broad Spectrum of Depressive-Like Phenotype. Int. J. Mol. Sci. 2021, 22, 937. [Google Scholar] [CrossRef]
- Ji, N.; Lei, M.; Chen, Y.; Tian, S.; Li, C.; Zhang, B. How Oxidative Stress Induces Depression? ASN Neuro 2023, 15, 17590914231181037. [Google Scholar]
- Zhao, Y.-T.; Zhang, L.; Yin, H.; Shen, L.; Zheng, W.; Zhang, K.; Zeng, J.; Hu, C.; Liu, Y. Hydroxytyrosol Alleviates Oxidative Stress and Neuroinflammation and Enhances Hippocampal Neurotrophic Signaling to Improve Stress-Induced Depressive Behaviors in Mice. Food Funct. 2021, 12, 5478–5487. [Google Scholar]
- Mahmoud, A.M.; Abd El-Ghafar, O.A.M.; Alzoghaibi, M.A.; Hassanein, E.H.M. Agomelatine Prevents Gentamicin Nephrotoxicity by Attenuating Oxidative Stress and TLR-4 Signaling, and Upregulating PPARγ and SIRT1. Life Sci. 2021, 278, 119600. [Google Scholar]
- Alruhaimi, R.S.; Hassanein, E.H.M.; Bin-Jumah, M.N.; Mahmoud, A.M. Cadmium Cardiotoxicity Is Associated with Oxidative Stress and Upregulated TLR-4/NF-kB Pathway in Rats; Protective Role of Agomelatine. Food Chem. Toxicol. 2023, 180, 114055. [Google Scholar] [PubMed]
- Xu, J.; Zhu, C.; Jin, P.; Sun, W.; Yu, E. Agomelatine Prevented Depression in the Chronic Restraint Stress Model through Enhanced Catalase Activity and Halted Oxidative Stress. PLoS ONE 2024, 19, e0289248. [Google Scholar]
- Wigner, P.; Synowiec, E.; Jóźwiak, P.; Czarny, P.; Bijak, M.; Barszczewska, G.; Białek, K.; Szemraj, J.; Gruca, P.; Papp, M.; et al. The Changes of Expression and Methylation of Genes Involved in Oxidative Stress in Course of Chronic Mild Stress and Antidepressant Therapy with Agomelatine. Genes 2020, 11, 644. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, D. Ferulic Acid as a Therapeutic Agent in Depression: Evidence from Preclinical Studies. CNS Neurosci. Ther. 2023, 29, 2397–2412. [Google Scholar]
- Bouvier, E.; Brouillard, F.; Molet, J.; Claverie, D.; Cabungcal, J.-H.; Cresto, N.; Doligez, N.; Rivat, C.; Do, K.Q.; Bernard, C.; et al. Nrf2-Dependent Persistent Oxidative Stress Results in Stress-Induced Vulnerability to Depression. Mol. Psychiatry 2017, 22, 1701–1713. [Google Scholar]
- Tang, F.; Zhao, L.; Yu, Q.; Liu, T.; Gong, H.; Liu, Z.; Li, Q. Upregulation of miR-215 Attenuates Propofol-Induced Apoptosis and Oxidative Stress in Developing Neurons by Targeting LATS2. Mol. Med. 2020, 26, 38. [Google Scholar]
- Lan, T.; Li, Y.; Fan, C.; Wang, L.; Wang, W.; Chen, S.; Yu, S.Y. MicroRNA-204-5p Reduction in Rat Hippocampus Contributes to Stress-Induced Pathology via Targeting RGS12 Signaling Pathway. J. Neuroinflamm. 2021, 18, 243. [Google Scholar]
- El-Demerdash, F.M. Lipid Peroxidation, Oxidative Stress and Acetylcholinesterase in Rat Brain Exposed to Organophosphate and Pyrethroid Insecticides. Food Chem. Toxicol. 2011, 49, 1346–1352. [Google Scholar]
- Liu, J.; Yang, L.; Niu, Y.; Su, C.; Wang, Y.; Ren, R.; Chen, J.; Ma, X. Potential Therapeutic Effects of Mi-Jian-Chang-Pu Decoction on Neurochemical and Metabolic Changes of Cerebral Ischemia-Reperfusion Injury in Rats. Oxid. Med. Cell Longev. 2022, 2022, 7319563. [Google Scholar]
