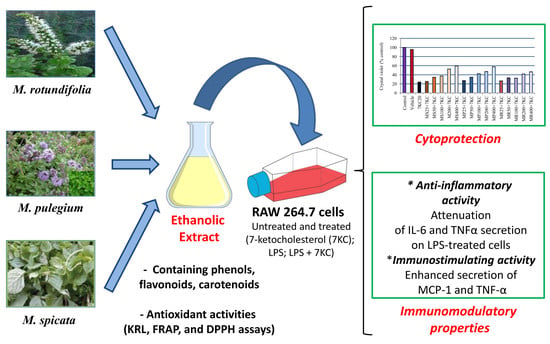
Evaluation of Antioxidant, Anti-Inflammatory and Cytoprotective Properties of Ethanolic Mint Extracts from Algeria on 7-Ketocholesterol-Treated Murine RAW 264.7 Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Herbal Material
2.2. Preparation of Ethanolic Mint Extracts
2.3. Quantification of Phenolic, Flavonoid and Carotenoid Contents of Ethanolic Mint Extracts
2.4. Determination of the Antioxidant Capacity of Ethanolic Mint Extracts
2.5. Cells and Cell Treatments
2.6. Evaluation of Cell Growth: Staining with Crystal Violet
2.7. Multiplexed Flow Cytometric Analysis of Inflammatory Cytokines
2.8. Statistical Analysis
3. Results
3.1. Quantification of Antioxidants and Determination of the Antioxidant Capacity
3.2. Effect of Mint Extracts on 7-Ketocholesterol-Induced Cell Growth Inhibition
3.3. Effect of Mint Extracts on Cytokine Secretion Induced by 7-Ketocholesterol (7KC) and Lipopolysaccharide (LPS) Associated or Not with 7KC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef]
- Iuliano, L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem. Phys. Lipids 2011, 164, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, C.; Iuliano, L. Cholesterol and related sterols autoxidation. Free Radic. Biol. Med. 2017, 111, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Rossin, D.; Poli, G.; Biasi, F.; Leonarduzzi, G. Implication of oxysterols in chronic inflammatory human diseases. Biochimie 2018, 153, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Monier, S.; Samadi, M.; Prunet, C.; Denance, M.; Laubriet, A.; Athias, A.; Berthier, A.; Steinmetz, E.; Jürgens, G.; Nègre-Salvayre, A.; et al. Impairment of the cytotoxic and oxidative activities of 7β-hydroxycholesterol and 7-ketocholesterol by esterification with oleate. Biochem. Biophys. Res. Commun. 2003, 303, 814–824. [Google Scholar] [CrossRef]
- Nury, T.; Samadi, M.; Zarrouk, A.; Riedinger, J.M.; Lizard, G. Improved synthesis and in vitro evaluation of the cytotoxic profile of oxysterols oxidized at C4 (4α-and 4β-hydroxycholesterol) and C7 (7-ketocholesterol, 7α-and 7β-hydroxycholesterol) on cells of the central nervous system. Eur. J. Med. Chem. 2013, 70, 558–567. [Google Scholar] [CrossRef]
- Nury, T.; Zarrouk, A.; Vejux, A.; Doria, M.; Riedinger, J.M.; Delage-Mourroux, R.; Lizard, G. Induction of oxiapoptophagy, a mixed mode of cell death associated with oxidative stress, apoptosis and autophagy, on 7-ketocholesterol-treated 158N murine oligodendrocytes: Impairment by α-tocopherol. Biochem. Biophys. Res. Commun. 2014, 446, 714–719. [Google Scholar] [CrossRef]
- Vejux, A.; Lizard, G. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Asp. Med. 2009, 30, 153–170. [Google Scholar] [CrossRef]
- Zarrouk, A.; Vejux, A.; Mackrill, J.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.; Lizard, G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 2014, 18, 148–162. [Google Scholar] [CrossRef]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in brain oxysterols at different stages of Alzheimer’s disease: Their involvement in neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef]
