Effects of Ammonia Stress on the Antioxidant, Ferroptosis, and Immune Response in the Liver of Golden Pompano Trachinotus ovatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Culture Conditions
2.2. Ammonia Stress Exposure and Sampling
2.3. Histomorphological Analysis
2.4. Biochemical Analysis
2.5. Gene Expression Analysis
2.6. Integrated Biomarker Response (IBR)
2.7. Statistical Analysis
3. Results
3.1. The Survival of the Fish
3.2. Histomorphological Changes of the Liver
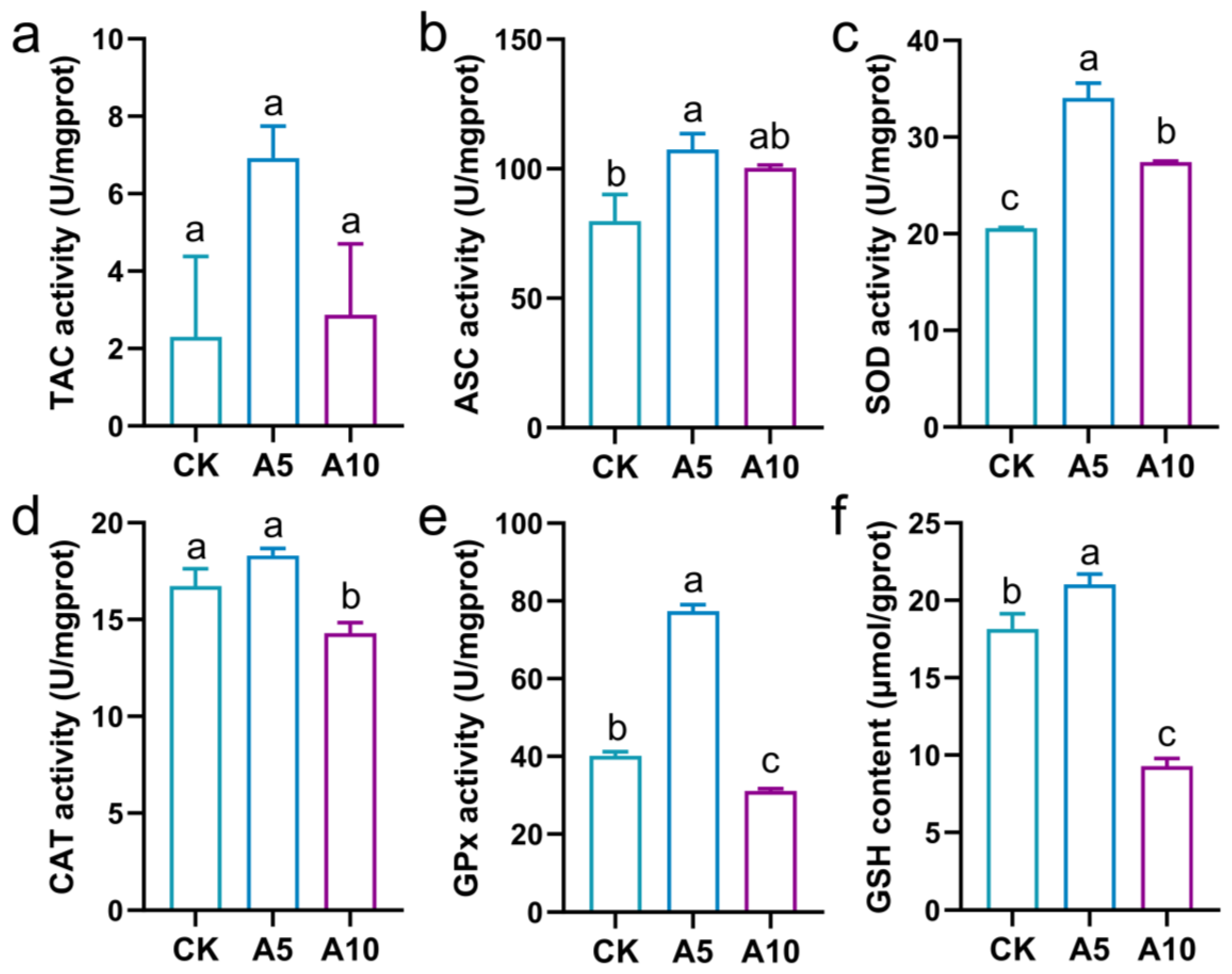
3.3. The Oxidative Stress Response in the Liver
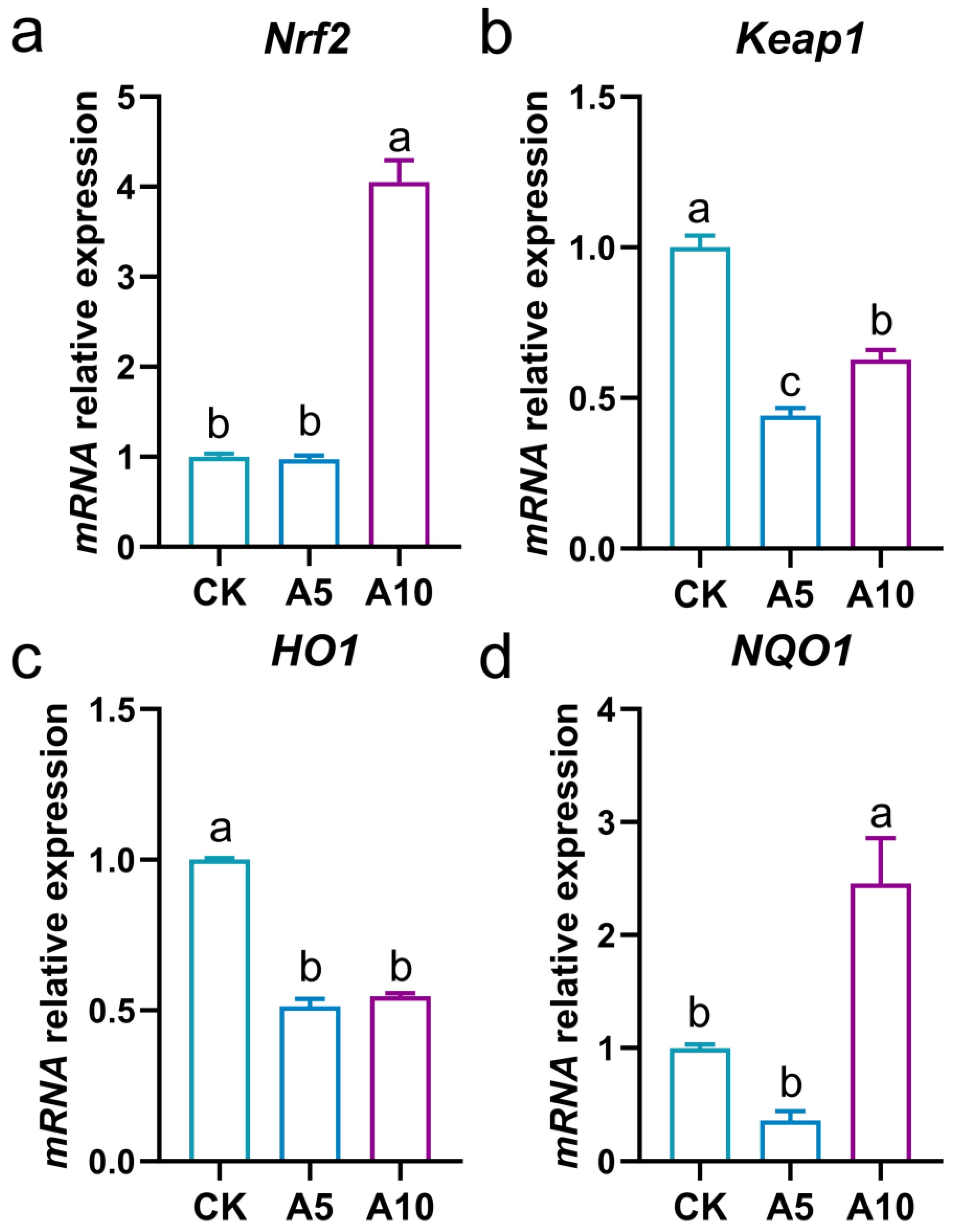
3.4. Changes of Ferroptosis-Related Gene Expression in the Liver
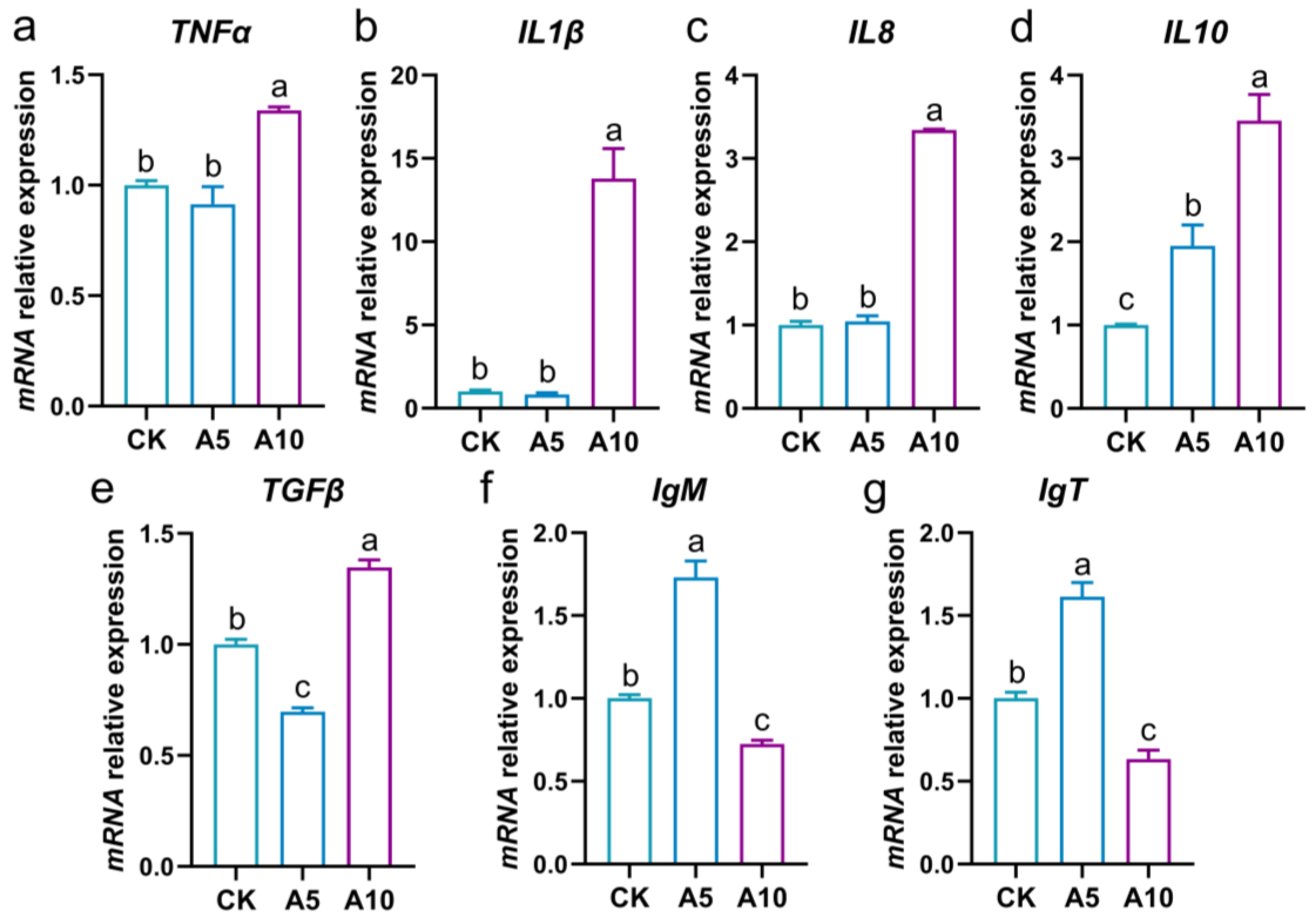
