A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, 8-Week Pilot Study of Tuna-Byproduct-Derived Novel Supplements for Managing Cellular Senescence and Cognitive Decline in Perimenopausal and Postmenopausal Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Functional Beverage Containing Tuna Oil
2.2. Study Design
2.3. Measurement of Body Mass Index (BMI) and Body Composition
2.4. Event-Related Potential (ERP) Assessment
2.5. Working Memory Assessment
2.6. Blood Collection and Preparation
2.7. Neurotransmitter Assessment
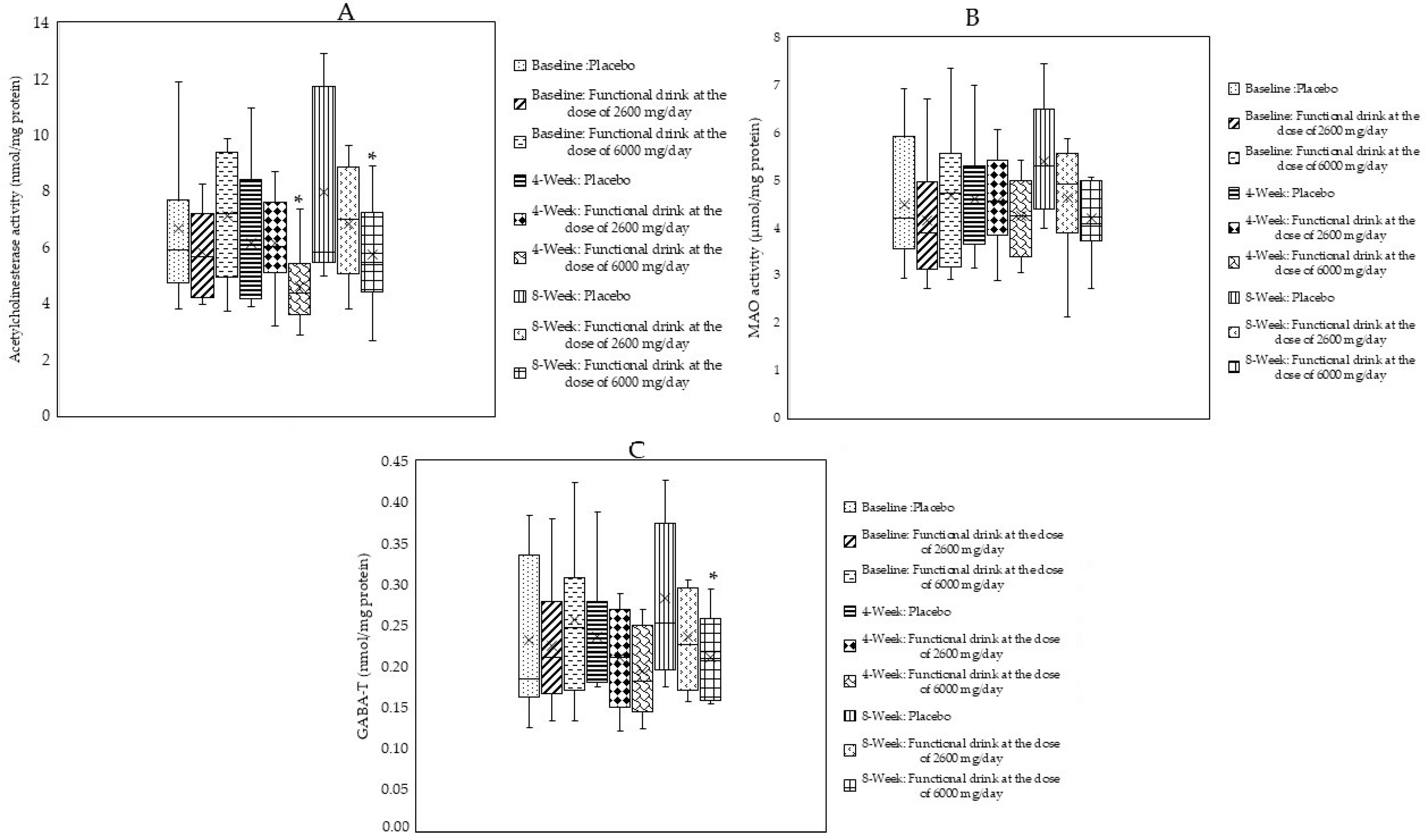
2.7.1. Acetylcholinesterase (AChE) Activity
2.7.2. Monoamine Oxidase (MAO) Levels
2.7.3. Gamma-Aminobutyric Acid-Transaminase (GABA-T) Activity
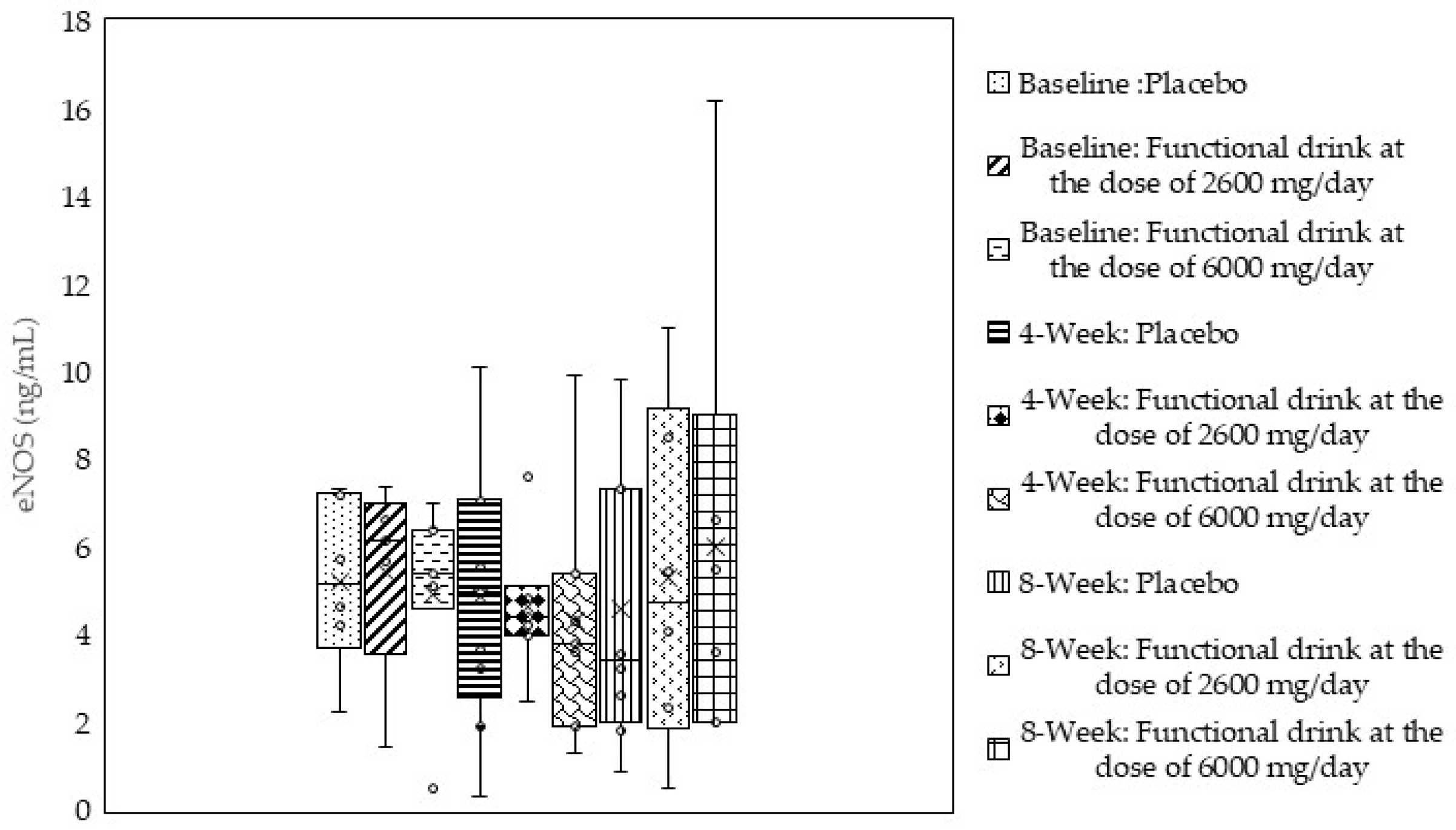
2.8. Endothelial Nitric Oxide Synthase (eNOS) Assessment
2.9. Oxidative Stress Status Assessment
2.9.1. Malondialdehyde (MDA) Measurement
2.9.2. Superoxide Dismutase (SOD) Activity Assessment
2.9.3. Catalase (CAT) Activity Assessment
2.9.4. Glutathione Peroxidase (GPx) Activity
2.10. Inflammatory Cytokine Assessment
2.11. Age-Related Biomarker Assessment
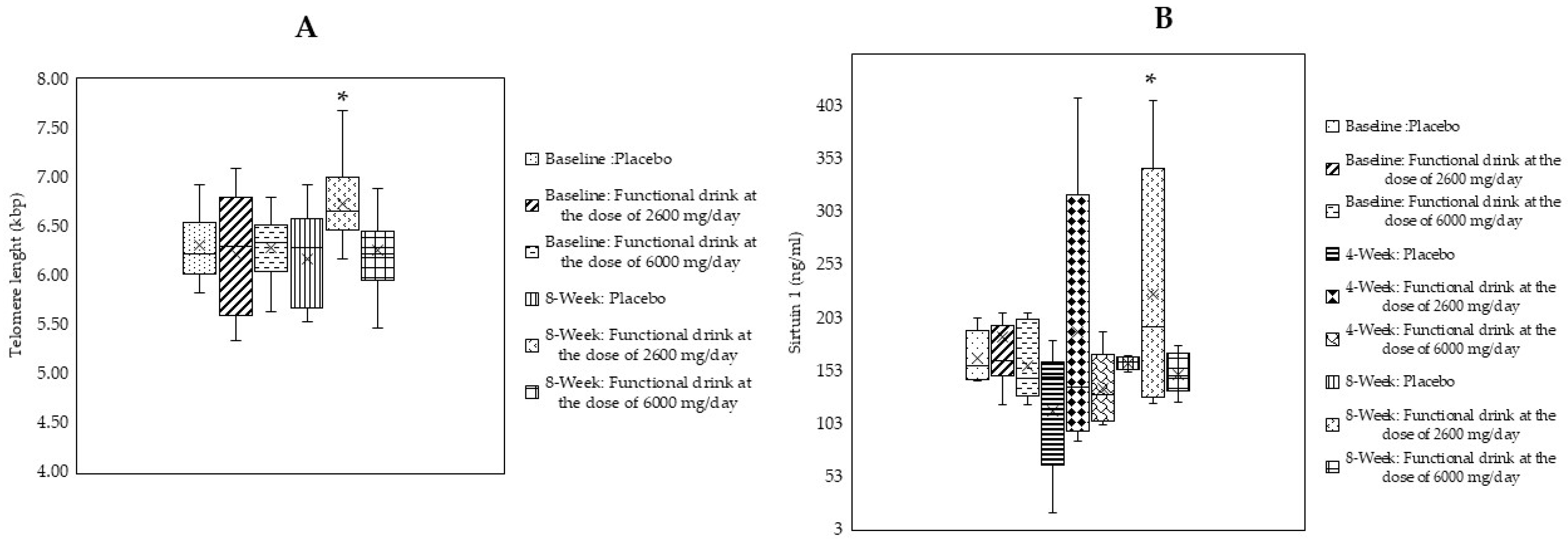
2.11.1. DNA Extraction and Assessment of Telomere Length
2.11.2. Assessment of Situin-1 (SIRT1)
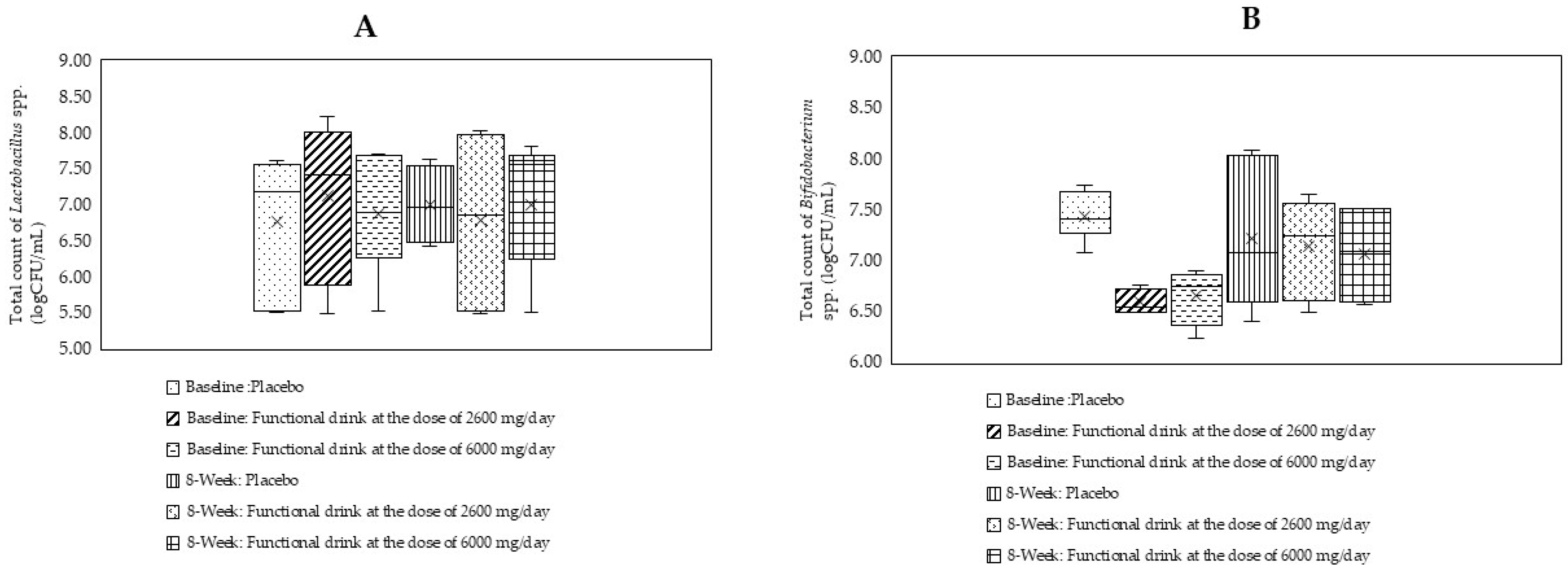
2.12. Lactobacillus and Bifidobacterium spp. Assessment
2.13. Determination of Docosahexaenoic Acid (DHA)
2.14. Determination of Total Phenolic Compounds (TPC) in Serum
2.15. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Subjects
3.2. Changes in Brain Wave Components
3.3. Working Memory Assessment via Recognition Tests
3.4. Changes in Neurotransmitters
3.5. Endothelial Nitric Oxide Synthase (eNOS)
3.6. Changes in Oxidative Stress Status and Inflammation
3.7. Changes in Age-Related Biomarkers
3.8. Changes in the Amount of Lactobacillus and Bifidobacterium spp.
3.9. Levels of Total Polyphenolic Compounds and Docosahexaenoic Acid (DHA) in Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
References
- Metcalf, C.A.; Duffy, K.A.; Page, C.E.; Novick, A.M. Cognitive Problems in Perimenopause: A Review of Recent Evidence. Curr. Psychiatry Rep. 2023, 510, 501–511. [Google Scholar] [CrossRef]
- Ramli, N.Z.; Yahaya, M.F.; Mohd Fahami, N.A.; Abdul Manan, H.; Singh, M.; Damanhuri, H.A. Brain volumetric changes in menopausal women and its association with cognitive function: A structured review. Front. Aging Neurosci. 2023, 15, 1158001. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Lin, C.H.; Lane, H.Y. From Menopause to Neurodegeneration-Molecular Basis and Potential Therapy. Int. J. Mol. Sci. 2021, 22, 8654. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Barman, B.; Thakur, M.K. Oxidative stress-mediated memory impairment during aging and its therapeutic intervention by natural bioactive compounds. Front. Aging Neurosci. 2022, 14, 944697. [Google Scholar] [CrossRef]
- McCarthy, M.; Raval, A.P. The peri-menopause in a woman’s life: A systemic inflammatory phase that enables later neurodegenerative disease. J. Neuroinflammation 2020, 17, 317. [Google Scholar] [CrossRef] [PubMed]
- Karolczak, K.; Watala, C. Estradiol as the Trigger of Sirtuin-1-Dependent Cell Signaling with a Potential Utility in Anti-Aging Therapies. Int. J. Mol. Sci. 2023, 24, 13753. [Google Scholar] [CrossRef]
- Yu, H.J.; Koh, S.H. Is Telomere Length Shortening a Risk Factor for Neurodegenerative Disorders? Dement. Neurocognitive Disord. 2022, 21, 83–92. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, Y.; Wang, L. Neurotransmitters in Prevention and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 3841. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Takano, H. Cognitive Function and Monoamine Neurotransmission in Schizophrenia: Evidence From Positron Emission Tomography Studies. Front. Psychiatry 2018, 9, 228. [Google Scholar] [CrossRef]
- Michels, L.; Martin, E.; Klaver, P.; Edden, R.; Zelaya, F.; Lythgoe, D.J.; Lüchinger, R.; Brandeis, D.; O’Gorman, R.L. Frontal GABA levels change during working memory. PLoS ONE 2012, 7, e31933. [Google Scholar] [CrossRef] [PubMed]
