Abstract
Advanced glycation end products (AGEs) are a group of compounds formed through non-enzymatic reactions between reducing sugars and proteins, lipids, or nucleic acids. AGEs can be generated in the body or introduced through dietary sources and smoking. Recent clinical and animal studies have highlighted the significant role of AGEs in various health conditions. These compounds accumulate in nearly all mammalian tissues and are associated with a range of diseases, including diabetes and its complications, cardiovascular disease, and neurodegeneration. This review summarizes the major diseases linked to AGE accumulation, presenting both clinical and experimental evidence. The pathologies induced by AGEs share common mechanisms across different organs, primarily involving oxidative stress, chronic inflammation, and direct protein cross-linking. Interventions targeting AGE-related diseases focus on inhibiting AGE formation using synthetic or natural antioxidants, as well as reducing dietary AGE intake through lifestyle modifications. AGEs are recognized as significant risk factors that impact health and accelerate aging, particularly in individuals with hyperglycemia. Monitoring AGE level and implementing nutritional interventions can help maintain overall health and reduce the risk of AGE-related complications.
1. Introduction
Advanced glycation end products (AGEs) are harmful compounds formed through non-enzymatic reactions involving reducing sugars and proteins [1]. Their discovery dates back to 1912, when non-enzymatic browning was observed in foods and beverages during the heating of glucose with glycine [2]. This reaction enhances the flavors and aromas of foods rich in sugar and protein.
However, AGEs have detrimental effects on tissues and organs. They can directly cross-link proteins, altering their structure and function, or indirectly contribute to disease by generating reactive oxygen species (ROS) and pro-inflammatory cytokines [3]. AGEs are naturally produced in the body as part of normal metabolism but can also form during food cooking and processing. Once ingested, they are absorbed through the gastrointestinal tract and may accumulate in excess [4]. Accumulated AGEs bind to their receptors (RAGEs), promoting oxidative stress and disrupting intracellular signaling. This contributes to the progression of chronic diseases, including diabetes [5], cardiovascular disease [6], cognitive impairment [7], and aging-related conditions [8], as well as disorders affecting the liver [9], lungs [10], eyes [11], intestines [12], and reproductive system [13]. Therapeutic strategies aimed at mitigating the impact of AGEs include inhibiting their formation, enhancing their clearance, breaking down established AGE-related protein cross-links, and blocking their interaction with RAGE.
This review examines recent advancements in AGE research, highlighting their role in various diseases and underlying pathological mechanisms, with a particular focus on clinical evidence. Additionally, it highlights recent studies identifying small-molecule inhibitors and natural compounds that target AGEs, offering insights into reducing AGE-related harm through nutritional interventions.
2. Advanced Glycation End Product (AGE) Introduction and Classification
2.1. AGE Formation
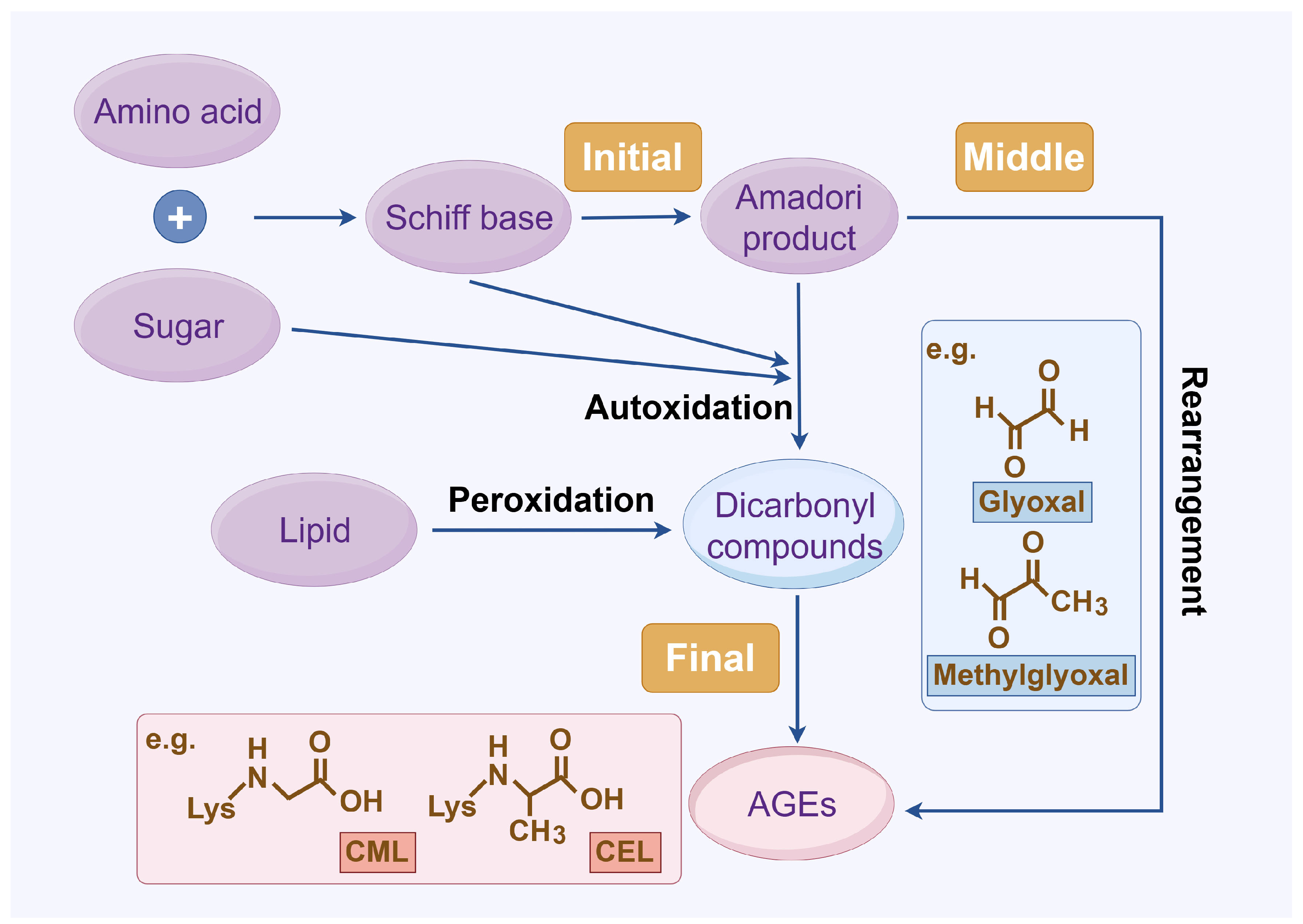
The complex series of reactions that contribute to AGE formation is collectively known as the Maillard reaction. In this process, the terminal amino groups of amino acids, as well as the side-chain amino groups of lysine and arginine residues in proteins, react non-enzymatically with the carbonyl groups of reducing sugars (e.g., glucose, ribose, and trioses) to form unstable Schiff bases (early AGEs). Under oxidative stress, these Schiff bases undergo rearrangement or glycoxidation, leading to the formation of final AGEs or highly reactive 1,2-dicarbonyl compounds, such as glyoxal and methylglyoxal. These dicarbonyl compounds, which also arise from glucose autoxidation, lipid peroxidation, and the polyol pathway, react with protein amino groups, leading to both intra- and inter-protein cross-linking, and then they undergo oxidation, dehydration, or polymerization [14]. The resulting stable protein modifications, along with the small molecular products formed from their degradation, are collectively known as AGEs, with carboxymethyl lysine (CML) and carboxyethyl lysine (CEL) being the most widely studied (Figure 1) [15]. While this process occurs naturally over time, it is promoted by hyperglycemia, oxidative stress, and metabolic imbalances.
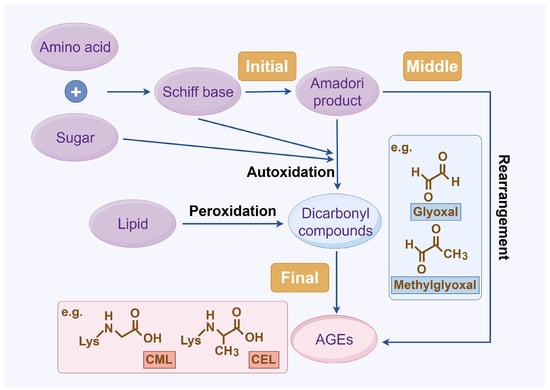
Figure 1.
AGE formation. Abbreviations: AGEs, advanced glycation end products; CEL, carboxy ethyl lysine; CML, carboxyl methyl lysine.
2.2. AGE Type
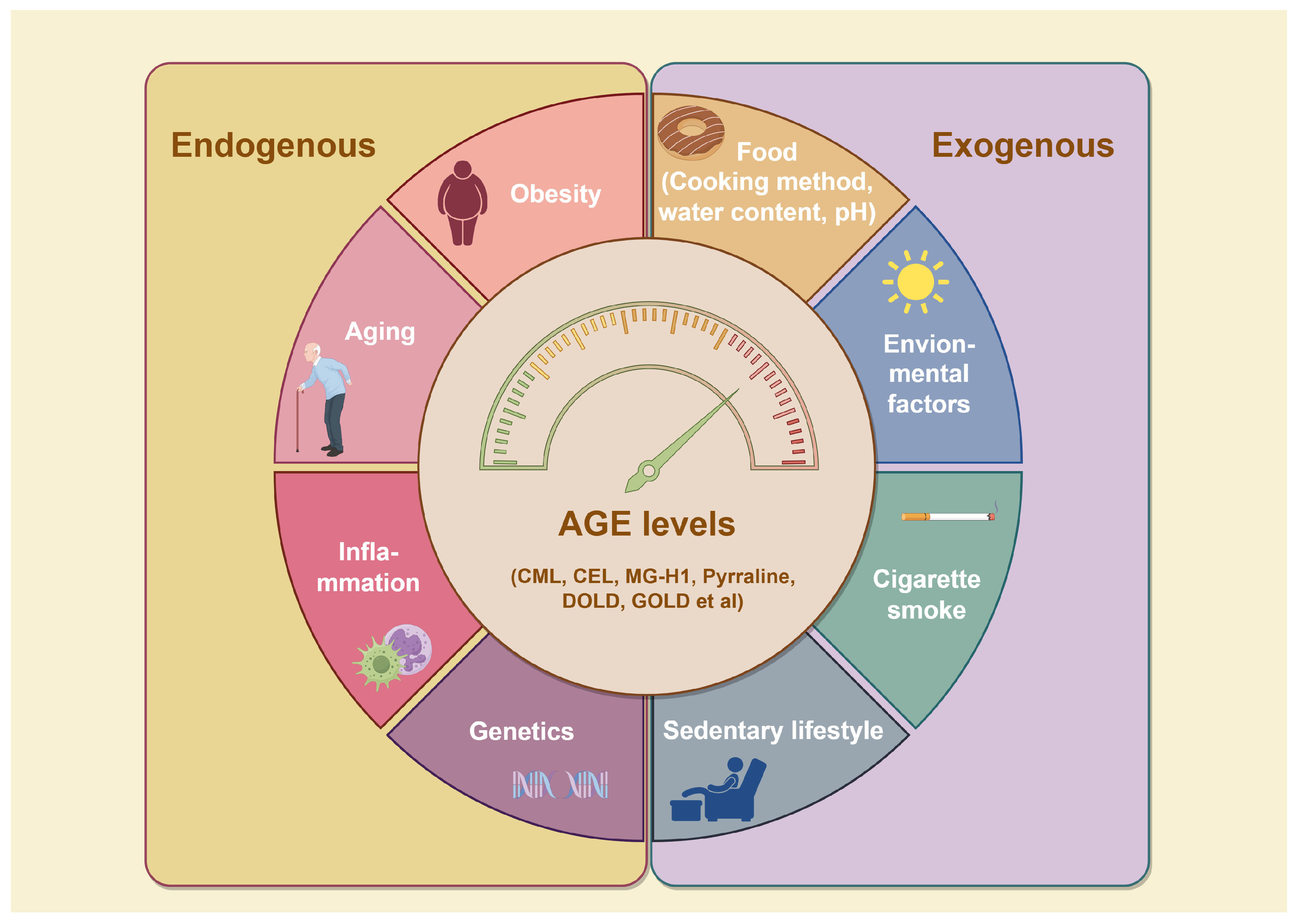
To date, over 20 distinct types of AGEs have been identified in the human body and dietary sources, each exhibiting a range of properties and chemical structures [15]. AGEs can form endogenously, either intracellularly or extracellularly, or be acquired exogenously, primarily from food and tobacco (Figure 2) [3]. The endogenous production of AGEs is influenced by carbohydrate precursor availability, ROS levels, and protein turnover rates [16]. Representative products include pentosidine, CML, and methylglyoxal (MGO), which can serve as markers of pathophysiological processes [17]. While exogenous AGEs are structurally and functionally identical to endogenous ones, they play a critical role in the accumulation and systemic circulation of AGEs [3]. Consequently, exogenous AGEs, primarily from dietary sources, are regarded as the predominant contributors to the body’s overall AGE burden [18]. The formation of AGEs in food primarily depends on factors such as cooking temperature, water content, pH, cooking time, and cooking method. Additionally, cigarette smoke contains highly reactive glycation products [8]. Together, systemic AGE concentrations are determined by a combination of dietary intake, genetics, age, and overall health status. Elevated AGEs can synergistically trigger inflammation and oxidative stress, resulting in various adverse health outcomes.
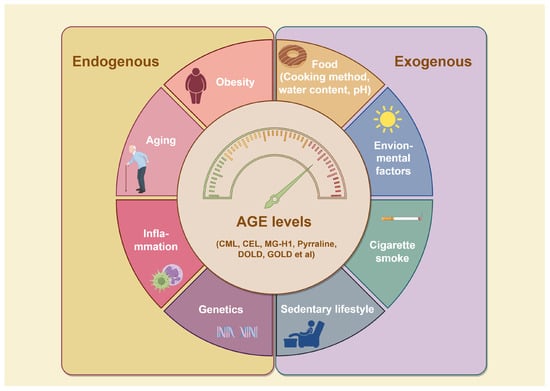
Figure 2.
Factors influencing AGE formation. Reducing sugars and amino compounds form Schiff bases through non-enzymatic reactions, which are subsequently converted into Amadori products through reduction and rearrangement. These Amadori products further degrade into highly reactive dicarbonyl intermediates, which react with amino groups in proteins and other biomolecules like lipids, leading to the formation of stable AGEs. AGE accumulation is influenced by both internal and external factors. Internally, genetic factors, inflammation, aging, and metabolic disorders like obesity accelerate AGE formation. Externally, factors such as a sedentary lifestyle, smoking, UV exposure, and consumption of AGE-rich foods further contribute to AGE buildup. Abbreviations: AGEs, advanced glycation end products; CEL, carboxy ethyl lysine; CML, carboxyl methyl lysine; DOLD, 1,3-di(Nε-lysino)-4-(2,3,4-trihy -droxybutyl)-imidazolium; GOLD, 6-{1-[(5S)-5-ammonio-6-oxido-6-oxohexyl] imidazolium-3-yl} -L-norleucine; MG-H1, Methylglyoxal-derived hydroimidazolone-1.
3. AGE-Related Diseases
3.1. Mechanisms of AGE-Induced Pathology
AGEs accumulate in almost all mammalian tissues and cause chronic pathology [19]. The primary mechanisms of AGE-induced pathology include direct protein trapping and cross-linking, which alter protein structure and function, as well as indirect activation of intracellular signaling through both receptor- and non-receptor-mediated pathways. These processes lead to increased production of inflammatory cytokines and ROS [3,5].
Representatively, covalent cross-linking of AGEs to collagen or elastin in the extracellular matrix (ECM) slows the hydrolytic breakdown of these proteins and reduces their flexibility. This process contributes to the thickening of the basement membrane, compromising the integrity of blood vessels and increasing the risk of hemorrhagic pathologies [20]. Additionally, AGE-induced skin aging is also governed by the simple act of covalently cross-linking to collagen and elastin, especially types I and IV collagen [21]. This process causes a loss of skin elasticity and hydration. Moreover, the accumulation of AGEs in skin tissue also leads to skin yellowness [22].
Besides direct trapping or cross-linking the proteins, AGEs also act by modifying proteins or forming adducts with them through binding with AGE ligand-gated receptors, such as the most well-known RAGE and its secreted soluble isoform, sRAGE [23]. RAGE, encoded by a gene on chromosome 6, is a transmembrane protein belonging to the immunoglobulin superfamily of cell surface receptors. It is distributed across various organs but is most enriched in the lung, heart, and skeletal muscle. RAGE is expressed at basal levels under normal conditions but is significantly elevated under pathological conditions or chronic inflammation [4]. The activation of RAGE induces a cascade of inflammatory reactions that start with the activation of transcription factor NF-κB. Then, it triggers a variety of downstream effectors, including mitogen-activated protein kinase (MAPK), p38, Ras-mediated extracellular signal-regulated kinase (ERK1/2), Janus kinase signal transducer and activator of transcription (JAK/STAT), and the stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) pathway, that in turn will lead to sustained activation of transcription factors such as NF-κB, HIF-1α, STAT3, and AP-1 [5]. These signaling pathways regulate various physiological processes, triggering proliferative, angiogenic, fibrotic, thrombogenic, and apoptotic responses, ultimately contributing to AGE-induced pathology in various tissues [24,25].
The AGE–RAGE interaction could also increase the ROS levels through the activation of mitochondrial and NADPH (nicotinamide adenine dinucleotide phosphate oxidase) pathways [26], which in turn leads to increased production of AGEs and a vicious cycle of intracellular damage [27].
The ROS is accumulated from the conversion of glucose to fructose through the NADPH pathway. The decreased endogenous antioxidant mechanisms, such as the depletion of GSH, increase the concentrations of glyoxal (GO) and MGO, thereby promoting the glycation process [28].
The soluble forms of AGEs, sRAGE, lacking the transmembrane and cytoplasmic domains, are distributed through the circulation [29]. The extracellular domain of RAGE released from the cells to the circulation is called endogenous secretory RAGE (esRAGE) [30]. It is reported that the esRAGE may act as a decoy, which cancels the effects of AGEs in cultured cells and reverses diabetic vascular dysfunction in mice [31]. The RAGE and esRAGE are suggested to be involved in feedback regulation of the toxic effects of RAGE-mediated signaling.
Therefore, more and more studies have been conducted to explore the role of sRAGE and esRAGE in various pathophysiologic conditions, and it has been found that they could potentially serve as promising prognostic markers. However, according to some research, the relationship between sRAGE and cardiovascular diseases is inconsistent, suggesting further studies are needed [32,33,34,35].
3.2. AGEs and Diabetes Mellitus
Diabetes mellitus refers to a group of chronic metabolic disorders characterized by elevated blood glucose levels (hyperglycemia) resulting from defects in insulin secretion, insulin action, or both [36,37]. Persistent hyperglycemia leads to both microvascular and macrovascular complications, making diabetes a major public health concern. One of the harmful consequences of hyperglycemia is the accelerated formation and accumulation of AGEs, which play a key role in the development of nearly all diabetic complications [38]. However, emerging research suggests that AGEs may contribute to the onset of diabetes rather than simply being a consequence of it. In healthy individuals, dietary intake of AGE-rich foods is the primary source of these compounds. AGEs act as pro-oxidants and appetite stimulants, contributing to overnutrition, inflammation, obesity, and ultimately, diabetes mellitus [39].
Although numerous studies have linked AGEs to the occurrence and severity of diabetes-related complications, few cohort studies have specifically investigated the relationship between serum AGE levels and the onset of diabetes itself. According to a stratified case-cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study, 543 middle-aged individuals who developed diabetes and 514 individuals who did not were followed over a median of 9 years. The study found that elevated levels of CML were associated with an increased risk of diabetes, particularly among those with impaired fasting glucose and among white participants. This suggests that elevated fasting CML may predict the development of incident diabetes [40]. Moreover, as consistently demonstrated by a large hospital-based case-control study and a nested case-control study within an ongoing community-based prospective cohort of Chinese adults, higher plasma AGEs and lower plasma sRAGE were significantly associated with increased risk of Type 2 diabetes (T2D) among adults without cardiovascular diseases. Specifically, positive associations were observed between plasma CML, CEL, and methyl glyoxal-derived hydroimidazolone 1 (MG-H1) levels and T2D, after adjusting for other factors [41]. Other studies have found that individuals of all ages who consume a diet high in AGEs may have elevated levels of high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor (TNF), fibrinogen, vascular cell adhesion molecule 1 (VCAM-1), and homeostatic model assessment (HOMA). These findings only indirectly suggest that AGEs may contribute to the development of diabetes. In animal studies, the evidence linking AGEs to diabetes is more direct. The nonobese diabetic (NOD) mouse, as a model of autoimmune type 1 diabetes mellitus (T1DM), usually becomes diabetic at the age of 4–5 months. However, an AGE-restricted diet could significantly reduce the incidence of T1DM in these NOD mice, even to less than 15% in the next two generations, as long as the AGE-restricted diet (the semipurified standard diet, AIN-93G, prepared without the second step of heating, containing fivefold less CML-like AGE and 4.5-fold less MG-derived epitopes compared to the identical chow mix processed under the standard procedure) was maintained in both dams and offspring [42]. Similar results were observed in type 2 diabetes mellitus (T2DM) model mice, when an AGE-restricted diet (produced with less exposure to heat and containing less CML, MG, or total AGEs measured by ELISA) was administered [43,44,45]. Several animal experiments have shown that simply reducing basal oxidative stress by dietary AGE restriction could sharply reduce the risk of diabetes mellitus and its complications [39].
In individuals with diabetes, AGE levels are strongly associated with disease severity and the extent of complications affecting various organs and tissues. This relationship will be explored in detail later in this review.
The pathophysiology of diabetes mellitus induced by AGEs primarily involves direct protein trapping and cross-linking, or indirect binding to cell surface receptors, with the RAGE being the most significant [46,47]. As reported, the elevated AGEs in diabetic individuals could interact with RAGE and trigger various downstream pathways, including SAPK/JNK, MAPK, p38, ERK1/2, JAK/STAT, and PKC, causing sustained activation of NF-κB, AP-1, CREB, and STAT3. This would create a vicious cycle, involving inflammation, ROS generation, mitochondrial dysfunction, beta cell impairment, and apoptosis, all of which contribute to the pathology of insulin resistance [5]. Furthermore, Islet amyloid polypeptide (IAPP), which is found to be heavily glycated and to form toxic amyloid-like aggregates, is another major factor contributing to beta cell death [48]. It could be selectively bound by RAGE, leading to NADPH oxidase-mediated ROS generation, inducing cellular stress, inflammation, islet amyloidosis, and finally decreased beta cell mass [49].
3.3. AGEs and Cardiovascular Diseases
Cardiovascular diseases (CVDs) are defined as life-threatening chronic conditions affecting the heart and vascular system and are categorized as microvascular and macrovascular diseases. Microvascular disease leads to nephropathy, retinopathy, and neuropathy; macrovascular disease is usually caused by atherosclerosis, which leads to the narrowing or occlusion of blood vessels, including coronary artery disease, cerebrovascular disease, and peripheral artery disease [50].