3.5. Changes of Inflammation-Related Gene Expression in the Liver
3.6. Changes of Immune-Related Gene Expression in the Liver
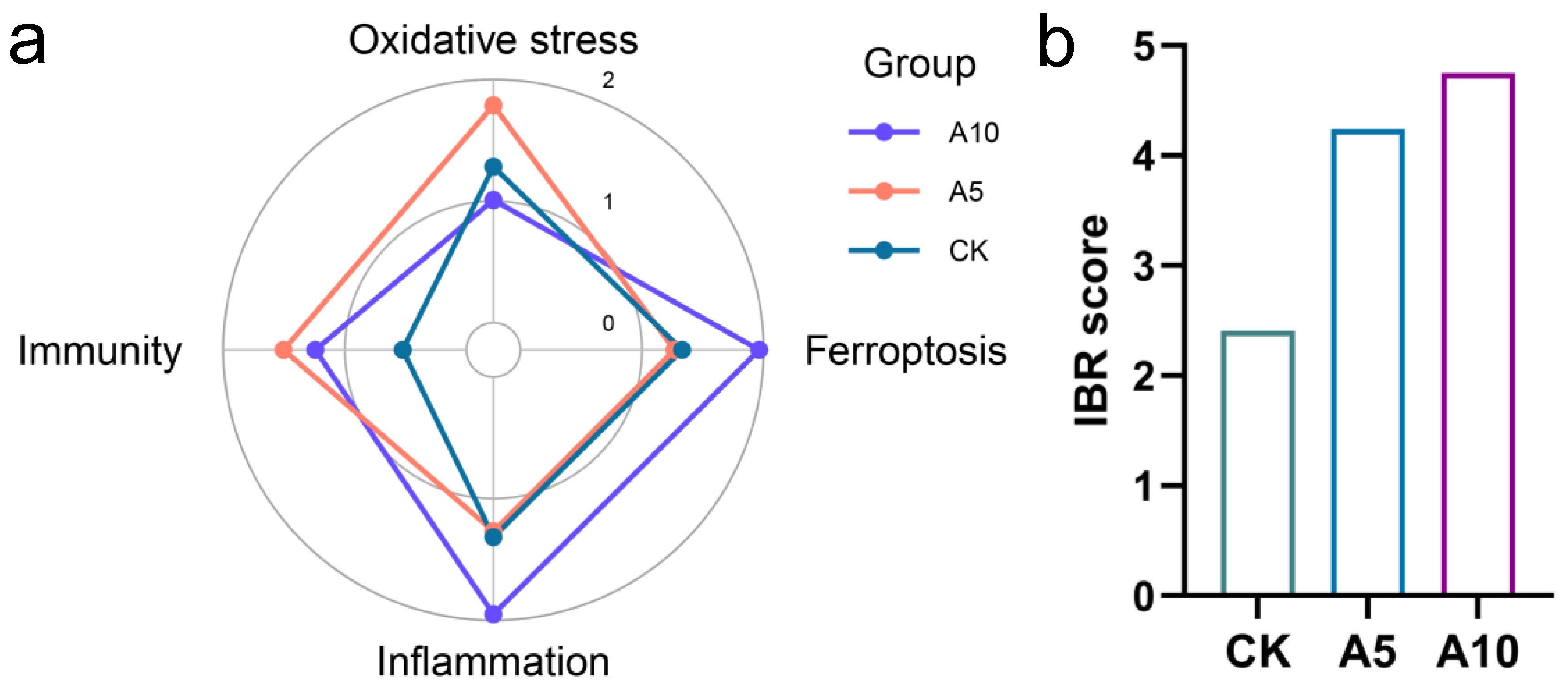
3.7. Integration of the Multi-Level Biomarker Responses
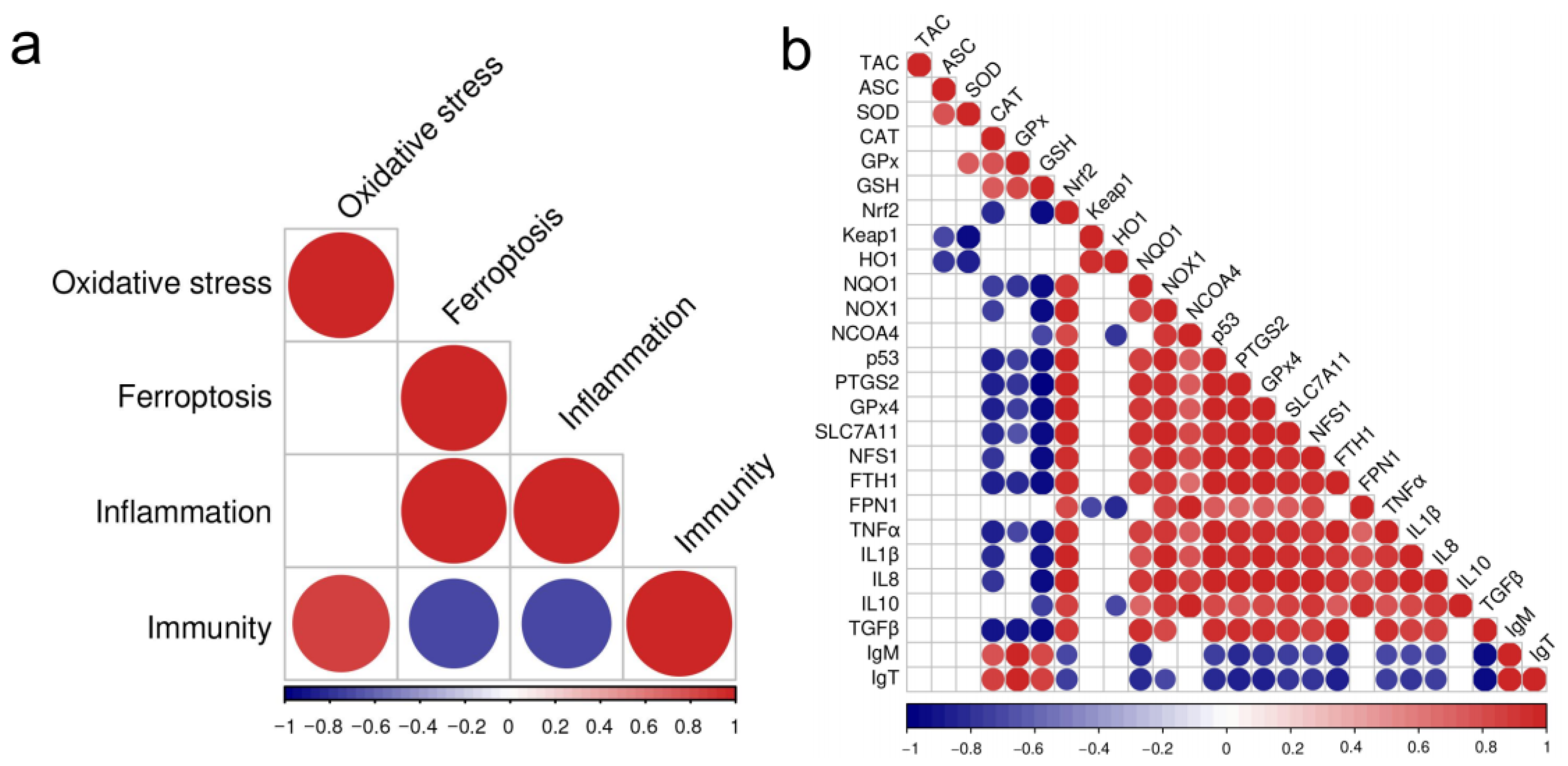