- Conde, D.M.; Verdade, R.C.; Valadares, A.L.R.; Mella, L.F.B.; Pedro, A.O.; Costa-Paiva, L. Menopause and cognitive impairment: A narrative review of current knowledge. World J. Psychiatry. 2021, 11, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Tavani, A.; La Vecchia, C. The adverse effects of hormone replacement therapy. Drugs Aging 1999, 14, 347–357. [Google Scholar] [CrossRef]
- Song, R.; Fan, X.; Seo, J. Physical and cognitive function to explain the quality of life among older adults with cognitive impairment: Exploring cognitive function as a mediator. BMC Psychol. 2023, 11, 51. [Google Scholar]
- Yu, Z.; Ke, L.; Lu, T.; Li, L.; Gu, H.; Rao, P. Implementing a food first strategy can transform preventive healthcare. NPJ Sci. Food 2024, 8, 57. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E. Brain Health across the Lifespan: A Systematic Review on the Role of Omega-3 Fatty Acid Supplements. Nutrients 2018, 10, 1094. [Google Scholar] [CrossRef]
- Külzow, N.; Witte, A.V.; Kerti, L.; Grittner, U.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Impact of Omega-3 Fatty Acid Supplementation on Memory Functions in Healthy Older Adults. J. Alzheimers Dis. 2016, 51, 713–725. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Thukham-mee, W. Omega-3-Rich Tuna Oil Derived from By-Products of the Canned Tuna Industry Enhances Memory in an Ovariectomized Rat Model of Menopause. Antioxidants 2024, 13, 637. [Google Scholar] [CrossRef]
- Sittiprapaporn, P.; Bumrungpert, A.; Suyajai, P.; Stough, C. Effectiveness of Fish Oil-DHA Supplementation for Cognitive Function in Thai Children: A Randomized, Doubled-Blind, Two-Dose, Placebo-Controlled Clinical Trial. Foods 2022, 11, 2595. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Castro, M.C.R.; Rezende, A.T.O.; Dos Santos Rodrigues, A.P.; Delpino, F.M.; Oliveira, E.S.; Corgosinho, F.C.; de Oliveira, C. Body composition assessment in individuals with class II/III obesity: A narrative review. BMC Nutr. 2024, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Wattanathorn, J.; Somboonporn, W.; Thukham-Mee, W.; Sungkamnee, S. Memory-Enhancing Effect of 8-Week Consumption of the Quercetin-Enriched Culinary Herbs-Derived Functional Ingredients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Foods 2022, 11, 2678. [Google Scholar] [CrossRef]
- Saenghong, N.; Wattanathorn, J.; Muchimapura, S.; Tongun, T.; Piyavhatkul, N.; Banchonglikitkul, C.; Kajsongkram, T. Zingiber officinale improves cognitive function of the middle-aged healthy women. Evid. Based Complement. Altern. Med. 2012, 2012, 383062. [Google Scholar] [CrossRef] [PubMed]
- Peth-Nui, T.; Wattanathorn, J.; Muchimapura, S.; Tong-Un, T.; Piyavhatkul, N.; Rangseekajee, P.; Ingkaninan, K.; Vittaya-Areekul, S. Effects of 12-Week Bacopa monnieri Consumption on Attention, Cognitive Processing, Working Memory, and Functions of Both Cholinergic and Monoaminergic Systems in Healthy Elderly Volunteers. Evid. Based Complement. Altern. Med. 2012, 2012, 606424. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Holt, D.F.; Sharman, G.B.B.; Palcic, M.M. A continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Anal Biochem. 1997, 244, 384–392. [Google Scholar] [CrossRef]
- Jacob, J.N.; Hesse, G.W.; Shashoua, V.E. Synthesis, brain uptake, and pharmacological properties of a glyceryl lipid containing GABA and the GABA-T inhibitor gamma-vinyl-GABA. J. Med. Chem. 1990, 33, 733–736. [Google Scholar] [CrossRef]
- Awad, R.; Muhammad, A.; Durst, T.; Trudeau, V.L.; Arnason, J.T. Bioassay-guided fractionation of lemon balm (Melissa officinailis L.) using an in vitro meansure of GABA transaminase activity. Phytother. Res. 2009, 23, 1075–1081. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Masaki, H.; Okano, Y.; Sakurai, H. Differential role of catalase and glutathione peroxidase in cultured human fibroblasts under exposure of H2O2 or ultraviolet B light. Arch. Dermatol. Res. 1998, 290, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Turapra, B.; Daduang, J.; Wongwattanakul, M.; Bae, C.-Y.; Jearanaikoon, P. Establishment of in-house telomere length measurement using qPCR. Arch. AHS 2022, 34, 44–52. [Google Scholar]