- Shakoor, M.U.; Tareen, F.K.; Rehman, Z.; Saghir, K.A.; Ashraf, W.; Anjum, S.M.M.; Ahmad, T.; Alqahtani, F.; Imran, I. Probiotics by Modulating Gut–Brain Axis Together with Brivaracetam Mitigate Seizure Progression, Behavioral Incongruities, and Prevented Neurodegeneration in Pentylenetetrazole-Kindled Mice. CNS Neurosci. Ther. 2024, 30, e70078. [Google Scholar]
- Fan, C.; Li, Y.; Lan, T.; Wang, W.; Mao, X.; Yu, S.Y. Prophylactic Treatment of Curcumin in a Rat Model of Depression by Attenuating Hippocampal Synaptic Loss. Food Funct. 2021, 12, 11202–11213. [Google Scholar] [PubMed]
- Rappeneau, V.; Blaker, A.; Petro, J.R.; Yamamoto, B.K.; Shimamoto, A. Disruption of the Glutamate-Glutamine Cycle Involving Astrocytes in an Animal Model of Depression for Males and Females. Front. Behav. Neurosci. 2016, 10, 231. [Google Scholar]
- Jovanović, P.; Žorić, L.; Stefanović, I.; Džunić, B.; Djordjević-Jocić, J.; Radenković, M.; Jovanović, M. Lactate dehydrogenase and oxidative stress activity in primary open-angle glaucoma aqueous humour. Bosn. J. Basic. Med. Sci. 2010, 10, 83–88. [Google Scholar] [PubMed]
- Amadio, P.; Sandrini, L.; Zarà, M.; Barbieri, S.S.; Ieraci, A. NADPH-Oxidases as Potential Pharmacological Targets for Thrombosis and Depression Comorbidity. Redox Biol. 2024, 70, 103060. [Google Scholar]
- Wang, L.; Wei, Y.; Sun, Z.; Tai, W.; Li, H.; Yin, Y.; Jiang, L.; Wang, J. Effectiveness and Mechanisms of Combined Use of Antioxidant Nutrients in Protecting against Oxidative Stress-induced Neuronal Loss and Related Neurological Deficits. CNS Neurosci. Ther. 2024, 30, e14886. [Google Scholar]
- Ren, Y.; Sun-Waterhouse, D.; Ouyang, F.; Tan, X.; Li, D.; Xu, L.; Li, B.; Wang, Y.; Li, F. Apple Phenolic Extracts Ameliorate Lead-Induced Cognitive Impairment and Depression- and Anxiety-like Behavior in Mice by Abating Oxidative Stress, Inflammation and Apoptosis via the miR-22-3p/SIRT1 Axis. Food Funct. 2022, 13, 2647–2661. [Google Scholar]
- Xu, B.; Yang, R.; Qiang, J.; Xu, X.; Zhou, M.; Ji, X.; Lu, Y.; Dong, Z. Gypenoside XLIX Attenuates Sepsis-Induced Splenic Injury through Inhibiting Inflammation and Oxidative Stress. Int. Immunopharmacol. 2024, 127, 111420. [Google Scholar]
- Liao, Y.; Huang, J.; Wang, Z.; Yang, Z.; Shu, Y.; Gan, S.; Wang, Z.; Lu, W. The Phosphokinase Activity of IRE1ɑ Prevents the Oxidative Stress Injury through miR-25/Nox4 Pathway after ICH. CNS Neurosci. Ther. 2023, 30, e14537. [Google Scholar]
- Yang, Y.; Yu, L.; Zhu, T.; Xu, S.; He, J.; Mao, N.; Liu, Z.; Wang, D. Neuroprotective Effects of Lycium barbarum Polysaccharide on Light-Induced Oxidative Stress and Mitochondrial Damage via the Nrf2/HO-1 Pathway in Mouse Hippocampal Neurons. Int. J. Biol. Macromol. 2023, 251, 126315. [Google Scholar]
- Sun, Y.-Y.; Zhu, H.-J.; Zhao, R.-Y.; Zhou, S.-Y.; Wang, M.-Q.; Yang, Y.; Guo, Z.-N. Remote Ischemic Conditioning Attenuates Oxidative Stress and Inflammation via the Nrf2/HO-1 Pathway in MCAO Mice. Redox. Biol. 2023, 66, 102852. [Google Scholar]
- Liu, Y.; Wang, S.; Jin, G.; Gao, K.; Wang, S.; Zhang, X.; Zhou, K.; Cai, Y.; Zhou, X.; Zhao, Z. Network Pharmacology-Based Study on the Mechanism of ShenKang Injection in Diabetic Kidney Disease through Keap1/Nrf2/Ho-1 Signaling Pathway. Phytomedicine 2023, 118, 154915. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, X.; Wang, Y.; Li, Z.; Wang, Y.; Shi, J.; Guan, F. MG53 Protein Rejuvenates hUC-MSCs and Facilitates Their Therapeutic Effects in AD Mice by Activating Nrf2 Signaling Pathway. Redox. Biol. 2022, 53, 102325. [Google Scholar] [CrossRef]
