Chrysophanol, Physcion, Hesperidin and Curcumin Modulate the Gene Expression of Pro-Inflammatory Mediators Induced by LPS in HepG2: In Silico and Molecular Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Plant Material
2.3. Extraction and Isolation
2.3.1. Isolation of Curcumin from Turmeric
2.3.2. Isolation of Anthraquinones from Rhubarb
2.3.3. Isolation of Hesperidin from Citrus Fruits
2.4. Cell Culture and Cytotoxicity Study
2.5. Induction the Production of Inflammatory Markers in HepG2
2.6. Quantitative Real Time PCR Analysis
2.7. In Silico Study for the Interaction of the Identified Compounds with MAPKs.
2.8. Preparation of Active Site for Molecular Docking
Molecular Docking of the Isolated Compounds in MAPKs Active Site
2.9. Statistical Analysis
3. Results and Discussion
3.1. Identifictation of the Isolated Compounds (1–4)
3.2. Cytotoxic Activity by MTT Method
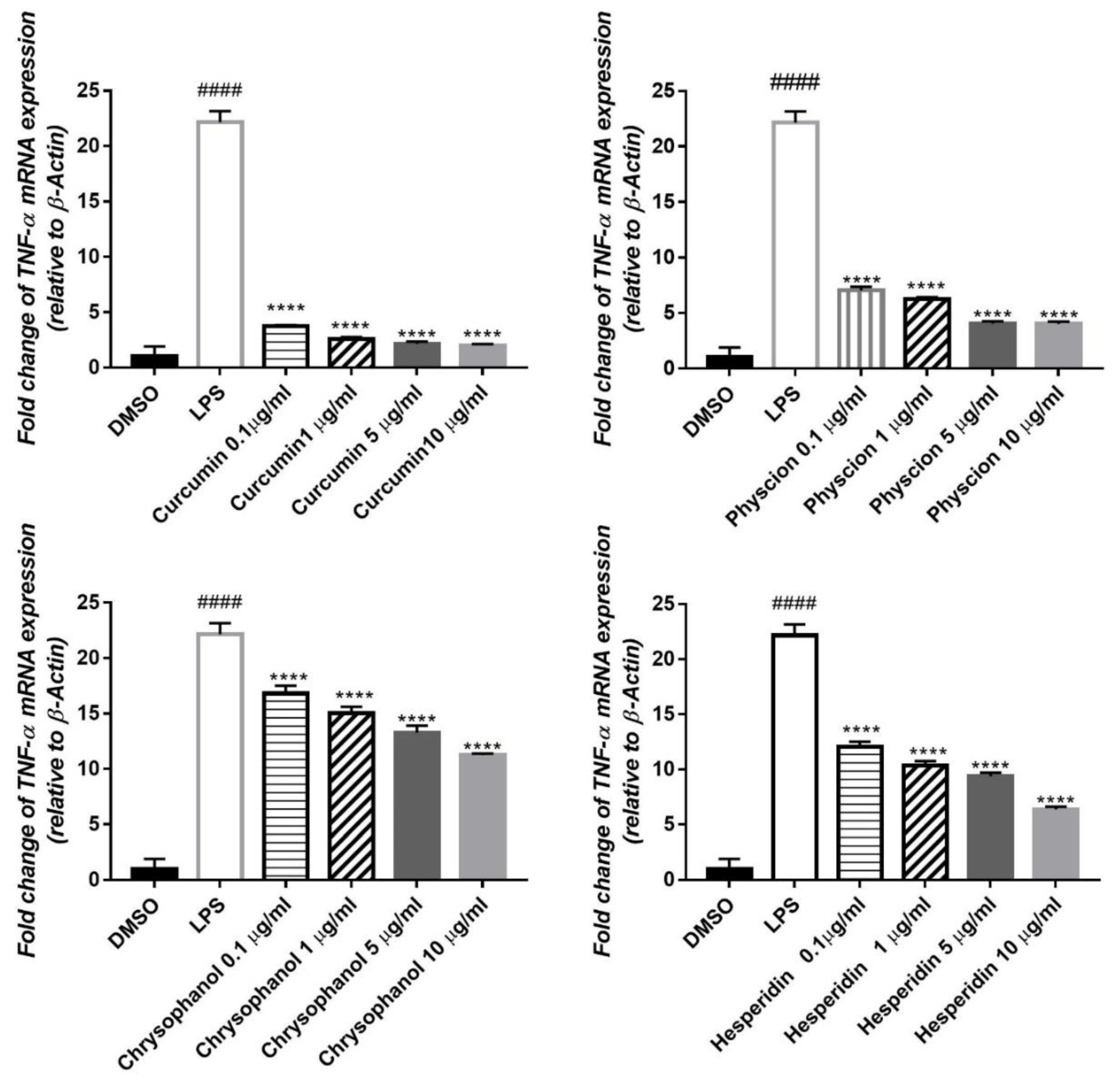
3.3. Effect of the Tested Compounds (1–4) on LPS-Induced Gene Expression of Different Inflammatory Mediators
3.4. In Silico Assessment of the Inhibitory Effect of Isolated Compounds (1–4) on MAPKs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blach, S.; Zeuzem, S.; Manns, M.; Altraif, I.; Duberg, A.-S.; Muljono, D.H.; Waked, I.; Alavian, S.M.; Lee, M.-H.; Negro, F.; et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- Zhang, L.; Schuppan, D. Traditional Chinese Medicine (TCM) for fibrotic liver disease: Hope and hype. J. Hepatol. 2014, 61, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; He, B.; Hui, L. Mitogen-activated protein kinases in hepatocellular carcinoma development. Semin. Cancer Biol. 2011, 21, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Klintman, D.; Li, X.; Santen, S.; Schramm, R.; Jeppsson, B.; Thorlacius, H. P38 mitogen-activated protein kinase-dependent chemokine production, leukocyte recruitment, and hepatocellular apoptosis in endotoxemic liver injury. Ann. Surg. 2005, 242, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, X.; Wang, J.; He, X.; Hu, Y.; Zhang, P.; Wang, R.; Li, R.; Gong, M.; Luo, S. Curcumin protects against CCl4-induced liver fibrosis in rats by inhibiting HIF-1α through an ERK-dependent pathway. Molecules 2014, 19, 18767–18780. [Google Scholar] [CrossRef] [PubMed]
- Scartezzini, P.; Speroni, E. Review on some plants of Indian traditional medicine with antioxidant activity. J. Ethnopharmacol. 2000, 71, 23–43. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Al-Elaiwi, A.M.; Athar, M.T.; Tariq, M.; Al Eid, A.; Al-Asmary, S.M. A review of hepatoprotective plants used in Saudi traditional medicine. Evid-Based Complement. Altern. Med. 2014, 2014, 890842. [Google Scholar] [CrossRef]
