Rubicon, a Key Molecule for Oxidative Stress-Mediated DNA Damage, in Ovarian Granulosa Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagents and Antibodies
2.3. Immunofluorescence Staining
2.4. Western Blotting
2.5. Cell Proliferation Assay
2.6. Reactive Oxygen Species (ROS) Assay
2.7. Transfection of siRNA or Plasmids
2.8. Collection of Ovarian Tissue
2.9. Statistical Analysis
3. Results
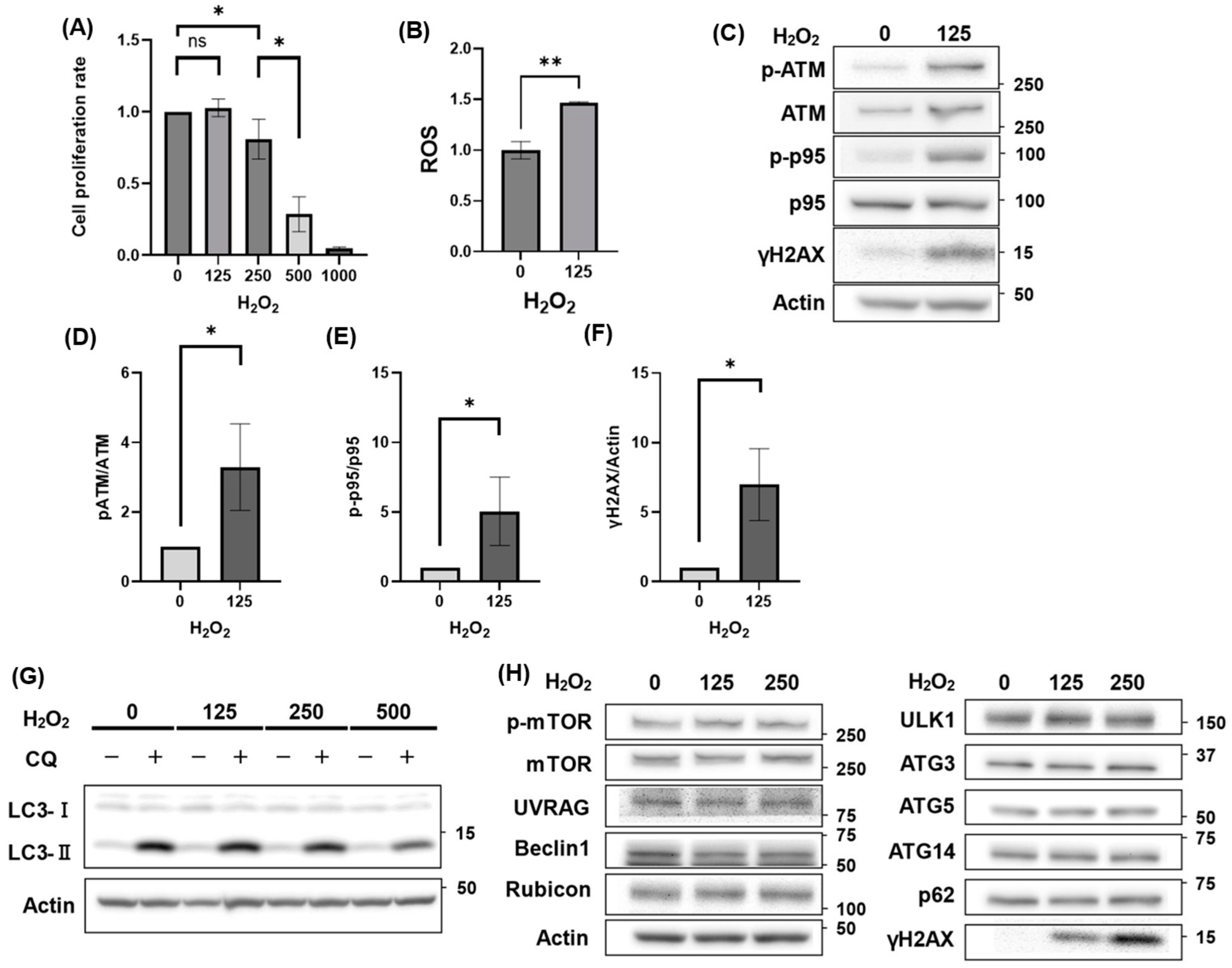
3.1. Autophagy Activity Unaffected by H2O2 Treatment
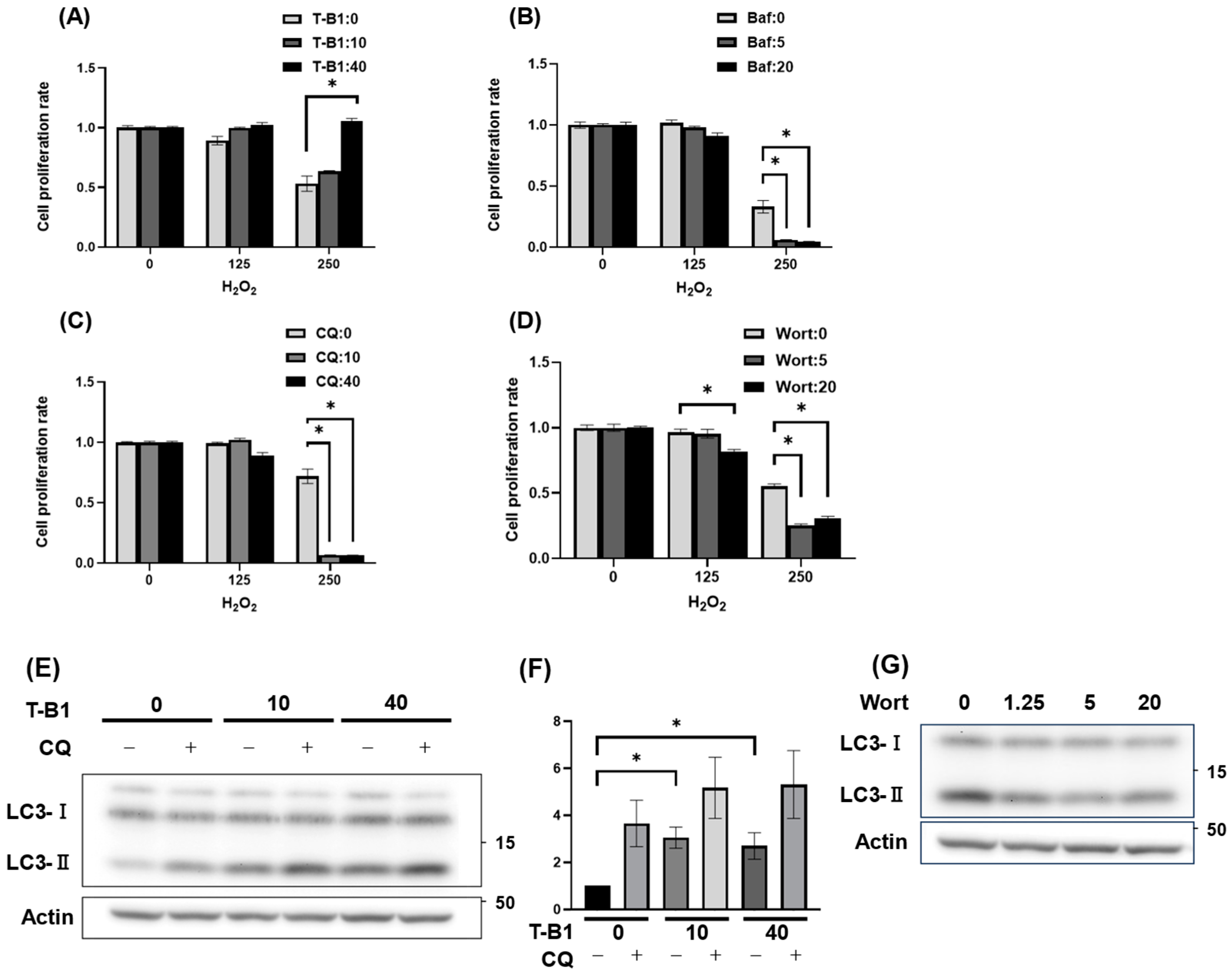
3.2. Autophagic Activation Enhanced HGrC1 Viability Against H2O2-Induced Oxidative Stress
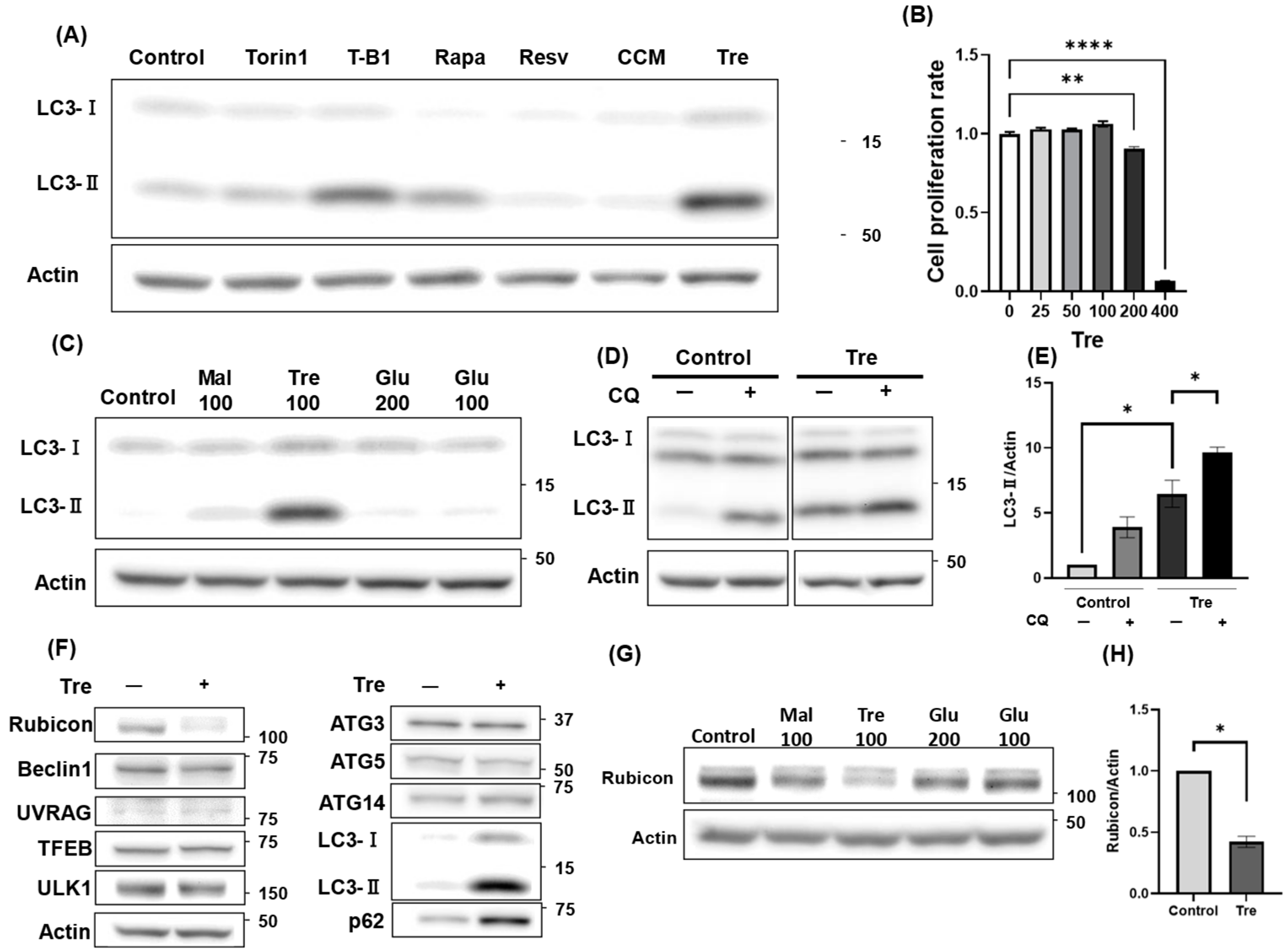
3.3. Trehalose Activated Autophagy and Decreased Rubicon Expression
3.4. Trehalose Enhanced Cell Viability Against H2O2-Mediated Oxidative Stress
3.5. Decreased Rubicon Expression, but Not Autophagic Activation, Was Involved in Reducing DNA Damage
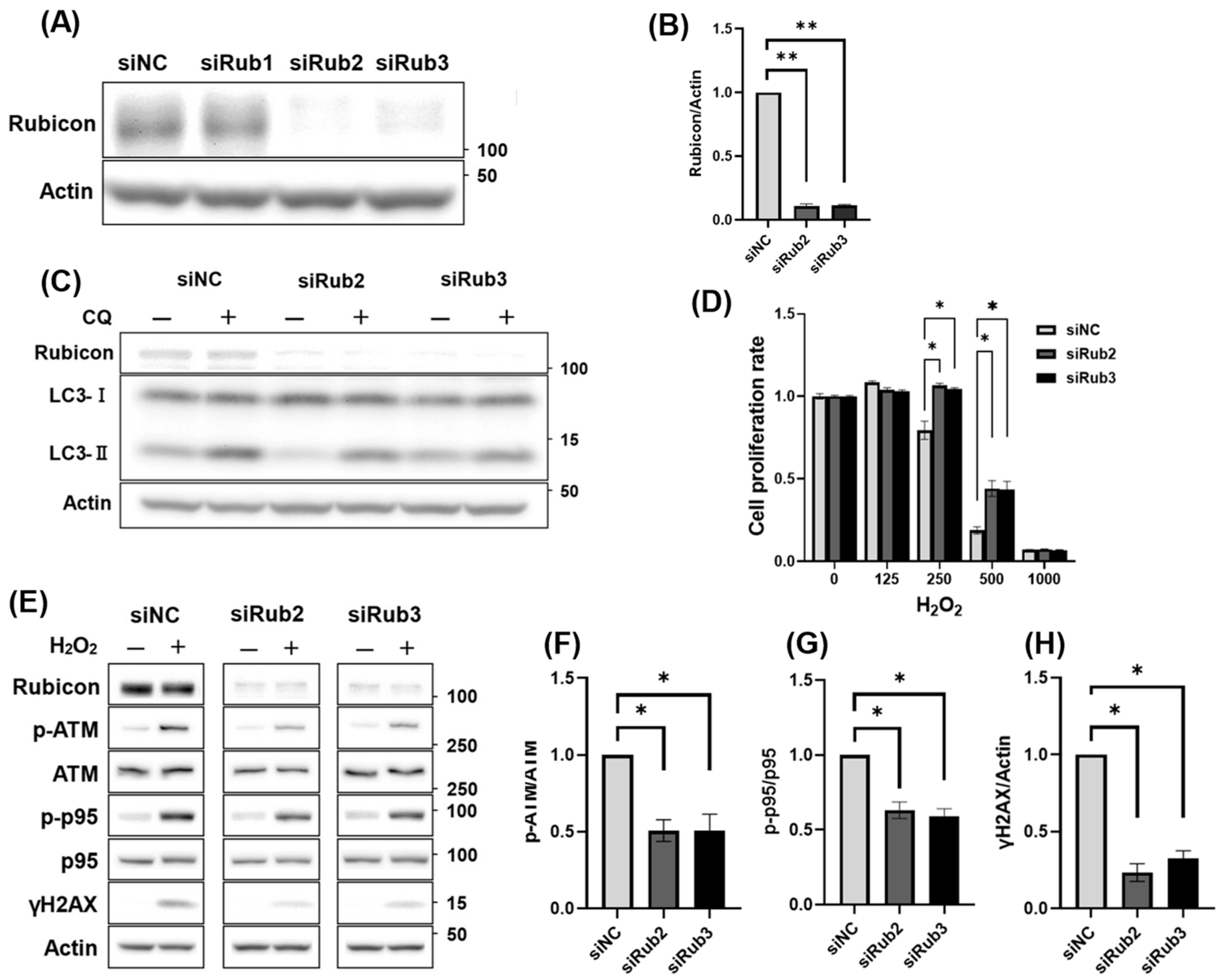
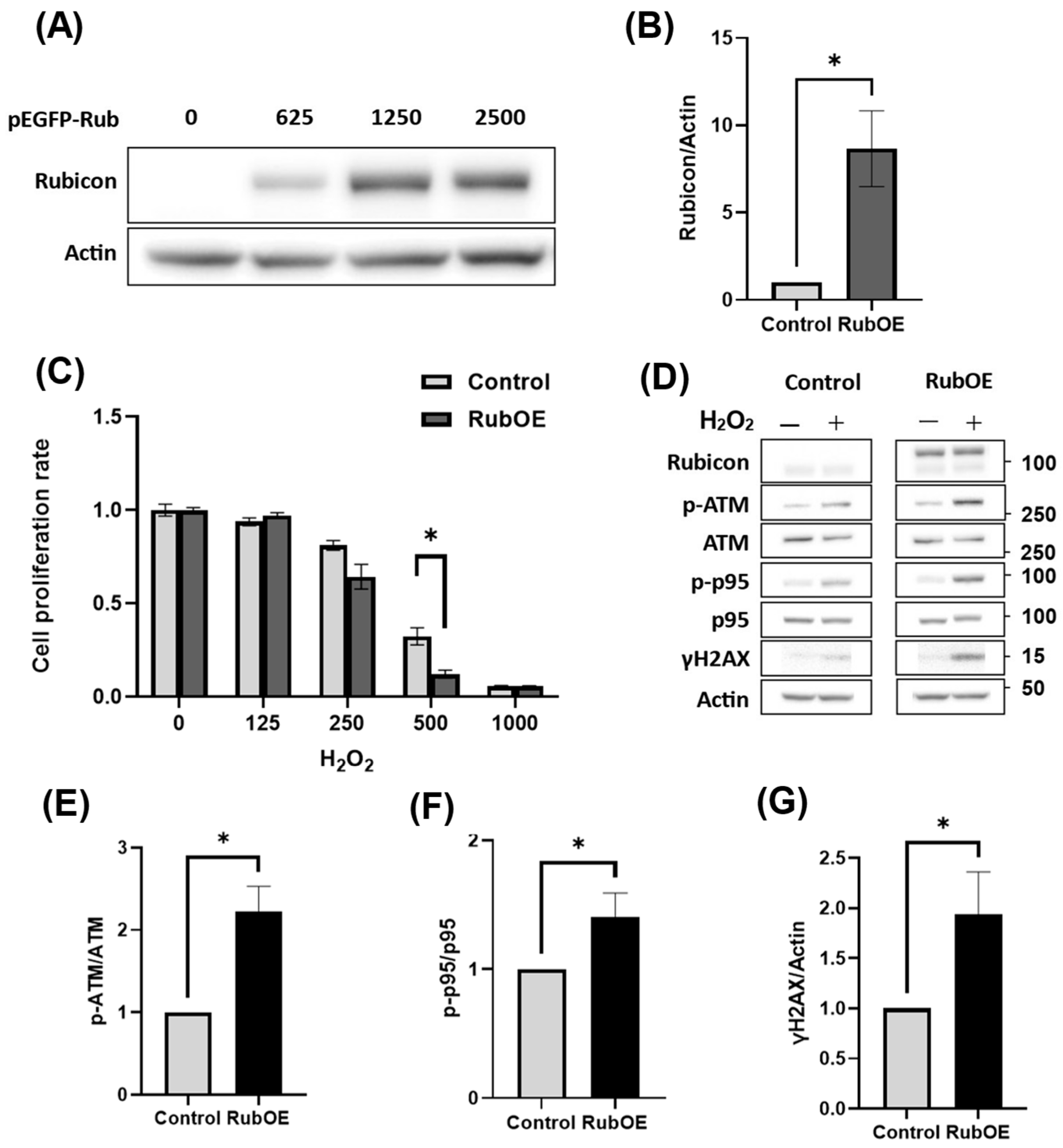
3.6. Increased Rubicon Expression Enhanced DNA Damage in Granulosa Cell Line
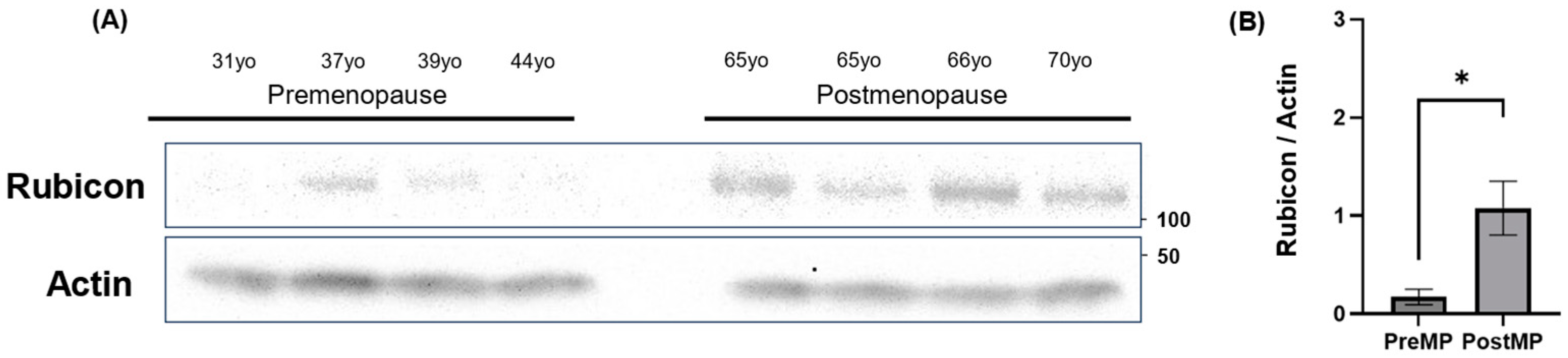
3.7. Increased Rubicon Expression in Ovaries of Postmenopausal Women
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATM | Ataxia Telangiectasia Mutated |
| Baf | Bafilomycin A1 |
| CARD9 | Caspase recruitment domain-containing protein 9 |
| CQ | Chloroquine |
| FSH | Follicle-stimulating Hormone |
| FSHR | FSH Receptor |
| GATA4 | GATA-binding protein 4 |
| GCs | Granulosa Cells |
| γH2AX | γH2A histone family member X |
| H2O2 | Hydrogen Peroxide |
| LC3 | Microtubule Associated Protein 1 Light Chain 3 beta |
| OS | Oxidative Stress |
| PCOS | Polycystic Ovary Syndrome |
| POR | Poor Ovarian Response |
| ROS | Reactive Oxygen Species |
| SIRT1 | Sirtuin 1 |
| T-B1 | Tat-Beclin1 D11 |
| TFEB | Transcription Factor EB |
| Wort | Wortmannin |
| YAP1 | Yes-associated protein 1 |
References
- Fitzpatrick, S.L.; Richards, J.S. Identification of a cyclic adenosine 3′,5′-monophosphate-response element in the rat aromatase promoter that is required for transcriptional activation in rat granulosa cells and R2C leydig cells. Mol. Endocrinol. 1994, 8, 1309–1319. [Google Scholar] [PubMed]
