Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress
Abstract
:1. Introduction
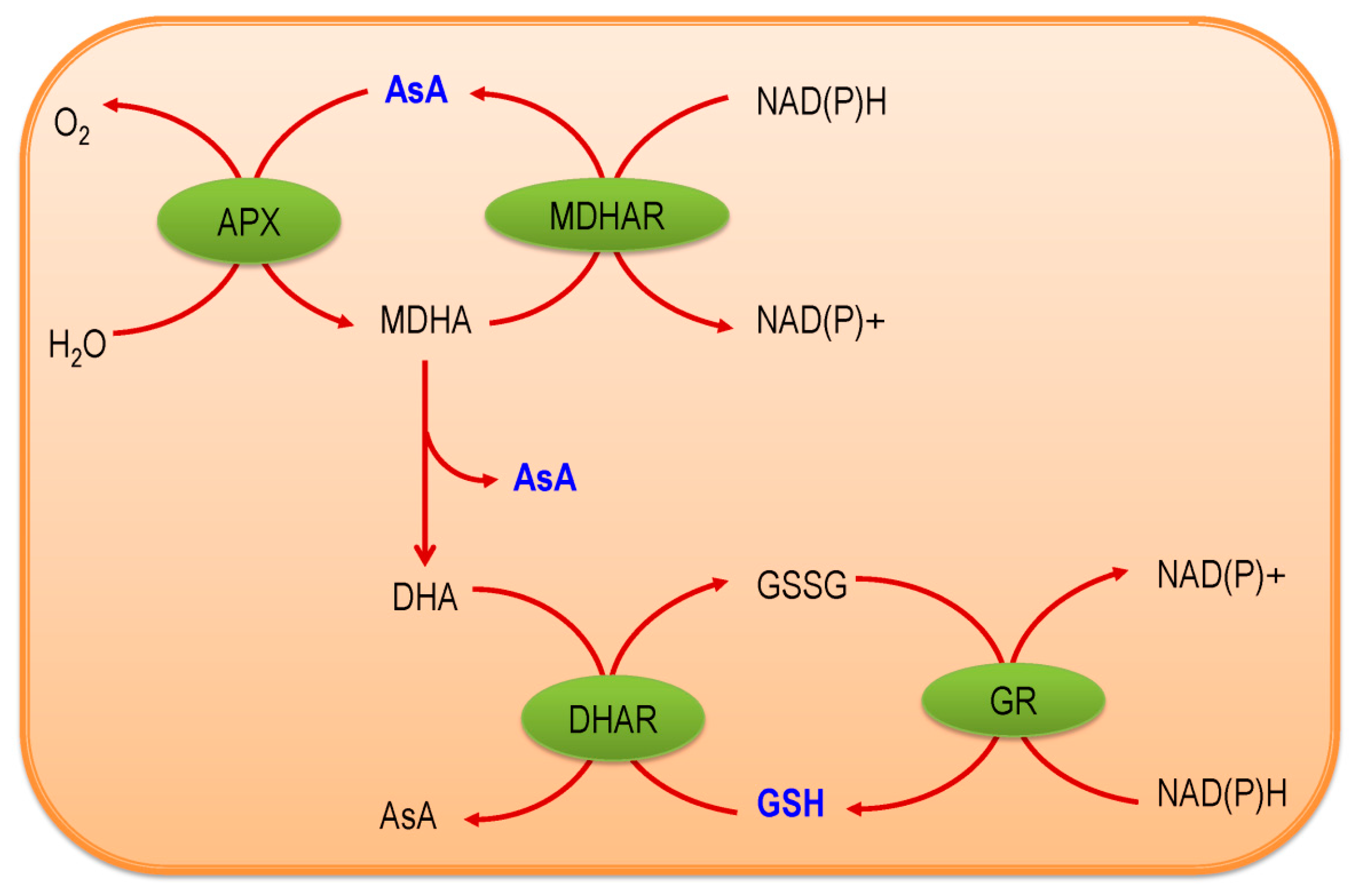
2. Ascorbate-Glutathione Pathway—An Overview
3. Components of AsA-GSH Pathway
3.1. Ascorbate
3.2. Glutathione
3.3. Ascorbate Peroxidase
3.4. Monodehydroascorbate Reductase
3.5. Dehydroascorbate Reductase
3.6. Glutathione Reductase
4. Ascorbate and glutathione Redox and its Role in Plant Metabolism
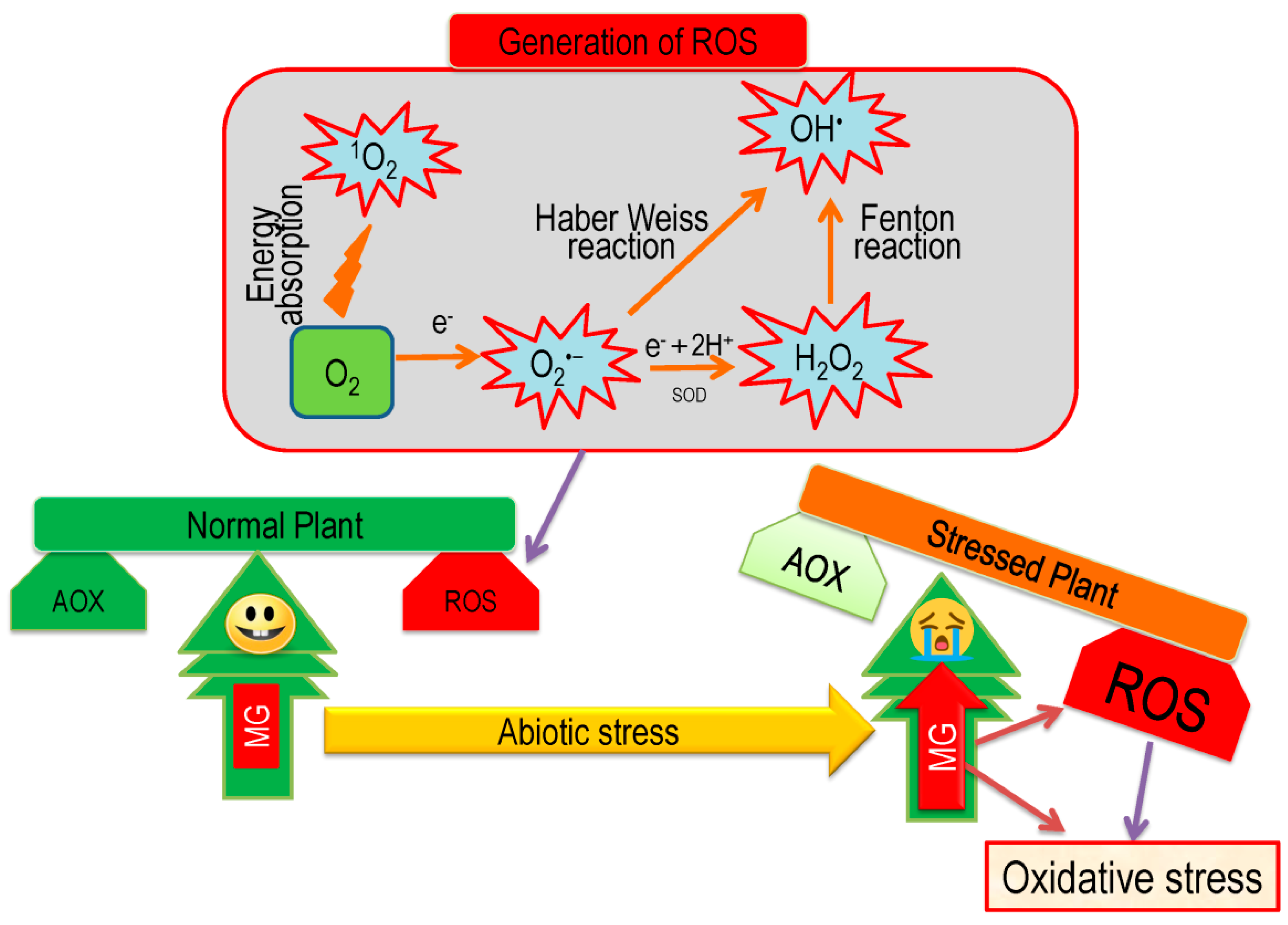
5. Overview of Oxidative Stress and Antioxidant Defense in Plants
6. Role of AsA-GSH in Regulating Oxidative Stress under Abiotic Stresses
6.1. Salinity
6.2. Drought
6.3. Toxic Metals/Metalloids
6.4. Extreme Temperature
6.5. Flooding
6.6. Atmospheric Pollutants
6.7. Other Stress
7. Exogenous Use of AsA and GSH in Conferring Abiotic Stress Tolerance
7.1. Exogenous AsA
7.2. Exogenous GSH
8. Interaction of Other Pathways with AsA-GSH Pathways in Regulating ROS Metabolism
8.1. Interaction of AsA-GSH Cycle with NO Metabolic Pathway
8.2. Signaling Role of AsA-GSH Cycle Components and Interaction with Other Pathways
8.3. AsA-GSH Cycle Interaction with Phytohormone Biosynthesis Pathways
8.4. Interaction of AsA-GSH Pathway with Glyoxalase Pathway
8.5. Interaction AsA-GSH Pathway with Xenobiotics Detoxification Pathways
8.6. AsA-GSH Cycle Interaction with Metal Chelation Process
9. Genetic Manipulation of AsA-GSH Pathway and Its Role in Abiotic Stress Tolerance
10. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. PMBP 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Mahmud, J.A.; Nahar, K.; Anee, T.I.; Inafuku, M.; Oku, H.; Fujita, M. Responses, adaptation, and ROS metabolism in plants exposed to waterlogging stress. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Antioxidant defences: Endogenous and diet derived. Free Radic. Biol. Med. 2007, 4, 79–186. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Teixeira da Silva, J.A.; Fujita, M. Plant responses and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and its Management: Perspectives and Strategies; Bandi, V., Shanker, A.K., Shanker, C., Mandapaka, M., Eds.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Luis, A. Role of peroxisomes as a source of reactive oxygen species (ROS) signaling molecules. In Peroxisomes and Their Key Role in Cellular Signaling and Metabolism; del Río, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 231–255. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front. Plant Sci. 2017, 8, 1061. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int. J. Mol. Sci. 2017, 18, 200. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Anee, T.I.; Parvin, K.; Fujita, M. Nitric oxide pretreatment enhances antioxidant defense and glyoxalase systems to confer PEG-induced oxidative stress in rapeseed. J. Plant Interact. 2017, 12, 323–331. [Google Scholar] [CrossRef]
- Zechmann, B. Compartment-specific importance of glutathione during abiotic and biotic stress. Front. Plant Sci. 2014, 5, 566. [Google Scholar] [CrossRef]
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int. J. Mol. Sci. 2012, 13, 4458–4483. [Google Scholar] [CrossRef]
- Tripathi, R.P.; Singh, B.; Bisht, S.S.; Pandey, J. L-Ascorbic acid in organic synthesis: An overview. Curr. Org. Chem. 2009, 13, 99–122. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Mellidou, I.; Keulemans, J.; Kanellis, A.K.; Davey, M.W. Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol. 2012, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Cronje, C.; George, G.M.; Fernie, A.R.; Bekker, J.; Kossmann, J.; Bauer, R. Manipulation of L-ascorbic acid biosynthesis pathways in Solanum lycopersicum: Elevated GDP-mannose pyrophosphorylase activity enhances L-ascorbate levels in red fruit. Planta 2012, 23, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Maruta, T.; Yoshimura, K.; Smirnoff, N. Biosynthesis and regulation of ascorbic acid in plants. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 163–179. [Google Scholar]
- Wheeler, G.; Ishikawa, T.; Pornsaksit, V.; Smirnoff, N. Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. Elife 2015, 4, e06369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallanca, J.E.; Smirnoff, N. The control of ascorbic acid synthesis and turnover in pea seedlings. J. Exp. Bot. 2000, 345, 669–674. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Miyake, C. Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: Molecular mechanisms and physiological functions. Plant Cell Physiol. 2010, 51, 1951–1963. [Google Scholar] [CrossRef]
- Loewus, F.A. Biosynthesis and metabolism of ascorbic acid and of analogs of ascorbic acid in fungi. Phytochemistry 1999, 52, 193–210. [Google Scholar] [CrossRef]
- Melino, V.J.; Soole, K.L.; Ford, C.M. Ascorbate metabolism and the developmental demand for tartaric and oxalic acids in ripening grape berries. BMC Plant Biol. 2009, 9, 145. [Google Scholar] [CrossRef]
- Truffault, V.; Fry, S.C.; Stevens, R.G.; Gautier, H. Ascorbate degradation in tomato leads to accumulation of oxalate, threonate and oxalyl threonate. Plant J. 2017, 89, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, R.A.; Clarkson, G.J.J.; Rothwell, S.D.; Fry, S.C. Novel insights into ascorbate retention and degradation during the washing and post-harvest storage of spinach and other salad leaves. Food Chem. 2017, 233, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Kärkönen, A.; Dewhirst, R.A.; Mackay, C.L.; Fry, S.C. Metabolites of 2,3-diketogulonate delay peroxidase action and induce non-enzymic H2O2 generation: Potential roles in the plant cell wall. Arch. Biochem. Biophys. 2017, 620, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trived, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione reductase and glutathione: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Queval, G.; Mhamdi, A.; Chaouch, S.; Foyer, C.H. Glutathione. The Arabidopsis Book. Am. Soc. Plant Biol. 2011, 9, e0142. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.I.; Neukermans, J.; Marquez-Garcia, B.E.L.E.N.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Fujita, M. Physiological roles of glutathione in conferring abiotic stress tolerance to plants. In Abiotic Stress Response in Plants; Gill, S.S., Tuteja, N., Eds.; Wiley: Weinheim, Germany, 2016; pp. 151–179. [Google Scholar]
- Zagorchev, L.; Seal, C.; Kranner, I.; Odjakova, M. A central role for thiols in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef]
- Mahmood, Q.; Ahmad, R.; Kwak, S.S.; Rashid, A.; Anjum, N.A. Ascorbate and glutathione: Protectors of plants in oxidative stress. In Ascorbate–Glutathione Pathway and Stress Tolerance in Plants; Mahmood, Q., Ahmad, R., Kwak, S.S., Rashid, A., Anjum, N.A., Eds.; Springer: Berlin, Germany, 2010; pp. 209–229. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Alharby, H.F.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Ferretti, M.; Destro, T.; Tosatto, S.C.E.; La Rocca, N.; Rascio, N.; Masi, A. Gamma-glutamyl transferase in the cell wall participates in extracellular glutathione salvage from the root apoplast. New Phytol. 2009, 181, 115–126. [Google Scholar] [CrossRef]
- Ohkama-Ohtsu, N.; Sasaki-Sekimoto, Y.; Oikawam, A.; Jikumaru, Y.; Shinoda, S.; Inoue, E.; Kamide, Y.; Yokoyama, T.; Hirai, M.Y.; Shirasu, K.; et al. 12-oxo-phytodienoic acid-glutathione conjugate is transported into the vacuole in Arabidopsis. Plant Cell Physiol. 2011, 52, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Xu, J.; Li, Y.; Lei, L.; Zhao, L.; Yang, H.; Feng, J.; Liu, G.; Ren, D. Glutathione-indole-3-acetonitrile is required for camalexin biosynthesis in Arabidopsis thaliana. Plant Cell 2011, 23, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, T.; Kumar, S.; Rani, B.; Jain, V.; Malhotra, S.P. Partial purification and characterization of ascorbate peroxidase from ripening ber (Ziziphus mauritiana L) fruits. Afr. J. Biotechnol. 2014, 13, 3323–3331. [Google Scholar]
- del Río, L.A.; López-Huertas, E. ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and ascorbate peroxidase—representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Guo, K.; Tu, L.; Wang, P.; Du, X.; Ye, S.; Luo, M.; Zhang, X. Ascorbate alleviates Fe deficiency-induced stress in cotton (Gossypium hirsutum) by modulating ABA levels. Front. Plant Sci. 2017, 7, 1997. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Samhan-Arias, A.K.; Gutierrez-Merino, C. Correlation between the potency of flavonoids for cytochrome c reduction and inhibition of cardiolipin-induced peroxidase activity. Biofactors 2017, 43, 451–468. [Google Scholar] [CrossRef]
- Ullah, S.; Kolo, Z.; Egbichi, I.; Keyster, M.; Ludidi, N. Nitric oxide influences glycine betaine content and ascorbate peroxidase activity in maize. S. Afr. J. Bot. 2016, 105, 218–225. [Google Scholar] [CrossRef]
- Leterrier, M.; Cagnac, O. Function of the various MDAR isoforms in higher plants. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 83–94. [Google Scholar]
- Begara-Morales, J.C.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.; Barroso, J.B. Transcriptional regulation of gene expression related to hydrogen peroxide (H2O2) and nitric oxide (NO). In Nitric Oxide and Hydrogen Peroxide Signaling in Higher Plants; Gupta, D., Palma, J., Corpas, F.J., Eds.; Springer: Cham, Switzerland, 2019; pp. 69–90. [Google Scholar]
- Park, A.K.; Kim, I.S.; Do, H.; Jeon, B.W.; Lee, C.W.; Roh, S.J.; Shin, S.C.; Park, H.; Kim, Y.S.; Kim, Y.H.; et al. Structure and catalytic mechanism of monodehydroascorbate reductase, MDHAR, from Oryza sativa L. japonica. Sci. Rep. 2016, 6, 33903. [Google Scholar] [CrossRef]
- Sano, S. Molecular and functional characterization of monodehydro-ascorbate and dehydroascorbate reductases. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 129–156. [Google Scholar]