- Cheli, S.; Napoli, A.; Clementi, E.; Montrasio, C. DNA extraction from fresh and frozen plasma: An alternative for real-time PCR genotyping in pharmacogenetics. Mol. Biol. Rep. 2020, 47, 6451–6455. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Wang, Y.; Savage, S.A.; Alsaggaf, R.; Aubert, G.; Dagnall, C.L.; Spellman, S.R.; Lee, S.J.; Hicks, B.; Jones, K.; Katki, H.A.; et al. Telomere Length Calibration from qPCR Measurement: Limitations of Current Method. Cells 2018, 7, 183. [Google Scholar] [CrossRef]
- Thapa, R.; Moglad, E.; Afzal, M.; Gupta, G.; Bhat, A.A.; Hassan Almalki, W.; Kazmi, I.; Alzarea, S.I.; Pant, K.; Singh, T.G.; et al. The role of sirtuin 1 in ageing and neurodegenerative disease: A molecular perspective. Ageing Res. Rev. 2024, 102, 102545. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic Laboratory Techniques: Plating Methods. J. Vis. Exp. 2012, 63, e3064. [Google Scholar] [CrossRef]
- Serafim, V.; Tiugan, D.A.; Andreescu, N.; Mihailescu, A.; Paul, C.; Velea, I.; Puiu, M.; Niculescu, M.D. Development and Validation of a LC-MS/MS-Based Assay for Quantification of Free and Total Omega 3 and 6 Fatty Acids from Human Plasma. Molecules 2019, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.; Borrero, M.; Sequeda, G.; Diez, O.; Castro1, V.; Angela Garcia, A.; Ruiz, A.; Pacetti, D.; Frega, N.; Gagliardi, N.R.; et al. Hybrid palm oil (Elaeis oleifera × Elaeis guineensis) supplementation improves plasma antioxidant capacity in humans. Eur. J. Lipid Sci. Technol. 2016, 119, 1600070. [Google Scholar] [CrossRef]
- An, L.; Shen, Y.; Chopp, M.; Zacharek, A.; Venkat, P.; Chen, Z.; Li, W.; Qian, Y.; Landschoot-Ward, J.; Chen, J. Deficiency of Endothelial Nitric Oxide Synthase (eNOS) Exacerbates Brain Damage and Cognitive Deficit in A Mouse Model of Vascular Dementia. Aging Dis. 2021, 12, 732–746. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar]
- Thornton, A.R.; Harmer, M.; Lavoie, B.A. Selective attention increases the temporal precision of the auditory N100 event-related potential. Hear. Res. 2007, 230, 73–79. [Google Scholar] [CrossRef]
- Guerrero, L.; Bouazzaoui, B.; Isingrini, M.; Angel, L. Impact of working memory capacity on predominance of parietal over frontal P300 amplitude. Brain Cogn. 2023, 170, 106056. [Google Scholar] [CrossRef] [PubMed]
- Casali, R.L.; Amaral, M.I.; Boscariol, M.; Lunardi, L.L.; Guerreiro, M.M.; Matas, C.G.; Colella-Santos, M.F. Comparison of auditory event-related potentials between children with benign childhood epilepsy with centrotemporal spikes and children with temporal lobe epilepsy. Epilepsy Behav. 2016, 59, 111–116. [Google Scholar] [CrossRef]
- Chevalier, N.; Kurth, S.; Doucette, M.R.; Wiseheart, M.; Deoni, S.C.; Dean, D.C., 3rd; O’Muircheartaigh, J.; Blackwell, K.A.; Munakata, Y.; LeBourgeois, M.K. Myelination Is Associated with Processing Speed in Early Childhood: Preliminary Insights. PLoS ONE 2015, 10, e0139897. [Google Scholar] [CrossRef]
- Javitt, D.C.; Freedman, R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am. J. Psychiatry 2015, 172, 17–31. [Google Scholar] [CrossRef]
- Feltham, B.A.; Louis, X.L.; Eskin, M.N.A.; Suh, M. Docosahexaenoic Acid: Outlining the Therapeutic Nutrient Potential to Combat the Prenatal Alcohol-Induced Insults on Brain Development. Adv. Nutr. 2020, 11, 724–735. [Google Scholar] [CrossRef]
- Vincent, C.; Inassi, J. Comparison of oxidative stress between premenopausal and postmenopausal women. Natl. J. Physiol. Pharm. Pharmacol. 2020, 10, 359–362. [Google Scholar] [CrossRef]
- Nsonwu-Anyanwu, A.C.; Ekong Eworo, R.; Chinonye Nsonwu, M.; Okon Akpan, U.; Adamma Kalu, F.; Ogbonna Nwevo, E. Age Related Changes in Indices of Bone Metabolism and Oxidative Stress in Postmenopausal Women in Southern Nigeria. J. Adv. Med. Biomed. Res. 2023, 31, 125–132. [Google Scholar] [CrossRef]
- Abildgaard, J.; Tingstedt, J.; Zhao, Y.; Hartling, H.J.; Pedersen, A.T.; Lindegaard, B.; Dam Nielsen, S. Increased systemic inflammation and altered distribution of T-cell subsets in postmenopausal women. PLoS ONE 2020, 15, e0235174. [Google Scholar] [CrossRef]
