A Comparison of Total Antioxidant Capacities of Concord, Purple, Red, and Green Grapes Using the CUPRAC Assay
Abstract
:1. Introduction
2. Materials and Methods
Statistics
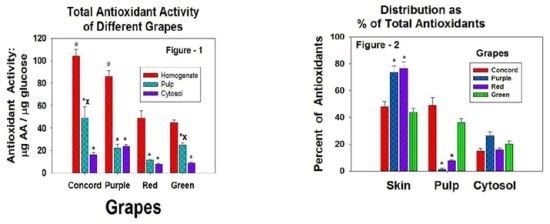
3. Results


4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lopez-Oliva, M.E.; Pozuelo, M.J.; Rotger, R.; Munoz-Martinez, E.; Goni, I. Grape antioxidant dietary fibre prevents mitochondrial apoptotic pathways by enhancing Bcl-2 and Bcl-xL expression and minimising oxidative stress in rat distal colonic mucosa. Br. J. Nutr. 2013, 109, 4–16. [Google Scholar] [CrossRef]
- Vislocky, L.M.; Fernandez, M.L. Biomedical effects of grape products. Nutr. Rev. 2010, 68, 656–670. [Google Scholar] [CrossRef]
- Folts, J.D. Potential health benefits from the flavonoids in grape products on vascular disease. Adv. Exp. Med. Biol. 2002, 505, 95–111. [Google Scholar] [CrossRef]
- Farrugia, G.; Balzan, R. Oxidative stress and programmed cell death in yeast. Front. Oncol. 2012, 2, 64. [Google Scholar] [PubMed]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665. [Google Scholar] [CrossRef]
- Salim, S.; Asghar, M.; Chugh, G.; Taneja, M.; Xia, Z.; Saha, K. Oxidative stress: A potential recipe for anxiety, hypertension and insulin resistance. Brain Res. 2010, 1359, 178–185. [Google Scholar] [CrossRef]
- Pimentel, C.; Batista-Nascimento, L.; Rodrigues-Pousada, C.; Menezes, R.A. Oxidative stress in Alzheimer’s and Parkinson’s diseases: Insights from the yeast Saccharomyces cerevisiae. Oxid. Med. Cell. Longev. 2012, 2012, 132146. [Google Scholar] [PubMed]
- Giasson, B.I.; Ischiropoulos, H.; Lee, V.M.; Trojanowski, J.Q. The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer’s and Parkinson’s diseases. Free Radic. Biol. Med. 2002, 32, 1264–1275. [Google Scholar] [CrossRef]
- Pashkow, F.J. Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int. J. Inflamm. 2011, 2011, 514623. [Google Scholar]
- Bowe, W.P.; Patel, N.; Logan, A.C. Acne vulgaris: The role of oxidative stress and the potential therapeutic value of local and systemic antioxidants. J. Drugs Dermatol. 2012, 11, 742–746. [Google Scholar] [PubMed]
- Dai, D.F.; Rabinovitch, P.S.; Ungvari, Z. Mitochondria and cardiovascular aging. Circ. Res. 2012, 110, 1109–1124. [Google Scholar] [CrossRef]
- Aoi, W.; Sakuma, K. Oxidative stress and skeletal muscle dysfunction with aging. Curr. Aging Sci. 2011, 4, 101–109. [Google Scholar] [CrossRef]
- Wei, Y.H.; Lu, C.Y.; Wei, C.Y.; Ma, Y.S.; Lee, H.C. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin. J. Physiol. 2001, 44, 1–11. [Google Scholar] [PubMed]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Torres, J.L.; Bobet, R. New flavanol derivatives from grape (Vitis vinifera) byproducts. Antioxidant aminoethylthio-flavan-3-ol conjugates from a polymeric waste fraction used as a source of flavanols. J. Agric. Food Chem. 2001, 49, 4627–4634. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Rowe, C.A.; Nantz, M.P.; Nieves, C., Jr.; West, R.L.; Percival, S.S. Regular consumption of concord grape juice benefits human immunity. J. Med. Food 2011, 14, 69–78. [Google Scholar] [CrossRef]
- Kalbasi, A.; Cisneros-Zevallos, L. Fractionation of monomeric and polymeric anthocyainins from Concord grape (Vitis labrusca L.) juice by membrane ultrafiltration. J. Agric. Food Chem. 2007, 55, 7036–7042. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Gastrointestinal stability and bioavailability of (poly)phenolic compounds following ingestion of Concord grape juice by humans. Mol. Nutr. Food Res. 2012, 56, 497–509. [Google Scholar] [CrossRef]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Identification of (poly)phenolic compounds in concord grape juice and their metabolites in human plasma and urine after juice consumption. J. Agric. Food Chem. 2011, 59, 9512–9522. [Google Scholar] [CrossRef]
- Bean, H.; Schuler, C.; Leggett, R.E.; Levin, R.M. Antioxidant levels of common fruits, vegetables, and juices versus protective activity against in vitro ischemia/reperfusion. Int. Urol. Nephrol. 2010, 42, 409–415. [Google Scholar] [CrossRef]
- Bean, H.; Radu, F.; De, E.; Schuler, C.; Leggett, R.E.; Levin, R.M. Comparative evaluation of antioxidant reactivity within obstructed and control rabbit urinary bladder tissue using FRAP and CUPRAC assays. Mol. Cell. Biochem. 2009, 323, 139–142. [Google Scholar] [CrossRef]
