Inhibition of Advanced Glycation End-Product Formation by High Antioxidant-Leveled Spices Commonly Used in European Cuisine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material Extraction Procedure
2.3. Determination of Total Phenolic (TP) Content
2.4. Determination of Antioxidant Activity
2.5. Advanced Glycation End Products (AGEs) Assay
2.5.1. BSA-Glucose Model System Preparation
2.5.2. BSA-MGO Model System Preparation
2.5.3. Measurement of AGE Fluorescence
2.5.4. Statistical Analysis
3. Results and Discussion
3.1. TP Content and Antioxidant Capacity Determination of Selected Herbs and Spices
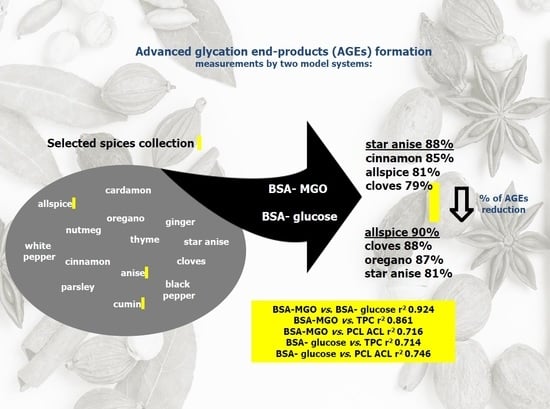
3.2. Results of AGE Inhibitory Ability Among Selected Herbs and Spices
3.3. Correlation Study and Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Effect of glycation inhibitors on aging and age-related diseases. Mech. Ageing Dev. 2016, 160, 1–18. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, K.W.; Ma, J.; Chen, B.; Ho, C.T.; Lo, C.; Chen, F.; Wang, M. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. J. Agric. Food Chem. 2008, 56, 1907–1911. [Google Scholar] [CrossRef]
- Jang, D.S.; Kim, J.M.; Kim, J.; Yoo, J.L.; Kim, Y.S.; Kim, J.S. Effects of compounds isolated from the fruits of Rumex japonicus on the protein glycation. Chem. Biodivers. 2008, 5, 2718–2723. [Google Scholar] [CrossRef] [PubMed]
- Guguicci, A.; Bastos, D.H.; Schulze, J.; Souza, M.F.F. Caffeic and chlorogenic acids in Ilex paraguarieusis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia 2009, 80, 339–344. [Google Scholar]
- Liu, Y.; Kakani, R.K.; Nair, M.G. Compounds in functional food fenugreek spice exhibit anti-inflammatory and antioxidant activity. Food Chem. 2012, 131, 1187–1192. [Google Scholar] [CrossRef]
- Bhattacherjee, A.; Chakraborti, A.S. Inhibitory effect of Piper betle Linn. Leaf extract on protein glycation- quantification and characterization of the antiglycation components. Indian J. Biochem. Biophys. 2013, 50, 529–536. [Google Scholar]
- Perez Gutierrez, R.M.; Lugrado diaz, S.; Cordova Reyes, I.; Neira Gonzalez, A.M. Evaluation of the antioxidant and anti-glication effects of the hexane extract from Piper auritum leaves in vitro and beneficial activity on oxidative stress and advanced glycation end-product-mediated renal injury in streptozotocin-treated diabetic rats. J. Nat. Prod. 2010, 3, 95–102. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Gołąb, K.; Gburek, J.; Wysokińska, H.; Markowski, A. Inhibition of Advanced Glycation End-Product Formation and Antioxidant Activity by Extracts and Polyphenols from Scutellaria alpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 2013, 6, 561–569. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid microplate high-throughput methodology for assessment of Folin-Ciocalteu reducing capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.; Lewin, G. Oxidants and Antioxidants; Part B; Packer, L., Ed.; Academic Press: New York, NY, USA; London, UK, 1999; pp. 96–100. [Google Scholar]
- Lucenford, N.; Gugliucci, A. Ilex paraguariensis extracts inhibit AGE formation more efficiently than green tea. Fitoterapia 2005, 76, 419–427. [Google Scholar]
- Szawara-Nowak, D.; Koutsidis, G.; Wiczkowski, W.; Zieliński, H. Evaluation of the in vitro inhibitory effects of buckwheat enhanced wheat bread extracts on the formation of advanced glycation end-products (AGEs). LWT-Food Sci. Technol. 2014, 58, 327–334. [Google Scholar] [CrossRef]
- Przygodzka, M.; Zielińska, D.; Ciesarová, Z.; Kristiná, K.; Zieliński, H. comparison of methods for evaluation of the antioxidant capacity and phenolic compound in common spices. LWT-Food Sci. Technol. 2014, 58, 321–326. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Peter, K.V. (Ed.) Handbook of Herbs and Spices; Woodhead Publishing Limited: Cambridge, UK, 2001; Volume 1, ISBN 978-1-85573-562-0. [Google Scholar]
