Optimized Sambucus nigra L., Epilobium hirsutum L., and Lythrum salicaria L. Extracts: Biological Effects Supporting Their Potential in Wound Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Harvesting of Plant Material and Preparation for Extraction
2.3. QbD Approach for Extract Development and Optimization
2.3.1. Quality Target Product Profile (QTPP) Definition
2.3.2. Risk Analysis
2.3.3. Design of Experiment (DoE)
2.3.4. Extraction Methods and Bioactive Compounds Screening
2.4. Characterization of OHEs
2.4.1. Identification and Quantification of Polyphenolic Compounds
2.4.2. Identification and Quantification of Phytosterols
2.4.3. Identification and Quantification of Tocopherols
2.4.4. Identification and Quantification of Procyanidins
2.5. Evaluation of Biological Activities of the OHEs
2.5.1. Antioxidant Activity of the OHEs
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Capacity
Ferric Reducing Antioxidant Power (FRAP) Assay
Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.5.2. Antibacterial Activity of the OHEs
2.5.3. In Vitro Cell Culture Assays for the OHEs
Preparation of OHE Solutions
Cell Cultures
Cell Viability
Antioxidant Activity in Cell Cultures
Anti-Inflammatory Activity in Cell Cultures
Measurement of Nitric Oxide (NO) Production
Wound Healing Assay in Cell Cultures
2.6. Statistical Analysis
3. Results and Discussion
3.1. QbD Approach for Extract Development and Optimization
3.1.1. Definition of QTPP
3.1.2. Results of the Risk Analysis
3.1.3. Summary of Fit
3.1.4. The Influences of Extraction Conditions on the TFC and TPC of the HEs
3.1.5. The Optimization of the HEs
3.2. Results of the Characterization of the OHEs
3.3. Results of Biological Activities Evaluation of the OHEs
3.3.1. Results of the Antioxidant Activity of the OHEs
3.3.2. Results of the Antibacterial Activity of the OHEs
3.3.3. Results of the In Vitro Cell Culture Assays for the OHEs
Results of the Cell Viability
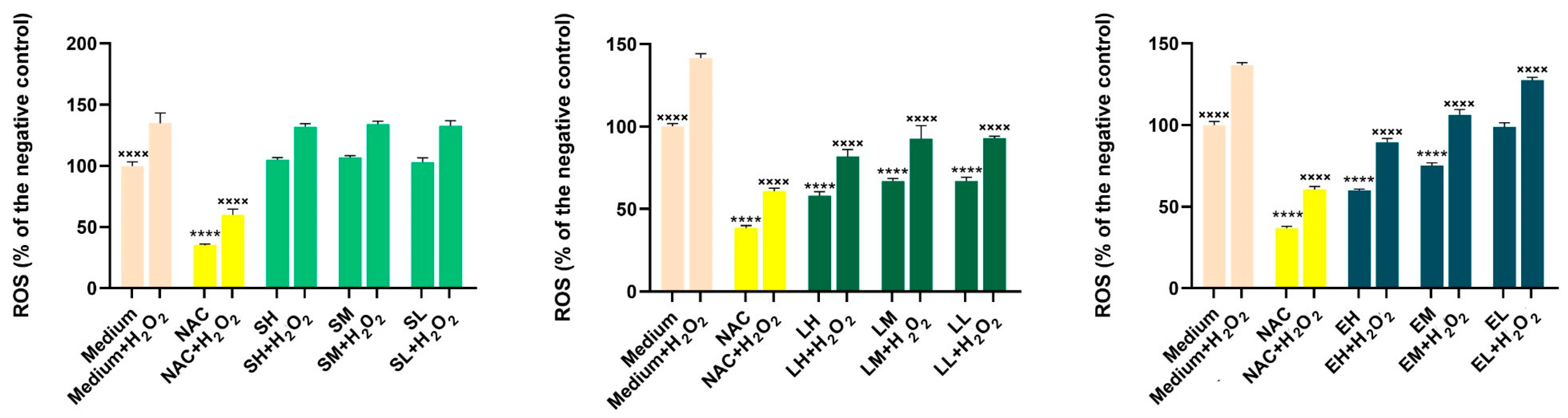
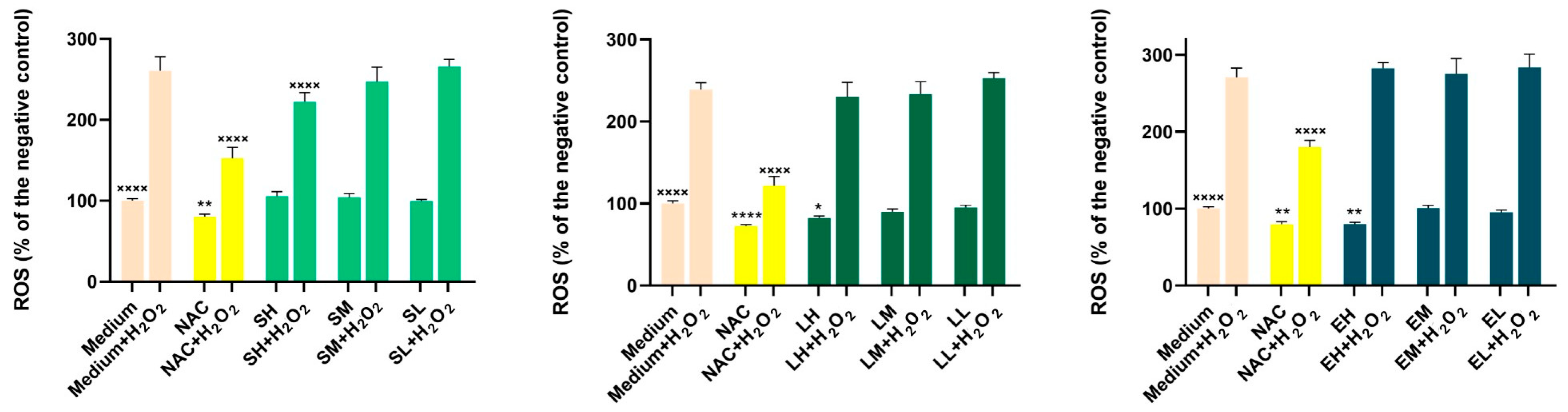
Results of the Antioxidant Activity in Cell Cultures
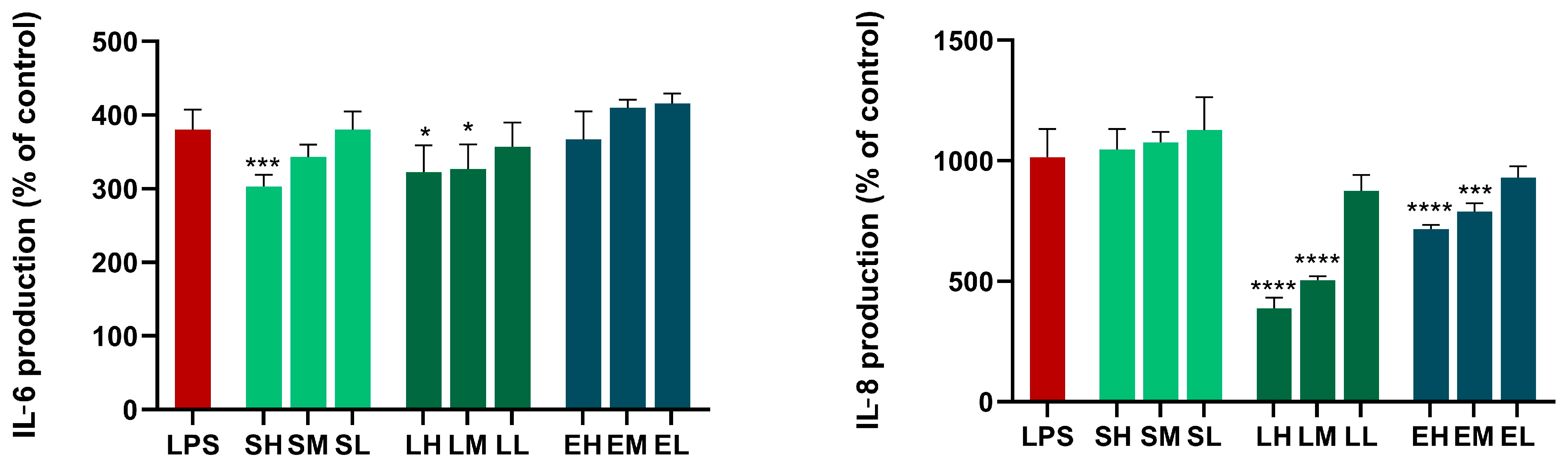
Results of the Anti-Inflammatory Activity in Cell Cultures
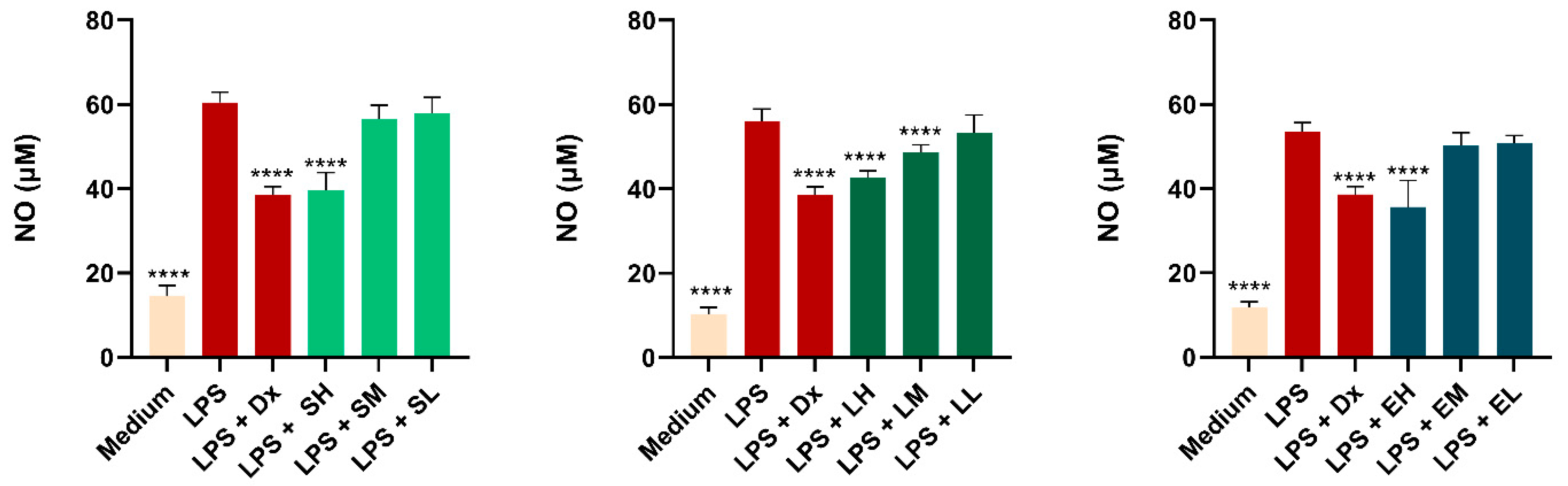
Results of the Measurement of Nitric Oxide (NO) Production
Results of the Wound Healing Assay
3.4. Study Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HEs | Herbal extracts of Sambucus nigra, Epilobium hirsutum, and Lythrum salicaria from this study before optimization or other extracts from these plant materials previously reported in the literature when used for comparison |
| OHEs | Optimized herbal extracts of Sambucus nigra, Epilobium hirsutum, and Lythrum salicaria after being optimized in this study |
| UTE | Ultra-turrax-assisted extraction |
| USE | Ultrasound-assisted extraction |
| TPC | Total polyphenol content, mg GAE/mL HE = mg gallic acid equivalents/mL of HE |
| TFC | Total flavonoid content, mM QAE/mL HE = mM quercetin equivalents/mL of HE |
| MIC | Minimum inhibitory concentration |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity, Trolox equivalents (TEs) mg/mL of OHE. |
| FRAP | Ferric reducing antioxidant power, Trolox equivalents (TEs) mM/OHE. |
| TEAC | Trolox equivalent antioxidant capacity, Trolox equivalents (TEs) mM/OHE. |
References
- Hoang, H.T.; Moon, J.Y.; Lee, Y.C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Safta, D.A.; Bogdan, C.; Moldovan, M.L. Vesicular Nanocarriers for Phytocompounds in Wound Care: Preparation and Characterization. Pharmaceutics 2022, 14, 991. [Google Scholar] [CrossRef] [PubMed]
- Safta, D.A.; Bogdan, C.; Moldovan, M.L. SLNs and NLCs for Skin Applications: Enhancing the Bioavailability of Natural Bioactives. Pharmaceutics 2024, 16, 1270. [Google Scholar] [CrossRef]
- Pathak, D.; Mazumder, A. A critical overview of challenging roles of medicinal plants in improvement of wound healing technology. DARU J. Pharm. Sci. 2024, 32, 379–419. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Hosseinkhani, A.; Falahatzadeh, M.; Raoofi, E.; Zarshenas, M.M. An Evidence-Based Review on Wound Healing Herbal Remedies From Reports of Traditional Persian Medicine. J. Evid. Based Complement. Altern. Med. 2016, 22, 334–343. [Google Scholar] [CrossRef]