- Johnston, E.J.; Rylott, E.L.; Beynon, E.; Lorenz, A.; Chechik, V.; Bruce, N.C. Monodehydroascorbate reductase mediates TNT toxicity in plants. Science 2015, 349, 1072–1075. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.; Rahman, A.; Mahmud, J.-A.; Suzuki, T.; Fujita, M. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: Osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma 2017, 254, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.H.M.B.; Hasanuzzaman, M.; Mahmud, J.A.; Hossain, M.S.; Bhuiyan, T.F.; Fijuta, F. Unraveling morphophysiological and biochemical responses of Triticum aestivum L. to extreme pH: Coordinated actions of antioxidant defense and glyoxalase systems. Plants 2019, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Khan, M.I.R.; Fujita, M. Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. S. Afr. J. Bot. 2018, 115, 50–57. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.; Carvajal, F.; Jamilena, M.; Garrido, D. Putrescine treatment increases the antioxidant response and carbohydrate content in zucchini fruit stored at low temperature. Postharvest Biol. Technol. 2016, 118, 68–70. [Google Scholar] [CrossRef]
- Krishna Das, B.; Kumar, A.; Maindola, P.; Mahanty, S.; Jain, S.K.; Reddy, M.K.; Arockiasamy, A. Non-native ligands define the active site of Pennisetum glaucum (L.) R. Br dehydroascorbate reductase. Biochem. Biophys. Res. Commun. 2016, 473, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Noshi, M.; Yamada, H.; Hatanaka, R.; Tanabe, N.; Tamoi, M.; Shigeoka, S. Arabidopsis dehydroascorbate reductase 1 and 2 modulate redox states of ascorbate-glutathione cycle in the cytosol in response to photooxidative stress. Biosci. Biotechnol. Biochem. 2017, 81, 523–533. [Google Scholar] [CrossRef]
- Do, H.; Kim, I.S.; Jeon, B.W.; Lee, C.W.; Park, A.K.; Wi, A.R.; Shin, S.C.; Park, H.; Kim, Y.S.; Yoon, H.S.; et al. Structural understanding of the recycling of oxidized ascorbate by dehydroascorbate reductase (OsDHAR) from Oryza sativa L. japonica. Sci. Rep. 2016, 6, 19498. [Google Scholar] [CrossRef]
- Kataya, A.R.; Reumann, S. Arabidopsis glutathione reductase 1 is dually targeted to peroxisomes and the cytosol. Plant Signal. Behav. 2010, 5, 171–175. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Hakeem, K.U.R.; Chandna, R.; Ahmad, P. Role of glutathione reductase in plant abiotic stress. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 149–158. [Google Scholar]
- Hanukoglu, I. Conservation of the enzyme–coenzyme interfaces in FAD and NADP binding adrenodoxin reductase—A ubiquitous enzyme. J. Mol. Evol. 2017, 85, 205–218. [Google Scholar] [CrossRef]
- Tang, X.; Webb, M.A. Soybean root nodule cDNA encoding glutathione reductase. Plant Physiol. 1994, 104, 1081–1082. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.H.; Wang, B.S. Role of ascorbate peroxidase and glutathione reductase in ascorbate–glutathione cycle and stress tolerance in plants. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Anjum, N., Chan, M.T., Umar, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 91–113. [Google Scholar]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Pellny, T.K.; Locato, V.; Hull, J.; De Gara, L. Analysis of redox relationships in the plant cell cycle: Determination of ascorbate, glutathione, and poly (ADPribose) polymerase (PARP) in plant cell cultures. In Redox-Mediated Signal Transduction, Methods in Molecular Biology; Hancock, J., Conway, M., Eds.; Springer: New York, NY, USA, 2019; pp. 165–181. [Google Scholar]
- Zhu, S.; He, L.; Zhang, F.; Li, M.; Jiao, S.; Li, Y.; Chen, M.; Zhao, X.E.; Wang, H. Fluorimetric evaluation of glutathione reductase activity and its inhibitors using carbon quantum dots. Talanta 2016, 161, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.B.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Suzuki, T.; Fujita, M. Polyamines-induced aluminum tolerance in mung bean: A study on antioxidant defense and methylglyoxal detoxification systems. Ecotoxicology 2017, 26, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Alam, M.M.; Nahar, K.; Mohsin, S.M.; Bhuyan, M.H.M.B.; Parvin, K.; Fujita, M. Silicon-induced antioxidant defense and methylglyoxal detoxification works coordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology 2019, 28, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Khan, N.A.; Sofo, A.; Baier, M.; Kizek, R. Redox homeostasis managers in plants under environmental stresses. Front. Environ. Sci. 2016, 4, 35. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Foyer, C.H. Redox homeostasis: Opening up ascorbate transport. Nat. Plants 2015, 1, 114012. [Google Scholar] [CrossRef]
- Debolt, S.; Melino, V.; Ford, C.M. Ascorbate as a biosynthetic precursor in plants. Ann. Bot. 2006, 99, 3–8. [Google Scholar] [CrossRef]
- Zechmann, B. Diurnal changes of subcellular glutathione content in Arabidopsis thaliana. Biol. Plant. 2017, 61, 791–796. [Google Scholar] [CrossRef]
- Hernández, L.E.; Sobrino-Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lallement, P.A.; Roret, T.; Tsan, P.; Gualberto, J.M.; Girardet, J.M.; Didierjean, C.; Rouhier, N.; Hecker, A. Insights into ascorbate regeneration in plants: Investigating the redox and structural properties of dehydroascorbate reductases from Populus trichocarpa. Biochem. J. 2016, 473, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Corpas, F.J.; Barroso, J.B. Protein S-nitrosylation and S-glutathionylation as regulators of redox homeostasis during abiotic stress response. In Redox State as a Central Regulator of Plant-Cell Stress Responses; Gupta, D., Palma, J., Corpas, F.J., Eds.; Springer: Cham, Switzerland, 2016; pp. 365–386. [Google Scholar]
- Miret, J.A.; Müller, M. AsA/DHA redox pair influencing plant growth and stress tolerance. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 297–319. [Google Scholar]
- Munné-Bosch, S.; Queval, G.; Foyer, C.H. The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol. 2013, 161, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Parsons, H.T.; Fry, S.C. Oxidation of dehydroascorbic acid and 2,3-diketogulonate under plant apoplastic conditions. Phytochemistry 2012, 75, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xin, S.; Tao, C.; Jin, X.; Li, H. Cotton ascorbate oxidase promotes cell growth in cultured tobacco bright yellow-2 cells through generation of apoplast oxidation. Int. J. Mol. Sci. 2017, 18, 1346. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chaouch, S.; Mhamdi, A.; Queval, G.; Zechmann, B.; Noctor, G. Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid. Redox Signal. 2013, 18, 2106–2121. [Google Scholar] [CrossRef] [PubMed]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Medic. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Nafees, M.; Fahad, S.; Shah, F.; Sha, A.M.; Bukhari, M.A.; Maryam; Ahmed, I.; Ahmad, S.; Hussain, S. Reactive Oxygen Species Signaling in Plants. In Reactive Oxygen Species Signaling in Plants; Hasanuzzaman, M., Hakeem, K., Nahar, K., Alharby, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 259–272. [Google Scholar]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Sem. Cell Develop. Biol. 2019, 80, 3–12. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012, 149, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Latowski, D.; Surówka, E.; Strzałka, K. Regulatory role of components of ascorbate–glutathione pathway in plant stress tolerance. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Anjum, N., Chan, M.T., Umar, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 1–53. [Google Scholar]
- Hernández, J.A.; Barba-Espín, G.; Diaz-Vivancos, P. Glutathione-mediated biotic stress tolerance in plants. In Glutathione in Plant Growth, Development, and Stress Tolerance; Hossain, M., Mostofa, M., Diaz-Vivancos, P., Burritt, D., Fujita, M., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 309–319. [Google Scholar]
- Rahman, A.K.; Al Mahmud, J.; Hasanuzzaman, M.; Hossain, M.S.; Fujita, M. Salt stress tolerance in rice: Emerging role of exogenous phytoprotectants. In Advances in International Rice Research; Li, Q., Ed.; InTech: Rijeka, Croatia, 2017; pp. 139–174. [Google Scholar]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.; Mohsin, S.M.; Fujita, M. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-induced cadmium stress tolerance in rice seedlings: Coordinated action of antioxidant defense, glyoxalase system and nutrient homeostasis. C. R. Biol. 2016, 339, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant. 2015, 59, 745–756. [Google Scholar] [CrossRef]
- Hossain, M.S.; Alam, M.U.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Mahmud, J.A.; Fujita, M. Use of iso-osmotic solution to understand salt stress responses in lentil (Lens culinaris Medik.). S. Afr. J. Bot. 2017, 113, 346–354. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Prasad, S.M. Nitrogen alleviates salinity toxicity in Solanum lycopersicum seedlings by regulating ROS homeostasis. Plant Physiol. Biochem. 2019, 141, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abd_Allah, E.F.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Siddique, K.H. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci. Rep. 2018, 8, 13515. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef]
- Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019, 161, 277–289. [Google Scholar] [CrossRef]
- Yan, Y.; Pan, C.; Du, Y.; Li, D.; Liu, W. Exogenous salicylic acid regulates reactive oxygen species metabolism and ascorbate–glutathione cycle in Nitraria tangutorum Bobr. under salinity stress. PMBP 2018, 24, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Hasanuzzaman, M.; Sohag, M.M.H.; Bhuyan, M.H.M.B.; Fujita, M. Acetate-induced modulation of ascorbate: Glutathione cycle and restriction of sodium accumulation in shoot confer salt tolerance in Lens culinaris Medik. PMBP Plants 2019, 25, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B. Effective microorganisms enhance the scavenging capacity of the ascorbate-glutathione cycle in common bean (Phaseolus vulgaris L.) plants grown in salty soils. Plant Physiol. Biochem. 2014, 80, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, T.F.; Ahamed, K.U.; Nahar, K.; Al Mahmud, J.; Bhuyan, M.B.; Anee, T.I.; Fujita, M.; Hasanuzzaman, M. Mitigation of PEG-induced drought stress in rapeseed (Brassica rapa L.) by exogenous application of osmolytes. Biocatal. Agric. Biotechnol. 2019, 20, 101197. [Google Scholar] [CrossRef]