The accumulation of AGEs (such as CML and 3-deoxyglucosone hydroimidazolone) is implicated in the onset and progression of cardiovascular complications in diabetic patients [6,51]. Serum AGE levels were higher in T2DM patients with either macrovascular or microvascular disease compared to those without complications [52]. As a pathologic basis for macrovascular disease, the progression and severity of atherosclerosis in diabetic patients have been reported to correlate with serum levels of AGEs [53]. In non-diabetic subjects with coronary heart disease, elevated AGE levels were associated with the number of vascular stenoses [54]. Additionally, tissue AGEs (skin autofluorescence) could serve as a predictor for diabetes-related heart failure and CVD mortality [55]. However, there is an inconsistency in the findings regarding the relationship between circulating sRAGE and CVDs. Some studies have found a negative correlation between atherosclerosis and circulating sRAGE, suggesting that sRAGE may be a protective factor against atherosclerosis [32,33]. However, other studies have shown a positive correlation between atherosclerosis and sRAGE [34,35]. The reason for the conflicting findings may be the differences in clinical study design and the strong correlation between serum sRAGE and other patient variables, such as age and diabetic background. As one of the common microvascular complications of diabetes, diabetic neuropathy could cause a range of symptoms, including foot ulcers [56]. Glycation has been reported to be an important pathophysiologic pathway leading to complications in foot ulcers [56]. The relevance of AGEs to diabetes-related nephropathy and retinopathy will be discussed in subsequent paragraphs.
In the circulatory system, the constituents of lipoproteins are particularly susceptible to advanced glycation, and glycated low-density lipoproteins (LDLs) may not be effectively recognized by their receptors [57,58]. These glycated particles can reach the arterial wall and be taken up by macrophage receptors, leading to lipid accumulation [57,58]. In addition, AGE–RAGE enhances the adhesion of macrophages and T-lymphocytes by upregulating adhesion proteins via the NF-κB signaling pathway [59]. This adhesion process induced continuous inflammatory responses, promoting plaque formation [60,61]. Furthermore, the AGE–RAGE axis regulates the expression of ATP-binding cassette transporter G1 (ABCG1) and cluster of differentiation 36 (CD36), facilitating the transformation of macrophages into foam cells [62,63]. The activation of transforming growth factor-β (TGF-β) by AGE–RAGE stimulated the proliferation and migration of arterial smooth muscle cells [64], which further disrupted the endothelial cell barrier and promoted plaque formation in response to inflammation [65]. Activation of AGE–RAGE could inhibit the expression of endothelial nitric oxide synthase (eNOS), thereby reducing the production, release, and bioactivity of nitric oxide (NO) [66]. This inhibition led to endothelial dysfunction and impaired vascular contraction and dilation [66].
Numerous studies have highlighted the role of the AGE–RAGE axis in cardiac dysfunction. Prolonged elevation of blood glucose levels led to increased non-enzymatic glycation of proteins involved in calcium (Ca2+) homeostasis, including sarcoplasmic reticulum Ca2+-ATPase (SERCA) and ryanodine receptors (RyR) [67,68]. The cross-linking of SERCA and RyR induced by AGEs disrupts Ca2+ handling, adversely affecting myocardial relaxation and contraction, which ultimately results in diastolic dysfunction [67,68].
3.4. AGEs and Chronic Kidney Diseases
As reported in many studies, chronic kidney disease (CKD) is marked by excessive production and accumulation of AGEs, which in turn promote CKD-related morbidities and mortality [69]. Urinary uromodulin (UMOD) is glycated in diabetic kidney disease (DKD) and forms AGEs, and glcUMOD may serve as a biomarker for DKD [70]. The free adduct of MG-H1 serves as a risk marker for CKD in both non-diabetic and diabetic patients [71]. Moreover, in poorly controlled diabetic patients, persistently low serum MG-H1 levels correlate with a reduced incidence of DKD [72]. Some early-type glycation products, as the precursors of AGEs, cause vascular damage through oxidative stress and have a close clinical association with CKD. The higher MGO concentrations were demonstrated to be associated with CKD and may be useful in determining the prognosis of the disease [73]. A recent prospective cohort study demonstrated that the glycated albumin (GA) level in CKD patients, an early Amadori-type glycation protein and a precursor to harmful AGEs, was independently associated with risks of end-stage kidney disease, CVDs, and mortality, regardless of diabetes status [74]. The AGEs could be evaluated using skin autofluorescence, and a recent study suggested the fluorophore AGE-to-NADH ratio to be a new biomarker for the presence of diabetic CKD [75]. In addition, elevated circulating esRAGE is independently associated with CKD and is an independent predictor of incident CKD in older community-dwelling adults [76]. The sRAGE was also reported to be associated with CKD incidence [77], and AGE/sRAGE can be an additional risk factor for CKD progression over a longer time [78].
The AGEs contribute to the pathology of CKD and CVD mainly as pro-oxidative and pro-inflammatory factors [79]. AGEs increase the levels of ROS through activation of NADPH oxidase and mitochondrial pathways in both a receptor-dependent and independent manner. Simultaneously, AGEs amplify inflammatory responses in patients with CKD through the AGE–RAGE axis, most significantly by activating the NF-kB. Additionally, several studies demonstrated that the expression of some Nrf2 target antioxidant genes is decreased in animal models and patients of CKD, which may consequently aggravate oxidative stress and inflammation [79]. Furthermore, AGE-induced direct modification and cross-linking of the proteins cause numerous structural changes, including alterations in surface charges, packing density, and structural integrity, leading to stiffening of the vasculature as well as the expansion of cellular basement membranes in diabetes and CKD [80].
3.5. AGEs in Joint Diseases
AGEs also accumulate in cartilage, bone, and synovium, particularly in individuals with type 2 diabetes, which disrupts normal cellular functions, alters collagen structure, promotes tissue deterioration, and leads to skeletal fragility [81]. Particularly, AGEs would cross-link collagen fibers in the cartilage, leading to stiffness and reduced elasticity. Studies in the Health, Aging, and Body Composition cohort showed that higher CML levels in T2D were associated with an increased risk of incident clinical fractures, independent of bone mineral density (BMD) and other factors. However, this association was not observed in non-diabetic individuals, and CML was not significantly linked to vertebral fractures in either group [82]. Pentosidine levels from urine are negatively associated with trabecular bone scores [83], and circulating pentosidine correlates with bone mechanical properties [84]. A meta-analysis involving 5878 participants reported that higher urinary pentosidine levels are linked to an increased fracture risk and, although less strongly, to lower bone mineral density, highlighting its potential as a biomarker of impaired bone health [85]. These findings suggest that AGEs, particularly CML and pentosidine, would contribute to the decreased resilience of cartilage and cause bone fragility in T2D, highlighting their role in the pathogenesis of bone-related complications in diabetic patients.
RAGE plays a pivotal role in the pathogenesis of joint diseases by mediating inflammatory responses and contributing to tissue damage, with high expression observed in joint tissues. Through their interaction with RAGE, AGEs can induce the expression of pro-inflammatory cytokines and osteoclastogenic markers in fibroblast-like synovial cells, leading to the structural and functional impairment of joints [86]. In rheumatoid arthritis (RA), higher expression of RAGE has been noted in synovial tissues, particularly in macrophages and endothelial cells, where it amplifies the inflammatory response by activating the NF-κB signaling pathway [87]. Additionally, CML was detected in both RA and osteoarthritis (OA) synovial tissues, where it may interact with RAGE on macrophages and T cells, potentially triggering autoimmune responses that worsen joint inflammation [88]. The prominent presence of RAGE in RA compared to OA suggests a more significant role in the inflammatory processes that characterize these conditions [89]. Moreover, soluble RAGE levels in plasma and synovial fluid are inversely related to the severity of knee osteoarthritis [90]. Together, these findings underscore the critical role of AGEs and RAGE in the propagation and exacerbation of joint diseases, highlighting their potential as therapeutic targets in arthritis.
Recent clinical studies have also confirmed the critical roles of AGEs and RAGE in the pathogenesis of joint diseases, especially in RA. AGEs and advanced oxidation protein products (AOPPs) were found to be significantly elevated in RA patients compared to healthy controls [91]. Additionally, the presence of anti-AGE antibodies was observed in nearly half of RA patients, while only 7.5% of healthy controls tested positive; they were associated with genetic risk factors, inflammation, and radiological progression, particularly in seronegative RA [92]. Moreover, the inhibition of AGEs with benfotiamine, pyridoxamine, and methylcobalamin has shown promising results in reducing endothelial dysfunction and inflammatory disease activity in RA, suggesting that AGEs contribute to the vascular and systemic inflammation characteristics of this condition [93]. Furthermore, low levels of sRAGE in female RA patients have been associated with increased cardiovascular risk, underscoring the need for metabolic health monitoring in these individuals [94]. Collectively, these findings suggest that targeting AGE formation or blocking RAGE signaling may present promising adjunctive strategies for managing arthritis, especially RA.
3.6. AGEs and Neurodegenerative Diseases
Neurodegenerative diseases (NDDs) encompass a diverse array of disorders characterized by irreversible neuronal damage and a progressive decline in neural function. These disorders include Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and prion diseases [95].
Over the past few decades, numerous studies have demonstrated a correlation between AGEs and brain dysfunction. The high oxygen consumption and glucose metabolism in the brain may contribute to the accumulation of MG-H1 and CEL [96]. AGEs have been detected in astrocytes exclusively in older individuals, including those with AD, but not in younger controls [97]. The number and proportion of AGE-positive neurons and astroglia are significantly elevated in AD patients [98,99]. Immunohistochemical studies show that AGEs accumulate notably in lipofuscin granules within the tissues of AD patients and the elderly [99]. Furthermore, increased levels of AGEs have been identified in Lewy bodies and toxic protein inclusions, which are recognized as pathological markers of PD [100], in neurons from PD patients [101]. In patients with ALS, AGEs levels, particularly CEL, CML, and pyrrole compounds, were significantly elevated in spinal cord astrocytes [102]. These findings indicate that the accumulation of AGEs in brain tissue is critical to the pathological progression of NDDs. Furthermore, increased levels of AGEs (such as CML and MG) have also been detected in the bodily fluids of patients with NGDs, including blood [103,104] and urine [105], alongside reduced levels of sRAGEs [106]. Elevated plasma AGEs have been associated with poorer cognitive function, with higher levels of CML correlating with a greater incidence of mild cognitive impairment [7]. These studies suggest that AGEs may serve as potential predictors for the onset and progression of NDDs.
The role of AGEs in NDDs is complex and multifaceted, involving several key mechanisms. Firstly, AGEs promoted the aggregation of amyloid-β peptides (Aβ) into insoluble fibrils, leading to the formation of amyloid plaques, a hallmark of AD pathology [107]. Additionally, the activation of glycogen synthase kinase-3 (GSK-3) by AGEs induced the excessive phosphorylation of Tau protein (p-Tau) [108]. In response to ROS disruption, Tau protein could interact with cyclin-dependent kinase 5 (CDK5) and GSK3β, leading to hyperphosphorylation [109]. Phosphorylated Tau specifically affected the activity of mitochondrial complex I, synergistically promoting Aβ-mediated mitochondrial dysfunction and ROS production [110]. These studies suggest that oxidative stress, mitochondrial disruption, and Aβ and p-Tau accumulation may form a vicious circle, and the causal relationship between them in AD needs to be further explored. The ROS production triggered by the AGE–RAGE axis could also amplify p-Tau and Aβ production, exacerbating their neurotoxicity [111]. Glycosylated α-synuclein and its oligomers were reported to induce toxicity in dopaminergic neurons in patients with PD [100]. Secondly, AGEs were involved in the disruption of the blood–brain barrier (BBB), which is primarily composed of endothelial cells, astrocytes, and pericytes [112]. Elevated AGE levels reduced the expression of integrin α1, platelet-derived growth factor receptor-1β (PDGF-R1β), and Connexin-43 (Cx-43), leading to pericyte dysfunction [113]. The upregulation of vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2 (MMP-2) in pericytes was induced by AGE-activated autocrine TGF-β signaling, resulting in basement membrane hypertrophy of the BBB [114]. Additionally, high levels of AGEs decreased the expression of tight junction proteins, such as occludin and claudin, which compromises the integrity of tight junctions [115]. Thirdly, neuroinflammation resulting from the dysregulation of the AGE–RAGE axis is a significant factor in the progression of NDDs. The RAGEs could recruit the adaptor protein mDia1 upon dimerization, thereby activating various signaling pathways, including the JAK/STAT pathway [116]. The translocation of activated STAT to the nucleus promoted expression of various cytokine-responsive genes and activated the inflammatory response [117]. Furthermore, the AGE–RAGE pathway modulated the apoptosis of various cell types in the brain, including microvascular cells and neuronal cells [118,119]. The interaction of AGEs with RAGE could lead to the release of IL-8, causing dephosphorylation of the cytoplasmic nuclear factor of activated T cells (NF-AT) [120]. The increased expression of Fas ligand (FasL), induced by the translocation of NF-AT, could increase caspase activity and ultimately result in cell death [120].
3.7. AGEs and Skin Disorders
AGEs are key contributors to skin aging, especially in photoaging, a process exacerbated by sun exposure. Numerous studies have shown that AGEs accumulate more in sun-exposed areas than in protected skin, indicating that UV exposure accelerates their formation [121]. This buildup of AGEs leads to the cross-linking of collagen and elastin fibers, reducing skin elasticity and increasing stiffness, which results in visible signs of aging such as wrinkles, sagging, and other signs of photoaging [122]. Moreover, AGEs intensify the skin’s inflammatory response, hastening the degradation of extracellular matrix proteins such as collagen and elastin, further amplifying their harmful effects and worsening visible signs of aging such as wrinkles and the loss of firmness [123].
Research has also demonstrated that exposure to ultraviolet radiation A (UVA) in the presence of AGEs triggers the generation of ROS such as superoxide, hydrogen peroxide, and hydroxyl radicals. These ROS further escalate oxidative stress, damaging dermal fibroblasts, reducing cell viability, and causing additional cellular damage, all of which compromise the structural integrity of the skin [124]. In addition to structural damage, AGEs are implicated in hyperpigmentation disorders. In particular, they activate the NLRP3 inflammasome in fibroblasts, promoting the release of pro-inflammatory cytokines like IL-18, driving melanogenesis, and elevating melanin levels, which are linked to hyperpigmentation disorders such as melasma and solar lentigo [125]. Clinically, higher AGE levels correlate with increased trans-epidermal water loss (TEWL), reduced hydration, and increased melanin and erythema, all of which deteriorate skin quality [126]. Given their roles in oxidative stress, inflammation, and collagen cross-linking, AGEs are pivotal in skin aging and represent promising targets for anti-skin aging therapies, particularly for those with extensive sun exposure.
AGE formation is accelerated in hyperglycemic conditions, such as diabetes, where it significantly hinders wound healing [127]. AGEs promote oxidative stress and chronic inflammation, both of which are detrimental to effective tissue repair. In diabetic wounds, the AGE–RAGE axis triggers excessive ROS production and an exaggerated inflammatory response, which delays healing and increases tissue damage. Furthermore, AGEs can induce apoptosis in key cell types involved in wound repair, such as fibroblasts, macrophages, and endothelial cells, thereby disrupting normal wound closure processes [128]. In diabetic keratopathy, for example, AGEs hyperactivate the NLRP3 inflammasome, leading to delayed corneal wound healing and impaired nerve regeneration [129]. Similarly, in diabetic foot ulcers, AGEs inhibit macrophage function and increase apoptosis, further impairing the healing process [130]. Targeting the AGE–RAGE axis, either by blocking RAGE or reducing AGE formation, has shown promise in improving wound healing outcomes, highlighting AGEs as critical factors in diabetic wound pathogenesis [131,132].
AGEs also play significant roles in the pathogenesis of skin diseases such as psoriasis, where they are involved in both inflammation and metabolic comorbidities. Multiple studies highlight the accumulation of AGEs in the skin and blood of psoriasis patients, particularly in severe cases, where they are associated with disease severity and progression [133]. The AGE–RAGE axis amplifies inflammatory responses through the release of cytokines, chemokines, and ROS, further aggravating psoriatic lesions and promoting keratinocyte proliferation [134]. This interaction, mediated by the STAT1/3 signaling pathway, stimulates the production of pro-inflammatory cytokines like IL-36α, enhancing Th17 immune responses [134]. Additionally, polymorphisms in the RAGE gene have been associated with a higher risk of psoriasis, particularly in patients without common comorbidities like cardiovascular disease and diabetes [135]. In addition to their role in psoriasis, AGEs have been implicated in other skin disorders linked to metabolic dysfunction. For instance, AGEs play a role in the development of systemic sclerosis, a disease characterized by excessive collagen deposition and fibrosis [136]. Elevated AGE levels have been observed in the skin of scleroderma patients [137], and their interaction with RAGE is thought to exacerbate fibrosis by promoting collagen cross-linking and perpetuating chronic inflammation.
3.8. AGEs and Liver Diseases
Metabolic-associated fatty liver disease (MAFLD) affects about 25% of the global population and is commonly associated with CVDs such as hypertension and atherosclerosis [138]. Research has shown elevated serum glyceraldehyde (GA)-derived AGEs in MAFLD patients compared to healthy individuals [139]. These GA-derived AGE levels could potentially serve as biomarkers for distinguishing metabolic dysfunction-associated steatohepatitis (MASH) from simple steatosis. For instance, a study with 43 MASH patients treated with atorvastatin showed a significant decrease in serum GA-derived AGEs levels over 12 months [140]. Additionally, high serum AGE levels have been identified as independent risk factors for severe MAFLD-related steatosis [141]. Genetic studies further indicate that certain RAGE polymorphisms in obese patients double the risk of MASH [9]. Other studies reported increased AGE levels and reduced sRAGE in MAFLD patients, which correlates with an increased glycation/sRAGE ratio and an elevated risk of MAFLD [142,143,144]. Further findings show that serum CML levels were increased in MAFLD patients and correlated with markers of liver injury [145].
In alcoholic liver disease (ALD), a major cause of chronic liver issues, AGEs are thought to contribute to liver injury through the ‘toxic AGE theory’, wherein AGEs derived from acetaldehyde or glyceraldehyde interact with RAGE, activating inflammation and oxidative stress. Nearly all chronic heavy drinkers develop ALD, with 10–35% progressing to alcoholic steatohepatitis and 8% to 20% to cirrhosis [146]. Alcoholic cirrhosis is linked to 47.9% of all cirrhosis-related deaths [147]. Research has observed acetaldehyde-derived AGEs in liver tissues of individuals with alcoholism [148]. A particular AGE, AGE10, was suggested as a diagnostic marker for alcoholic steatohepatitis [149]. In addition, elevated RAGE expression in ALD patients correlates with levels of transferrin, hepcidin, and ferritin, underscoring the potential role of AGEs in ALD progression [150].
Regarding liver cancer, hepatocellular carcinoma (HCC) is the predominant form and is particularly prevalent worldwide [151]. Studies on Finnish and Japanese populations show an inverse correlation between serum sRAGE and CML-AGE levels and liver cancer risk [152,153]. Another study on Taiwanese individuals associated the RAGE gene polymorphism rs1800625 with early-stage HCC [154]. The expression of RAGE is higher in well-differentiated tumors among HCC patients and is associated with inflammation, carcinogenesis, and poor post-surgery outcomes, pointing to RAGE reduction as a possible therapeutic target [155]. In other liver diseases, a study on chronic hepatitis B patients found decreased serum sRAGE and tissue RAGE levels, correlated with hepatic necroinflammation [156]. Evidence suggests that AGEs influence liver cirrhosis by disrupting collagen architecture, with studies indicating elevated plasma AGE concentrations in cirrhosis patients, and increased serum CML serves as a marker for cirrhosis.
The liver plays a central role in clearing AGEs, primarily through hepatic sinusoidal endothelial cells and Kupffer cells, which handle 60% and 25% of this process, respectively [157]. Liver diseases impair this process, while AGE–RAGE interactions induce oxidative stress, affecting gene expression in hepatocytes, hepatic stellate cells, and Kupffer cells as well as activating pathways such as MAPK, PI3-K/AKT, and JAK2/STAT1 [158,159,160]. This leads to the release of profibrotic cytokines, transforming quiescent stellate cells into myofibroblasts, promoting fibrosis and cirrhosis [159]. The RAGE–ligand axis regulates MGO-mediated oxidative stress, contributing to hepatic steatosis, inflammation, fibrosis, and HCC [161]. Elevated levels of MGO-induced AGEs and reduced Glyoxalase-I in cirrhotic livers exacerbate liver injury and perpetuate the progression of cirrhosis [162]. Fatty acids stimulate CML accumulation, activating RAGE and increasing PAI-1, IL-8, and CRP production [163].
3.9. AGEs and Eye Diseases
AGEs accumulate in various regions of the eye, including the cornea, lens, retina, Bruch’s membrane, sclera, and optic nerve [164,165,166]. They are primarily associated with age-related or hyperglycemia-related macular degeneration, retinopathy, and cataracts [167,168,169]. For example, human lenses contain both crystallin monomers and polymers of argpyrimidine, with increased levels detected in cataractous lenses compared to noncataractous ones [170]. Moreover, accumulated argpyrimidine would induce apoptosis in lens epithelial cells [171]. High oxygen levels and oxidative stress in vascularized eye regions accelerate the formation of CML and other AGEs, making these areas particularly vulnerable [166]. Since retinal pigment epithelial cells and most lens cells lack proliferative capacity, AGE (such as CML and CEL)-induced damage is difficult to repair, leading to rapid accumulation [172]. Extracellular CML and MG-H1 alter the biochemical properties of ECM, contributing to retinal aging and age-related macular degeneration (AMD) [173]. Intracellular AGEs induce protein crosslinking, disrupting protein structure and function, which is cytotoxic and disrupts the inner blood-retinal barrier (iBRB) [5]. This upregulates VEGF and downregulates platelet-derived growth factor (PEDF), leading to oxidative stress, inflammation, and vascular dysfunction in the retina [174].
Elderly individuals with AMD have higher plasma levels of CML and pentosidine [175]. Elevated AGE levels have also been found in individuals with cataracts or retinopathy, both diabetic and non-diabetic [168,169]. In diabetic patients, glucosepane levels in the lens capsule are significantly higher, regardless of retinopathy status [165]. AGEs may also play a role in ocular surface diseases, such as diabetic keratopathy, by promoting inflammation and ROS pathways [176]. Additionally, serum high mobility group box-1 (HMGB1) levels are elevated in children with spring catarrhal conjunctivitis [177].
Intra-lenticular AGEs induce lens epithelial cell senescence, triggering a mesenchymal transition in adjacent cells, leading to posterior capsule opacification (PCO), a common complication after cataract surgery [178]. The interaction of AGEs with structural proteins such as myelin, tubulin, and lens proteins is linked to cataracts and other ocular disorders in aging and diabetes [179]. AGEs also affect collagen binding, reducing endothelial cell adhesion and causing vascular dysregulation, which is related to diabetic microvascular complications [180]. Accumulation of CML and CEL-modified albumin in retinal endothelial cells raises cell adhesion molecule levels, leading to capillary blockage and retinal ischemia [181]. Furthermore, the AGE–RAGE axis may contribute to diabetic retinopathy through the involvement of adiponectin [182].
