Involvement of Nuclear Receptors PPAR-α, PPAR-γ, and the Transcription Factor Nrf2 in Cellular Protection Against Oxidative Stress Regulated by H2S and Induced by Hypoxia–Reoxygenation and High Glucose in Primary Cardiomyocyte Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Neonatal Rat Cardiomyocytes (NRCMs) Isolation and Culture
2.3. HIF1-α Expression
2.4. Cell Viability
2.5. Antioxidant Capacity Assay
2.6. ROS Production
2.7. Quantification of Malondialdehyde (MDA)
2.8. Quantification of 8-Hydroxy-2′-Deoxyguanosine (8-OH-2dG)
2.9. Capillary Zone Electrophoresis for Determination of BH4 and BH2
2.10. Palmitoyl CoA Oxidase Activity
2.11. Protein Expression by Western Blot
2.12. Mitochondria Ultrastructure
2.13. Statistical Analysis
3. Results
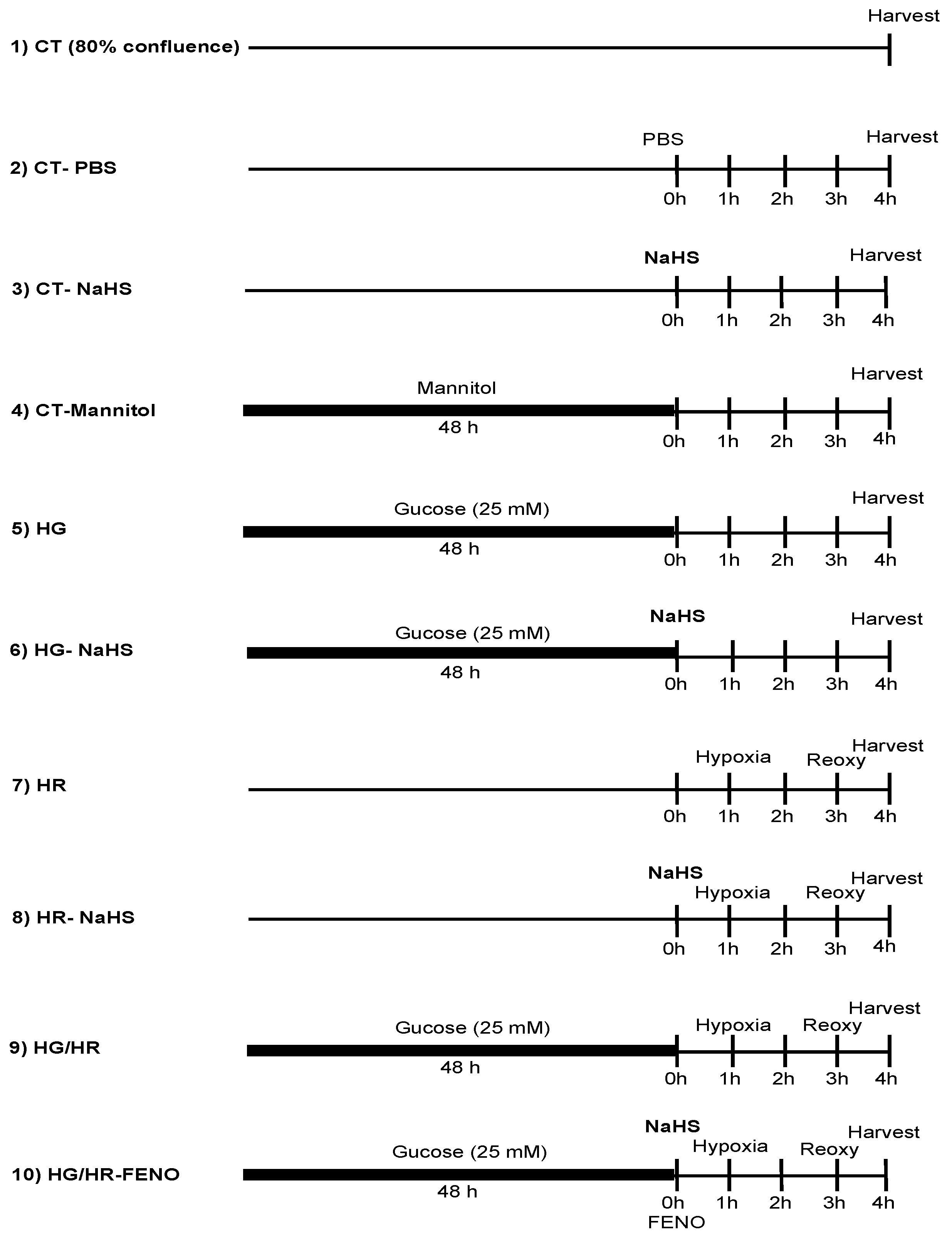
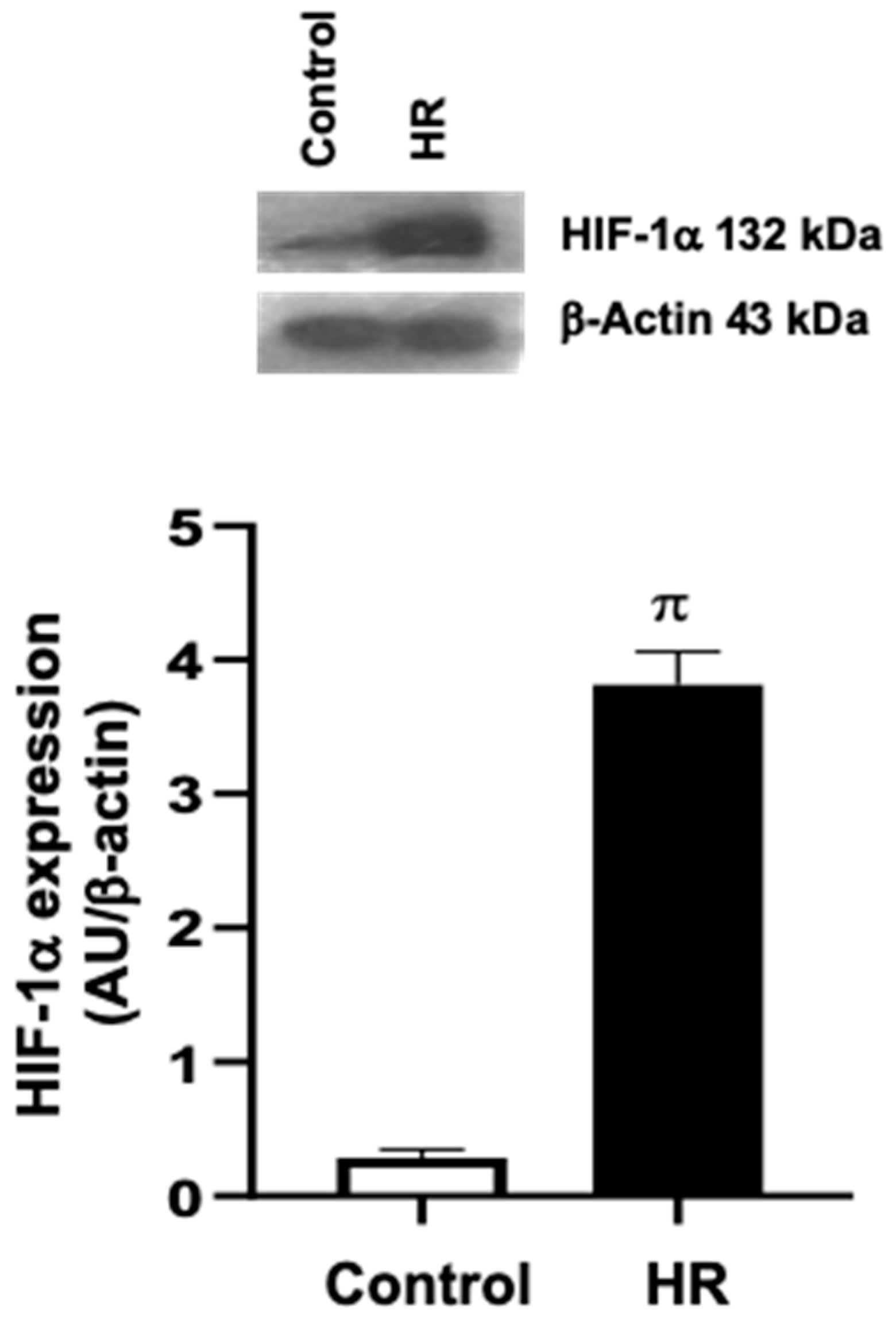
3.1. Evaluation of the Hypoxia–Reoxygenation (HR) Model in Primary Cardiomyocyte Cultures
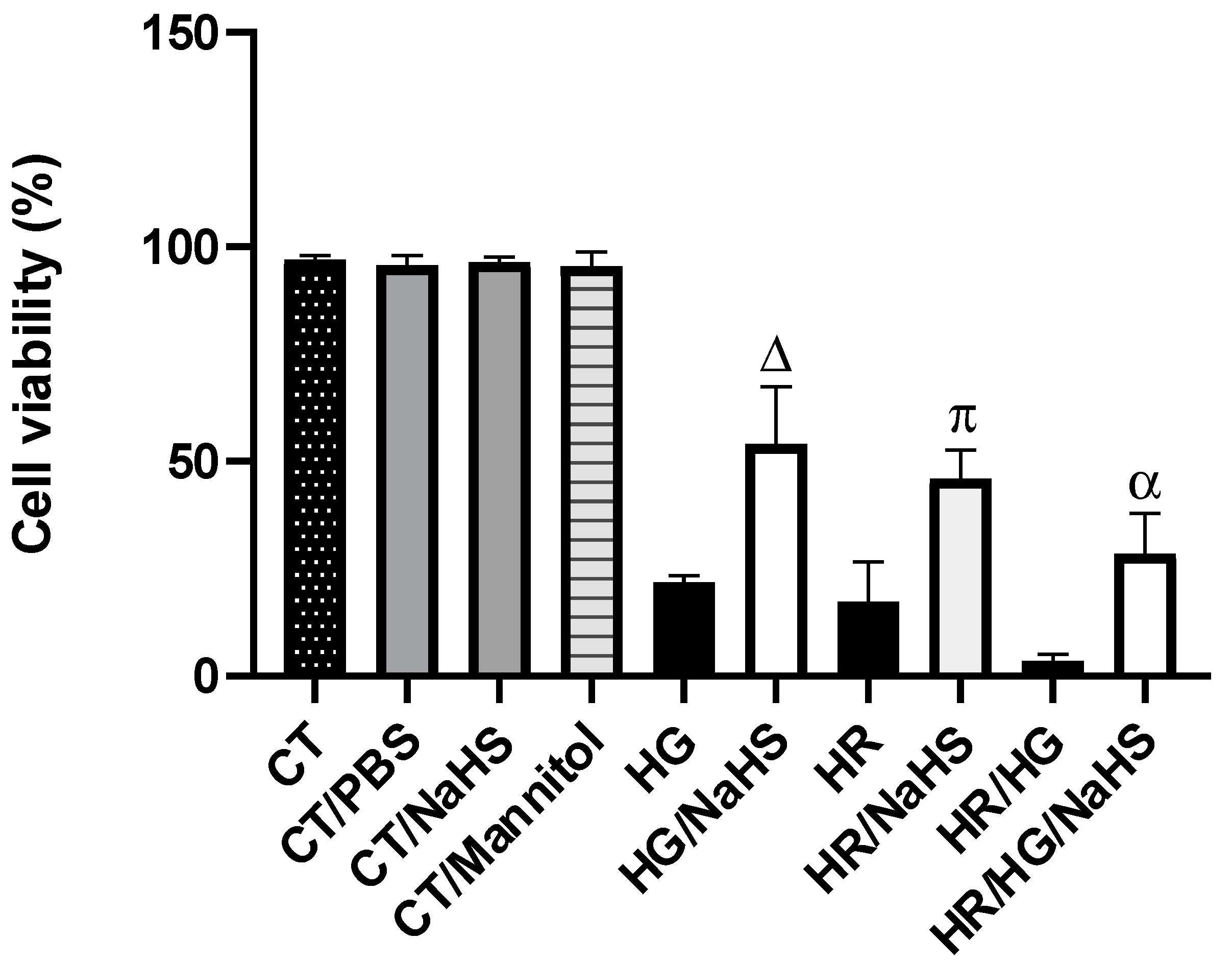
3.2. Evaluation of Cell Viability
3.3. Total Antioxidant Capacity (TAC)
3.4. ROS Production
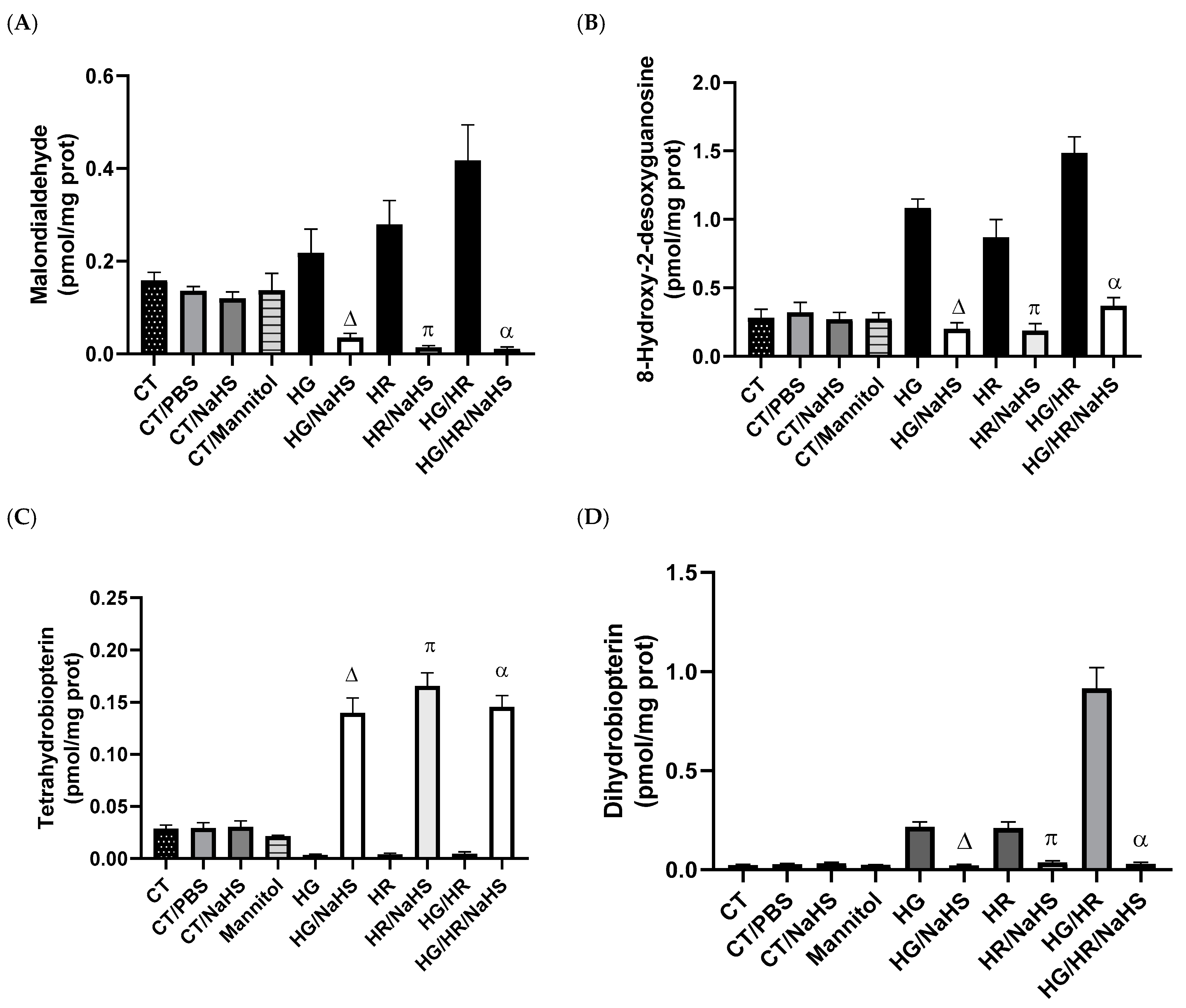
3.5. Evaluation of Oxidative Stress
3.6. Evaluation of Cofactor for eNOS, Tetrahydrobiopterin (BH4), and Its Oxidation Product (BH2)
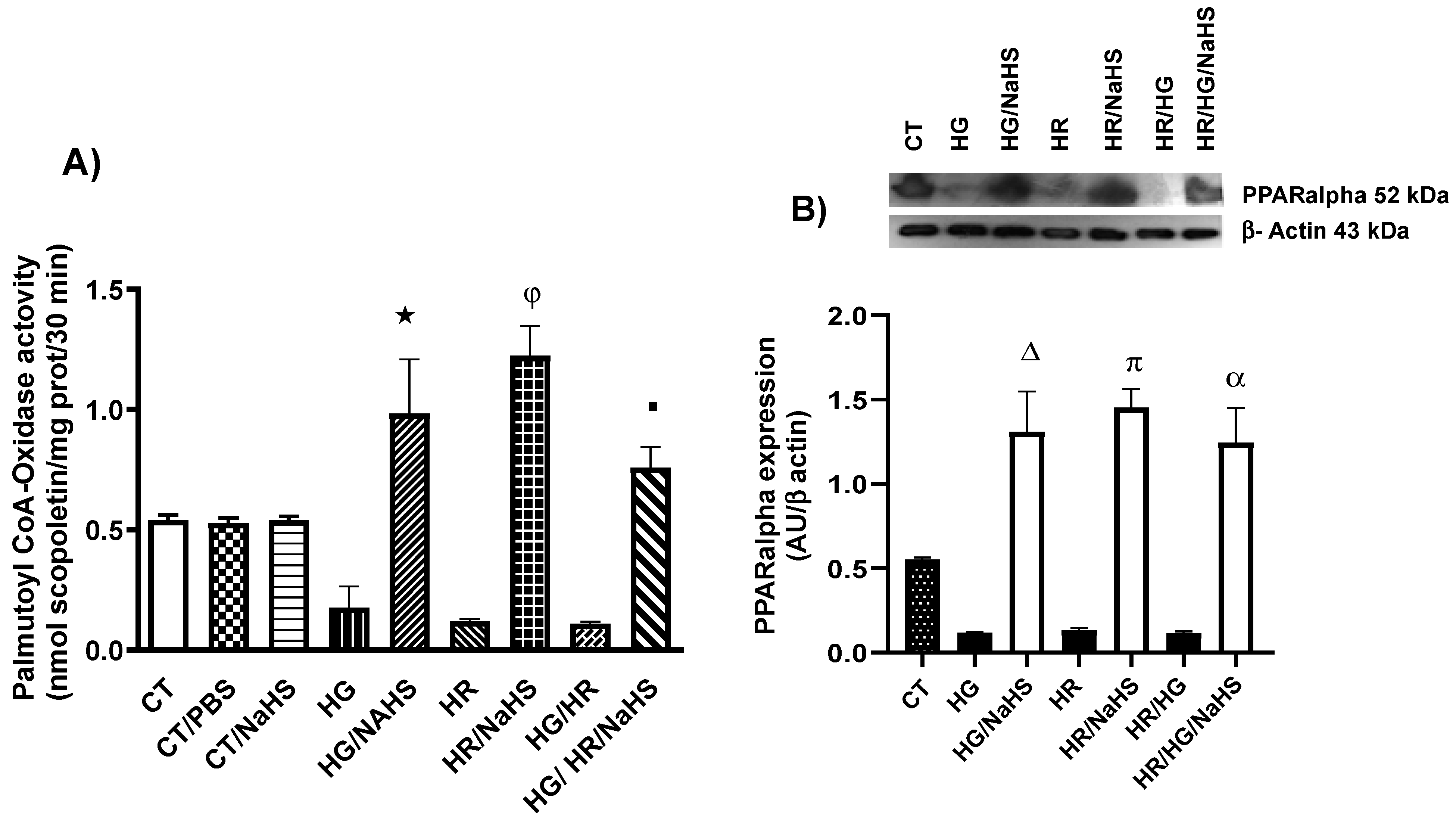
3.7. Evaluation of Palmitoyl-CoA Oxidase Activity and PPAR-α Expression
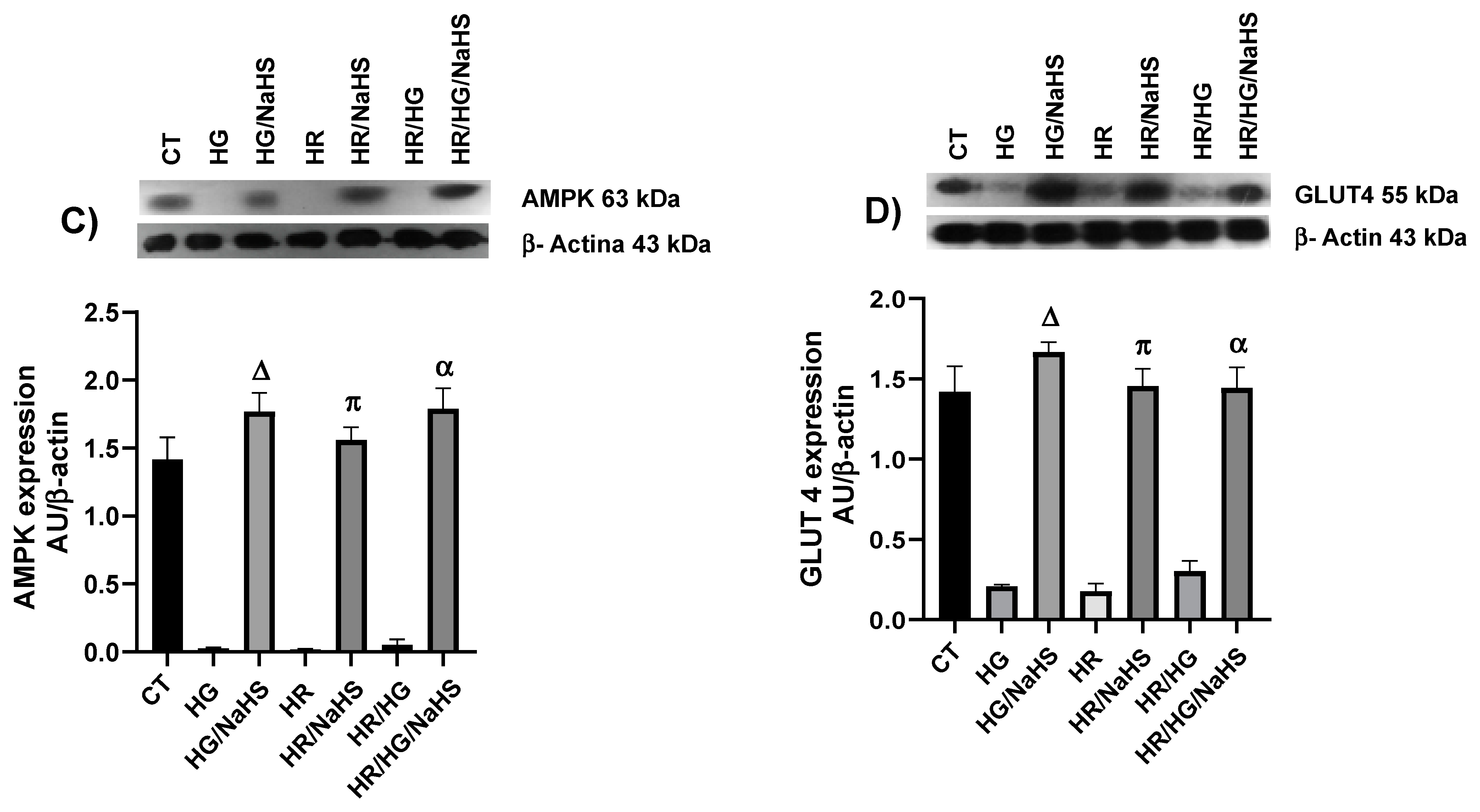
3.8. Evaluation of PPAR-γ, PGC-1α, AMPK, GLUT4, Keap1, Nrf2, and p-eNOS Ser1177 Expression
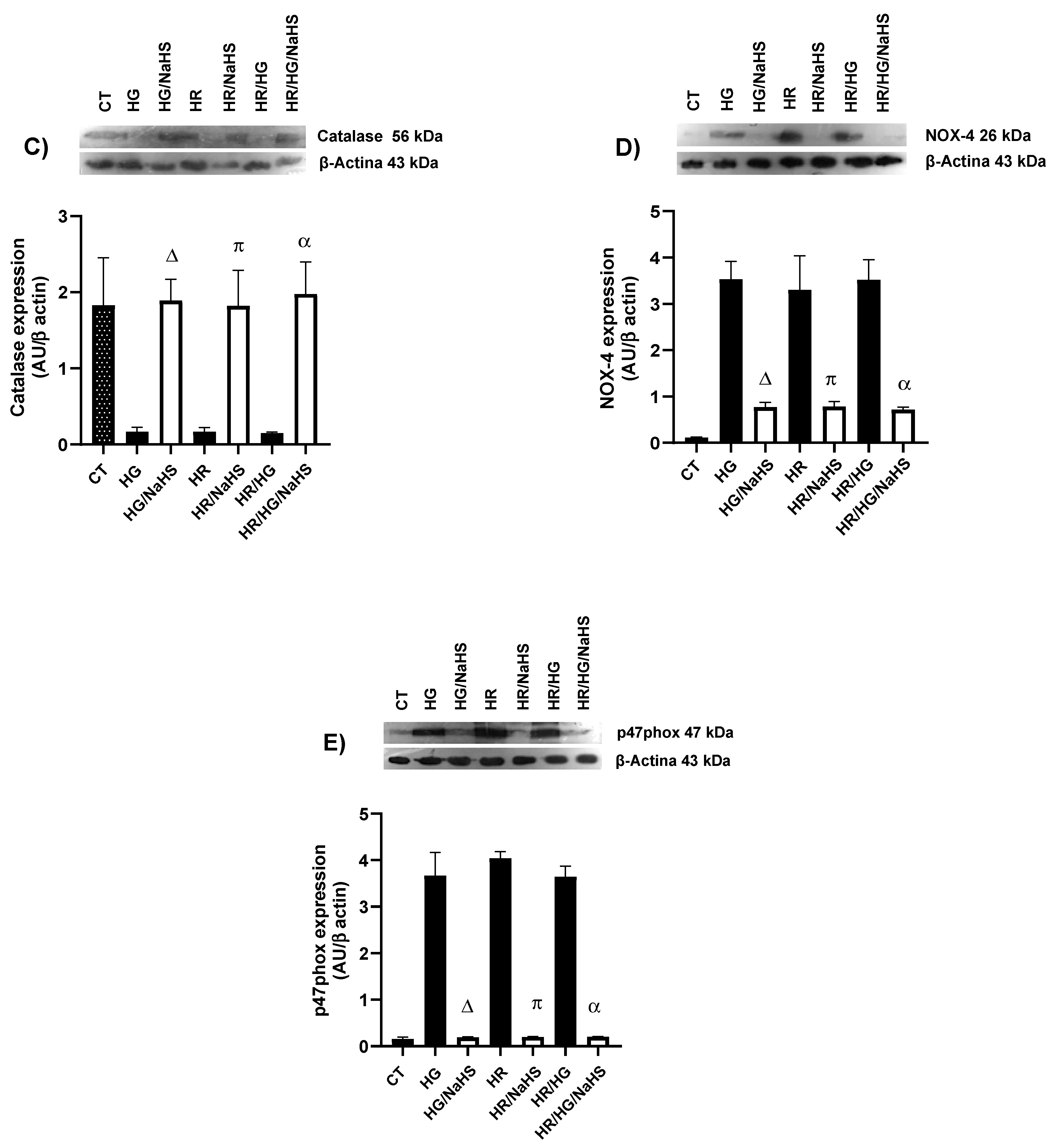
3.9. Evaluation of the Expression of SOD-Cu2+/Zn2+, SOD-Mn2+, Catalase, NOX4, and p47phox
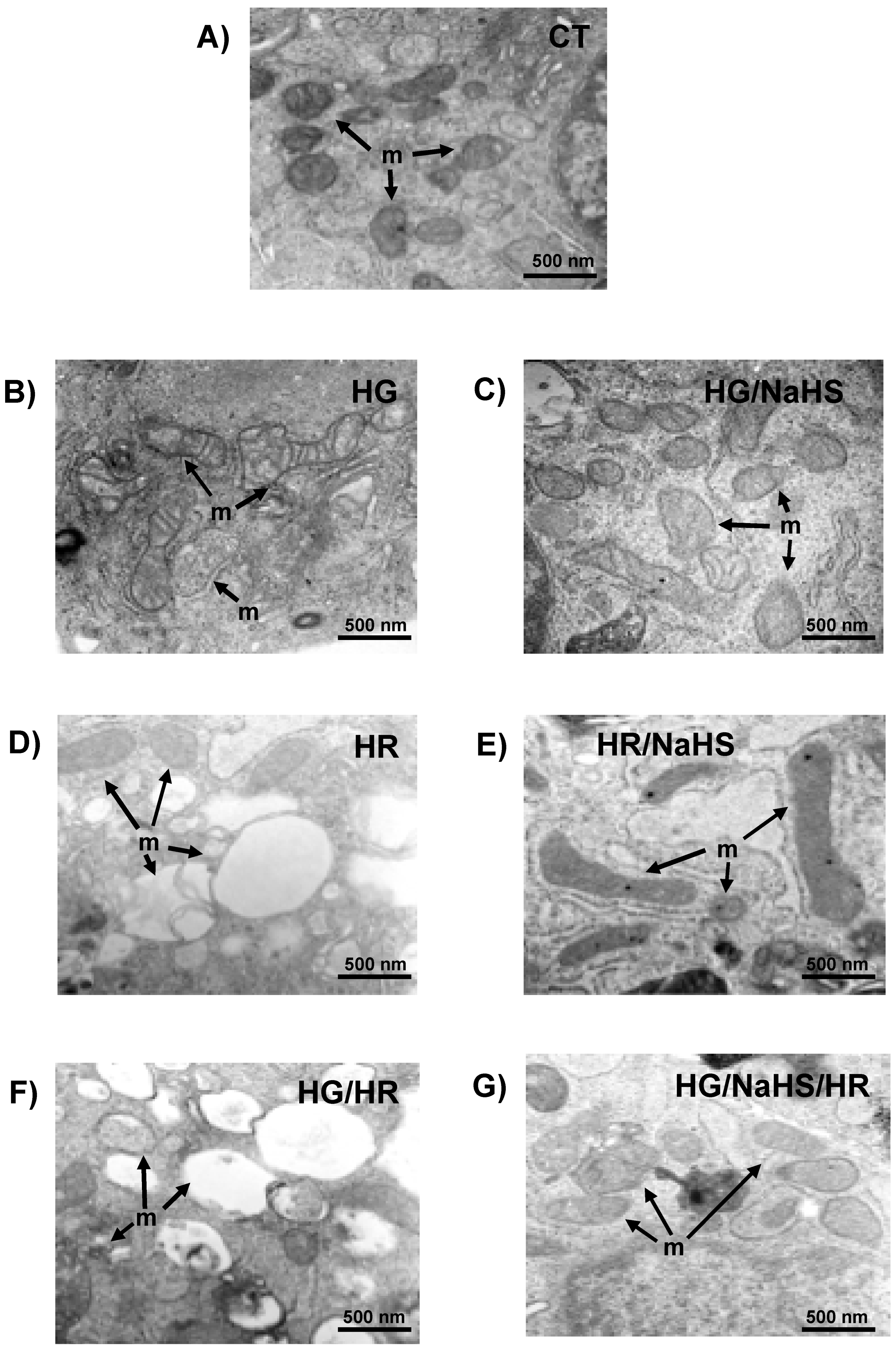