- Jie, L.; Yaping, L.; Klaassen, C.D. The effect of Chinese hepatoprotective medicines on experimental liver injury in mice. J. Ethnopharmacol. 1994, 42, 183–191. [Google Scholar] [CrossRef]
- Li, J.W.-H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Elgazar, A.A.; Selim, N.M.; Abdel-Hamid, N.M.; El-Magd, M.A.; El Hefnawy, H.M. Isolates from Alpinia officinarum Hance attenuate LPS-induced inflammation in HepG2: Evidence from in silico and in vitro studies. Phytother. Res. PTR 2018, 32, 1273–1288. [Google Scholar] [CrossRef]
- Arumanayagam, S.; Arunmani, M. Hepatoprotective and antibacterial activity of Lippia nodiflora Linn. against lipopolysaccharides on HepG2 cells. Pharmacogn. Mag. 2015, 11, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, S.H.; Shalaby, S.M.; Abd-Elbary, E.; Saleh, A.A.; El-Magd, M.A. Human peripheral blood CD34+ cells attenuate oleic acid–induced acute lung injury in rats. Cytotherapy 2015, 17, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- El-Magd, M.A.; Abdo, W.S.; El-Maddaway, M.; Nasr, N.M.; Gaber, R.A.; El-Shetry, E.S.; Saleh, A.A.; Alzahrani, F.A.A.; Abdelhady, D.H. High doses of S-methylcysteine cause hypoxia-induced cardiomyocyte apoptosis accompanied by engulfment of mitochondaria by nucleus. Biomed. Pharmacother. 2017, 94, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.; Rarey, M.; Lengauer, T. Evaluation of the FLEXX incremental construction algorithm for protein–ligand docking. Proteins Struct. Funct. Bioinform. 1999, 37, 228–241. [Google Scholar]
- Li, W.; Wang, S.; Feng, J.; Xiao, Y.; Xue, X.; Zhang, H.; Wang, Y.; Liang, X. Structure elucidation and NMR assignments for curcuminoids from the rhizomes of Curcuma longa. Magn. Reson. Chem. MRC 2009, 47, 902–908. [Google Scholar] [CrossRef]
- Ngoc, T.M.; Minh, P.T.H.; Hung, T.M.; Thuong, P.T.; Lee, I.; Min, B.-S.; Bae, K. Lipoxygenase inhibitory constituents from rhubarb. Arch. Pharm. Res. 2008, 31, 598–605. [Google Scholar]
- Cornejo, A.; Salgado, F.; Caballero, J.; Vargas, R.; Simirgiotis, M.; Areche, C. Secondary Metabolites in Ramalina terebrata Detected by UHPLC/ESI/MS/MS and Identification of Parietin as Tau Protein Inhibitor. Int. J. Mol. Sci. 2016, 17, 1303. [Google Scholar] [CrossRef]
- Dewick, P.M. The Acetate Pathway: Fatty Acids and Polyketides. In Medicinal Natural Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001; pp. 35–120. [Google Scholar]
- Choi, S.Z.; Lee, S.O.; Jang, K.U.; Chung, S.H.; Park, S.H.; Kang, H.C.; Yang, E.Y.; Cho, H.J.; Lee, K.R. Antidiabetic stilbene and anthraquinone derivatives fromRheum undulatum. Arch. Pharm. Res. 2005, 28, 1027–1030. [Google Scholar] [CrossRef]