3.10. AGEs and Lung Diseases
AGEs are increasingly recognized as key players in the pathogenesis of various lung diseases, including pulmonary fibrosis, acute lung injury (ALI), cancer, and infectious diseases. AGEs accumulate in lung tissues and interact with RAGE, triggering multiple signaling pathways that drive inflammation and fibrosis, ultimately leading to lung functional decline. Notably, RAGE is highly expressed in the lungs, particularly in alveolar epithelial cells, making its dysregulation a significant factor in the development of lung disorders [10].
In idiopathic pulmonary fibrosis (IPF) lungs, both pentosidine and CML were significantly increased [183]. Additionally, in the induced sputum of asthma patients, pentosidine levels were markedly increased, suggesting a potential role in impaired pulmonary function [184]. Furthermore, lower plasma levels of sRAGE have been associated with greater disease severity and poorer outcomes, suggesting that sRAGE plays a protective role by neutralizing the harmful effects of AGEs [185]. Moreover, studies using RAGE-deficient mice have shown that these animals are largely protected from bleomycin-induced lung fibrosis, further emphasizing the role of the AGE–RAGE axis in promoting fibrotic changes. The absence of RAGE in these mice leads to reduced pulmonary levels of pro-fibrotic cytokines such as TGF-β and PDGF, which are critical mediators of fibrosis [186]. Additionally, in cystic fibrosis-related diabetes (CFRD), elevated plasma levels of AGEs correlate with decreased lung function, indicating the potential for AGEs to exacerbate lung damage in this context [187]. Collectively, these studies suggest that the AGE–RAGE axis not only contributes to the progression of fibrosis but that sRAGE functions as a crucial protective factor, potentially offering a therapeutic target to mitigate the advancement of fibrosis in lung diseases.
RAGE is highly expressed in lung tissue under normal conditions, with its expression intensifying during inflammatory conditions such as ALI and acute respiratory distress syndrome (ARDS). Matrix metalloproteinase-9 (MMP-9) plays a role in ALI by influencing the release of sRAGE, and its knockdown exacerbates sepsis-induced ALI and inflammation by decreasing sRAGE levels. Conversely, administration of sRAGE mitigates inflammation and oxidative stress, improving overall outcomes [188]. In the context of infectious diseases like COVID-19, the AGE–RAGE axis has been implicated in the heightened morbidity and mortality observed in elderly patients and those with pre-existing conditions such as diabetes, obesity, and cardiovascular diseases, which are all associated with elevated AGE levels [189]. DM has been linked to a higher risk of severe complications and increased hospitalization, with pre-existing conditions in DM patients, such as endothelial dysfunction and a prothrombotic state, amplifying RAGE signaling, worsening lung inflammation, and thus increasing COVID-19 mortality [190]. Moreover, a significant association between COVID-19 severity and serum sRAGE levels has been established, with higher sRAGE levels observed in patients with severe COVID-19 compared to those with non-severe cases [191]. Blood samples from symptomatic COVID-19 patients revealed that elevated levels of RAGE and the viral antigens were strongly associated with the development of severe disease. The findings suggest that both biomarkers could help identify high-risk patients in emergency settings [192].
The AGE–RAGE axis plays a complex and multifaceted role in lung cancer progression, as CML has been shown to increase in both glycation and cancer models, suggesting a connection between glycation processes and cancer development [193]. While RAGE is highly expressed in normal lung tissue, its expression is often downregulated in cancerous tissues. This downregulation is associated with a significant reduction in sRAGE levels in the serum of lung cancer patients [194]. A study confirmed that serum sRAGE levels, as well as RAGE expression, are lower in lung cancer patients compared to healthy controls, with lower sRAGE levels correlating with increased lymph node involvement [195]. Concurrently, RAGE ligands such as HMGB1 and S100 proteins are upregulated in lung cancer tissue, contributing to the disease’s pathogenesis [194]. Additionally, the presence of RAGE gene polymorphisms has been linked to both the initiation and progression of lung cancer, indicating its potential as a diagnostic and prognostic biomarker [194].
The downregulation of RAGE in lung cancer cells reduces cell-to-cell and cell-to-substrate communication, thereby facilitating tumor progression [196]. Moreover, RAGE overexpression has been shown to inhibit cancer cell growth through p53-dependent mechanisms, although this overexpression also promotes tumor metastasis and the accumulation of tumor-associated macrophages via ERK signaling [197]. RAGE has also been implicated in the resistance of brain metastases to whole-brain radiotherapy through the S100A9-RAGE-NF-κB-JunB pathway, with targeting this pathway showing potential to improve treatment outcomes [198]. Overall, the intricate role of RAGE in lung cancer highlights its potential as both a biomarker and a therapeutic target in the treatment of lung cancer and its metastases.
3.11. AGEs and Obesity
Obesity, defined as excessive fat accumulation with a body mass index (BMI) of 30 or higher in adults, is not only a global epidemic but also a significant risk factor for various fetal diseases [199].
In recent years, the role of AGE accumulation in the development of obesity has garnered widespread attention. Early studies indicated that plasma AGE levels were lower in obese children [200]. A meta-analysis also observed an inverse association between circulating AGEs and BMI in adults [201]. However, circulating AGEs were reported to be elevated in unhealthy obese individuals at risk for metabolic syndrome [202]. The relationship between circulating AGE levels and obesity following in-body metabolism remains controversial. Notably, obese patients exhibited significant accumulation of AGEs, particularly CML, and higher RAGE expression in adipose tissue (AT), compared to non-obese subjects [203]. Due to the strong influence of serum CML concentrations on AT, CML might preferentially accumulate in AT [204], leading to lower circulating levels of CML in obese individuals [203]. Recent research revealed high AGE-to-sRAGE ratios in overweight and obese children [205]. This finding implies that monitoring the ratio of AGEs to sRAGEs could enhance our understanding of the mechanisms behind obesity and its associated complications, providing valuable insights for potential interventions.
Increasing research suggests that the AGE–RAGE axis is involved in AT dysfunction and the development of obesity. First of all, AGE formation impaired the proliferation and osteogenic differentiation potential of adipose-derived stem cells (ASCs) by suppressing the Wnt/β-catenin signaling pathway and increasing DNA methylation [206]. In contrast, in vitro studies have shown that exposure to AGEs inhibited the adipogenesis differentiation of human mesenchymal stem cells, with minimal impact on osteogenic development [207]. In addition to modulating the fate of ASCs, the AGE–RAGE axis has the potential to regulate lipolysis and adaptive thermogenesis by inhibiting the protein kinase A (PKA)-mediated phosphorylation of hormone-sensitive lipase (HSL) and the p38 MAPK pathway [208]. AGEs also suppressed apolipoprotein E expression in adipocytes, thereby reducing intracellular triglyceride synthesis both in vitro and in vivo [209]. Furthermore, the accumulation of AGEs in the ECM impaired glucose uptake and led to insulin resistance in human primary adipocytes by activating the AGE/RAGE/Diaphanous 1 axis [210,211]. AGE-stimulated adipocyte hypertrophy was accompanied by the downregulation of insulin-sensitive genes, such as glucose transporter type 4 (GLUT4) and adiponectin, which further impaired glucose uptake, insulin signaling and adipokine secretion [212].
3.12. AGEs and Intestinal Diseases
The gastrointestinal tract harbors trillions of microorganisms, collectively known as the gut microbiota, which play a critical role in maintaining host health [213]. Approximately 10% of dAGEs are absorbed by the small intestine and enter circulation, with some excreted in the urine [214]. The remaining AGEs, along with amino acids and peptides released during epithelial desquamation, are eliminated through feces [215]. These AGEs, such as CML, may be partially degraded and metabolized by the gut microbiota, serving as potential nutrients that influence host health by modulating inflammatory responses, metabolic processes, and immunoregulation [216]. This interaction can also affect the composition and function of the microbiome, thereby impacting overall health through changes to gut architecture [217].
Inflammatory bowel disease (IBD), encompassing conditions like ulcerative colitis and Crohn’s disease, is strongly linked to AGE/RAGE accumulation in the gastrointestinal tract [218]. Elevated pentose glycosides are observed in the gastrointestinal tissues of IBD patients, with urinary concentrations rising during active disease phases [219]. The high levels of AGE/RAGE in IBD may accelerate intestinal fibrosis, often associated with epithelial-mesenchymal transition [220]. Recent studies also suggest that AGE/RAGE signaling may serve as predictors for postoperative recurrence in Crohn’s disease [221,222]. Specifically, RAGE expression is significantly increased in the inflamed intestinal tissues of Crohn’s disease patients [223]. Dietary intake of CEL has been associated with an increased risk of Crohn’s disease [224]. Conversely, in ulcerative colitis or patients with penetrating behavior, sRAGE levels are reduced and inversely correlate with disease activity [225].
The interaction between RAGE polymorphisms, elevated RAGE levels, oxidative stress, and inflammation may contribute to the progression of IBD to colorectal cancer (CRC) [218]. A retrospective study of 133 colorectal adenocarcinoma cases found that AGE expression correlated with cancer histological grade, suggesting that AGEs may play a role in both early and late colorectal carcinogenesis [226]. Circulating glyceraldehyde-derived AGEs are significantly correlated with CRC-specific mortality [227]. Interestingly, pre-diagnosis sRAGE levels are negatively associated with CRC risk in men [228]. In women with a BMI of 25 or greater, those with the highest sRAGE levels exhibited a reduced risk of CRC [229]. However, other studies have found a positive correlation between sRAGE and CRC mortality, highlighting the complex role of RAGE in cancer outcomes [230]. In advanced CRC tumors, glyoxalase 1 (GLO1) levels are depleted, while methylglyoxal remains elevated, further implicating AGEs in cancer progression [231].
Dietary interventions have shown that AGE restriction can alter the gut microbiota. A study of peritoneal dialysis patients revealed that one month of AGE restriction (measured by CML and MG levels) led to a significant reduction in the abundance of Prevotella copri and Bifidobacterium animalis and an increase in Clostridium citroniae, Clostridium hathewayi, Alistipes indistinctus, and Ruminococcus gauvreauii [232]. This microbiota shift may contribute to cardiovascular events. Similarly, a Dutch trial with 718 participants found that higher dietary AGE (quantified by CML, CEL, and MG-H1 levels) intake was linked to a lower abundance of Barnesiella, Colidextribacter, and Terrisporobacter, while increasing Coprococcus, Dorea, and Blautia [214]. Studies on adolescents suggest that Maillard reaction products reduce lactobacilli and bifidobacterial levels, further supporting the notion that AGEs can significantly reshape the microbiota [233].
AGEs have been associated with a reduction in the abundance of beneficial microbes that produce short-chain fatty acids (SCFAs), potentially contributing to insulin resistance [234]. However, some glycated products also serve as substrates for SCFA production [235]. AGEs may disrupt intestinal homeostasis by interacting with intestinal glial cells, promoting inflammation and oxidative stress [236]. They also upregulate SP1 expression, potentially promoting invasion and metastasis in colorectal cancer through the RAGE/ERK/MMP2 signaling pathway [237]. Moreover, methylglyoxal-derived AGEs can compromise the integrity of the intestinal epithelial cell barrier, increasing permeability, triggering inflammatory responses, and further exacerbating oxidative stress, all of which contribute to gastrointestinal disorders and cancer progression [238].
3.13. AGEs and Reproductive Diseases
AGEs are also strongly linked to reproductive disorders, a connection that is often overlooked. The involvement of AGEs in ovarian dysfunction was demonstrated in the study of polycystic ovary syndrome (PCOS) [239,240]. Elevated AGE levels could be detected in both the serum and the ovary of PCOS patients. Further evidence from the human granulosa cell line model (KGN) showed that the AGEs in vitro could interfere with LH action and lead to sustained abnormal activation of the ERK1/2 pathway, which leads to impaired follicular development and hence ovulatory dysfunction associated with PCOS. Another experiment on the culture of the KGN cells with insulin or human glycated albumin (HGA; rich with AGEs), alone or in combination, demonstrated that AGEs within the ovary alter glucose metabolism and folliculogenesis [241]. Besides PCOS, AGEs may also contribute to the formation of endometriosis [242], although the mechanisms are not well understood. For reproductive dysfunction during aging, the AGE-RAGE-VEGF signaling may lead to reproductive environment changes and affect reproductive function [243]. More directly, the relationship between AGEs and infertility has been documented in several studies, suggesting the important role of AGE accumulation in ovarian dysfunction and poorer outcomes in women with elevated AGEs and undergoing in vitro fertilization (IVF) [243]. As reported, the toxic AGEs (TAGEs) pentosidine and CML accumulate in the follicular fluid, and the TAGE level in serum is negatively correlated with follicular growth, fertilization, and embryonic development. Lower concentrations of pentosidine in the follicular fluid and TAGEs in the serum were even taken as the most significant predictors for achieving pregnancy in those patients undergoing IVF [244].
The deleterious effects of AGEs on the ovary have also been evidenced in animal experiments. Female mice subjected to a diet high in AGEs had longer diestrus phases, significant alterations in genes involved in steroidogenesis and folliculogenesis, and fewer corpora lutea in their ovaries, suggesting the dysfunction of the ovary [245].
As they accumulate in female ovaries, AGEs can also deposit in the male testes, epididymis, and sperm, especially in men with diabetes [246], and possibly play a role in diabetes-related infertility [13]. Levels of AGEs were significantly increased in the spermatozoa of diabetic men, and RAGE expression was also elevated in both spermatozoa and seminal plasma [247,248]. However, the direct clinical evidence of AGE-induced infertility is limited. In animal studies, an AGE-rich diet induced histopathological damage in the testes and epididymides of mice, accompanied by a decrease in normal sperm morphology, epididymal sperm reserve [249], and sperm function [250]. Oxidative stress is one of the key factors in AGEs involved in male infertility [251]. As reported, AGEs can inhibit testosterone production and secretion in rat Leydig cells by inducing oxidative stress and endoplasmic reticulum stress [252].
4. AGEs and Interventions
4.1. AGE Inhibitors
Compounds that inhibit AGEs hold potential as therapeutic approaches for treating various AGE-related diseases. They act through multiple mechanisms, including preventing the interaction between reducing sugars and amino acids in early glycation, scavenging free radicals, sequestering Amadori intermediates to block late glycation stages, and breaking AGE crosslinks [253,254]. These AGE inhibitors can be categorized into two main groups based on the sources: naturally occurring polyphenolic compounds and synthetic small molecules [253].
Currently, most research is conducted at the in vitro and animal levels, with only a limited number of compounds undergoing clinical studies. Additionally, some drugs (such as aspirin and diclofenac) are widely used in clinical practice. However, there are no specific clinical studies focused on inhibiting AGEs to improve associated diseases, therefore, their clinical dosages have not been established. The following table outlines the mechanisms, indications, and clinical dosages (where available) of synthetic compounds (Table 1). Clinical studies on these inhibitors have primarily focused on diabetes and its complications, as well as related metabolic disorders. However, whether these small molecules can be applied to other AGE-related diseases (skin, joints, gut, and aging) requires further evidence.
Several widely used medications, including metformin, benfotiamine, and pyridoxamine, have also been shown to inhibit AGE formation, presenting potential new therapeutic applications. Despite over two decades of research into AGE inhibitors, clinical trials involving compounds like aminoguanidine and pyridoxamine have shown significant side effects [1,255]. To date, the FDA has not approved any treatments specifically for AGE-related diseases. However, recent advances in newly synthesized small molecules with potent AGE-inhibitory effects offer promising prospects for future therapies.

Table 1.
The mechanisms and indications of synthetic compounds as AGE inhibitors.
Table 1.
The mechanisms and indications of synthetic compounds as AGE inhibitors.
| Structure | Name | Mechanisms | Potential Indications | Clinical Dosage | References |
|---|---|---|---|---|---|
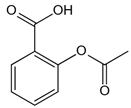 | Aspirin | Inhibits the glycation process via acetylating free amino groups of proteins, thereby blocking the attachment of reducing sugars | Diabetes and its late-stage complications | / | [256,257,258] |
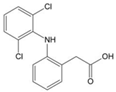 | Diclofenac | Protects proteins from sugar attachment due to its non-covalent interaction with proteins (such as serum albumin) | Anti-inflammation | / | [259] |
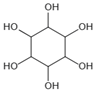 | Inositol | Scavenging of glucose | Diabetes, cataracts, and diabetic retinopathy | / | [260,261,262] |
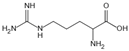 | Arginine/lysine | Protein-glycation inhibitor, competitive attachment with glucose | Cataracts | / | [263,264] |
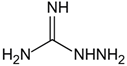 | Aminoguanidine | Carbonyl scavenger, reacts with β-dicarbonyl intermediates induced by glycation | Diabetic complication | / | [265,266,267] |
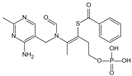 | Benfotiamine | Accelerates the precursors of AGEs toward the pentose phosphate pathway | Alzheimer’s disease | 600 mg/d | [268,269,270] |
| type 2 diabetes | 900 mg/d | ||||
| diabetic neuropathy | 150–320 mg/d | ||||
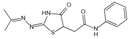 | 2- Isopropylidenehydrazono-4-oxo-thiazolidine-5-ylacetanilide (OPB-9195) | Carbonyl scavenger, metal-ion chelation | Diabetic complication | / | [271] |
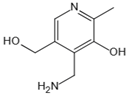 | Pyridoxamine | Mental-ion chelation, radical scavenging properties, sequestering the ROS and RNS | Diabetic retinopathy | 200 mg/d | [272,273,274,275,276] |
| Atherosclerosis | 1200–2400 mg/d | ||||
| Diabetic nephropathy | 100–500 mg/d | ||||
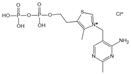 | Thiamine pyrophosphate | Dicarbonyl scavenger | Vascular complications of diabetes | / | [277,278] |
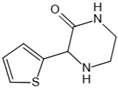 | Tenilsetam | Dicarbonyl scavenger, restrains the polymerization of lysozyme with 3-DG, transition metal ion chelator | Age-related neurodegenerative diseases | / | [265,279] |
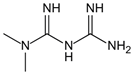 | Metformin | Protects proteins against glycation and cross-linking, captures discarbonyls produced | Type 2 diabetes | 1500–2550 mg/d | [280,281,282] |
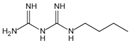 | Buformin | Protects proteins against glycation and cross-linking, traps carbonyls of ammonia and MGO | Type 2 diabetes | / | [281] |
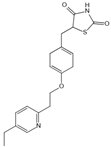 | Pioglitazone | Carbonyl scavenger | Type 2 diabetes | 30 mg/d | [283,284] |
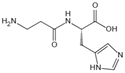 | Carnosine | Reacts with sugars to prevent the formation of AGEs, protects proteins against glycation and cross-linking, and scavenges ROS | Vascular complications of diabetes | / | [285,286,287,288] |
| Type 2 diabetes and diabetic nephropathy | 1000 mg/d | ||||
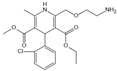 | Amlodipine | Radical scavenging properties | Atherosclerotic lesions | / | [289,290,291] |
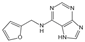 | Kinetin | Radical scavenging properties | Alzheimer’s disease | / | [292,293] |
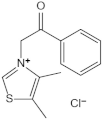 | Alagebrium chloride (ALT-711) | AGE breaker, breaks down established AGE-related protein cross-link | Arteriosclerosis, hypertension, diastolic heart failure | 420 mg/d | [294,295,296,297] |
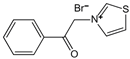 | N-phenacylthiazolium bromide (PTB) | AGE breaker, cleaving α-diketone structure | Diabetic vascular complications, diabetic periodontitis | / | [298,299,300] |
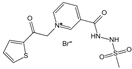 | 3-[[2-(Methylsulfonyl)hydrazinyl]carbonyl]-1-[2-oxo-2-(2-thienyl)ethyl]pyridinium bromide (TRC-4149) | AGE breaker, free radical scavenging activity | Diabetic vascular complications | / | [301,302,303] |
4.2. Natural Products Inhibiting AGE Formation
Chemical compounds extracted from plants or animals, known as ‘natural compounds’, have exhibited unique pharmacological effects. Currently, natural compounds from plants and foods have shown excellent inhibitory activity against the formation of AGEs, with minimal toxicity [304]. Therefore, the screening of natural compounds that can be used as glycation inhibitors has been of great interest. This paper presents natural compounds with the potential to inhibit the formation of AGEs, categorized into six groups based on their structural properties: polyphenols, polysaccharides, terpenoids, vitamins, alkaloids, and peptides.
4.2.1. Polyphenols
Polyphenols are classified into flavonoids, phenolic acids, stilbenes, and lignans based on the number of phenolic rings and the structural elements that connect them [305]. This paper will focus on flavonoids that can suppress the formation and accumulation of AGEs.
Flavonoids
Flavonols: Quercetin, as a flavonol glycoside, has been found to reduce plasma MGO concentrations in healthy (prehypertensive) adults, indicating its therapeutic potential for MGO-mediated diseases [306]. Quercetin could inhibit AGE formation by scavenging MGO and GO in vitro [307]. Another flavonol glycoside, rutin, could not only scavenge free radicals but also inhibit the formation of AGEs by chelating metal ions to form complexes [308]. Studies on rutin supplementation have demonstrated its ability to improve metabolic parameters and oxidative stress factors in T2DM patients, confirming the in vivo antiglycation capacity of rutin [309]. Other flavonols, such as kaempferol and myricetin, also play a role in preventing the formation and accumulation of AGEs through various mechanisms and have shown beneficial effects on antioxidant capacity in vivo [310].
Flavanonols: As one of the active ingredients in silymarin, taxifolin has been shown to trap endogenous MGO by forming mono-MGO adducts in diabetic mice, highlighting its potential for treating chronic diseases associated with AGEs [311]. The derivative of aromadendrin, aromadendrin-8-C-glucopyranoside, was found to inhibit the formation of AGEs in a dose-dependent manner [312], warranting further in-depth investigation.
Flavones: Luteolin, apigenin, and diosmin have been reported to inhibit AGE formation through various mechanisms, including the trapping of active dicarbonyl intermediates and the inhibition of ROS generation [310].
Flavanones: Naringenin and eriodictyol have the potential to suppress the formation of AGEs and mitigate AGE-induced oxidative stress and inflammation [313,314]. The enhanced inhibitory activity of eriodictyol, compared to naringenin, is attributed to the presence of an additional hydroxyl group in the B ring of eriodictyol [314].
Flavanols: Significant inhibition of AGE formation and accumulation through the trapping of MGO by (+)-catechin, (−)-epicatechin, and their derivatives, (−)-epicatechin-gallate and (−)-epigallocatechin-3-gallate (EGCG), has been demonstrated both in vitro and in vivo [310].
Chalcones: Curcumin has been suggested to inhibit the formation of AGEs by capturing MGO and preventing collagen cross-linking [315]. Apple polyphenols, including phloretin and its derivative phloridzin, could effectively trap dicarbonyls produced by late-stage glycosylation end products [316,317], potentially mitigating secondary complications associated with diabetes [318].
Isoflavonoids and Biflavonoids: One of the isoflavonoids, genistein, has been reported to be a potent aldose reductase inhibitor that traps MGO to form mono- and dual-MGO adducts in vitro, thereby reducing the formation of AGEs [319]. In addition, amentoflavone, which belongs to the class of biflavonoids, exerts its anti-AGE activity by scavenging free radicals, chelating divalent metal ions, and trapping dicarbonyl groups [307].