3.10. Evaluation of Mitochondrial Ultrastructure in Cardiomyocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Nesto, R.W. Correlation between cardiovascular disease and diabetes mellitus: Current concepts. Am. J. Med. 2004, 116, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell. Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Kinugawa, S.; Utsumi, H.; Kang, D.; Hattori, N.; Uchida, K.; Arimura, K.; Egashira, K.; Takeshita, A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res. 1999, 85, 357–363. [Google Scholar] [CrossRef]
- Dhalla, A.K.; Singal, P.K. Antioxidant changes in hypertrophied and failing guinea pig hearts. Am. J. Physiol. 1994, 266 Pt 2, H1280-5. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Let. 2023, 28, 21–26. [Google Scholar] [CrossRef]
- Kuroda, J.; Ago, T.; Matsushima, S.; Zhai, P.; Schneider, M.D.; Sadoshima, J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA 2010, 107, 15565–15570. [Google Scholar] [CrossRef]
- Ibarra-Lara, L.; Hong, E.; Soria-Castro, E.; Torres-Narváez, J.C.; Pérez-Severiano, F.; Del Valle-Mondragón, L.; Cervantes-Pérez, L.G.; Ramírez-Ortega, M.; Pastelín-Hernández, G.S.; Sánchez-Mendoza, A. Clofibrate PPAR-α activation reduces oxidative stress and improves ultrastructure and ventricular hemodynamics in no-flow myocardial ischemia. J. Cardiovasc. Pharmacol. 2012, 60, 323–334. [Google Scholar] [CrossRef]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The Novel Role of γ-Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Jun, H.S. Effects of glucagon-like peptide-1 on oxidative stress and Nrf2 signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- Bian, J.S.; Yong, Q.C.; Pan, T.T.; Feng, Z.N.; Ali, M.Y.; Zhou, S.; Moore, P.K. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J. Pharmacol. Exp. Ther. 2006, 316, 670–678. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, H.; Liu, P.; Tang, C.; Wang, J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening KATP channels. Can. J. Physiol. Pharmacol. 2007, 85, 1248–1253. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Sun, W.; Li, L.; Wu, B.; Bai, S.; Li, H.; Zhong, X.; Wang, R.; Wu, L.; et al. Exogenous hydrogen sulfide restores cardioprotection of ischemic post-conditioning via inhibition of mPTP opening in the aging cardiomyocytes. Cell. Biosci. 2015, 5, 43. [Google Scholar] [CrossRef]
- Ehler, E.; Moore-Morris, T.; Lange, S. Isolation and culture of neonatal mouse cardiomyocytes. J. Vis. Exp. 2013, 15, 50154. [Google Scholar]
- Brewer, J.H.; Allgeier, D.L. Disposable Hydrogen Generator. Science 1965, 147, 1033–1034. [Google Scholar] [CrossRef]
- Brewer, J.H.; Allgeier, D.L. Safe Self-contained Carbon Dioxide-Hydrogen Anaerobic System. Appl. Microbiol. 1966, 14, 985–988. [Google Scholar] [CrossRef]
- Karki, P.; Fliegel, L. Overexpression of the NHE1 isoform of the Na+/H+ exchanger causes elevated apoptosis in isolated cardiomyocytes after hypoxia/reoxygenation challenge. Mol. Cell. Biochem. 2010, 338, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.K.; Pedram, A.; Razandi, M.; Levin, E.R. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J. Biol. Chem. 2006, 28, 6760–6767. [Google Scholar]
- Zou, C.; Li, W.; Pan, Y.; Khan, Z.A.; Li, J.; Wu, X.; Wang, Y.; Deng, L.; Liang, G.; Zhao, Y. 11β-HSD1 inhibition ameliorates diabetes-induced cardiomyocyte hypertrophy and cardiac fibrosis through modulation of EGFR activity. Oncotarget 2017, 8, 96263–96275. [Google Scholar] [CrossRef]