- De Medina, P.; Paillasse, M.R.; Ségala, G.; Khallouki, F.; Brillouet, S.; Dalenc, F.; Courbon, F.; Record, M.; Poirot, M.; Silvente-Poirot, S. Importance of cholesterol and oxysterols metabolism in the pharmacology of tamoxifen and other AEBS ligands. Chem. Phys. Lipids 2011, 164, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Kloudova, A.; Guengerich, F.P.; Soucek, P. The Role of Oxysterols in Human Cancer. Trends Endocrinol. Metab. 2017, 28, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Biasi, F.; Leonarduzzi, G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013, 1, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Mossalayi, M.; Rambert, J.; Renouf, E.; Micouleau, M.; Mérillon, J. Grape polyphenols and propolis mixture inhibits inflammatory mediator release from human leukocytes and reduces clinical scores in experimental arthritis. Phytomedicine 2014, 21, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Rossin, D.; Rossin, D.; Calfapietra, S.; Sottero, B.; Poli, G.; Biasi, F. HNE and cholesterol oxidation products in colorectal inflammation and carcinogenesis. Free Radic. Biol. Med. 2017. [Google Scholar] [CrossRef]
- Vejux, A.; Malvitte, L.; Lizard, G. Side effects of oxysterols: Cytotoxicity, oxidation, inflammation, and phospholipidosis. Braz. J. Med. Biol. Res. 2008, 41, 545–556. [Google Scholar] [CrossRef]
- Brahmi, F.; Vejux, A.; Sghaier, R.; Zarrouk, A.; Nury, T.; Meddeb, W.; Rezig, L.; Namsi, A.; Sassi, K.; Yammine, A.; et al. Prevention of 7-ketocholesterol-induced side effects by natural compounds. Crit. Rev. Food Sci. Nutr. 2018. [Google Scholar] [CrossRef]
- Nury, T.; Zarrouk, A.; Ragot, K.; Debbabi, M.; Riedinger, J.M.; Vejux, A.; Aubourg, P.; Lizard, G. 7-Ketocholesterol is increased in the plasma of X-ALD patients and induces peroxisomal modifications in microglial cells: Potential roles of 7-ketocholesterol in the pathophysiology of X-ALD. J. Steroid Biochem. Mol. Biol. 2017, 169, 123–136. [Google Scholar] [CrossRef]
- Sottero, B.; Rossin, D.; Poli, G.; Biasi, F. Lipid oxidation products in the pathogenesis of inflammation-related gut diseases. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef]
- Lizard, G.; Lemaire, S.; Monier, S.; Gueldry, S.; Néel, D.; Gambert, P. Induction of apoptosis and of interleukin-1beta secretion by 7beta-hydroxycholesterol and 7-ketocholesterol: Partial inhibition by Bcl-2 overexpression. FEBS Lett. 1997, 15, 276–280. [Google Scholar] [CrossRef]
- Lemaire, S.; Lizard, G.; Monier, S.; Miguet, C.; Gueldry, S.; Volot, F.; Gambert, P.; Néel, D. Different patterns of IL-1beta secretion, adhesion molecule expression and apoptosis induction in human endothelial cells treated with 7alpha-, 7beta-hydroxycholesterol, or 7-ketocholesterol. FEBS Lett. 1998, 4, 434–439. [Google Scholar] [CrossRef]
- Debbabi, M.; Nury, T.; Zarrouk, A.; Mekahli, N.; Bezine, M.; Sghaier, R.; Grégoire, S.; Martine, L.; Durand, P.; Camus, E. Protective effects of α-tocopherol, γ-tocopherol and oleic acid, three compounds of olive oils, and no effect of trolox, on 7-Ketocholesterol-induced mitochondrial and peroxisomal dysfunction in microglial BV-2 Cells. Int. J. Mol. Sci. 2016, 17, 1973. [Google Scholar] [CrossRef] [PubMed]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Mertz, C.; Gancel, A.-L.; Gunata, Z.; Alter, P.; Dhuique-Mayer, C.; Vaillant, F.; Perez, A.M.; Ruales, J.; Brat, P. Phenolic compounds, carotenoids and antioxidant activity of three tropical fruits. J. Food Compos. Anal. 2009, 22, 381–387. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Bozin, B.; Mentha, L. Species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr. Pharm. Des. 2008, 14, 3141–3150. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Priya, N.G.; Subathra, M.; Ramesh, A. Anti-inflammatory activity of four solvent fractions of ethanol extract of Mentha spicata L. investigated on acute and chronic inflammation induced rats. Environ. Toxicol. Pharmacol. 2008, 26, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jain, D.K.; Balekar, N. In-Vivo Antioxidant activity of ethanolic extract of Mentha pulegium leaf against CCl4 induced toxicity in rats. Asian Pac. J. Trop. Biomed. 2012, 2, 737–740. [Google Scholar] [CrossRef]
- Brahmi, F.; Hauchard, D.; Guendouze, N.; Madani, K.; Kiendrebeogo, M.; Kamagaju, L.; Stévigny, C.; Chibane, M.; Duez, P. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae). Ind. Crop. Prod. 2015, 74, 722–730. [Google Scholar] [CrossRef]
- Dorman, H.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef]
- Brahmi, F.; Hadj-Ahmed, S.; Zarrouk, A.; Bezine, M.; Nury, T.; Madani, K.; Chibane, M.; Vejux, A.; Andreoletti, P.; Boulrkbache-Makhlouf, L.; et al. Evidence of biological activity of Mentha species extracts on apoptotic and authophagic targets on murine RAW 264.7 and human U 937 monocytic cells. Pharm. Biol. 2017, 55, 293. [Google Scholar] [CrossRef]
- Prunet, C.; Montange, T.; Véjux, A.; Laubriet, A.; Rohmer, J.F.; Riedinger, J.M.; Athias, A.; Lemaire-Ewing, S.; Néel, D.; Petit, J.M. Multiplexed flow cytometric analyses of pro-and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytom. Part A 2006, 69, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Kitanaka, S. Chemical Compounds in Natural Medicines That Affect Macropharges and Adipocyte Cells. Yakugaku Zasshi 2016, 136, 1195–1216. [Google Scholar] [CrossRef] [PubMed]
- Sass-Kiss, A.; Kiss, J.; Milotay, P.; Kerek, M.; Toth-Markus, M. Differences in anthocyanin and carotenoid ontent of fruits and vegetables. Food Res. Int. 2005, 38, 1023–1029. [Google Scholar] [CrossRef]
- Rifler, J.P.; Lorcerie, F.; Durand, P.; Delmas, D.; Ragot, K.; Limagne, E.; Mazué, F.; Riedinger, J.M.; d’Athis, P.; Hudelot, B.; et al. A moderate red wine intake improves blood lipid parameters and erythrocytes membrane fluidity in post myocardial infarct patients. Mol. Nutr. Food Res. 2012, 56, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Pastorelli, G.; Corino, C. Application of KRL test to assess total antioxidant activity in pigs: Sensitivity to dietary antioxidants. Res. Vet. Sci. 2013, 94, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Prost, M. Process for the Determination by Means of Free Radicals of the Antioxidant Properties of a Living Organism or a Potentially Aggressive Age. U.S. Patent 5,135,850, 4 August 1992. [Google Scholar]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim. Pol. 2010, 57, 139–142. [Google Scholar]
- Rossi, R.; Corino, C.; Pastorelli, G.; Durand, P.; Prost, M. Assessment of antioxidant activity of natural extracts. Ital. J. Anim. Sci. 2009, 8, 655–657. [Google Scholar] [CrossRef]
- El Kamouni, S.; El Kebbaj, R.; Andreoletti, P.; El Ktaibi, A.; Rharrassi, I.; Essamadi, A.; El Kebbaj, M.S.; Mandard, S.; Latruffe, N.; Vamecq, J.; et al. Protective effect of argan and olive oils against LPS-induced oxidative stress and inflammation in mice livers. Int. J. Mol. Sci. 2017, 18, 2181. [Google Scholar] [CrossRef] [PubMed]