Phenolic Acids, Stilbenes, and Lignans
Phenolic acids are common plant secondary products with higher concentrations than polyphenolic flavonoids and are mainly divided into two groups: hydroxybenzoic acids and hydroxycinnamic acids [320]. An example of hydroxybenzoic acids is 7-O-galloyl-D-sedoheptulose, which reduced levels of AGEs and ROS in diabetic mice, demonstrating a protective effect in the early stages of diabetic kidney disease [321]. Among the well-studied hydroxycinnamic acids, caffeic acid, ellagic acid [322], and ferulic acid [323] have been shown to reduce the AGE formation and accumulation in diabetic rats. Rosmarinic acid could inhibit AGE-mediated crosslinking and alleviate liver fibrosis progression in mice [324]. These studies suggest that phenolic acids are potential anti-glycation compounds.
Resveratrol, a kind of stilbene, has been shown to inhibit the carbohydrate enzymes α-glucosidase and α-amylase while also reducing the formation of AGEs [325]. Resveratrol supplementation reduced glycated hemoglobin and had a protective effect in patients with T2DM [326]. Lignans extracted from Cortex Eucommiae protected against AGE-induced endothelial cell dysfunction in vivo and in vitro by modulating Nrf2/HO-1 signaling [327].
Anthocyanins
The anthocyanins from fruits and vegetables have been mostly reported to have antiglycative activities. Blueberry (Vaccinium corymbosum) anthocyanins extract (BAE) exhibits strong anti-glycation activity by scavenging glycosylated intermediates, like Schiff base, fructosamine, and α-dicarbonyl compounds, attenuating the molecular aggregation and amyloid-like fibril formation, preventing conformational modification, and inhibiting AGE-stimulated inflammation in RAW264.7 cells [328]. Similar activity was reported in other berries, like Chinese bayberry (Myrica rubra) [329], cranberry (Vaccinium Oxycoccos), strawberry (Fragaria ananassa Duch.), raspberry (Rubus corchorifolius), blackberry (Rubus grandifolius Lowe) [330], blackcurrant (Ribes nigrum L.) [331], sea buckthorn (Hippophae rhamnoides) [332], black chokeberry (Aronia melanocarpa) [333], goji berry (Lycium barbarum L.), and grape [334], which are all rich in anthocyanins. Bilberry (Vaccinium myrtillus L.) also contains vast amounts of anthocyanins and is reported to have anti-diabetic activities [335]. It has long been used for the prevention and treatment of diabetes [336]. In China, the drug Difrarel, which contains bilberry extracts as its primary ingredient, was approved for the treatment of diabetic retinopathy. In a clinical study of seventy-four healthy subjects, plasma protein-bound AGEs can be reduced partly by 3-month Vaccinium myrtillus extract supplementation [337]. In another more in-depth animal experiment, the rats were fed an AGE-rich diet with or without bilberry extracts for 80 weeks. The results showed that exposure to the AGE-rich diet led to varying degrees of accumulation of AGEs (free-CML, free-CEL, bound-CML, and bound-CEL) in different tissues. Notably, the addition of bilberry extracts significantly reduced AGE accumulation, especially in the kidney, skin, and brain. These results suggested that Vaccinium Myrtillus could be a promising natural product for the prevention of AGE accumulation [19]. In addition to berries, other plants such as purple corn, red rice, and Clitoria ternatea L. flowers also demonstrate anti-glycation activity due to their rich anthocyanin content.
The commonly consumed berry fruits had excellent MGO-trapping and antiglycative activities, which are positively associated with their total phenolic contents, as exhibited by some ex vivo experiments [331]. Moreover, certain anthocyanins from these anti-glycation plant extracts exhibit strong efficacy and unique mechanisms. Cyanidin-3-rutinoside can attenuate MGO-induced protein glycation and DNA damage via carbonyl trapping ability and scavenging ROS [338]. Cyanidin-3-O-glucoside exhibits masking-like function, carbonyl scavenging, and chemical chaperone activity in the inhibition of early and advanced glycation [339].
4.2.2. Terpenes
Terpenoids, which are derivatives of terpenes, are widely distributed among various plant species and exhibit antioxidant and anti-glycation bioactivities [304]. Among the most extensively studied pentacyclic triterpenoids, oleanolic acid and ursolic acid have shown promise for clinical applications in the prevention or mitigation of glycation-related diseases [340]. Research indicates that ursolic acid may inhibit the activities of aldose reductase and sorbitol dehydrogenase, resulting in a reduction in hyperglycemia and hepatic glucose production in diabetic mice [341]. Aucubin, which belongs to another class of terpenoids known as iridoids, has been shown to directly trap MGO in vitro and prevent the formation and accumulation of AGEs in vivo, indicating its potential for treating AGE-related chronic diseases [342].
4.2.3. Polysaccharides
Polysaccharides are macromolecules composed of at least ten monosaccharides that are polymerized into straight or branched chains via glycosidic bonds, exhibiting antioxidant and hypoglycemic properties [343]. Astragalus polysaccharides have been shown to decrease the formation of AGEs by protecting insulin activity, stimulating insulin secretion, and improving glucose and lipid metabolism [344]. Recently, the degraded polysaccharides from Auricularia auricula-judae have been shown to inhibit the formation of AGEs in a dose-dependent manner [345]. Due to the relatively low toxicity of most polysaccharides extracted from plants, they are expected to be a promising source of glycosylation inhibitors. However, further research is required to delineate the molecular mechanisms involved.
4.2.4. Alkaloids
As a class of nitrogenous organic compounds, alkaloids exhibit a diverse range of pharmacological and biological activities due to their various structures. One of the most extensively studied alkaloids, berberine, has been shown to increase the production of nitrogen oxides and promote endothelium-dependent vasodilation [346]. Additionally, it exerted protective effects against endothelial dysfunction induced by high glucose levels in vitro [346]. Berberine can reduce blood glucose levels and inhibit the accumulation of AGEs in diabetic rats [347]. A meta-analysis revealed that berberine exerted a comparable therapeutic effect on T2DM without serious side effects [348], suggesting that berberine has a promising clinical application in anti-glycation.
4.2.5. Vitamins
Vitamin C is a kind of water-soluble vitamin that competes with glucose in binding to proteins, thereby inhibiting the production of AGEs in vitro [349]. In addition to inhibiting the sorbitol pathway, vitamin C also reduces fructose production in diabetic patients [349]. Vitamin E, a lipid-soluble vitamin, has been shown to suppress hemoglobin glycosylation and the formation of AGEs [350]. Pyridoxamine, a member of the vitamin B6 family, has demonstrated the ability to scavenge free radicals and chelate metal ions, making it a commonly used glycation inhibitor in clinical settings [351].
4.2.6. Peptides
Carnosine, a natural dipeptide synthesized in the body, was found in high concentrations in brain and muscle tissues, with levels ranging from approximately 1 to 20 mmol/kg [352]. Carnosine exhibited antioxidant and anti-glycation properties, effectively preventing oxidative stress and the formation of AGEs in D-gal-induced aging rats [353]. In addition, research indicates that glutathione is more effective than carnosine in preventing glucose glycation [354].
4.3. Health Interventions for AGEs
Various environmental factors, such as high-carbohydrate and high-calorie diets, high-temperature cooking, cigarette smoking, and a sedentary lifestyle, may contribute to the formation of AGEs [4]. To summarize, AGEs and metabolic disorders are established on the concept of the ‘common soil’ [14]. Although ultraviolet rays, ionizing radiation, and air pollution are unavoidable, preventive action against glycation by lifestyle adjustment may be warranted [4]. For a healthier diet, it is advisable to limit highly processed food and instead choose options rich in whole grains, dairy products, nuts, and legumes [355,356], prioritizing foods with a low glycemic index and low fat content, along with a variety of natural fresh ingredients [357]. Moreover, adopting a Mediterranean diet, which is rich in monounsaturated fatty acids and minimally processed natural foods, can help reduce AGE levels by mitigating postprandial oxidative stress and inflammation [358]. Cooking strategies to minimize exposure to food AGEs include avoiding the use of repeatedly heated oils, opting for lower cooking temperatures when possible, and incorporating acidic ingredients like vinegar or lemon juice to lower AGE formation [359]. Additionally, it is advisable to choose cooking methods such as steaming, stewing, and boiling rather than frying or grilling [360]. In addition to dietary restrictions, enhancing physical activity and quitting smoking are also crucial interventions and practical strategies.
5. Discussion
Over the past few decades, AGEs have garnered widespread attention as crucial factors associated with aging and various chronic diseases. AGEs are a class of compounds formed through non-enzymatic glycation (i.e., the Maillard reaction), and their progressive accumulation in tissues and organs can lead to cellular damage, disrupt normal physiological functions, and contribute to the onset of multiple diseases, including diabetes, cardiovascular diseases, neurodegenerative diseases, chronic kidney disease, skin aging, metabolic disorders, reproductive disorders, and degenerative bone diseases.
Traditionally, AGE levels have been most closely linked to diabetes and its complications, such as CVDs and CKD, as their severity is positively correlated with AGE accumulation. This is primarily because the persistent hyperglycemic environment in diabetic patients naturally facilitates AGE formation. However, as research on AGEs has deepened, new theories have emerged, suggesting that AGEs are not merely byproducts of diabetes but may also play a causal role in its development. Additionally, many prediabetic individuals exhibit significantly elevated AGE levels, which may be closely related to certain complications that appear even before a formal diabetes diagnosis.
Furthermore, AGE-induced skin aging has become a significant health concern in recent years. The cross-linking of collagen and elastin caused by AGE leads to a decline in skin elasticity, while AGE-induced melanin deposition results in a dull complexion.
Given these findings, studying the formation mechanisms and pathogenic effects of AGEs is of great significance for understanding aging and chronic diseases, particularly the onset and progression of diabetes and its related syndromes.
The formation of AGEs involves multiple chemical processes. Changes in metabolic levels, along with lifestyle factors such as hyperglycemia, high-calorie diets, smoking, and a sedentary lifestyle, can accelerate the synthesis and accumulation of AGEs. AGEs primarily exert their pathological effects by trapping and crosslinking the protein or by interacting with their receptor RAGE, triggering oxidative stress, inflammatory responses, and apoptosis, which further exacerbate tissue and organ damage. As a result, reducing the formation and accumulation of AGEs has become a key focus in current biomedical research.
Currently, interventions targeting AGEs mainly fall into two categories: chemically synthesized small-molecule inhibitors and natural compounds. Chemically synthesized small-molecule inhibitors can reduce the harmful effects of AGEs by interfering with their formation pathways or breaking down already-formed AGEs. However, due to the potential side effects associated with some small-molecule inhibitors, their clinical applications have been limited. As a result, researchers have increasingly focused on bioactive compounds derived from natural foods and plants in recent years.
Natural compounds, particularly polyphenolic compounds from plants, such as anthocyanins, quercetin, and catechins, have been proven to possess strong anti-glycation properties. These compounds could inhibit the formation of AGEs through mechanisms such as lowering the blood glucose level, trapping reactive carbonyl compounds, inhibiting oxidative stress, and blocking protein glycation sites. At the same time, they may also promote the degradation of AGEs and protect tissues from AGE-induced pathological damage.
To date, many natural compounds or plant extracts have been clinically tested for their ability to reduce AGEs and protect against AGE-related damage. Three months of intervention with Vaccinium myrtillus extract (600 mg/day) in healthy subjects reduced the CML level, though the CEL level was not significantly changed [337]. Two months of resveratrol supplementation (1000 mg/day) can effectively reduce Hb1Ac levels in the blood of T1D patients and exert anti-diabetic effects [361]. Supplementation of vitamin B (133 mg/day), C (500 mg/day), and E (400 mg/day) in combination with anti-diabetic drugs can improve the rate of development of retinopathy in patients with T2DM and reduce circulating AGEs [362]. Many plant extracts, such as Moringa oleifera leaf (2400 mg/day) and green tea extract (800 mg/day), also reduce glycemic outcomes [363,364]. Currently, the combination of small-molecule inhibitors and herbal medicines is also being used in the treatment of diabetes, which is expected to be an effective option for lowering blood glucose in the clinic [365,366]. At the same time, numerous animal studies have also demonstrated the effectiveness of these natural compounds in reducing AGE levels and protecting against related dysfunction.
Although natural compounds exhibit great potential in inhibiting AGE formation, their clinical application remains somewhat limited due to their low bioavailability. For instance, polyphenolic compounds are often easily degraded in the digestive tract, leading to low absorption efficiency in the body. Therefore, future research should focus on improving the bioavailability of these compounds by enhancing their stability and absorption efficiency through techniques such as nanocarriers, liposomal encapsulation, or formulation optimization.
Additionally, to further advance the application of natural anti-AGE compounds, a thorough evaluation of their safety and efficacy is necessary. While some natural compounds have shown promising effects in animal studies and clinical research, more randomized controlled trials (RCTs) are needed to verify their long-term safety and therapeutic effectiveness.
From the perspective of developing drugs to reduce AGEs, we still have a long way to go. However, some natural products that serve both as food and medicine have a long history of consumption, demonstrating good safety profiles while also exhibiting strong AGE-lowering activity. These natural products, such as bilberry extract, green tea extract, and resveratrol, can be prioritized as nutritional interventions to help protect against the health risks associated with AGEs.
Author Contributions
Conceptualization, Y.Z. and R.H.; software, Z.Z.; writing—original draft preparation, Y.Z., Z.Z., C.T. and X.C.; writing—review and editing, Y.Z., Z.Z., C.T., X.C. and R.H.; supervision, R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Y.Z., Z.Z., C.T., X.C., and R.H. are associated with By-Health Co., Ltd. company. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today 2006, 11, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Bansal, S.; Burman, A.; Tripathi, A.K. Advanced glycation end products: Key mediator and therapeutic target of cardiovascular complications in diabetes. World J. Diabetes 2023, 14, 1146–1162. [Google Scholar] [CrossRef]
- Deng, S.; He, R.; Yue, Z.; Li, B.; Li, F.; Xiao, Q.; Wang, X.; Li, Y.; Chen, R.; Rong, S. Association of Advanced Glycation End Products with Cognitive Function: HealthyDance Study. J. Alzheimers Dis. 2024, 100, 551–562. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef]
- Mehta, R.; Shaw, G.; Masschelin, P.; Felix, S.; Otgonsuren, M.; Baranova, A.; Goodman, Z.; Younossi, Z. Polymorphisms in the receptor for advanced glycation end-products (RAGE) gene and circulating RAGE levels as a susceptibility factor for non-alcoholic steatohepatitis (NASH). PLoS ONE 2018, 13, e0199294. [Google Scholar] [CrossRef]
- Oczypok, E.A.; Perkins, T.N.; Oury, T.D. All the “RAGE” in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr. Respir. Rev. 2017, 23, 40–49. [Google Scholar] [CrossRef]
- Glenn, J.V.; Stitt, A.W. The role of advanced glycation end products in retinal ageing and disease. Biochim. Biophys. Acta 2009, 1790, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Tan, S.M.; Clarke, R.E.; de Pasquale, C.; Thallas-Bonke, V.; Nguyen, T.V.; Penfold, S.A.; Harcourt, B.E.; Sourris, K.C.; Lindblom, R.S.; et al. Processed foods drive intestinal barrier permeability and microvascular diseases. Sci. Adv. 2021, 7, eabe4841. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Cai, Y.Q.; Long, S.L.; Chen, Z.; Mo, Z.C. The role of advanced glycation end products in human infertility. Life Sci. 2020, 255, 117830. [Google Scholar] [CrossRef]
- Ruiz, H.H.; Ramasamy, R.; Schmidt, A.M. Advanced Glycation End Products: Building on the Concept of the “Common Soil” in Metabolic Disease. Endocrinology 2020, 161, bqz006. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef]
- Selvaraj, N.; Bobby, Z.; Sridhar, M.G. Oxidative stress: Does it play a role in the genesis of early glycated proteins? Med. Hypotheses 2008, 70, 265–268. [Google Scholar] [CrossRef]
- Cho, S.J.; Roman, G.; Yeboah, F.; Konishi, Y. The road to advanced glycation end products: A mechanistic perspective. Curr. Med. Chem. 2007, 14, 1653–1671. [Google Scholar] [CrossRef]
- Phuong-Nguyen, K.; McNeill, B.A.; Aston-Mourney, K.; Rivera, L.R. Advanced Glycation End-Products and Their Effects on Gut Health. Nutrients 2023, 15, 405. [Google Scholar] [CrossRef]
- Mo, X.; Shen, L.; Wang, X.; Sun, Y.; Cheng, R.; Chen, W.; Chen, J.; He, R.; Liu, L. European bilberry extract reduces high-temperature baked food-induced accumulation of Nε-carboxymethyllysine and Nε-carboxyethyllysine in vivo. Food Res. Int. 2024, 197, 115157. [Google Scholar] [CrossRef]
- Nicholl, I.D.; Stitt, A.W.; Moore, J.E.; Ritchie, A.J.; Archer, D.B.; Bucala, R. Increased levels of advanced glycation endproducts in the lenses and blood vessels of cigarette smokers. Mol. Med. 1998, 4, 594–601. [Google Scholar] [CrossRef]
- Danby, F.W. Nutrition and aging skin: Sugar and glycation. Clin. Dermatol. 2010, 28, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Li, L.; Winget, J.; Laughlin, T.; Hakozaki, T. Identification of Yellow Advanced Glycation End Products in Human Skin. Int. J. Mol. Sci. 2024, 25, 5596. [Google Scholar] [CrossRef] [PubMed]
- Sessa, L.; Gatti, E.; Zeni, F.; Antonelli, A.; Catucci, A.; Koch, M.; Pompilio, G.; Fritz, G.; Raucci, A.; Bianchi, M.E. The receptor for advanced glycation end-products (RAGE) is only present in mammals, and belongs to a family of cell adhesion molecules (CAMs). PLoS ONE 2014, 9, e86903. [Google Scholar] [CrossRef] [PubMed]
- Gasiorowski, K.; Brokos, B.; Echeverria, V.; Barreto, G.E.; Leszek, J. RAGE-TLR Crosstalk Sustains Chronic Inflammation in Neurodegeneration. Mol. Neurobiol. 2018, 55, 1463–1476. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Naka, Y.; Schmidt, A.M. Glycation, inflammation, and RAGE: A scaffold for the macrovascular complications of diabetes and beyond. Circ. Res. 2003, 93, 1159–1169. [Google Scholar] [CrossRef]
- Cai, W.; He, J.C.; Zhu, L.; Peppa, M.; Lu, C.; Uribarri, J.; Vlassara, H. High levels of dietary advanced glycation end products transform low-density lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation 2004, 110, 285–291. [Google Scholar] [CrossRef]
- Lopes-Virella, M.F.; Baker, N.L.; Hunt, K.J.; Cleary, P.A.; Klein, R.; Virella, G.; Group, D.E.R. Baseline markers of inflammation are associated with progression to macroalbuminuria in type 1 diabetic subjects. Diabetes Care 2013, 36, 2317–2323. [Google Scholar] [CrossRef]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A Role for Advanced Glycation End Products in Molecular Ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef]
- Fujisawa, K.; Katakami, N.; Kaneto, H.; Naka, T.; Takahara, M.; Sakamoto, F.; Irie, Y.; Miyashita, K.; Kubo, F.; Yasuda, T.; et al. Circulating soluble RAGE as a predictive biomarker of cardiovascular event risk in patients with type 2 diabetes. Atherosclerosis 2013, 227, 425–428. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. The RAGE axis: A fundamental mechanism signaling danger to the vulnerable vasculature. Circ. Res. 2010, 106, 842–853. [Google Scholar] [CrossRef]