- Cortes-Lopez, F.; Sanchez-Mendoza, A.; Centurion, D.; Cervantes-Perez, L.G.; Castrejon-Tellez, V.; Del Valle-Mondragon, L.; Soria-Castro, E.; Ramirez, V.; Sanchez-Lopez, A.; Pastelin-Hernandez, G.; et al. Fenofibrate Protects Cardiomyocytes from Hypoxia/Reperfusion- And High Glucose-Induced Detrimental Effects. PPAR Res. 2021, 9, 8895376. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3. B.1–A3.B.3. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Christensen, M.E.; Waterhouse, N.J. Measuring cell death by trypan blue uptake and light microscopy. Cold. Spring. Harb. Protoc. 2016, 2016, 643–646. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E.; Altun, M. Total antioxidant capacity assay of human serum using copper (II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free. Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef]
- Sung, H.K.; Song, E.; Jahng, J.W.S.; Pantopoulos, K.; Sweeney, G. Iron induces insulin resistance in cardiomyocytes via regulation of oxidative stress. Sci. Rep. 2019, 9, 4668. [Google Scholar] [CrossRef]
- Sánchez-Aguilar, M.; Ibarra-Lara, L.; Del Valle-Mondragón, L.; Rubio-Ruiz, M.E.; Aguilar-Navarro, A.G.; Zamorano-Carrillo, A.; Ramírez-Ortega, M.D.C.; Pastelín-Hernández, G.; Sánchez-Mendoza, A. Rosiglitazone, a Ligand to PPAR-γ, Improves Blood Pressure and Vascular Function through Renin-Angiotensin System Regulation. PPAR Res. 2019, 2019, 1371758. [Google Scholar] [CrossRef]
- Kvasnicová, V.; Samcová, E.; Jursová, A.; Jelínek, I. Determination of 8-hydroxy-2′-deoxyguanosine in untreated urine by capillary electrophoresis with UV detection. J. Chromatogr. A 2003, 985, 513–517. [Google Scholar] [CrossRef]