- Nieto, J.; Gutierrez, A. 1H NMR spectra at 360 MHz of diosmin and hesperidin in DMSO solution. Spectrosc. Lett. 1986, 19, 427–434. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. Reagents and procedures for the ultraviolet spectral analysis of flavonoids. In The Systematic Identification of Flavonoids; Springer: Berlin, Germany, 1970; pp. 35–40. [Google Scholar]
- Sobeh, M.; Mahmoud, M.F.; Hasan, R.A.; Abdelfattah, M.A.; Sabry, O.M.; Ghareeb, M.A.; El-Shazly, A.M.; Wink, M. Tannin-rich extracts from Lannea stuhlmannii and Lannea humilis (Anacardiaceae) exhibit hepatoprotective activities in vivo via enhancement of the anti-apoptotic protein Bcl-2. Sci. Rep. 2018, 8, 9343. [Google Scholar]
- Elgazar, A.A.; Knany, H.R.; Ali, M.S.; Medicine, C. Insights on the molecular mechanism of anti-inflammatory effect of formula from Islamic traditional medicine: An in silico study. J. Tradit. Complement. Med. 2018, 9, 353–363. [Google Scholar] [CrossRef]
- Fang, J.; Liu, C.; Wang, Q.; Lin, P.; Cheng, F. In silico polypharmacology of natural products. Brief. Bioinform. 2017, 19, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Stepanić, V.; Matijašić, M.; Horvat, T.; Verbanac, D.; Kučerová-Chlupáčová, M.; Saso, L.; Žarković, N.J.A. Antioxidant Activities of Alkyl Substituted Pyrazine Derivatives of Chalcones—In Vitro and In Silico Study. Antioxidants 2019, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Castillo, J.; Perugorria, M.; Latasa, M.; Prieto, J.; Avila, M.A. Inflammation and liver cancer: New molecular links. Ann. N. Y. Acad. Sci. 2009, 1155, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A. The inflammation and liver cancer. In Inflammation and Cancer; Springer: Berlin, Germany, 2014; pp. 401–435. [Google Scholar]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.-J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 1997, 185, 1661–1670. [Google Scholar] [CrossRef]
- Lee, J.; Mira-Arbibe, L.; Ulevitch, R.J. TAK1 regulates multiple protein kinase cascades activated by bacterial lipopolysaccharide. J. Leukoc. Biol. 2000, 68, 909–915. [Google Scholar]
- Granado, A.B.; Martín, M.Á.; Bravo, L.; Goya, L.; Ramos, S. Quercetin attenuates TNF-induced inflammation in hepatic cells by inhibiting the NF-κB pathway. Nutr. Cancer 2012, 64, 588–598. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Tsai, P.-M.; Chou, Y.-H.; Lee, Y.-C.; Lin, H.-H.; Chen, J.