- Vazzana, N.; Santilli, F.; Cuccurullo, C.; Davì, G. Soluble forms of RAGE in internal medicine. Intern. Emerg. Med. 2009, 4, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Grauen Larsen, H.; Marinkovic, G.; Nilsson, P.M.; Nilsson, J.; Engström, G.; Melander, O.; Orho-Melander, M.; Schiopu, A. High Plasma sRAGE (Soluble Receptor for Advanced Glycation End Products) Is Associated With Slower Carotid Intima-Media Thickness Progression and Lower Risk for First-Time Coronary Events and Mortality. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Moriya, S.; Yamazaki, M.; Murakami, H.; Maruyama, K.; Uchiyama, S. Two soluble isoforms of receptors for advanced glycation end products (RAGE) in carotid atherosclerosis: The difference of soluble and endogenous secretory RAGE. J. Stroke Cerebrovasc. Dis. 2014, 23, 2540–2546. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Castagnini, M.; Del Turco, S.; Epistolato, M.C.; Righini, P.; Sangiorgi, G.M.; De Caterina, R.; Tanganelli, P. High plasma levels of the soluble receptor for advanced glycation endproducts in patients with symptomatic carotid atherosclerosis. Eur. J. Clin. Investig. 2009, 39, 1065–1072. [Google Scholar] [CrossRef]
- Reichert, S.; Triebert, U.; Santos, A.N.; Hofmann, B.; Schaller, H.G.; Schlitt, A.; Schulz, S. Soluble form of receptor for advanced glycation end products and incidence of new cardiovascular events among patients with cardiovascular disease. Atherosclerosis 2017, 266, 234–239. [Google Scholar] [CrossRef]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef]
- Pleus, S.; Tytko, A.; Landgraf, R.; Heinemann, L.; Werner, C.; Muller-Wieland, D.; Ziegler, A.G.; Muller, U.A.; Freckmann, G.; Kleinwechter, H.; et al. Definition, Classification, Diagnosis and Differential Diagnosis of Diabetes Mellitus: Update 2023. Exp. Clin. Endocrinol. Diabetes 2024, 132, 112–124. [Google Scholar] [CrossRef]
- Peppa, M.; Uribarri, J.; Vlassara, H. Glucose, Advanced Glycation End Products, and Diabetes Complications: What Is New and What Works. Clin. Diabetes 2003, 21, 186–187. [Google Scholar] [CrossRef]
- Vlassara, H.; Striker, G.E. AGE restriction in diabetes mellitus: A paradigm shift. Nat. Rev. Endocrinol. 2011, 7, 526–539. [Google Scholar] [CrossRef]
- Luft, V.C.; Duncan, B.B.; Schmidt, M.I.; Chambless, L.E.; Pankow, J.S.; Hoogeveen, R.C.; Couper, D.J.; Heiss, G. Carboxymethyl lysine, an advanced glycation end product, and incident diabetes: A case-cohort analysis of the ARIC Study. Diabet. Med. 2016, 33, 1392–1398. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Lv, Y.; Xu, W.; Jiang, G.; Li, Y.; Luo, P.; He, R.; Liu, L. Association of plasma advanced glycation end-products and their soluble receptor with type 2 diabetes among Chinese adults. Diabetes Metab. Res. Rev. 2024, 40, e3735. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; He, C.; Hattori, M.; McEvoy, R.; Zheng, F.; Vlassara, H. Fetal or neonatal low-glycotoxin environment prevents autoimmune diabetes in NOD mice. Diabetes 2003, 52, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Wallenstein, S.; Striker, G.E.; Vlassara, H. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: Association with increased AGER1 expression. Am. J. Pathol. 2007, 170, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.M.; Dong, H.J.; Li, Z.; Cai, W.; Altomonte, J.; Thung, S.N.; Zeng, F.; Fisher, E.A.; Vlassara, H. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes 2002, 51, 2082–2089. [Google Scholar] [CrossRef]
- Sandu, O.; Song, K.; Cai, W.; Zheng, F.; Uribarri, J.; Vlassara, H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes 2005, 54, 2314–2319. [Google Scholar] [CrossRef]
- Garay-Sevilla, M.E.; Rojas, A.; Portero-Otin, M.; Uribarri, J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients 2021, 13, 2802. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef]
- Milordini, G.; Zacco, E.; Percival, M.; Puglisi, R.; Dal Piaz, F.; Temussi, P.; Pastore, A. The Role of Glycation on the Aggregation Properties of IAPP. Front. Mol. Biosci. 2020, 7, 104. [Google Scholar] [CrossRef]
- Abedini, A.; Cao, P.; Plesner, A.; Zhang, J.; He, M.; Derk, J.; Patil, S.A.; Rosario, R.; Lonier, J.; Song, F.; et al. RAGE binds preamyloid IAPP intermediates and mediates pancreatic beta cell proteotoxicity. J. Clin. Investig. 2018, 128, 682–698. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Koska, J.A.-O.; Saremi, A.; Howell, S.; Bahn, G.; De Courten, B.A.-O.; Ginsberg, H.; Beisswenger, P.J.; Reaven, P.D. Advanced Glycation End Products, Oxidation Products, and Incident Cardiovascular Events in Patients With Type 2 Diabetes. Diabetes Care 2018, 41, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Chawla, D.; Fau-Siddarth, M.; Siddarth, M.; Fau-Banerjee, B.D.; Banerjee Bd Fau-Madhu, S.V.; Madhu Sv Fau-Tripathi, A.K.; Tripathi, A.K. A study on serum advanced glycation end products and its association with oxidative stress and paraoxonase activity in type 2 diabetic patients with vascular complications. Clin. Biochem. 2013, 46, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, K.; Nejima, J.; Fau-Takano, T.; Takano, T.; Fau-Ohta, M.; Ohta, M.; Fau-Hashimoto, H.; Hashimoto, H. Increased serum concentrations of advanced glycation end products: A marker of coronary artery disease activity in type 2 diabetic patients. Heart 2001, 85, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, M.; Tsujimoto, N.; Hashimoto, T. Advanced glycation end products in nondiabetic patients with coronary artery disease. Diabetes Care 2001, 24, 1620–1623. [Google Scholar] [CrossRef]
- Willemsen, S.; Hartog, J.W.; Hummel, Y.M.; van Ruijven, M.H.; van der Horst, I.C.; van Veldhuisen, D.J.; Voors, A.A. Tissue advanced glycation end products are associated with diastolic function and aerobic exercise capacity in diabetic heart failure patients. Eur. J. Heart Fail. 2011, 13, 76–82. [Google Scholar] [CrossRef]
- Huijberts, M.S.; Schaper, N.C.; Schalkwijk, C.G. Advanced glycation end products and diabetic foot disease. Diabetes Metab. Res. Rev. 2008, 24 (Suppl. S1), S19–S24. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.R.; Li, P.C.; Feng, B. Advanced glycation end products increase lipids accumulation in macrophages through upregulation of receptor of advanced glycation end products: Increasing uptake, esterification and decreasing efflux of cholesterol. Lipids Health Dis. 2016, 15, 161. [Google Scholar] [CrossRef]
- Borén, J.; Williams, K.J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef]
- Stassen, M.; Müller, C.; Arnold, M.; Hültner, L.; Klein-Hessling, S.; Neudörfl, C.; Reineke, T.; Serfling, E.; Schmitt, E. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-kappa B is decisively involved in the expression of IL-9. J. Immunol. 2001, 166, 4391–4398. [Google Scholar] [CrossRef]
- Motawi, T.M.; Abou-Seif, M.A.; Bader, A.M.; Mahmoud, M.O. Effect of glycemic control on soluble RAGE and oxidative stress in type 2 diabetic patients. BMC Endocr. Disord. 2013, 13, 32. [Google Scholar] [CrossRef]
- McNair, E.; Qureshi, M.; Prasad, K.; Pearce, C. Atherosclerosis and the Hypercholesterolemic AGE-RAGE Axis. Int. J. Angiol. 2016, 25, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, L.; López-Díez, R.; Shekhtman, A.; Ramasamy, R.; Schmidt, A.M. Glycation and a Spark of ALEs (Advanced Lipoxidation End Products)—Igniting RAGE/Diaphanous-1 and Cardiometabolic Disease. Front. Cardiovasc. Med. 2022, 9, 937071. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Yao, H.P.; Sun, Z. N(ε)-(carboxymethyl)lysine promotes lipid uptake of macrophage via cluster of differentiation 36 and receptor for advanced glycation end products. World J. Diabetes 2023, 14, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.G.; Rasmussen, L.M.; Ruoslahti, E. Antibodies against transforming growth factor-beta 1 suppress intimal hyperplasia in a rat model. J. Clin. Investig. 1994, 93, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Bidasee, K.R.; Nallani, K.; Yu, Y.; Cocklin, R.R.; Zhang, Y.; Wang, M.; Dincer, U.D.; Besch, H.R., Jr. Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes 2003, 52, 1825–1836. [Google Scholar] [CrossRef]
- Bidasee, K.R.; Zhang, Y.; Shao, C.H.; Wang, M.; Patel, K.P.; Dincer, U.D.; Besch, H.R., Jr. Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 2004, 53, 463–473. [Google Scholar] [CrossRef]
- Dozio, E.; Vettoretti, S.; Caldiroli, L.; Nerini-Molteni, S.; Tacchini, L.; Ambrogi, F.; Messa, P.; Corsi Romanelli, M.M. Advanced Glycation End Products (AGE) and Soluble Forms of AGE Receptor: Emerging Role as Mortality Risk Factors in CKD. Biomedicines 2020, 8, 638. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.Y.; Huang, C.H.; Wu, C.L.; Wu, H.M.; Chiu, P.F.; Kor, C.T.; Chen, T.H.; Chang, G.D.; Kuo, C.C.; et al. Urinary glycated uromodulin in diabetic kidney disease. Clin. Sci. 2017, 131, 1815–1829. [Google Scholar] [CrossRef]
- Rabbani, N.; Adaikalakoteswari, A.; Larkin, J.R.; Panagiotopoulos, S.; MacIsaac, R.J.; Yue, D.K.; Fulcher, G.R.; Roberts, M.A.; Thomas, M.; Ekinci, E.; et al. Analysis of Serum Advanced Glycation Endproducts Reveals Methylglyoxal-Derived Advanced Glycation MG-H1 Free Adduct Is a Risk Marker in Non-Diabetic and Diabetic Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 24, 152. [Google Scholar] [CrossRef]
- Nakamura, T.; Tsujimoto, T.; Yasuda, K.; Kajio, H.; Ueki, K. Consistently low serum levels of MG-H1 is associated with a lower risk of diabetic kidney disease. J. Clin. Endocrinol. Metab. 2025, dgaf098. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, Y.; Nakaya, I.; Nakayama, K.; Nakayama, M.; Yahata, M.; Soma, J. Methylglyoxal as a prognostic factor in patients with chronic kidney disease. Nephrology 2019, 24, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Berg, A.H.; Zheng, H.; Rhee, E.P.; Allegretti, A.S.; Nigwekar, S.U.; Karumanchi, S.A.; Lash, J.P.; Kalim, S. Glycated Albumin and Adverse Clinical Outcomes in Patients With CKD: A Prospective Cohort Study. Am. J. Kidney Dis. 2024, 84, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, D.M.; Olar, L.E.; Stefan, R.; Veresiu, I.A.; Bala, C.G.; Mircea, P.A.; Roman, G. Fluorophores advanced glycation end products (AGEs)-to-NADH ratio is predictor for diabetic chronic kidney and cardiovascular disease. J. Diabetes Complicat. 2015, 29, 893–897. [Google Scholar] [CrossRef]
- Dalal, M.; Semba, R.D.; Sun, K.; Crasto, C.; Varadhan, R.; Bandinelli, S.; Fink, J.C.; Guralnik, J.M.; Ferrucci, L. Endogenous secretory receptor for advanced glycation end products and chronic kidney disease in the elderly population. Am. J. Nephrol. 2011, 33, 313–318. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Astor, B.C.; Grams, M.E.; Halushka, M.K.; Lazo, M.; Hoogeveen, R.C.; Ballantyne, C.M.; Coresh, J.; Selvin, E. Association of plasma levels of soluble receptor for advanced glycation end products and risk of kidney disease: The Atherosclerosis Risk in Communities study. Nephrol. Dial. Transplant. 2015, 30, 77–83. [Google Scholar] [CrossRef]
- Molinari, P.; Caldiroli, L.; Dozio, E.; Rigolini, R.; Giubbilini, P.; Romanelli, M.M.C.; Messa, P.; Vettoretti, S. AGEs and sRAGE Variations at Different Timepoints in Patients with Chronic Kidney Disease. Antioxidants 2021, 10, 1994. [Google Scholar] [CrossRef]
- Stinghen, A.E.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef]
- Fotheringham, A.K.; Gallo, L.A.; Borg, D.J.; Forbes, J.M. Advanced Glycation End Products (AGEs) and Chronic Kidney Disease: Does the Modern Diet AGE the Kidney? Nutrients 2022, 14, 2675. [Google Scholar] [CrossRef]
- Wang, B.; Vashishth, D. Advanced glycation and glycoxidation end products in bone. Bone 2023, 176, 116880. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, R.; Ewing, S.K.; Vashishth, D.; Semba, R.D.; Schwartz, A.V. Greater Carboxy-Methyl-Lysine Is Associated With Increased Fracture Risk in Type 2 Diabetes. J. Bone Miner. Res. 2022, 37, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Ock, S.Y.; Jin, Y.; Lee, J.S.; Kim, S.H.; Chung, Y. Urinary Pentosidine levels negatively associates with trabecular bone scores in patients with type 2 diabetes mellitus. Osteoporos. Int. 2018, 29, 907–915. [Google Scholar] [CrossRef]
- Kawamura, M.; Masaki, C.; Shibata, Y.; Kondo, Y.; Mukaibo, T.; Miyazaki, T.; Hosokawa, R. Pentosidine correlates with nanomechanical properties of human jaw bone. J. Mech. Behav. Biomed. Mater. 2019, 98, 20–25. [Google Scholar] [CrossRef]
- Shirinezhad, A.; Azarboo, A.; Mafhoumi, A.; Islampanah, M.; Mohammadi, S.; Ghaseminejad-Raeini, A.; Hoveidaei, A.H. Urinary pentosidine as a potential biomarker of impaired bone health: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2025, 24, 6. [Google Scholar] [CrossRef]
- Franke, S.; Sommer, M.; Ruster, C.; Bondeva, T.; Marticke, J.; Hofmann, G.; Hein, G.; Wolf, G. Advanced glycation end products induce cell cycle arrest and proinflammatory changes in osteoarthritic fibroblast-like synovial cells. Arthritis Res. Ther. 2009, 11, R136. [Google Scholar] [CrossRef]
- Sunahori, K.; Yamamura, M.; Yamana, J.; Takasugi, K.; Kawashima, M.; Makino, H. Increased expression of receptor for advanced glycation end products by synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 97–104. [Google Scholar] [CrossRef]
- Drinda, S.; Franke, S.; Canet, C.C.; Petrow, P.; Brauer, R.; Huttich, C.; Stein, G.; Hein, G. Identification of the advanced glycation end products N(epsilon)-carboxymethyllysine in the synovial tissue of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2002, 61, 488–492. [Google Scholar] [CrossRef]
- Drinda, S.; Franke, S.; Ruster, M.; Petrow, P.; Pullig, O.; Stein, G.; Hein, G. Identification of the receptor for advanced glycation end products in synovial tissue of patients with rheumatoid arthritis. Rheumatol. Int. 2005, 25, 411–413. [Google Scholar] [CrossRef]
- Chayanupatkul, M.; Honsawek, S. Soluble receptor for advanced glycation end products (sRAGE) in plasma and synovial fluid is inversely associated with disease severity of knee osteoarthritis. Clin. Biochem. 2010, 43, 1133–1137. [Google Scholar] [CrossRef]
- Najafizadeh, S.R.; Amiri, K.; Moghaddassi, M.; Khanmohammadi, S.; Mirmiranpour, H.; Nakhjavani, M. Advanced glycation end products, advanced oxidation protein products, and ferric reducing ability of plasma in patients with rheumatoid arthritis: A focus on activity scores. Clin. Rheumatol. 2021, 40, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- van den Beukel, M.D.; van Wesemael, T.J.; Hoogslag, A.T.W.; Borggreven, N.V.; Huizinga, T.W.; van der Helm-van Mil, A.H.; Toes, R.E.; van der Woude, D.; Trouw, L.A. Antibodies against advanced glycation end-products and malondialdehyde-acetaldehyde adducts identify a new specific subgroup of hitherto patients with seronegative arthritis with a distinct clinical phenotype and an HLA class II association. RMD Open 2023, 9, e003480. [Google Scholar] [CrossRef] [PubMed]
- Syngle, A.; Vohra, K.; Garg, N.; Kaur, L.; Chand, P. Advanced glycation end-products inhibition improves endothelial dysfunction in rheumatoid arthritis. Int. J. Rheum. Dis. 2012, 15, 45–55. [Google Scholar] [CrossRef]
- Nadali, M.; Lyngfelt, L.; Erlandsson, M.C.; Silfversward, S.T.; Andersson, K.M.E.; Bokarewa, M.I.; Pullerits, R. Low Soluble Receptor for Advanced Glycation End Products Precedes and Predicts Cardiometabolic Events in Women With Rheumatoid Arthritis. Front. Med. 2020, 7, 594622. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Pollet, E.; Palavicini, J.P.; Kellogg, D.L., Jr.; Kraig, E.; Orr, M.E. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Investig. 2022, 132, e158453. [Google Scholar] [CrossRef]
- Akhter, F.; Chen, D.; Akhter, A.; Sosunov, A.A.; Chen, A.; McKhann, G.M.; Yan, S.F.; Yan, S.S. High Dietary Advanced Glycation End Products Impair Mitochondrial and Cognitive Function. J. Alzheimers Dis. 2020, 76, 165–178. [Google Scholar] [CrossRef]
- Krautwald, M.; Münch, G. Advanced glycation end products as biomarkers and gerontotoxins—A basis to explore methylglyoxal-lowering agents for Alzheimer’s disease? Exp. Gerontol. 2010, 45, 744–751. [Google Scholar] [CrossRef]
- Lüth, H.J.; Ogunlade, V.; Kuhla, B.; Kientsch-Engel, R.; Stahl, P.; Webster, J.; Arendt, T.; Münch, G. Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer’s disease brains. Cereb. Cortex 2005, 15, 211–220. [Google Scholar] [CrossRef]
- Srikanth, V.; Maczurek, A.; Phan, T.; Steele, M.; Westcott, B.; Juskiw, D.; Münch, G. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 763–777. [Google Scholar] [CrossRef]
- Guerrero, E.; Vasudevaraju, P.; Hegde, M.L.; Britton, G.B.; Rao, K.S. Recent advances in α-synuclein functions, advanced glycation, and toxicity: Implications for Parkinson’s disease. Mol. Neurobiol. 2013, 47, 525–536. [Google Scholar] [CrossRef]
- Juranek, J.; Ray, R.; Banach, M.; Rai, V. Receptor for advanced glycation end-products in neurodegenerative diseases. Rev. Neurosci. 2015, 26, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Hirano, A.; Hedley-Whyte, E.T.; Dal Canto, M.C.; Nagai, R.; Uchida, K.; Horiuchi, S.; Kawaguchi, M.; Yamamoto, T.; Kobayashi, M. Selective formation of certain advanced glycation end products in spinal cord astrocytes of humans and mice with superoxide dismutase-1 mutation. Acta Neuropathol. 2002, 104, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Damasiewicz-Bodzek, A.; Łabuz-Roszak, B.; Kumaszka, B.; Tyrpień-Golder, K. Carboxymethyllysine and carboxyethyllysine in multiple sclerosis patients. Arch. Med. Sci. 2024, 20, 736–742. [Google Scholar] [CrossRef]
- Beeri, M.S.; Uribarri, J.; Cai, W.; Buchman, A.S.; Haroutunian, V. Human Brain and Serum Advanced Glycation End Products are Highly Correlated: Preliminary Results of Their Role in Alzheimer Disease and Type 2 Diabetes. Endocr. Pract. 2020, 26, 576–577. [Google Scholar] [CrossRef]
- Yaffe, K.; Lindquist, K.; Schwartz, A.V.; Vitartas, C.; Vittinghoff, E.; Satterfield, S.; Simonsick, E.M.; Launer, L.; Rosano, C.; Cauley, J.A.; et al. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology 2011, 77, 1351–1356. [Google Scholar] [CrossRef]
- Xu, X.Y.; Deng, C.Q.; Wang, J.; Deng, X.J.; Xiao, Q.; Li, Y.; He, Q.; Fan, W.H.; Quan, F.Y.; Zhu, Y.P.; et al. Plasma levels of soluble receptor for advanced glycation end products in Alzheimer’s disease. Int. J. Neurosci. 2017, 127, 454–458. [Google Scholar] [CrossRef]
- Münch, G.; Mayer, S.; Michaelis, J.; Hipkiss, A.R.; Riederer, P.; Müller, R.; Neumann, A.; Schinzel, R.; Cunningham, A.M. Influence of advanced glycation end-products and AGE-inhibitors on nucleation-dependent polymerization of beta-amyloid peptide. Biochim. Biophys. Acta 1997, 1360, 17–29. [Google Scholar] [CrossRef]
- Li, X.H.; Lv, B.L.; Xie, J.Z.; Liu, J.; Zhou, X.W.; Wang, J.Z. AGEs induce Alzheimer-like tau pathology and memory deficit via RAGE-mediated GSK-3 activation. Neurobiol. Aging 2012, 33, 1400–1410. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Mondragón-Rodríguez, S.; Perry, G.; Zhu, X.; Moreira, P.I.; Acevedo-Aquino, M.C.; Williams, S. Phosphorylation of tau protein as the link between oxidative stress, mitochondrial dysfunction, and connectivity failure: Implications for Alzheimer’s disease. Oxid. Med. Cell Longev. 2013, 2013, 940603. [Google Scholar] [CrossRef]
- Xue, D.; Zhao, M.; Wang, Y.J.; Wang, L.; Yang, Y.; Wang, S.W.; Zhang, R.; Zhao, Y.; Liu, R.T. A multifunctional peptide rescues memory deficits in Alzheimer’s disease transgenic mice by inhibiting Aβ42-induced cytotoxicity and increasing microglial phagocytosis. Neurobiol. Dis. 2012, 46, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2019, 4, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Rom, S.; Heldt, N.A.; Gajghate, S.; Seliga, A.; Reichenbach, N.L.; Persidsky, Y. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci. Rep. 2020, 10, 7274. [Google Scholar] [CrossRef]
- Shimizu, F.; Sano, Y.; Tominaga, O.; Maeda, T.; Abe, M.A.; Kanda, T. Advanced glycation end-products disrupt the blood-brain barrier by stimulating the release of transforming growth factor-β by pericytes and vascular endothelial growth factor and matrix metalloproteinase-2 by endothelial cells in vitro. Neurobiol. Aging 2013, 34, 1902–1912. [Google Scholar] [CrossRef]
- Li, W.; Maloney, R.E.; Aw, T.Y. High glucose, glucose fluctuation and carbonyl stress enhance brain microvascular endothelial barrier dysfunction: Implications for diabetic cerebral microvasculature. Redox Biol. 2015, 5, 80–90. [Google Scholar] [CrossRef]
- Derk, J.; MacLean, M.; Juranek, J.; Schmidt, A.M. The Receptor for Advanced Glycation Endproducts (RAGE) and Mediation of Inflammatory Neurodegeneration. J. Alzheimers Dis. Park. 2018, 8, 421. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Alam, M.R.; Kamal, M.A.; Seo, K.J.; Singh, L.R. AGE-RAGE axis culminates into multiple pathogenic processes: A central road to neurodegeneration. Front. Mol. Neurosci. 2023, 16, 1155175. [Google Scholar] [CrossRef]
