Evaluation of Efficacy of Water-Soluble Fraction of Rhus semialata Gall Extract and Penta-O-Galloyl-β-D-Glucose on Mitigation of Hair Loss: An In Vitro and Randomized Double-Blind Placebo-Controlled Clinical Study
Abstract
:1. Introduction
2. Materials and Methods
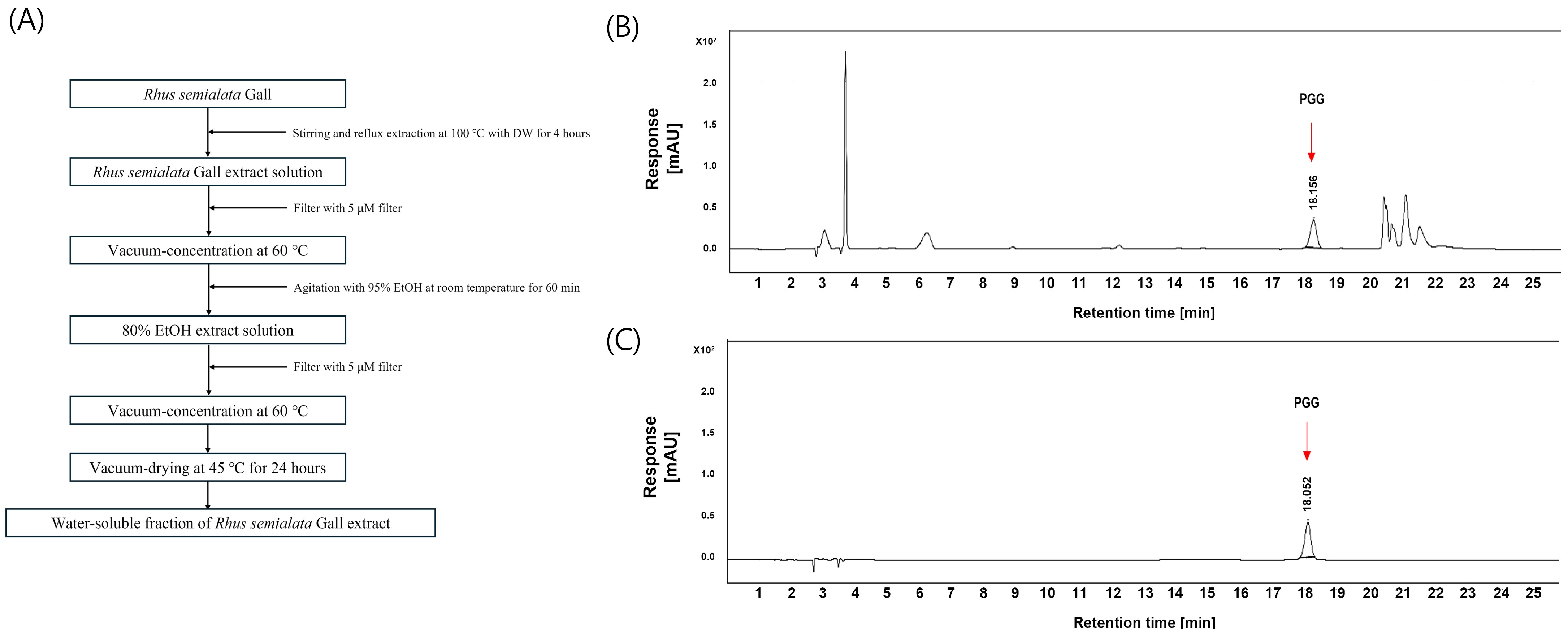
2.1. Preparation of WRGE
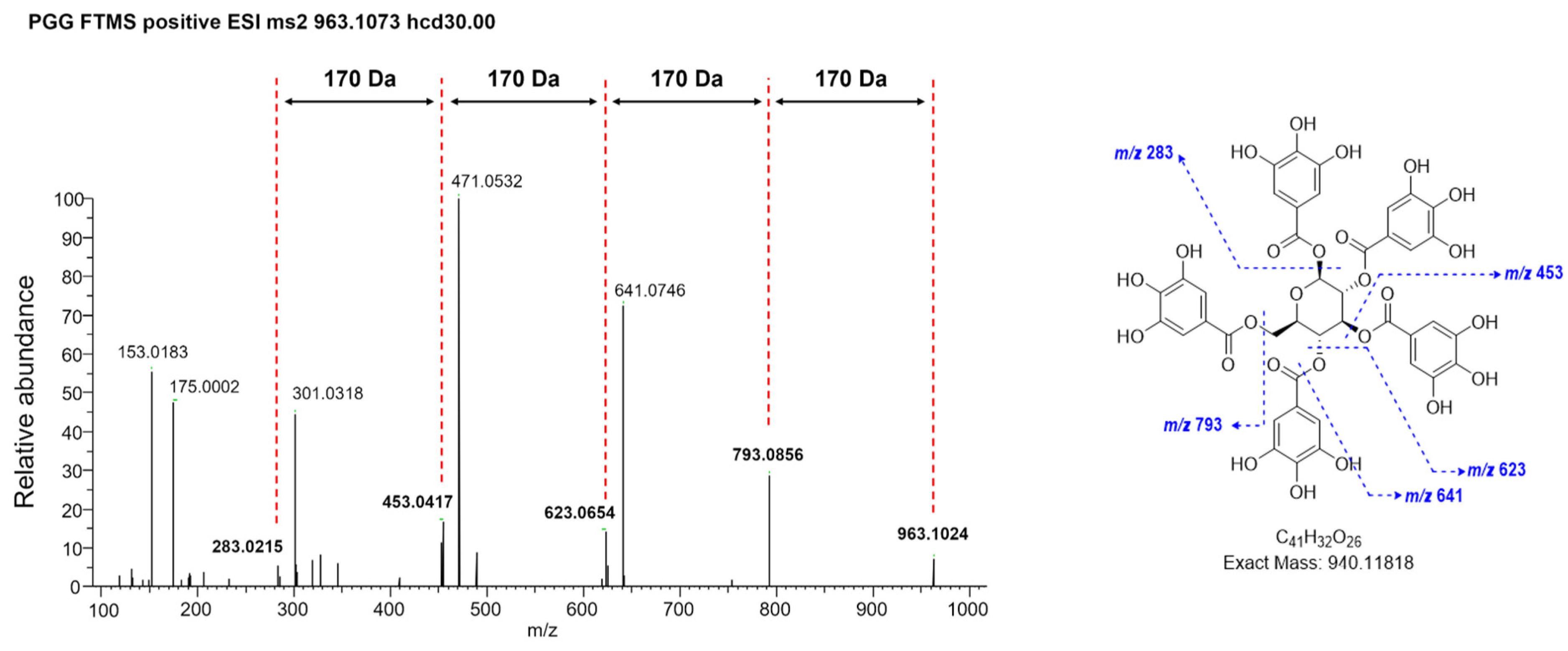
2.2. HPLC and HRESIMS Analysis of WRGE
2.3. DPPH Radical Scavenging Activity
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. H2O2-Induced Antioxidant Capacity Assay
2.7. Intracellular ROS Measurement
2.8. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.9. Western Blot
2.10. DKK-1 ELISA
2.11. Clinical Trial
2.11.1. Evaluation Method
2.11.2. Hair Density Measurement (Phototrichogram)
2.11.3. Hair Photography and Researchers’ Visual Evaluations
2.12. Statistical Analysis
3. Results
3.1. Analysis of PGG in WRGE Using HPLC and HRESIMS
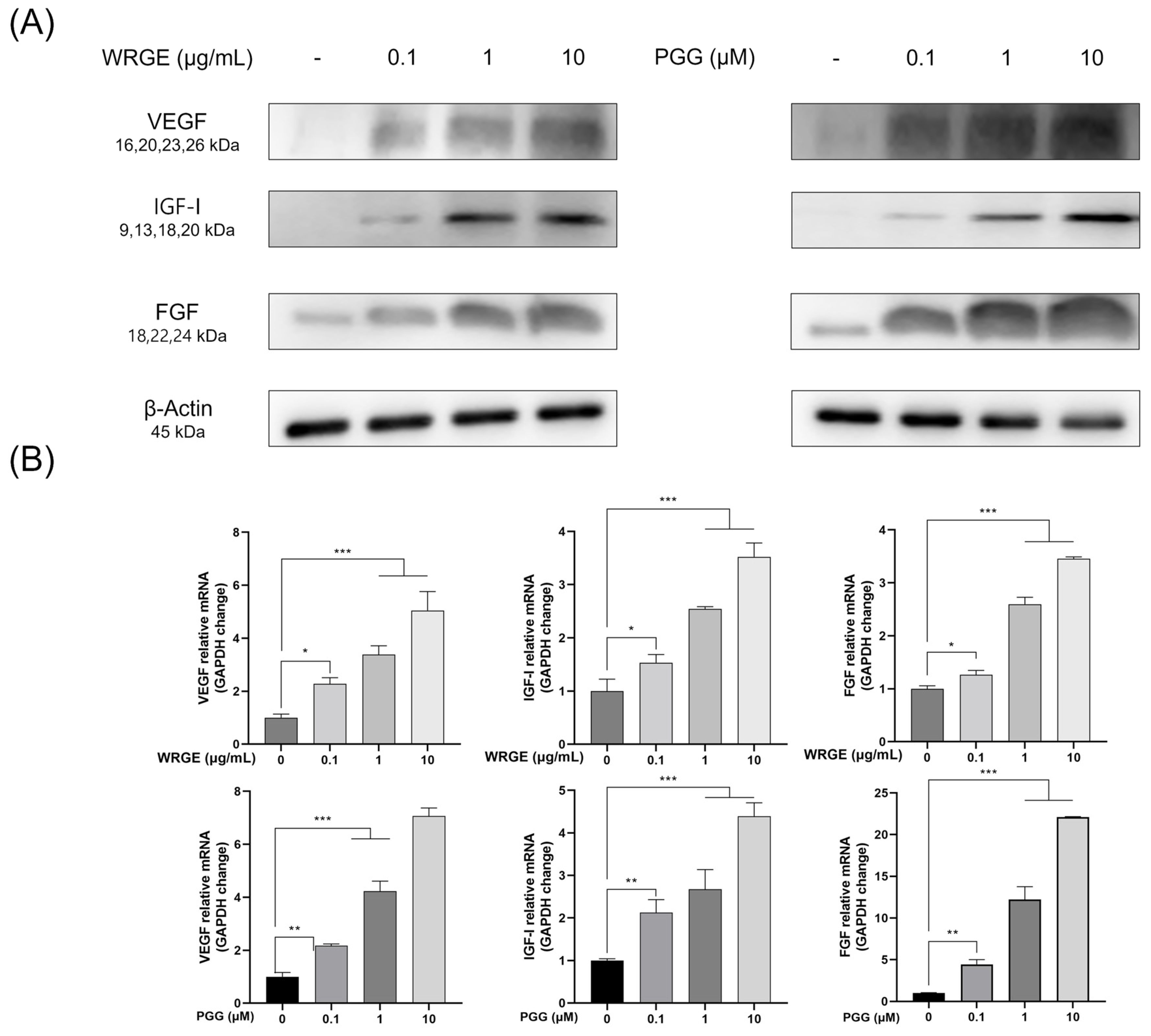
3.2. Hair-Inductive Properties of WRGE and PGG in HDPCs
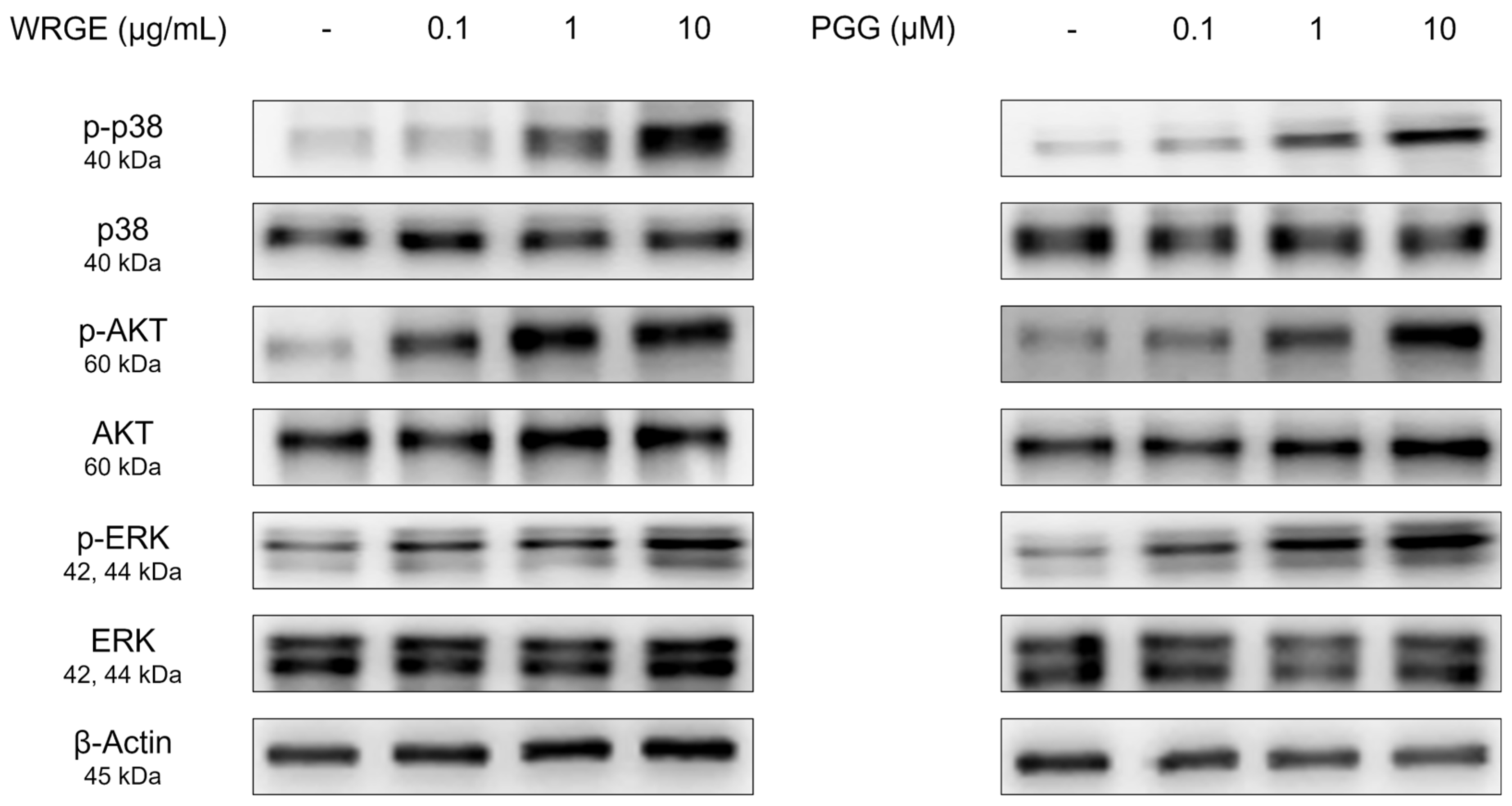
3.3. Effects of WRGE and PGG on the Wnt/β-Catenin Pathway and MAPK/AKT Pathway in HDPCs
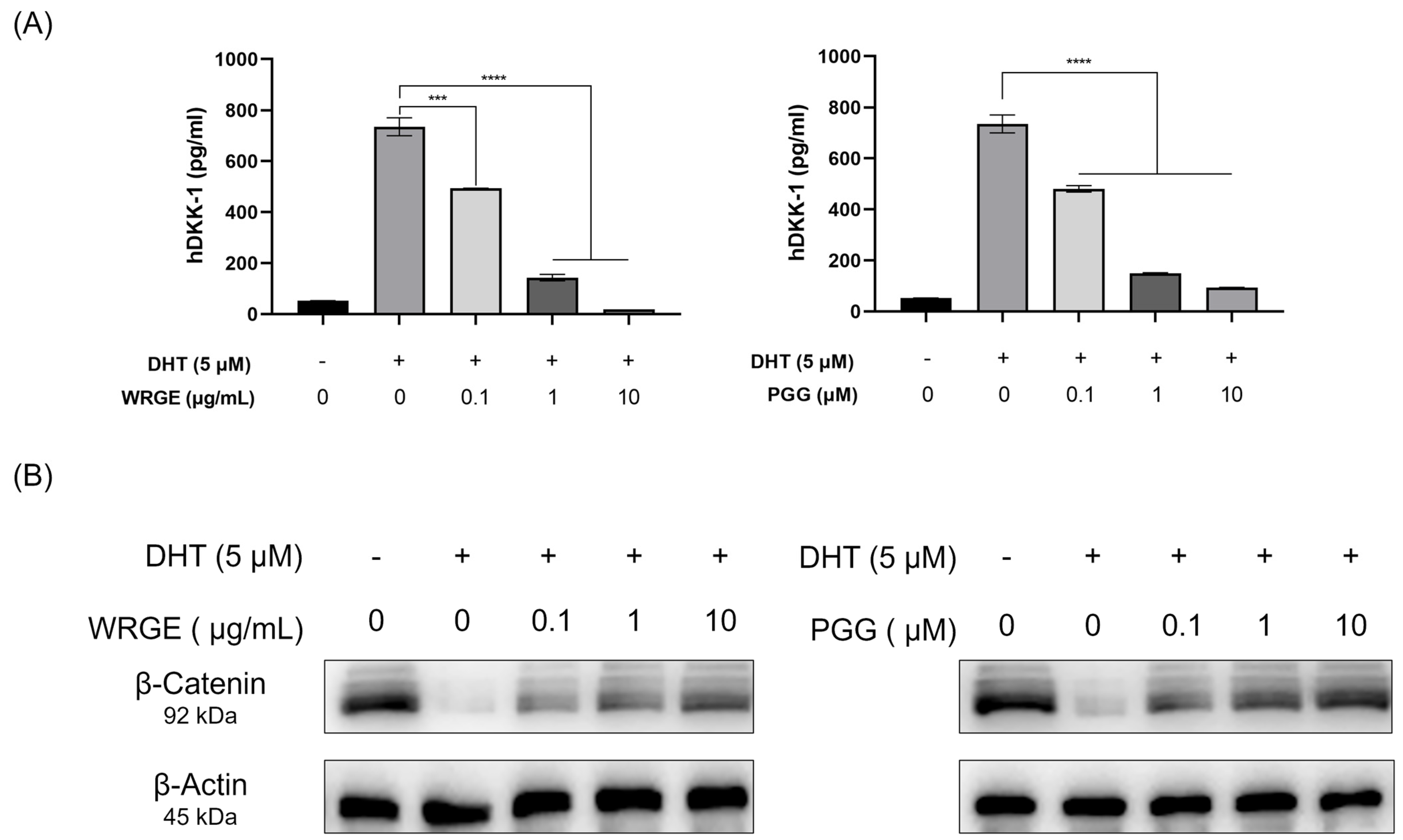
3.4. WRGE and PGG Modulation of DHT-Induced DKK-1 and β-Catenin in HDPCs
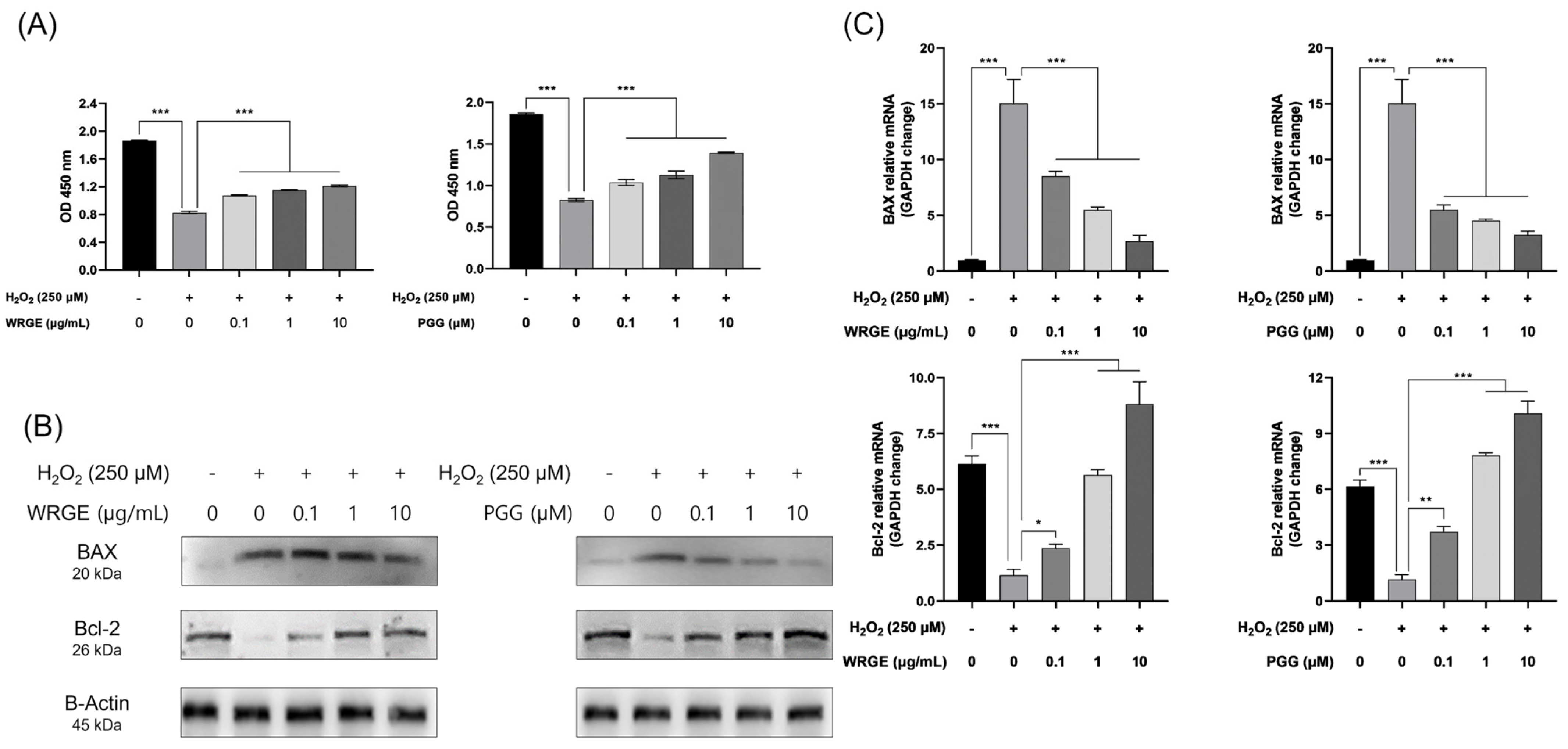
3.5. Antioxidant and Protective Effects of WRGE and PGG on H2O2-Induced Apoptosis in HDPCs
3.6. Homogeneity Test
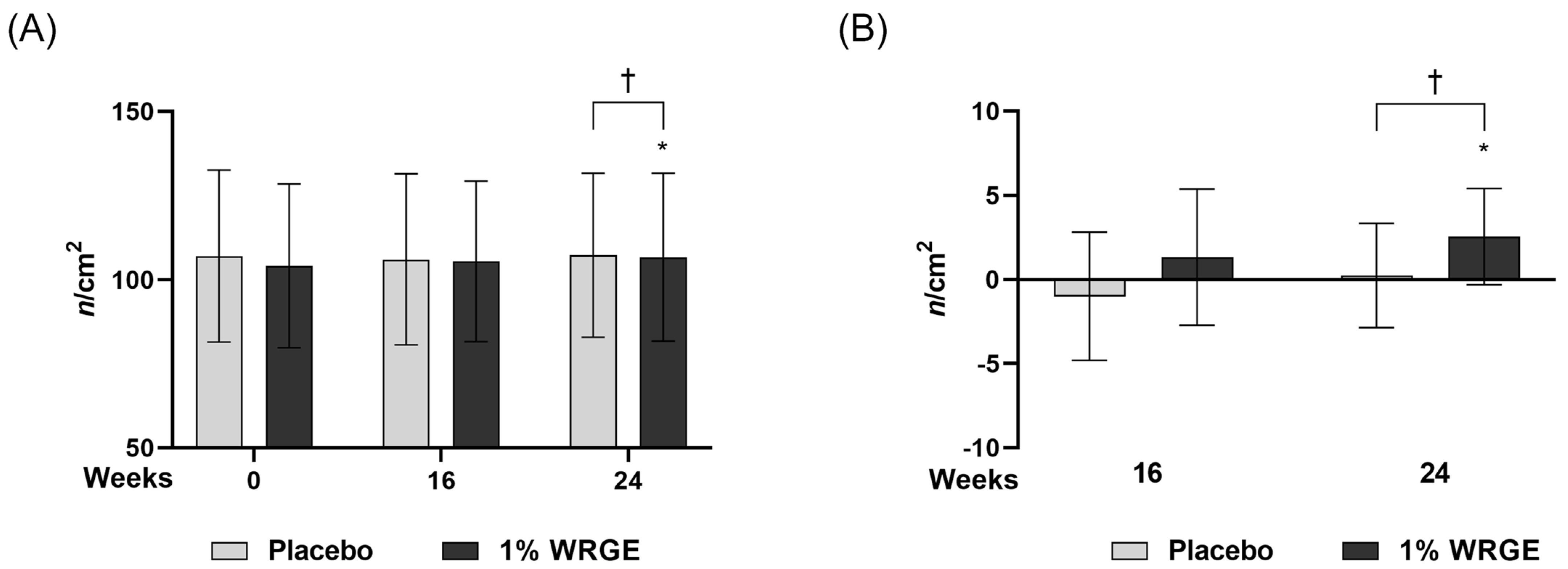
3.7. Measurement of Hair Density
3.7.1. Measurement of Hair Density in the Placebo Treatment Group
3.7.2. Measurement of Hair Density in the 1% WRGE Treatment Group
3.8. Visual Evaluation
3.8.1. Researchers’ Visual Evaluations (ICC)
3.8.2. Researcher’s Visual Evaluation for the Placebo Treatment Group
3.8.3. Researchers’ Visual Evaluation for the 1% WRGE Treatment Group
3.9. Safety Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGA | Androgenetic alopecia |
| BSA | Bovine serum albumin |
| DCFDA | 2′, 7′–dichlorofluorescein diacetate |
| DHT | Dihydrotestosterone |
| DKK-1 | Dickkopf-related protein 1 |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ELISA | Enzyme-linked immunosorbent assay |
| FGF | Fibroblast growth factor |
| GSK3β | Glycogen synthase kinase 3β |
| H2O2 | Hydrogen peroxide |
| HDPCs | Human dermal papilla cells |
| HF | Hair follicle |
| HPLC | High-performance liquid chromatography |
| HRESIMS | High-resolution electrospray ionization mass spectrometry |