- Shafin, N.; Zakaria, R.; Hussain, N.H.; Othman, Z. Association of oxidative stress and memory performance in postmenopausal women receiving estrogen-progestin therapy. Menopause 2013, 20, 661–666. [Google Scholar] [CrossRef]
- Ramos-González, E.J.; Bitzer-Quintero, O.K.; Ortiz, G.; Hernández-Cruz, J.J.; Ramírez-Jirano, L.J. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurologia 2024, 39, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Klaiber, E.L.; Broverman, D.M.; Vogel, W.; Peterson, L.G.; Snyder, M.B. Relationships of serum estradiol levels, menopausal duration, and mood during hormonal replacement therapy. Psychoneuroendocrinology 1997, 22, 549–558. [Google Scholar] [CrossRef]
- Monteiro, S.C.; Stefanello, F.M.; Vianna, L.P.; Matte, C.; Barp, J.; Belló-Klein, A.; Trindade, V.M.; Wyse, A.T. Ovariectomy enhances acetylcholinesterase activity but does not alter ganglioside content in cerebral cortex of female adult rats. Metab. Brain Dis. 2005, 20, 35–44. [Google Scholar] [CrossRef]
- Noudoost, B.; Moore, T. The role of neuromodulators in selective attention. Trends Cogn. Sci. 2011, 15, 585–591. [Google Scholar] [CrossRef]
- Jiménez-Balado, J.; Eich, T.S. GABAergic dysfunction, neural network hyperactivity and memory impairments in human aging and Alzheimer’s disease. Semin. Cell Dev. Biol. 2021, 116, 146–159. [Google Scholar] [CrossRef]
- Meital, L.T.; Windsor, M.T.; Perissiou, M.; Schulze, K.; Magee, R.; Kuballa, A.; Golledge, J.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci. Rep. 2019, 9, 12978. [Google Scholar] [CrossRef]
- Wu, L.; Xie, J.; Jiang, L.; Su, T.; Ye, L.; Zhou, W.; Jiang, Y.; Zhang, C.; Ning, G.; Wang, W. Feminizing Adrenocortical Carcinoma: The Source of Estrogen Production and the Role of Adrenal-Gonadal Dedifferentiation. J. Clin. Endocrinol. Metab. 2018, 103, 3706–3713. [Google Scholar] [CrossRef] [PubMed]
- Huun, M.U.; Garberg, H.T.; Escobar, J.; Chafer, C.; Vento, M.; Holme, I.M.; Saugstad, O.D.; Solberg, R. DHA reduces oxidative stress following hypoxia-ischemia in newborn piglets: A study of lipid peroxidation products in urine and plasma. J. Perinat. Med. 2018, 46, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fernández, B.; Sánchez-Benavides, G.; Genius, P.; Minguillon, C.; Fauria, K.; De Vivo, I.; Navarro, A.; Molinuevo, J.L.; Gispert, J.D.; Sala-Vila, A.; et al. Association between telomere length and cognitive function among cognitively unimpaired individuals at risk of Alzheimer’s disease. Neurobiol. Aging. 2024, 141, 140–150. [Google Scholar] [CrossRef]
- Gampawar, P.; Schmidt, R.; Schmidt, H. Telomere length and brain aging: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 80, 101679. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Tomita, K. How long does telomerase extend telomeres? Regulation of telomerase release and telomere length homeostasis. Curr. Genet. 2018, 64, 1177–1181. [Google Scholar] [CrossRef]
- Ali, S.; Scapagnini, G.; Davinelli, S. Effect of omega-3 fatty acids on the telomere length: A mini meta-analysis of clinical trials. Biomol. Concepts 2022, 13, 25–33. [Google Scholar] [CrossRef]
- Shang, T.; Liu, L.; Zhou, J.; Zhang, M.; Hu, Q.; Fang, M.; Wu, Y.; Yao, P.; Gong, Z. Protective effects of various ratios of DHA/EPA supplementation on high-fat diet-induced liver damage in mice. Lipids Health Dis. 2017, 16, 65. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Brown, R.E.; Zhang, P.C.; Zhao, Y.T.; Ju, X.H.; Song, C. DHA, EPA and their combination at various ratios differently modulated Aβ25–35-induced neurotoxicity in SH-SY5Y cells. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 85–94. [Google Scholar] [CrossRef]
- Hubbard, B.P.; Sinclair, D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014, 35, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Michán, S.; Li, Y.; Chou, M.M.; Parrella, E.; Ge, H.; Long, J.M.; Allard, J.S.; Lewis, K.; Miller, M.; Xu, W.; et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J. Neurosci. 2010, 30, 9695–9707. [Google Scholar] [CrossRef] [PubMed]