- Sun, J.; Gu, J.; Zeng, J.; Han, S.; Song, A.P.; Chen, F.; Fang, W.; Jiang, J.; Chen, S. Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci. Hortic. 2013, 161, 249–258. [Google Scholar] [CrossRef]
- Lou, L.; Li, X.; Chen, J.; Li, Y.; Tang, Y.; Lv, J. Photosynthetic and ascorbate-glutathione metabolism in the flag leaves as compared to spikes under drought stress of winter wheat (Triticum aestivum L.). PLoS ONE 2018, 13, e0194625. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, S.; Ou, X. The roles of H2S and H2O2 in regulating AsA-GSH cycle in the leaves of wheat seedlings under drought stress. Protoplasma 2018, 255, 1257–1262. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Mostofa, M.G.; Watanabe, Y.; Tran, C.D.; Rahman, M.M.; Tran, L.S.P. Overexpression of GmNAC085 enhances drought tolerance in Arabidopsis by regulating glutathione biosynthesis, redox balance and glutathione-dependent detoxification of reactive oxygen species and methylglyoxal. Environ. Exp. Bot. 2019, 161, 242–254. [Google Scholar] [CrossRef]
- Sreeharsha, R.V.; Mudalkar, S.; Sengupta, D.; Unnikrishnan, D.K.; Reddy, A.R. Mitigation of drought-induced oxidative damage by enhanced carbon assimilation and an efficient antioxidative metabolism under high CO2 environment in pigeonpea (Cajanus cajan L.). Photosynth. Res. 2019, 139, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Glutathione-induced drought stress tolerance in mung bean: Coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants 2015, 7, plv069. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.S.; Ferreira-Silva, S.L.; Carvalho, F.E.L.; Neto, M.C.L.; Aragão, R.M.; Silva, E.N.; Sousa, R.M.J.; Silveira, J.A.G. Antioxidant protection and PSII regulation mitigate photo-oxidative stress induced by drought followed by high light in cashew plants. Environ. Exp. Bot. 2018, 149, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Al Mahmud, J.; Biswas, P.K.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Exogenous application of gibberellic acid mitigates drought-induced damage in spring wheat. Acta Agrobot. 2019, 72, 1776. [Google Scholar]
- Hasanuzzaman, M.; Alam, M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed. Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1704–1721. [Google Scholar] [CrossRef]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Alleviation of osmotic stress in Brassica napus, B. campestris, and B. juncea by ascorbic acid application. Biol. Plant. 2014, 58, 697–708. [Google Scholar] [CrossRef]
- Alam, M.M.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2013, 7, 1053–1063. [Google Scholar]
- Jin, X.; Liu, T.; Xu, J.; Gao, Z.; Hu, X. Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol. 2019, 19, 48. [Google Scholar] [CrossRef]
- García-Martí, M.; Piñero, M.C.; García-Sanchez, F.; Mestre, T.C.; López-Delacalle, M.; Martínez, V.; Rivero, R.M. Amelioration of the oxidative stress generated by simple or combined abiotic stress through the K+ and Ca2+ supplementation in tomato plants. Antioxidants 2019, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef] [PubMed]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Hossain, M.S.; Fujita, M. Maleic acid assisted improvement of metal chelation and antioxidant metabolism confers chromium tolerance in Brassica juncea L. Ecotoxicol. Environ. Saf. 2017, 144, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Alharby, H.F.; Razafindrabe, B.H.N.; Fujita, M. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: An intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci. 2017, 8, 115. [Google Scholar] [CrossRef]
- Bharwana, S.A.; Ali, S.; Farooq, M.A.; Iqbal, N.; Abbas, F.; Ahmad, M.S.A. Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J. Bioremed. Biodeg. 2013, 4, 187. [Google Scholar]
- Abd_Allah, E.F.; Hashem, A.; Alam, P.; Ahmad, P. Silicon alleviates nickel-induced oxidative stress by regulating antioxidant defense and glyoxalase systems in mustard plants. J. Plant Growth Regul. 2019, 1–14. [Google Scholar] [CrossRef]
- Rahman, A.; Mostofa, M.G.; Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. BioMed. Res. Int. 2015, 2015, 340812. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef]
- Gill, R.A.; Zang, L.; Ali, B.; Farooq, M.A.; Cui, P.; Yang, S.; Ali, S.; Zhou, W. Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 2015, 120, 154–164. [Google Scholar] [CrossRef]
- Awasthi, J.P.; Saha, B.; Panigrahi, J.; Yanase, E.; Koyama, H.; Panda, S.K. Redox balance, metabolic fingerprint and physiological characterization in contrasting North East Indian rice for aluminum stress tolerance. Sci. Rep. 2019, 9, 8681. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ. Sci. Pollut. Res. Int. 2016, 23, 21206–21218. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mungbean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Mostofa, M.G.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems. Braz. J. Bot. 2016, 39, 393–407. [Google Scholar] [CrossRef]
- Rahman, A.; Hossain, M.S.; Mahmud, J.A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-induced salt stress tolerance in rice seedlings: Regulation of ion homeostasis, antioxidant defense and glyoxalase systems. PMBP 2016, 22, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, K.; Liu, Z.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on the subcellular distribution of cadmium and oxidative stress induced by cadmium in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2019, 26, 16220. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Siddique, A.; Ashraf, M.A.; Rasheed, R.; Ibrahim, M.; Iqbal, M.; Akbar, S.; Imran, M. Does exogenous application of ascorbic acid modulate growth, photosynthetic pigments and oxidative defense in okra (Abelmoschus esculentus (L.) Moench) under lead stress? Acta Physiol. Plant. 2017, 39, 144. [Google Scholar] [CrossRef]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Fujita, M. Relative tolerance of different species of Brassica to cadmium toxicity: Coordinated role of antioxidant defense and glyoxalase systems. Plant Omics J. 2017, 10, 107–117. [Google Scholar] [CrossRef]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Fujita, M. EDTA reduces cadmium toxicity in mustard (Brassica juncea L.) by enhancing metal chelation, antioxidant defense and glyoxalase systems. Acta Agrobot. 2019, 72, 1722. [Google Scholar] [CrossRef]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Bhuyan, M.H.M.B.; Fujita, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018, 147, 990–1001. [Google Scholar] [CrossRef]
- Dias, M.C.; Mariz-Ponte, N.; Santos, C. Lead induces oxidative stress in Pisum sativum plants and changes the levels of phytohormones with antioxidant role. Plant Physiol. Biochem. 2019, 137, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Akram, N.A.; Sürücü, A.; Ashraf, M. Alleviating effect of nitric oxide on oxidative stress and antioxidant defence system in pepper (Capsicum annuum L.) plants exposed to cadmium and lead toxicity applied separately or in combination. Sci. Hortic. 2019, 255, 52–60. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, G.; Bao, M.; Wang, L.; Xie, X. Exogenous application of ascorbic acid mitigates cadmium toxicity and uptake in Maize (Zea mays L.). Environ. Sci. Pollut. Res. 2019, 26, 19261–19271. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, V.M.; Soengas, P.; Alonso-Villaverde, V.; Sotelo, T.; Cartea, M.E.; Velasco, M.P. Effect of temperature stress on the early vegetative development of Brassica oleracea L. BMC Plant Biol. 2015, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Gao, F.; Ni, Z.; Lin, L.; Deng, Q.; Tang, Y.; Wang, X.; Luo, X.; Xia, H. Melatonin improves heat tolerance in kiwifruit seedlings through promoting antioxidant enzymatic activity and glutathione S-transferase transcription. Molecules 2018, 23, 584. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant. 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Vasseur, F.; Pantin, F.; Vile, D. Changes in light intensity reveal a major role for carbon balance in Arabidopsis responses to high temperature. Plant Cell Environ. 2011, 34, 1563–1576. [Google Scholar] [CrossRef]
- Khanna-Chopra, R.; Chauhan, S. Wheat cultivars differing in heat tolerance show a differential response to oxidative stress during monocarpic senescence under high temperature stress. Protoplasma 2015, 25, 1241–1251. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, K.; Gupta, A.K. Salicylic acid induces differential antioxidant response in spring maize under high temperature stress. Indian J. Exp. Biol. 2016, 54, 386–393. [Google Scholar]
- Jin, S.H.; Li, X.Q.; Wang, G.G.; Zhu, X.T. Brassinosteroids alleviate high-temperature injury in Ficus concinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. AoB Plants 2015, 7, plv009. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wen, X.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2018, 161, 303–311. [Google Scholar] [CrossRef]
- Sang, Q.Q.; Shu, S.; Shana, X.; Guo, S.R.; Sun, J. Effects of exogenous spermidine on antioxidant system of tomato seedlings exposed to high temperature stress. Russ. J. Plant Physiol. 2016, 63, 645–655. [Google Scholar] [CrossRef]
- Sgobba, A.; Paradiso, A.; Dipierro, S.; Gara, L.B.; de Pinto, M.C. Changes in antioxidants are critical in determining cell responses to short- and long-term heat stress. Physiol. Plant. 2015, 153, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Damanik, R.I.; Maziah, M.; Ismail, M.R.; Ahmad, S.; Zain, A.M. Responses of the antioxidative enzymes in Malaysian rice (Oryza sativa L.) cultivars under submergence condition. Acta Physiol. Plant 2010, 32, 739–747. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, C.W.; Khan, A.L.; Mun, B.G.; Shahzad, R.; Ko, J.W.; Yun, B.W.; Park, S.K.; Lee, I.J. Exo-ethylene application mitigates waterlogging stress in soybean (Glycine max L.). BMC Plant Biol. 2018, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Hu, W.; Snider, J.L.; Zhou, Z. Short-term soil-waterlogging contributes to cotton cross tolerance to chronic elevated temperature by regulating ROS metabolism in the subtending leaf. Plant Physiol. Biochem. 2019, 139, 333–341. [Google Scholar] [CrossRef]