-H. In Vitro and In Vivo Protective Effects of Flavonoid-Enriched Lotus Seedpod Extract on Lipopolysaccharide-Induced Hepatic Inflammation. Am. J. Chin. Med 2019, 47, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qian, Y.; Chen, F.; Chen, X.; Chen, Z.; Zheng, M. EGCG attenuates pro-inflammatory cytokines and chemokines production in LPS-stimulated L02 hepatocyte. Acta Biochim. Biophys. Sin. 2014, 46, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pinsetta, F.R.; Taft, C.A.; de Paula da Silva, C.H.T. Structure-and ligand-based drug design of novel p38-alpha MAPK inhibitors in the fight against the Alzheimer’s disease. J. Biomol. Struct. Dyn. 2014, 32, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Gum, R.J.; McLaughlin, M.M.; Kumar, S.; Wang, Z.; Bower, M.J.; Lee, J.C.; Adams, J.L.; Livi, G.P.; Goldsmith, E.J.; Young, P.R. Acquisition of sensitivity of stress-activated protein kinases to the p38 inhibitor, SB 203580, by alteration of one or more amino acids within the ATP binding pocket. J. Biol. Chem. 1998, 273, 15605–15610. [Google Scholar] [PubMed]
- Heo, Y.S.; Kim, S.K.; Seo, C.I.; Kim, Y.K.; Sung, B.J.; Lee, H.S.; Lee, J.I.; Park, S.Y.; Kim, J.H.; Hwang, K.Y.; et al. Structural basis for the selective inhibition of JNK1 by the scaffolding protein JIP1 and SP600125. EMBO J. 2004, 23, 2185–2195. [Google Scholar] [PubMed]
- Ward, R.A.; Bethel, P.; Cook, C.; Davies, E.; Debreczeni, J.E.; Fairley, G.; Feron, L.; Flemington, V.; Graham, M.A.; Greenwood, R. Structure-Guided Discovery of Potent and Selective Inhibitors of ERK1/2 from a Modestly Active and Promiscuous Chemical Start Point. J. Med. Chem. 2017, 60, 3438–3450. [Google Scholar] [CrossRef]
- Araújo, P.M.; da Silva, L.P.; Esteves da Silva, J.C. Theoretical Analysis of the Binding of Potential Inhibitors to Protein Kinases MK2 and MK3. Med. Chem. 2015, 11, 573–579. [Google Scholar] [CrossRef]
- Cheng, R.; Felicetti, B.; Palan, S.; Toogood-Johnson, I.; Scheich, C.; Barker, J.; Whittaker, M.; Hesterkamp, T. High-resolution crystal structure of human Mapkap kinase 3 in complex with a high affinity ligand. Protein. Sci. 2010, 19, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 2001, 56, 683–687. [Google Scholar]
- Chainani-Wu, N.; Medicine, C. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, M.-C.; Lee, B.-J.; Park, D.-H.; Hong, S.-H.; Um, J.-Y. Anti-Inflammatory activity of chrysophanol through the suppression of NF-kB/caspase-1 activation in vitro and in vivo. Molecules 2010, 15, 6436–6451. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das Sarma, M.; Patra, A.; Hazra, B. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn. Pharmacology 2010, 62, 1158–1166. [Google Scholar]