- Berbaum, K.; Shanmugam, K.; Stuchbury, G.; Wiede, F.; Körner, H.; Münch, G. Induction of novel cytokines and chemokines by advanced glycation endproducts determined with a cytometric bead array. Cytokine 2008, 41, 198–203. [Google Scholar] [CrossRef]
- Glushkova, O.V.; Parfenyuk, S.B.; Novoselova, T.V.; Khrenov, M.O.; Lunin, S.M.; Novoselova, E.G. The Role of p38 and CK2 Protein Kinases in the Response of RAW 264.7 Macrophages to Lipopolysaccharide. Biochemistry 2018, 83, 746–754. [Google Scholar] [CrossRef]
- Soumaya, K.-J.; Zied, G.; Nouha, N.; Mounira, K.; Kamel, G.; Genviève, F.D.M.; Leila, G.C. Evaluation of in vitro antioxidant and apoptotic activities of Cyperus rotundus. Asian Pac. J. Trop. Med. 2014, 7, 105–112. [Google Scholar] [CrossRef]
- Conforti, F.; Sosa, S.; Marrelli, M.; Menichini, F.; Statti, G.A.; Uzunov, D.; Tubaro, A.; Menichini, F.; Della Loggia, R. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J. Ethnopharmacol. 2008, 116, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995, 62, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Ramamurthy, P.; Ramesh, A. Antioxidant and cytotoxic activities of lipophilic and hydrophilic fractions of Mentha spicata L. (Lamiaceae). Int. J. Food Prop. 2010, 13, 23–31. [Google Scholar] [CrossRef]
- Ansorena, D.; Barriuso, B.; Cardenia, V.; Astiasarán, I.; Lercker, G.; Rodriguez-Estrada, M.T. Thermo-oxidation of cholesterol: Effect of the unsaturation degree of the lipid matrix. Food Chem. 2013, 141, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Lizard, G.; Gueldry, S.; Sordet, O.; Monier, S.; Athias, A.; Miguet, C.; Bessede, G.; Lemaire, S.; Solary, E.; Gambert, P. Glutathione is implied in the control of 7-ketocholesterol-induced apoptosis, which is associated with radical oxygen species production. FASEB J. 1998, 12, 1651–1663. [Google Scholar] [CrossRef]
- Lizard, G.; Miguet, C.; Besséde, G.; Monier, S.; Gueldry, S.; Neel, D.; Gambert, P. Impairment with various antioxidants of the loss of mitochondrial transmembrane potential and of the cytosolic release of cytochrome c occuring during 7-ketocholesterol-induced apoptosis. Free Radic. Biol. Med. 2000, 28, 743–753. [Google Scholar] [CrossRef]
- Moreno, L.; Bello, R.; Primo-Yúfera, E.; Esplugues, J. Pharmacological properties of the methanol extract from Mentha suaveolens Ehrh. Phytother. Res. 2002, 16, 10–13. [Google Scholar] [CrossRef]
- Janoszka, B. 7-Ketocholesterol and 7-hydroxycholesterol in pork meat and its gravy thermally treated without additives and in the presence of onion and garlic. Meat Sci. 2010, 86, 976–984. [Google Scholar] [CrossRef]
- Brahmi, F.; Madani, K.; Stévigny, C.; Chibane, M.; Duez, P. Algerian mint species: High performance thin layer chromatography quantitative determination of rosmarinic acid and in vitro inhibitory effects on linoleic acid peroxidation. J. Coast. Life Med. 2014, 2, 986–992. [Google Scholar] [CrossRef]