- Ji, R.; Jia, F.; Chen, X.; Gao, Y.; Yang, J. Carnosol Inhibits KGN Cells Oxidative Stress and Apoptosis and Attenuates Polycystic Ovary Syndrome Phenotypes in Mice through Keap1-Mediated Nrf2/HO-1 Activation. Phytother. Res. 2023, 37, 1405–1421. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Wang, G.; Huang, J.; Liang, L.; Zheng, Y.; Wei, Y.; Wang, H.; Xiao, L.; Wang, H. Dihydrolipoic Acid Protects against Lipopolysaccharide-Induced Behavioral Deficits and Neuroinflammation via Regulation of Nrf2/HO-1/NLRP3 Signaling in Rat. J. Neuroinflamm. 2020, 17, 166. [Google Scholar] [CrossRef]
- Chumboatong, W.; Khamchai, S.; Tocharus, C.; Govitrapong, P.; Tocharus, J. Agomelatine Protects against Permanent Cerebral Ischaemia via the Nrf2-HO-1 Pathway. Eur. J. Pharmacol. 2020, 874, 173028. [Google Scholar] [CrossRef] [PubMed]
- Firdoos, S.; Dai, R.; Younas, Z.; Shah, F.A.; Gul, M.; Rasheed, M. Agomelatine-Loaded Nanostructured Lipid Carriers Alleviate Neuropathic Pain in Rats by Nrf2/HO-1 Signalling Pathway. Clin. Exp. Pharmacol. Physiol. 2024, 51, e13922. [Google Scholar] [CrossRef]
- Alruhaimi, R.S.; Hassanein, E.H.M.; Bin-Jumah, M.N.; Mahmoud, A.M. Cadmium-Induced Lung Injury Is Associated with Oxidative Stress, Apoptosis, and Altered SIRT1 and Nrf2/HO-1 Signaling; Protective Role of the Melatonin Agonist Agomelatine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 2335–2345. [Google Scholar] [CrossRef]
- Alruhaimi, R.S.; Hassanein, E.H.M.; Abd El-Aziz, M.K.; Siddiq Abduh, M.; Bin-Ammar, A.; Kamel, E.M.; Mahmoud, A.M. The Melatonin Receptor Agonist Agomelatine Protects against Acute Pancreatitis Induced by Cadmium by Attenuating Inflammation and Oxidative Stress and Modulating Nrf2/HO-1 Pathway. Int. Immunopharmacol. 2023, 124, 110833. [Google Scholar] [CrossRef]
- Scuto, M.; Majzúnová, M.; Torcitto, G.; Antonuzzo, S.; Rampulla, F.; Di Fatta, E.; Trovato Salinaro, A. Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health. Int. J. Mol. Sci. 2024, 25, 12155. [Google Scholar] [CrossRef]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef]
- Deng, M.; Sun, J.; Peng, L.; Huang, Y.; Jiang, W.; Wu, S.; Zhou, L.; Chung, S.K.; Cheng, X. Scutellarin Acts on the AR-NOX Axis to Remediate Oxidative Stress Injury in a Mouse Model of Cerebral Ischemia/Reperfusion Injury. Phytomedicine 2022, 103, 154214. [Google Scholar] [PubMed]
- Xiong, F.; Jiang, K.; Wu, Y.; Lou, C.; Ding, C.; Zhang, W.; Zhang, X.; Li, C.; Zheng, H.; Gao, H. Intermittent Fasting Alleviates Type 1 Diabetes-Induced Cognitive Dysfunction by Improving the Frontal Cortical Metabolic Disorder. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2023, 1869, 166725. [Google Scholar]
- Shakil, S.; Masjoan Juncos, J.X.; Mariappan, N.; Zafar, I.; Amudhan, A.; Amudhan, A.; Aishah, D.; Siddiqui, S.; Manzoor, S.; Santana, C.M.; et al. Behavioral and Neuronal Effects of Inhaled Bromine Gas: Oxidative Brain Stem Damage. Int. J. Mol. Sci. 2021, 22, 6316. [Google Scholar] [CrossRef]