- Schoukens, G. Bioactive dressings to promote wound healing. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing: Cambridge, UK; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–167. [Google Scholar]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 341–370. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Balderas-Cordero, D.; Canales-Alvarez, O.; Sánchez-Sánchez, R.; Cabrera-Wrooman, A.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. Anti-Inflammatory and Histological Analysis of Skin Wound Healing through Topical Application of Mexican Propolis. Int. J. Mol. Sci. 2023, 24, 11831. [Google Scholar] [CrossRef]
- Allaw, M.; Manca, M.L.; Gómez-Fernández, J.C.; Pedraz, J.L.; Terencio, M.C.; Sales, O.D.; Nacher, A.; Manconi, M. Oleuropein Multicompartment Nanovesicles Enriched with Collagen as A Natural Strategy for the Treatment of Skin Wounds Connected with Oxidative Stress. Nanomedicine 2021, 16, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Nasab, M.E.; Takzaree, N.; Saffaria, P.M.; Partoazar, A. In vitro antioxidant activity and in vivo wound-healing effect of lecithin liposomes: A comparative study. J. Comp. Eff. Res. 2019, 8, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Ajmal, G.; Upadhyay, S.N.; Mishra, P.K. Nano-fibrous scaffold with curcumin for anti-scar wound healing. Int. J. Pharm. 2020, 589, 119858. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Manca, M.L.; Marongiu, F.; Caddeo, C.; Castangia, I.; Petretto, G.L.; Pintoreb, G.; Saraisa, G.; D’hallewinc, G.; Zarud, M.; et al. Chemical characterization of Citrus limon var. pompia and incorporation in phospholipid vesicles for skin delivery. Int. J. Pharm. 2016, 506, 449–457. [Google Scholar] [CrossRef]
- Moulaoui, K.; Caddeo, C.; Manca, M.L.; Castangia, I.; Valenti, D.; Escribano, E.; Atmani, D.; Fadda, A.M.; Manconi, M. Identification and nanoentrapment of polyphenolic phytocomplex from Fraxinus angustifolia: In vitro and in vivo wound healing potential. Eur. J. Med. Chem. 2015, 89, 179–188. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Antibacterial activity of medicinal plants and their role in wound healing. Future J. Pharm. Sci. 2024, 10, 68. [Google Scholar] [CrossRef]
- Kassam, N.A.; Damian, D.J.; Kajeguka, D.; Nyombi, B.; Kibiki, G.S. Spectrum and antibiogram of bacteria isolated from patients presenting with infected wounds in a Tertiary Hospital, northern Tanzania. BMC Res. Notes 2017, 10, 757. [Google Scholar] [CrossRef]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A. An Overview on the Recent Advances in the Treatment of Infected Wounds: Antibacterial Wound Dressings. Macromol. Biosci. 2022, 22, e2200014. [Google Scholar] [CrossRef]
- Arip, M.; Selvaraja, M.; Mogana, R.; Tan, L.F.; Leong, M.Y.; Tan, P.L.; Yap, V.L.; Chinnapan, S.; Tat, N.C.; Abdullah, M.; et al. Review on Plant-Based Management in Combating Antimicrobial Resistance-Mechanistic Perspective. Front. Pharmacol. 2022, 13, 879495. [Google Scholar] [CrossRef]
- Srivastava, J.; Chandra, H.; Nautiyal, A.R.; Kalra, S.J.S. Antimicrobial resistance (AMR) and plant-derived antimicrobials (PDAms) as an alternative drug line to control infections. Biotech 2014, 4, 451–460. [Google Scholar] [CrossRef]
- Sibanda, T.; Okoh, A.I. The challenges of overcoming antibiotic resistance: Plant extracts as potential sources of antimicrobial and resistance modifying agents. Afr. J. Biotechnol. 2007, 6, 2886–2896. [Google Scholar]
- Safta, D.A.; Ielciu, I.; Șuștic, R.; Hanganu, D.; Niculae, M.; Cenariu, M.; Pall, E.; Moldovan, M.L.; Achim, M.; Bogdan, C.; et al. Chemical Profile and Biological Effects of an Herbal Mixture for the Development of an Oil-in-Water Cream. Plants 2023, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Vlase, A.M.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizesan, I.; Nadas, G.C.; Novac, C.Ș.; Tamas, M.; et al. Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants 2023, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Solcan, M.B.; Fizeșan, I.; Vlase, L.; Vlase, A.M.; Rusu, M.E.; Mateș, L.; Petru, A.-E.; Creștin, I.-V.; Tomuță, I.; Popa, D.-S. Phytochemical Profile and Biological Activities of Extracts Obtained from Young Shoots of Blackcurrant (Ribes nigrum L.), European Blueberry (Vaccinium myrtillus L.), and Mountain Cranberry (Vaccinium vitis-idaea L.). Horticulturae 2023, 9, 1163. [Google Scholar] [CrossRef]