- Walusimbi-Kisitu, M.; Harrison, E.H. Fluorometric assay for rat liver peroxisomal fatty acyl-coenzyme A oxidase activity. J. Lipid. Res. 1983, 24, 1077–1084. [Google Scholar] [CrossRef]
- González-Morán, M.G.; Soria-Castro, E. Changes in the tubular compartment of the testis of Gallus domesticus during development. Br. Poult. Sci. 2010, 51, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Invest. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free. Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Verbeuren, T.J.; Van De Voorde, J.; Lameire, N.H.; Vanhoutte, P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000, 130, 963–974. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Yin, J.; Wu, S.; Zhou, X. Protective mechanisms of hydrogen sulfide in myocardial ischemia. J. Cell. Physiol. 2020, 235, 9059–9070. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- King, A.L.; Polhemus, D.J.; Bhushan, S.; Otsuka, H.; Kondo, K.; Nicholson, C.K.; Bradley, J.M.; Islam, K.N.; Calvert, J.W.; Tao, Y.X.; et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 3182–3187. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, L.; Wang, Y.; Dong, S.; Leng, X.; Jia, J.; Zhao, Y.; Le, H.; Zhang, X.; Xu, C.; et al. Exogenous hydrogen sulfide attenuates diabetic myocardial injury through cardiac mitochondrial protection. Mol. Cell. Biochem. 2012, 371, 187–198. [Google Scholar] [CrossRef]
- Searcy, D.G.; Whitehead, J.P.; Maroney, M.J. Interaction of Cu, Zn superoxide dismutase with hydrogen sulfide. Arch. Bichem. Biophys. 1995, 8, 251–263. [Google Scholar] [CrossRef]