- Chien, J.-T.; Hsu, D.-J.; Chen, B.-H. Kinetic model for studying the effect of quercetin on cholesterol oxidation during heating. J. Agric. Food Chem. 2006, 54, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Dugas, B.; Charbonnier, S.; Baarine, M.; Ragot, K.; Delmas, D.; Ménétrier, F.; Lherminier, J.; Malvitte, L.; Khalfaoui, T.; Bron, A.; et al. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: Cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur. J. Nutr. 2010, 49, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H.; Kiderlen, A.F. Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells. Phytochemistry 2005, 66, 2056–2071. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.S.; Carneiro, T.C.B.; Cerqueira-Lima, A.T.; Queiroz, N.V.; Alcântara-Neves, N.M.; Pontes-de-Carvalho, L.C.; da Silva Velozo, E.; Oliveira, E.J.; Figueiredo, C.A. Ocimum gratissimum Linn. and rosmarinic acid, attenuate eosinophilic airway inflammation in an experimental model of respiratory allergy to Blomia tropicalis. Int. Immunopharmacol. 2012, 13, 126–134. [Google Scholar] [CrossRef]
- Sanbongi, C.; Takano, H.; Osakabe, N.; Sasa, N.; Natsume, M.; Yanagisawa, R.; Inoue, K.-I.; Sadakane, K.; Ichinose, T.; Yoshikawa, T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin. Exp. Allergy 2004, 34, 971–977. [Google Scholar] [CrossRef]



| Extracts | Extraction Yield (%) | TPC (*) (Eq. mg Gallic Acid/g) | TFC (*) (Eq. mg Quercetin/g) | TCC (*) (Eq. mg β-Carotene/g) |
|---|---|---|---|---|
| MS | 27.3 | 30.8 ± 3.0 a | 5.2 ± 0.4 b | 3.4 ± 0.1 b |
| MP | 24.3 | 28.3 ± 1.5 a | 5.7 ± 0.2 b | 3.3 ± 0.2 b |
| MR | 29.5 | 23. 8 ± 3.3 b | 7.1 ± 0.3 a | 4.2 ± 0.2 a |
| Antioxidant Assays | Antioxidant Activity (mg of Trolox equivalent/g) | ||
|---|---|---|---|
| MS | MP | MR | |
| KRL | 796.78 ± 2.72 a | 554.19 ± 2.46 c | 606.34 ± 1.09 b |
| FRAP | 1430.13 ± 221.66 a | 776.48 ± 39.48 b | 364.79 ± 32.52 c |
| DPPH | 207.96 ± 10.98 a | 81.98 ± 6.44 b | 38.50 ± 1.96 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brahmi, F.; Nury, T.; Debbabi, M.; Hadj-Ahmed, S.; Zarrouk, A.; Prost, M.; Madani, K.; Boulekbache-Makhlouf, L.; Lizard, G. Evaluation of Antioxidant, Anti-Inflammatory and Cytoprotective Properties of Ethanolic Mint Extracts from Algeria on 7-Ketocholesterol-Treated Murine RAW 264.7 Macrophages. Antioxidants 2018, 7, 184. https://doi.org/10.3390/antiox7120184
Brahmi F, Nury T, Debbabi M, Hadj-Ahmed S, Zarrouk A, Prost M, Madani K, Boulekbache-Makhlouf L, Lizard G. Evaluation of Antioxidant, Anti-Inflammatory and Cytoprotective Properties of Ethanolic Mint Extracts from Algeria on 7-Ketocholesterol-Treated Murine RAW 264.7 Macrophages. Antioxidants. 2018; 7(12):184. https://doi.org/10.3390/antiox7120184
Chicago/Turabian StyleBrahmi, Fatiha, Thomas Nury, Meryam Debbabi, Samia Hadj-Ahmed, Amira Zarrouk, Michel Prost, Khodir Madani, Lila Boulekbache-Makhlouf, and Gérard Lizard. 2018. "Evaluation of Antioxidant, Anti-Inflammatory and Cytoprotective Properties of Ethanolic Mint Extracts from Algeria on 7-Ketocholesterol-Treated Murine RAW 264.7 Macrophages" Antioxidants 7, no. 12: 184. https://doi.org/10.3390/antiox7120184
APA StyleBrahmi, F., Nury, T., Debbabi, M., Hadj-Ahmed, S., Zarrouk, A., Prost, M., Madani, K., Boulekbache-Makhlouf, L., & Lizard, G. (2018). Evaluation of Antioxidant, Anti-Inflammatory and Cytoprotective Properties of Ethanolic Mint Extracts from Algeria on 7-Ketocholesterol-Treated Murine RAW 264.7 Macrophages. Antioxidants, 7(12), 184. https://doi.org/10.3390/antiox7120184