Protein Redox State Monitoring Studies of Thiol Reactivity
Abstract
1. Introduction
2. Methods
2.1. Protein Expression and Purification
2.2. Cell Culture
2.3. Protein Redox State Monitoring
2.4. Statistical Analysis
3. Results
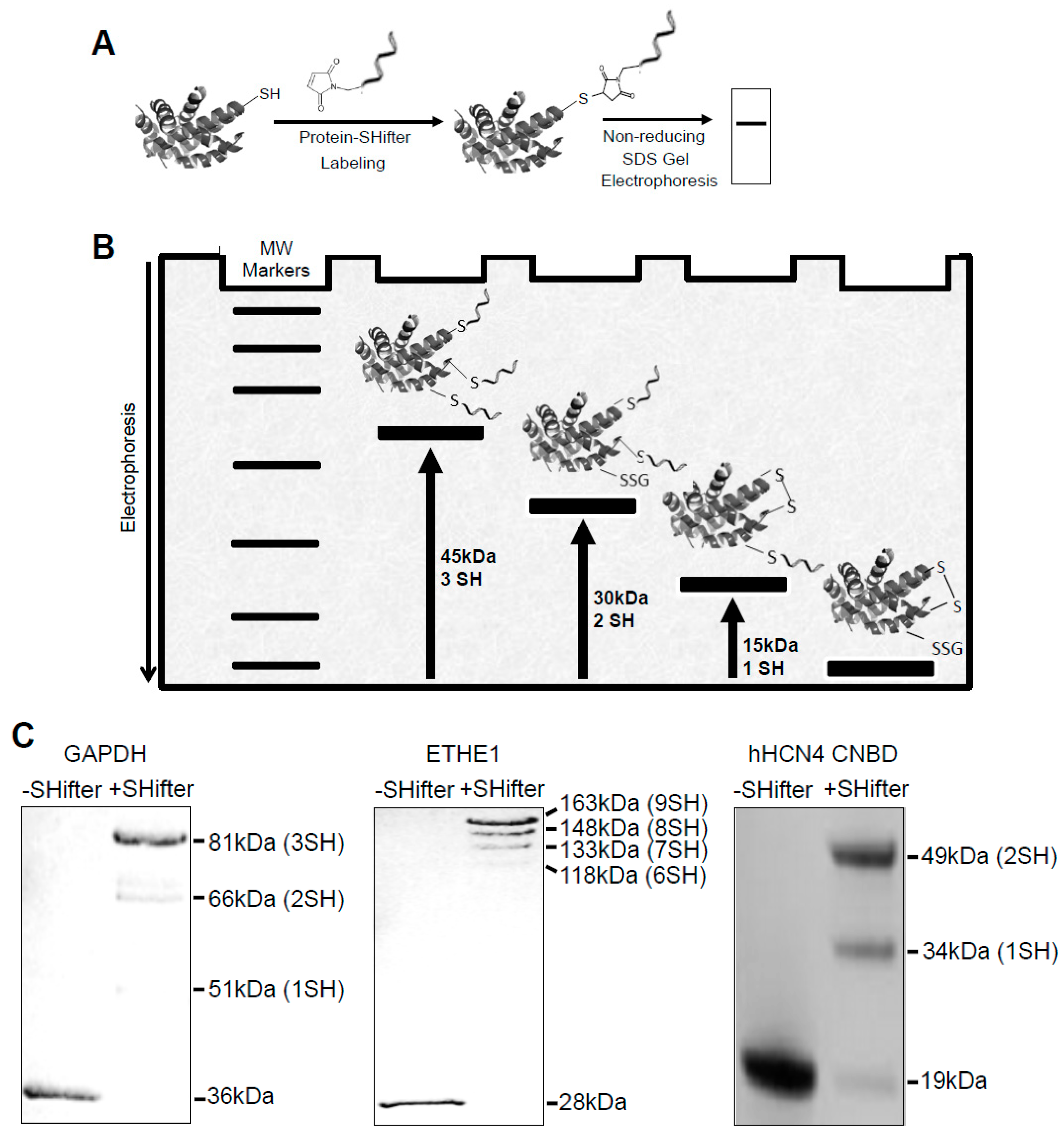
3.1. -SulfoBiotics- Protein Redox State Monitoring Kit to Assess Thiol States of Purified Proteins
3.2. Redox Studies of Prx6 in Cultured Cells Using -Sulfobiotics- Protein Redox State Monitoring Kit Plus
3.3. H2O2 Can Decrease Disulfide Bonds in the Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boronat, S.; Domènech, A.; Hidalgo, E. Proteomic characterization of reversible thiol oxidations in proteomes and proteins. Antioxid. Redox Signal. 2017, 26, 329–344. [Google Scholar] [CrossRef]
- Kasamatsu, S.; Nishimura, A.; Morita, M.; Matsunaga, T.; Abdul Hamid, H.; Akaike, T. Redox signaling regulated by cysteine persulfide and protein polysulfidation. Molecules 2016, 21, 1721. [Google Scholar] [CrossRef]
- Chung, H.S.; Wang, S.B.; Venkatraman, V.; Murray, C.I.; Van Eyk, J.E. Cysteine oxidative posttranslational modifications: Emerging regulation in the cardiovascular system. Circ. Res. 2013, 112, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Mieyal, J.J.; Chock, P.B. Posttranslational modification of cysteine in redox signaling and oxidative stress: Focus on s-glutathionylation. Antioxid. Redox Signal. 2012, 16, 471–475. [Google Scholar] [CrossRef]
- Brandes, N.; Schmitt, S.; Jakob, U. Thiol-based redox switches in eukaryotic proteins. Antioxid. Redox Signal. 2009, 11, 997–1014. [Google Scholar] [CrossRef]
- Sen, C.K. Cellular thiols and redox-regulated signal transduction. Curr. Top. Cell. Regul. 2000, 36, 1–30. [Google Scholar] [PubMed]
- Suzuki, Y.J.; Forman, H.J.; Sevanian, A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1997, 22, 269–285. [Google Scholar] [CrossRef]
- Rudyk, O.; Eaton, P. Biochemical methods for monitoring protein thiol redox states in biological systems. Redox Biol. 2014, 2, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Kil, I.S. Multiple Functions and Regulation of Mammalian Peroxiredoxins. Annu. Rev. Biochem. 2017, 86, 749–775. [Google Scholar] [CrossRef]
- Rhee, S.G. Overview on Peroxiredoxin. Mol. Cells 2016, 39, 1–5. [Google Scholar] [CrossRef]
- Kim, K.; Kim, I.H.; Lee, K.Y.; Rhee, S.G.; Stadtman, E.R. The isolation and purification of a specific ‘protector’ protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J. Biol. Chem. 1988, 263, 4704–4711. [Google Scholar]
- Seo, M.S.; Kang, S.W.; Kim, K.; Baines, I.C.; Lee, T.H.; Rhee, S.G. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J. Biol. Chem. 2000, 275, 20346–20354. [Google Scholar] [CrossRef]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef]
- Fisher, A.B. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox Signal. 2011, 15, 831–844. [Google Scholar] [CrossRef]
- Wahl-Schott, C.; Biel, M. HCN channels: Structure, cellular regulation and physiological function. Cell. Mol. Life Sci. 2009, 66, 470–494. [Google Scholar] [CrossRef] [PubMed]