- Sairam, R.K.; Kumutha, D.; Ezhilmathi, K.; Chinnusamy, V.; Meena, R.C. Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol. Plant. 2009, 53, 493–504. [Google Scholar] [CrossRef]
- Sairam, R.K.; Dharmar, K.; Lekshmy, S.; Chinnusamy, V. Expression of antioxidant defense genes in mung bean (Vigna radiata L.) roots under water-logging is associated with hypoxia tolerance. Acta Physiol. Plant. 2011, 3, 735–744. [Google Scholar] [CrossRef]
- Yiu, J.-C.; Liu, C.-W.; Fang, D.Y.; Lai, Y.-S. Waterlogging tolerance of Welsh onion (Allium fistulosum L.) enhanced by exogenous spermidine and spermine. Plant Physiol. Biochem. 2009, 47, 710–716. [Google Scholar] [CrossRef]
- Simova-Stoilova, L.; Demirevska, K.; Kingston-Smith, A.; Feller, U. Involvement of the leaf antioxidant system in the response to soil flooding in two Trifolium genotypes differing in their tolerance to waterlogging. Plant Sci. 2012, 183, 43–49. [Google Scholar] [CrossRef]
- Jaiswal, A.; Srivastava, J.P. Changes in reactive oxygen scavenging system and protein profiles in maize roots in response to nitric oxide under waterlogging stress. Indian J. Biochem. Biophys. 2018, 55, 26–33. [Google Scholar]
- Woo, S.Y.; Lee, D.K.; Lee, Y.K. Net photosynthetic rate, ascorbate peroxidase and glutathione reductase activities of Erythrina orientalis in polluted and non-polluted areas. Photosynthetica 2007, 45, 293–295. [Google Scholar] [CrossRef]
- Seyyednejad, S.M.; Koochak, H.; Vaezi, J. Changes in anti-oxidative enzymes activity, protein content and ascorbic acid level in Prosopis juliflora exposed to industrial air pollution. J. Biol. Today World 2013, 2, 482–492. [Google Scholar]
- Lucas, J.A.; Gutierrez-Albanchez, E.; Alfaya, T.; Feo-Brito, F.; Gutiérrez-Mañero, F.J. Oxidative stress in ryegrass growing under different air pollution levels and its likely effects on pollen allergenicity. Plant Physiol. Biochem. 2019, 135, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zeng, Q.; Zhu, J.G.; Liu, G.; Tang, H.Y. Dissimilarity of ascorbate–glutathione (AsA–GSH) cycle mechanism in two rice (Oryza sativa L.) cultivars under experimental free-air ozone exposure. Agric. Ecosyst. Environ. 2013, 165, 39–49. [Google Scholar] [CrossRef]
- Dusart, N.; Gérard, J.; Thiec, D.L.; Collignon, C.; Jolivet, Y.; Vaultier, M.N. Integrated analysis of the detoxification responses of two Euramerican poplar genotypes exposed to ozone and water deficit: Focus on the ascorbate-glutathione cycle. Sci. Total Environ. 2019, 651, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Kim, T.H.; Choi, B.C.; Lee, B.S.; Lee, J.H. Effect of CO, NOx and SO2 on ROS production, photosynthesis and ascorbate–glutathione pathway to induce Fragaria x annasa as a hyperaccumulator. Redox Biol. 2014, 2, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, B.; Zhang, R.; Gao, Y.; Zhang, X.; Li, Y.; Zuo, Z. Initial simulated acid rain impacts reactive oxygen species metabolism and photosynthetic abilities in Cinnamonum camphora undergoing high temperature. Ind. Crops Prod. 2019, 135, 352–361. [Google Scholar] [CrossRef]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Khan, M.N.; Ali, H.M.; Alakeel, K.A. Nitric oxide-mediated cross-talk of proline and heat shock proteins induce thermotolerance in Vicia faba L. Environ. Exp. Bot. 2019, 161, 290–302. [Google Scholar] [CrossRef]
- Anee, T.I.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef]
- Lin, K.H.R.; Weng, C.C.; Lo, H.F.; Chen, J.T. Study of the root antioxidative system of tomatoes and eggplants under waterlogged conditions. Plant Sci. 2004, 167, 355–365. [Google Scholar] [CrossRef]
- Bhuyan, M.B.; Hasanuzzaman, M.; Al Mahmud, J.; Hossain, M.S.; Alam, M.U.; Fujita, M. Explicating physiological and biochemical responses of wheat cultivars under acidity stress: Insight into the antioxidant defense and glyoxalase systems. PMBP 2019, 25, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Conklin, P.L.; Saracco, S.A.; Norris, S.R.; Last, R.L. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 2000, 154, 847–856. [Google Scholar] [PubMed]
- Gao, Q.; Zhang, L. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Srivastava, P.K.; Prasad, S.M. Differential effect of UV-B radiation on growth, oxidative stress and ascorbate-glutathione cycle in two cyanobacteria under copper toxicity. Plant Physiol. Biochem. 2012, 61, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Shiu, C.-T.; Lee, T.-M. Ultraviolet-B-induced oxidative stress and responses of the ascorbate–glutathione cycle in a marine macroalga Ulva fasciata. J. Exp. Bot. 2005, 56, 2851–2865. [Google Scholar] [CrossRef] [PubMed]
- Noshi, M.; Hatanaka, R.; Tanabe, N.; Terai, Y.; Maruta, T.; Shigeoka, S. Redox regulation of ascorbate and glutathione by a chloroplastic dehydroascorbate reductase is required for high-light stress tolerance in Arabidopsis. Biosci. Biotechnol. Biochem. 2016, 80, 870–877. [Google Scholar] [CrossRef]
- Zheng, L.-D.; Li, M.; Chow, W.S.; Peng, C.-L. Susceptibility of an ascorbate-deficient mutant of Arabidopsis to high-light stress. Photosynthetica 2018, 56, 427–432. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Devireddy, A.R.; Azad, R.K.; Shulaev, V.; Mittler, R. Rapid accumulation of glutathione during light stress in Arabidopsis. Plant Cell Physiol. 2018, 59, 1817–1826. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Riaz, M.; Arif, M.S.; Rasheed, R.; Iqbal, M.; Hussain, I.; Salman, M. The role of non-enzymatic antioxidants in improving abiotic stress tolerance in plants. In Plant Tolerance to Environmental Stress: Role of Phytoprotectants; Hasanuzzaman, M., Fujita, M., Oku, H., Islam, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 129–143. [Google Scholar]
- Naz, H.; Akram, N.A.; Ashraf, M. Impact of ascorbic acid on growth and some physiological attributes of cucumber (Cucumis sativus) plants under water-deficit conditions. Pak. J. Bot. 2016, 48, 877–883. [Google Scholar]
- Xu, Y.; Huang, B. Exogenous ascorbic acid mediated abiotic stress tolerance in plants. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 233–253. [Google Scholar]
- Billah, M.; Rohman, M.; Hossain, N.; Uddin, M.S. Exogenous ascorbic acid improved tolerance in maize (Zea mays L.) by increasing antioxidant activity under salinity stress. Afr. J. Agric. Res. 2017, 12, 1437–1446. [Google Scholar]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; Arshad, A. Synergistic effects of drought and ascorbic acid on growth, mineral nutrients and oxidative defense system in canola (Brassica napus L.) plants. Acta Physiol. Plant. 2014, 36, 1539–1553. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, R.; Kaur, N.; Bhandhari, K.; Kaushal, N.; Gupta, K.; Bains, T.; Nayyar, H. Heat-stress induced inhibition in growth and chlorosis in mungbean (Phaseolus aureus Roxb.) is partly mitigated by ascorbic acid application and is related to reduction in oxidative stress. Acta Physiol. Plant. 2011, 33, 2091–2101. [Google Scholar] [CrossRef]
- Kobayakawa, H.; Imai, K. Exogenous ascorbic acid scarcely ameliorates inhibition of photosynthesis in rice leaves by O3. Plant Prod. Sci. 2017, 20, 83–89. [Google Scholar] [CrossRef]
- Wang, J.; Wu, B.; Yin, H.; Fan, Z.; Li, X.; Ni, S.; He, L.; Li, J. Overexpression of CaAPX induces orchestrated reactive oxygen scavenging and enhances cold and heat tolerances in tobacco. BioMed. Res. Int. 2017, 2017, 4049534. [Google Scholar] [CrossRef] [PubMed]
- Saeidi-Sar, S.; Abbaspour, H.; Afshari, H.; Yaghoobi, S.R. Effects of ascorbic acid and gibberellin A3 on alleviation of salt stress in common bean (Phaseolus vulgaris L.) seedlings. Acta Physiol. Plant. 2013, 35, 667–677. [Google Scholar] [CrossRef]
- Rehman, R.U.; Zia, M.; Abbasi, B.H.; Lu, G.; Chaudhary, M.F. Ascorbic acid and salicylic acid mitigate NaCl stress in Caralluma tuberculata Calli. Appl. Biochem. Biotechnol. 2014, 173, 968–979. [Google Scholar] [CrossRef]
- Wang, R.; Liu, S.; Zhou, F.; Ding, C.; Hua, C. Exogenous ascorbic acid and glutathione alleviate oxidative stress induced by salt stress in the chloroplasts of Oryza sativa L. Z. Naturforsch. C 2014, 69, 226–236. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Sequenced application of ascorbate-proline-glutathione improves salt tolerance in maize seedlings. Ecotoxicol. Environ. Saf. 2016, 133, 252–259. [Google Scholar] [CrossRef]
- Terzi, R.; Kalaycıoglu, E.; Demiralay, M.; Saglam, A.; Kadioglu, A. Exogenous ascorbic acid mitigates accumulation of abscisic acid, proline and polyamine under osmotic stress in maize leaves. Acta Physiol. Plant. 2015, 37, 43. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Q.; Huang, B. Ascorbic acid mitigation of water stress-inhibition of root growth in association with oxidative defense in tall fescue (Festuca arundinacea Schreb. ) Front. Plant Sci. 2015, 6, 807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Zhang, Y.; Cai, X.; Gong, P.; Zhang, J.; Wang, T.; Li, H.; Ye, Z. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 2011, 30, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, L.; Xie, J.; Li, T.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Herbicide isoproturon aggravates the damage of low temperature stress and exogenous ascorbic acid alleviates the combined stress in wheat seedlings. Plant Growth Regul. 2018, 84, 293–301. [Google Scholar] [CrossRef]
