The Methanol Extract of Allium cepa L. Protects Inflammatory Markers in LPS-Induced BV-2 Microglial Cells and Upregulates the Antiapoptotic Gene and Antioxidant Enzymes in N27-A Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BV-2 and N27-A Cell Cultures
2.3. Cell Viability and Nitrite Assay
2.4. Isolation of Total RNA and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
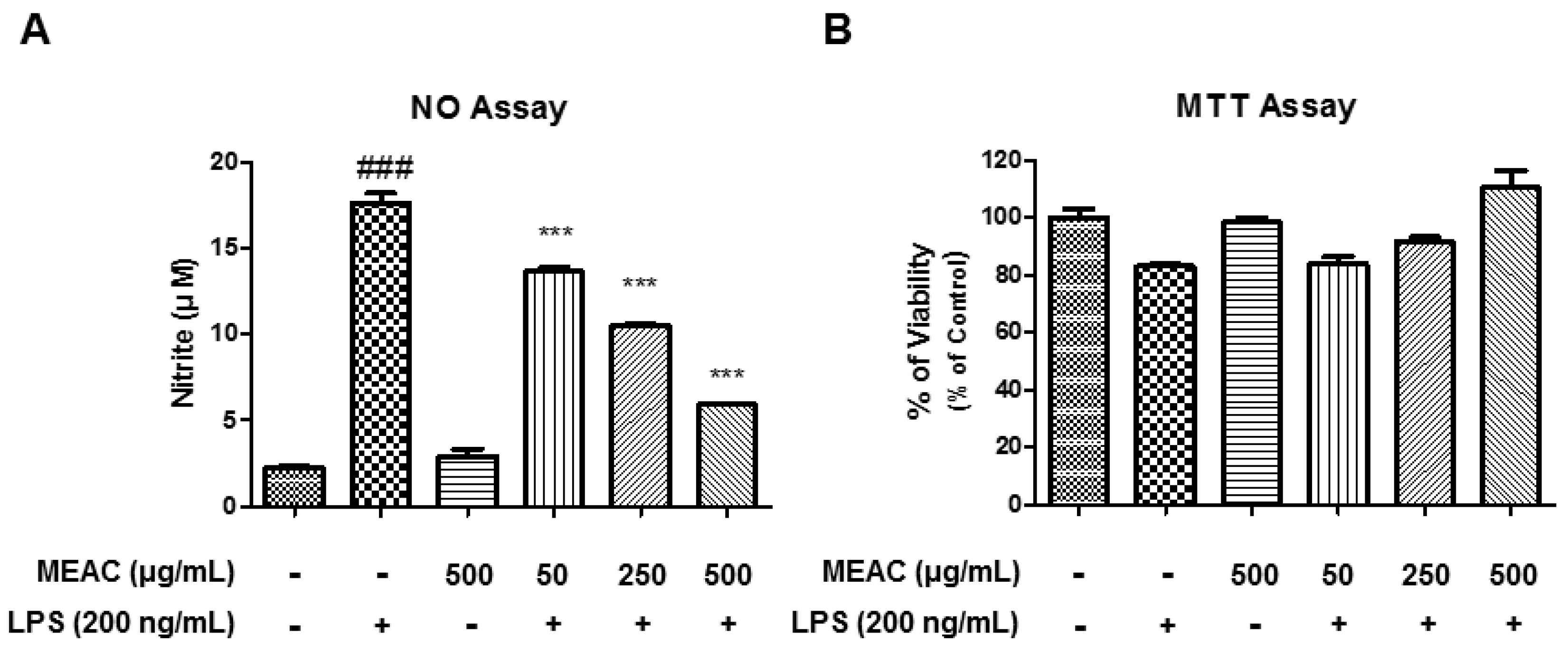
3.1. Action of MEAC Treatment on LPS-Induced Cell Viability and NO Production
3.2. Action of MEAC on LPS-Induced iNOS Expressions in BV-2 Microglial Cells at mRNA and Protein Levels
3.3. Action of MEAC on LPS-Induced COX-2 Expressions in BV-2 Microglial Cells at mRNA and Protein Levels
3.4. Action of MEAC on LPS-Induced Expressions of Inflammatory Cytokines in BV-2 Microglial Cells at mRNA Level
3.5. Protective Activity of MEAC Against MPP+-Induced N27-A Cells
3.6. Regulatory Effect of MEAC on Bcl-2, HO-1, NQO1, and Catalase Expression in N27-A Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Allium cepa |
| Bcl-2 | B-cell lymphoma 2 |
| COX-2 | Cyclooxygenase-2 |
| HO-1 | Hemeoxygenase-1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| iNOS | Nitric oxide synthase |
| LPS | Lipopolysaccharide |
| MEAC | Methanol extract of A. cepa |
| MPP+ | 1-methyl-4-phenylpyridinium |
| NDDs | Neurodegenerative diseases |
| NO | Nitric oxide |
| NQO1 | NAD(P)H Quinone Dehydrogenase 1 |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-α |
References
- Jakaria, M.; Kim, J.; Karthivashan, G.; Park, S.Y.; Ganesan, P.; Choi, D.K. Emerging signals modulating potential of ginseng and its active compounds focusing on neurodegenerative diseases. J. Ginseng Res. 2019, 43, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.Y.; Ko, H.; Kim, J.; Kim, B.W.; Yun, Y.S.; Park, J.I.; Ganesan, P.; Lee, J.T.; Choi, D.K. Scoparone inhibits LPS-simulated inflammatory response by suppressing IRF3 and ERK in BV-2 microglial cells. Molecules 2016, 21, 1718. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Nam, J.H.; Yoon, G.; Lee, J.Y.; Nam, Y.; Kang, H.J.; Cho, H.J.; Kim, J.; Hoe, H.S. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J. Neuroinflamm. 2018, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.E.; Kim, I.S.; Jakaria, M.; Akther, M.; Choi, D.K. Importance of GPCR-Mediated Microglial Activation in Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 258. [Google Scholar] [CrossRef]