| IGG | Intraclass correlation coefficient |
| IGF-1 | Insulin-like growth factor 1 |
| MAPK | Mitogen-activated protein kinase |
| PGG | Penta-O-Galloyl-β-D-Glucose |
| qRT-PCR | Quantitative reverse transcription-polymerase chain reaction |
| ROS | Reactive oxygen species |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless |
| WRGE | Water-soluble fraction of Rhus semialata gall extract |
References
- Adams, J.U. Raising hairs. Nat. Biotechnol. 2011, 29, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.L.; Katta, R. Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M.P.; Sanders, D.; Westgate, G.E.; Kealey, T. Human hair growth in vitro: A model for the study of hair follicle biology. J. Dermatol. Sci. 1994, 7, S55–S72. [Google Scholar] [CrossRef]
- Williamson, D.; Gonzalez, M.; Finlay, A.Y. The effect of hair loss on quality of life. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 137–139. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-N. The effect on hair loss for impression formation and interpersonal anxiety. Korean J. Aesthet. Cosmetol. 2010, 8, 247–258. [Google Scholar]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Yang, C.-C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010, 57, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Bae, J.; Lee, D.S.; Kim, S. Hair growth-promoting effects of Enz_MoriL on human dermal papilla cells through modulation of the Wnt/β-Catenin and JAK-STAT signaling pathways. Arch. Dermatol. Res. 2024, 316, 290. [Google Scholar] [CrossRef]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Kim, Y.-r.; Park, J.; Choi, S.; Kim, G.-U.; Kim, E.; Hwang, Y.; Kim, H.; Bak, S.S.; Lee, J.E. Wnt/β-catenin signaling activator restores hair regeneration suppressed by diabetes mellitus. BMB Rep. 2022, 55, 559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, M.; Yang, Y.; Yang, K.; Wickett, R.R.; Andl, T.; Millar, S.E.; Zhang, Y. Activation of β-catenin signaling in CD133-positive dermal papilla cells drives postnatal hair growth. PLoS ONE 2016, 11, e0160425. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, G.; Cárdenas, Y.; Rodríguez, Y.; Morales, L.C.; Matheus, L.; Arboleda, H. Differential regulation of AKT, MAPK and GSK3β during C2-ceramide-induced neuronal death. Neurotoxicology 2010, 31, 687–693. [Google Scholar] [CrossRef]
- Thornton, T.M.; Pedraza-Alva, G.; Deng, B.; Wood, C.D.; Aronshtam, A.; Clements, J.L.; Sabio, G.; Davis, R.J.; Matthews, D.E.; Doble, B.; et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science 2008, 320, 667–670. [Google Scholar] [CrossRef]