- Shehzad, A.; Qureshi, M.; Anwar, M.N.; Lee, Y.S. Multifunctional curcumin mediate multitherapeutic effects. J. Food Sci. 2017, 82, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wu, R.; Xie, W.; Xie, C.; Wu, J.; Geng, S.; Li, X.; Zhu, M.; Zhu, W.; Zhu, J.; et al. Effects of curcumin on tobacco smoke-induced hepatic MAPK pathway activation and epithelial–mesenchymal transition in vivo. Phytother. Res. 2017, 31, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Gangwal, R.P.; Bhadauriya, A.; Damre, M.V.; Dhoke, G.V.; Sangamwar, A.T. p38 Mitogen-activated protein kinase inhibitors: A review on pharmacophore mapping and QSAR studies. Curr. Top. Med. Chem. 2013, 13, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Wei, Q.; Wang, S.; Qi, H.; Zhu, Q.; Liu, X.; Shi, X.; Wen, S. Physcion 8-O-β-glucopyranoside extracted from Polygonum cuspidatum exhibits anti-proliferative and anti-inflammatory effects on MH7A rheumatoid arthritis-derived fibroblast-like synoviocytes through the TGF-β/MAPK pathway. Int. J. Mol. Med. 2018, 42, 745–754. [Google Scholar]
- Moon, P.-D.; Han, N.-R.; Lee, J.S.; Hong, S.; Yoo, M.-S.; Kim, H.-J.; Kim, J.-H.; Kang, S.; Jee, H.-W.; Kim, H.-M.; et al. Use of Physcion to Improve Atopic Dermatitis-Like Skin Lesions through Blocking of Thymic Stromal Lymphopoietin. Molecules 2019, 24, 1484. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Kim, H.-Y.; Kim, H.-M. Molecular mechanisms of anti-inflammatory effect of chrysophanol, an active component of AST2017-01 on atopic dermatitis in vitro models. Int. Immunopharmacol. 2018, 54, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 0411110005. [Google Scholar] [CrossRef]












| Gene | Forward Primer (‘5 ------ ‘3) | Reverse Primer (‘5 ------ ‘3) |
|---|---|---|
| TNF-α | CCCAGGGACCTCTCTCTAATC | ATGGGCTACAGGCTTGTCACT |
| IL-1β | ACAGATGAAGTGCTCCTTCCA | GTCGGAGATTCGTAGCTGGAT |
| IL-6 | GGTACATCCTCGACGGCATCT | GTGCCTCTTTGCTGCTTTCAC |
| β-actin | CGACATCAGGAAGGACCTGTATGCC | GAAGATTCGTCGTGAAAGTCG |
| Compound | HepG2 IC50 [µg/mL] |
|---|---|
| Chrysophanol | 34.22 ± 0.10 |
| Curcumin | 80.73 ± 0.05 |
| Hesperidin | 95.66 ± 0.85 |
| Physcion | 172.6 ± 0.59 |
| Compound | P38 α MAPK | ERK1/2 | JNK1 | MK3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FlexX Score | Amino Acids Interactions | Hyde Assessment/Predicted Ki | FlexX Score | Amino Acids Interactions | Hyde Assessment/Predicted Ki | FlexX Score | Amino Acids Interactions | Hyde Assessment/Predicted Ki | FlexX Score | Amino Acids Interactions | Hyde Assessment/Predicted Ki | |
| Curcumin | −24.639 | Gly110, Gly033 Met109, Thr106, Asp 168, Asn155, His107 | −34/µM | −21.8 | MET 108, Gln105, Glu33, Thr 110, Lys114 | −28 /µM | −17.72 | Asn 114, Met111, Lys 55, Ile32 | −23 /µM | −24.63 | Lys73, Met121, Glu119, Glu125 | −36/ nM |