- Jeppesen, J.V.; Kristensen, S.G.; Nielsen, M.E.; Humaidan, P.; Dal Canto, M.; Fadini, R.; Schmidt, K.T.; Ernst, E.; Yding Andersen, C. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J. Clin. Endocrinol. Metab. 2012, 97, E1524–E1531. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Ferraretti, A.P.; La Marca, A.; Fauser, B.C.; Tarlatzis, B.; Nargund, G.; Gianaroli, L.; on behalf of the ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum. Reprod. 2011, 26, 1616–1624. [Google Scholar] [CrossRef]
- Buffenstein, R.; Edrey, Y.H.; Yang, T.; Mele, J. The oxidative stress theory of aging: Embattled or invincible? Insights from non-traditional model organisms. Age 2008, 30, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ra, K.; Park, S.C.; Lee, B.C. Female Reproductive Aging and Oxidative Stress: Mesenchymal Stem Cell Conditioned Medium as a Promising Antioxidant. Int. J. Mol. Sci. 2023, 24, 5053. [Google Scholar] [CrossRef] [PubMed]
- Immediata, V.; Ronchetti, C.; Spadaro, D.; Cirillo, F.; Levi-Setti, P.E. Oxidative Stress and Human Ovarian Response-from Somatic Ovarian Cells to Oocytes Damage: A Clinical Comprehensive Narrative Review. Antioxidants 2022, 11, 1335. [Google Scholar] [CrossRef]
- Tatone, C.; Carbone, M.C.; Falone, S.; Aimola, P.; Giardinelli, A.; Caserta, D.; Marci, R.; Pandolfi, A.; Ragnelli, A.M.; Amicarelli, F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol. Hum. Reprod. 2006, 12, 655–660. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Nakashima, A.; Furuta, A.; Yamada, K.; Yoshida-Kawaguchi, M.; Yamaki-Ushijima, A.; Yasuda, I.; Ito, M.; Yamashita, S.; Tsuda, S.; Yoneda, S.; et al. The Role of Autophagy in the Female Reproduction System: For Beginners to Experts in This Field. Biology 2023, 12, 373. [Google Scholar] [CrossRef]