- Yin, M.; Liu, Z.; Wang, J.; Gao, W. Buyang Huanwu Decoction Alleviates Oxidative Injury of Cerebral Ischemia-Reperfusion through PKCε/Nrf2 Signaling Pathway. J. Ethnopharmacol. 2023, 303, 115953. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.; Huo, H.; Ma, F.; Zhao, M.; Han, Q.; Hu, L.; Li, Y.; Zhang, H.; Pan, J.; et al. N-Acetylcysteine Combined with Insulin Alleviates the Oxidative Damage of Cerebrum via Regulating Redox Homeostasis in Type 1 Diabetic Mellitus Canine. Life Sci. 2022, 308, 120958. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld-Klatta, K.; Jiers, W.; Rzepczyk, S.; Nowicki, F.; Łukasik-Głębocka, M.; Świderski, P.; Zielińska-Psuja, B.; Żaba, Z.; Żaba, C. The Effect of Neuropsychiatric Drugs on the Oxidation-Reduction Balance in Therapy. Int. J. Mol. Sci. 2024, 25, 7304. [Google Scholar] [CrossRef]
- Mariani, N.; Everson, J.; Pariante, C.M.; Borsini, A. Modulation of Microglial Activation by Antidepressants. J. Psychopharmacol. 2022, 36, 131–150. [Google Scholar]
- Ștefan, M.-G.; Kiss, B.; Gutleb, A.C.; Loghin, F. Redox Metabolism Modulation as a Mechanism in SSRI Toxicity and Pharmacological Effects. Arch. Toxicol. 2020, 94, 1417–1441. [Google Scholar]
- Cherngwelling, R.; Pengrattanachot, N.; Swe, M.T.; Thongnak, L.; Promsan, S.; Phengpol, N.; Sutthasupha, P.; Lungkaphin, A. Agomelatine Protects against Obesity-Induced Renal Injury by Inhibiting Endoplasmic Reticulum Stress/Apoptosis Pathway in Rats. Toxicol. Appl. Pharmacol. 2021, 425, 115601. [Google Scholar]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Safa, M.; Ghaznavi, H.; Naseripour, M. Diabetic Retinopathy Pathogenesis and the Ameliorating Effects of Melatonin; Involvement of Autophagy, Inflammation and Oxidative Stress. Life Sci. 2018, 193, 20–33. [Google Scholar] [CrossRef]
- Kasahara, T.; Abe, K.; Mekada, K.; Yoshiki, A.; Kato, T. Genetic Variation of Melatonin Productivity in Laboratory Mice under Domestication. Proc. Natl. Acad. Sci. USA 2010, 107, 6412–6417. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J. Melatonin Research in Mice: A Review. Chronobiol. Int. 2019, 36, 1167–1183. [Google Scholar] [CrossRef]
- Zhang, Z.; Silveyra, E.; Jin, N.; Ribelayga, C.P. A Congenic Line of the C57BL/6J Mouse Strain That Is Proficient in Melatonin Synthesis. J. Pineal. Res. 2018, 65, e12509. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, X.; Ge, M.; Hu, H.; Xu, C.; Wen, S.; Deng, H.; Mei, X. Zinc Defends against Parthanatos and Promotes Functional Recovery after Spinal Cord Injury through SIRT3-mediated Anti-oxidative Stress and Mitophagy. CNS Neurosci. Ther. 2023, 29, 2857–2872. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, A.; He, H.; She, X.; He, Y.; Li, S.; Liu, L.; Luo, T.; Huang, N.; Luo, H.; et al. Trametenolic Acid B Protects against Cerebral Ischemia and Reperfusion Injury through Modulation of microRNA-10a and PI3K/Akt/mTOR Signaling Pathways. Biomed. Pharmacother. 2019, 112, 108692. [Google Scholar] [CrossRef]
- Wang, M.; Bi, Y.; Zeng, S.; Liu, Y.; Shao, M.; Liu, K.; Deng, Y.; Wen, G.; Sun, X.; Zeng, P.; et al. Modified Xiaoyao San Ameliorates Depressive-like Behaviors by Triggering Autophagosome Formation to Alleviate Neuronal Apoptosis. Biomed. Pharmacother. 2019, 111, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Akhter, F.; Xue, R.; Sosunov, A.A.; Wu, L.; Chen, D.; Arancio, O.; Yan, S.F.; Yan, S.S. Synaptic Mitochondria Glycation Contributes to Mitochondrial Stress and Cognitive Dysfunction. Brain 2025, 148, 262–275. [Google Scholar] [CrossRef]