3.8. The Correlation Between the Changes of the Biomarkers
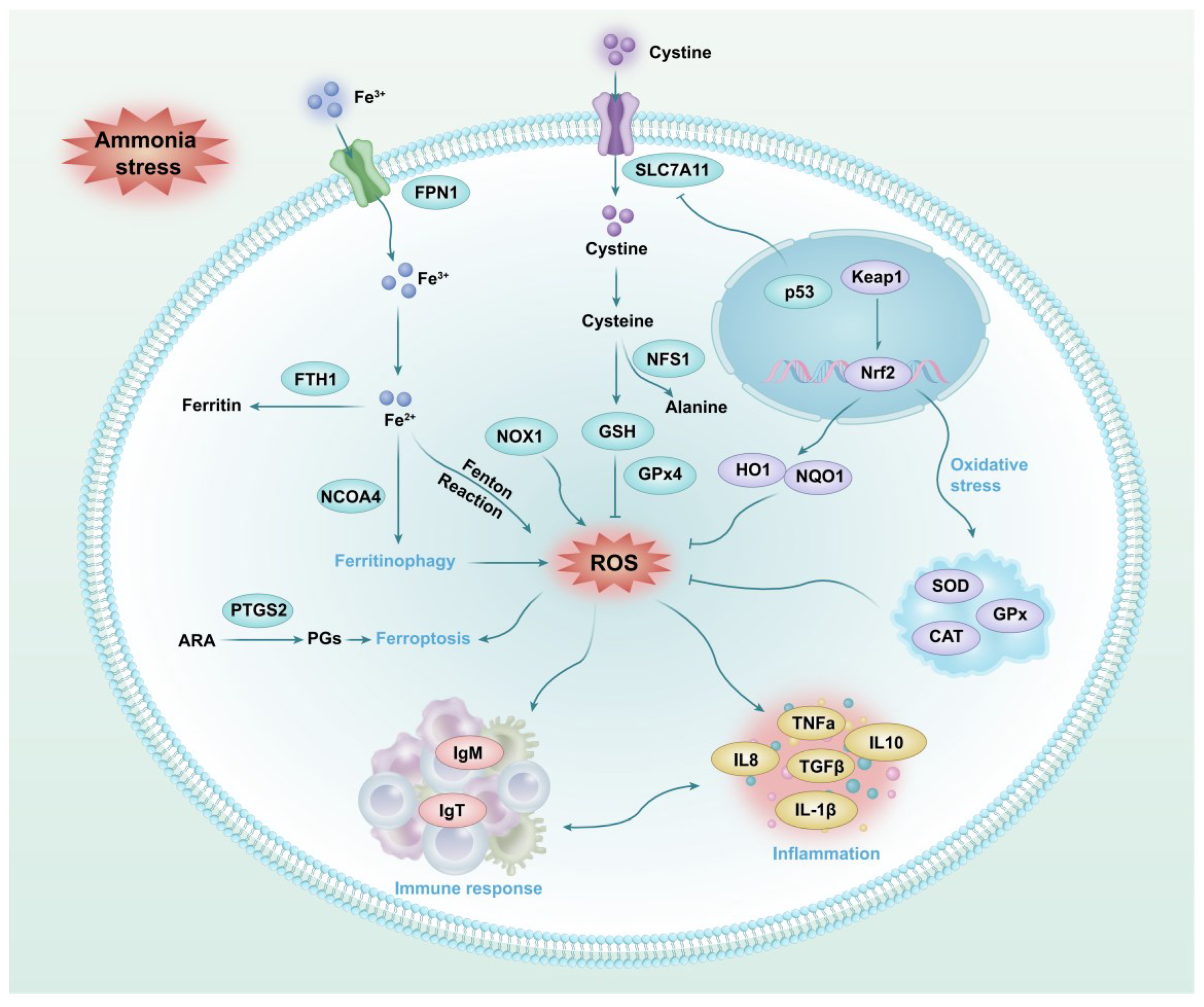
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- China Fishery Statistical Yearbook. In 2-2 Aquaculture; China Agriculture Press: Beijing, China, 2023; p. 22.