- Li, Q.; Cai, M.; Wang, J.; Gao, Q.; Guo, X.; Jia, X.; Xu, S.; Zhu, H. Decreased ovarian function and autophagy gene methylation in aging rats. J. Ovarian Res. 2020, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.H.; Yu, H.Y.; Wang, P.; Mao, G.K.; Liu, W.X.; Li, M.N.; Wang, H.N.; Shang, Y.L.; Liu, C.; Xu, Z.L.; et al. Germ cell-specific Atg7 knockout results in primary ovarian insufficiency in female mice. Cell Death Dis. 2015, 6, e1589. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, X.; Mei, S. Autophagy in Ovarian Follicular Development and Atresia. Int. J. Biol. Sci. 2019, 15, 726–737. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Kuma, A.; Akashi, T.; Hara, T.; Yamamoto, A.; Kurikawa, Y.; Itakura, E.; Tsukamoto, S.; Shitara, H.; Eishi, Y.; et al. Systemic Analysis of Atg5-Null Mice Rescued from Neonatal Lethality by Transgenic ATG5 Expression in Neurons. Dev. Cell 2016, 39, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, H.; Yang, F.; Zhang, H.; Zeng, S. FSH Promotes Progesterone Synthesis by Enhancing Autophagy to Accelerate Lipid Droplet Degradation in Porcine Granulosa Cells. Front. Cell Dev. Biol. 2021, 9, 626927. [Google Scholar] [CrossRef] [PubMed]
- Gawriluk, T.R.; Ko, C.; Hong, X.; Christenson, L.K.; Rucker, E.B., 3rd. Beclin-1 deficiency in the murine ovary results in the reduction of progesterone production to promote preterm labor. Proc. Natl. Acad. Sci. USA 2014, 111, E4194–E4203. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, J.; Wang, X.; Yang, J.; Chen, D.; Huff, V.; Liu, Y.X. Wt1 functions in ovarian follicle development by regulating granulosa cell differentiation. Hum. Mol. Genet. 2014, 23, 333–341. [Google Scholar] [CrossRef]
- Wu, Y.; Ghosh, S.; Nishi, Y.; Yanase, T.; Nawata, H.; Hu, Y. The orphan nuclear receptors NURR1 and NGFI-B modulate aromatase gene expression in ovarian granulosa cells: A possible mechanism for repression of aromatase expression upon luteinizing hormone surge. Endocrinology 2005, 146, 237–246. [Google Scholar] [CrossRef]
- Liu, W.; Chen, M.; Liu, C.; Wang, L.; Wei, H.; Zhang, R.; Ren, Z.; Chen, Y.; Luo, M.; Zhao, J.; et al. Epg5 deficiency leads to primary ovarian insufficiency due to WT1 accumulation in mouse granulosa cells. Autophagy 2023, 19, 644–659. [Google Scholar] [CrossRef]