- Verstreken, P.; Ly, C.V.; Venken, K.J.T.; Koh, T.-W.; Zhou, Y.; Bellen, H.J. Synaptic Mitochondria Are Critical for Mobilization of Reserve Pool Vesicles at Drosophila Neuromuscular Junctions. Neuron 2005, 47, 365–378. [Google Scholar] [CrossRef]
- Sun, T.; Qiao, H.; Pan, P.-Y.; Chen, Y.; Sheng, Z.-H. Motile Axonal Mitochondria Contribute to the Variability of Presynaptic Strength. Cell Rep. 2013, 4, 413–419. [Google Scholar] [CrossRef]
- Evans, R.J.; Derkach, V.; Surprenant, A. ATP Mediates Fast Synaptic Transmission in Mammalian Neurons. Nature 1992, 357, 503–505. [Google Scholar] [CrossRef]
- Amaro, A.; Sousa, D.; Sá-Rocha, M.; Ferreira-Junior, M.D.; Rosendo-Silva, D.; Saavedra, L.P.J.; Barra, C.; Monteiro-Alfredo, T.; Gomes, R.M.; de Freitas Mathias, P.C.; et al. Postnatal Overfeeding in Rodents Induces a Neurodevelopment Delay and Anxious-like Behaviour Accompanied by Sex- and Brain-Region-Specific Synaptic and Metabolic Changes. Nutrients 2023, 15, 3581. [Google Scholar] [CrossRef] [PubMed]
- Hasantari, I.; Nicolas, N.; Alzieu, P.; Leval, L.; Shalabi, A.; Grolleau, S.; Dinet, V. Factor H’s Control of Complement Activation Emerges as a Significant and Promising Therapeutic Target for Alzheimer’s Disease Treatment. Int. J. Mol. Sci. 2024, 25, 2272. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Pandey, S.; Li, Y.; Badger, J.D.; Lu, W.; Roche, K.W. PSD-95 Binding Dynamically Regulates NLGN1 Trafficking and Function. Proc. Natl. Acad. Sci. USA 2019, 116, 12035–12044. [Google Scholar] [CrossRef] [PubMed]
- Maria-Belen, P.-R.; Isabel, P.; David, A.P. Structural and Functional Abnormalities in Thalamic Neurons Following Neocortical Focal Status Epilepticus. Neurobiol. Dis. 2023, 176, 105934. [Google Scholar] [CrossRef]
- Li, H.; Graber, K.D.; Jin, S.; McDonald, W.; Barres, B.A.; Prince, D.A. Gabapentin Decreases Epileptiform Discharges in a Chronic Model of Neocortical Trauma. Neurobiol. Dis. 2012, 48, 429–438. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Li, Y.; Yu, Z.; Chen, X.; Lan, T.; Wang, M.; Yu, S. Agomelatine Alleviates Depressive-like Behaviors by Suppressing Hippocampal Oxidative Stress in the Chronic Social Defeat Stress Model. Antioxidants 2025, 14, 410. https://doi.org/10.3390/antiox14040410
Zhu Y, Li Y, Yu Z, Chen X, Lan T, Wang M, Yu S. Agomelatine Alleviates Depressive-like Behaviors by Suppressing Hippocampal Oxidative Stress in the Chronic Social Defeat Stress Model. Antioxidants. 2025; 14(4):410. https://doi.org/10.3390/antiox14040410
Chicago/Turabian StyleZhu, Yan, Ye Li, Zhaoying Yu, Xiao Chen, Tian Lan, Meijian Wang, and Shuyan Yu. 2025. "Agomelatine Alleviates Depressive-like Behaviors by Suppressing Hippocampal Oxidative Stress in the Chronic Social Defeat Stress Model" Antioxidants 14, no. 4: 410. https://doi.org/10.3390/antiox14040410
APA StyleZhu, Y., Li, Y., Yu, Z., Chen, X., Lan, T., Wang, M., & Yu, S. (2025). Agomelatine Alleviates Depressive-like Behaviors by Suppressing Hippocampal Oxidative Stress in the Chronic Social Defeat Stress Model. Antioxidants, 14(4), 410. https://doi.org/10.3390/antiox14040410