- Bektasoglu, B.; Esin Celik, S.; Ozyurek, M.; Guclu, K.; Apak, R. Novel hydroxyl radical scavenging antioxidant activity assay for water-soluble antioxidants using a modified CUPRAC method. Biochem. Biophys. Res. Commun. 2006, 345, 1194–1200. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Demirata, B.; Ozyurek, M.; Celik, S.E.; Bektasoglu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Pekal, A.; Drozdz, P.; Biesaga, M.; Pyrzynska, K. Evaluation of the antioxidant properties of fruit and flavoured black teas. Eur. J. Nutr. 2011, 50, 681–688. [Google Scholar] [CrossRef]
- Jastrzebski, Z.; Tashma, Z.; Katrich, E.; Gorinstein, S. Biochemical characteristics of the herb mixture Prolipid as a plant food supplement and medicinal remedy. Plant Foods Hum. Nutr. 2007, 62, 145–150. [Google Scholar] [CrossRef]
- Sariburun, E.; Sahin, S.; Demir, C.; Turkben, C.; Uylaser, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef]
- Toledo, F.; Arancibia-Avila, P.; Park, Y.S.; Jung, S.T.; Kang, S.G.; Heo, B.G.; Drzewiecki, J.; Zachwieja, Z.; Zagrodzki, P.; Pasko, P.; et al. Screening of the antioxidant and nutritional properties, phenolic contents and proteins of five durian cultivars. Int. J. Food Sci. Nutr. 2008, 59, 415–427. [Google Scholar] [CrossRef]
- Hui, C.; Bin, Y.; Xiaoping, Y.; Long, Y.; Chunye, C.; Mantian, M.; Wenhua, L. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer 2010, 62, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.; Berry, N.M.; Misan, G.; Coates, A.M.; Buckley, J.D.; Howe, P.R. Dose-related effects of flavanol-rich cocoa on blood pressure. J. Hum. Hypertens. 2010, 24, 568–576. [Google Scholar] [CrossRef]
- Farouque, H.M.; Leung, M.; Hope, S.A.; Baldi, M.; Schechter, C.; Cameron, J.D.; Meredith, I.T. Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: A randomized double-blind placebo-controlled study. Clin. Sci. (Lond.) 2006, 111, 71–80. [Google Scholar] [CrossRef]
- Pearson, D.A.; Paglieroni, T.G.; Rein, D.; Wun, T.; Schramm, D.D.; Wang, J.F.; Holt, R.R.; Gosselin, R.; Schmitz, H.H.; Keen, C.L. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb. Res. 2002, 106, 191–197. [Google Scholar] [CrossRef]
- Agartan, C.A.; Whitbeck, C.; Sokol, R.; Chichester, P.; Levin, R.M. Protection of urinary bladder function by grape suspension. Phytother. Res. 2004, 18, 1013–1018. [Google Scholar] [CrossRef]
- Lin, A.D.; Mannikarottu, A.; Chaudhry, A.; Whitbeck, C.; Kogan, B.A.; Chichester, P.; Levin, R.M. Protective effects of grape suspension on in vivo ischaemia/reperfusion of the rabbit bladder. BJU Int. 2005, 96, 1397–1402. [Google Scholar] [CrossRef]
- Lin, A.D.; Mannikarottu, A.; Kogan, B.A.; Whitbeck, C.; Leggett, R.E.; Levin, R.M. Effect of bilateral in vivo ischemia/reperfusion on the activities of superoxide dismutase and catalase: Response to a standardized grape suspension. Mol. Cell. Biochem. 2007, 296, 11–16. [Google Scholar] [CrossRef]
- Venugopal, V.; Leggett, R.E.; Schuler, C.; Levin, R.M. Effect of hydrogen peroxide on rabbit urinary bladder citrate synthase activity in the presence and absence of a grape suspension. Int. Braz. J. Urol. 2010, 36, 749–757. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Callaghan, C.M.; Leggett, R.E.; Levin, R.M. A Comparison of Total Antioxidant Capacities of Concord, Purple, Red, and Green Grapes Using the CUPRAC Assay. Antioxidants 2013, 2, 257-264. https://doi.org/10.3390/antiox2040257
Callaghan CM, Leggett RE, Levin RM. A Comparison of Total Antioxidant Capacities of Concord, Purple, Red, and Green Grapes Using the CUPRAC Assay. Antioxidants. 2013; 2(4):257-264. https://doi.org/10.3390/antiox2040257
Chicago/Turabian StyleCallaghan, Connor M., Robert E. Leggett, and Robert M. Levin. 2013. "A Comparison of Total Antioxidant Capacities of Concord, Purple, Red, and Green Grapes Using the CUPRAC Assay" Antioxidants 2, no. 4: 257-264. https://doi.org/10.3390/antiox2040257
APA StyleCallaghan, C. M., Leggett, R. E., & Levin, R. M. (2013). A Comparison of Total Antioxidant Capacities of Concord, Purple, Red, and Green Grapes Using the CUPRAC Assay. Antioxidants, 2(4), 257-264. https://doi.org/10.3390/antiox2040257