- Ito, M.; Yoshino, O.; Ono, Y.; Yamaki-Ushijima, A.; Tanaka, T.; Shima, T.; Orisaka, M.; Iwase, A.; Nakashima, A.; Saito, S. Bone morphogenetic protein-2 enhances gonadotropin-independent follicular development via sphingosine kinase 1. Am. J. Reprod. Immunol. 2021, 85, e13374. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yoshino, O.; Nakashima, A.; Ito, M.; Nishio, K.; Ono, Y.; Kusabiraki, T.; Kunitomi, C.; Takahashi, N.; Harada, M.; et al. Inhibition of autophagy in theca cells induces CYP17A1 and PAI-1 expression via ROS/p38 and JNK signalling during the development of polycystic ovary syndrome. Mol. Cell Endocrinol. 2020, 508, 110792. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Oba, M.; Suzuki, M.; Takahashi, A.; Yamamuro, T.; Fujiwara, M.; Ikenaka, K.; Minami, S.; Tabata, N.; Yamamoto, K.; et al. Suppression of autophagic activity by Rubicon is a signature of aging. Nat. Commun. 2019, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Shima, T.; Yoshida-Kawaguchi, M.; Yamada, K.; Yasuda, I.; Tsuda, S.; Yamaki-Ushijima, A.; Yoneda, S.; Higashisaka, K.; Cheng, S.B.; et al. Chloroquine is a safe autophagy inhibitor for sustaining the expression of antioxidant enzymes in trophoblasts. J. Reprod. Immunol. 2023, 155, 103766. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef]
- Sanchez-Martin, P.; Sou, Y.S.; Kageyama, S.; Koike, M.; Waguri, S.; Komatsu, M. NBR1-mediated p62-liquid droplets enhance the Keap1-Nrf2 system. EMBO Rep. 2020, 21, e48902. [Google Scholar] [CrossRef]
- Rawat, L.; Balan, M.; Sasamoto, Y.; Sabarwal, A.; Pal, S. A novel combination therapy with Cabozantinib and Honokiol effectively inhibits c-Met-Nrf2-induced renal tumor growth through increased oxidative stress. Redox Biol. 2023, 68, 102945. [Google Scholar] [CrossRef]
- Regier, D.S.; Waite, K.A.; Wallin, R.; McPhail, L.C. A phosphatidic acid-activated protein kinase and conventional protein kinase C isoforms phosphorylate p22(phox), an NADPH oxidase component. J. Biol. Chem. 1999, 274, 36601–36608. [Google Scholar] [CrossRef]