- Acharya, P.; Mathur, M.C. Oxidative stress in alopecia areata: A systematic review and meta-analysis. Int. J. Dermatol. 2020, 59, 434–440. [Google Scholar] [CrossRef]
- Huang, W.Y.; Huang, Y.C.; Huang, K.S.; Chan, C.C.; Chiu, H.Y.; Tsai, R.Y.; Chan, J.Y.; Lin, S.J. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial-mesenchymal interaction. J. Dermatol. Sci. 2017, 86, 114–122. [Google Scholar] [CrossRef]
- Kaya Erdogan, H.; Bulur, I.; Kocaturk, E.; Yildiz, B.; Saracoglu, Z.N.; Alatas, O. The role of oxidative stress in early-onset androgenetic alopecia. J. Cosmet. Dermatol. 2017, 16, 527–530. [Google Scholar] [CrossRef]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Cicero, N.; Gangemi, S. Alopecia Areata: A Review of the Role of Oxidative Stress, Possible Biomarkers, and Potential Novel Therapeutic Approaches. Antioxidants 2023, 12, 135. [Google Scholar] [CrossRef]
- Prie, B.E.; Iosif, L.; Tivig, I.; Stoian, I.; Giurcaneanu, C. Oxidative stress in androgenetic alopecia. J. Med. Life 2016, 9, 79–83. [Google Scholar]
- Prie, B.E.; Voiculescu, V.M.; Ionescu-Bozdog, O.B.; Petrutescu, B.; Iosif, L.; Gaman, L.E.; Clatici, V.G.; Stoian, I.; Giurcaneanu, C. Oxidative stress and alopecia areata. J. Med. Life 2015, 8, 43–46. [Google Scholar] [PubMed]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef]
- Ali, E.; Owais, R.; Sheikh, A.; Shaikh, A. Olumniant (Baricitinib) oral tablets: An insight into FDA-approved systemic treatment for Alopecia Areata. Ann. Med. Surg. 2022, 80, 104157. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Woo, J.; Roh, K.B.; Jeon, K.; Jang, Y.; Choi, S.A.; Ryu, D.; Cho, E.; Park, D.; Lee, J.; et al. Evaluation of efficacy of Silybum marianum flower extract on the mitigating hair loss in vitro and in vivo. J. Cosmet. Dermatol. 2024, 23, 529–542. [Google Scholar] [CrossRef]
- Park, S.; Han, N.; Lee, J.M.; Lee, J.H.; Bae, S. Effects of Allium hookeri Extracts on Hair-Inductive and Anti-Oxidative Properties in Human Dermal Papilla Cells. Plants 2023, 12, 1919. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.C.; Nam, G.W.; Jeong, N.H.; Choi, B.Y. Hair growth promotion by extracts of Inula Helenium and Caesalpinia Sappan Bark in patients with androgenetic alopecia: A pre-clinical study using phototrichogram analysis. Cosmetics 2019, 6, 66. [Google Scholar] [CrossRef]