- Chao, Y.Y.; Hong, C.Y.; Kao, C.H. The decline in ascorbic acid content is associated with cadmium toxicity of rice seedlings. Plant Physiol. Bioch. 2010, 48, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Nasir Khan, M.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A.; Al-Rabiah, H.; Mateen, M. Ascorbic acid improves the tolerance of wheat plants to lead toxicity. J. Plant Interact. 2018, 13, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Jiang, Y.; He, L.; Zhou, Q.; Yu, J.; Hui, D.; Huang, D. Exogenous glutathione improves high root-zone temperature tolerance by modulating photosynthesis, antioxidant and osmolytes systems in cucumber seedlings. Sci. Rep. 2016, 6, 35424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, F.; Liu, L.; Ibrahim, W.; Cai, Y.; Wu, F. Alleviating effects of exogenous glutathione, glycinebetaine, brassinosteroids and salicylic acid on cadmium toxicity in rice seedlings (Oryza sativa). Agrotechnology 2013, 2, 107. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, Y.; Huang, S.; Yang, Y.; Gu, C. Effects of exogenous glutathione and cysteine on growth, lead accumulation, and tolerance of Iris lactea var. chinensis. Environ. Sci. Pollut. Res. 2015, 22, 2808–2816. [Google Scholar] [CrossRef]
- Kim, Y.-O.; Bae, H.-J.; Cho, E.; Kang, H. Exogenous Glutathione Enhances Mercury Tolerance by Inhibiting Mercury Entry into Plant Cells. Front. Plant Sci. 2017, 8, 683. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wen, Z.; Zhang, J.; Chen, X.; Cui, J.; Xu, W.; Liu, H. Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci. Hortic. 2017, 220, 90–101. [Google Scholar] [CrossRef]
- Akram, S.; Siddiqui, M.N.; Hussain, B.N.; Al Bari, M.A.; Mostofa, M.G.; Hossain, M.A.; Tran, L.S.P. Exogenous glutathione modulates salinity tolerance of soybean [Glycine max (L.) Merrill] at reproductive stage. J. Plant Growth Regul. 2017, 36, 877–888. [Google Scholar] [CrossRef]
- Zhou, Y.; Diao, M.; Cui, J.; Chen, X.; Wen, Z.; Zhang, J.; Liu, H. Exogenous GSH protects tomatoes against salt stress by modulating photosystem II efficiency, absorbed light allocation and H2O2-scavenging system in chloroplasts. J. Integr. Agric. 2018, 17, 2257–2272. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Sandalio, L.M. Nitric oxide level is self-regulating and also regulates its ROS partners. Front. Plant Sci. 2016, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mu, J.; Chen, L.; Feng, J.; Hu, J.; Li, L.; Zhou, J.M.; Zuo, J. S-Nitrosylation positively regulates ascorbate peroxidase activity during plant stress responses. Plant Physiol. 2015, 167, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Mata-Pérez, C.; Valderrama, R.; Padilla, M.N.; López-Jaramillo, J.; Luque, F.; Corpas, F.J.; Barroso, J.B. Differential molecular response of monodehydroascorbate reductase and glutathione reductase by nitration and S-nitrosylation. J. Exp. Bot. 2015, 66, 5983–5996. [Google Scholar] [CrossRef]
- Tanou, G.; Filippou, P.; Belghazi, M.; Job, D.; Diamantidis, G.; Fotopoulos, V.; Molassiotis, A. Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J. 2012, 72, 585–599. [Google Scholar] [CrossRef]
- Groß, F.; Durner, J.; Gaupels, F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013, 4, 419. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V.; Shabala, S. Mechanisms of cytosolic calcium elevation in plants: The role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS-Ca2+ Hub’. Funct. Plant Biol. 2018, 45, 9–27. [Google Scholar] [CrossRef]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Plant Sci. 2000, 19, 267–290. [Google Scholar] [CrossRef]
- Gallie, D.R. L-ascorbic acid: A multifunctional molecule supporting plant growth and development. Scientifica 2013, 2013, 795964. [Google Scholar] [CrossRef] [PubMed]
- Makavitskaya, M.; Svistunenko, D.; Navaselsky, I.; Hryvusevich, P.; Mackievic, V.; Rabadanova, C.; Tyutereva, E.; Samokhina, V.; Straltsova, D.; Sokolik, A.; et al. Novel roles of ascorbate in plants: Induction of cytosolic Ca2+ signals and efflux from cells via anion channels. J. Exp. Bot. 2018, 69, 3477–3489. [Google Scholar] [CrossRef] [PubMed]
- Kuźniak, E.; Kaźmierczak, A.; Wielanek, M.; Głowacki, R.; Kornas, A. Involvement of salicylic acid, glutathione and protein S-thiolation in plant cell death-mediated defence response of Mesembryanthemum crystallinum against Botrytis cinerea. Plant Physiol. Biochem. 2013, 63, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bedhomme, M.; Adamo, M.; Marchand, C.H.; Couturier, J.; Rouhier, N.; Lemaire, S.D.; Zaffagnini, M.; Trost, P. Glutathionylation of cytosolic glyceraldehyde-3-phosphate dehydrogenase from the model plant Arabidopsis thaliana is reversed by both glutaredoxins and thioredoxins in vitro. Biochem. J. 2012, 445, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Akter, N.; Okuma, E.; Sobahan, M.A.; Uraji, M.; Munemasa, S.; Nakamura, Y.; Mori, I.C.; Murata, Y. Negative regulation of methyl jasmonate-induced stomatal closure by glutathione in Arabidopsis. J. Plant Growth Regul. 2013, 32, 208–215. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Ma, C.; Zhao, Y.; Wang, Y.; Hasi, A.; Qi, Z. Glutamate receptor-like channel 3.3 is involved in mediating glutathione-triggered cytosolic calcium transients, transcriptional changes, and innate immunity responses in Arabidopsis. Plant Physiol. 2013, 162, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Liebthal, M.; Maynard, D.; Dietz, K.J. Peroxiredoxins and redox signaling in plants. Antioxid. Redox Signal. 2018, 28, 609–624. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Treberg, J.R. Protein S-glutathionlyation links energy metabolism to redox signaling in mitochondria. Redox Biol. 2016, 8, 110–118. [Google Scholar] [CrossRef]
- Czerniawski, P.; Bednarek, P. Glutathione S-Transferases in the Biosynthesis of Sulfur-Containing Secondary Metabolites in Brassicaceae Plants. Front. Plant Sci. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Benjeddou, H.; Ahmed, C.B.; Rouina, B.B. Influence of antioxidative enzymes, phytohormones and pigments in alternate bearing of three olive cultivars. Sci. Hortic. 2019, 253, 17–23. [Google Scholar] [CrossRef]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of phytohormone signaling: A primary function of flavonoids in plant–environment interactions. Front. Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, J.; Li, S.; Kakan, X.; Zhou, Y.; Miao, Y.; Wang, F.; Qin, H.; Huang, R. Ascorbic acid integrates the antagonistic modulation of ethylene and abscisic acid in the accumulation of reactive oxygen species. Plant Physiol. 2019, 179, 1861–1875. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chang, X.; Guo, X. Dynamic changes of ascorbic acid, phenolics biosynthesis and antioxidant activities in mung beans (Vigna radiata) until maturation. Plants 2019, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Huo, J.; Zhang, J.; Wang, C.; Wang, B.; Fang, H.; Liao, W. Protein S-nitrosylation in programmed cell death in plants. Cell. Mol. Life Sci. 2019, 76, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Kumar, D.; Sultana, A.; Hazra, S.; Bhattacharyya, D.; Chattopadhyay, S. Glutathione regulates 1-aminocyclopropane-1-carboxylate synthase transcription via WRKY33 and 1-aminocyclopropane-1-carboxylate oxidase by modulating messenger RNA stability to induce ethylene synthesis during stress. Plant Physiol. 2015, 169, 2963–2981. [Google Scholar] [PubMed]
- Singh, A.P.; Dixit, G.; Mishra, S.; Dwivedi, S.; Tiwari, M.; Mallick, S.; Pandey, V.; Trivedi, P.K.; Chakrabarty, D.; Tripathi, R.D. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 340. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Ko, K.; Chang, W.L.; Kuo, W.C.; Chen, G.H.; Lin, T.P. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J. 2015, 83, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Labrou, N.E.; Papageorgiou, A.C.; Pavli, O.; Flemetakis, E. Plant GSTome: Structure and functional role in xenome network and plant stress response. Curr. Opin. Biotechnol. 2015, 32, 186–194. [Google Scholar] [CrossRef]
- Sevilla, F.; Camejo, D.; Ortiz-Espín, A.; Calderón, A.; Lázaro, J.J.; Jiménez, A. The thioredoxin/peroxiredoxin/sulfiredoxin system: Current overview on its redox function in plants and regulation by reactive oxygen and nitrogen species. J. Exp. Bot. 2015, 66, 2945–2955. [Google Scholar] [CrossRef]
- Cao, S.; Du, X.-H.; Li, L.-H.; Liu, Y.-D.; Zhang, L.; Pan, X.; Li, Y.; Li, H. Overexpression of Populus tomentosa cytosolic ascorbate peroxidase enhances abiotic stress tolerance in tobacco plants. Russ. J. Plant Physiol. 2017, 64, 224–234. [Google Scholar] [CrossRef]
- Chin, D.-C.; Kumar, R.S.; Suen, C.-S.; Chien, C.-Y.; Hwang, M.-J.; Hsu, C.-H.; Xuhan, X.; Lai, Z.X.; Yeh, K.W. Plant cytosolic ascorbate peroxidase with dual catalytic activity modulates abiotic stress tolerances. iScience 2019, 16, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Eltelib, H.A.; Fujikawa, Y.; Esaka, M. Overexpression of the acerola (Malpighia glabra) monodehydroascorbate reductase gene in transgenic tobacco plants results in increased ascorbate levels and enhanced tolerance to salt stress. S. Afr. J. Bot. 2012, 78, 295–301. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Kim, M.-H.; Kim, Y.-H.; Park, H.M.; Yoon, H.-S. Co-Expression of monodehydroascorbate reductase and dehydroascorbate reductase from Brassica rapa effectively confers tolerance to freezing-induced oxidative stress. Mol. Cells 2013, 36, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Mano, J.; Tanaka, K.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S. High level of reduced glutathione contributes to detoxification of lipid peroxide-derived reactive carbonyl species in transgenic Arabidopsis overexpressing glutathione reductase under aluminum stress. Physiol. Plant. 2017, 161, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Ding, J.; Zhang, B.; Xi, D.; Ming, F. OsNAC2 positively affects salt-induced cell death and binds to the OsAP37 and OsCOX11 promoters. Plant J. 2018, 94, 454–468. [Google Scholar] [CrossRef]
- Borgohain, P.; Saha, B.; Agrahari, R.; Chowardhara, B.; Sahoo, S.; van der Vyver, C.; Panda, S.K. SlNAC2 overexpression in Arabidopsis results in enhanced abiotic stress tolerance with alteration in glutathione metabolism. Protoplasma 2019, 256, 1065. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, C.; Xue, X.; Xu, M.; Li, J.; Wu, J.-X. Overexpression of a cytosolic sscorbate peroxidase gene, OsAPX2, increases salt tolerance in transgenic Alfalfa. J. Integr. Agric. 2014, 13, 2500–2507. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Wu, W.; Guo, J.; Yang, Y. Cadmium stress tolerance in wheat seedlings induced by ascorbic acid was mediated by NO signaling pathways. Ecotoxicol. Environ. Saf. 2017, 135, 75–81. [Google Scholar] [CrossRef]