- Kirkley, K.S.; Popichak, K.A.; Afzali, M.F.; Legare, M.E.; Tjalkens, R.B. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J. Neuroinflamm. 2017, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Jakaria, M.; Kim, I.S.; Kim, J.; Haque, M.E.; Choi, D.K. Regulation of Toll-Like Receptor (TLR) Signaling Pathway by Polyphenols in the Treatment of Age-Linked Neurodegenerative Diseases: Focus on TLR4 Signaling. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [CrossRef] [Green Version]
- Dansokho, C.; Heneka, M.T. Neuroinflammatory responses in Alzheimer’s disease. J. Neural Transm. 2018, 125, 771–779. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+ T cells in neurodegenerative diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef]
- Bhanot, A.; Shri, R. A comparative profile of methanol extracts of Allium cepa and Allium sativum in diabetic neuropathy in mice. Pharmacogn. Res. 2010, 2, 374–384. [Google Scholar] [Green Version]
- Fredotović, Ž.; Šprung, M.; Soldo, B.; Ljubenkov, I.; Budić-Leto, I.; Bilušić, T.; Čikeš-Čulić, V.; Puizina, J. Chemical Composition and Biological Activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) Methanolic Extracts. Molecules 2017, 22, 448. [Google Scholar] [CrossRef] [PubMed]
- Shri, R.; Bora, K.S. Neuroprotective effect of methanolic extracts of Allium cepa on ischemia and reperfusion-induced cerebral injury. Fitoterapia 2008, 79, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Goel, R.K. Neuroprotective effect of Allium cepa L. in aluminium chloride induced neurotoxicity. Neurotoxicology 2015, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Jung, Y.S. Allium cepa extract and quercetin protect neuronal cells from oxidative stress via PKC-ε inactivation/ERK1/2 activation. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; More, S.V.; Yun, Y.S.; Ko, H.M.; Kwak, J.H.; Lee, H.; Suk, K.; Kim, I.S.; Choi, D.K. A novel synthetic compound MCAP suppresses LPS-induced murine microglial activation in vitro via inhibiting NF-kB and p38 MAPK pathways. Acta Pharmacol. Sin. 2016, 37, 334–343. [Google Scholar] [CrossRef]
- Ko, H.M.; Koppula, S.; Kim, B.W.; Kim, I.S.; Hwang, B.Y.; Suk, K.; Park, E.J.; Choi, D.K. Inflexin attenuates proinflammatory responses and nuclear factor-kappaB activation in LPS-treated microglia. Eur. J. Pharmacol. 2010, 633, 98–106. [Google Scholar] [CrossRef]
- Park, S.Y.; Karthivashan, G.; Ko, H.M.; Cho, D.Y.; Kim, J.; Cho, D.J.; Ganesan, P.; Su-Kim, I.; Choi, D.K. Aqueous Extract of Dendropanaxmorbiferus Leaves Effectively Alleviated Neuroinflammation and Behavioral Impediments in MPTP-Induced Parkinson’s Mouse Model. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Jakaria, M.; Park, S.Y.; Haque, M.E.; Karthivashan, G.; Kim, I.S.; Ganesan, P.; Choi, D.K. Neurotoxic Agent-Induced Injury in Neurodegenerative Disease Model: Focus on Involvement of Glutamate Receptors. Front. Mol. Neurosci. 2018, 11, 307. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.H.; Uddin, M.S.; Kim, I.S.; Choi, D.K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Cho, D.Y.; EzazulHaque, M.; Karthivashan, G.; Kim, I.S.; Ganesan, P.; Choi, D.K. Neuropharmacological Potential and Delivery Prospects of Thymoquinone for Neurological Disorders. Oxid. Med. cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Jakaria, M.; Mathew, B.; Barreto, G.E.; Ashraf, G.M. Nootropic and Anti-Alzheimer’s Actions of Medicinal Plants: Molecular Insight into Therapeutic Potential to Alleviate Alzheimer’s Neuropathology. Mol. Neurobiol. 2019, 56, 4925–4944. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Haque, M.E.; Kim, J.; Cho, D.Y.; Kim, I.S.; Choi, D.K. Active ginseng components in cognitive impairment: Therapeutic potential and prospects for delivery and clinical study. Oncotarget 2018, 9, 33601–33620. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Haque, M.E.; Cho, D.Y.; Azam, S.; Kim, I.S.; Choi, D.K. Molecular Insights into NR4A2(Nurr1): An Emerging Target for Neuroprotective Therapy Against Neuroinflammation and Neuronal Cell Death. Mol. Neurobiol. 2019, 56, 5799–5814. [Google Scholar] [CrossRef]
- Hyun, S.W.; Jang, M.; Park, S.W.; Kim, E.J.; Jung, Y.S. Onion (Allium cepa) extract attenuates brain edema. Nutrition 2013, 29, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.M.; Kim, S.Y.; Joo, S.H.; Cheong, J.H.; Yang, S.I.; Shin, C.Y.; Koo, B.N. Synergistic activation of lipopolysaccharide-stimulated glial cells by propofol. Biochem. Biophys. Res. Commun. 2013, 438, 420–426. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42, S125–S152. [Google Scholar] [CrossRef]
- Gorman, A.M.; McGowan, A.; O’Neill, C.; Cotter, T. Oxidative stress and apoptosis in neurodegeneration. J. Neurol. Sci. 1996, 139, 45–52. [Google Scholar] [CrossRef]
- Anilkumar, U.; Prehn, J.H.M. Anti-apoptotic BCL-2 family proteins in acute neural injury. Front. Cell. Neurosci. 2014, 8, 281. [Google Scholar] [CrossRef]
- Phatak, N.R.; Stankowska, D.L.; Krishnamoorthy, R.R. Bcl-2, Bcl-xL, and p-AKT are involved in neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma. Mol. Vis. 2016, 22, 1048–1061. [Google Scholar] [PubMed]