- Feng, Y.W.; Su, X.G.; Sun, H.M.; Lin, H.P.; Chen, Q.H.; Shu, H. Identification and denitrification performance of a high ammonia nitrogen-resistant aerobic denitrifying bacteria. South China Fish. Sci. 2023, 19, 107–115. [Google Scholar]
- Gan, L.; Yao, L.; Zhang, M.Z.; He, K.W.; Zhang, H.; Lin, Y.H.; Qin, C.J.; Shao, J.; Jiang, H.B.; Wen, M. Ammonia triggers ferroptosis in macrophages of yellow catfish Pelteobagrus fulvidraco. Aquac. Res. 2022, 53, 568–575. [Google Scholar]
- Liu, M.J.; Guo, H.Y.; Liu, B.; Zhu, K.C.; Guo, L.; Liu, B.S.; Zhang, N.; Yang, J.W.; Jiang, S.G.; Zhang, D.C. Gill oxidative damage caused by acute ammonia stress was reduced through the HIF-1α/NF-κB signaling pathway in golden pompano (Trachinotus ovatus). Ecotoxicol. Environ. Saf. 2021, 222, 112504. [Google Scholar]
- Liu, M.J.; Guo, H.Y.; Zhu, K.C.; Liu, B.S.; Liu, B.; Guo, L.; Zhang, N.; Yang, J.W.; Jiang, S.G.; Zhang, D.C. Effects of acute ammonia exposure and recovery on the antioxidant response and expression of genes in the Nrf2-Keap1 signaling pathway in the juvenile golden pompano (Trachinotus ovatus). Aquat. Toxicol. 2021, 240, 105969. [Google Scholar]
- Wu, Y.W.; Xia, Y.T.; Hu, A.; Xiong, G.Q.; Wu, W.J.; Shi, L.; Chen, L.; Guo, X.J.; Qiao, Y.; Liu, C.S.; et al. Difference in muscle metabolism caused by metabolism disorder of rainbow trout liver exposed to ammonia stress. Sci. Total Environ. 2024, 924, 171576. [Google Scholar] [PubMed]
- Xu, G.F.; Pan, Y.C.; Gu, W.; Huang, T.Q.; Liu, E.H.; Wang, G.C. Evaluation of the acute toxic effects of ammonia on juvenile ussuri cisco (Coregonus ussuriensis) based on histopathology, antioxidant enzyme activity, immune response and the integrated biomarker response. Mar. Pollut. Bull. 2024, 209, 117215. [Google Scholar]
- Li, Y.W.; Guo, H.H.; Ge, H.; Sha, H.; Wu, Y.D.; Zou, G.W.; Liang, H.W. A time-dependent interactive effect of nitrite and ammonia on inflammatory and immune response in the head kidney of silver carp (Hypophthalmichthys molitrix). Comp. Biochem. Phys. C 2025, 288, 110078. [Google Scholar]
- Deng, L.; He, S.; Guo, N.; Tian, W.; Zhang, W.; Luo, L. Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res. 2023, 72, 281–299. [Google Scholar]
- Liu, J.B.; Li, Z.F.; Lu, L.; Wang, Z.Y.; Wang, L. Glyphosate damages blood-testis barrier via NOX1-triggered oxidative stress in rats: Long-term exposure as a potential risk for male reproductive health. Environ. Int. 2022, 159, 107038. [Google Scholar]
- Jiang, X.Q.; Peng, Z.Y.; He, B.M.; Li, S.Q.; Huang, Q. A comprehensive review of ferroptosis in environmental pollutants-induced chronic obstructive pulmonary disease. Sci. Total Environ. 2024, 957, 177534. [Google Scholar]
- Alvarez, S.W.; Sviderskiy, V.O.; Terzi, E.M.; Papagiannakopoulos, T.; Moreira, A.L.; Adams, S.; Sabatini, D.M.; Birsoy, K.; Possemato, R. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 2017, 551, 639–643. [Google Scholar] [PubMed]
- Hu, H.; Zhang, G.D.; Tian, M.X.; Yin, Y.; Bao, Y.Q.; Guan, X.; Ding, C.; Yu, S.Q. Brucella rough RB51 infection activates P53-Slc7a11-Gpx4/GSH pathway to induce ferroptosis to attenuate the intracellular survival on macrophages. Vet. Microbiol. 2024, 298, 110224. [Google Scholar]