- Yang, C.S.; Lee, J.S.; Rodgers, M.; Min, C.K.; Lee, J.Y.; Kim, H.J.; Lee, K.H.; Kim, C.J.; Oh, B.; Zandi, E.; et al. Autophagy protein Rubicon mediates phagocytic NADPH oxidase activation in response to microbial infection or TLR stimulation. Cell Host Microbe 2012, 11, 264–276. [Google Scholar] [CrossRef]
- Kim, Y.R.; Koh, H.J.; Kim, J.S.; Yun, J.S.; Jang, K.; Lee, J.Y.; Jung, J.U.; Yang, C.S. Peptide inhibition of p22phox and Rubicon interaction as a therapeutic strategy for septic shock. Biomaterials 2016, 101, 47–59. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, J.S.; Gu, S.J.; Jo, S.; Kim, S.; Young Kim, S.; Lee, D.; Jang, K.; Choo, H.; Kim, T.H.; et al. Identification of highly potent and selective inhibitor, TIPTP, of the p22phox-Rubicon axis as a therapeutic agent for rheumatoid arthritis. Sci. Rep. 2020, 10, 4570. [Google Scholar] [CrossRef]
- Li, Y.; Liang, P.; Jiang, B.; Tang, Y.; Liu, X.; Liu, M.; Sun, H.; Chen, C.; Hao, H.; Liu, Z.; et al. CARD9 promotes autophagy in cardiomyocytes in myocardial ischemia/reperfusion injury via interacting with Rubicon directly. Basic. Res. Cardiol. 2020, 115, 29. [Google Scholar] [CrossRef]
- Claude-Taupin, A.; Isnard, P.; Bagattin, A.; Kuperwasser, N.; Roccio, F.; Ruscica, B.; Goudin, N.; Garfa-Traore, M.; Regnier, A.; Turinsky, L.; et al. The AMPK-Sirtuin 1-YAP axis is regulated by fluid flow intensity and controls autophagy flux in kidney epithelial cells. Nat. Commun. 2023, 14, 8056. [Google Scholar] [CrossRef] [PubMed]
- Claude-Taupin, A.; Terzi, F.; Codogno, P.; Dupont, N. Yapping at the autophagy door? The answer is flowing in the kidney proximal tubule. Autophagy 2024, 20, 1465–1466. [Google Scholar] [CrossRef]
- Nakashima, A.; Cheng, S.B.; Ikawa, M.; Yoshimori, T.; Huber, W.J.; Menon, R.; Huang, Z.; Fierce, J.; Padbury, J.F.; Sadovsky, Y.; et al. Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy 2020, 16, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yamanaka-Tatematsu, M.; Fujita, N.; Koizumi, K.; Shima, T.; Yoshida, T.; Nikaido, T.; Okamoto, A.; Yoshimori, T.; Saito, S. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy 2013, 9, 303–316. [Google Scholar] [CrossRef]