- Bogdan, C.; Safta, D.A.; Iurian, S.; Petrușcă, D.R.; Moldovan, M.L. QbD Approach in Cosmetic Cleansers Research: The Development of a Moisturizing Cleansing Foam Focusing on Thickener, Surfactants, and Polyols Content. Gels 2024, 10, 484. [Google Scholar] [CrossRef]
- Skowrońska, W.; Granica, S.; Piwowarski, J.P.; Jakupović, L.; Zovko Končić, M.; Bazylko, A. Wound healing potential of extract from Sambucus nigra L. leaves and its fractions. J. Ethnopharmacol. 2024, 320, 117423. [Google Scholar] [CrossRef]
- Sala, G.; Pasta, S.; Maggio, A.; La Mantia, T. Sambucus nigra L. (fam. Viburnaceae) in Sicily: Distribution, Ecology, Traditional Use and Therapeutic Properties. Plants 2023, 12, 3457. [Google Scholar] [CrossRef]
- Tiboc Schnell, C.N.; Filip, G.A.; Decea, N.; Moldovan, R.; Opris, R.; Man, S.C.; Moldovan, B.; David, L.; Tabăran, F.; Olteanu, D.; et al. The impact of Sambucus nigra L. extract on inflammation, oxidative stress and tissue remodeling in a rat model of lipopolysaccharide-induced subacute rhinosinusitis. Inflammopharmacology 2021, 29, 753–769. [Google Scholar] [CrossRef]
- Wójciak, M.; Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z.; Szczepanek, D.; Oniszczuk, T.; Sowa, I. Anti-Inflammatory and Protective Effects of Water Extract and Bioferment from Sambucus nigra Fruit in LPS-Induced Human Skin Fibroblasts. Int. J. Mol. Sci. 2023, 24, 10286. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Paczkowska-Walendowska, M.; Woźna, Z.; Plech, T.; Szulc, P.; Cielecka-Piontek, J. Elderberry Leaves with Antioxidant and Anti-Inflammatory Properties as a Valuable Plant Material for Wound Healing. Pharmaceuticals 2024, 17, 618. [Google Scholar] [CrossRef]
- Seymenska, D.; Teneva, D.; Nikolova, I.; Benbassat, N.; Denev, P. In Vivo Anti-Inflammatory and Antinociceptive Activities of Black Elder (Sambucus nigra L.) Fruit and Flower Extracts. Pharmaceuticals 2024, 17, 409. [Google Scholar] [CrossRef] [PubMed]
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Health-Promoting Properties: Anti-Inflammatory and Anticancer Properties of Sambucus nigra L. Flowers and Fruits. Molecules 2023, 28, 6235. [Google Scholar] [CrossRef] [PubMed]
- Santin, J.R.; Benvenutti, L.; Broering, M.F.; Nunes, R.; Goldoni, F.C.; Patel, Y.B.K.; de Souza, J.A.; Kopp, M.A.T.; de Souza, P.; da Silva, R.d.C.V.; et al. Sambucus nigra: A traditional medicine effective in reducing inflammation in mice. J. Ethnopharmacol. 2022, 283, 114736. [Google Scholar] [CrossRef]
- Laurutis, A.; Liobikas, J.; Stanciauskaite, M.; Marksa, M.; Ramanauskiene, K.; Majiene, D. Comparison of the Formulation, Stability and Biological Effects of Hydrophilic Extracts from Black Elder Flowers (Sambucus nigra L.). Pharmaceutics 2022, 14, 2831. [Google Scholar] [CrossRef]
- Karakaya, S.; Süntar, I.; Yakinci, O.F.; Sytar, O.; Ceribasi, S.; Dursunoglu, B.; Ozbek, H.; Guvenalp, Z. In vivo bioactivity assessment on Epilobium species: A particular focus on Epilobium angustifolium and its components on enzymes connected with the healing process. J. Ethnopharmacol. 2020, 262, 113207. [Google Scholar] [CrossRef]
- Nowak, A.; Zagórska-Dziok, M.; Ossowicz-Rupniewska, P.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Adamiak-Giera, U.; Prowans, P.; Czapla, N.; Bargiel, P.; et al. Epilobium angustifolium L. Extracts as Valuable Ingredients in Cosmetic and Dermatological Products. Molecules 2021, 26, 3456. [Google Scholar] [CrossRef]
- Nowak, A.; Zagórska-Dziok, M.; Perużyńska, M.; Cybulska, K.; Kucharska, E.; Ossowicz-Rupniewska, P.; Piotrowska, K.; Duchnik, W.; Kucharski, Ł.; Sulikowski, T.; et al. Assessment of the Anti-Inflammatory, Antibacterial and Anti-Aging Properties and Possible Use on the Skin of Hydrogels Containing Epilobium angustifolium L. Extracts. Front. Pharmacol. 2022, 13, 896706. [Google Scholar]
- Nowak, A.; Duchnik, W.; Makuch, E.; Kucharski, Ł.; Ossowicz-rupniewska, P.; Cybulska, K.; Sulikowski, T.; Moritz, M.; Klimowicz, A. Epilobium angustifolium L. Essential Oil—Biological Activity and Enhancement of the Skin Penetration of Drugs—In Vitro Study. Molecules 2021, 26, 7188. [Google Scholar] [CrossRef]
- Kiss, A.K.; Bazylko, A.; Filipek, A.; Granica, S.; Jaszewska, E.; Kiarszys, U.; Kośmider, A.; Piwowarski, J. Oenothein B’s contribution to the anti-inflammatory and antioxidant activity of Epilobium sp. Phytomedicine 2011, 18, 557–560. [Google Scholar] [CrossRef]