- Yin, B.W.; Ren, J.Y.; Liu, X.Y.; Lu, M.M.; Huang, D.; Zhang, Y.D.; Zuo, J.S.; Wen, R.; Pei, H.T.; Zhu, S.Q.; et al. Astaxanthin attenuates doxorubicin-induced liver injury via suppression of ferroptosis in rats. J. Funct. Foods 2024, 121, 106437. [Google Scholar]
- Long, X.R.; He, K.W.; Zhang, M.Z.; Jiang, H.B.; Dong, X.H.; Wang, C.G.; Shao, J.; Gan, L.; Hu, X.J.; Li, M. Ferroptosis preceded the onset of oxidative stress under acute ammonia exposure and quercetin relieved ammonia-induced ferroptosis of yellow catfish (Pelteobagrus fulvidraco). Aquac. Rep. 2023, 33, 101766. [Google Scholar]
- Long, X.R.; He, K.W.; Zhang, M.Z.; Li, M.; Wang, Z.L.; Wang, C.G.; Dong, X.H.; Shao, J.; Gan, L.; Hu, X.J.; et al. Temporal correlations of ferroptosis, inflammation and oxidative stress under acute ammonia exposure in brain tissue of yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 271, 109693. [Google Scholar]
- Wu, L.Y.; Dong, B.; Chen, Q.Z.; Wang, Y.; Han, D.; Zhu, X.M.; Liu, H.K.; Zhang, Z.M.; Yang, X.Y.; Xie, S.Q.; et al. Effects of curcumin on oxidative stress and ferroptosis in acute ammonia stress-induced liver injury in gibel carp (Carassius gibelio). Int. J. Mol. Sci. 2023, 24, 6441. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.F.; Yang, Y.K.; Li, H.; Zhang, Z.; Chen, X.Y.; Xiao, M.; Nan, Y.X. The toxic effects of tetracycline exposure on the physiological homeostasis of the gut-liver axis in grouper. Environ. Res. 2024, 258, 119402. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-ΔΔC(T)) method. Methods 2001, 25, 402–408. [Google Scholar]
- Duan, Y.; Zhong, G.; Nan, Y.; Yang, Y.; Xiao, M.; Li, H. Effects of nitrite stress on the antioxidant, immunity, energy metabolism, and microbial community status in the intestine of Litopenaeus vannamei. Antioxidants 2024, 13, 1318. [Google Scholar] [CrossRef]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322. [Google Scholar]
- Słowińska, M.; Nynca, J.; Cejko, B.I.; Dietrich, M.A.; Horváth, Á.; Urbányi, B.; Kotrik, L.; Ciereszko, A. Total antioxidant capacity of fish seminal plasma. Aquaculture 2013, 400–401, 101–104. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhou, S.J.; Wang, Y.G.; Sun, Y.Y.; Li, M.H.; Ma, Z.H. Effects of acidification stress on antioxidant and immunity in juvenile yellowfin tuna (Thunnus albacares). South China Fish. Sci. 2024, 20, 85–91. [Google Scholar]
- Ferreira, M.J.; Rodrigues, T.A.; Pedrosa, A.G.; Gales, L.; Salvador, A.; Francisco, T.; Azevedo, J.E. The mammalian peroxisomal membrane is permeable to both GSH and GSSG-implications for intraperoxisomal redox homeostasis. Redox Biol. 2023, 63, 102764. [Google Scholar] [CrossRef]
- Battino, M.; Giampieri, F.; Pistollato, F.; Sureda, A.; de Oliveira, M.R.; Pittalà, V.; Fallarino, F.; Atanasov, A.G.; Nabavi, S.M. Nrf2 as a regulator of innate immunity: A molecular Swiss army knife! Biotechnol. Adv. 2018, 36, 358–370. [Google Scholar] [CrossRef] [PubMed]
- El-Mahrouk, S.R.; El-Ghiaty, M.A.; El-Kadi, A.O.S. The role of nuclear factor erythroid 2-related factor 2 (NRF2) in arsenic toxicity. J. Environ. Sci. 2025, 150, 632–644. [Google Scholar] [CrossRef]
- Cui, W.W.; Hao, M.; Yang, X.; Yin, C.Q.; Chu, B. Gut microbial metabolism in ferroptosis and colorectal cancer. Trends Cell Biol. 2024; in press. [Google Scholar] [CrossRef]