- Waghela, B.N.; Vaidya, F.U.; Ranjan, K.; Chhipa, A.S.; Tiwari, B.S.; Pathak, C. AGE-RAGE synergy influences programmed cell death signaling to promote cancer. Mol. Cell Biochem. 2021, 476, 585–598. [Google Scholar] [CrossRef]
- Fang, Y.; Doyle, M.F.; Chen, J.; Alosco, M.L.; Mez, J.; Satizabal, C.L.; Qiu, W.Q.; Murabito, J.M.; Lunetta, K.L. Association between inflammatory biomarkers and cognitive aging. PLoS ONE 2022, 17, e0274350. [Google Scholar] [CrossRef]
- Mahali, S.; Raviprakash, N.; Raghavendra, P.B.; Manna, S.K. Advanced glycation end products (AGEs) induce apoptosis via a novel pathway: Involvement of Ca2+ mediated by interleukin-8 protein. J. Biol. Chem. 2011, 286, 34903–34913. [Google Scholar] [CrossRef]
- Crisan, M.; Taulescu, M.; Crisan, D.; Cosgarea, R.; Parvu, A.; Catoi, C.; Drugan, T. Expression of advanced glycation end-products on sun-exposed and non-exposed cutaneous sites during the ageing process in humans. PLoS ONE 2013, 8, e75003. [Google Scholar] [CrossRef] [PubMed]
- Gkogkolou, P.; Bohm, M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinol 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Y.; Zhao, C. The effects of advanced glycation end-products on skin and potential anti-glycation strategies. Exp. Dermatol. 2024, 33, e15065. [Google Scholar] [CrossRef]
- Masaki, H.; Okano, Y.; Sakurai, H. Generation of active oxygen species from advanced glycation end-products (AGE) under ultraviolet light A (UVA) irradiation. Biochem. Biophys. Res. Commun. 1997, 235, 306–310. [Google Scholar] [CrossRef]
- Fang, J.; Ouyang, M.; Qu, Y.; Wang, M.; Huang, X.; Lan, J.; Lai, W.; Xu, Q. Advanced Glycation End Products Promote Melanogenesis by Activating NLRP3 Inflammasome in Human Dermal Fibroblasts. J. Investig. Dermatol. 2022, 142, 2591–2602.e2598. [Google Scholar] [CrossRef]
- Martinovic, D.; Tokic, D.; Usljebrka, M.; Lupi-Ferandin, S.; Cigic, L.; Vanjaka Rogosic, L.; Ercegovic, S.; Kontic, M.; Kumric, M.; Rusic, D.; et al. The Association between the Level of Advanced Glycation End Products and Objective Skin Quality Parameters. Life 2023, 13, 256. [Google Scholar] [CrossRef]
- Van Putte, L.; De Schrijver, S.; Moortgat, P. The effects of advanced glycation end products (AGEs) on dermal wound healing and scar formation: A systematic review. Scars Burn. Heal. 2016, 2, 2059513116676828. [Google Scholar] [CrossRef]
- Shaikh-Kader, A.; Houreld, N.N.; Rajendran, N.K.; Abrahamse, H. The link between advanced glycation end products and apoptosis in delayed wound healing. Cell Biochem. Funct. 2019, 37, 432–442. [Google Scholar] [CrossRef]
- Wan, L.; Bai, X.; Zhou, Q.; Chen, C.; Wang, H.; Liu, T.; Xue, J.; Wei, C.; Xie, L. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int. J. Biol. Sci. 2022, 18, 809–825. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, Y.; Zeng, G.; Hu, J.; Li, M.; Tian, M.; Lei, T.; Huang, R. Advanced glycation end products regulate macrophage apoptosis and influence the healing of diabetic foot wound through miR-361-3p/CSF1R and PI3K/AKT pathway. Heliyon 2024, 10, e24598. [Google Scholar] [CrossRef]
- Goova, M.T.; Li, J.; Kislinger, T.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Nowygrod, S.; Wolf, B.M.; Caliste, X.; Yan, S.F.; et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am. J. Pathol. 2001, 159, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Tsai, S.C.; Jheng, Y.H.; Lin, Y.F.; Chen, C.C. Soft-tissue wound healing by anti-advanced glycation end-products agents. J. Dent. Res. 2014, 93, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Papagrigoraki, A.; Del Giglio, M.; Cosma, C.; Maurelli, M.; Girolomoni, G.; Lapolla, A. Advanced Glycation End Products are Increased in the Skin and Blood of Patients with Severe Psoriasis. Acta Derm. Venereol. 2017, 97, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Chen, J.; Wang, S.; Zhang, S.; Li, S.; Guo, S.; Song, P.; Liu, L.; Wang, G.; Gao, T.; et al. Advanced Glycation End Products-Induced Activation of Keratinocytes: A Mechanism Underlying Cutaneous Immune Response in Psoriasis. J. Innate Immun. 2023, 15, 876–892. [Google Scholar] [CrossRef]
- Vasku, V.; Kankova, K.; Vasku, A.; Muzik, J.; Izakovicova Holla, L.; Semradova, V.; Vacha, J. Gene polymorphisms (G82S, 1704G/T, 2184A/G and 2245G/A) of the receptor of advanced glycation end products (RAGE) in plaque psoriasis. Arch. Dermatol. Res. 2002, 294, 127–130. [Google Scholar] [CrossRef]
- Davies, C.A.; Herrick, A.L.; Cordingley, L.; Freemont, A.J.; Jeziorska, M. Expression of advanced glycation end products and their receptor in skin from patients with systemic sclerosis with and without calcinosis. Rheumatology 2009, 48, 876–882. [Google Scholar] [CrossRef]
- Dadoniene, J.; Cypiene, A.; Ryliskyte, L.; Rugiene, R.; Ryliskiene, K.; Laucevicius, A. Skin Autofluorescence in Systemic Sclerosis Is Related to the Disease and Vascular Damage: A Cross-Sectional Analytic Study of Comparative Groups. Dis. Markers 2015, 2015, 837470. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef]
- Hyogo, H.; Yamagishi, S.; Iwamoto, K.; Arihiro, K.; Takeuchi, M.; Sato, T.; Ochi, H.; Nonaka, M.; Nabeshima, Y.; Inoue, M.; et al. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2007, 22, 1112–1119. [Google Scholar] [CrossRef]
- Kimura, Y.; Hyogo, H.; Yamagishi, S.; Takeuchi, M.; Ishitobi, T.; Nabeshima, Y.; Arihiro, K.; Chayama, K. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: Clinical usefulness of AGEs as a biomarker for the attenuation of NASH. J. Gastroenterol. 2010, 45, 750–757. [Google Scholar] [CrossRef]
- Pereira, E.; Paula, D.P.; de Araujo, B.P.; da Fonseca, M.J.M.; Diniz, M.; Daliry, A.; Griep, R.H. Advanced glycation end product: A potential biomarker for risk stratification of non-alcoholic fatty liver disease in ELSA-Brasil study. World J. Gastroenterol. 2021, 27, 4913–4928. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Blardi, P.; Scapellato, C.; Bocchia, M.; Guazzi, G.; Terzuoli, L.; Tabucchi, A.; Silvietti, A.; Lucani, B.; Gioffrè, W.R.; et al. Decreased plasma endogenous soluble RAGE, and enhanced adipokine secretion, oxidative stress and platelet/coagulative activation identify non-alcoholic fatty liver disease among patients with familial combined hyperlipidemia and/or metabolic syndrome. Vascul. Pharmacol. 2015, 72, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Ulukaya, E.; Gul, O.O.; Arabul, M.; Gul, C.B.; Atug, O.; Oral, A.Y.; Aker, S.; Dolar, E. Decreased plasma levels of soluble receptor for advanced glycation endproducts (sRAGE) in patients with nonalcoholic fatty liver disease. Clin. Biochem. 2009, 42, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Priken, K.; Tapia, G.; Cadagan, C.; Quezada, N.; Torres, J.; D’Espessailles, A.; Pettinelli, P. Higher hepatic advanced glycation end products and liver damage markers are associated with nonalcoholic steatohepatitis. Nutr. Res. 2022, 104, 71–81. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Salomone, F.; Kolodkin-Gal, I.; Erez, N.; Buch, A.; Yeshua, H.; Webb, M.; Halpern, Z.; Shibolet, O. Protective role of soluble receptor for advanced glycation end-products in patients with non-alcoholic fatty liver disease. Dig. Liver Dis. 2017, 49, 523–529. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef]
- Hayashi, N.; George, J.; Takeuchi, M.; Fukumura, A.; Toshikuni, N.; Arisawa, T.; Tsutsumi, M. Acetaldehyde-derived advanced glycation end-products promote alcoholic liver disease. PLoS ONE 2013, 8, e70034. [Google Scholar] [CrossRef]
- Litwinowicz, K.; Waszczuk, E.; Kuzan, A.; Bronowicka-Szydełko, A.; Gostomska-Pampuch, K.; Naporowski, P.; Gamian, A. Alcoholic Liver Disease Is Associated with Elevated Plasma Levels of Novel Advanced Glycation End-Products: A Preliminary Study. Nutrients 2022, 14, 5266. [Google Scholar] [CrossRef]
- Li, Y.; Qin, M.; Zhong, W.; Liu, C.; Deng, G.; Yang, M.; Li, J.; Ye, H.; Shi, H.; Wu, C.; et al. RAGE promotes dysregulation of iron and lipid metabolism in alcoholic liver disease. Redox Biol. 2023, 59, 102559. [Google Scholar] [CrossRef]
- Zhu, R.X.; Seto, W.K.; Lai, C.L.; Yuen, M.F. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver 2016, 10, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Moy, K.A.; Jiao, L.; Freedman, N.D.; Weinstein, S.J.; Sinha, R.; Virtamo, J.; Albanes, D.; Stolzenberg-Solomon, R.Z. Soluble receptor for advanced glycation end products and risk of liver cancer. Hepatology 2013, 57, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Nakashima, Y.; Yamakawa, M.; Hori, A.; Seishima, M.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; Nagata, C. Dietary advanced glycation end products and cancer risk in Japan: From the Takayama study. Cancer Sci. 2022, 113, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Hsieh, M.J.; Chou, Y.E.; Fan, W.L.; Yeh, C.B.; Yang, S.F. Effects of RAGE Gene Polymorphisms on the Risk and Progression of Hepatocellular Carcinoma. Medicine 2015, 94, e1396. [Google Scholar] [CrossRef]
- Ito, R.; Ishii, Y.; Wakiyama, S.; Shiba, H.; Fujioka, S.; Misawa, T.; Ishida, Y.; Hano, H.; Yanaga, K. Prognostic significance of receptor for advanced glycation end products expression in hepatocellular carcinoma after hepatectomy. J. Surg. Res. 2014, 192, 503–508. [Google Scholar] [CrossRef]
- Zhang, X.; You, Y.; Liu, Q.; Sun, X.; Chen, W.; Duan, L. Reduced Circulating Soluble Receptor for Advanced Glycation End-products in Chronic Hepatitis B Are Associated with Hepatic Necroinflammation. Inflammation 2022, 45, 2559–2569. [Google Scholar] [CrossRef]
- Smedsrød, B.; Melkko, J.; Araki, N.; Sano, H.; Horiuchi, S. Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem. J. 1997, 322 Pt 2, 567–573. [Google Scholar] [CrossRef]
- Santos, J.C.; Valentim, I.B.; de Araújo, O.R.; Ataide Tda, R.; Goulart, M.O. Development of nonalcoholic hepatopathy: Contributions of oxidative stress and advanced glycation end products. Int. J. Mol. Sci. 2013, 14, 19846–19866. [Google Scholar] [CrossRef]
- Iwamoto, K.; Kanno, K.; Hyogo, H.; Yamagishi, S.; Takeuchi, M.; Tazuma, S.; Chayama, K. Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J. Gastroenterol. 2008, 43, 298–304. [Google Scholar] [CrossRef]
- He, Y.; Zhu, J.; Huang, Y.; Gao, H.; Zhao, Y. Advanced glycation end product (AGE)-induced hepatic stellate cell activation via autophagy contributes to hepatitis C-related fibrosis. Acta Diabetol. 2015, 52, 959–969. [Google Scholar] [CrossRef]
- Hu, X.; Yang, X.; He, Q.; Chen, Q.; Yu, L. Glyoxalase 1 is up-regulated in hepatocellular carcinoma and is essential for HCC cell proliferation. Biotechnol. Lett. 2014, 36, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Hollenbach, M. The Role of Glyoxalase-I (Glo-I), Advanced Glycation Endproducts (AGEs), and Their Receptor (RAGE) in Chronic Liver Disease and Hepatocellular Carcinoma (HCC). Int. J. Mol. Sci. 2017, 18, 2466. [Google Scholar] [CrossRef] [PubMed]
- Gaens, K.H.; Niessen, P.M.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.; Driessen, A.; Wolfs, M.G.; Hofker, M.H.; Bloemen, J.G.; Dejong, C.H.; et al. Endogenous formation of Nε-(carboxymethyl)lysine is increased in fatty livers and induces inflammatory markers in an in vitro model of hepatic steatosis. J. Hepatol. 2012, 56, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Ladea, L.; Zemba, M.; Calancea, M.I.; Călțaru, M.V.; Dragosloveanu, C.D.M.; Coroleucă, R.; Catrina, E.L.; Brezean, I.; Dinu, V. Corneal Epithelial Changes in Diabetic Patients: A Review. Int. J. Mol. Sci. 2024, 25, 3471. [Google Scholar] [CrossRef]
- Rankenberg, J.; Rakete, S.; Wagner, B.D.; Patnaik, J.L.; Henning, C.; Lynch, A.; Glomb, M.A.; Nagaraj, R.H. Advanced glycation end products in human diabetic lens capsules. Exp. Eye Res. 2021, 210, 108704. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.J.; Yu, J.; Wang, H.J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cell Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef]
- Bejarano, E.; Domenech-Bendaña, A.; Avila-Portillo, N.; Rowan, S.; Edirisinghe, S.; Taylor, A. Glycative stress as a cause of macular degeneration. Prog. Retin. Eye Res. 2024, 101, 101260. [Google Scholar] [CrossRef]
- Khare, K.; Mendonca, T.; Rodrigues, G.; Kamath, M.; Hegde, A.; Nayak, S.; Kamath, A.; Kamath, S. Aldose reductase and glutathione in senile cataract nucleus of diabetics and non-diabetics. Int. Ophthalmol. 2023, 43, 3673–3680. [Google Scholar] [CrossRef]
- Sharma, Y.; Saxena, S.; Saxena, A.; Mishra, A.; Natu, S.M. Interrelationship of elevated serum Advanced Glycation End-product levels and malnutrition (Subjective Global Assessment) scores with the severity of retinopathy in type II diabetes. Clin. Nutr. ESPEN 2015, 10, e42–e48. [Google Scholar] [CrossRef]
- Padayatti, P.S.; Ng, A.S.; Uchida, K.; Glomb, M.A.; Nagaraj, R.H. Argpyrimidine, a blue fluorophore in human lens proteins: High levels in brunescent cataractous lenses. Invest. Ophthalmol. Vis. Sci. 2001, 42, 1299–1304. [Google Scholar]
- Kim, J.; Kim, O.S.; Kim, C.S.; Sohn, E.; Jo, K.; Kim, J.S. Accumulation of argpyrimidine, a methylglyoxal-derived advanced glycation end product, increases apoptosis of lens epithelial cells both in vitro and in vivo. Exp. Mol. Med. 2012, 44, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cross, S.D.; Stanton, J.B.; Marmorstein, A.D.; Le, Y.Z.; Marmorstein, L.Y. Early AMD-like defects in the RPE and retinal degeneration in aged mice with RPE-specific deletion of Atg5 or Atg7. Mol. Vis. 2017, 23, 228–241. [Google Scholar] [PubMed]
- Bejarano, E.; Taylor, A. Too sweet: Problems of protein glycation in the eye. Exp. Eye Res. 2019, 178, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kandarakis, S.A.; Piperi, C.; Moschonas, D.P.; Korkolopoulou, P.; Papalois, A.; Papavassiliou, A.G. Dietary glycotoxins induce RAGE and VEGF up-regulation in the retina of normal rats. Exp. Eye Res. 2015, 137, 1–10. [Google Scholar] [CrossRef]
- Ni, J.; Yuan, X.; Gu, J.; Yue, X.; Gu, X.; Nagaraj, R.H.; Crabb, J.W. Plasma protein pentosidine and carboxymethyllysine, biomarkers for age-related macular degeneration. Mol. Cell Proteom. 2009, 8, 1921–1933. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Wang, Q.; Li, Y.; Wei, C.; Xie, L. Mechanistic investigations of diabetic ocular surface diseases. Front. Endocrinol. 2022, 13, 1079541. [Google Scholar] [CrossRef]
- Zicari, A.M.; Zicari, A.; Nebbioso, M.; Mari, E.; Celani, C.; Lollobrigida, V.; Cesoni Marcelli, A.; Occasi, F.; Duse, M. High-mobility group box-1 (HMGB-1) and serum soluble receptor for advanced glycation end products (sRAGE) in children affected by vernal keratoconjunctivitis. Pediatr. Allergy Immunol. 2014, 25, 57–63. [Google Scholar] [CrossRef]
- Cooksley, G.; Nam, M.H.; Nahomi, R.B.; Rankenberg, J.; Smith, A.J.O.; Wormstone, Y.M.; Wormstone, I.M.; Nagaraj, R.H. Lens capsule advanced glycation end products induce senescence in epithelial cells: Implications for secondary cataracts. Aging Cell 2024, 23, e14249. [Google Scholar] [CrossRef]
- Kandarakis, S.A.; Piperi, C.; Topouzis, F.; Papavassiliou, A.G. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog. Retin. Eye Res. 2014, 42, 85–102. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Oshitari, T. Advanced Glycation End-Products and Diabetic Neuropathy of the Retina. Int. J. Mol. Sci. 2023, 24, 2927. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhengran, L.; Yingli, L.; Tong, W.; Liyang, C.; Xi, G.; Xiongyi, Y.; Mingzhe, C.; Guoguo, Y. The contribution of adiponectin to diabetic retinopathy progression: Association with the AGEs-RAGE pathway. Heliyon 2024, 10, e36111. [Google Scholar] [CrossRef] [PubMed]
- Machahua, C.; Montes-Worboys, A.; Llatjos, R.; Escobar, I.; Dorca, J.; Molina-Molina, M.; Vicens-Zygmunt, V. Increased AGE-RAGE ratio in idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Tochino, Y.; Kyoh, S.; Ichimaru, Y.; Asai, K.; Hirata, K. Potential roles of pentosidine in age-related and disease-related impairment of pulmonary functions in patients with asthma. J. Allergy Clin. Immunol. 2011, 127, 899–904. [Google Scholar] [CrossRef]
- Manichaikul, A.; Sun, L.; Borczuk, A.C.; Onengut-Gumuscu, S.; Farber, E.A.; Mathai, S.K.; Zhang, W.; Raghu, G.; Kaufman, J.D.; Hinckley-Stukovsky, K.D.; et al. Plasma Soluble Receptor for Advanced Glycation End Products in Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2017, 14, 628–635. [Google Scholar] [CrossRef]
- He, M.; Kubo, H.; Ishizawa, K.; Hegab, A.E.; Yamamoto, Y.; Yamamoto, H.; Yamaya, M. The role of the receptor for advanced glycation end-products in lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L1427–L1436. [Google Scholar] [CrossRef]
- Hunt, W.R.; Helfman, B.R.; McCarty, N.A.; Hansen, J.M. Advanced glycation end products are elevated in cystic fibrosis-related diabetes and correlate with worse lung function. J. Cyst. Fibros. 2016, 15, 681–688. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, Y.F.; Zhao, Y.; Xu, D.F.; Wang, Y.; Xu, C.F.; Dong, W.W.; Zhu, X.Y.; Ding, N.; Jiang, L.; et al. Upregulation of Matrix Metalloproteinase-9 Protects against Sepsis-Induced Acute Lung Injury via Promoting the Release of Soluble Receptor for Advanced Glycation End Products. Oxid. Med. Cell Longev. 2021, 2021, 8889313. [Google Scholar] [CrossRef]
- Sellegounder, D.; Zafari, P.; Rajabinejad, M.; Taghadosi, M.; Kapahi, P. Advanced glycation end products (AGEs) and its receptor, RAGE, modulate age-dependent COVID-19 morbidity and mortality. A review and hypothesis. Int. Immunopharmacol. 2021, 98, 107806. [Google Scholar] [CrossRef]
- Rojas, A.; Lindner, C.; Gonzalez, I.; Morales, M.A. Advanced-glycation end-products axis: A contributor to the risk of severe illness from COVID-19 in diabetes patients. World J. Diabetes 2021, 12, 590–602. [Google Scholar] [CrossRef]
- Saputra, G.N.R.; Yudhawati, R.; Fitriah, M. Association of soluble receptor for advanced glycation end-products (sRAGE) serum on COVID-19 severity: A cross-sectional study. Ann. Med. Surg. 2022, 74, 103303. [Google Scholar] [CrossRef] [PubMed]
- Matthay, Z.A.; Fields, A.T.; Wick, K.D.; Jones, C.; Lane, H.C.; Herrera, K.; Nunez-Garcia, B.; Gennatas, E.; Hendrickson, C.M.; Kornblith, A.E.; et al. Association of SARS-CoV-2 nucleocapsid viral antigen and the receptor for advanced glycation end products with development of severe disease in patients presenting to the emergency department with COVID-19. Front. Immunol. 2023, 14, 1130821. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Khan, M.S.; Ahmad, S. The in vivo and in vitro approaches for establishing a link between advanced glycation end products and lung cancer. J. Cell Biochem. 2018, 119, 9099–9109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Yu, W.; Ma, L.; Ji, X.; Xiao, W. Expression of the receptor for advanced glycation end-products and frequency of polymorphism in lung cancer. Oncol. Lett. 2015, 10, 51–60. [Google Scholar] [CrossRef]
- Jing, R.; Cui, M.; Wang, J.; Wang, H. Receptor for advanced glycation end products (RAGE) soluble form (sRAGE): A new biomarker for lung cancer. Neoplasma 2010, 57, 55–61. [Google Scholar] [CrossRef]
- Bartling, B.; Demling, N.; Silber, R.E.; Simm, A. Proliferative stimulus of lung fibroblasts on lung cancer cells is impaired by the receptor for advanced glycation end-products. Am. J. Respir. Cell Mol. Biol. 2006, 34, 83–91. [Google Scholar] [CrossRef]
- Chen, M.C.; Chen, K.C.; Chang, G.C.; Lin, H.; Wu, C.C.; Kao, W.H.; Teng, C.J.; Hsu, S.L.; Yang, T.Y. RAGE acts as an oncogenic role and promotes the metastasis of human lung cancer. Cell Death Dis. 2020, 11, 265. [Google Scholar] [CrossRef]
- Monteiro, C.; Miarka, L.; Perea-Garcia, M.; Priego, N.; Garcia-Gomez, P.; Alvaro-Espinosa, L.; de Pablos-Aragoneses, A.; Yebra, N.; Retana, D.; Baena, P.; et al. Stratification of radiosensitive brain metastases based on an actionable S100A9/RAGE resistance mechanism. Nat. Med. 2022, 28, 752–765. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Sebeková, K.; Somoza, V.; Jarcusková, M.; Heidland, A.; Podracká, L. Plasma advanced glycation end products are decreased in obese children compared with lean controls. Int. J. Pediatr. Obes. 2009, 4, 112–118. [Google Scholar] [CrossRef]
- Turki Jalil, A.; Alameri, A.A.; Iqbal Doewes, R.; El-Sehrawy, A.A.; Ahmad, I.; Ramaiah, P.; Kadhim, M.M.; Kzar, H.H.; Sivaraman, R.; Romero-Parra, R.M.; et al. Circulating and dietary advanced glycation end products and obesity in an adult population: A paradox of their detrimental effects in obesity. Front. Endocrinol. 2022, 13, 966590. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Woodward, M.; Tripp, E.; Goldberg, L.; Pyzik, R.; Yee, K.; Tansman, L.; Chen, X.; Mani, V.; et al. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: A link between healthy and unhealthy obesity? J. Clin. Endocrinol. Metab. 2015, 100, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Gaens, K.H.; Goossens, G.H.; Niessen, P.M.; van Greevenbroek, M.M.; van der Kallen, C.J.; Niessen, H.W.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.; Blaak, E.E.; et al. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Sohouli, M.H.; Sharifi-Zahabi, E.; Lari, A.; Fatahi, S.; Shidfar, F. The impact of low advanced glycation end products diet on obesity and related hormones: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 22194. [Google Scholar] [CrossRef]
