Optimized Fermentation Conditions of Pulses Increase Scavenging Capacity and Markers of Anti-Diabetic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
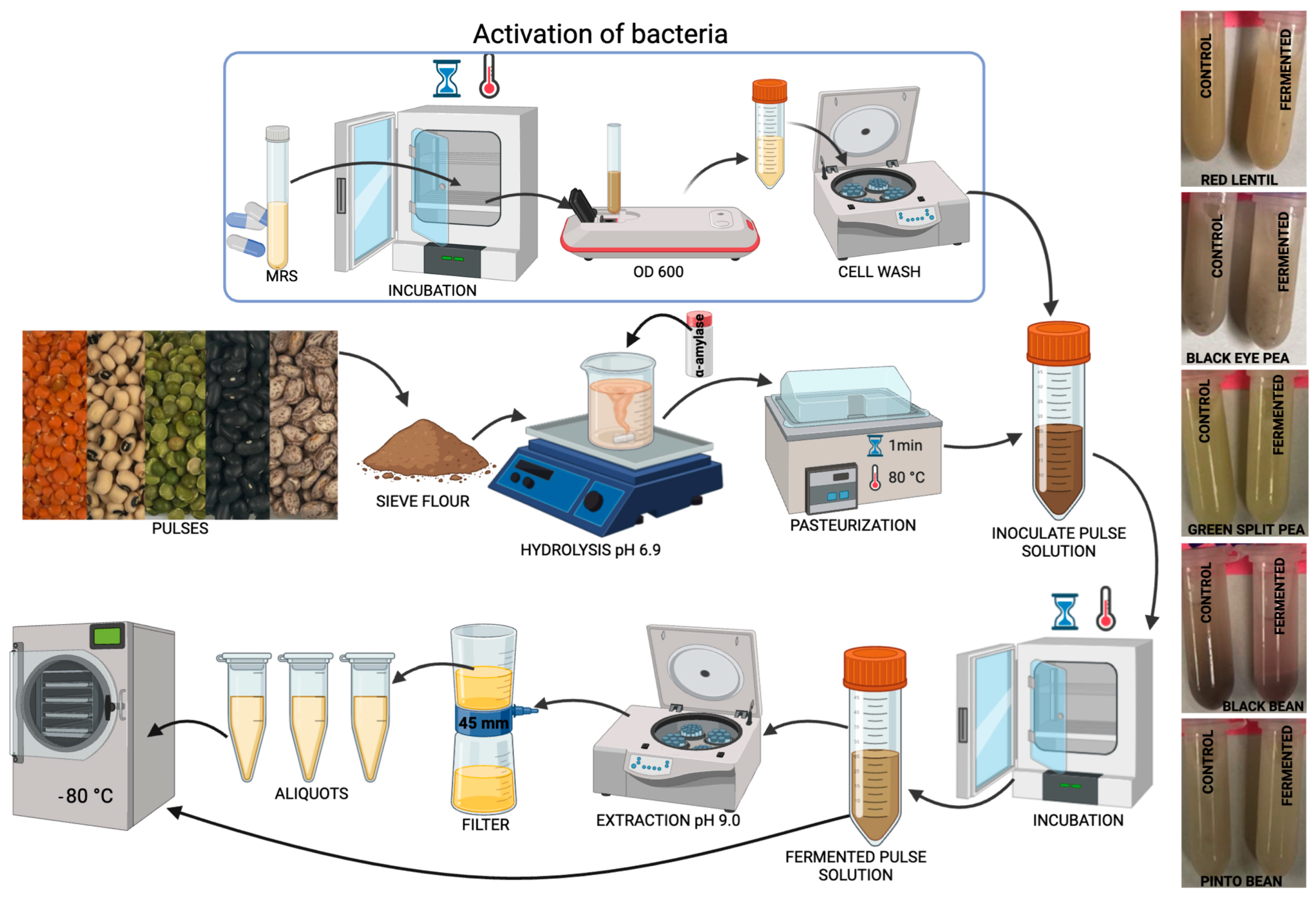
2.2. Fermentation of Pulses with Lp299v
2.3. Antioxidant Assays
2.3.1. Measurement of 2,2-diphenyl-1-picrylhydrazyl (DPPH)-Radical-Scavenging Capacity
2.3.2. Measurement of Nitric Oxide (NO)-Scavenging Capacity
2.4. Protein and Peptide Profile
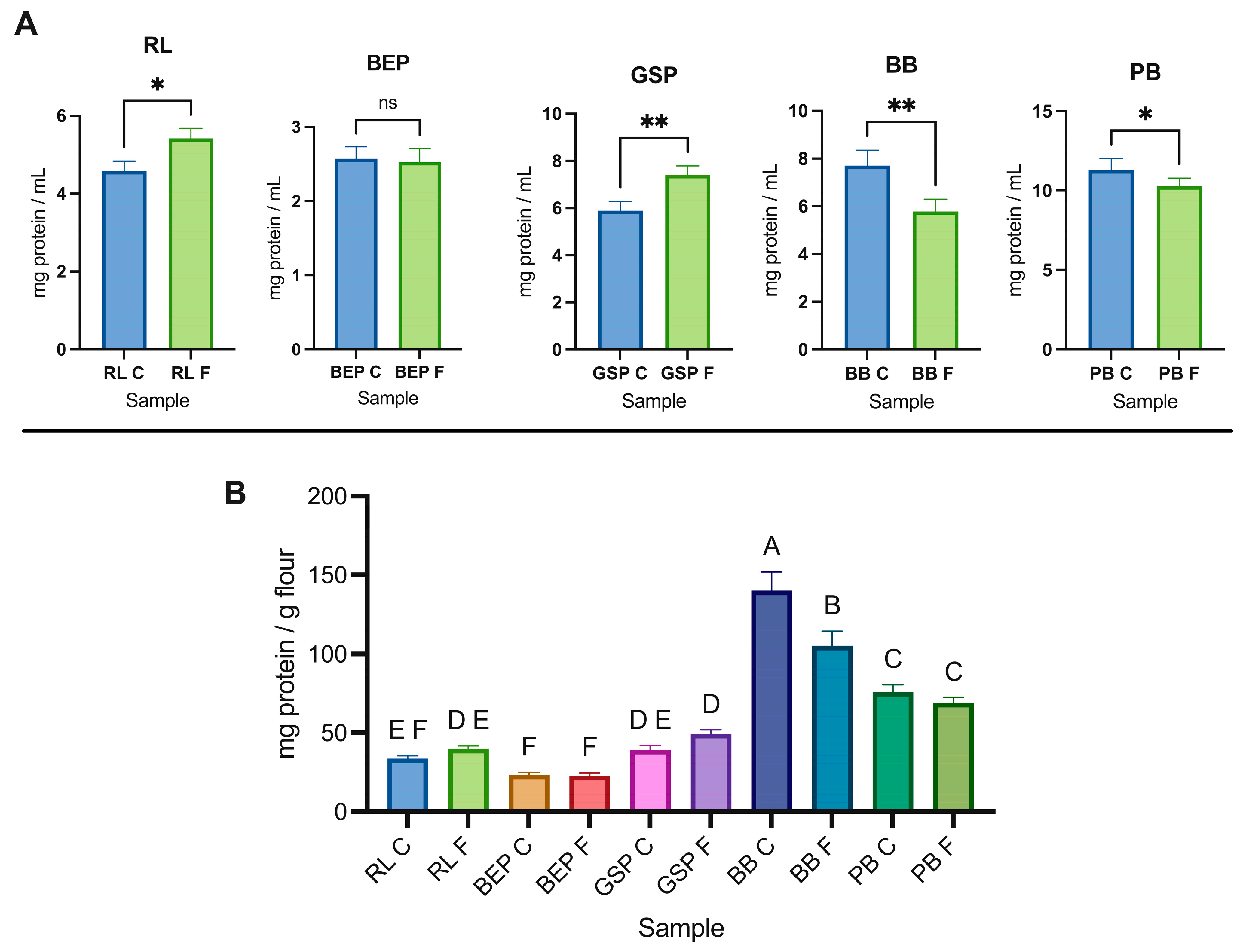
2.4.1. Protein Quantification
2.4.2. Protein Profile Based on Gel Electrophoresis Analysis (SDS-PAGE)
2.4.3. Proteolytic Activity Determination of Commercial and High-Purity α-Amylase
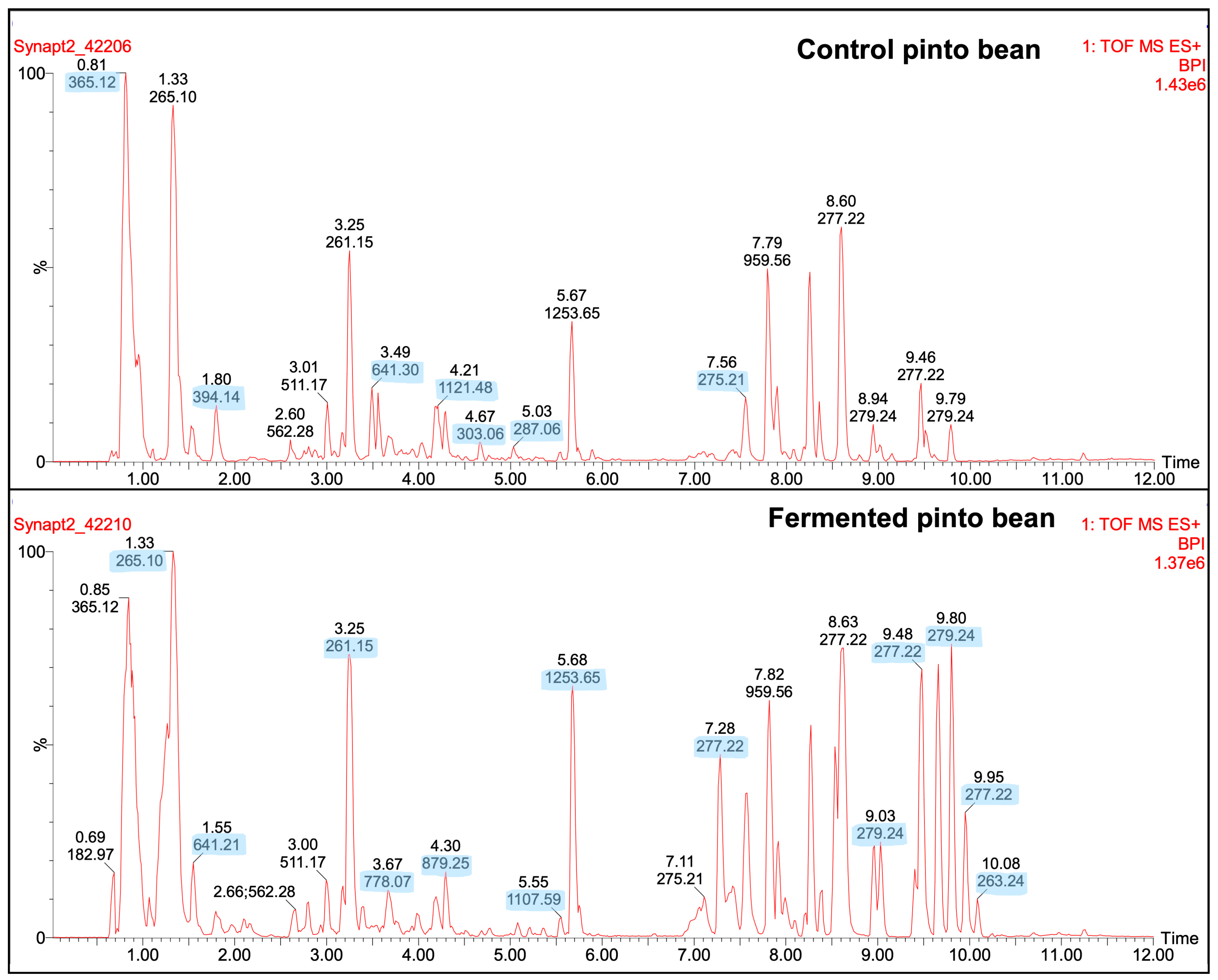
2.4.4. LC-MSMS Peptide Profile
2.5. T2D Markers
2.5.1. DPP-IV Inhibition
2.5.2. α-Glucosidase Inhibition
2.6. In Vitro Assays
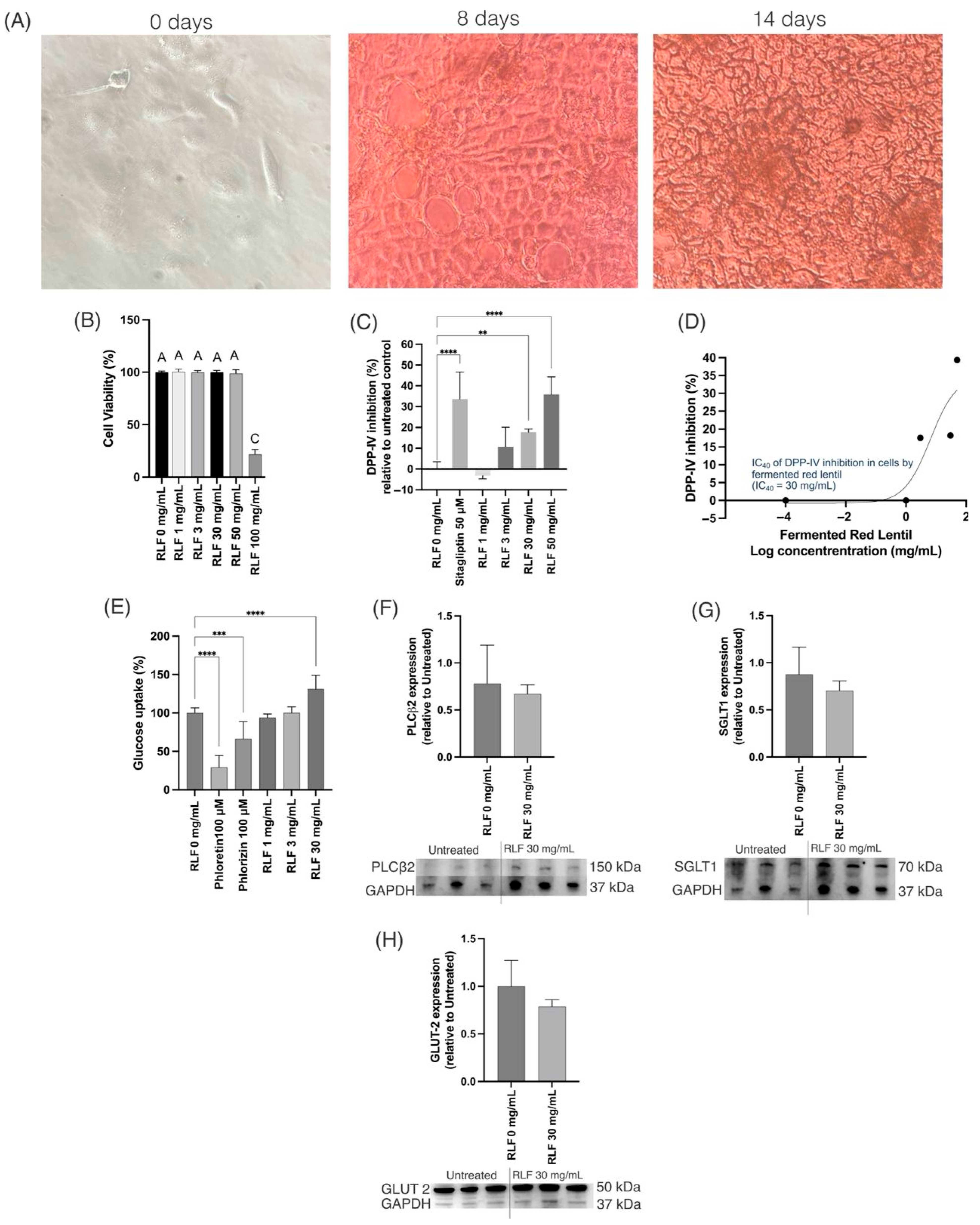
2.6.1. Differentiated Caco2 Cells
2.6.2. Glucose Uptake, Expression of Glucose Absorption-Related Markers Based with Western Blot, and DPP-IV Inhibition
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Kinetics Based on pH Changes
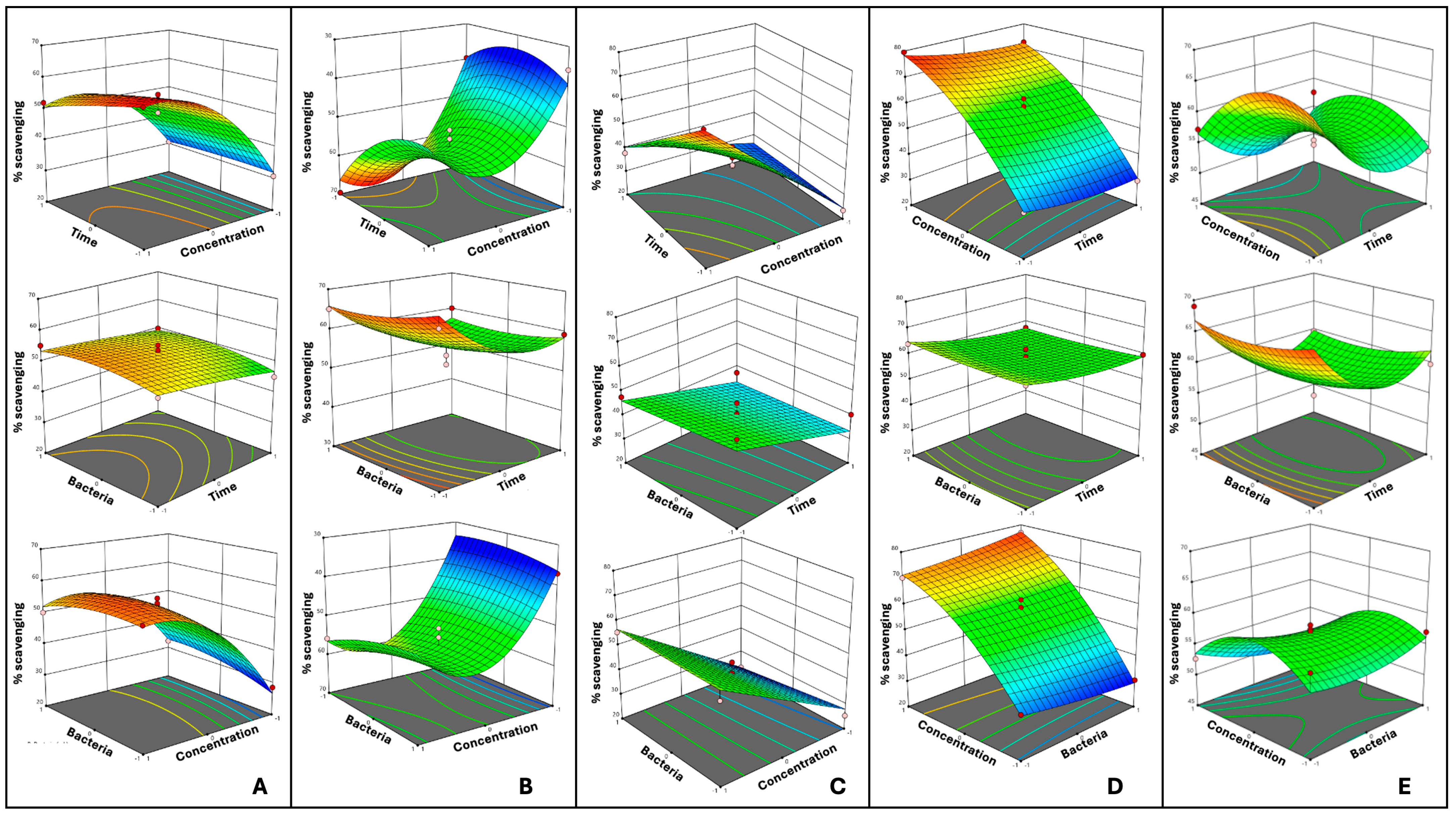
3.2. Optimized Fermentation Conditions
| Factor | DPPH (%) | Final pH | ||||
|---|---|---|---|---|---|---|
| X1: Time (h) | X2: Bacteria (CFU/mL) | X3: Flour Concentration (g/100 mL) | Predicted | Observed Verification | Observed Condition | |
| RL | 9 | 1.34 × 109 | 13.6 | 60.44 ± 2. 43 | 56.65 ± 2.84 | 4.07 ± 0.00 |
| BEP | 8 | 1.70 × 109 | 11.0 | 69.74 ± 4.01 | 67.77 ± 1.47 | 3.88 ± 0.02 |
| GSP | 8 | 0.76 × 109 | 15.0 | 73.12 ± 4.45 | 82.67 ± 6.03 | 4.02 ± 0.02 |
| BB | 8 | 3.50 × 109 | 5.5 | 68.61 ± 3.01 | 72.35 ± 5.48 | 3.95 ± 0.02 |
| PB | 8 | 2.58 × 109 | 14.9 | 79.61 ± 1.90 | 70.87 ± 3.68 | 3.90 ± 0.00 |
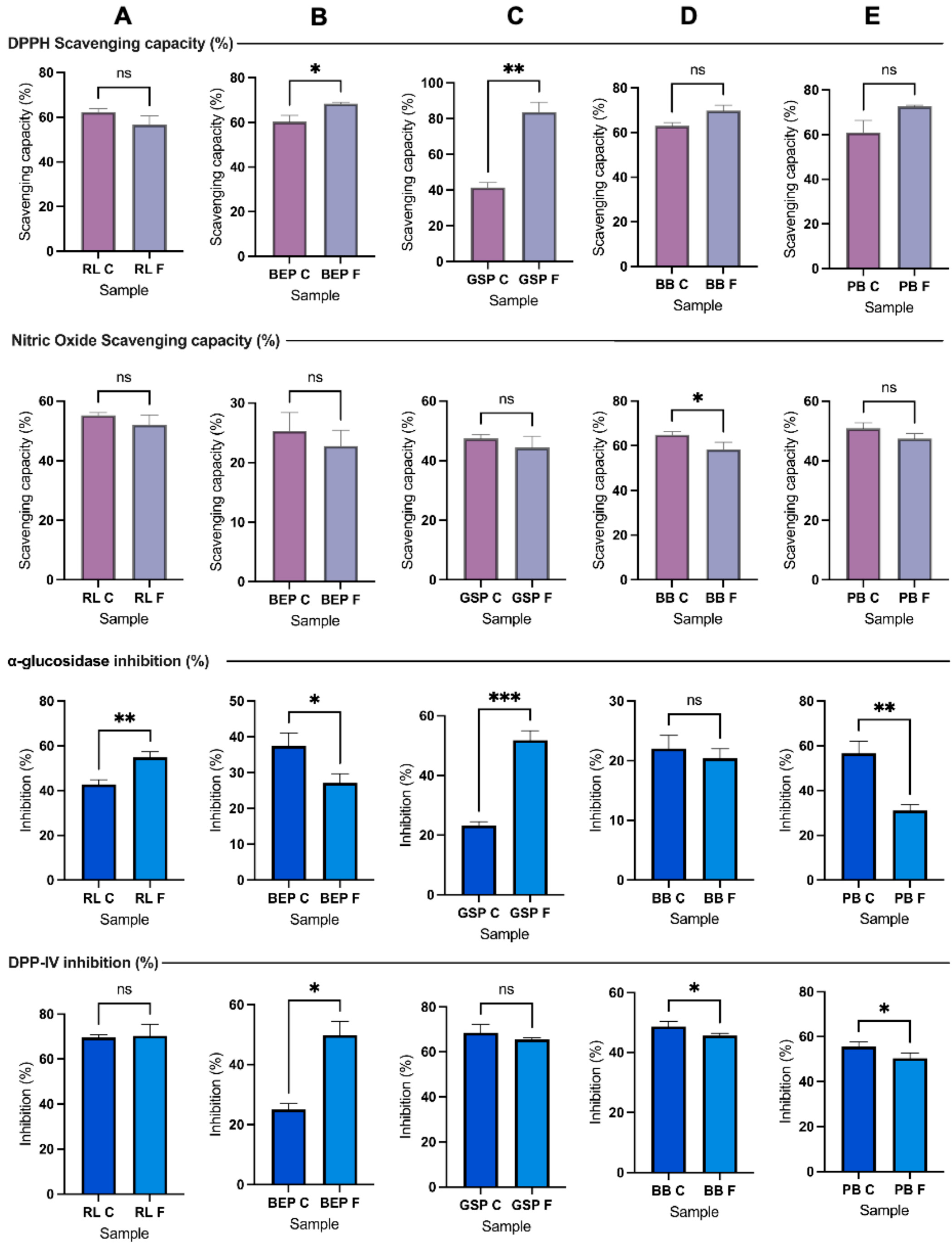
3.3. DPPH-Scavenging Capacity
3.4. Nitric Oxide (NO)-Scavenging Capacity
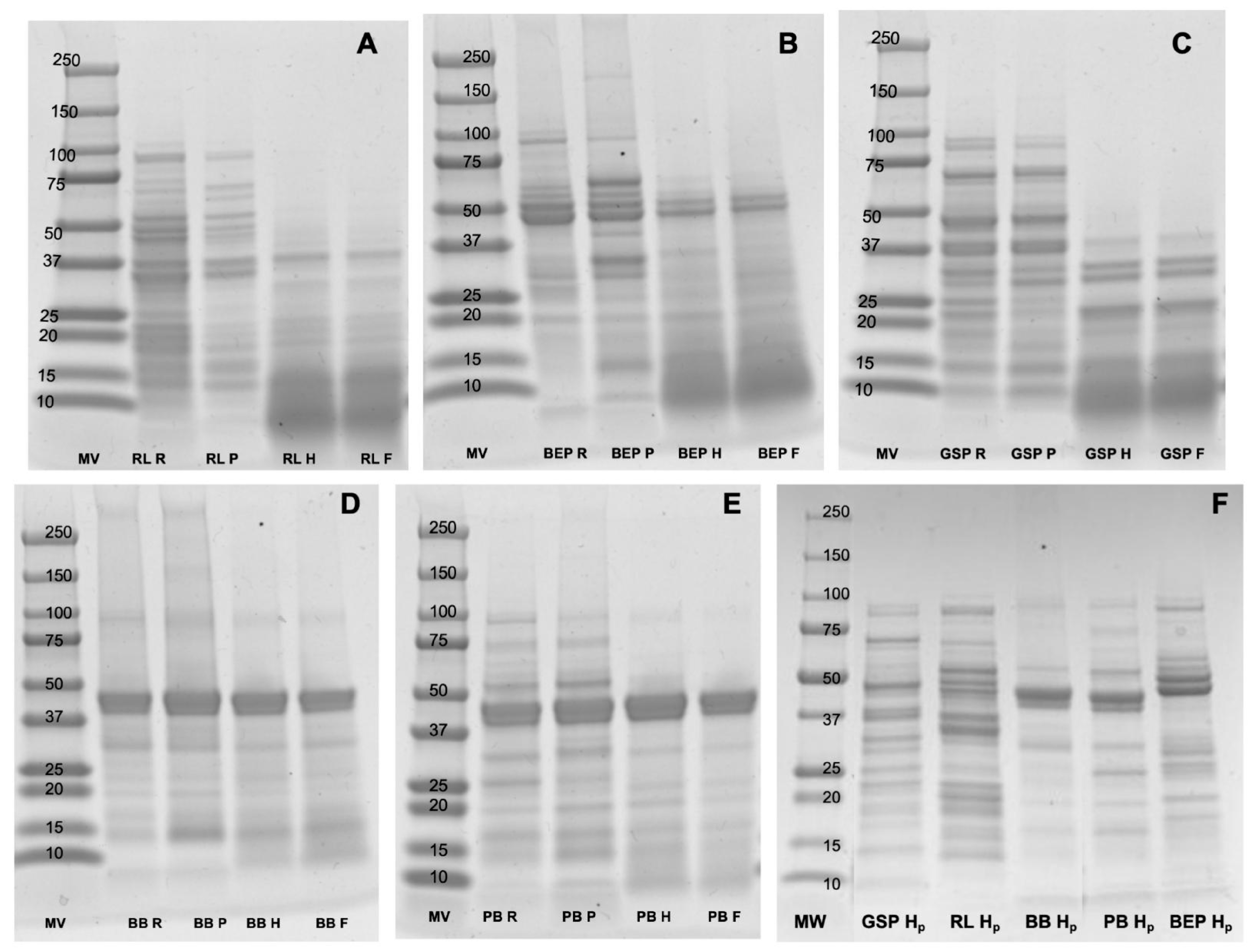
3.5. Protein Profiles and Quantification
3.6. DPP-IV and AG Inhibition Under Optimal Fermentation Conditions
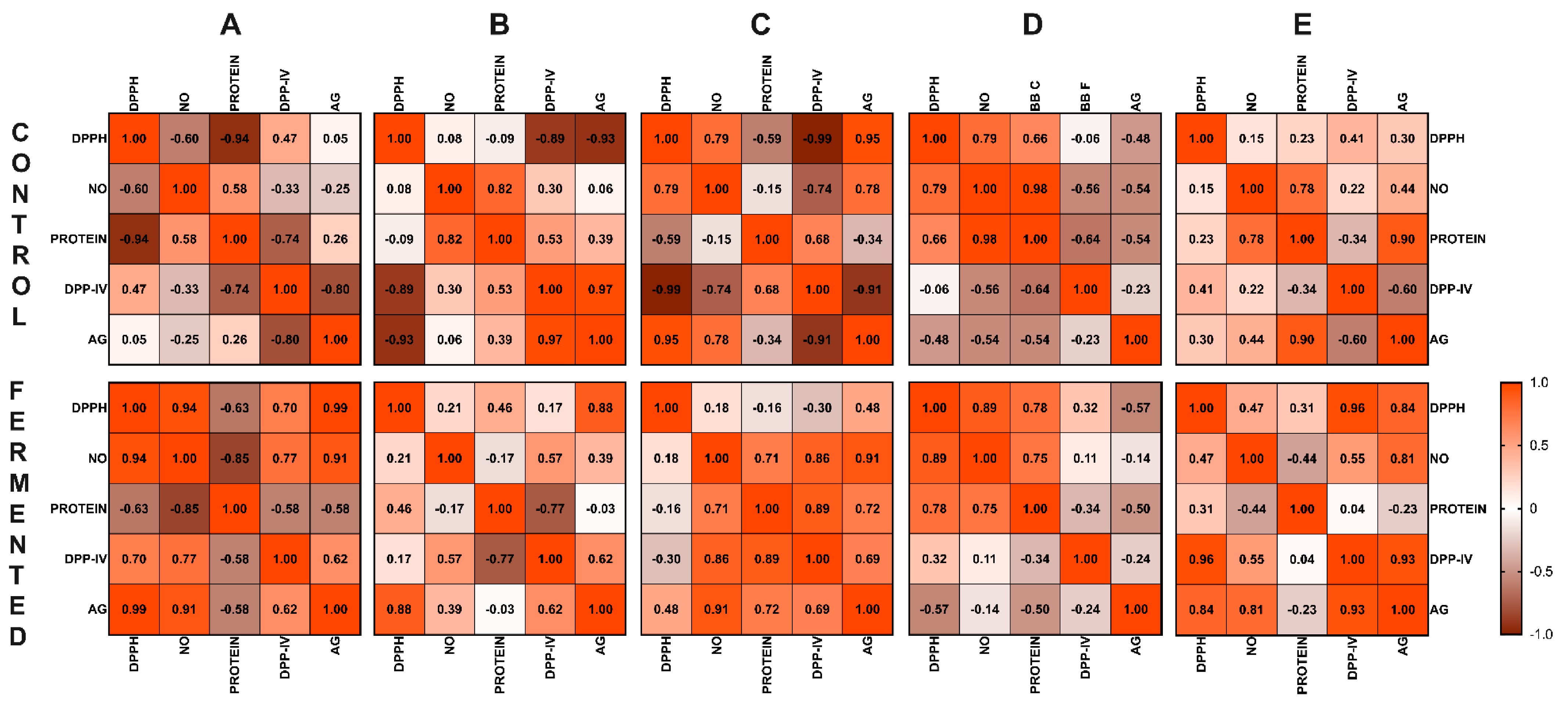
3.7. Correlation Analysis
3.8. In Vitro Caco-2 Cells Studies
3.9. General Observations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- International Diabetes Federation IDF Diabetes Atlas 11th Edition—2025. Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 14 April 2025).
- CDC Type 2 Diabetes|CDC. Available online: https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html (accessed on 7 April 2024).
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes Mellitus: Classification, Mediators, and Complications; A Gate to Identify Potential Targets for the Development of New Effective Treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Huang, H.; Li, H.; Wei, Y.; Yao, C. Legume-Derived Bioactive Peptides in Type 2 Diabetes: Opportunities and Challenges. Nutrients 2023, 15, 1096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Han, H.; Hu, Y.; Zhu, L.; Rimm, E.B.; Hu, F.B.; Sun, Q. Associations between Plant-Based Dietary Patterns and Risks of Type 2 Diabetes, Cardiovascular Disease, Cancer, and Mortality—A Systematic Review and Meta-Analysis. Nutr. J. 2023, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Mullins, A.P.; Arjmandi, B.H. Health Benefits of Plant-Based Nutrition: Focus on Beans in Cardiometabolic Diseases. Nutrients 2021, 13, 519. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Lawrence, F.R.; Hartman, T.J.; Curran, J.M. Consumption of Dry Beans, Peas, and Lentils Could Improve Diet Quality in the US Population. J. Am. Diet. Assoc. 2009, 109, 909–913. [Google Scholar] [CrossRef]
- 2025 Dietary Guidelines Advisory Committee. Scientific Report of the 2025 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and Secretary of Agriculture; U.S. Department of Health and Human Services: Washington, DC, USA, 2024.
- Singhal, P.; Kaushik, G.; Mathur, P. Antidiabetic Potential of Commonly Consumed Legumes: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 655–672. [Google Scholar] [CrossRef]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The Role of Oxidative Stress and Antioxidants in Diabetic Complications. Sultan Qaboos Univ. Med. J. 2012, 12, 5–18. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Zhu, J.; Shen, G.; Li, Z.; Dong, J. Long Noncoding RNAs in the Regulation of Oxidative Stress. Oxid. Med. Cell Longev. 2019, 2019, 1318795. [Google Scholar] [CrossRef]
- Permenter, M.G.; McDyre, B.C.; Ippolito, D.L.; Stallings, J.D. Alterations in Tissue MicroRNA after Heat Stress in the Conscious Rat: Potential Biomarkers of Organ-Specific Injury. BMC Genom. 2019, 20, 141. [Google Scholar] [CrossRef]
- Ren, S.; Wang, J.; Wang, Y.; Luo, Q.; Pu, W.; Meng, X.; Liu, S. Oxidative Stress and Type 2 Diabetes: A Review of Lactic Acid Bacteria as Potential Prophylactic and Therapeutic Interventions. Food Sci. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Farag, M.A.; Aboul Naser, A.F.; Zayed, A.; Sharaf El-Dine, M.G. Comparative Insights into Four Major Legume Sprouts Efficacies for Diabetes Management and Its Complications: Untargeted versus Targeted NMR Biochemometrics Approach. Metabolites 2022, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kadyan, S.; Ukhanov, V.; Cheng, J.; Nagpal, R.; Cui, L. Recent Advances in the Health Benefits of Pea Protein (Pisum sativum): Bioactive Peptides and the Interaction with the Gut Microbiome. Curr. Opin. Food Sci. 2022, 48, 100944. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant Food Anti-Nutritional Factors and Their Reduction Strategies: An Overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Haji, A.; Teka, T.A.; Bereka, T.Y.; Astatkie, T.; Woldemariam, H.W.; Urugo, M.M. Effect of Processing Methods on the Nutrient, Antinutrient, Functional, and Antioxidant Properties of Pigeon Pea (Cajanus cajan (L.) Millsp.) Flour. J. Agric. Food Res. 2024, 18, 101493. [Google Scholar] [CrossRef]
- Arbab Sakandar, H.; Chen, Y.; Peng, C.; Chen, X.; Imran, M.; Zhang, H. Impact of Fermentation on Antinutritional Factors and Protein Degradation of Legume Seeds: A Review. Food Rev. Int. 2023, 39, 1227–1249. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Prasanth, M.I.; Chaiyasut, C. A Mini Review on Antidiabetic Properties of Fermented Foods. Nutrients 2018, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Emkani, M.; Oliete, B.; Saurel, R. Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review. Fermentation 2022, 8, 244. [Google Scholar] [CrossRef]