- Goto, A. Synaptic plasticity during systems memory consolidation. Neurosci. Res. 2022, 183, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef]


| Ingredients | Percent by Weight (mL) | ||
|---|---|---|---|
| Placebo | Functional Beverage at a Dose of 2600 mg/Serving (150 mL) | Functional Beverage at a Dose of 6000 mg/Serving (150 mL) | |
| Active Ingredients | |||
| Soy protein extract | 5.000 | 5.000 | 5.000 |
| Tuna oil | 0.000 | 1.700 | 3.900 |
| Sunflower oil | 3.900 | 2.200 | 0.000 |
| Sesame powder | 2.000 | 2.000 | 2.000 |
| Total Vitamin B (B1, B2, B3, B5, B6, B7, B9, and B12) | 0.020 | 0.020 | 0.020 |
| Inactive Ingredients | |||
| Sugar | 6.126 | 6.126 | 6.126 |
| Water | 82.934 | 82.934 | 82.934 |
| Nutritional Fact per 150 mL | Placebo Containing Sunflower Oil | Tuna-Oil-Containing Beverage (2600 mg/Serving) | Tuna-Oil-Containing Beverage (6000 mg/Serving) |
|---|---|---|---|
| Energy (Kcal) | 135 | 140 | 140 |
| Protein (g) | 7 | 7 | 7 |
| Carbohydrate (g) | 10 | 11 | 10 |
| Sugar | 8 | 8 | 8 |
| Fat (g) | 8 | 8 | 8 |
| Omega 3 (mg) | 32 | 860 | 1941 |
| DHA (mg) | Less than 10 | 630 | 1424 |
| EPA (mg) | Less than 10 | 130 | 295 |
| Sodium (mg) | 128 | 128 | 118 |
| Vitamin B1 (mg) | 1.43 | 1.47 | 1.46 |
| Vitamin B2 (mg) | 0.95 | 0.96 | 0.94 |
| Niacin (mg NE) | 4.96 | 5.39 | 5.19 |
| Pantothenic acid (mg) | 4.58 | 4.74 | 4.47 |
| Vitamin B6 (mg) | 1.81 | 1.85 | 1.84 |
| Biotin (µg) | 66.8 | 63.4 | 63.3 |
| Folate (µg) | 128 | 135 | 124 |
| Vitamin B12 (µg) | 1.53 | 1.63 | 1.63 |
| Vitamin E (mg alpha-TE) | 6.70 | 6.16 | 6.01 |
| PCR Primers | Oligomer Sequence (5′-3′) | Amplicon Size |
|---|---|---|
| teloF | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGG TTTGGGTT | >76 bp |
| teloR | GGCTTGCCTTACCCTTACCCTTACCC TTACCCTTACCCT | |
| 36B4F | CAGCAAGTGGGAAGGTGTAATCC | 75 bp |
| 36B4R | CCCATTCTATCATCAACGGGTACAA |
| Parameters | Baseline | ||
| Placebo (n = 20) | Functional Beverage at a Dose of 2600 mg/day (n = 20) | Functional Beverage at a Dose of 6000 mg/day (n = 20) | |
| Age (year) | 49.36 ± 4.25 | 49.61 ± 4.37 (p = 0.884) | 49.41 ± 3.78 (p = 0.852) |
| Body temperature (°C) | 36.05 ± 0.38 | 36.15 ± 0.22 (p = 0.953) | 36.16 ± 0.26 (p = 0.685) |
| Heart rate (beats/min) | 70.18 ± 6.24 | 71.61 ± 7.77 (p = 0.640) | 70.50 ± 7.99 (p = 0.919) |
| Respiratory rate (breaths/min) | 16.18 ± 0.40 | 16.07 ± 0.28 (p = 0.449) | 16.33 ± 0.49 (p = 0.419) |
| Systolic blood pressure (mmHg) | 119.90 ± 22.66 | 114.69 ± 9.94 (p = 0.954) | 111.00 ± 11.82 (p = 0.478) |
| Diastolic blood pressure (mmHg) | 67.90 ± 9.01 | 69.30 ± 6.49 (p = 0.670) | 63.58 ± 8.31 (p = 0.200) |
| Body weight (kg) | 54.71 ± 6.44 | 54.73 ± 7.12 (p = 0.993) | 56.44 ± 3.55 (p = 0.491) |
| Body height (cm) | 156.63 ± 3.14 | 156.23 ± 6.26 (p = 0.844) | 155.91 ± 4.81 (p = 0.732) |
| Body mass index (BMI) (kg/m2) | 22.26 ± 2.17 | 22.35 ± 1.79 (p = 0.977) | 23.22 ± 1.15 (p = 0.460) |
| Parameters | 4 weeks | ||
| Placebo (n = 20) | Functional Beverage at a Dose of 2600 mg/day (n = 20) | Functional Beverage at a Dose of 6000 mg/day (n = 20) | |
| Age (year) | 49.36 ± 4.25 | 49.61 ± 4.37 (p = 0.884) | 49.41 ± 3.78 (p = 0.852) |
| Body temperature (°C) | 36.02 ± 0.33 | 36.17 ± 0.21 (p = 0.112) | 36.15 ± 0.12 (p = 0.100) |
| Heart rate (beats/min) | 72.09 ± 8.68 | 70.61 ± 8.91 (p = 0.681) | 70.66 ± 8.45 (p = 0.697) |
| Respiratory rate (breaths/min) | 16.27 ± 0.79 | 16.53 ± 0.78 (p = 0.470) | 16.66 ± 0.49 (p = 0.209) |
| Systolic blood pressure (mmHg) | 112.18 ± 10.57 | 115.38 ± 10.45 (p = 0.454) | 110.50 ± 9.92 (p = 0.699) |
| Diastolic blood pressure (mmHg) | 69.63 ± 8.37 | 69.69 ± 5.25 (p = 0.985) | 66.16 ± 7.86 (p = 0.257) |
| Body weight (kg) | 55.36 ± 7.28 | 54.55 ± 7.59 (p = 0.769) | 55.90 ± 3.76 (p = 0.836) |
| Body height (cm) | 156.63 ± 3.14 | 156.23 ± 6.26 (p = 0.845) | 156.00 ± 4.86 (p = 0.763) |
| Body mass index (BMI) (kg/m2) | 22.51 ± 2.52 | 22.26 ± 1.86 (p = 0.755) | 22.97 ± 1.19 (p = 0.572) |
| Parameters | 8 weeks | ||
| Placebo (n = 20) | Functional Beverage at a Dose of 2600 mg/day (n = 20) | Functional Beverage at a Dose of 6000 mg/day (n = 20) | |