- Iwai-Kanai, E.; Yuan, H.; Huang, C.; Sayen, M.R.; Perry-Garza, C.N.; Kim, L.; Gottlieb, R.A. A method to measure cardiac autophagic flux in vivo. Autophagy 2008, 4, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, M.; Janjetovic, K.; Paunovic, V.; Ciric, D.; Kravic-Stevovic, T.; Trajkovic, V. Comparative analysis of cell death mechanisms induced by lysosomal autophagy inhibitors. Eur. J. Pharmacol. 2019, 859, 172540. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Ma, L.Z.; Liu, S.J.; Zhang, C.T.; Meng, R.; Chen, Y.Z.; Jiang, Z.L. Protective effects of trehalose on frozen-thawed ovarian granulosa cells of cattle. Anim. Reprod. Sci. 2019, 200, 14–21. [Google Scholar] [CrossRef]
- Diez, J.; Diederichs, K.; Greller, G.; Horlacher, R.; Boos, W.; Welte, W. The crystal structure of a liganded trehalose/maltose-binding protein from the hyperthermophilic Archaeon Thermococcus litoralis at 1.85 A. J. Mol. Biol. 2001, 305, 905–915. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, S.; Jash, S.; Fierce, J.; Agudelo, A.; Higashiyama, T.; Hanna, N.; Nakashima, A.; Saito, S.; Padbury, J.; et al. Exploiting sweet relief for preeclampsia by targeting autophagy-lysosomal machinery and proteinopathy. Exp. Mol. Med. 2024, 56, 1206–1220. [Google Scholar] [CrossRef]
- Yamamuro, T.; Kawabata, T.; Fukuhara, A.; Saita, S.; Nakamura, S.; Takeshita, H.; Fujiwara, M.; Enokidani, Y.; Yoshida, G.; Tabata, K.; et al. Age-dependent loss of adipose Rubicon promotes metabolic disorders via excess autophagy. Nat. Commun. 2020, 11, 4150. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Odle, A.K.; Aykin-Burns, N.; Allen, A.R. Chemotherapy induced oxidative stress in the ovary: Drug-dependent mechanisms and potential interventionsdagger. Biol. Reprod. 2023, 108, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.A.; El Shourbagy, S.; St John, J.C. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil. Steril. 2006, 85, 584–591. [Google Scholar] [CrossRef]