- Woo, H.; Kim, H.; Shin, S.; Shin, J.H.; Ryu, D.; Park, D.; Jung, E. Rhus semialata M. extract ameliorate para-phenylenediamine-induced toxicity in keratinocytes. Toxicol. Rep. 2021, 8, 96–105. [Google Scholar] [CrossRef]
- Cha, B.-C.; Lee, S.-B. Antioxidative and free radical scavenging effects of Rhus javanica Linne. Korean J. Med. Crop Sci. 1998, 6, 181–187. [Google Scholar]
- Cha, B.-C.; Lee, S.-B.; Rhim, T.-J.; Lee, K.-H. Antioxidative and hepatoprotective effect of compounds isolated from galla rhois (Rhus javanica Linne). Korean J. Med. Crop Sci. 2000, 8, 157–164. [Google Scholar]
- Satoh, K.; Nagai, F.; Ushiyama, K.; Yasuda, I.; Seto, T.; Kano, I. Inhibition of Na+,K(+)-ATPase by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose, a major constituent of both moutan cortex and Paeoniae radix. Biochem. Pharmacol. 1997, 53, 611–614. [Google Scholar] [CrossRef]
- Lee, K.-E.; Nam, J.-J.; Kim, S.-M.; Kim, H.-K.; Moon, S.; Youm, J.K. Anti-Inflammatory Effects of the Mixture of Sorbus commixta, Urtica dioica, Phyllostachys nigra, and Rhus semialata Gall Extracts on LPS-induced Inflammation in HaCaT Cells. J. Soc. Cosmet. Sci. Korea 2014, 40, 45–54. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Warleta, F.; Quesada, C.S.; Campos, M.; Allouche, Y.; Beltrán, G.; Gaforio, J.J. Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients 2011, 3, 839–857. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Lee, Y.-K.; Park, J.-H.; Kim, S.-H.; Park, C.-S.; Kim, K.; Lee, C.-K. Therapeutic efficacy and mechanism of solubilized sturgeon oil in a mouse model of house dust mite-induced atopic dermatitis. J. Funct. Foods 2024, 115, 106093. [Google Scholar] [CrossRef]
- Kim, D.H.; Yu, J.E.; Lee, D.H.; Kim, M.J.; Jeon, S.H.; Yun, J.; Son, D.J.; Kim, B.; Yong, Y.J.; Lim, Y.-s.; et al. Anti-arthritis Effect of Anti-chitinase-3-like 1 Antibody Through Inhibition of MMP3. Immune Netw. 2025, 25, e5. [Google Scholar]
- Shim, E.-H.; Kim, S.-H.; Kim, D.-J.; Jang, Y.-S. Complement C5a Receptor Signaling in Macrophages Enhances Trained Immunity Through mTOR Pathway Activation. Immune Netw. 2024, 24, e24. [Google Scholar] [CrossRef]
- Cicchetti, D.V.; Sparrow, S.A. Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. Am. J. Ment. Defic. 1981, 86, 127–137. [Google Scholar]