- Flores-Medellín, S.A.; Camacho-Ruiz, R.M.; Guízar-González, C.; Rivera-Leon, E.A.; Llamas-Covarrubias, I.M.; Mojica, L. Protein Hydrolysates and Phenolic Compounds from Fermented Black Beans Inhibit Markers Related to Obesity and Type-2 Diabetes. Legume Sci. 2021, 3, e64. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of Air Classification and Fermentation by Lactobacillus plantarum VTT E-133328 on Faba Bean (Vicia faba L.) Flour Nutritional Properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef]
- Nordström, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus plantarum 299v (LP299V®): Three Decades of Research. Benef. Microbes 2021, 12, 441–466. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Folwarski, M.; Witkowski, J.M.; Bryl, E.; Makarewicz, W. The Role of Lactobacillus plantarum 299v in Supporting Treatment of Selected Diseases. Cent. Eur. J. Immunol. 2020, 45, 488–493. [Google Scholar] [CrossRef]
- Criste, A.D.; Urcan, A.C.; Coroian, C.O.; Copolovici, L.; Copolovici, D.M.; Burtescu, R.F.; Oláh, N.K. Plant-Based Beverages from Germinated and Ungerminated Seeds, as a Source of Probiotics, and Bioactive Compounds with Health Benefits—Part 1: Legumes. Agriculture 2023, 13, 1185. [Google Scholar] [CrossRef]
- Skrzypczak, K.; Teterycz, D.; Gustaw, W.; Domagała, D.; Mielczarek, P.; Kasprzyk-Pochopień, J. The Possibility of Using Lactobacillus plantarum 299v to Reinforce the Bioactive Properties of Legume-Derived Beverages. Appl. Sci. 2024, 14, 5187. [Google Scholar] [CrossRef]
- Antony, P.; Vijayan, R. Bioactive Peptides as Potential Nutraceuticals for Diabetes Therapy: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 9059. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Hernández, D.; Lugo-Cervantes, E.D.C.; Escobedo-Reyes, A.; Mojica, L. Black Bean (Phaseolus vulgaris L.) Polyphenolic Extract Exerts Antioxidant and Antiaging Potential. Molecules 2021, 26, 6716. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Digested Total Protein and Protein Fractions from Chia Seed (Salvia hispanica L.) Had High Scavenging Capacity and Inhibited 5-LOX, COX-1-2, and INOS Enzymes. Food Chem. 2019, 289, 204–214. [Google Scholar] [CrossRef]
- Merheb-Dini, C.; Gomes, E.; Boscolo, M.; da Silva, R. Production and Characterization of a Milk-Clotting Protease in the Crude Enzymatic Extract from the Newly Isolated Thermomucor Indicae-Seudaticae N31. Food Chem. 2010, 120, 87–93. [Google Scholar] [CrossRef]
- Acevedo-Martínez, K.A.; de Mejia, E.G. Comparison of Five Chickpea Varieties, Optimization of Hydrolysates Production and Evaluation of Biomarkers for Type 2 Diabetes. Food Res. Int. 2021, 147, 110572. [Google Scholar] [CrossRef]
- Kusumah, J.; Castañeda-Reyes, E.D.; Bringe, N.A.; de Mejia, E.G. Soybean (Glycine Max) INFOGEST Colonic Digests Attenuated Inflammatory Responses Based on Protein Profiles of Different Varieties. Int. J. Mol. Sci. 2023, 24, 12396. [Google Scholar] [CrossRef]