| Age (year) | 49.36 ± 4.25 | 49.61 ± 4.37 (p = 0.884) | 49.41 ± 3.78 (p = 0.852) |
| Body temperature (°C) | 35.90 ± 0.37 | 36.19 ± 0.26 (p = 0.213) | 36.02 ± 0.29 (p = 0.521) |
| Heart rate (beats/min) | 72.72 ± 10.04 | 72.07 ± 8.40 (p = 0.864) | 72.88 ± 9.29 (p = 0.978) |
| Respiratory rate (breaths/min) | 16.54 ± 0.69 | 16.46 ± 0.66 (p = 0.974) | 16.75 ± 0.87 (p = 0.612) |
| Systolic blood pressure (mmHg) | 106.90 ± 12.19 | 114.00 ± 11.28 (p = 0.132) | 109.75 ± 10.17 (p = 0.548) |
| Diastolic blood pressure (mmHg) | 70.18 ± 9.72 | 70.53 ± 5.35 (p = 0.910) | 67.00 ± 7.51 (p = 0.324) |
| Body weight (kg) | 54.92 ± 7.33 | 54.83 ± 8.03 (p = 0.974) | 55.45 ± 3.85 (p = 0.850) |
| Body height (cm) | 156.63 ± 3.14 | 156.23 ± 6.26 (p = 0.844) | 155.91 ± 4.81 (p = 0.732) |
| Body mass index (BMI) (kg/m2) | 22.35 ± 2.57 | 22.37 ± 2.05 (p = 0.973) | 22.81 ± 1.36 (p = 0.585) |
| Location | Wave | Treatment Group | Baseline | 4 Weeks | 8 Weeks |
|---|---|---|---|---|---|
| Fz | N100 Latency (ms) | Placebo | 114.18 ± 6.62 | 111.54 ± 7.45 | 107.45 ± 5.79 |
| Functional beverage at a dose of 2600 mg/day | 111.61 ± 8.03 (p = 0.418) | 112.07 ± 9.44 (p = 0.882) | 107.07 ± 7.83 (p = 0.904) | ||
| Functional beverage at a dose of 6000 mg/day | 111.83 ± 8.07 (p = 0.467) | 112.50 ± 8.86 (p = 0.794) | 108.75 ± 8.71 (p = 0.685) | ||
| N100 Amplitude (µV) | Placebo | 6.98 ± 3.72 | 6.13 ± 2.64 | 7.45 ± 3.05 | |
| Functional beverage at a dose of 2600 mg/day | 8.92 ± 4.46 (p = 0.259) | 6.84 ± 2.98 (p = 0.258) | 7.71 ± 2.78 (p = 0.830) | ||
| Functional beverage at a dose of 6000 mg/day | 8.17 ± 3.43 (p = 0.424) | 6.40 ± 3.48 (p = 0.460) | 7.78 ± 2.98 (p = 0.787) | ||
| P300 Latency (ms) | Placebo | 345.63 ± 12.13 | 345.90 ± 10.55 | 346.36 ± 15.40 | |
| Functional beverage at a dose of 2600 mg/day | 342.30 ± 13.69 (p = 0.542) | 343.76 ± 12.97 (p = 0.661) | 340.61 ± 10.41 (p = 0.311) | ||
| Functional beverage at a dose of 6000 mg/day | 340.91 ± 13.55 (p = 0.397) | 344.41 ± 11.54 (p = 0.764) | 332.00 ± 15.01 * (p = 0.017) | ||
| P300 Amplitude (µV) | Placebo | 21.65 ± 5.22 | 22.39 ± 4.13 | 23.44 ± 4.53 | |
| Functional beverage at a dose of 2600 mg/day | 25.26 ± 7.81 (p = 0.171) | 24.84 ± 5.94 (p = 0.381) | 28.71± 5.35 * (p = 0.020) | ||
| Functional beverage at a dose of 6000 mg/day | 22.55 ± 5.22 (p = 0.736) | 29.39 ± 8.60 * (p = 0.022) | 29.16 ± 4.60 * (p = 0.017) | ||
| Cz | N100 Latency (ms) | Placebo | 113.81 ± 6.05 | 113.72 ± 6.71 | 109.54 ± 7.90 |
| Functional beverage at a dose of 2600 mg/day | 112.00 ± 5.92 (p = 0.514) | 111.76 ± 8.68 (p = 0.563) | 108.00 ± 7.40 (p = 0.612) | ||
| Functional beverage at a dose of 6000 mg/day | 110.33 ± 8.02 (p = 0.223) | 113.00 ± 8.82 (p = 0.833) | 111.41 ± 6.82 (p = 0.547) | ||
| N100 Amplitude (µV) | Placebo | 7.28 ± 2.90 | 5.06 ± 2.01 | 7.73 ± 3.06 | |
| Functional beverage at a dose of 2600 mg/day | 7.78 ± 3.52 (p = 0.401) | 8.51 ± 2.77 ** (p = 0.001) | 8.02 ± 3.59 (p = 0.827) | ||
| Functional beverage at a dose of 6000 mg/day | 7.55 ± 1.42 (p = 0.268) | 7.20 ± 3.15 * (p = 0.029) | 7.39 ± 2.91 (p = 0.803) | ||
| P300 Latency (ms) | Placebo | 341.90 ± 12.43 | 348.00 ± 11.58 | 346.54 ± 16.74 | |
| Functional beverage at a dose of 2600 mg/day | 344.07 ± 10.81 (p = 0.653) | 347.76 ± 10.10 (p = 0.960) | 343.53 ± 9.42 (p = 0.604) | ||
| Functional beverage at a dose of 6000 mg/day | 337.41 ± 11.80 (p = 0.362) | 346.33 ± 11.95 (p = 0.724) | 337.41 ± 15.38 (p = 0.128) | ||
| P300 Amplitude (µV) | Placebo | 20.40 ± 4.78 | 19.65 ± 5.84 | 22.83 ± 7.24 | |
| Functional beverage at a dose of 2600 mg/day | 24.46 ± 9.17 (p = 0.118) | 24.56 ± 5.77 (p = 0.091) | 20.67 ± 5.01 (p = 0.436) | ||
| Functional beverage at a dose of 6000 mg/day | 23.34 ± 6.80 (p = 0.235) | 26.28 ± 7.03 * (p = 0.028) | 25.97 ± 6.45 (p = 0.260) |
| Cognitive Domains | Test Items | Treatment Group | Baseline | 4 Weeks | 8 Weeks |
|---|---|---|---|---|---|
| Word Recognition | Time | Placebo | 1150.43 ± 234.14 | 1186.94 ± 224.87 | 1179.63 ± 211.74 |
| Functional beverage at a dose of 2600 mg/day | 1228.28 ± 291.10 (p = 0.430) | 1129.06 ± 178.61 (p = 0.447) | 1071.68 ± 145.15 (p = 0.107) | ||