- Liu, F.; Huang, N.; Wang, L.; Ling, H.; Sun, T.; Ahmad, W.; Muhammad, K.; Guo, J.; Xu, L.; Gao, S.; et al. A novel L-ascorbate peroxidase 6 gene, ScAPX6, plays an important role in the regulation of response to biotic and abiotic stresses in sugarcane. Front. Plant Sci. 2018, 8, 2262. [Google Scholar] [CrossRef]
- Chin, D.C.; Hsieh, C.C.; Lin, H.Y.; Yeh, K.W. A low glutathione redox state couples with a decreased ascorbate redox ratio to accelerate flowering in Oncidium orchid. Plant Cell Physiol. 2016, 57, 423–436. [Google Scholar] [CrossRef]
- Kavitha, K.; George, S.; Venkataraman, G.; Parida, A. A salt-inducible chloroplastic monodehydroascorbate reductase from halophyte Avicennia marina confers salt stress tolerance on transgenic plants. Biochimie 2010, 92, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Choi, S.M.; Ahn, Y.O.; Lee, H.S.; Lee, H.B.; Park, Y.M.; Kwak, S.S. Enhanced stress–tolerance of transgenic plants expressing a human dehydroascorbate reductase gene. J. Plant Physiol. 2003, 160, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, T.; Nakagawa, T.; Fujioka, Y.; Daicho, K.; Naito, M.; Yamauchi, Y.; Nonaka, H.; Amako, K.; Yamawaki, K.; Murata, N. Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J. Plant Physiol. 2006, 163, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Kim, S.H.; Bang, J.W.; Lee, H.S.; Kwak, S.S.; Kwon, S.Y. Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Rep. 2007, 26, 591–598. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.S.; Yu, C.; Zhu, Z.J.; Yu, X.C. Overexpression in tobacco of a tomato GMPase gene improves tolerance to both low and high temperature stress by enhancing antioxidation capacity. Plant Cell Rep. 2011, 30, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Le Martret, B.; Poage, M.; Shiel, K.; Nugent, G.D.; Dix, P.J. Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol. J. 2011, 9, 661–673. [Google Scholar] [CrossRef] [PubMed]







| Plant Species | Stress Levels | Status of AsA-GSH Component(s) | ROS Regulation | References |
|---|---|---|---|---|
| Triticum aestivum L. | 100 mM NaCl | GSH content increased by 15%; Stimulated APX and GR activities by 78% and 56%, respectively | Increased H2O2 content about 79% | [100] |
| T. aestivum L. cv. BARI Gom-21 | 12% PEG for 48 and 72 h | Decreased AsA content at 48 h, but after 72 h, AsA content again enhanced; Increased GSH and GSSG content where GSH/GSSG ratio decreased time-dependently; Enhanced the activities of APX, MDHAR, and GR | Enhanced the H2O2 content by 62% and increased O2− accumulation | [113] |
| T. aestivum L. | 10% PEG | Reduced AsA/DHA and GSH/GSSG redox; Increased enzymatic antioxidants actions of AsA-GSH cycle | Increased H2O2 production | [107] |
| T. aestivum L. | 35–40% field capacity (FC) water | Increased GSH/GSSG by 64% while decreased AsA/DHA by 52% respective with a duration of stress; Enhanced APX, MDHAR, DHAR and GR activities | Increased H2O2 along with stress duration | [106] |
| T. aestivum cv. Pradip | 150 and 300 mM NaCl | Reduced AsA content upto 52%; Increased reduced and oxidized GSH accumulation by 55% and 18%, respectively with 32% higher GSH/GSSG ratio; Increased APX activity with 29% reduction of GR activity; Slightly increased MDHAR and DHAR activity | Enhanced H2O2 generation by 60% | [28] |
| Oryza sativa L. cv. BRRI dhan47 | 150 mM NaCl | Increased GSH accumulation while reduced AsA content by 49% Increased GSH content and lowered the redox status of both AsA/DHA and GSH/GSSG; Upregulated the activity of APX, MDHAR, DHAR, and GR | Increased the production of O2− with 82% higher H2O2 accumulation | [93] |
| O. sativa L. cv. BRRI dhan49 | 300 mM NaCl | Reduced AsA and GSH accumulation by 51% and 57%, respectively; Decrease GSH/GSSG redox by 87%; Showed lowered APX (27%), MDHAR (24%), DHAR and GR (25%) activities | Increased H2O2 content upto 69% | [114] |
| O. sativa L. cv. BRRI dhan54 | 300 mM NaCl | Improved AsA content by 51% with higher GSH content; Decreased GSH/GSSG ratio by 53%; Showed higher APX (27%) and DHAR activities while decreased both GR (23%) and MDHAR activities | Accumulated 63% higher H2O2 content | [114] |
| Brassica napus L. cv. BINA sharisha 3 | 100 and mM NaCl | Reduced the AsA content by 22%; Increased GSH content by 72% and GSSG content by 88%; Unaltered the GSH/GSSG ratio; Amplified APX activity by 32%, decreased DHAR activity by 17%; Slightly increased GR activity | Accumulated higher H2O2 content by 76% | [115] |
| B. napus L. cv. BINA sharisha 3 | 200 mM NaCl | Reduced the AsA content (40%) along with increased GSH (43%) and GSSG (136%) contents; Decreased the GSH/GSSG ratio (40%); Amplified the APX activity (39%) and reduced the MDHAR (29%) and DHAR (35%) activities; Improved GR activity (18%) | Showed 90% more H2O2 content | [115] |
| B. napus L. | 15% PEG | The AsA accumulation remained unaltered and reduced the AsA/DHA ratio; Enhanced GSH content by 19% and GSSG by 67% and decreased GSH/GSSG ratio; Increased APX, MDHAR, DHAR and GR activities | Higher accumulation of H2O2 by 55% | [116] |
| B. campestris L. | 15% PEG | Decreased AsA content by 27% with a decrease of AsA/DHA ratio; Increased GSH content by 33% with higher GSSG content by 79% and lowered GSH/GSSG ratio; Decreased DHAR activity | Higher accumulation of H2O2 about 109% | [116] |
| B. juncea L. | 15% PEG | Increased the AsA content and did not affect the AsA/DHA ratio; Increased GSH content by 48% and GSSG by 83% and decreased GSH/GSSG ratio; Increased APX, MDHAR, DHAR and GR activities | Accumulation of 37% higher H2O2 | [116] |
| B. juncea L. cv. BARI Sharisha 11 | 10% PEG | Reduced AsA content (14%) while increased both GSH (32%) and GSSG (48%) contents; Enhanced APX activity (24%); Decreased MDHAR and DHAR (33%) activities along with 31% increased GR activity | Acute generation of H2O2 (41%) | [117] |
| B. juncea L. cv. BARI Sharisha 11 | 20% PEG | Decreased AsA content by 34% while increased the content of GSH by 25% and GSSG by 101%; Up-regulated APX activity by 33%; Decreased activity of MDHAR and DHAR (30%) | Extreme generation of H2O2 by 95% | [117] |
| B. napus L. cv. BINA Sarisha 3 | 10% PEG | Increased AsA (21%), GSH (55%) and GSSG contents while decreased GSH/GSSG ratio Unaltered the activities of APX, and increased the activity of MDHAR, DHAR, and GR (26%) | Elevated the H2O2 production | [11] |
| B. napus L. cv. BINA Sarisha 3 | 20% PEG | Unaltered AsA content along with higher content of GSH (46%) and GSSG and reduced GSH/GSSG ratio; Reduced the APX and MDHAR activities along with the higher activity of DHAR and GR (23%) | Showed higher H2O2 production | [11] |
| B. napus L. cv. BINA sharisha 3 | 10% PEG | Increased AsA, GSH (31%) and GSSG (83%) accumulation with lowered GSH/GSSG ratio; Increased APX activity while reduced MDHAR and DHAR activities, but GR activity remained unaltered | Increased H2O2 content by 53% | [52] |
| B. napus L. cv. BINA Sharisha 3 | 20% PEG | Slightly increased AsA content with 26% and 225% increase of GSH and GSSG content, respectively; Reduced GSH/GSSG ratio; Increased APX activity while decreased the activity of MDHAR, DHAR, and GR (30%) | Increased about 93% H2O2 content | [52] |
| B. rapa L. cv. BARI Sharisha-15 | 20% PEG | Slightly increased AsA content with 72% and 178% increase of GSH and GSSG content, respectively; Reduced GSH/GSSG ratio by 38%; Increased APX, MDHAR, DHAR, and GR activity | Increased about 131% H2O2 content | [104] |
| Cucumis melo L. cv. Yipintianxia No. 208 | 50 mM of NaCl:Na2SO4:NaHCO3:Na2CO3 (1:9:9:1 M) | Improved AsA, GSSG and DHA contents; Lowered GSH content; Reduced the ratio of AsA/DHA and GSH/GSSG; Stimulated the activity of APX by 96% and DHAR by 38% while reducing the activity of MDHAR and GR by 48% and 34%, respectively | Increased H2O2 accumulation | [118] |
| Solanum lycopersicum L., var. Lakshmi | 0.3 and 0.5 g NaCl kg−1 soil | Reduced AsA and AsA/DHA ratio; Lowered GSH and GSSG accumulation with decreased GSH/GSSG redox; Increased APX activity by 28%, DHAR activity by 28% and GR activity by 14% | Enhanced H2O2 and O2− accumulation | [97] |
| S. lycopersicum L.cv. Boludo | 60 mM NaCl, 30 days | Reduced the activities of APX, DHAR, and GR; Increased MDHAR activity | Higher H2O2 generation | [119] |
| S. lycopersicum L. var. Pusa Ruby | 150 mM NaCl | Decreased AsA and GSH content with a higher content of DHA and GSSG; Increased APX, MDHAR, DHAr and GR activities | Higher generation of H2O2 and O2− | [92] |
| S. lycopersicum L. var. Pusa Rohini | 150 mM NaCl | Reduced AsA content by 42%; Increased both GSH and GSSG accumulation; Enhanced the activity of APX and GR by 86% and 29%, respectively with reduction of the activity of MDHAR and DHAR by 38% and 32%, respectively | Accumulated about 3 fold higher H2O2 content | [99] |
| S. lycopersicon L. cv.K-21 | 150 mM NaCl | Reduced AsA content by 40% with 50% higher GSH content; Lowered GSSG content by 23% while increased GSH/GSSG ratio by 112%; Increased APX (86%) and GR (92%) activity along with the lowered activity of MDHAR (32%) and DHAR (30%) | Elevated H2O2 content about 175% | [98] |
| Nitraria Tangutorum Bobr. | 100,200, 300 and 400 mM NaCl | Increased AsA, DHA, GSH and GSSG accumulation decreased their redox status; Enhanced the activity of APX and GR; Unvaried the activity of DHAR and MDHAR but increased DHAR activity only at 300 mM NaCl | Increased O2−and H2O2 content by 38–98 and 49–102% respectively | [101] |
| Camellia sinensis (L.) O.Kuntze | 300 mM NaCl | Enhanced the AsA and GSH content; Increased APX activity | Elevated H2O2 and O2− content | [120] |
| Phaseolus vulgaris L. cv. Nebraska | 2.5 and 5.0 dS m–1 prepared from a mixture of NaCl, CaCl2, and MgSO4 | Increased AsA, GSH, DHA and GSSG accumulations; Enhanced AsA/DHA and GSH/GSSG status; Stimulated the enzymatic activity of APX, MDHAR, DHAR and GR activities | Accumulated higher H2O2 content | [103] |