- Di Stefano, E.; Tsopmo, A.; Oliviero, T.; Fogliano, V.; Udenigwe, C.C. Bioprocessing of Common Pulses Changed Seed Microstructures, and Improved Dipeptidyl Peptidase-IV and α-Glucosidase Inhibitory Activities. Sci. Rep. 2019, 9, 15308. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Inhibition of Dipeptidyl Peptidase (DPP)-IV and α-Glucosidase Activities by Pepsin-Treated Whey Proteins. J. Agric. Food Chem. 2013, 61, 7500–7506. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; de Mejia, E.G. Optimization, Identification, and Comparison of Peptides from Germinated Chickpea (Cicer arietinum) Protein Hydrolysates Using Either Papain or Ficin and Their Relationship with Markers of Type 2 Diabetes. Food Chem. 2022, 374, 131717. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.P.; Thevelein, J.M.; Luyten, W. Characterization of SGLT1-Mediated Glucose Transport in Caco-2 Cell Monolayers, and Absence of Its Regulation by Sugar or Epinephrine. Eur. J. Pharmacol. 2021, 897, 173925. [Google Scholar] [CrossRef]
- Mojica, L.; Luna-Vital, D.A.; de Mejia, E.G. Black Bean Peptides Inhibit Glucose Uptake in Caco-2 Adenocarcinoma Cells by Blocking the Expression and Translocation Pathway of Glucose Transporters. Toxicol. Rep. 2018, 5, 552–560. [Google Scholar] [CrossRef]
- Xu, F.; Xu, B.; Chen, H.; Ju, X.; Gonzalez de Mejia, E. Enhancement of DPP-IV Inhibitory Activity and the Capacity for Enabling GLP-1 Secretion through RADA16-Assisted Molecular Designed Rapeseed Peptide Nanogels. Food Funct. 2022, 13, 5215–5228. [Google Scholar] [CrossRef]
- Hurtado-Murillo, J.; Franco, W.; Contardo, I. Impact of Homolactic Fermentation Using Lactobacillus Acidophilus on Plant-Based Protein Hydrolysis in Quinoa and Chickpea Flour Blended Beverages. Food Chem. 2025, 463, 141110. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Y.; Zhang, Y.; Cao, H.; Luo, D.; Yi, C.; Guan, X. Formulation of Plant-Based Yoghurt from Soybean and Quinoa and Evaluation of Physicochemical, Rheological, Sensory and Functional Properties. Food Biosci. 2022, 49, 101831. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Trząskowska, M.; Kołożyn-Krajewska, D. The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation 2024, 10, 298. [Google Scholar] [CrossRef]
- Andrade, V.T.; de Castro, R.J.S. Fermented Grain-Based Beverages as Probiotic Vehicles and Their Potential Antioxidant and Antidiabetic Properties. Biocatal. Agric. Biotechnol. 2023, 53, 102873. [Google Scholar] [CrossRef]
- Liang, Z.; Yi, M.; Sun, J.; Zhang, T.; Wen, R.; Li, C.; Reshetnik, E.I.; Gribanova, S.L.; Liu, L.; Zhang, G. Physicochemical Properties and Volatile Profile of Mung Bean Flour Fermented by Lacticaseibacillus casei and Lactococcus lactis. LWT 2022, 163, 113565. [Google Scholar] [CrossRef]