- Ak, G.; Zengin, G.; Mahomoodally, M.F.; Llorent-Martínez, E.; Orlando, G.; Chiavaroli, A.; Brunetti, L.; Recinella, L.; Leone, S.; Di Simone, S.C.; et al. Shedding light into the connection between chemical components and biological effects of extracts from Epilobium hirsutum: Is it a potent source of bioactive agents from natural treasure? Antioxidants 2021, 10, 1389. [Google Scholar] [CrossRef]
- Nowak, A.; Zielonka-Brzezicka, J.; Perużyńska, M.; Klimowicz, A. Epilobium angustifolium L. as a Potential Herbal Component of Topical Products for Skin Care and Treatment—A Review. Molecules 2022, 27, 3536. [Google Scholar] [CrossRef] [PubMed]
- Piwowarski, J.P.; Kiss, A.K. Contribution of C-glucosidic ellagitannins to Lythrum salicaria L. influence on pro-inflammatory functions of human neutrophils. J. Nat. Med. 2014, 69, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Piwowarski, J.P.; Granica, S.; Kiss, A.K. Lythrum salicaria L.—Underestimated medicinal plant from European traditional medicine. A review. J. Ethnopharmacol. 2015, 170, 226–250. [Google Scholar] [CrossRef] [PubMed]
- Tunalier, Z.; Koşar, M.; Küpeli, E.; Çaliş, I.; Başer, K.H.C. Antioxidant, anti-inflammatory, anti-nociceptive activities and composition of Lythrum salicaria L. extracts. J. Ethnopharmacol. 2007, 110, 539–547. [Google Scholar] [CrossRef]
- Vafi, F.; Bahramsoltani, R.; Abdollahi, M.; Manayi, A.; Abdolghaffari, A.H.; Samadi, N.; Amin, G.; Hassanzadeh, G.; Jamalifar, H.; Baeeri, M.; et al. Burn Wound Healing Activity of Lythrum salicaria L. and Hypericum scabrum L. Index Wounds 2016, 28, 448–458. [Google Scholar]
- Jouravel, G.; Guénin, S.; Bernard, F.X.; Elfakir, C.; Bernard, P.; Himbert, F. New Biological Activities of Lythrum salicaria L.: Effects on Keratinocytes, Reconstructed Epidermis and Reconstructed Skins, Applications in Dermo-Cosmetic Sciences. Cosmetics 2017, 4, 52. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Ivanauskas, L.; Bezruk, I.; Petrikaitė, V.; Georgiyants, V. Application of Quality by Design Approach to the Pharmaceutical Development of Anticancer Crude Extracts of Crocus sativus Perianth. Sci. Pharm. 2022, 90, 19. [Google Scholar] [CrossRef]
- Pașca, D.; Frangiamone, M.; Mangiapelo, L.; Vila-Donat, P.; Mîrza, O.; Vlase, A.M.; Miere, D.; Filip, L.; Mañes, J.; Loghin, F.; et al. Phytochemical Characterization of Bilberries and Their Potential as a Functional Ingredient to Mitigate Ochratoxin A Toxicity in Cereal-Based Products. Nutrients 2024, 16, 3137. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 2, 208. [Google Scholar] [CrossRef]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic compounds of blackthorn (Prunus spinosa L.) and influence of in vitro digestion on their antioxidant capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.M.; Nadăș, G.C.; Matei, I.A.; Filip, G.A.; Vlase, L.; Crișan, G. The Effect of Extraction Methods on Phytochemicals and Biological Activities of Green Coffee Beans Extracts. Plants 2023, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Oniga, I.; Hanganu, D.; Gheldiu, A.M.; Puşcaş, C.; Silaghi-Dumitrescu, R.; Duma, M.; Tiperciuc, B.; Vârban, R.; Vlase, L. Sources for developing new medicinal products: Biochemical investigations on alcoholic extracts obtained from aerial parts of some Romanian Amaryllidaceae species. BMC Complement. Altern. Med. 2018, 18, 226. [Google Scholar] [CrossRef] [PubMed]
- Carpa, R.; Drăgan-Bularda, M.; Muntean, V. Microbiologie Generala—Lucrari Practice; Presa Universitara Clujeana: Cluj-Napoca, Romania, 2014. [Google Scholar]
- Ungurean, C.; Carpa, R.; Campean, R.; Maior, M.C.; Olah, N.K. Phytochemical and microbial analyses of Berberis sp. extracts. Rom Biotechnol. Lett. 2020, 25, 2132–2139. [Google Scholar] [CrossRef]
- Pop, A.; Bogdan, C.; Fizesan, I.; Iurian, S.; Carpa, R.; Bacali, C.; Vlase, L.; Benedec, D.; Moldovan, M.L. In Vitro Evaluation of Biological Activities of Canes and Pomace Extracts from Several Varieties of Vitis vinifera L. for Inclusion in Freeze-Drying Mouthwashes. Antioxidants 2022, 11, 218. [Google Scholar] [CrossRef]
- EUCAST: MIC Determination of Non-Fastidious and Fastidious Organisms [Internet]. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 7 March 2025).
- Pop, A.; Fizesan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L. Cell Viability Assays. 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/?report=reader (accessed on 7 March 2025).