- Comhaire, F.; Zalata, A.; Mahmoud, A.; Depuydt, C.; Dhooghe, W.; Christophe, A. Which efforts towards conservative treatment of male infertility will be successful? Reactive oxygen species, antioxidants, and sperm phospholipids. Andrologia 1999, 31, 295–296. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: A target for new therapies against cardiovascular diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of hydrogen sulfide in Nrf2-and sirtuin-dependent maintenance of cellular redox balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef]
- Hassan, M.I.; Boosen, M.; Schaefer, L.; Kozlowska, J.; Eisel, F.; von Knethen, A.; Beck, M.; Hemeida, R.A.; El-Moselhy, M.A.; Hamada, F.M.; et al. Platelet-derived growth factor-BB induces cystathionine Iγ-lyase expression in rat mesangial cells via a redox-dependent mechanism. Br. J. Pharmacol. 2012, 166, 2231–2242. [Google Scholar] [CrossRef]
- Hourihan, J.M.; Kenna, J.G.; Hayes, J.D. The Gasotransmitter Hydrogen Sulfide Induces Nrf2-Target Genes by Inactivating the Keap1 Ubiquitin Ligase Substrate Adaptor Through Formation of a Disulfide Bond Between Cys-226 and Cys-613. Antioxid. Redox. Signall. 2012, 19, 465–481. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, Y.; Zhang, L.; Xu, W.; Zhou, X. Diagnostic and prognostic value of biomarkers in acute myocardial infarction. Postgrad. Med. J. 2019, 95, 210–216. [Google Scholar] [CrossRef]
- Liang, D.; Huang, A.; Jin, Y.; Lin, M.; Xia, X.; Chen, X.; Airong, H. Protective effects of exogenous nahs against sepsis-induced myocardial mitochondrial injury by enhancing the PGC-1α/NRF2 pathway and mitochondrial biosynthesis in mice. Am. J. Transl. Res. 2018, 10, 1422–1430. [Google Scholar]