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Landis, J. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and criteria changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.M.; Jang, Y.N.; Park, A.Y.; Kim, S.Y.; Kim, B.J.; Lee, J.O. Irisin promotes hair growth and hair cycle transition by activating the GSK-3β/β-catenin pathway. Exp. Dermatol. 2024, 33, e15155. [Google Scholar] [CrossRef]
- Park, S.; Erdogan, S.; Hwang, D.; Hwang, S.; Han, E.H.; Lim, Y.H. Bee Venom Promotes Hair Growth in Association with Inhibiting 5α-Reductase Expression. Biol. Pharm. Bull. 2016, 39, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Hyun, J.; Kim, S.W.; Bhang, S.H. Enhancing the Angiogenic and Proliferative Capacity of Dermal Fibroblasts with Mulberry (Morus alba. L) Root Extract. Tissue Eng. Regen. Med. 2022, 19, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Y.; Song, Z.; Hao, F.; Yang, X. Identification of Wnt/β-catenin signaling pathway in dermal papilla cells of human scalp hair follicles: TCF4 regulates the proliferation and secretory activity of dermal papilla cell. J. Dermatol. 2014, 41, 84–91. [Google Scholar] [CrossRef]
- Shin, D.W. The Molecular Mechanism of Natural Products Activating Wnt/β-Catenin Signaling Pathway for Improving Hair Loss. Life 2022, 12, 1856. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, J.M.; Leirós, G.J.; Balañá, M.E. Androgens and androgen receptor action in skin and hair follicles. Mol. Cell Endocrinol. 2018, 465, 122–133. [Google Scholar] [CrossRef]
- Sadgrove, N.; Batra, S.; Barreto, D.; Rapaport, J. An updated etiology of hair loss and the new cosmeceutical paradigm in therapy: Clearing ‘the big eight strikes’. Cosmetics 2023, 10, 106. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Jia, K.; Xu, X.; Li, Y.; Zhao, Y.; Zhang, X.; Zhang, J.; Liu, G.; Deng, S.; et al. Crosstalk between androgen and Wnt/β-catenin leads to changes of wool density in FGF5-knockout sheep. Cell Death Dis. 2020, 11, 407. [Google Scholar] [CrossRef]
- Liu, N.; Wang, L.H.; Guo, L.L.; Wang, G.Q.; Zhou, X.P.; Jiang, Y.; Shang, J.; Murao, K.; Chen, J.W.; Fu, W.Q.; et al. Chronic restraint stress inhibits hair growth via substance P mediated by reactive oxygen species in mice. PLoS ONE 2013, 8, e61574. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, S.H.; Yang, W.M. Beneficial effects of Astragaloside IV for hair loss via inhibition of Fas/Fas L-mediated apoptotic signaling. PLoS ONE 2014, 9, e92984. [Google Scholar] [CrossRef]
- Lindner, G.; Botchkarev, V.A.; Botchkareva, N.V.; Ling, G.; van der Veen, C.; Paus, R. Analysis of apoptosis during hair follicle regression (catagen). Am. J. Pathol. 1997, 151, 1601–1617. [Google Scholar]