- Rusu, M.E.; Fizeșan, I.; Pop, A.; Gheldiu, A.M.; Mocan, A.; Crișan, G.; Vlase, L.; Loghin, F.; Popa, D.-S.; Tomuta, I. Enhanced recovery of antioxidant compounds from hazelnut (Corylus avellana L.) involucre based on extraction optimization: Phytochemical profile and biological activities. Antioxidants 2019, 8, 460. [Google Scholar] [CrossRef]
- Hwang, J.H.; Ma, J.N.; Park, J.H.; Jung, H.W.; Park, Y.-K. Anti-inflammatory and antioxidant effects of MOK, a polyherbal extract, on lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Mol. Med. 2018, 43, 26–36. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 1, e0232565. [Google Scholar] [CrossRef]
- Cedillo-Cortezano, M.; Martinez-Cuevas, L.R.; López, J.A.M.; Barrera López, I.L.; Escutia-Perez, S.; Petricevich, V.L. Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals 2024, 17, 303. [Google Scholar] [CrossRef]
- Qazimi, B.; Stanoeva, J.P.; Cvetanoska, M.; Geskovski, N.; Dragusha, S.; Koraqi, H.; Qazimi, V.; Ejupi, V. Phenolic Compound Composition of Sambucus nigra L. Wild-Growing Plants from Kosovo. Turk. J. Pharm. Sci. 2023, 20, 380–389. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Hassan, N.D.; Mamat, S.N.H.; Nawi, N.M.; Rashid, W.A.; Tan, N.A. Extraction Technologies and Solvents of Phytocompounds from Plant Materials: Physicochemical Characterization and Identification of Ingredients and Bioactive Compounds from Plant Extract Using Various Instrumentations. In Ingredients Extraction by Physicochemical Methods in Food Handbook of Food Bioengineering; Academic Press: Cambridge, MA, USA, 2016; pp. 523–560. [Google Scholar]
- Xu, W.J.; Zhai, J.W.; Cui, Q.; Liu, J.Z.; Luo, M.; Fu, Y.J.; Zu, Y.-G. Ultra-turrax based ultrasound-assisted extraction of five organic acids from honeysuckle (Lonicera japonica Thunb.) and optimization of extraction process. Sep. Purif. Technol. 2016, 166, 73–82. [Google Scholar] [CrossRef]
- Rusu, M.E.; Gheldiu, A.M.; Mocan, A.; Moldovan, C.; Popa, D.S.; Tomuta, I.; Vlase, L. Process optimization for improved phenolic compounds recovery from walnut (Juglans regia L.) Septum: Phytochemical profile and biological activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.; Amparo, T.R.; Almeida, T.C.; Costa, F.S.F.; Brandão, G.C.; dos Santos, O.D.H.; da Silva, G.N.; Bianco de Souza, G.H. Cytotoxic activity of butanolic extract from Sambucus nigra L. flowers in natura and vehiculated in micelles in bladder cancer cells and fibroblasts. Nat. Prod. Res. 2022, 36, 1100–1104. [Google Scholar] [CrossRef]
- Chen, L.Y.; Huang, C.N.; Liao, C.K.; Chang, H.M.; Kuan, Y.H.; Tseng, T.J.; Yen, K.-J.; Yang, K.-L.; Lin, H.-C. Effects of rutin on wound healing in hyperglycemic rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef]
- Moghadam, S.E.; Ebrahimi, S.N.; Salehi, P.; Farimani, M.M.; Hamburger, M.; Jabbarzadeh, E. Wound healing potential of chlorogenic acid and myricetin-3-o-β-rhamnoside isolated from parrotia persica. Molecules 2017, 22, 1501. [Google Scholar] [CrossRef]
- Song, L.; Yang, H.; Liang, D.; Chu, D.; Yang, L.; Li, M.; Yang, B.; Shi, Y.; Chen, Z.; Yu, Z.; et al. A chlorogenic acid-loaded hyaluronic acid-based hydrogel facilitates anti-inflammatory and pro-healing effects for diabetic wounds. J. Drug Deliv. Sci. Technol. 2022, 70, 103232. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, N.Y.; Topal, A.; Tas, S.; Ozyigit, M.O.; Cinkilic, N.; Gul, Z.; Cam Etoz, B.; Ziyanok, S.; Inan, S.; et al. Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 1101–1116. [Google Scholar] [CrossRef]
- Chen, W.C.; Liou, S.S.; Tzeng, T.F.; Lee, S.L.; Liu, I.M. Effect of topical application of chlorogenic acid on excision wound healing in rats. Planta Med. 2013, 79, 616–621. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Identification of Quercitrin as a Potential Therapeutic Agent for Periodontal Applications. J. Periodontol. 2014, 85, 966–974. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Quercitrin for periodontal regeneration: Effects on human gingival fibroblasts and mesenchymal stem cells. Sci. Rep. 2015, 5, 16593. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Kaur, G.; Soni, V.; Kataria, J.; Dhawan, R.K. Evaluation of the wound healing potential of isoquercetin-based cream on scald burn injury in rats. Burn. Trauma 2016, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Hoff, J.; Karl, B.; Gerstmeier, J.; Beekmann, U.; Schmölz, L.; Börner, F.; Kralisch, D.; Bauer, M.; Werz, O.; Fischer, D.; et al. Controlled release of the α-tocopherol-derived metabolite α-13′-carboxychromanol from bacterial nanocellulose wound cover improves wound healing. Nanomaterials 2021, 11, 1939. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani-Nasab, M.; Akbari-Gharalari, N.; Rahmani Del Bakhshayesh, A.; Ghotaslou, A.; Ebrahimi-kalan, A.; Mahdipour, M.; Mehdipour, A. Synergistic effect of chitosan-alginate composite hydrogel enriched with ascorbic acid and alpha-tocopherol under hypoxic conditions on the behavior of mesenchymal stem cells for wound healing. Stem Cell Res. Ther. 2023, 14, 326. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Na, Y.; Woo, J.; Choi WIl Lee, J.H.; Hong, J.; Sung, D. α-Tocopherol-loaded reactive oxygen species-scavenging ferrocene nanocapsules with high antioxidant efficacy for wound healing. Int. J. Pharm. 2021, 596, 120205. [Google Scholar] [CrossRef]
- Liang, Q.; Yang, J.; He, J.; Chen, X.; Zhang, H.; Jia, M.; Liu, K.; Jia, C.; Pan, Y.; Wei, J. Stigmasterol alleviates cerebral ischemia/reperfusion injury by attenuating inflammation and improving antioxidant defenses in rats. Biosci. Rep. 2020, 40, BSR20192133. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Nazir, S.; Ahmad, I.; Mobashar, A.; Sharif, A.; Shabbir, A.; Chaudhary, W.A. Mechanistic evaluation of antiarthritic and anti-inflammatory effect of campesterol ester derivatives in complete Freund’s adjuvant-induced arthritic rats. Front. Pharmacol. 2023, 14, 1346054. [Google Scholar] [CrossRef]
- Khan, Z.; Nath, N.; Rauf, A.; Emran TBin Mitra, S.; Islam, F.; Chandran, D.; Barua, J.; Khandaker, M.U.; Idris, A.M.; Wilairatana, P.; et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem. Biol. Interact. 2022, 365, 110117. [Google Scholar] [CrossRef]