- Wu, S.F.; Ga, Y.; Ma, D.Y.; Hou, S.L.; Hui, Q.Y.; Hao, Z.H. The role of ferroptosis in environmental pollution-induced male reproductive system toxicity. Environ. Pollut. 2024, 363, 125118. [Google Scholar] [CrossRef]
- Fu, W.J.; Amenyogbe, E.; Luo, J.; Yang, E.J.; Huang, J.S.; Chen, Y.M.; Chen, G. Influences of ferulic acid on intestinal digestive and antioxidant enzymes, immune, antioxidant gene and tight junction protein expression and microbiota in hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus polyphekadion ♂). Aquacul. Rep. 2022, 27, 101348. [Google Scholar] [CrossRef]
- Zhang, Q.; Geng, M.; Li, K.; Gao, H.; Jiao, X.Y.; Ai, K.; Wei, X.M.; Yang, J.L. TGF-β1 suppresses the T-cell response in teleost fish by initiating Smad3- and Foxp3-mediated transcriptional networks. J. Biol. Chem. 2023, 299, 102843. [Google Scholar] [CrossRef]
- Chen, L.; Xu, J.X.; Li, L.J.; Zhao, C.F.; Long, X.W. Effects of dietary fishmeal replacement by Periplaneta americana meal on biochemical indexes, disease resistance and metabolomics of juvenile Oncorhynchus mykiss. South China Fish. Sci. 2023, 19, 86–97. [Google Scholar]
- Salinas, I.; Fernández-Montero, Á.; Ding, Y.; Sunyer, J.O. Mucosal immunoglobulins of teleost fish: A decade of advances. Dev. Comp. Immunol. 2021, 121, 104079. [Google Scholar]
- Xu, H.Y.; Wang, Z.X.; Li, Y.Q.; Xu, Z. The distribution and function of teleost IgT. Fish Shellfish Immunol. 2024, 144, 109281. [Google Scholar] [PubMed]
- Han, Q.; Hu, Y.T.; Lu, Z.J.; Wang, J.L.; Chen, H.P.; Mo, Z.Q.; Luo, X.C.; Li, A.X.; Dan, X.M.; Li, Y.W. Study on the characterization of grouper (Epinephelus coioides) immunoglobulin T and its positive cells. Fish Shellfish Immunol. 2021, 118, 102–110. [Google Scholar] [PubMed]
- Han, Q.; Mo, Z.Q.; Lai, X.L.; Guo, W.J.; Hu, Y.T.; Chen, H.P.; He, Z.C.; Dan, X.M.; Li, Y.W. Mucosal immunoglobulin response in Epinephelus coioides after Cryptocaryon irritans infection. Fish Shellfish Immunol. 2022, 128, 436–446. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Xiao, M.; Zhu, R.; Nan, Y.; Yang, Y.; Huang, X.; Zhang, D. Effects of Ammonia Stress on the Antioxidant, Ferroptosis, and Immune Response in the Liver of Golden Pompano Trachinotus ovatus. Antioxidants 2025, 14, 419. https://doi.org/10.3390/antiox14040419
Duan Y, Xiao M, Zhu R, Nan Y, Yang Y, Huang X, Zhang D. Effects of Ammonia Stress on the Antioxidant, Ferroptosis, and Immune Response in the Liver of Golden Pompano Trachinotus ovatus. Antioxidants. 2025; 14(4):419. https://doi.org/10.3390/antiox14040419
Chicago/Turabian StyleDuan, Yafei, Meng Xiao, Ruijie Zhu, Yuxiu Nan, Yukai Yang, Xiaohua Huang, and Dianchang Zhang. 2025. "Effects of Ammonia Stress on the Antioxidant, Ferroptosis, and Immune Response in the Liver of Golden Pompano Trachinotus ovatus" Antioxidants 14, no. 4: 419. https://doi.org/10.3390/antiox14040419
APA StyleDuan, Y., Xiao, M., Zhu, R., Nan, Y., Yang, Y., Huang, X., & Zhang, D. (2025). Effects of Ammonia Stress on the Antioxidant, Ferroptosis, and Immune Response in the Liver of Golden Pompano Trachinotus ovatus. Antioxidants, 14(4), 419. https://doi.org/10.3390/antiox14040419