- Stringer, J.M.; Alesi, L.R.; Winship, A.L.; Hutt, K.J. Beyond apoptosis: Evidence of other regulated cell death pathways in the ovary throughout development and life. Hum. Reprod. Update 2023, 29, 434–456. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, T.; Nakamura, S.; Yamano, Y.; Endo, T.; Yanagawa, K.; Tokumura, A.; Matsumura, T.; Kobayashi, K.; Mori, H.; Enokidani, Y.; et al. Rubicon prevents autophagic degradation of GATA4 to promote Sertoli cell function. PLoS Genet. 2021, 17, e1009688. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Hu, Y.; Xu, L.; Jiang, Y.; Zhang, C.; Ding, L.; Jiang, R.; Sun, J.; Sun, H.; et al. miR-181a increases FoxO1 acetylation and promotes granulosa cell apoptosis via SIRT1 downregulation. Cell Death Dis. 2017, 8, e3088. [Google Scholar] [CrossRef]
- Sun, T.; Li, X.; Zhang, P.; Chen, W.D.; Zhang, H.L.; Li, D.D.; Deng, R.; Qian, X.J.; Jiao, L.; Ji, J.; et al. Acetylation of Beclin 1 inhibits autophagosome maturation and promotes tumour growth. Nat. Commun. 2015, 6, 7215. [Google Scholar] [CrossRef]
- Dahm-Daphi, J.; Sass, C.; Alberti, W. Comparison of biological effects of DNA damage induced by ionizing radiation and hydrogen peroxide in CHO cells. Int. J. Radiat. Biol. 2000, 76, 67–75. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Liang, G.; Geng, G.; Zhao, F.; Yin, P.; Nowsheen, S.; Wu, C.; Li, Y.; Li, L.; et al. CHK2-FOXK axis promotes transcriptional control of autophagy programs. Sci. Adv. 2020, 6, eaax5819. [Google Scholar] [CrossRef]
- Tanaka, S.; Hikita, H.; Tatsumi, T.; Sakamori, R.; Nozaki, Y.; Sakane, S.; Shiode, Y.; Nakabori, T.; Saito, Y.; Hiramatsu, N.; et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 2016, 64, 1994–2014. [Google Scholar] [CrossRef]
- Tanaka, T.; Urata, Y.; Harada, M.; Kunitomi, C.; Kusamoto, A.; Koike, H.; Xu, Z.; Sakaguchi, N.; Tsuchida, C.; Komura, A.; et al. Cellular senescence of granulosa cells in the pathogenesis of polycystic ovary syndrome. Mol. Hum. Reprod. 2024, 30, gaae015. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, T.; Nakamura, S.; Yanagawa, K.; Tokumura, A.; Kawabata, T.; Fukuhara, A.; Teranishi, H.; Hamasaki, M.; Shimomura, I.; Yoshimori, T. Loss of RUBCN/rubicon in adipocytes mediates the upregulation of autophagy to promote the fasting response. Autophagy 2022, 18, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Karalay, O.; Jager, P.S.; Horikawa, M.; Klein, C.; Nakamura, K.; Latza, C.; Templer, S.E.; Dieterich, C.; Antebi, A. Mondo complexes regulate TFEB via TOR inhibition to promote longevity in response to gonadal signals. Nat. Commun. 2016, 7, 10944. [Google Scholar] [CrossRef]