- Baskar, A.A.; Al Numair, K.S.; Gabriel Paulraj, M.; Alsaif, M.A.; Muamar, M.A.; Ignacimuthu, S. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J. Med. Food 2012, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, M.; Moreno, J.J. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic. Biol. Med. 2005, 39, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hammam, W.E.; Gad, A.M.; Gad, M.K.; Kirollos, F.N.; Yassin, N.A.; Tantawi, M.E.E.; El Hawary, S.S.E. Pyrus communis L. (Pear) and Malus domestica Borkh. (apple) leaves lipoidal extracts as sources for beta-sitosterol rich formulae and their wound healing evaluation. Nat. Prod. Res. 2022, 37, 2613–2617. [Google Scholar] [CrossRef]
- Dighe, S.B.; Kuchekar, B.S.; Wankhede, S.B.; Santosh, M.; Dighe Head, B. Analgesic and anti-inflammatory activity of β-sitosterol isolated from leaves of Oxalis corniculata. Int. J. Pharmacol. Res. 2016, 6, 109–113. [Google Scholar]
- Ododo, M.M.; Choudhury, M.K.; Dekebo, A.H. Structure elucidation of β-sitosterol with antibacterial activity from the root bark of Malva parviflora. Springerplus 2016, 5, 1210. [Google Scholar] [CrossRef]
- Petkova, D.T.; Mihaylova, D.S.; Deseva, I.N.; Denev, P.N.; Krastanov, A.I. Green approach to obtain extracts of seven edible flowers. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1031, 012101. [Google Scholar] [CrossRef]
- Tundis, R.; Ursino, C.; Bonesi, M.; Loizzo, M.R.; Sicari, V.; Pellicanò, T.; Manfredi, I.L.; Figoli, A.; Cassano, A. Flower and leaf extracts of Sambucus nigra L.: Application of membrane processes to obtain fractions with antioxidant and antityrosinase properties. Membranes 2019, 9, 127. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Jamshidi, M.; Shabani, E.; Hashemi, Z.; Ebrahimzadeh, M.A. Evaluation of three methods for the extraction of antioxidants from leaf and aerial parts of Lythrum salicaria L. (Lythraceae). Int. Food Res. J. 2014, 21, 783–788. [Google Scholar]
- Kustova, T.; Karpenyuk, T.; Goncharova, A.; Mamonov, L.; Ross, S. Herbal extracts in the treatment of Diabetic Foot Syndrome. Cent. Asian J. Glob. Health 2014, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Nácher, A.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Díez-Sales, O.; Ruiz-Saurí, A.; Manconi, M. Fabrication of quercetin and curcumin bionanovesicles for the prevention and rapid regeneration of full-thickness skin defects on mice. Acta Biomater. 2014, 10, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Milkova-Tomova, I.; Kazakova, Z.; Buhalova, D.; Gentscheva, G.; Nikolova, K.; Minkova, S. Antioxidant Properties and Antibacterial Activity of Water Extracts from Sambucus nigra L. under Different Conditions. Folia Med. 2023, 65, 295–300. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Badim, H.; Salvador, Â.C.; Silvestre, A.J.D.; Santos, S.A.O.; Rocha, S.M.; Sousa, A.M.; Pereira, M.O.; Wilson, C.P.; Rocha, C.M.R.; et al. Chemical characterization of Sambucus nigra L. Flowers aqueous extract and its biological implications. Biomolecules 2021, 11, 1222. [Google Scholar] [CrossRef]
- Haș, I.M.; Teleky, B.E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.-C.; Tit, D.M.; Nițescu, M. Bioactive Potential of Elderberry (Sambucus nigra L.): Antioxidant, Antimicrobial Activity, Bioaccessibility and Prebiotic Potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra extracts–natural antioxidants and antimicrobial compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef]
- Hearst, C.; Mccollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial activity of elder (Sambucus nigra L.) flower or berry against hospital pathogens. J. Med. Plants Res. 2010, 4, 1805–1809. [Google Scholar]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Becker, H.; Scher, J.M.; Speakman, J.B.; Zapp, J. Bioactivity guided isolation of antimicrobial compounds from Lythrum salicaria. Fitoterapia 2005, 76, 580–584. [Google Scholar] [CrossRef]
- Wyse, D.; Fulcher, R.G.; Ehlke, N.J.; Biesboer, D. Antimicrobial activity of native and naturalized plants of Minnesota and Wisconsin. J. Med. Plants Res. 2008, 2, 98–110. [Google Scholar]
- Guclu, E.; Genc, H.; Zengin, M.; Karabay, O. Antibacterial Activity of Lythrum salicaria against Multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Annu. Res. Rev. Biol. 2014, 4, 1099–1105. [Google Scholar] [CrossRef]
- Battinelli, L.; Tita, B.; Evandri, M.G.; Mazzanti, G. Antimicrobial activity of Epilobium spp. extracts. Farmaco 2001, 56, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pastor, R.; Carrera-Pacheco, S.E.; Zúñiga-Miranda, J.; Rodríguez-Pólit, C.; Mayorga-Ramos, A.; Guamán, L.P.; Barba-Ostria, C. Current Landscape of Methods to Evaluate Antimicrobial Activity of Natural Extracts. Molecules 2023, 28, 1068. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Hwang, E.; Ngo, H.T.T.; Seo, S.A.; Yi, T.H. Sambucus nigra L. ameliorates UVB-induced photoaging and inflammatory response in human skin keratinocytes. Cytotechnology 2019, 71, 1003–1017. [Google Scholar] [CrossRef]
- Filip, G.A.; Florea, A.; Olteanu, D.; Clichici, S.; David, L.; Moldovan, B.; Cenariu, M.; Scrobota, I.; Potara, M.; Baldea, I. Biosynthesis of silver nanoparticles using Sambucus nigra L. fruit extract for targeting cell death in oral dysplastic cells. Mater. Sci. Eng. C 2021, 123, 111974. [Google Scholar] [CrossRef]
- Skowrońska, W.; Granica, S.; Czerwińska, M.E.; Osińska, E.; Bazylko, A. Sambucus nigra L. leaves inhibit TNF-α secretion by LPS-stimulated human neutrophils and strongly scavenge reactive oxygen species. J. Ethnopharmacol. 2022, 290, 115116. [Google Scholar] [CrossRef]
- Zielińska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczyński, R. Elderberry (Sambucus nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem. X 2022, 15, 100437. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lim, S.H.; Park, M.H.; Park, Y.H.; Ham, H.J.; Lee, K.Y.; Park, D.S.; Kim, K.H.; Kim, S.M. Biological Activities in the Leaf Extract of Lythrum salicaria L. Korean J. Med. Crop Sci. 2010, 18, 409–415. [Google Scholar] [CrossRef]