- Staels, B.; Fruchart, J.C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 2005, 54, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Lee, W.H. AMPK-dependent metabolic regulation by PPAR agonists. PPAR Res. 2010, 2010, 549110. [Google Scholar]
- Lin, F.; Yang, Y.; Wei, S.; Huang, X.; Peng, Z.; Ke, X. Hydrogen sulfide protects against high glucose-induced human umbilical vein endothelial cell injury through activating PI3K/Akt/eNOS pathway. Drug Des. Devel. Ther. 2020, 14, 621–633. [Google Scholar] [CrossRef]
- Li, D.; Xiong, Q.; Peng, J.; Hu, B.; Li, W.; Zhu, Y. Hydrogen sulfide up-regulates the expression of ATP-binding cassette transporter A1 via promoting nuclear translocation of PPAR-α. Int. J. Mol. Sci. 2016, 17, 635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, J.; Huang, S.; Chen, X.; Chia, A.; Chang, Y. Redox Biology Hydrogen sulfide protects cardiomyocytes from doxorubicin-induced ferroptosis through the SLC7A11/GSH/GPx4 pathway by Keap1 S-sulfhydration and Nrf2 activation. Redox. Biol. 2024, 70, 103066. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, J.; Zhang, S.; Pang, K.; Zhao, Y.; Liu, N.; Huang, J.; Kang, J.; Dong, S.; Li, H.; et al. Exogenous H2S initiating Nrf2/GPx4/GSH pathway through promoting Syvn1-Keap1 interaction in diabetic hearts. Cell. Death. Discov. 2023, 9, 394. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarra-Lara, L.; Sánchez-López, A.; del Valle-Mondragon, L.; Soria-Castro, E.; Zarco-Olvera, G.; Patlán, M.; Guarner-Lans, V.; Torres-Narváez, J.C.; Ruiz-Ramírez, A.; Díaz de León-Sánchez, F.; et al. Involvement of Nuclear Receptors PPAR-α, PPAR-γ, and the Transcription Factor Nrf2 in Cellular Protection Against Oxidative Stress Regulated by H2S and Induced by Hypoxia–Reoxygenation and High Glucose in Primary Cardiomyocyte Cultures. Antioxidants 2025, 14, 482. https://doi.org/10.3390/antiox14040482