- Corica, D.; Aversa, T.; Ruggeri, R.M.; Cristani, M.; Alibrandi, A.; Pepe, G.; De Luca, F.; Wasniewska, M. Could AGE/RAGE-Related Oxidative Homeostasis Dysregulation Enhance Susceptibility to Pathogenesis of Cardio-Metabolic Complications in Childhood Obesity? Front. Endocrinol. 2019, 10, 426. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, M.; Huang, K.; Yao, Z.; Rao, P.; Cai, X.; Xiao, J. Advanced glycation end products inhibit the osteogenic differentiation potential of adipose-derived stem cells by modulating Wnt/β-catenin signalling pathway via DNA methylation. Cell Prolif. 2020, 53, e12834. [Google Scholar] [CrossRef]
- Kume, S.; Kato, S.; Yamagishi, S.; Inagaki, Y.; Ueda, S.; Arima, N.; Okawa, T.; Kojiro, M.; Nagata, K. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J. Bone Miner. Res. 2005, 20, 1647–1658. [Google Scholar] [CrossRef]
- Hurtado Del Pozo, C.; Ruiz, H.H.; Arivazhagan, L.; Aranda, J.F.; Shim, C.; Daya, P.; Derk, J.; MacLean, M.; He, M.; Frye, L.; et al. A Receptor of the Immunoglobulin Superfamily Regulates Adaptive Thermogenesis. Cell Rep. 2019, 28, 773–791.e777. [Google Scholar] [CrossRef]
- Espiritu, D.J.; Huang, Z.H.; Zhao, Y.; Mazzone, T. Hyperglycemia and advanced glycosylation end products suppress adipocyte apoE expression: Implications for adipocyte triglyceride metabolism. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E615–E623. [Google Scholar] [CrossRef]
- Strieder-Barboza, C.; Baker, N.A.; Flesher, C.G.; Karmakar, M.; Neeley, C.K.; Polsinelli, D.; Dimick, J.B.; Finks, J.F.; Ghaferi, A.A.; Varban, O.A.; et al. Advanced glycation end-products regulate extracellular matrix-adipocyte metabolic crosstalk in diabetes. Sci. Rep. 2019, 9, 19748. [Google Scholar] [CrossRef]
- Ruiz, H.H.; Nguyen, A.; Wang, C.; He, L.; Li, H.; Hallowell, P.; McNamara, C.; Schmidt, A.M. AGE/RAGE/DIAPH1 axis is associated with immunometabolic markers and risk of insulin resistance in subcutaneous but not omental adipose tissue in human obesity. Int. J. Obes. 2021, 45, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Monden, M.; Koyama, H.; Otsuka, Y.; Morioka, T.; Mori, K.; Shoji, T.; Mima, Y.; Motoyama, K.; Fukumoto, S.; Shioi, A.; et al. Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: Involvement of Toll-like receptor 2. Diabetes 2013, 62, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Zhong, Y.; Wu, Y.; Zhang, Z.; Yan, Z.; Liu, B.; Wang, W. Unveiling the dynamic processes of dietary advanced glycation end-products (dAGEs) in absorption, accumulation, and gut microbiota metabolism. Food Funct. 2024, 15, 9024–9036. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Radjabzadeh, D.; Medina-Gomez, C.; Voortman, T.; van Meurs, J.B.J.; Ikram, M.A.; Uitterlinden, A.G.; Kraaij, R.; Zillikens, M.C. Advanced Glycation End Products (AGEs) in Diet and Skin in Relation to Stool Microbiota: The Rotterdam Study. Nutrients 2023, 15, 2567. [Google Scholar] [CrossRef]
- Xu, D.; Li, L.; Zhang, X.; Yao, H.; Yang, M.; Gai, Z.; Li, B.; Zhao, D. Degradation of Peptide-Bound Maillard Reaction Products in Gastrointestinal Digests of Glyoxal-Glycated Casein by Human Colonic Microbiota. J. Agric. Food Chem. 2019, 67, 12094–12104. [Google Scholar] [CrossRef]
- Hellwig, M.; Auerbach, C.; Müller, N.; Samuel, P.; Kammann, S.; Beer, F.; Gunzer, F.; Henle, T. Metabolization of the Advanced Glycation End Product N-ε-Carboxymethyllysine (CML) by Different Probiotic E. coli Strains. J. Agric. Food Chem. 2019, 67, 1963–1972. [Google Scholar] [CrossRef]
- Hellwig, M.; Bunzel, D.; Huch, M.; Franz, C.M.; Kulling, S.E.; Henle, T. Stability of Individual Maillard Reaction Products in the Presence of the Human Colonic Microbiota. J. Agric. Food Chem. 2015, 63, 6723–6730. [Google Scholar] [CrossRef]
- Moura, F.A.; Goulart, M.O.F.; Campos, S.B.G.; da Paz Martins, A.S. The Close Interplay of Nitro-Oxidative Stress, Advanced Glycation end Products and Inflammation in Inflammatory Bowel Diseases. Curr. Med. Chem. 2020, 27, 2059–2076. [Google Scholar] [CrossRef]
- Kato, S.; Itoh, K.; Ochiai, M.; Iwai, A.; Park, Y.; Hata, S.; Takeuchi, K.; Ito, M.; Imaki, J.; Miura, S.; et al. Increased pentosidine, an advanced glycation end-product, in urine and tissue reflects disease activity in inflammatory bowel diseases. J. Gastroenterol. Hepatol. 2008, 23 (Suppl. S2), S140–S145. [Google Scholar] [CrossRef]
- Pompili, S.; Vetuschi, A.; Latella, G.; Smakaj, A.; Sferra, R.; Cappariello, A. PPAR-Gamma Orchestrates EMT, AGE, and Cellular Senescence Pathways in Colonic Epithelium and Restrains the Progression of IBDs. Int. J. Mol. Sci. 2023, 24, 8952. [Google Scholar] [CrossRef]
- Luceri, C.; Bigagli, E.; Agostiniani, S.; Giudici, F.; Zambonin, D.; Scaringi, S.; Ficari, F.; Lodovici, M.; Malentacchi, C. Analysis of Oxidative Stress-Related Markers in Crohn’s Disease Patients at Surgery and Correlations with Clinical Findings. Antioxidants 2019, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; D’Ambrosio, M.; Cinci, L.; Fiorindi, C.; Agostiniani, S.; Bruscoli, E.; Nannoni, A.; Lodovici, M.; Scaringi, S.; Giudici, F.; et al. Impact of Preoperative Immunonutrition on Oxidative Stress and Gut Barrier Function in Surgical Patients with Crohn’s Disease. Nutrients 2023, 15, 882. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Vanoli, A.; Klersy, C.; Imbesi, V.; Boccaccio, V.; Manca, R.; Betti, E.; Cangemi, G.C.; Strada, E.; Besio, R.; et al. Role of the advanced glycation end products receptor in Crohn’s disease inflammation. World J. Gastroenterol. 2013, 19, 8269–8281. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yang, W.; Cao, Y.; Cao, X.; Zhang, Y.; Yao, L.; Cao, Q. The contribution of dietary advanced glycation end-products and genetic risk in the development of inflammatory bowel disease: A prospective cohort study. Aliment. Pharmacol. Ther. 2024, 60, 1075–1086. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Imbesi, V.; Betti, E.; Boccaccio, V.; Kruzliak, P.; Gallia, A.; Cangemi, G.C.; Maffe, G.C.; Vanoli, A.; Merante, S.; et al. The Circulating Level of Soluble Receptor for Advanced Glycation End Products Displays Different Patterns in Ulcerative Colitis and Crohn’s Disease: A Cross-Sectional Study. Dig. Dis. Sci. 2015, 60, 2327–2337. [Google Scholar] [CrossRef]
- Sakellariou, S.; Fragkou, P.; Levidou, G.; Gargalionis, A.N.; Piperi, C.; Dalagiorgou, G.; Adamopoulos, C.; Saetta, A.; Agrogiannis, G.; Theohari, I.; et al. Clinical significance of AGE-RAGE axis in colorectal cancer: Associations with glyoxalase-I, adiponectin receptor expression and prognosis. BMC Cancer 2016, 16, 174. [Google Scholar] [CrossRef]
- Mao, Z.; Baker, J.R.; Takeuchi, M.; Hyogo, H.; Tjønneland, A.; Eriksen, A.K.; Severi, G.; Rothwell, J.; Laouali, N.; Katzke, V.; et al. Prediagnostic serum glyceraldehyde-derived advanced glycation end products and mortality among colorectal cancer patients. Int. J. Cancer 2023, 152, 2257–2268. [Google Scholar] [CrossRef]
- Aglago, E.K.; Rinaldi, S.; Freisling, H.; Jiao, L.; Hughes, D.J.; Fedirko, V.; Schalkwijk, C.G.; Weiderpass, E.; Dahm, C.C.; Overvad, K.; et al. Soluble Receptor for Advanced Glycation End-products (sRAGE) and Colorectal Cancer Risk: A Case-Control Study Nested within a European Prospective Cohort. Cancer Epidemiol. Biomark. Prev. 2021, 30, 182–192. [Google Scholar] [CrossRef]
- Chen, L.; Duan, Z.; Tinker, L.; Sangi-Haghpeykar, H.; Strickler, H.; Ho, G.Y.; Gunter, M.J.; Rohan, T.; Logsdon, C.; White, D.L.; et al. A prospective study of soluble receptor for advanced glycation end-products and colorectal cancer risk in postmenopausal women. Cancer Epidemiol. 2016, 42, 115–123. [Google Scholar] [CrossRef]
- Li, J.; Roshelli Baker, J.; Aglago, E.K.; Zhao, Z.; Jiao, L.; Freisling, H.; Hughes, D.J.; Eriksen, A.K.; Tjønneland, A.; Severi, G.; et al. Pre-diagnostic plasma advanced glycation end-products and soluble receptor for advanced glycation end-products and mortality in colorectal cancer patients. Int. J. Cancer 2024, 155, 1982–1995. [Google Scholar] [CrossRef]
- Chiavarina, B.; Nokin, M.J.; Bellier, J.; Durieux, F.; Bletard, N.; Sherer, F.; Lovinfosse, P.; Peulen, O.; Verset, L.; Dehon, R.; et al. Methylglyoxal-Mediated Stress Correlates with High Metabolic Activity and Promotes Tumor Growth in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 213. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, R.; Nugent, M.; Cai, W.; Nadkarni, G.N.; Chaves, L.D.; Abyad, S.; Honan, A.M.; Thomas, S.A.; Zheng, W.; Valiyaparambil, S.A.; et al. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS ONE 2017, 12, e0184789. [Google Scholar] [CrossRef] [PubMed]
- Seiquer, I.; Rubio, L.A.; Peinado, M.J.; Delgado-Andrade, C.; Navarro, M.P. Maillard reaction products modulate gut microbiota composition in adolescents. Mol. Nutr. Food Res. 2014, 58, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, W.; Yu, J.; Liu, H.; He, S.; Zhu, L.; Xu, J. Dietary Advanced Glycation End Products Shift the Gut Microbiota Composition and Induce Insulin Resistance in Mice. Diabetes Metab. Syndr. Obes. 2022, 15, 427–437. [Google Scholar] [CrossRef]
- Bui, T.P.; Ritari, J.; Boeren, S.; de Waard, P.; Plugge, C.M.; de Vos, W.M. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 2015, 6, 10062. [Google Scholar] [CrossRef]
- Ochoa-Cortes, F.; Turco, F.; Linan-Rico, A.; Soghomonyan, S.; Whitaker, E.; Wehner, S.; Cuomo, R.; Christofi, F.L. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 433–449. [Google Scholar] [CrossRef]
- Deng, R.; Wu, H.; Ran, H.; Kong, X.; Hu, L.; Wang, X.; Su, Q. Glucose-derived AGEs promote migration and invasion of colorectal cancer by up-regulating Sp1 expression. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1065–1074. [Google Scholar] [CrossRef]
- Yu, G.; He, J.; Gao, Z.; Fu, L.; Zhang, Q. Protein-bound AGEs derived from methylglyoxal induce pro-inflammatory response and barrier integrity damage in epithelial cells by disrupting the retinol metabolism. Food Funct. 2024, 15, 11650–11666. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Piperi, C.; Patsouris, E.; Korkolopoulou, P.; Panidis, D.; Pawelczyk, L.; Papavassiliou, A.G.; Duleba, A.J. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem. Cell Biol. 2007, 127, 581–589. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Katsikis, I.; Piperi, C.; Kandaraki, E.; Piouka, A.; Papavassiliou, A.G.; Panidis, D. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2008, 69, 634–641. [Google Scholar] [CrossRef]
- Merhi, Z. Advanced glycation end products and their relevance in female reproduction. Hum. Reprod. 2014, 29, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Fujii, E.Y.; Nakayama, M.; Nakagawa, A. Concentrations of receptor for advanced glycation end products, VEGF and CML in plasma, follicular fluid, and peritoneal fluid in women with and without endometriosis. Reprod. Sci. 2008, 15, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Fujii, E.Y.; Nakayama, M. The measurements of RAGE, VEGF, and AGEs in the plasma and follicular fluid of reproductive women: The influence of aging. Fertil. Steril. 2010, 94, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Jinno, M.; Takeuchi, M.; Watanabe, A.; Teruya, K.; Hirohama, J.; Eguchi, N.; Miyazaki, A. Advanced glycation end-products accumulation compromises embryonic development and achievement of pregnancy by assisted reproductive technology. Hum. Reprod. 2011, 26, 604–610. [Google Scholar] [CrossRef]
- Thornton, K.; Merhi, Z.; Jindal, S.; Goldsammler, M.; Charron, M.J.; Buyuk, E. Dietary Advanced Glycation End Products (AGEs) could alter ovarian function in mice. Mol. Cell Endocrinol. 2020, 510, 110826. [Google Scholar] [CrossRef]
- Karimi, J.; Goodarzi, M.T.; Tavilani, H.; Khodadadi, I.; Amiri, I. Relationship between advanced glycation end products and increased lipid peroxidation in semen of diabetic men. Diabetes Res. Clin. Pract. 2011, 91, 61–66. [Google Scholar] [CrossRef]
- Mallidis, C.; Agbaje, I.M.; Rogers, D.A.; Glenn, J.V.; Pringle, R.; Atkinson, A.B.; Steger, K.; Stitt, A.W.; McClure, N. Advanced glycation end products accumulate in the reproductive tract of men with diabetes. Int. J. Androl. 2009, 32, 295–305. [Google Scholar] [CrossRef]
- Mallidis, C.; Agbaje, I.; Rogers, D.; Glenn, J.; McCullough, S.; Atkinson, A.B.; Steger, K.; Stitt, A.; McClure, N. Distribution of the receptor for advanced glycation end products in the human male reproductive tract: Prevalence in men with diabetes mellitus. Hum. Reprod. 2007, 22, 2169–2177. [Google Scholar] [CrossRef]
- Chen, M.C.; Lin, J.A.; Lin, H.T.; Chen, S.Y.; Yen, G.C. Potential effect of advanced glycation end products (AGEs) on spermatogenesis and sperm quality in rodents. Food Funct. 2019, 10, 3324–3333. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, F.; Rahmani, M.; Tavalaee, M.; Abedpoor, N.; Taki, M.; Ghaedi, K.; Nasr-Esfahani, M.H. Effect of Different High-Fat and Advanced Glycation End-Products Diets in Obesity and Diabetes-Prone C57BL/6 Mice on Sperm Function. Int. J. Fertil. Steril. 2021, 15, 226–233. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Ji, H.; Ji, Y.; Yang, J.; Huang, J.; Sun, D. Involvement of hypoxia-inducible factor-1alpha in the oxidative stress induced by advanced glycation end products in murine Leydig cells. Toxicol. Vitr. 2016, 32, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Qi, Y.W.; Hu, C.Y.; Chen, S.H.; Liu, Y. Advanced glycation end products inhibit testosterone secretion by rat Leydig cells by inducing oxidative stress and endoplasmic reticulum stress. Int. J. Mol. Med. 2016, 38, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Darkwah, E.K. Advanced Glycation End Products in Health and Disease. Microorganisms 2022, 10, 1848. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct. 2011, 2, 289–301. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G. Diabetes and Pancreatic Cancer-A Dangerous Liaison Relying on Carbonyl Stress. Cancers 2021, 13, 313. [Google Scholar] [CrossRef]
- Malik, N.S.; Meek, K.M. The inhibition of sugar-induced structural alterations in collagen by aspirin and other compounds. Biochem. Biophys. Res. Commun. 1994, 199, 683–686. [Google Scholar] [CrossRef]
- Caballero, F.; Gerez, E.; Batlle, A.; Vazquez, E. Preventive aspirin treatment of streptozotocin induced diabetes: Blockage of oxidative status and revertion of heme enzymes inhibition. Chem. Biol. Interact. 2000, 126, 215–225. [Google Scholar] [CrossRef]
- Shastri, G.V.; Thomas, M.; Victoria, A.J.; Selvakumar, R.; Kanagasabapathy, A.S.; Thomas, K.; Lakshmi. Effect of aspirin and sodium salicylate on cataract development in diabetic rats. Indian J. Exp. Biol. 1998, 36, 651–657. [Google Scholar]
- Indurthi, V.S.; Leclerc, E.; Vetter, S.W. Calorimetric investigation of diclofenac drug binding to a panel of moderately glycated serum albumins. Eur. J. Pharm. Sci. 2014, 59, 58–68. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sulochana, K.N.; Punitham, R. Two new functions of inositol in the eye lens: Antioxidation and antiglycation and possible mechanisms. Indian. J. Biochem. Biophys. 1999, 36, 129–133. [Google Scholar]
- Sulochana, K.N.; Ramprasad, S.; Coral, K.; Lakshmi, S.; Punitham, R.; Narayanasamy, A.; Ramakrishnan, S. Glycation and glycoxidation studies in vitro on isolated human vitreous collagen. Med. Sci. Monit. 2003, 9, Br220–Br224. [Google Scholar] [PubMed]
- Sanchis, P.; Rivera, R.; Berga, F.; Fortuny, R.; Adrover, M.; Costa-Bauza, A.; Grases, F.; Masmiquel, L. Phytate Decreases Formation of Advanced Glycation End-Products in Patients with Type II Diabetes: Randomized Crossover Trial. Sci. Rep. 2018, 8, 9619. [Google Scholar] [CrossRef] [PubMed]
- Chilukuri, H.; Kulkarni, M.J.; Fernandes, M. Revisiting amino acids and peptides as anti-glycation agents. MedChemComm 2018, 9, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xiaoqin, L.; Potts, B.; Strauch, C.M.; Nemet, I.; Monnier, V.M. Topical application of L-arginine blocks advanced glycation by ascorbic acid in the lens of hSVCT2 transgenic mice. Mol. Vis. 2011, 17, 2221–2227. [Google Scholar]
- Nenna, A.; Nappi, F.; Avtaar Singh, S.S.; Sutherland, F.W.; Di Domenico, F.; Chello, M.; Spadaccio, C. Pharmacologic Approaches Against Advanced Glycation End Products (AGEs) in Diabetic Cardiovascular Disease. Res. Cardiovasc. Med. 2015, 4, e26949. [Google Scholar] [CrossRef]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M.S.; Alouffi, S.; Khan, S.; Khan, M.; Akashah, R.; Faisal, M.; Shahab, U. Gold Nanoparticle-Bioconjugated Aminoguanidine Inhibits Glycation Reaction: An In Vivo Study in a Diabetic Animal Model. Biomed. Res. Int. 2021, 2021, 5591851. [Google Scholar] [CrossRef]
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial. J. Alzheimers Dis. 2020, 78, 989–1010. [Google Scholar] [CrossRef]
- Alkhalaf, A.; Kleefstra, N.; Groenier, K.H.; Bilo, H.J.; Gans, R.O.; Heeringa, P.; Scheijen, J.L.; Schalkwijk, C.G.; Navis, G.J.; Bakker, S.J. Effect of benfotiamine on advanced glycation endproducts and markers of endothelial dysfunction and inflammation in diabetic nephropathy. PLoS ONE 2012, 7, e40427. [Google Scholar] [CrossRef]
- Winkler, G.; Pál, B.; Nagybéganyi, E.; Ory, I.; Porochnavec, M.; Kempler, P. Effectiveness of different benfotiamine dosage regimens in the treatment of painful diabetic neuropathy. Arzneimittelforschung 1999, 49, 220–224. [Google Scholar] [CrossRef][Green Version]
- Miyata, T.; Ueda, Y.; Asahi, K.; Izuhara, Y.; Inagi, R.; Saito, A.; de Strihou, C.V.Y.; Kurokawa, K. Mechanism of the inhibitory effect of OPB-9195 [(+/-)-2-isopropylidenehydrazono-4-oxo-thiazolidin-5-yla cetanilide] on advanced glycation end product and advanced lipoxidation end product formation. J. Am. Soc. Nephrol. 2000, 11, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Adrover, M.; Vilanova, B.; Frau, J.; Muñoz, F.; Donoso, J. The pyridoxamine action on Amadori compounds: A reexamination of its scavenging capacity and chelating effect. Bioorg Med. Chem. 2008, 16, 5557–5569. [Google Scholar] [CrossRef] [PubMed]
- Ramis, R.; Ortega-Castro, J.; Caballero, C.; Casasnovas, R.; Cerrillo, A.; Vilanova, B.; Adrover, M.; Frau, J. How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of Its Primary Antioxidant Activity. Antioxidants 2019, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, M.D.G.; Houben, A.; Scheijen, J.; Linkens, A.M.A.; Niessen, P.M.; Simons, N.; Hanssen, N.M.J.; Kusters, Y.; Eussen, S.; Miyata, T.; et al. Pyridoxamine reduces methylglyoxal and markers of glycation and endothelial dysfunction, but does not improve insulin sensitivity or vascular function in abdominally obese individuals: A randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2023, 25, 1280–1291. [Google Scholar] [CrossRef]
- Itokawa, M.; Miyashita, M.; Arai, M.; Dan, T.; Takahashi, K.; Tokunaga, T.; Ishimoto, K.; Toriumi, K.; Ichikawa, T.; Horiuchi, Y.; et al. Pyridoxamine: A novel treatment for schizophrenia with enhanced carbonyl stress. Psychiatry Clin. Neurosci. 2018, 72, 35–44. [Google Scholar] [CrossRef]
- Williams, M.E.; Bolton, W.K.; Khalifah, R.G.; Degenhardt, T.P.; Schotzinger, R.J.; McGill, J.B. Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am. J. Nephrol. 2007, 27, 605–614. [Google Scholar] [CrossRef]
- Booth, A.A.; Khalifah, R.G.; Todd, P.; Hudson, B.G. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs). Novel inhibition of post-Amadori glycation pathways. J. Biol. Chem. 1997, 272, 5430–5437. [Google Scholar] [CrossRef]
- Booth, A.A.; Khalifah, R.G.; Hudson, B.G. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: Comparison with aminoguanidine. Biochem. Biophys. Res. Commun. 1996, 220, 113–119. [Google Scholar] [CrossRef]
- Webster, J.; Urban, C.; Berbaum, K.; Loske, C.; Alpar, A.; Gärtner, U.; de Arriba, S.G.; Arendt, T.; Münch, G. The carbonyl scavengers aminoguanidine and tenilsetam protect against the neurotoxic effects of methylglyoxal. Neurotox. Res. 2005, 7, 95–101. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression. Horm. Metab. Res. 2012, 44, 891–895. [Google Scholar] [CrossRef]