| Functional beverage at a dose of 6000 mg/day | 1141.21 ± 164.94 (p = 0.927) | 1026.17 ± 128.86 * (p = 0.046) | 1024.64 ± 111.11 * (p = 0.026) | ||
| %Accuracy | Placebo | 87.87 ± 7.64 | 85.75 ± 8.18 | 90.00 ± 8.69 | |
| Functional beverage at a dose of 2600 mg/day | 84.87 ± 10.60 (p = 0.465) | 89.48 ± 8.15 (p = 0.319) | 86.66 ± 9.91 (p = 0.980) | ||
| Functional beverage at a dose of 6000 mg/day | 92.22 ± 6.41 (p = 0.225) | 93.33 ± 4.71 * (p = 0.020) | 90.00 ± 9.21 (p = 0.925) | ||
| Picture Recognition | Time | Placebo | 1227.98 ± 203.11 | 1232.52 ± 167.04 | 1219.79 ± 212.98 |
| Functional beverage at a dose of 2600 mg/day | 1326.06 ± 352.77 (p = 0.543) | 1180.59 ± 197.54 (p = 0.480) | 1206.40 ± 260.07 (p = 0.882) | ||
| Functional beverage at a dose of 6000 mg/day | 1246.29 ± 201.84 (p = 0.854) | 1139.84 ± 162.30 (p = 0.219) | 1113.67 ± 170.02 (p = 0.254) | ||
| %Accuracy | Placebo | 86.81 ± 8.15 | 89.09 ± 5.39 | 88.63 ± 6.74 | |
| Functional beverage at a dose of 2600 mg/day | 90.00 ± 10.41 (p = 0.289) | 88.46 ± 7.18 (p = 0.785) | 89.23 ± 8.13 (p = 0.404) | ||
| Functional beverage at a dose of 6000 mg/day | 92.50 ± 5.44 (p = 0.091) | 92.50 ± 5.84 (p = 0.161) | 91.25 ± 5.28 (p = 0.213) | ||
| Simple Reaction | Time | Placebo | 620.10 ± 114.57 | 668.66 ± 109.58 | 654.99 ± 164.52 |
| Functional beverage at a dose of 2600 mg/day | 649.34 ± 117.93 (p = 0.530) | 593.40 ± 70.39 * (p = 0.035) | 624.16 ± 100.14 (p = 0.581) | ||
| Functional beverage at a dose of 6000 mg/day | 641.42 ± 104.44 (p = 0.653) | 568.79 ± 57.36 ** (p = 0.007) | 631.08 ± 137.80 (p = 0.674) | ||
| Digit Vigilance | Time | Placebo | 631.92 ± 46.53 | 662.61 ± 57.83 | 642.35 ± 53.98 |
| Functional beverage at a dose of 2600 mg/day | 620.92 ± 56.16 (p = 0.931) | 644.25 ± 52.91 (p = 0.401) | 642.32 ± 39.41 (p = 0.839) | ||
| Functional beverage at a dose of 6000 mg/day | 657.18 ± 40.21 (p = 0.140) | 641.63 ± 47.06 (p = 0.346) | 649.28 ± 46.23 (p = 0.498) | ||
| %Accuracy | Placebo | 95.10 ± 5.49 | 94.05 ± 5.99 | 95.22 ± 5.53 | |
| Functional beverage at a dose of 2600 mg/day | 96.25 ± 3.83 (p = 0.765) | 95.85 ± 5.00 (p = 0.374) | 95.06 ± 4.28 (p = 0.598) | ||
| Functional beverage at a dose of 6000 mg/day | 95.62 ± 3.81 (p = 0.950) | 95.51 ± 2.81 (p = 0.876) | 94.65 ± 4.30 (p = 0.556) | ||
| Choice Reaction Time | Time | Placebo | 800.03 ± 116.80 | 816.85 ± 104.14 | 801.82 ± 107.78 |
| Functional beverage at a dose of 2600 mg/day | 812.59 ± 105.03 (p = 0.768) | 794.97 ± 107.00 (p = 0.604) | 814.06 ± 72.50 (p = 0.354) | ||
| Functional beverage at a dose of 6000 mg/day | 799.58 ± 85.96 (p = 0.992) | 786.04 ± 94.40 (p = 0.475) | 785.19 ± 89.79 (p = 0.865) | ||
| %Accuracy | Placebo | 98.54 ± 1.57 | 97.45 ± 2.02 | 98.90 ± 1.64 | |
| Functional beverage at a dose of 2600 mg/day | 97.38 ± 2.22 (p = 0.178) | 98.92 ± 1.32 (p = 0.052) | 98.92 ± 1.75 (p = 0.973) | ||
| Functional beverage at a dose of 6000 mg/day | 98.00 ± 2.26 (p = 0.643) | 98.33 ± 2.23 (p = 0.177) | 97.50 ± 2.28 (p = 0.095) | ||
| Spatial Memory | Time | Placebo | 1390.59 ± 314.27 | 1348.38 ± 253.38 | 1471.43 ± 235.28 |
| Functional beverage at a dose of 2600 mg/day | 1460.04 ± 410.53 (p = 0.582) | 1320.48 ± 158.46 (p = 0.797) | 1272.64 ± 236.83 * (p = 0.049) | ||
| Functional beverage at a dose of 6000 mg/day | 1226.30 ± 191.46 (p = 0.196) | 1258.69 ± 349.11 (p = 0.420) | 1163.61 ± 226.35 ** (p = 0.004) | ||
| %Accuracy | Placebo | 87.50 ± 7.20 | 92.17 ± 7.01 | 91.91 ± 9.66 | |
| Functional beverage at a dose of 2600 mg/day | 87.00 ± 15.25 (p = 0.921) | 92.50 ± 13.15 (p = 0.201) | 94.23 ± 7.21 (p = 0.857) | ||
| Functional beverage at a dose of 6000 mg/day | 93.74 ± 10.27 (p = 0.223) | 95.13 ± 5.70 (p = 0.245) | 94.44 ± 7.95 (p = 0.652) | ||
| Numeric Working Memory | Time | Placebo | 1199.77 ± 295.98 | 1117.28 ± 152.77 | 1107.26 ± 212.83 |
| Functional beverage at a dose of 2600 mg/day | 1101.40 ± 126.64 (p = 0.258) | 1019.02 ± 136.67 (p = 0.110) | 1005.46 ± 153.27 (p = 0.182) | ||
| Functional beverage at a dose of 6000 mg/day | 1104.45 ± 182.70 (p = 0.282) | 1037.64 ± 149.55 (p = 0.200) | 1013.33 ± 181.12 (p = 0.226) | ||