- Norris, D.A.; Duke, R.; Whang, K.; Middleton, M. Immunologic cytotoxicity in alopecia areata: Apoptosis of dermal papilla cells in alopecia areata. J. Investig. Dermatol. 1995, 104, 8s–9s. [Google Scholar] [CrossRef] [PubMed]
- Soma, T.; Ogo, M.; Suzuki, J.; Takahashi, T.; Hibino, T. Analysis of apoptotic cell death in human hair follicles in vivo and in vitro. J. Investig. Dermatol. 1998, 111, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Müller-Röver, S.; Rossiter, H.; Lindner, G.; Peters, E.M.J.; Kupper, T.S.; Paus, R. Hair Follicle Apoptosis and Bcl-2. J. Investig. Dermatol. Symp. Proc. 1999, 4, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Fukamachi, Y.; Karasaki, Y.; Sugiura, T.; Itoh, H.; Abe, T.; Yamamura, K.; Higashi, K. Zinc suppresses apoptosis of U937 cells induced by hydrogen peroxide through an increase of the Bcl-2/Bax ratio. Biochem. Biophys. Res. Commun. 1998, 246, 364–369. [Google Scholar] [CrossRef]
- Lee, J.E.; Sohn, J.; Lee, J.H.; Lee, K.C.; Son, C.S.; Tockgo, Y.C. Regulation of bcl-2 family in hydrogen peroxide-induced apoptosis in human leukemia HL-60 cells. Exp. Mol. Med. 2000, 32, 42–46. [Google Scholar] [CrossRef]
- Hwang, S.B.; Park, H.J.; Lee, B.H. Hair-Growth-Promoting Effects of the Fish Collagen Peptide in Human Dermal Papilla Cells and C57BL/6 Mice Modulating Wnt/β-Catenin and BMP Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 11904. [Google Scholar] [CrossRef]
- Kang, J.I.; Choi, Y.K.; Han, S.C.; Kim, H.G.; Hong, S.W.; Kim, J.; Kim, J.H.; Hyun, J.W.; Yoo, E.S.; Kang, H.K. Limonin, a Component of Immature Citrus Fruits, Activates Anagen Signaling in Dermal Papilla Cells. Nutrients 2022, 14, 5358. [Google Scholar] [CrossRef]
- Wang, C.; Zang, K.; Tang, Z.; Yang, T.; Ye, X.; Dang, Y. Hordenine Activated Dermal Papilla Cells and Promoted Hair Regrowth by Activating Wnt Signaling Pathway. Nutrients 2023, 15, 694. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef]
- Mecklenburg, L.; Tobin, D.J.; Müller-Röver, S.; Handjiski, B.; Wendt, G.; Peters, E.M.J.; Pohl, S.; Moll, I.; Paus, R. Active Hair Growth (Anagen) is Associated with Angiogenesis. J. Investig. Dermatol. 2000, 114, 909–916. [Google Scholar] [CrossRef]
- Detmar, M. The role of VEGF and thrombospondins in skin angiogenesis. J. Dermatol. Sci. 2000, 24 (Suppl. S1), S78–S84. [Google Scholar] [CrossRef] [PubMed]