- Marková, L.; Ciesarová, Z.; Kukurová, K.; Zieliński, H.; Przygodzka, M.; Bednáriková, A.; Šimko, P. Influence of various spices on acrylamide content in buckwheat ginger cakes. Chem. Pap. 2012, 66, 949–954. [Google Scholar] [CrossRef]
- Assefa, A.D.; Keum, Y.S.; Saini, R.K. A comprehensive study of polyphenols contents and antioxidant potential of 39 widely used spices and food condiments. J. Food Meas. Charact. 2018, 12, 1548–1555. [Google Scholar] [CrossRef]
- Agbor, G.A.; Vinson, J.A.; Oben, J.E.; Ngogang, J.Y. Comparative analysis of the in vitro antioxidant activity of white and black pepper. Nutr. Res. 2006, 26, 659–663. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Dvorackova, E.; Snoblova, M.; Chromcova, L.; Hrdlicka, P. Effect of extraction methods on the phenolic compounds contents and antioxidant capacities of cinnamon extracts. Food Sci. Biotechnol. 2015, 24, 1201–1207. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants-a mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects: A review. J. Funct. Food 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Dearlove, R.P.; Greenspan, P.; Hartle, D.K.; Swanson, R.B.; Hargrove, J.L. Inhibition of protein glycation by extracts of culinary herbs and spices. J. Med. Food 2008, 11, 275–281. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Sompong, W.; Meeprom, A.; Ngamukote, S.; Yibchok-anun, S. Cinnamic acid and its derivatives inhibit fructose-mediated protein glycation. Int. J. Mol. Sci. 2012, 13, 1778–1789. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Akanji, M.A.; Hafizur Rahman, M.; Choudhary, M.I. Antiglycation, antioxidant and toxicological potential of polyphenol extracts of alligator pepper, ginger and nutmeg from Nigeria. Asian Pac. J. Trop. Biomed. 2012, 2, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with antiglycation activity and mechanism of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, B. Kinetics of glycoxidation of bovine serum albumin by methylglyoxal and glyoxal and its prevention by various compounds. Molecules 2014, 19, 4880–4896. [Google Scholar] [CrossRef]
- Bhattacherjee, A.; Datta, A. Mechanism of antiglycating properties of syringic and chlorogenic acids in in vitro glycation system. Food Res. Int. 2015, 77, 540–548. [Google Scholar] [CrossRef]
- Xia, S.; Li, Y.; Xia, Q.; Zhang, X.; Huang, Q. Glycosylation of bovine serum albumin via Maillard reaction prevents epigallocatechin-3-gallate-induced protein aggregation. Food Hydrocoll. 2015, 43, 228–235. [Google Scholar] [CrossRef]
- Yoon, S.-R.; Shim, S.-M. Inhibitory effect of polyphenols in Houttuynia cordata on advanced glycation end-products (AGEs) by trapping methylglyoxal. LWT-Food Sci. Technol. 2015, 61, 158–163. [Google Scholar] [CrossRef]
- Przygodzka, M.; Zieliński, H. Evaluation of in vitro inhibitory activity of rye-buckwheat ginger cakes with rutin on the formation of advanced glycation end-products (AGEs). Pol. J. Food Nutr. Sci. 2015, 65, 191–198. [Google Scholar] [CrossRef]
- Harris, C.S.; Cuerrier, A.; Lamont, E.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.L.; Johns, T. Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: Chemical correlates of in vitro antiglycation activity. Plant Foods Hum. Nutr. 2014, 69, 71–77. [Google Scholar] [CrossRef]
- Deetae, P.; Parichanon, P.; Trakunleewatthana, P.; Chanseetis, C.; Lertsiri, S. Antioxidant and anti-glycation properties of Thai herbal teas in comparison with conventional teas. Food Chem. 2012, 133, 953–959. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Wang, M. Antioxidant and antiglycation activity of selected dietary polyphenols in a cookie model. J. Agric. Food Chem. 2014, 62, 1643–1648. [Google Scholar] [CrossRef]