| Vigna radiate L. cv. BINA moog-1 | 25% PEG | Reduced AsA content along with higher GSH content of 92%; Increased GSSG content by 236% and reduced GSH/GSSG ratio; Amplified the activity of APX (21%) and GR while reduced MDHAR and DHAR activities | Elevated H2O2 content by 114% with higher O2− generation | [111] |
| V. radiata L. | 200 mM NaCl | Reduced AsA content; Increased GSSG and GSH accumulation and lowered GSH/GSSG ratio; Amplified the activity of APX, MDHAR, DHAR, and GR | Increased H2O2 content by 80% and O2− generation by 86% | [95] |
| V. radiata L. cv. BARI Mung-2 | 5% PEG | Reduced AsA content where decreased AsA/DHA ratio by 54%; Increased GSSG content; Upregulated the activity of APX and GR (42%) while downregulated the MDHAR (26%) and DHAR activities | Elevated H2O2 and O2− accumulation | [50] |
| Lens culinaris Medik cv. BARI Lentil-7 | 20% PEG | Lowered AsA content with higher total GSH content; Unaltered the APX and GR activities while the increased activity of MDHAR and DHAR (64%) | Accumulated higher H2O2 content | [96] |
| L. culinaris Medik cv. BARI Lentil-7 | 100 mM NaCl | Reduced AsA content by 87% while increased total GSH content by 260%; Improved the activity of APX, MDHAR, DHAR (286%) and GR (162%) | Increased H2O2 content by 15% | [96] |
| Anacardium occidentale L. | 21-day water withdrawal | Enhanced total AsA and GSH content; Increased APX activity | Reduced H2O2 generation | [112] |
| Arabidopsis | 12-day water withhold | Showed higher GSH and GSSG accumulation; Reduced GSH/GSSG ratio; Increased GR activity | Increased H2O2 accumulation rate | [108] |
| Cajanus cajan L. | Complete water withholding for 3, 6 and 9 days | Decreased GSH/GSSG ratio; Increased the activity of APX, DHAR, and GR | Higher H2O2 content | [109] |
| Amaranthus tricolor L.cv. VA13 | 30% FC | Increased AsA and GSH contents by 286% and 98%, respectively; Improved APX, MDHAR, DHAR, and GR activity by 371%, 379%, 375%, and 375%, respectively | No increment of H2O2 content | [110] |
| A. tricolor L.cv. VA15 | 30% FC | Increased AsA and GSH contents along with higher redox status of AsA/total AsA and GSH/total GSH; Enhanced the activity of APX, MDHAR, DHAR, and GR by 37%, 45%, 40%, and 2%, respectively | Accumulated higher H2O2 content by 137% | [110] |
| C. sinensis (L.) O. Kuntze | 20% PEG | Higher contents of both AsA and GSH; Enhanced the APX activity | Higher accumulation of H2O2 and O2− | [120] |
| Plant Species | Stress Levels | Status of AsA-GSH Component(s) | ROS Regulation | References |
|---|---|---|---|---|
| Brassica napus L. cv. BINA sharisha 3 | Cd (0.5 mM and 1.0 mM CdCl2), 48 h | Reduced AsA content by 20% under 0.5 mM and 32% under 1.0 mM CdCl2 treatment; Increased GSH content only under 0.5 mM CdCl2 stress but enhanced level of GSSG by 34% under 0.5 mM and 65% under 1.0 mM CdCl2 treatment; Increased function of APX by 39% and 43% under 0.5 mM and 1.0 mM CdCl2 treatment but MDHAR and DHAR activity were diminished in dose dependant fashion; GR activity increased by 66% due to 0.5 mM CdCl2 treatment but reduced by 24% due to 1.0 mM CdCl2 treatment | Enhanced H2O2 content by 37% under 0.5 mM and 60% under 1.0 mM CdCl2 treatment | [88] |
| Gossypium spp. (genotype MNH 886) | Pb [50 and 100 μM Pb(NO3)2], 6 weeks | Increased APX activity | Increased H2O2 content | [124] |
| T. aestivum L. cv. Pradip | As (0.25 and 0.5 mM Na2HAsO47H2O), 72 h | Reduced AsA content by 14% under 0.25 and 34% underd 0.5 mM Na2HAsO4·7H2O treatment; Increased GSH content by 46% and 34%, GSSG content by 50 and 101% under 0.25 and 0.5 mM Na2HAsO4·7H2O stress; Enhanced APX function by 39% and 43% but decreased DHAR function by 33% and 30% under 0.25 and 0.5 mM Na2HAsO4·7H2O treatment; Increased GR function by 31% under 0.25 mM | Increased H2O2 content by 41% under 0.25 and 95% under 0.5 mM Na2HAsO4·7H2O treatment | [127] |
| B. napus L. viz. ZS 758, Zheda619, ZY 50 and Zheda 622 | Cr (400 µM), 15 days | Increased GSH and GSSG content; Increased APX activity | Increased H2O2 content | [128] |
| Oryza sativa L. cv. BRRI dhan29 | As (0.5 mM and 1 mM Na2HAsO4), 5 days | Decreased AsA content by 33 and 51% and increased DHA content by 27% and 40% under 0.5mM and 1mM Na2HAsO4 treatment, respectively; Decreased ratio of AsA/DHA; Enhanced GSH content by 48 and 82% under 0.5mM and 1mM Na2HAsO4 treatment, respectively; Enhanced GSSG content whereas lessened GSH/GSSG ratio by 25% under 0.5mM and 41% under 1mM Na2HAsO4 treatment; Augmented the function of APX, MDHAR, and GR, however, reduced the activity of DHAR | Increased H2O2 content by 65% and 89% under 0.5mM and 1mM Na2HAsO4 treatment, respectively | [126] |
| O. sativa L. cv. Disang (tolerant) | 100 µM AlCl3, 48 h | Increased AsA content in both roots and shoots; Enhanced the GSH content in shoots; Higher activities of APX, MDHAR, DHAR, and GR, | Elevated the generation of H2O2 and O2− | [129] |
| O. sativa L. cv. Joymati (sensitive) | 100 µM AlCl3, 48 h | Higher accumulation of AsA in both roots and shoots; Reduced the GSH content in roots while shoots content was unaltered; Increased APX, MDHAR, DHAR activities; Slightly increased GR activities | Higher accumulation of H2O2 and O2− | [129] |
| V. radiata L. cv. BARI Mung-2 | Cd (mild: 1.0 mM CdCl2, severer: 1.5 mM CdCl2), 48 h | Declined AsA content by 31% due to mild and 41% due to severe stress; Enhanced DHA level and reduced AsA/DHA ratio; GSH content did not change due to mild stress but enhanced owing to stress severity; GSSG level enhanced, and GSH/GSSG ratio decreased in dose-dependent manner; Increased function of APX but lessened MDHAR and DHAR function due to both level of stress; GR activity increased only due to severe stress | H2O2 level and O2− generation rate was augmented by 73% and 127% due to mild and 69% and 120% due to severe Cd stresses | [130] |
| V. radiata L. cv. BARI Mung-2 | Cd (1.5 mM CdCl2), 48 h | AsA content decreased by 27%, and the ratio of AsA/DHA reduced by 80% whereas DHA content increased considerably; Augmented the function of APX and GR however lessened function of MDHAR and DHAR | Increased H2O2 level and O2− generation rate | [131] |
| O. sativa L. cv. BRRI dhan29 | Cd (0.25 mM and 0.5 mM CdCl2), 3 days | AsA content and AsA/DHA ratio reduced by 37% and 57% due to 0.25 mM CdCl2 and reduced by 51% and 68% due to 0.5 mM CdCl2, respectively; DHA content increased significantly; GSH content enhanced due to 0.25 mM CdCl2 stress, but reduced due to 0.5 mM CdCl2 stress; GSSG content enhanced by 76% under 0.25 mM and 108% under 0.5 mM CdCl2 stress; Reduced ratio of GSH/GSSG in dose dependant manner; Enhanced APX, MDHAR and GR activity | Enhenced H2O2 by 46% under 0.25 mM CdCl2 and 84% under 0.5 mM CdCl2 treatmen whereas O2− generation rate increased in dose dependant manner | [132] |
| O. sativa L. cv. BRRI dhan29 | Cd (0.3 mM CdCl2), 3 days | Lessened level of AsA and AsA/DHA ratio but enhanced DHA level; Enhanced the level of GSH and GSSG however lessened GSH/GSSG ratio; Enhanced the action of APX, MDHAR, and GR whereas declined DHAR function | Overproduced ROS (H2O2 and O2−) | [133] |
| O. sativa L. Zhunliangyou 608 | Cd (5 μM Cd(NO3)2·4H2O), 6 days | Reduced AsA content; Increased GSH content; Slightly reduced the APX activity | H2O2 content increased by 22.73% | [134] |
| Abelmoschus esculentus L. Moench | Pb (100 mg L−1), 21 days | Increased AsA content | Enhanced H2O2 content | [135] |
| B. juncea L. cv. BARI Sharisha-11 | Cr (mild: 0.15 mM K2CrO4, severe: 0.3 mM K2CrO4), 5 days | AsA content lessened by 19% due to mild and 32% due to severe stress whereas DHA level enhanced by 83% due to mild and 133% due to severe stress as well as AsA/DHA ratio lessened by 47% due to mild and 82% due to severe stress; GSH content did not change considerably but GSSG content enhanced by 42% due to mild and 67% due to severe stress as well as GSH/GSSG ratio lessened by 26% due to mild and 41% due to severe stress; The function of APX enhanced by 21% due to mild and 28% due to severe stress; The activity of MDHAR and DHAR reduced by 25 and 32% under mild and 31 and 50%, under severe stress, respectively; Mild stress increased the activity of GR by 19% while severe stress increased by 16% | H2O2 level enhanced by 24% and 46% due to mild and severe stress. Similarly, O2− generation rate also raised in a dose-dependent manner | [122] |
| B. campestris L. cv. BARISharisha 9, B. napus L. cv. BARI Sharisha 13 and B. juncea L. cv. BARI Sharisha 16 | Cd (mild: 0.25 mM CdCl2, severer: 0.5 mM CdCl2), 3 days | Decreased level ofAsA, augmented level of DHA as well as decreased AsA/DHA ratio in all studied cultivars; GSH and GSSG level enhanced, but GSH/GSSG ratio lessened in all studied cultivars; APX and GR activities of all species increased significantly under both levels of Cd toxicity | Enhanced H2O2 level and O2− production rate in all tested cultivars in a concentration-dependent fashion | [136] |
| B. juncea L. BARI Sharisha-11 | Cd (mild: 0.5 mM CdCl2, severer: 1.0 mM CdCl2), 3 days | Reduced AsA content with higher DHA content and thus decreased AsA/DHA ratio; Increased GSH and GSSG levels as well as declined GSH/GSSG ratio; APX activity increased where GR increased at mild stress but remained unaltered at severe stress; Decreased MDHAR and DHAR activities | Enhanced the H2O2 and O2−level | [137] |
| V. radiata L. cv. BARI Mung-2 | Al (AlCl3, 0.5 mM), 48 and 72 h | Enhanced DHA content but reduced AsA level and AsA/DHA ratio; Increased level of GSH and GSSG but the diminished ratio of GSH/GSSG; Augmented APX activity but decreased MDHAR and DHAR activity | Enhanced H2O2 level by 83% and O2− generation rate by 110% | [50] |
| T. aestivum L. cv. Pradip | Pb [mild: 0.5 mM Pb(NO3)2, severer: 1.0 mM Pb(NO3)2], 2 days | AsA decreased in a dose-dependent manner; Mild stress improved the GSH level, but severe stress reduced it; Increased GSSG content; Increased APX activity; Diminished activity of MDHAR and DHAR in a concentration-dependent fashion; Mild stress improved GR activity but severe stress reduced it | Mild stress increased H2O2 levels by 41%, but severe stress enhanced it by 95% while O2− generation rate also increased in a dose-dependent manner | [35] |