- Su, H.Y.; Hickford, J.G.; Bickerstaffe, R.; Palmer, B.R. Insulin-like growth factor 1 and hair growth. Dermatol. Online J. 1999, 5, 1. [Google Scholar] [CrossRef]
- Rosenquist, T.A.; Martin, G.R. Fibroblast growth factor signalling in the hair growth cycle: Expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev. Dyn. 1996, 205, 379–386. [Google Scholar] [CrossRef]
- Escamilla-Cruz, M.; Magaña, M.; Escandón-Perez, S.; Bello-Chavolla, O.Y. Use of 5-Alpha Reductase Inhibitors in Dermatology: A Narrative Review. Dermatol. Ther. 2023, 13, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- Papaccio, F.; D′Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. The impact of oxidative stress on hair. Int. J. Cosmet. Sci. 2015, 37 (Suppl. S2), 25–30. [Google Scholar] [CrossRef]
- Trüeb, R.M. Oxidative stress and its impact on skin, scalp and hair. Int. J. Cosmet. Sci. 2021, 43 (Suppl. S1), S9–S13. [Google Scholar] [CrossRef]
- Choi, Y.S.; Zhang, Y.; Xu, M.; Yang, Y.; Ito, M.; Peng, T.; Cui, Z.; Nagy, A.; Hadjantonakis, A.K.; Lang, R.A.; et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 2013, 13, 720–733. [Google Scholar] [CrossRef]





| Gene | Primer Sequences (5′→3′) | Accession Number |
|---|---|---|
| VEGF | F: TCCTGGAGCGTGTACGTTG R: ACACGTCTGCGGATCTTGTA | NM_001171623.2 |
| IGF-1 | F: TCAACAAGCCCACAGGGTAT R: ACTCGTGCAGAGCAAAGGAT | NM_001111283.3 |
| FGF | F: CTGTACTGCAAAAACGGGGG R: CGAAATGGAAATGAGGCGGA | X04431.1 |
| BAX | F: GGGAGCAGCCCAGAGG R: ATTCGCCCTGCTCGATCC | NM_001291428.2 |
| Bcl-2 | F: GGATAACGGAGGCTGGGATG R: TTGTGGCTCAGATAGGCACC | NM_000633.3 |
| GAPDH | F: ACTTTTGGTATCGTGGAAGGAC R: GCAGGGATGATGTTCTGGAG | NM_002046.7 |
| Characteristic | 1% WRGE Group | Placebo Group |
|---|---|---|
| Age (years) mean ± SD | 46.3 ± 8.6 | 43.1 ± 7.6 |
| Sex, n (%) | ||
| Male | 11 (42.3) | 9 (34.6) 17 (65.4) |
| Female | 15 (57.7) |
| Hair Density (Mean ± SD) | ||
|---|---|---|
| Group Name | Total Hair Count (n/cm2) | p-Value a |
| Placebo | 107.048 ± 25.586 | 0.743 |
| 1% WRGE | 104.136 ± 24.337 | |
| Hair Density (Mean ± SD) | |||
|---|---|---|---|
| Measurement Time Point | n/cm2 | Variation a | p-Value b |
| 0 weeks | 107.048 ± 25.586 | - | - |
| 16 weeks | 106.048 ± 25.471 | −1.000 ± 3.808 | 0.243 |
| 24 weeks | 107.286 ± 24.350 | 0.238 ± 3.097 | 0.728 |
| Hair Density (Mean ± SD) | ||||
|---|---|---|---|---|
| Measurement Time | n/cm2 | Variation a | p-Value | |
| Compared Within Groups b | Compared Between Groups c | |||
| 0 weeks | 104.136 ± 24.337 | - | - | - |