- Palmieri, M.; Pal, R.; Nelvagal, H.R.; Lotfi, P.; Stinnett, G.R.; Seymour, M.L.; Chaudhury, A.; Bajaj, L.; Bondar, V.V.; Bremner, L.; et al. mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat. Commun. 2017, 8, 14338. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, M.; Cornuau, M.; Cloix, L.; Rame, C.; Guerif, F.; Royere, D.; Dupont, J. Visfatin is expressed in human granulosa cells: Regulation by metformin through AMPK/SIRT1 pathways and its role in steroidogenesis. Mol. Hum. Reprod. 2013, 19, 313–326. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, K.; Ito, M.; Nunomura, H.; Nishigori, T.; Furuta, A.; Yoshida, M.; Yamaki, A.; Nakamura, T.; Iwase, A.; Shima, T.; et al. Rubicon, a Key Molecule for Oxidative Stress-Mediated DNA Damage, in Ovarian Granulosa Cells. Antioxidants 2025, 14, 470. https://doi.org/10.3390/antiox14040470
Yamada K, Ito M, Nunomura H, Nishigori T, Furuta A, Yoshida M, Yamaki A, Nakamura T, Iwase A, Shima T, et al. Rubicon, a Key Molecule for Oxidative Stress-Mediated DNA Damage, in Ovarian Granulosa Cells. Antioxidants. 2025; 14(4):470. https://doi.org/10.3390/antiox14040470
Chicago/Turabian StyleYamada, Kiyotaka, Masami Ito, Haruka Nunomura, Takashi Nishigori, Atsushi Furuta, Mihoko Yoshida, Akemi Yamaki, Tomoko Nakamura, Akira Iwase, Tomoko Shima, and et al. 2025. "Rubicon, a Key Molecule for Oxidative Stress-Mediated DNA Damage, in Ovarian Granulosa Cells" Antioxidants 14, no. 4: 470. https://doi.org/10.3390/antiox14040470
APA StyleYamada, K., Ito, M., Nunomura, H., Nishigori, T., Furuta, A., Yoshida, M., Yamaki, A., Nakamura, T., Iwase, A., Shima, T., & Nakashima, A. (2025). Rubicon, a Key Molecule for Oxidative Stress-Mediated DNA Damage, in Ovarian Granulosa Cells. Antioxidants, 14(4), 470. https://doi.org/10.3390/antiox14040470