- Mousavi, M.; Gharekhani, M.; Alirezalu, K.; Roufegarinejad, L.; Azadmard-Damirchi, S. Production and Characterization of Nondairy Gluten-free Fermented Beverage Based on Buckwheat and Lentil. Food Sci. Nutr. 2023, 11, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Saeed, S.M.G.; Sohail, M.; Aloufi, A.S.; Yehia, H.M. Nano-Imaging by Atomic Force Microscopy, Thermogravimetric Characteristics, and Bioactive Compounds of Thermally Treated and Fermented Mash Beans. LWT 2024, 199, 116131. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, C.; Zhu, Y.; Zhou, C.; Xiong, Z.; Samy Eweys, A.; Zhou, H.; Dong, Y.; Xiao, X. Metabolomics Strategy for Revealing the Components in Fermented Barley Extracts with Lactobacillus plantarum Dy-1. Food Res. Int. 2021, 139, 109808. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Abouelenein, D.; Acquaticci, L.; Alessandroni, L.; Angeloni, S.; Borsetta, G.; Caprioli, G.; Nzekoue, F.K.; Sagratini, G.; Vittori, S. Polyphenols, Saponins and Phytosterols in Lentils and Their Health Benefits: An Overview. Pharmaceuticals 2022, 15, 1225. [Google Scholar] [CrossRef]
- Dhull, S.B.; Punia, S.; Kidwai, M.K.; Kaur, M.; Chawla, P.; Purewal, S.S.; Sangwan, M.; Palthania, S. Solid-State Fermentation of Lentil (Lens culinaris L.) with Aspergillus Awamori: Effect on Phenolic Compounds, Mineral Content, and Their Bioavailability. Legume Sci. 2020, 2, e37. [Google Scholar] [CrossRef]
- Almeida, B.; Rogers, K.E.; Nag, O.K.; Delehanty, J.B. Sensing Nitric Oxide in Cells: Historical Technologies and Future Outlook. ACS Sens. 2021, 6, 1695–1703. [Google Scholar] [CrossRef]
- Can, Z.; Keskin, B.; Üzer, A.; Apak, R. Detection of Nitric Oxide Radical and Determination of Its Scavenging Activity by Antioxidants Using Spectrophotometric and Spectrofluorometric Methods. Talanta 2022, 238, 122993. [Google Scholar] [CrossRef]
- Fonseca-Hernandez, D.; Cervantes, E.D.C.L.; Mojica, L. Bioactive Compounds in Fermented Chickpeas and Common Beans. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1406, pp. 115–133. [Google Scholar]
- Emkani, M.; Oliete, B.; Saurel, R. Pea Protein Extraction Assisted by Lactic Fermentation: Impact on Protein Profile and Thermal Properties. Foods 2021, 10, 549. [Google Scholar] [CrossRef]
- Ma, M.; Xu, Z.; Li, P.; Sui, Z.; Corke, H. Removal of Starch Granule-Associated Proteins Affects Amyloglucosidase Hydrolysis of Rice Starch Granules. Carbohydr. Polym. 2020, 247, 116674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Xu, J.; Yuan, J.; Fan, X. Effect of Starch Chain Structure and Non-Starch Components on the Hydrolysis of Starch by A-Amylase. Starch 2022, 74, 2100107. [Google Scholar] [CrossRef]
- Scott, G.; Awika, J.M. Effect of Protein–Starch Interactions on Starch Retrogradation and Implications for Food Product Quality. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2081–2111. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zou, W.; Dhital, S.; Wu, P.; Gidley, M.J.; Fox, G.P.; Gilbert, R.G. The Adsorption of α-Amylase on Barley Proteins Affects the in Vitro Digestion of Starch in Barley Flour. Food Chem. 2018, 241, 493–501. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, B.; Zhao, J.; Liao, S.; Han, J.; Fang, J.; Liu, P.; Ding, W.; Che, Z.; Xu, M. Insight into the Protein Degradation during the Broad Bean Fermentation Process. Food Sci. Nutr. 2022, 10, 2760–2772. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic Metabolism Revisited: Metabolism of Lactic Acid Bacteria in Food Fermentations and Food Spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Ali, S.A.; Saeed, S.M.G.; Sohail, M.; Alzahrani, A.; Yehia, H.M. Gelatinization and Fermentation Synergy: Investigating the Protein Digestibility, Mineral Bioaccessibility and Microstructural Transformations of Black Mash Beans through Saccharomyces Cerevisiae and Lactobacillus Spp. Int. J. Food Prop. 2024, 27, 674–688. [Google Scholar] [CrossRef]
- Li, W.; Wang, T. Effect of Solid-State Fermentation with Bacillus Subtilis Lwo on the Proteolysis and the Antioxidative Properties of Chickpeas. Int. J. Food Microbiol. 2021, 338, 108988. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, S.; Li, Y.; Sun, F.; Huang, D.; Chen, X. Alternations in the Multilevel Structures of Chickpea Protein during Fermentation and Their Relationship with Digestibility. Food Res. Int. 2023, 165, 112453. [Google Scholar] [CrossRef]
- Kravchenko, I.V.; Furalyov, V.A.; Pshennikova, E.S.; Fedorov, A.N.; Popov, V.O. The Effect of Fermentation by Lactobacilli on the Functional–Technological Properties of Pea Protein Isolates. Appl. Biochem. Microbiol. 2024, 60, 1388–1397. [Google Scholar] [CrossRef]
- Acevedo-Martínez, K.A.; de Mejia, E.G. Fortification of Maize Tortilla with an Optimized Chickpea Hydrolysate and Its Effect on DPP-IV Inhibition Capacity and Physicochemical Characteristics. Foods 2021, 10, 1835. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Chen, K.; de Mejía, E.G. Impact of Commercial Precooking of Common Bean (Phaseolus vulgaris) on the Generation of Peptides, After Pepsin–Pancreatin Hydrolysis, Capable to Inhibit Dipeptidyl Peptidase-IV. J. Food Sci. 2015, 80, H188–H198. [Google Scholar] [CrossRef] [PubMed]
- Farias, T.C.; de Souza, T.S.P.; Fai, A.E.C.; Koblitz, M.G.B. Critical Review for the Production of Antidiabetic Peptides by a Bibliometric Approach. Nutrients 2022, 14, 4275. [Google Scholar] [CrossRef]
- Di Stefano, E.; Hüttmann, N.; Dekker, P.; Tomassen, M.M.M.; Oliviero, T.; Fogliano, V.; Udenigwe, C.C. Solid-State Fermentation of Green Lentils by Lactiplantibacillus plantarum Leads to Formation of Distinct Peptides That Are Absorbable and Enhances DPP-IV Inhibitory Activity in an Intestinal Caco-2 Cell Model. Food Funct. 2024, 15, 11220–11235. [Google Scholar] [CrossRef]
- Bhatia, R.; Singh, S.; Maurya, R.; Bhadada, S.K.; Bishnoi, M.; Chopra, K.; Joshi, S.R.; Kondepudi, K.K. In Vitro Characterization of Lactic Acid Bacterial Strains Isolated from Fermented Foods with Anti-Inflammatory and Dipeptidyl Peptidase-IV Inhibition Potential. Braz. J. Microbiol. 2023, 54, 293–309. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, J.; Kim, D. Resistant Starch and Type 2 Diabetes Mellitus: Clinical Perspective. J. Diabetes Investig. 2024, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Preinfalk, V.; Kimmeswenger, I.; Somoza, V.; Lieder, B. Dipeptidyl-Peptidase 4 (DPP4) Mediates Fatty Acid Uptake Inhibition by Glucose via TAS1R3 and GLUT-2 in Caco-2 Enterocytes. Heliyon 2024, 10, e30329. [Google Scholar] [CrossRef]