- Mohamed, S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovascular disease. Trends Food Sci. Technol. 2014, 35, 114–128. [Google Scholar] [CrossRef]
- Mesías, M.; Navarro, M.; Gökmen, V.; Morales, F.J. Antiglycative effect of fruit and vegetable seed extracts: Inhibition of AGE formation and carbonyl-trapping abilities. J. Sci. Food Agric. 2013, 93, 2037–2044. [Google Scholar] [CrossRef]
- Del Castillo, M.D.; Fernandez-Gomez, B.; Martinez-Saez, N.; Iriondo-DeHond, A.; Martirosyan, M.D.; Mesa, M.D. Coffee silverskin extract for aging and chronic diseases. In Functional Foods for Chronic Diseases; Food Science Publisher: Dallas, TX, USA, 2016; pp. 386–409. [Google Scholar]
- Sri Harsha, P.S.; Lavelli, V.; Scarafoni, A. Protective ability of phenolics from white grape vinification by-products against structural damage of bovine serum albumin induced by glycation. Food Chem. 2014, 156, 220–226. [Google Scholar] [CrossRef] [PubMed]


| No. | Spices | Botanical Name | Total Phenolics (mg of GAE g−1 DM) | Antioxidant Capacity | ||
|---|---|---|---|---|---|---|
| ABTS (μmol TE g−1 DM) | DPPH (% of Inhibition) | PCL ACL (μmol TE g−1 DM) | ||||
| 1 | Anise | Pimpinella anisum L. | 8.2 ± 0.4 e | 61.6 ± 0.2 i | 65.9 ± 5.9 c | 21.8 ± 0.2 j |
| 2 | Cumin | Cuminum cyminum L. | 28.1 ± 2.3 d | 39.4 ± 2.2 k | 93.4 ± 3.2 a | 46.0 ± 2.6 f |
| 3 | Parsley | Petroselinum crispum Mill. | 13.5 ± 0.5 d | 40.3 ± 1.8 k | 48.3 ± 0.7 d | 18.3 ± 0.8 k |
| 4 | Cardamom | Elettaria cardamomum L. | 3.3 ± 0.1 f | 46.1 ± 2.1 j | 31.7 ± 0.1 e | 13.7 ± 0.4 l |
| 5 | Ginger | Zingiber officinale Rosc. | 11.3 ± 0.1 d | 39.4 ± 0.8 k | 60.7 ± 2.8 c | 92.5 ± 7.6 e |
| 6 | Allspice | Pimenta dioica L. | 183.9 ± 1.3 a | 718.8 ± 10.8 e | 6.6 ± 1.6 g | 143.7 ± 8.8 c |
| 7 | Cloves | Syzygium aromaticum L. | 156.7 ± 3.5 b | 2071.1 ± 75.5 a | 88.3 ± 2.4 b | 896.5 ± 4.3 a |
| 8 | Black pepper | Piper nigrum L. | 43.1 ± 0.1 c | 43.1 ± 0.1 k | 43.0 ± 2.3 d | 33.5 ± 0.4 g |
| 9 | White pepper | Piper nigrum L. | 2.5 ± 0.1 g | 83.0 ± 2.3 h | 27.2 ± 3.3 f | 23.6 ± 0.1 i |
| 10 | Oregano | Origanum vulgare L. | 26.6 ± 6.4 d | 106.8 ± 0.9 f | 93.2 ± 0.9 a | 116.0 ± 5.5 d |
| 11 | Thyme | Thymus vulgaris L. | 24.8 ± 2.7 d | 94.1 ± 5.1 g | 88.1 ± 2.2 b | 94.3 ± 13.0 e |
| 12 | Star anise | Illicum verum L. | 190.7 ± 17.5 a | 500.4 ± 14.7 d | 43.2 ± 3.7 d | 24.8 ± 0.8 h |
| 13 | Nutmeg | Myristica fragrans H. | 11.8 ± 0.6 e | 289.8 ± 14.1 e | 27.1 ± 4.0 f | 45.1 ± 6.1 f |
| 14 | Cinnamon | Cinnamomum verum J. | 180.6 ± 14.2 a | 1119.9 ± 199.2 b | 90.7 ± 0.1 b | 512.0 ± 19.3 b |
| BSA-MGO | BSA-glucose | TPC | ABTS | DPPH | PCL ACL | |
|---|---|---|---|---|---|---|
| BSA-MGO | 1.000 | |||||
| BSA-glucose | 0.924 | 1.000 | ||||
| TPC | 0.861 | 0.714 | 1.000 | |||
| ABTS | 0.621 | 0.571 | 0.752 | 1.000 | ||
| DPPH | 0.526 | 0.498 | 0.314 | 0.417 | 1.000 | |
| PCL ACL | 0.716 | 0.746 | 0.762 | 0.642 | 0.471 | 1.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starowicz, M.; Zieliński, H. Inhibition of Advanced Glycation End-Product Formation by High Antioxidant-Leveled Spices Commonly Used in European Cuisine. Antioxidants 2019, 8, 100. https://doi.org/10.3390/antiox8040100
Starowicz M, Zieliński H. Inhibition of Advanced Glycation End-Product Formation by High Antioxidant-Leveled Spices Commonly Used in European Cuisine. Antioxidants. 2019; 8(4):100. https://doi.org/10.3390/antiox8040100
Chicago/Turabian StyleStarowicz, Małgorzata, and Henryk Zieliński. 2019. "Inhibition of Advanced Glycation End-Product Formation by High Antioxidant-Leveled Spices Commonly Used in European Cuisine" Antioxidants 8, no. 4: 100. https://doi.org/10.3390/antiox8040100
APA StyleStarowicz, M., & Zieliński, H. (2019). Inhibition of Advanced Glycation End-Product Formation by High Antioxidant-Leveled Spices Commonly Used in European Cuisine. Antioxidants, 8(4), 100. https://doi.org/10.3390/antiox8040100