- Merighi, S.; Travagli, A.; Tedeschi, P.; Marchetti, N.; Gessi, S. Antioxidant and antiinflammatory effects of Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum plant extracts in macrophage and microglial cells. Cells 2021, 10, 2691. [Google Scholar] [CrossRef]


| Plant Material | Voucher Specimen No. | Harvest Period and Location |
|---|---|---|
| Sambucus nigra flowers | 139.1.2.1/05.2022 | May 2022, Stremț, Alba County |
| Lythrum salicaria aerial parts | 60.3.1.1/06.2022 | June 2022, Săndulești, Cluj County |
| Epilobium hirsutum aerial parts | 62.5.1.1/07.2022 | July 2022, Săndulești, Cluj County |
| Input Variables and Variation Levels (Independent Variables, Factors) | Output Variables (Dependent Variables, Responses) | ||
|---|---|---|---|
| Qualitative variable | Extraction method—X1 | UTE | TPC—Y1 μg GAE/mL HE |
| USE | |||
| Quantitative variable | Extraction time—X2 | 3, 5, 10 min | TFC—Y2 μM QAE/HE |
| Ethanol ratio in the extraction solvent—X3 | 30%, 50%, 70% | ||
| Parameter | Justification [3,31,63,64] | Target |
|---|---|---|
| Antioxidant capacity | To regulate the redox environment from the wound to combat oxidative damage, which can otherwise hinder the healing process, leading to delayed healing or chronic wounds. | Maximize |
| Antibacterial activity | To prevent or treat the infections caused by the most common bacteria in wounds, like Staphylococcus aureus and resistant strains (e.g., MRSA, methicillin-resistant Staphylococcus aureus), Escherichia coli (frequently met in chronic wounds), Pseudomonas aeruginosa (commonly isolated from wounds following surgeries and burns). | |
| Anti-inflammatory activity | To reduce excessive inflammation, minimize tissue damage, decrease the associated pain, overcome the inflammatory phase of the physiological process of wound healing, and to prevent the chronicity of the wound and scar formation. | |
| Cell viability | To ensure the biocompatibility with implied cell lines (HaCaT, BJ), increase fibroblast and keratinocytes proliferation, and stimulate the growth factors involved in the process of healing (e.g., FGF—fibroblast growth factor, EGF—epidermal growth factor). | |
| Wound-healing activity | To stimulate the growth factors involved in the process of healing and the migration of the cells and to hasten the wound closure. | |
| All the above may be enhanced by increasing the content of polyphenols and flavonoids. | ||
| Total polyphenol content (TPC) | To exert the antioxidant, antibacterial, anti-inflammatory, and wound-healing activities. | Maximize |
| Total flavonoid content (TFC) | To exert the antioxidant, antibacterial, anti-inflammatory, and wound-healing activities. | |
| HEs | Response | R2 | Q2 | p-Value | Lack of Fit | Model Validity | Reproducibility |
|---|---|---|---|---|---|---|---|
| Sambucus nigra | Y1-TPC | 0.562 | −0.797 | 0.386 | 0.001 | −0.200 | 0.999 |
| Y2-TFC | 0.672 | 0.430 | 0.004 | 0.165 | 0.549 | 0.913 | |
| Lythrum salicaria | Y1-TPC | 0.698 | 0.418 | 0.010 | 0.285 | 0.685 | 0.835 |
| Y2-TFC | 0.589 | 0.335 | 0.012 | 0.108 | 0.443 | 0.931 | |
| Epilobium hirsutum | Y1-TPC | 0.917 | 0.690 | 0.000 | 0.440 | 0.795 | 0.912 |
| Y2-TFC | 0.870 | 0.794 | 0.000 | 0.124 | 0.477 | 0.975 |
| Exp. No. | Input Variables | Obtained Output Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All HEs | Sambucus nigra HE | Lythrum salicaria HE | Epilobium hirsutum HE | ||||||
| X1 | X2 | X3 | Y1-TPC | Y2-TFC | Y1-TPC | Y2-TFC | Y1-TPC | Y2-TFC | |
| N1 | UTE | 3 | 30 | 4889.61 ± 195.58 | 2296.96 ± 45.93 | 6591.62 ± 131.83 | 638.97 ± 25.55 | 6530.52 ± 195.91 | 1554.6 ± 31.09 |
| N2 | USE | 3 | 30 | 4749.95 ± 427.49 | 1032.13 ± 20.64 | 6059.20 ± 121.18 | 328.26 ± 29.54 | 5587.87 ± 167.63 | 729.71 ± 14.59 |
| N3 | UTE | 10 | 30 | 5282.38 ± 369.76 | 1969.75 ± 39.39 | 6757.46 ± 405.44 | 471.24 ± 9.42 | 6705.09 ± 536.41 | 1714.08 ± 68.56 |
| N4 | USE | 10 | 30 | 4802.32 ± 240.11 | 1897.16 ± 56.91 | 6094.11 ± 243.76 | 306.27 ± 18.37 | 5552.96 ± 166.58 | 823.19 ± 57.62 |
| N5 | USE | 5 | 30 | 5230.01 ± 104.60 | 1695.89 ± 33.91 | 5344.48 ± 213.77 | 157.79 ± 4.73 | 5413.31 ± 108.26 | 677.46 ± 20.32 |
| N6 | UTE | 3 | 70 | 4976.89 ± 447.92 | 2481.18 ± 173.68 | 7158.96 ± 143.17 | 773.7 ± 38.68 | 6582.89 ± 65.82 | 1906.55 ± 38.13 |
| N7 | USE | 3 | 70 | 5718.79 ± 285.93 | 2663.76 ± 159.82 | 6478.15 ± 583.03 | 1023.92 ± 40.95 | 5491.86 ± 439.34 | 1131.16 ± 56.55 |
| N8 | UTE | 10 | 70 | 5046.72 ± 252.33 | 3642.62 ± 218.55 | 6713.82 ± 268.55 | 913.93 ± 36.55 | 5692.61 ± 170.77 | 2068.78 ± 186.19 |
| N9 | USE | 10 | 70 | 4907.07 ± 196.28 | 2143.53 ± 150.04 | 5989.37 ± 59.89 | 473.99 ± 23.69 | 5291.11 ± 211.64 | 759.95 ± 68.39 |
| N10 | UTE | 5 | 50 | 4959.44 ± 297.56 | 2931.02 ± 205.17 | 7246.25 ± 434.77 | 575.733 ± 34.54 | 6635.27 ± 597.17 | 1606.84 ± 16.06 |
| N11 | USE | 5 | 50 | 4872.15 ± 389.77 | 1778.47 ± 124.49 | 5178.39 ± 268.91 | 148.78 ± 1.48 | 5238.74 ± 52.38 | 612.23 ± 24.48 |
| N12 | USE | 5 | 50 | 4854.70 ± 97.09 | 1921.36 ± 115.28 | 5665.67 ± 54.65 | 185.28 ± 11.12 | 5247.47 ± 367.32 | 522.73 ± 47.04 |
| N13 | USE | 5 | 50 | 4872.15 ± 146.16 | 2125.85 ± 148.81 | 5422.04 ± 271.10 | 210.78 ± 6.32 | 5509.32 ± 220.37 | 575.73 ± 34.54 |
| Extraction conditions for obtaining the OHEs | ||||||
| Variation factors | Sambucus nigra | Lythrum salicaria | Epilobium hirsutum | |||
| X1 | UTE | UTE | UTE | |||
| X2 (min) | 6 | 3 | 3 | |||
| X3 (% EtOH) | 70 | 70 | 70 | |||
| Results of analysis of the OHEs | ||||||
| Sambucus nigra | Lythrum salicaria | Epilobium hirsutum | ||||
| TPC | TFC | TPC | TFC | TPC | TFC | |
| Predicted maximal values | 5459.17 | 3208.05 | 7067.61 | 923.85 | 6628.25 | 1925.44 |
| Experimental values | 5750.01 ± 173.21 | 3030.39 ± 42.21 | 7653.11 ± 974.226 | 1025.74 ± 39.01 | 6950.35 ± 790.811 | 1973.03 ± 21.31 |
| Bioactive Compounds | Sambucus nigra | Epilobium hirsutum | Lythrum salicaria | |
|---|---|---|---|---|
| Phenolic acids (µg/mL) | Caftaric acid | - | <LOQ | - |
| Chlorogenic acid | 598.838 ± 35.930 | <LOQ | 3.422 ± 0.239 | |
| 4-O-caffeoylquinic acid | 40.811 ± 2.856 | - | - | |