- Franke, C.; Nöldner, M.; Abdel-Kader, R.; Johnson-Anuna, L.N.; Gibson Wood, W.; Müller, W.E.; Eckert, G.P. Bcl-2 upregulation and neuroprotection in guinea pig brain following chronic simvastatin treatment. Neurobiol. Dis. 2007, 25, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Huang, B.; Zhang, X.; Zhu, Y.; Chen, X. Astaxanthin protects against MPP(+)-induced oxidative stress in PC12 cells via the HO-1/NOX2 axis. BMC Neurosci. 2012, 13, 156. [Google Scholar] [CrossRef] [PubMed]





| Gene Target. | Primer Sequence | Size (bp) |
|---|---|---|
| iNOS | F 5′-GAG GTA CTC AGC GTC CTC CA -3′ R 5′-AGG GAG GAA AGG GAG AGA GG-3′ | 444 |
| COX-2 | F 5′-TGA GTG GTA GCC AGC AAA GC-3′ R 5′-CTG CAG TCC AGG TTC AAT GG -3′ | 319 |
| TNF-α | F 5′-TTC GAG TGA CAA GCC TGT AGC-3′ R 5′-AGA TTG ACC TCA GCG CTG AGT-3′ | 390 |
| IL-1β | F 5′-CAA GGA GAA CCA AGC AAC GA-3′ R 5′-TTG GCC GAG GAC TAA GGA GT-3′ | 428 |
| IL-6 | F 5′-GGA GGC TTA ATT ACA CAT GTT-3′ R 5′-TGA TTT CAA GAT GAA TTG GAT-3′ | 435 |
| Bcl-2 | F-5′-CCA GGC CTT CAA CCA TTA TC-3′ R-5′-CTC ATT GAA CTC GTC TCC GA-3′ | 127 |
| HO-1 | F-5′ TGT CAC CCT GTG CTT GAC CT-3′ R-5′-ATA CCC GCT ACC TGG GTG AC-3′ | 200 |
| NQO1 | F-5′-AGA GCC CTG ATT GTA TTG GC-3′ R-AGG TCA GAT TCG ACC ACC TC-3’ | 113 |
| Catalase | F-5′-CCT GAC ATG GTC TGG GAC TT-3′ R-5′-CAA GTT TTT GAT GCC GTG GT-3′ | 201 |
| GAPDH | F 5′- ACC ACA GTC CAT GCC ATC AC -3′ R 5′- CCA CCA CCC TGT TGC TGT AG-3′ | 472 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakaria, M.; Azam, S.; Cho, D.-Y.; Haque, M.E.; Kim, I.-S.; Choi, D.-K. The Methanol Extract of Allium cepa L. Protects Inflammatory Markers in LPS-Induced BV-2 Microglial Cells and Upregulates the Antiapoptotic Gene and Antioxidant Enzymes in N27-A Cells. Antioxidants 2019, 8, 348. https://doi.org/10.3390/antiox8090348
Jakaria M, Azam S, Cho D-Y, Haque ME, Kim I-S, Choi D-K. The Methanol Extract of Allium cepa L. Protects Inflammatory Markers in LPS-Induced BV-2 Microglial Cells and Upregulates the Antiapoptotic Gene and Antioxidant Enzymes in N27-A Cells. Antioxidants. 2019; 8(9):348. https://doi.org/10.3390/antiox8090348
Chicago/Turabian StyleJakaria, Md., Shofiul Azam, Duk-Yeon Cho, Md. Ezazul Haque, In-Su Kim, and Dong-Kug Choi. 2019. "The Methanol Extract of Allium cepa L. Protects Inflammatory Markers in LPS-Induced BV-2 Microglial Cells and Upregulates the Antiapoptotic Gene and Antioxidant Enzymes in N27-A Cells" Antioxidants 8, no. 9: 348. https://doi.org/10.3390/antiox8090348
APA StyleJakaria, M., Azam, S., Cho, D. -Y., Haque, M. E., Kim, I. -S., & Choi, D. -K. (2019). The Methanol Extract of Allium cepa L. Protects Inflammatory Markers in LPS-Induced BV-2 Microglial Cells and Upregulates the Antiapoptotic Gene and Antioxidant Enzymes in N27-A Cells. Antioxidants, 8(9), 348. https://doi.org/10.3390/antiox8090348