| 16 weeks | 105.455 ± 23.876 | 1.318 ± 4.052 | 0.166 | 0.048 |
| 24 weeks | 106.682 ± 24.952 | 2.545 ± 2.857 | 0.001 * | 0.009 † |
| 8 Weeks | 16 Weeks | 24 Weeks | |
|---|---|---|---|
| Researcher 1 | 0.163 ± 0.485 | 0.349 ± 0.529 | 0.372 ± 0.691 |
| Researcher 2 | 0.000 ± 0.309 | 0.116 ± 0.498 | 0.163 ± 0.574 |
| ICC | 0.815 | ||
| Hair Density (Mean ± SD) | ||||
|---|---|---|---|---|
| Measurement Time | Grade | Variation a | p-Value | |
| Compared Within Groups b | Compared Between Groups c | |||
| 0 weeks | 0.000 ± 0.000 | - | - | - |
| 8 weeks | 0.045 ± 0.213 | 0.045 ± 0.213 | 0.317 | 0.071 |
| 16 weeks | 0.364 ± 0.492 | 0.364 ± 0.492 | 0.005 * | 0.000 † |
| 24 weeks | 0.500 ± 0.512 | 0.500 ± 0.512 | 0.001 * | 0.000 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-S.; Han, J.S.; Park, J.-H.; Lee, M.-H.; Seo, Y.-J.; Jeon, S.Y.; Hong, H.R.; Kim, M.; Do, S.G.; Hwang, B.Y.; et al. Evaluation of Efficacy of Water-Soluble Fraction of Rhus semialata Gall Extract and Penta-O-Galloyl-β-D-Glucose on Mitigation of Hair Loss: An In Vitro and Randomized Double-Blind Placebo-Controlled Clinical Study. Antioxidants 2025, 14, 477. https://doi.org/10.3390/antiox14040477
Lee H-S, Han JS, Park J-H, Lee M-H, Seo Y-J, Jeon SY, Hong HR, Kim M, Do SG, Hwang BY, et al. Evaluation of Efficacy of Water-Soluble Fraction of Rhus semialata Gall Extract and Penta-O-Galloyl-β-D-Glucose on Mitigation of Hair Loss: An In Vitro and Randomized Double-Blind Placebo-Controlled Clinical Study. Antioxidants. 2025; 14(4):477. https://doi.org/10.3390/antiox14040477
Chicago/Turabian StyleLee, Hee-Sung, Jae Sang Han, Ji-Hyun Park, Min-Hyeok Lee, Yu-Jin Seo, Se Yeong Jeon, Hye Ryeong Hong, Miran Kim, Seon Gil Do, Bang Yeon Hwang, and et al. 2025. "Evaluation of Efficacy of Water-Soluble Fraction of Rhus semialata Gall Extract and Penta-O-Galloyl-β-D-Glucose on Mitigation of Hair Loss: An In Vitro and Randomized Double-Blind Placebo-Controlled Clinical Study" Antioxidants 14, no. 4: 477. https://doi.org/10.3390/antiox14040477
APA StyleLee, H.-S., Han, J. S., Park, J.-H., Lee, M.-H., Seo, Y.-J., Jeon, S. Y., Hong, H. R., Kim, M., Do, S. G., Hwang, B. Y., & Park, C.-S. (2025). Evaluation of Efficacy of Water-Soluble Fraction of Rhus semialata Gall Extract and Penta-O-Galloyl-β-D-Glucose on Mitigation of Hair Loss: An In Vitro and Randomized Double-Blind Placebo-Controlled Clinical Study. Antioxidants, 14(4), 477. https://doi.org/10.3390/antiox14040477