- Kiho, T.; Kato, M.; Usui, S.; Hirano, K. Effect of buformin and metformin on formation of advanced glycation end products by methylglyoxal. Clin. Chim. Acta 2005, 358, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Safiah, M.; Hyassat, D.; Khader, Y.; Farahid, O.; Batieha, A.; El-Khateeb, M.; Ajlouni, K. Effect of Metformin on Anthropometric Measurements and Hormonal and Biochemical Profile in Patients with Prediabetes. J. Diabetes Res. 2021, 2021, 8275303. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, S.; Natarajan, R.; Yerneni, K.; Scott, S.; Gonzales, N.; Nadler, J.L. Evidence that pioglitazone, metformin and pentoxifylline are inhibitors of glycation. Clin. Chim. Acta 2000, 301, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Oz Gul, O.; Tuncel, E.; Yilmaz, Y.; Ulukaya, E.; Gul, C.B.; Kiyici, S.; Oral, A.Y.; Guclu, M.; Ersoy, C.; Imamoglu, S. Comparative effects of pioglitazone and rosiglitazone on plasma levels of soluble receptor for advanced glycation end products in type 2 diabetes mellitus patients. Metabolism 2010, 59, 64–69. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Fantauzzi, C.B.; Pugliese, G. L-carnosine and its Derivatives as New Therapeutic Agents for the Prevention and Treatment of Vascular Complications of Diabetes. Curr. Med. Chem. 2020, 27, 1744–1763. [Google Scholar] [CrossRef]
- Lavilla, C.J.; Billacura, M.P.; Hanna, K.; Boocock, D.J.; Coveney, C.; Miles, A.K.; Foulds, G.A.; Murphy, A.; Tan, A.; Jackisch, L.; et al. Carnosine protects stimulus-secretion coupling through prevention of protein carbonyl adduction events in cells under metabolic stress. Free Radic. Biol. Med. 2021, 175, 65–79. [Google Scholar] [CrossRef]
- Houjeghani, S.; Kheirouri, S.; Faraji, E.; Jafarabadi, M.A. l-Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor-α levels in patients with type 2 diabetes: A double-blind placebo-controlled randomized clinical trial. Nutr. Res. 2018, 49, 96–106. [Google Scholar] [CrossRef]
- Elbarbary, N.S.; Ismail, E.A.R.; El-Naggar, A.R.; Hamouda, M.H.; El-Hamamsy, M. The effect of 12 weeks carnosine supplementation on renal functional integrity and oxidative stress in pediatric patients with diabetic nephropathy: A randomized placebo-controlled trial. Pediatr. Diabetes 2018, 19, 470–477. [Google Scholar] [CrossRef]
- Sobal, G.; Menzel, E.J.; Sinzinger, H. Calcium antagonists as inhibitors of in vitro low density lipoprotein oxidation and glycation. Biochem. Pharmacol. 2001, 61, 373–379. [Google Scholar] [CrossRef]
- Akira, K.; Amano, M.; Okajima, F.; Hashimoto, T.; Oikawa, S. Inhibitory effects of amlodipine and fluvastatin on the deposition of advanced glycation end products in aortic wall of cholesterol and fructose-fed rabbits. Biol. Pharm. Bull. 2006, 29, 75–81. [Google Scholar] [CrossRef][Green Version]
- Kang, M.K.; Chung, W.B.; Hong, S.K.; Kim, O.R.; Ihm, S.H.; Chang, K.; Seung, K.B. Effects of candesartan cilexetil and amlodipine orotate on receptor for advanced glycation end products expression in the aortic wall of Otsuka Long-Evans Tokushima Fatty (OETFF) type 2 diabetic rats. Arch. Pharm. Res. 2016, 39, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, P.; Siboska, G.E.; Clark, B.F.; Rattan, S.I. Kinetin inhibits protein oxidation and glycoxidation in vitro. Biochem. Biophys. Res. Commun. 2000, 276, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, D.; Zheng, Y.; Li, H.; Hao, C.; Ouyang, W. Protective effects of kinetin against aluminum chloride and D-galactose induced cognitive impairment and oxidative damage in mouse. Brain Res. Bull. 2017, 134, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Wolffenbuttel, B.H.; Boulanger, C.M.; Crijns, F.R.; Huijberts, M.S.; Poitevin, P.; Swennen, G.N.; Vasan, S.; Egan, J.J.; Ulrich, P.; Cerami, A.; et al. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc. Natl. Acad. Sci. USA 1998, 95, 4630–4634. [Google Scholar] [CrossRef]
- Price, D.L.; Rhett, P.M.; Thorpe, S.R.; Baynes, J.W. Chelating activity of advanced glycation end-product inhibitors. J. Biol. Chem. 2001, 276, 48967–48972. [Google Scholar] [CrossRef]
- Bakris, G.L.; Bank, A.J.; Kass, D.A.; Neutel, J.M.; Preston, R.A.; Oparil, S. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. Am. J. Hypertens. 2004, 17, 23s–30s. [Google Scholar] [CrossRef]
- Thomas, M.C.; Baynes, J.W.; Thorpe, S.R.; Cooper, M.E. The role of AGEs and AGE inhibitors in diabetic cardiovascular disease. Curr. Drug Targets 2005, 6, 453–474. [Google Scholar] [CrossRef]
- Vasan, S.; Zhang, X.; Zhang, X.; Kapurniotu, A.; Bernhagen, J.; Teichberg, S.; Basgen, J.; Wagle, D.; Shih, D.; Terlecky, I.; et al. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature 1996, 382, 275–278. [Google Scholar] [CrossRef]
- Cooper, M.E.; Thallas, V.; Forbes, J.; Scalbert, E.; Sastra, S.; Darby, I.; Soulis, T. The cross-link breaker, N-phenacylthiazolium bromide prevents vascular advanced glycation end-product accumulation. Diabetologia 2000, 43, 660–664. [Google Scholar] [CrossRef]
- Chang, P.C.; Tsai, S.C.; Chong, L.Y.; Kao, M.J. N-Phenacylthiazolium bromide inhibits the advanced glycation end product (AGE)-AGE receptor axis to modulate experimental periodontitis in rats. J. Periodontol. 2014, 85, e268–e276. [Google Scholar] [CrossRef]
- Pathak, P.; Gupta, R.; Chaudhari, A.; Shiwalkar, A.; Dubey, A.; Mandhare, A.B.; Gupta, R.C.; Joshi, D.; Chauthaiwale, V. TRC4149 a novel advanced glycation end product breaker improves hemodynamic status in diabetic spontaneously hypertensive rats. Eur. J. Med. Res. 2008, 13, 388–398. [Google Scholar] [PubMed]
- Joshi, D.; Gupta, R.; Dubey, A.; Shiwalkar, A.; Pathak, P.; Gupta, R.C.; Chauthaiwale, V.; Dutt, C. TRC4186, a novel AGE-breaker, improves diabetic cardiomyopathy and nephropathy in Ob-ZSF1 model of type 2 diabetes. J. Cardiovasc. Pharmacol. 2009, 54, 72–81. [Google Scholar] [CrossRef]
- Chandra, K.P.; Shiwalkar, A.; Kotecha, J.; Thakkar, P.; Srivastava, A.; Chauthaiwale, V.; Sharma, S.K.; Cross, M.R.; Dutt, C. Phase I clinical studies of the advanced glycation end-product (AGE)-breaker TRC4186: Safety, tolerability and pharmacokinetics in healthy subjects. Clin. Drug Investig. 2009, 29, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.; Castegna, A.; Pocernich, C.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444. [Google Scholar] [CrossRef]
- Van den Eynde, M.D.G.; Geleijnse, J.M.; Scheijen, J.; Hanssen, N.M.J.; Dower, J.I.; Afman, L.A.; Stehouwer, C.D.A.; Hollman, P.C.H.; Schalkwijk, C.G. Quercetin, but Not Epicatechin, Decreases Plasma Concentrations of Methylglyoxal in Adults in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial with Pure Flavonoids. J. Nutr. 2018, 148, 1911–1916. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef]
- Muthenna, P.; Akileshwari, C.; Saraswat, M.; Bhanuprakash Reddy, G. Inhibition of advanced glycation end-product formation on eye lens protein by rutin. Br. J. Nutr. 2012, 107, 941–949. [Google Scholar] [CrossRef]
- Bazyar, H.; Moradi, L.; Zaman, F.; Zare Javid, A. The effects of rutin flavonoid supplement on glycemic status, lipid profile, atherogenic index of plasma, brain-derived neurotrophic factor (BDNF), some serum inflammatory, and oxidative stress factors in patients with type 2 diabetes mellitus: A double-blind, placebo-controlled trial. Phytother. Res. 2023, 37, 271–284. [Google Scholar] [CrossRef]
- Velichkova, S.; Foubert, K.; Pieters, L. Natural Products as a Source of Inspiration for Novel Inhibitors of Advanced Glycation Endproducts (AGEs) Formation. Planta Med. 2021, 87, 780–801. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, L.; Wen, Q.; Feng, Y.; Luo, Y.; Tan, T. Trapping Methylglyoxal by Taxifolin and Its Metabolites in Mice. J. Agric. Food Chem. 2022, 70, 5026–5038. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; El-Bassossy, H.; Mohamed, G.A.; El-Halawany, A.M.; Alshali, K.Z.; Banjar, Z.M. Phenolics from Garcinia mangostana Inhibit Advanced Glycation Endproducts Formation: Effect on Amadori Products, Cross-Linked Structures and Protein Thiols. Molecules 2016, 21, 251. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Li, Y.; Yu, W.; Zhao, Y.; Hu, X.; Tao, N.P.; Wang, M. Naringenin, a common flavanone, inhibits the formation of AGEs in bread and attenuates AGEs-induced oxidative stress and inflammation in RAW264.7 cells. Food Chem. 2018, 269, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Z.; Cheng, Y.; Wu, Q.; He, Y.; Li, Q.; Cao, X. Eriodictyol and naringenin inhibit the formation of AGEs: An in vitro and molecular interaction study. J. Mol. Recognit. 2020, 33, e2814. [Google Scholar] [CrossRef]
- Sun, Y.P.; Gu, J.F.; Tan, X.B.; Wang, C.F.; Jia, X.B.; Feng, L.; Liu, J.P. Curcumin inhibits advanced glycation end product-induced oxidative stress and inflammatory responses in endothelial cell damage via trapping methylglyoxal. Mol. Med. Rep. 2016, 13, 1475–1486. [Google Scholar] [CrossRef]
- Shao, X.; Bai, N.; He, K.; Ho, C.T.; Yang, C.S.; Sang, S. Apple polyphenols, phloretin and phloridzin: New trapping agents of reactive dicarbonyl species. Chem. Res. Toxicol. 2008, 21, 2042–2050. [Google Scholar] [CrossRef]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of Apple Phytochemicals, Phloretin and Phloridzin, in Modulating Processes Related to Intestinal Inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef]
- Masumoto, S.; Akimoto, Y.; Oike, H.; Kobori, M. Dietary phloridzin reduces blood glucose levels and reverses Sglt1 expression in the small intestine in streptozotocin-induced diabetic mice. J. Agric. Food Chem. 2009, 57, 4651–4656. [Google Scholar] [CrossRef]
- Lv, L.; Shao, X.; Chen, H.; Ho, C.T.; Sang, S. Genistein inhibits advanced glycation end product formation by trapping methylglyoxal. Chem. Res. Toxicol. 2011, 24, 579–586. [Google Scholar] [CrossRef]
- Choudhury, R.; Srai, S.K.; Debnam, E.; Rice-Evans, C.A. Urinary excretion of hydroxycinnamates and flavonoids after oral and intravenous administration. Free Radic. Biol. Med. 1999, 27, 278–286. [Google Scholar] [CrossRef]
- Park, C.H.; Noh, J.S.; Tanaka, T.; Roh, S.S.; Lee, J.C.; Yokozawa, T. Polyphenol isolated from Corni Fructus, 7-O-galloyl-D-sedoheptulose, modulates advanced glycation endproduct-related pathway in type 2 diabetic db/db mice. Arch. Pharm. Res. 2015, 38, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Mong, M.C.; Chan, K.C.; Yin, M.C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.B.; Patil, K.N. SIRT1/AMPK-mediated pathway: Ferulic acid from sugar beet pulp mitigating obesity-induced diabetes-linked complications and improving metabolic health. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159511. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Kong, W.; Liu, Z.; Wang, S.; Zhao, P.; Liang, K.; Niu, Y.; Yang, W.; Xiang, C.; Hu, X.; et al. Advanced glycation end-products as mediators of the aberrant crosslinking of extracellular matrix in scarred liver tissue. Nat. Biomed. Eng. 2023, 7, 1437–1454. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Z.; Sheng, Z. Ability of resveratrol to inhibit advanced glycation end product formation and carbohydrate-hydrolyzing enzyme activity, and to conjugate methylglyoxal. Food Chem. 2017, 216, 153–160. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M. Resveratrol supplementation and type 2 diabetes: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4465–4480. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.P.; Wang, W.Q.; Song, S.G.; Liu, X.M. Lignans Extracted from Eucommia Ulmoides Oliv. Protects Against AGEs-Induced Retinal Endothelial Cell Injury. Cell Physiol. Biochem. 2016, 39, 2044–2054. [Google Scholar] [CrossRef]
- Peng, J.; Liang, G.; Wen, W.; Huang, W.; Qiu, Y.; Xiao, G.; Wang, Q. Blueberry anthocyanins extract inhibits advanced glycation end-products (AGEs) production and AGEs-stimulated inflammation in RAW264.7 cells. J. Sci. Food Agric. 2024, 104, 75–82. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z.; Wang, Y.; Wang, Y.; Fu, L.; Su, L. Chinese bayberry (Myrica rubra) phenolics mitigated protein glycoxidation and formation of advanced glycation end-products: A mechanistic investigation. Food Chem. 2021, 361, 130102. [Google Scholar] [CrossRef]
- Spinola, V.; Pinto, J.; Llorent-Martinez, E.J.; Tomas, H.; Castilho, P.C. Evaluation of Rubus grandifolius L. (wild blackberries) activities targeting management of type-2 diabetes and obesity using in vitro models. Food Chem. Toxicol. 2019, 123, 443–452. [Google Scholar] [CrossRef]
- Hsiao, Y.W.; Hsia, S.M.; Pan, M.H.; Ho, C.T.; Hung, W.L. Berry anthocyanins prevent alpha-dicarbonyls and advanced glycation end product formation in phosphate-buffered saline-based model systems, cookie and ground pork. J. Food Sci. 2024, 89, 3745–3758. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.L.; Lee, C.J.; Choi, S.Y.; Kim, Y.; Hur, J. Inhibitory effect of sea buckthorn extracts on advanced glycation endproduct formation. Food Chem. 2022, 373, 131364. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Cui, B.; Zheng, K.; Gao, N.; An, X.; Zhang, Y.; Cheng, Z.; Nie, Y.; Zhu, J.; Wang, L.; et al. Novel inhibitory effect of black chokeberry (Aronia melanocarpa) from selected eight berries extracts on advanced glycation end-products formation and corresponding mechanism study. Food Chem. X 2024, 21, 101032. [Google Scholar] [CrossRef] [PubMed]
- Coelho, O.G.L.; Ribeiro, P.V.M.; Alfenas, R.C.G. Can grape polyphenols affect glycation markers? A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1208–1218. [Google Scholar] [CrossRef]
- Chehri, A.; Yarani, R.; Yousefi, Z.; Shakouri, S.K.; Ostadrahimi, A.; Mobasseri, M.; Araj-Khodaei, M. Phytochemical and pharmacological anti-diabetic properties of bilberries (Vaccinium myrtillus), recommendations for future studies. Prim. Care Diabetes 2022, 16, 27–33. [Google Scholar] [CrossRef]
- Herbalist, R.U. Bilberry Fruit Vaccinium myrtillus L. Standards of Analysis, Quality Control, and Therapeutics; American Herbal Pharmacopoeia and Therapeutic Compendium: Santa Cruz, CA, USA, 2001. [Google Scholar]
- Chen, L.; Zhang, X.; Wang, Q.; Li, W.; Liu, L. Effect of Vaccinium Myrtillus Extract Supplement on Advanced Glycation End-products: A Pilot Study (P06-098-19). Curr. Dev. Nutr. 2019, 3, nzz031.P006-098-019. [Google Scholar] [CrossRef]
- Thilavech, T.; Ngamukote, S.; Belobrajdic, D.; Abeywardena, M.; Adisakwattana, S. Cyanidin-3-rutinoside attenuates methylglyoxal-induced protein glycation and DNA damage via carbonyl trapping ability and scavenging reactive oxygen species. BMC Complement. Altern. Med. 2016, 16, 138. [Google Scholar] [CrossRef]
- Prasanna, G.; Jing, P. Cyanidin-3-O-glucoside functions like chemical chaperone and attenuates the glycation mediated amyloid formation in albumin. Arch. Biochem. Biophys. 2018, 643, 50–56. [Google Scholar] [CrossRef]
- Silva, F.S.; Oliveira, P.J.; Duarte, M.F. Oleanolic, Ursolic, and Betulinic Acids as Food Supplements or Pharmaceutical Agents for Type 2 Diabetes: Promise or Illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef]
- Jang, S.M.; Kim, M.J.; Choi, M.S.; Kwon, E.Y.; Lee, M.K. Inhibitory effects of ursolic acid on hepatic polyol pathway and glucose production in streptozotocin-induced diabetic mice. Metabolism 2010, 59, 512–519. [Google Scholar] [CrossRef]
- Jung, E.; Park, S.B.; Jung, W.K.; Kim, H.R.; Kim, J. Antiglycation Activity of Aucubin In Vitro and in Exogenous Methylglyoxal Injected Rats. Molecules 2019, 24, 3653. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.C.; Yang, H.H.; Chiang, S.C.; Chou, Y.X.; Yang, H.T. Auricularia polytricha aqueous extract supplementation decreases hepatic lipid accumulation and improves antioxidative status in animal model of nonalcoholic fatty liver. Biomedicine 2014, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S60–S84. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Fang, Z.; Chen, Y.; Chen, Y.; Xiao, B.; Guo, L.; Xu, Y.; Wang, G.; Wang, W.; Zhang, Y. Hypoglycemic Effect of the Degraded Polysaccharides from the Wood Ear Medicinal Mushroom Auricularia auricula-judae (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 1033–1042. [Google Scholar] [CrossRef]
- Hao, M.; Li, S.Y.; Sun, C.K.; Jingyu, X.; Lin, Y.; Liu, K.X.; Wang, L.; Li, C.X.; Zhou, Q.; Du, J.L.; et al. Amelioration effects of berberine on diabetic microendothelial injury model by the combination of high glucose and advanced glycation end products in vitro. Eur. J. Pharmacol. 2011, 654, 320–325. [Google Scholar] [CrossRef]
- Wu, D.; Wen, W.; Qi, C.L.; Zhao, R.X.; Lü, J.H.; Zhong, C.Y.; Chen, Y.Y. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine 2012, 19, 712–718. [Google Scholar] [CrossRef]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Hongsprabhas, P.; Thottiam Vasudevan, R. In vitro and in vivo inhibition of maillard reaction products using amino acids, modified proteins, vitamins, and genistein: A review. J. Food Biochem. 2019, 43, e13089. [Google Scholar] [CrossRef]
- Jain, S.K.; Palmer, M. The effect of oxygen radicals metabolites and vitamin E on glycosylation of proteins. Free Radic. Biol. Med. 1997, 22, 593–596. [Google Scholar] [CrossRef]
- Chen, J.L.; Francis, J. Pyridoxamine, advanced glycation inhibition, and diabetic nephropathy. J. Am. Soc. Nephrol. 2012, 23, 6–8. [Google Scholar] [CrossRef]
- Alhamdani, M.S.; Al-Kassir, A.H.; Abbas, F.K.; Jaleel, N.A.; Al-Taee, M.F. Antiglycation and antioxidant effect of carnosine against glucose degradation products in peritoneal mesothelial cells. Nephron Clin. Pract. 2007, 107, c26–c34. [Google Scholar] [CrossRef] [PubMed]
- Aydın, A.F.; Küçükgergin, C.; Çoban, J.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Uysal, M.; Koçak-Toker, N. Carnosine prevents testicular oxidative stress and advanced glycation end product formation in D-galactose-induced aged rats. Andrologia 2018, 50, e12939. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Lin, Y.S.; Lin, S.Y.; Hou, W.C. Antioxidant and antiglycation activities of the synthesised dipeptide, Asn-Trp, derived from computer-aided simulation of yam dioscorin hydrolysis and its analogue, Gln-Trp. Food Chem. 2014, 147, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, A.; Bhatia, A.; Ram, A.K.; Goel, S. Increased advanced glycation end product specific fluorescence in repeatedly heated used cooking oil. J. Food Sci. Technol. 2017, 54, 2602–2606. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Deo, P.; Clifton, P.M. Differential Effects of Dietary Patterns on Advanced Glycation end Products: A Randomized Crossover Study. Nutrients 2020, 12, 1767. [Google Scholar] [CrossRef]
- Del Castillo, M.D.; Iriondo-DeHond, A.; Iriondo-DeHond, M.; Gonzalez, I.; Medrano, A.; Filip, R.; Uribarri, J. Healthy eating recommendations: Good for reducing dietary contribution to the body’s advanced glycation/lipoxidation end products pool? Nutr. Res. Rev. 2021, 34, 48–63. [Google Scholar] [CrossRef]
- Lopez-Moreno, J.; Quintana-Navarro, G.M.; Delgado-Lista, J.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Camargo, A.; Perez-Martinez, P.; Tinahones, F.J.; Striker, G.E.; et al. Mediterranean Diet Supplemented With Coenzyme Q10 Modulates the Postprandial Metabolism of Advanced Glycation End Products in Elderly Men and Women. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 340–346. [Google Scholar] [CrossRef]
- Sharma, C.; Kaur, A.; Thind, S.S.; Singh, B.; Raina, S. Advanced glycation End-products (AGEs): An emerging concern for processed food industries. J. Food Sci. Technol. 2015, 52, 7561–7576. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Movahed, A.; Raj, P.; Nabipour, I.; Mahmoodi, M.; Ostovar, A.; Kalantarhormozi, M.; Netticadan, T. Efficacy and Safety of Resveratrol in Type 1 Diabetes Patients: A Two-Month Preliminary Exploratory Trial. Nutrients 2020, 12, 161. [Google Scholar] [CrossRef]
- Pramanik, S.; Banerjee, K.; Mondal, L.K. The Amelioration of Detrimental Biochemical Anomalies by Supplementing B, C, and E Vitamins in Subjects with Type 2 Diabetes Mellitus May Reduce the Rate of Development of Diabetic Retinopathy. J. Diabetes Res. 2022, 2022, 3886710. [Google Scholar] [CrossRef]
- Gómez-Martínez, S.; Díaz-Prieto, L.E.; Vicente Castro, I.; Jurado, C.; Iturmendi, N.; Martín-Ridaura, M.C.; Calle, N.; Dueñas, M.; Picón, M.J.; Marcos, A.; et al. Moringa oleifera Leaf Supplementation as a Glycemic Control Strategy in Subjects with Prediabetes. Nutrients 2021, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Barocio-Pantoja, M.; Quezada-Fernández, P.; Cardona-Müller, D.; Jiménez-Cázarez, M.B.; Larios-Cárdenas, M.; González-Radillo, O.I.; García-Sánchez, A.; Carmona-Huerta, J.; Chávez-Guzmán, A.N.; Díaz-Preciado, P.A.; et al. Green Tea Extract Increases Soluble RAGE and Improves Renal Function in Patients with Diabetic Nephropathy. J. Med. Food 2021, 24, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, X.; Liu, P.; Wang, B.; Duan, D.M.; Guo, D.H. A comparison study of metformin only therapy and metformin combined with Chinese medicine jianyutangkang therapy in patients with type 2 diabetes: A randomized placebo-controlled double-blind study. Complement. Ther. Med. 2016, 24, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Tian, J.; Chen, X.; Li, Z.; Piao, C.; Guo, J.; Ma, L.; Zhao, L.; Xia, C.; Wang, C.Z.; et al. The Efficacy and Safety of Chinese Herbal Medicine Jinlida as Add-On Medication in Type 2 Diabetes Patients Ineffectively Managed by Metformin Monotherapy: A Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial. PLoS ONE 2015, 10, e0130550. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).