- James, Z.M.; Zagotta, W.N. Structural insights into the mechanisms of CNBD channel function. J. Gen. Physiol. 2018, 150, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Hayoz, S.; Tiwari, P.B.; Piszczek, G.; Uren, A.; Brelidze, T.I. Investigating cyclic nucleotide and cyclic dinucleotide binding to HCN channels by surface plasmon resonance. PLoS ONE 2017, 12, e0185359. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Nojima, T.; Seio, K.; Yoshida, M.; Hisabori, T. DNA-maleimide: An improved maleimide compound for electrophoresis-based titration of reactive thiols in a specific protein. Biochim. Biophys. Acta 2013, 1830, 3077–3081. [Google Scholar] [CrossRef]
- Hara, S.; Tatenaka, Y.; Ohuchi, Y.; Hisabori, T. Direct determination of the redox status of cysteine residues in proteins in vivo. Biochem. Biophys. Res. Commun. 2015, 456, 339–343. [Google Scholar] [CrossRef]
- Sundaresan, M.; Yu, Z.X.; Ferrans, V.J.; Irani, K.; Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 1995, 270, 296–299. [Google Scholar] [CrossRef]
- Thamsen, M.; Jakob, U. The redoxome: Proteomic analysis of cellular redox networks. Curr. Opin. Chem. Biol. 2011, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Carroll, K.S.; Liebler, D.C. The Expanding Landscape of the Thiol Redox Proteome. Mol. Cell. Proteom. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Couvertier, S.M.; Zhou, Y.; Weerapana, E. Chemical-proteomic strategies to investigate cysteine posttranslational modifications. Biochim. Biophys. Acta 2014, 1844, 2315–2330. [Google Scholar] [CrossRef]
- Leichert, L.I.; Gehrke, F.; Gudiseva, H.V.; Blackwell, T.; Ilbert, M.; Walker, A.K.; Strahler, J.R.; Andrews, P.C.; Jakob, U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 8197–8202. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Pescitelli, R.; Camici, G.; Manao, G.; Ramponi, G. Hydrogen peroxide triggers the formation of a disulfide dimer of muscle acylphosphatase and modifies some functional properties of the enzyme. J. Biol. Chem. 2001, 276, 41862–41869. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wijk, T.; Overvoorde, J.; den Hertog, J. H2O2-induced intermolecular disulfide bond formation between receptor protein-tyrosine phosphatases. J. Biol. Chem. 2004, 279, 44355–44361. [Google Scholar] [CrossRef] [PubMed]
- Bindoli, A.; Fukuto, J.M.; Forman, H.J. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid. Redox Signal. 2008, 10, 1549–1564. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, Y.J.; Marcocci, L.; Shimomura, T.; Tatenaka, Y.; Ohuchi, Y.; Brelidze, T.I. Protein Redox State Monitoring Studies of Thiol Reactivity. Antioxidants 2019, 8, 143. https://doi.org/10.3390/antiox8050143
Suzuki YJ, Marcocci L, Shimomura T, Tatenaka Y, Ohuchi Y, Brelidze TI. Protein Redox State Monitoring Studies of Thiol Reactivity. Antioxidants. 2019; 8(5):143. https://doi.org/10.3390/antiox8050143
Chicago/Turabian StyleSuzuki, Yuichiro J., Lucia Marcocci, Takashi Shimomura, Yuki Tatenaka, Yuya Ohuchi, and Tinatin I. Brelidze. 2019. "Protein Redox State Monitoring Studies of Thiol Reactivity" Antioxidants 8, no. 5: 143. https://doi.org/10.3390/antiox8050143
APA StyleSuzuki, Y. J., Marcocci, L., Shimomura, T., Tatenaka, Y., Ohuchi, Y., & Brelidze, T. I. (2019). Protein Redox State Monitoring Studies of Thiol Reactivity. Antioxidants, 8(5), 143. https://doi.org/10.3390/antiox8050143