- Clapham, J.C. Sixty Years of Drug Discovery for Type 2 Diabetes: Where Are We Now? Methods Mol. Biol. 2020, 2076, 1–30. [Google Scholar] [CrossRef]
- Jin, R.; Shang, J.; Teng, X.; Zhang, L.; Liao, M.; Kang, J.; Meng, R.; Wang, D.; Ren, H.; Liu, N. Characterization of DPP-IV Inhibitory Peptides Using an In Vitro Cell Culture Model of the Intestine. J. Agric. Food Chem. 2021, 69, 2711–2718. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the Two Incretin Hormones: Similarities and Differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef]
- García-Curiel, L.; Berenice, O.L.; Elizabeth, C.A.; Emmanuel, P.; Pérez-Flores, J.G.; Guillermo, G.L. DPP-IV Inhibitory Peptides from Whey Proteins: Production, Functional Mechanisms, Bibliometric Insights, and Future Directions for Type 2 Diabetes Therapy. Pept. Sci. 2025, 117, e70000. [Google Scholar] [CrossRef]
- Wu, P.; He, P.; Zhao, S.; Huang, T.; Lu, Y.; Zhang, K. Effects of Ursolic Acid Derivatives on Caco-2 Cells and Their Alleviating Role in Streptozocin-Induced Type 2 Diabetic Rats. Molecules 2014, 19, 12559–12576. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shen, J.; Zhu, X.; Ju, T.; Willing, B.P.; Wu, X.; Lu, Q.; Liu, R. Peanut Skin Procyanidins Reduce Intestinal Glucose Transport Protein Expression, Regulate Serum Metabolites and Ameliorate Hyperglycemia in Diabetic Mice. Food Res. Int. 2023, 173, 113471. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Zou, J.; Qin, X.; Lv, Q.; Li, B. Effects of Lactobacillus plantarum Fermentation on the Structure and Digestion of Resistant Starch Type 3 and Properties of Fermented Starch in the Simulated Digestion System. Carbohydr. Polym. 2025, 353, 123264. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Mehren, L.; Elliger, L.; May, H.; Schieber, A.; Schulze-Kaysers, N. Foaming Properties and Olfactory Profile of Fermented Chickpea Aquafaba and Its Application in Vegan Chocolate Mousse. Curr. Res. Food Sci. 2025, 10, 100988. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.-X.; Mata, A.; Corke, H.; Gan, R.-Y.; Fang, Y. Physicochemical and PH-Dependent Functional Properties of Proteins Isolated from Eight Traditional Chinese Beans. Food Hydrocoll. 2021, 112, 106288. [Google Scholar] [CrossRef]
| Factor | Final pH | Response: DPPH-Scavenging Capacity (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1: Time | X2: Bacteria | X3: Flour Concentration | RL | BEP | GSP | BB | PB | RL | BEP | GSP | BB | PB |
| −1 | −1 | 0 | 3.92 | 4.10 | 3.97 | 4.28 | 4.03 | 52.33 | 66.62 | 55.06 | 68.28 | 63.92 |
| 1 | −1 | 0 | 3.76 | 3.80 | 3.75 | 3.88 | 3.75 | 45.42 | 59.44 | 40.96 | 59.99 | 60.11 |
| −1 | 1 | 0 | 3.85 | 3.83 | 3.80 | 4.06 | 3.81 | 55.44 | 65.11 | 48.29 | 69.06 | 64.20 |
| 1 | 1 | 0 | 3.71 | 3.70 | 3.70 | 3.88 | 3.79 | 50.26 | 60.30 | 37.69 | 59.92 | 57.76 |
| −1 | 0 | −1 | 3.85 | 3.87 | 3.82 | 4.08 | 3.85 | 30.92 | 39.34 | 23.67 | 62.38 | 36.84 |
| 1 | 0 | −1 | 3.80 | 3.88 | 3.75 | 3.98 | 3.80 | 30.92 | 35.61 | 22.79 | 53.87 | 29.92 |