| p-coumaric acid | <LOQ | <LOQ | - | |
| Gentisic acid | <LOQ | - | - | |
| Gallic acid | 2.199 ± 0.087 | 36.827 ± 1.104 | 29.366 ± 2.348 | |
| Protocatechuic acid | 8.395 ± 0.755 | - | 0.167 ± 0.009 | |
| Vanillic acid | 0.155 ± 0.012 | - | - | |
| Flavanols (µg/mL) | (+)-Epicatechin | 0.331 ± 0.003 | 0.081 ± 0.001 | 0.082 ± 0.002 |
| (-)-Catechin | 0.042 ± 0.001 | 0.213 ± 0.008 | 0.022 ± 0.001 | |
| Epigallocatechin | 0.415 ± 0.004 | 1.147 ± 0.068 | 0.126 ± 0.009 | |
| Epigallocatechin gallate | - | 0.538 ± 0.021 | - | |
| Procyanidin A1 | 0.216 ± 0.014 | 0.067 ± 0.004 | - | |
| Procyanidin B1 | 0.138 ± 0.010 | - | - | |
| Procyanidin B2 | 0.321 ± 0.006 | 0.113 ± 0.009 | 0.147 ± 0.007 | |
| Flavonols (µg/mL) | Hyperoside | - | 33.160 ± 1.658 | 0.507 ± 0.045 |
| Isoquercitrin | 151.530 ± 10.607 | 5.744 ± 0.344 | 10.830 ± 0.758 | |
| Rutin | 916.193 ± 27.485 | 1.302 ± 0.091 | <LOQ | |
| Myricetin | - | 22.618 ± 0.227 | - | |
| Quercitrin | 250.889 ± 12.544 | 82.627 ± 2.478 | <LOQ | |
| Quercetol | 1.935 ± 0.135 | <LOQ | - | |
| Kaempferol-3-Rhamnoside | - | 5.943 ± 0.118 | - | |
| Flavones (µg/mL) | Luteolin | 88.966 ± 5.338 | - | 1.781 ± 0.053 |
| Apigenin | - | - | 0.285 ± 0.014 | |
| Sterols (μg/mL) | Ergosterol | 0.441 ± 0.022 | - | - |
| Stigmasterol | 11.219 ± 0.561 | - | 2.714 ± 0.108 | |
| Beta-Sitosterol | 417.593 ± 12.527 | 638.215 ± 51.057 | 256.391 ± 12.819 | |
| Campesterol | 11.296 ± 0.225 | 1.883 ± 0.094 | 1.104 ± 0.066 | |
| Brassicasterol | 1.163 ± 0.034 | 3.210 ± 0.096 | 0.845 ± 0.059 | |
| Tocopherols (ng/mL) | α-tocopherol | 2273.811 ± 181.904 | 290.202 ± 17.412 | 837.842 ± 50.268 |
| δ-tocopherol | 29.419 ± 0.295 | 159.665 ± 4.788 | 8.212 ± 0.656 | |
| γ-tocopherol | 202.909 ± 2.031 | 847.473 ± 25.422 | 103.864 ± 2.076 | |
| OHEs | DPPH Assay | FRAP Assay | TEAC Assay |
|---|---|---|---|
| Sambucus nigra | 7.5394 ± 0.3982 | 29.5620 ± 1.0730 | 48.8131 ± 15.6024 |
| Lythrum salicaria | 12.0192 ± 0.9553 | 69.1414 ± 9.0016 | 65.8586 ± 4.9098 |
| Epilobium hirsutum | 11.6666 ± 0.5266 | 21.5528 ± 5.1290 | 156.8182 ± 0.9185 |
| Bacterial Strains | Sambucus nigra | Lythrum salicaria | Epilobium hirsutum | NC | PC |
|---|---|---|---|---|---|
| Zone of Inhibition (mm) | |||||
| Escherichia coli, ATCC 25922 | 9.67 ± 0.58 **** | 16.33 ± 1.15 ** | 20.00 ± 0.00 * | 0 | 18.5 ± 0.00 |
| Staphylococcus aureus MRSA, ATCC 700699 | 8.33 ± 0.58 **** | 20.17 ± 0.29 | 18.00 ± 1.00 ** | 0 | 20.0 ± 0.00 |
| Staphylococcus aureus MSSA, ATCC 25923 | 9.00 ± 1.00 **** | 20.33 ± 0.57 | 18.00 ± 0.50 ** | 0 | 20.0 ± 0.00 |
| Pseudomonas aeruginosa, ATCC 27853 | 9.33 ± 1.15 **** | 15.00 ± 1.73 * | 15.67 ± 1.52 * | 0 | 18.5 ± 0.00 |
| Bacterial Strains | OHEs | Concentration mg/mL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.6 | 0.8 | 0.4 | 0.2 | 0.1 | C+ | ||
| S. aureus MSSA ATCC 25923 | Sambucus nigra | − | − | − | + | + | + | + | + | + | + | + | + |
| Epilobium hirsutum | − | − | − | − | + | + | + | + | + | + | + | + | |
| Lythrum salicaria | − | − | − | − | − | + | + | + | + | + | + | + | |
| S. aureus MRSA ATCC 700699 | Sambucus nigra | − | − | + | + | + | + | + | + | + | + | + | + |
| Epilobium hirsutum | − | − | − | − | + | + | + | + | + | + | + | + | |
| Lythrum salicaria | − | − | − | − | − | + | + | + | + | + | + | + | |
| E. coli ATCC 25922 | Sambucus nigra | − | − | − | + | + | + | + | + | + | + | + | + |
| Epilobium hirsutum | − | − | − | − | − | + | + | + | + | + | + | + | |
| Lythrum salicaria | − | − | − | − | + | + | + | + | + | + | + | + | |
| P. aeruginosa ATCC 27853 | Sambucus nigra | − | − | − | + | + | + | + | + | + | + | + | + |
| Epilobium hirsutum | − | − | − | + | + | + | + | + | + | + | + | + | |
| Lythrum salicaria | − | − | − | − | + | + | + | + | + | + | + | + | |
| Levels | Sambucus nigra | Lythrum salicaria | Epilobium hirsutum |
|---|---|---|---|
| Non-toxic concentrations of OHEs on HaCaT (µg/mL) | |||
| H | 400 | 150 | 50 |
| M | 100 | 75 | 25 |
| L | 25 | 25 | 1 |
| Non-toxic concentrations of OHEs on BJ (µg/mL) | |||
| H | 400 | 50 | 25 |
| M | 100 | 25 | 10 |
| L | 25 | 1 | 1 |
| Non-toxic concentrations of OHEs on RAW 264.7 (µg/mL) | |||
| H | 400 | 50 | 150 |
| M | 100 | 25 | 75 |
| L | 25 | 1 | 25 |
| Wound Closure After 24 h (%) | Cell Migration After 24 h (mm/h) | |||
|---|---|---|---|---|
| OHEs | HaCaT | BJ | HaCaT | BJ |
| Sambucus nigra | 59.87 ± 13.33 * | 53.62 ± 11.70 | 4.50 ± 0.83 * | 3.85 ± 1.76 |
| Lythrum salicaria | 49.54 ± 5.13 | 40.16 ± 16.34 | 3.97 ± 0.67 | 4.26 ± 1.44 |
| Epilobium hirsutum | 98.49 ± 2.60 *** | 76.49 ± 8.20 * | 5.94 ± 0.64 * | 4.66 ± 1.73 |
| NC | 44.01 ± 17.32 | 37.31 ± 6.25 | 3.26 ± 1.31 | 4.29 ± 3.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safta, D.A.; Vlase, A.-M.; Pop, A.; Cherfan, J.; Carpa, R.; Iurian, S.; Bogdan, C.; Vlase, L.; Moldovan, M.-L. Optimized Sambucus nigra L., Epilobium hirsutum L., and Lythrum salicaria L. Extracts: Biological Effects Supporting Their Potential in Wound Care. Antioxidants 2025, 14, 521. https://doi.org/10.3390/antiox14050521
Safta DA, Vlase A-M, Pop A, Cherfan J, Carpa R, Iurian S, Bogdan C, Vlase L, Moldovan M-L. Optimized Sambucus nigra L., Epilobium hirsutum L., and Lythrum salicaria L. Extracts: Biological Effects Supporting Their Potential in Wound Care. Antioxidants. 2025; 14(5):521. https://doi.org/10.3390/antiox14050521
Chicago/Turabian StyleSafta, Diana Antonia, Ana-Maria Vlase, Anca Pop, Julien Cherfan, Rahela Carpa, Sonia Iurian, Cătălina Bogdan, Laurian Vlase, and Mirela-Liliana Moldovan. 2025. "Optimized Sambucus nigra L., Epilobium hirsutum L., and Lythrum salicaria L. Extracts: Biological Effects Supporting Their Potential in Wound Care" Antioxidants 14, no. 5: 521. https://doi.org/10.3390/antiox14050521
APA StyleSafta, D. A., Vlase, A.-M., Pop, A., Cherfan, J., Carpa, R., Iurian, S., Bogdan, C., Vlase, L., & Moldovan, M.-L. (2025). Optimized Sambucus nigra L., Epilobium hirsutum L., and Lythrum salicaria L. Extracts: Biological Effects Supporting Their Potential in Wound Care. Antioxidants, 14(5), 521. https://doi.org/10.3390/antiox14050521






_Cherfan.png)