Ibarra-Lara L, Sánchez-López A, del Valle-Mondragon L, Soria-Castro E, Zarco-Olvera G, Patlán M, Guarner-Lans V, Torres-Narváez JC, Ruiz-Ramírez A, Díaz de León-Sánchez F, et al. Involvement of Nuclear Receptors PPAR-α, PPAR-γ, and the Transcription Factor Nrf2 in Cellular Protection Against Oxidative Stress Regulated by H2S and Induced by Hypoxia–Reoxygenation and High Glucose in Primary Cardiomyocyte Cultures. Antioxidants. 2025; 14(4):482. https://doi.org/10.3390/antiox14040482
Chicago/Turabian StyleIbarra-Lara, Luz, Araceli Sánchez-López, Leonardo del Valle-Mondragon, Elizabeth Soria-Castro, Gabriela Zarco-Olvera, Mariana Patlán, Verónica Guarner-Lans, Juan Carlos Torres-Narváez, Angélica Ruiz-Ramírez, Fernando Díaz de León-Sánchez, and et al. 2025. "Involvement of Nuclear Receptors PPAR-α, PPAR-γ, and the Transcription Factor Nrf2 in Cellular Protection Against Oxidative Stress Regulated by H2S and Induced by Hypoxia–Reoxygenation and High Glucose in Primary Cardiomyocyte Cultures" Antioxidants 14, no. 4: 482. https://doi.org/10.3390/antiox14040482
APA StyleIbarra-Lara, L., Sánchez-López, A., del Valle-Mondragon, L., Soria-Castro, E., Zarco-Olvera, G., Patlán, M., Guarner-Lans, V., Torres-Narváez, J. C., Ruiz-Ramírez, A., Díaz de León-Sánchez, F., Oidor-Chan, V. H., & Castrejón-Téllez, V. (2025). Involvement of Nuclear Receptors PPAR-α, PPAR-γ, and the Transcription Factor Nrf2 in Cellular Protection Against Oxidative Stress Regulated by H2S and Induced by Hypoxia–Reoxygenation and High Glucose in Primary Cardiomyocyte Cultures. Antioxidants, 14(4), 482. https://doi.org/10.3390/antiox14040482