| −1 | 0 | 1 | 3.88 | 3.92 | 3.91 | 4.05 | 3.97 | 60.10 | 69.63 | 72.99 | 57.45 | 79.57 |
| 1 | 0 | 1 | 3.76 | 3.79 | 3.77 | 3.94 | 3.85 | 52.16 | 47.45 | 38.41 | 57.81 | 72.51 |
| 0 | −1 | −1 | 3.84 | 3.86 | 3.68 | 3.99 | 3.77 | 28.67 | 36.25 | 25.50 | 57.95 | 36.77 |
| 0 | 1 | −1 | 3.83 | 3.79 | 3.74 | 3.99 | 3.74 | 32.64 | 36.90 | 22.95 | 57.24 | 30.82 |
| 0 | −1 | 1 | 3.66 | 3.83 | 3.81 | 4.03 | 3.88 | 56.13 | 55.64 | 52.43 | 52.88 | 70.43 |
| 0 | 1 | 1 | 3.83 | 3.79 | 3.78 | 3.96 | 3.87 | 50.26 | 46.59 | 55.94 | 46.20 | 76.80 |
| 0 | 0 | 0 | 3.92 | 3.77 | 3.74 | 3.88 | 3.85 | 49.91 | 51.62 | 37.61 | 58.37 | 62.26 |
| 0 | 0 | 0 | 3.92 | 3.78 | 3.76 | 3.92 | 3.81 | 53.71 | 53.91 | 38.01 | 57.67 | 57.27 |
| 0 | 0 | 0 | 3.92 | 3.77 | 3.75 | 3.92 | 3.82 | 55.61 | 58.29 | 41.27 | 54.92 | 59.28 |
| 0 | 0 | 0 | 3.92 | 3.77 | 3.76 | 3.90 | 3.83 | 54.06 | 59.80 | 45.74 | 57.38 | 57.76 |
| Fit statistics | ||||||||||||
| Standard deviation | 2.43 | 4.01 | 4.45 | 3.00 | 1.90 | |||||||
| Mean | 47.41 | 52.66 | 41.21 | 58.21 | 57.26 | |||||||
| Coefficient of variance (%) | 5.12 | 7.61 | 10.80 | 5.17 | 3.32 | |||||||
| R2 | 0.98 | 0.95 | 0.94 | 0.88 | 0.99 | |||||||
| Adjusted R2 | 0.95 | 0.87 | 0.90 | 0.70 | 0.98 | |||||||
| Predicted R2 | 0.81 | 0.51 | 0.74 | −0.73 | 0.96 | |||||||
| Adeq precision | 17.27 | 11.26 | 16.92 | 8.00 | 32.32 | |||||||
| p-Value | |||||
|---|---|---|---|---|---|
| RL | BEP | GSP | BB | PB | |
| Model | 0.000 | 0.003 | 0.000 | 0.034 | 0.000 |
| X1-Time | 0.027 | 0.016 | 0.001 | 0.024 | 0.004 |
| X2-Bacteria | 0.413 | 0.455 | 0.489 | 0.462 | 0.769 |
| X3-Flour concentration | 0.000 | 0.001 | 0.000 | 0.091 | 0.000 |
| X1 X2 | 0.734 | 0.777 | 0.703 | 0.892 | 0.515 |
| X1 X3 | 0.153 | 0.061 | 0.004 | 0.191 | 0.972 |
| X2 X 3 | 0.089 | 0.272 | 0.513 | 0.359 | 0.018 |
| X12 | 0.735 | 0.032 | - | 0.009 | 0.128 |
| X22 | 0.146 | 0.511 | - | 0.370 | 0.504 |
| X32 | 0.000 | 0.000 | - | 0.016 | 0.001 |
| Lack of Fit | 0.495 | 0.435 | 0.376 | 0.072 | 0.746 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés-Alvarado, A.J.; Castañeda-Reyes, E.D.; Gonzalez de Mejia, E. Optimized Fermentation Conditions of Pulses Increase Scavenging Capacity and Markers of Anti-Diabetic Properties. Antioxidants 2025, 14, 523. https://doi.org/10.3390/antiox14050523
Valdés-Alvarado AJ, Castañeda-Reyes ED, Gonzalez de Mejia E. Optimized Fermentation Conditions of Pulses Increase Scavenging Capacity and Markers of Anti-Diabetic Properties. Antioxidants. 2025; 14(5):523. https://doi.org/10.3390/antiox14050523
Chicago/Turabian StyleValdés-Alvarado, Andrea Jimena, Erick Damián Castañeda-Reyes, and Elvira Gonzalez de Mejia. 2025. "Optimized Fermentation Conditions of Pulses Increase Scavenging Capacity and Markers of Anti-Diabetic Properties" Antioxidants 14, no. 5: 523. https://doi.org/10.3390/antiox14050523
APA StyleValdés-Alvarado, A. J., Castañeda-Reyes, E. D., & Gonzalez de Mejia, E. (2025). Optimized Fermentation Conditions of Pulses Increase Scavenging Capacity and Markers of Anti-Diabetic Properties. Antioxidants, 14(5), 523. https://doi.org/10.3390/antiox14050523