| %Accuracy | Placebo | 96.36 ± 6.23 | 97.57 ± 3.97 | 95.15 ± 6.03 | |
| Functional beverage at a dose of 2600 mg/day | 92.56 ± 10.73 (p = 0.319) | 92.56 ± 10.11 (p = 0.262) | 92.82 ± 12.16 (p = 0.576) | ||
| Functional beverage at a dose of 6000 mg/day | 93.33 ± 9.75 (p = 0.443) | 94.16 ± 8.89 (p = 0.502) | 96.11 ± 5.29 (p = 0.600) |
| Parameters | Treatment Group | Baseline | 4 Weeks | 8 Weeks |
|---|---|---|---|---|
| MDA (umol/mg protein) | Placebo | 8.45 ± 5.41 | 7.72 ± 4.43 | 4.04 ± 1.28 |
| Functional beverage at a dose of 2600 mg/day | 9.04 ± 6.35 (p = 0.822) | 5.45 ± 2.23 (p = 0.088) | 5.11 ± 1.47 (p = 0.156) | |
| Functional beverage at a dose of 6000 mg/day | 10.64 ± 6.30 (p = 0.401) | 4.98 ± 1.47 * (p = 0.041) | 5.76 ±2.46(p = 0.385) | |
| SOD (U/mg protein) | Placebo | 12.68 ± 4.06 | 15.46 ± 1.78 | 18.64 ± 8.21 |
| Functional beverage at a dose of 2600 mg/day | 11.76 ± 2.87 (p = 0.740) | 12.94 ± 4.11 (p = 0.180) | 16.04 ± 8.34 (p = 0.535) | |
| Functional beverage at a dose of 6000 mg/day | 14.43± 5.66 (p = 0.514) | 13.74 ± 2.07 (p = 0.368) | 25.46 ± 1.99 * (p = 0.037) | |
| CAT (U/mg protein) | Placebo | 23.52 ± 0.83 | 23.86 ±4.74 | 21.07 ± 2.39 |
| Functional beverage at a dose of 2600 mg/day | 22.11 ± 1.86 (p = 0.127) | 24.38 ± 8.05 (p = 0.974) | 23.69 ± 5.46 (p = 0.388) | |
| Functional beverage at a dose of 6000 mg/day | 22.46 ± 1.92(p = 0.304) | 21.92 ± 2.88 (p = 0.703) | 24.24 ± 5.64 (p = 0.269) | |
| GPx. (U/mg protein) | Placebo | 0.09 ± 0.07 | 0.07 ± 0.05 | 0.17 ± 0.02 |
| Functional beverage at a dose of 2600 mg/day | 0.08 ± 0.05 (p = 0.671) | 0.07 ± 0.05 (p = 0.938) | 0.18 ± 0.12 (p = 0.937) | |
| Functional beverage at a dose of 6000 mg/day | 0.10 ± 0.06 (p = 0.793) | 0.13 ± 0.08 * (p = 0.042) | 0.18 ± 0.07 (p = 0.839) | |
| TNF-α (ng/mL) | Placebo | 1.13 ± 0.82 | 0.83 ± 0.57 | 1.23 ± 0.72 |
| Functional beverage at a dose of 2600 mg/day | 0.97 ± 0.38 (p = 0.599) | 0.99 ± 0.50 (p = 0.469) | 0.93 ± 0.59 (p = 0.281) | |
| Functional beverage at a dose of 6000 mg/day | 0.92 ± 0.44 (p = 0.509) | 0.52 ± 0.26 * (p = 0.044) | 0.58 ± 0.24 * (p = 0.029) | |
| IL-6 (pg/mL) | Placebo | 31.40 ± 4.59 | 36.64 ± 4.08 | 38.04 ± 5.90 |
| Functional beverage at a dose of 2600 mg/day | 31.65 ± 6.88 (p = 0.975) | 29.96 ± 4.28 (p = 0.380) | 29.28 ± 4.42 (p = 0.276) | |
| Functional beverage at a dose of 6000 mg/day | 24.77 ± 5.21 (p = 0.447) | 26.22 ± 6.71 (p = 0.202) | 24.28 ± 4.62 * (p = 0.046) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattanathorn, J.; Thukham-mee, W.; Tong-un, T.; Sangartit, W.; Somboonporn, W.; Paholpak, P. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, 8-Week Pilot Study of Tuna-Byproduct-Derived Novel Supplements for Managing Cellular Senescence and Cognitive Decline in Perimenopausal and Postmenopausal Women. Antioxidants 2025, 14, 520. https://doi.org/10.3390/antiox14050520
Wattanathorn J, Thukham-mee W, Tong-un T, Sangartit W, Somboonporn W, Paholpak P. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, 8-Week Pilot Study of Tuna-Byproduct-Derived Novel Supplements for Managing Cellular Senescence and Cognitive Decline in Perimenopausal and Postmenopausal Women. Antioxidants. 2025; 14(5):520. https://doi.org/10.3390/antiox14050520
Chicago/Turabian StyleWattanathorn, Jintanaporn, Wipawee Thukham-mee, Terdthai Tong-un, Weerapon Sangartit, Woraluck Somboonporn, and Pongsatorn Paholpak. 2025. "A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, 8-Week Pilot Study of Tuna-Byproduct-Derived Novel Supplements for Managing Cellular Senescence and Cognitive Decline in Perimenopausal and Postmenopausal Women" Antioxidants 14, no. 5: 520. https://doi.org/10.3390/antiox14050520
APA StyleWattanathorn, J., Thukham-mee, W., Tong-un, T., Sangartit, W., Somboonporn, W., & Paholpak, P. (2025). A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, 8-Week Pilot Study of Tuna-Byproduct-Derived Novel Supplements for Managing Cellular Senescence and Cognitive Decline in Perimenopausal and Postmenopausal Women. Antioxidants, 14(5), 520. https://doi.org/10.3390/antiox14050520