| Physcion | −27.99 | Gly110, Met109, Thr106, Ala 111, His107 | −33/µM | −22.6 | Met 108, Gln 105, Lys 54 | −23/ µM | −24.07 | Glu 109, Met111, Ile32 | −32 /µM | −27.99 | Met121, Glu119, Thr186 | −24/ µM |
| Chrysophanol | −27.65 | Gly110, Met109, Thr106, Ala 111, His107 | −32/µM | −22.5 | Gln 105, Asp 106, Met 108, Lys54, Asp167, Tyr36, Gly34, Ile56 | −17/ mM | −24.44 | Glu 109, Met111, Ile32, Ala53 | −30 /µM | −27.65 | Met121, Glu119, leu50, leu173 | −35 /µM |
| Hesperidin | −20.03 | Gly110, Met109, Thr106, Asp 168, Ser 154 | −38/nM | −21.9 | Asp 106, Met108, Lys114, Asp111, Tyr36, Glu33, Asn15, Gln105, Ile31 | −14/ mM | −13.12 | Glu 109, Met111, Ile32, Ser34, Lys55 | −24/ µM | −20.03 | Glu125, Cys120, Met121, Asp187, Glu 170, Lys 73 | −22/ µM |
| (Co-crystallized ligand) | −30.7 | Gly110, Met109 Thr106, Ala 157 | −41/nM | −39 | Lys 54, Asp 167, Met 108, Thr 110, Tyr-36, Gly34, Ile56 | −45/ nM | −29 | Ile 32, Met111, Glu 109, Val158, Leu168, Ala53, Val40 | −39/ nM | −43.17 | Met121, Cys120, Glu 170, Glu 125, leu52 | −18 /mM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, N.M.; Elgazar, A.A.; Abdel-Hamid, N.M.; Abu El-Magd, M.R.; Yasri, A.; El Hefnawy, H.M.; Sobeh, M. Chrysophanol, Physcion, Hesperidin and Curcumin Modulate the Gene Expression of Pro-Inflammatory Mediators Induced by LPS in HepG2: In Silico and Molecular Studies. Antioxidants 2019, 8, 371. https://doi.org/10.3390/antiox8090371
Selim NM, Elgazar AA, Abdel-Hamid NM, Abu El-Magd MR, Yasri A, El Hefnawy HM, Sobeh M. Chrysophanol, Physcion, Hesperidin and Curcumin Modulate the Gene Expression of Pro-Inflammatory Mediators Induced by LPS in HepG2: In Silico and Molecular Studies. Antioxidants. 2019; 8(9):371. https://doi.org/10.3390/antiox8090371
Chicago/Turabian StyleSelim, Nabil Mohamed, Abdullah Abdurrahman Elgazar, Nabil Mohie Abdel-Hamid, Mohammed Rizk Abu El-Magd, Aziz Yasri, Hala Mohamed El Hefnawy, and Mansour Sobeh. 2019. "Chrysophanol, Physcion, Hesperidin and Curcumin Modulate the Gene Expression of Pro-Inflammatory Mediators Induced by LPS in HepG2: In Silico and Molecular Studies" Antioxidants 8, no. 9: 371. https://doi.org/10.3390/antiox8090371
APA StyleSelim, N. M., Elgazar, A. A., Abdel-Hamid, N. M., Abu El-Magd, M. R., Yasri, A., El Hefnawy, H. M., & Sobeh, M. (2019). Chrysophanol, Physcion, Hesperidin and Curcumin Modulate the Gene Expression of Pro-Inflammatory Mediators Induced by LPS in HepG2: In Silico and Molecular Studies. Antioxidants, 8(9), 371. https://doi.org/10.3390/antiox8090371