| B. juncea L. cv. BARI Sharisha-11 | Cd (mild: 0.5 mM CdCl2, severer: 1.0 mM CdCl2), 3 days | AsA content decreased by 24% due to mild and 42% due to severe stress whereas DHA level enhanced by 79% due mild and 200% due to severe stress; Decreased AsA/DHA ratio in dose-dependent manner; GSH and GSSG content enhanced by 19% and 44%, respectively, due to mild stress, while only GSSG content enhanced due to severe stress by 72%; The ratio of GSH/GSSG declined by 17% due to mild and 43% due to severe stress; Enhanced APX by 15% due to mild and 24% due to severe stress; The activity of MDHAR and DHAR reduced by 12% and 14% due to mild stress whereas 17% and 24%, due to severe stress, respectively; The activity of GR enhanced under mild stress by 16% and lessened under severe stress by 9% | Level of H2O2 enhanced by 43% due to mild and 54% due to severe stress. Augmented O2− generation rate in a dose-dependent manner | [138] |
| B. juncea L. cv. varuna | Ni, (150 μM NiCl2.6H2O), 1 week | AsA content decreased by 61% whereas GSH and GSSG content increased by 75% and 151%, respectively; Enhanced function of APX by 60% and GR by 72%; DHAR and MDHAR activities were decreased by 62% and 65%, respectively | Increased H2O2 by 3.23-fold | [125] |
| Pisum sativum L. cv. Corne de Bélier | Pb (500 mg PbCl2 kg−1), 28 days | Increased APX and GR activity | Increased H2O2 content | [139] |
| O. sativa L. cv. BRRI dhan54 | Ni (0.25 mMand 0.5 mM NiSO4·7H2O) | Diminished content of AsA and enhanced content of DHA as well as the lessened ratio of AsA/DHA by 73% and 92% under 0.25 mM and 0.5 mM NiSO4·7H2O stress; GSH and GSSG level enhanced in a dose-dependent manner. However, the GSH/GSSG ratio reduced only under 0.5 mM NiSO4·7H2O treatment; Increased APX, MDHAR, DHAR and GR activity by 70%, 61%, 19% and 37% under 0.25 mM NiSO4·7H2O and 114%, 115%, 31% and 104% under 0.5 mM NiSO4·7H2O treatment, respectively | Increased H2O2 content by 28% and 35% due to 0.25 mM and 0.5 mM NiSO4·7H2O treatment | [68] |
| Capsicum annuum L.cv. Semerkand | Cd (0.1 mM CdCl2), 3 weeks | Enhanced AsA and GSH content | Increased H2O2 content | [140] |
| C. annuum L. cv. Semerkand | Pb (0.1 mM PbCl2), 3 weeks | Enhanced AsA and GSH content | Increased H2O2 content | [140] |
| Zea mays L. cv. Run Nong 35 and Wan Dan 13 | Cd (50 mg 3CdSO4·8H2O kg−1 soil), 6 months | Decreased GSH content | Increased accumulation of H2O2 | [141] |
| Plant Species | Stress Levels | Status of AsA-GSH Component(s) | ROS Mitigation | References |
|---|---|---|---|---|
| Actinidia deliciosa | 45 °C, 8 h | Increased content of AsA; Higher activity of APX, MDHAR, DHAR, and GR | Increased H2O2 content | [143] |
| Zea mays L. cv. Ludan No. 8 | 46 °C, 16 h | Decreased GSH, and GSSG content, but interestingly GSH/(GSH + GSSG) ratio increased; Reduced GR activity | - | [154] |
| Cinnamonum camphora | 40 °C, 2 days | Reduced AsA content with higher DHA content; Increased GSH and GSSG content; Enhanced the activities of APX, MDHAr, DHAR, and GR | Higher content of H2O2 and O2− | [166] |
| S. lycopersicum L. cv. Ailsa Craig | 40 °C, 9 h | Higher APX and GR activities by 74% and 45%, respectively | H2O2 content increased by 49% | [149] |
| S. lycopersicum L.cv. Boludo | 35 °C, 30 days | Increased the APX, DHAR and GR activities; Reduced the MDHAR activity | Increased H2O2 content | [119] |
| Vicia faba L. cv. C5 | 42 °C, 48 h | Enhanced the AsA, GSH ans GSSG content significantly; The enzymatic activity of APX and GR also enhanced | Extreme accumulation of O2− and H2O2 | [167] |
| V.radiata L. cv. BARI Mung-2 | 40 °C, 48 h | Decreased 64% in AsA/DHA ratio; GSSG pool increased; Higher APX (42%) and GR (50%) activities but declined activities of MDHAR (17%) and DHAR | Higher H2O2 content and O2−production rate | [50] |
| Z. mays cv. CML-32 and LM-11 | 40 °C, 72 h | Increased AsA content in both shoot and root of tolerant (CML-32) one, but unaffected in the susceptible (LM-11) one; Both APX and GR activity increased in roots of CML-32 but reduced in the shoot | Higher H2O2 accumulation, especially in shoots | [147] |
| L. esculentum Mill. cv. Puhong 968 | 38/28 °C day/night, 7 days | AsA+DHA and DHA increased by 220% and 99% respectively; AsA/DHA ratio decreased by 33%.; Higher GSSG (25%), but reduced GSH content (23.4%) and GSH/GSSG ratio (39%); APX, MDHAR, DHAR and GR activities declined | Enhanced O2− generation rate and H2O2 content by 129% and 33% respectively | [150] |
| Nicotiana tabacum cv. BY-2 | 35 °C, 7 days | Total GSH and AsA contents rose after 7 days heat stress; Increased MDHAR. DHAR and GR activities up to 72 h | The increasing trend of H2O2 generation was observed up to 72 h, and then a sharp decline occurred | [151] |
| Ficus concinna var. subsessilis | 35 °C and 40 °C, 48 h | AsA content reduced at 40 °C but GSH content similar to control at both 35 and 40 °C; DHA content enhanced by 49% at 35 °C and by 70% at 40 °C; APX activity increased by 51% and 30% at 35 °C and 40 °C; Activities of MDHAR, DHAR, and GR increased at 35 °C, but GR activity decreased by 34% at 40 °C | At 35 °C, 103% higher H2O2 content and 58% higher O2−production rate and at 40 °C those were 3.3- and 2.2-fold respectively | [148] |
| T. aestivum cv. Hindi62 and PBW343 | Heat stress environment, Late sown (Mid-January) | Higher activities of MDHAR and DHAR was observed in heat-tolerant (Hindi62) one whereas other enzyme activities seemed mostly to decline with time | The content of H2O2 was higher up to 14 DAA compared to non-stressed seedlings | [147] |
| G. hirsutum cv. Siza | Waterlogged pot for 3 days and 6 days | Increased content of AsA by 20% at 3 days and 30% at 6 days of waterlogging; Lower APX, MDHAR and GR activities | Enhanced O2− generation rate by 22 and 53% and H2O2 content by 10 and 39% at 3 and 6 days of waterlogging, respectively | [154] |
| Sesamum indicum L. cv. BARI Til-4 | Waterlogged pot by 2 cm standing water on the soil surface for 2, 4, 6 and 8 days | Reduced AsA content upto 38%; Enhanced GSH and GSSG content significantly; Increased APX and MDHAR activities; Reduced DHAR activity upto 59%; GR activity decreased upto 23% | Increased H2O2content sharply | [168] |
| Z. mays cv. Huzum-265 and Huzum-55 | Root portions waterlogged for 21 h | Reduced AsA content in both cultivars; Increased APX activity in both cultivars | - | [159] |
| Glycine max L. | Waterlogged pot for 14 days | GSH activity declined sharply in roots but shoots unaffected; Reduced GR activity in shoots but roots unaffected | - | [153] |
| Trifolium repens L. cv. Rivendel and T. pratense L. cv. Raya | 2 cm standing water on the soil surface for 14 days and 21 days | Increased contents of both oxidized and reduced AsA observed in both genotypes | Higher H2O2 generation in both genotypes | [158] |
| V. radiata L. cvs. T-44 and Pusa Baisakhi; and V. luteola | Pot filled with water to 1–2 cm height below the soil level, 8 days | Increased activities of both APX and GR in tolerant genotypes but in susceptible one, activities reduced | Reduced contents of O2−and H2O2 in susceptible (Pusa Baisakhi) cultivar | [156] |
| O. sativa L. MR219-4, MR219-9 and FR13A | Complete submergence for 4, 8 and 12 days | APX activity declined by 88% in FR13A under 4 days of submergence but decreased about 64 and 83% under 8 and 12 days of submergence; GR activity increased in FR13A and MR219-4 cultivars by 10- and 13-fold respectively after 8 days | - | [152] |
| Allium fistulosum L. cv. Erhan | Waterlogging (5 cm) at substrate surface for 10 days | Lower APX and GR activities | Increased rate of O2− generation by 240.4% and 289.8% higher H2O2 content | [157] |
| C. cajan L. genotypes ICPL 84,023 and ICP 7035 | Soil surface waterlogged (1–2 cm) for 6 days | Reduced APX and GR activities in susceptible genotype, which was higher in tolerant one | Lower accumulation of H2O2 and rate of O2− generation | [155] |
| S. melongena L. cv. EG117 and EG203 | Flooding with a water level of 5 cm, 72 h | Increased AsA content in susceptible EG117 genotype GSH content in both genotypes; Increased APX activity but decreased GR activity | - | [169] |
| S. lycopersicum cv. ASVEG and L4422 | Flooding with a water level of 5 cm, 72 h | Increase in both AsA and GSH contents; Non-significant changes in APX and GR activities | - | [169] |
| Lolium perenne | Grown in an area with high air pollution | APX and DHAR activities decreased while MDHAR and GR activities increased | A higher concentration of H2O2 in pollens | [162] |
| Populus deltoides × Populus nigra cvs. Carpaccio and Robusta | O3 treatment (120 nmol mol−1 for 13 h), 17 days | No impact on AsA and GSH contents; DHAR activity decreased while GR and MDHAR activity increased | - | [164] |
| Fragaria x anansa | High dose of carbon monoxide (CO) nitroxide (NOx) and sulfur dioxide (SO2) | The activity of both APX and GR increased upto medium dose but reduced under high dose | H2O2 content as well as O2− generation rate increased | [165] |
| O. sativa L. cvs. SY63 and WXJ14 | Continuous O3 exposure for up to 79 days | Both AsA and GSH contents are more likely to decrease; APX, MDHAR, DHAR, and GR activity increased up to 70 days of O3 exposure | Both O2− generation rate and H2O2 contents increased | [163] |
| Prosopis juliflora | Grown in the polluted industrial region | The content of AsA and APX activity increased under polluted environment | - | [161] |
| Erythrina orientalis | Grown in a polluted industrial area | Increased activities of both APX and GR enzymes recorded | - | [160] |
| T. aestivum L. cv. BARI Gom-26 | Acidic pH (4.5) of growing media | Increased AsA and GSH content; Improved redox balance of GSH/GSSG; Increased activity of APX, MDHAR, DHR, and GR | H2O2 contents increased by 209% | [170] |
| Enzymes | Gene | Gene Source | Target Plants | Regulatory Effects | References |
|---|---|---|---|---|---|
| APX | OsAPX2 | Rice | Alfalfa | Decreased H2O2 and MDA contents; Enhanced salt tolerance | [237] |
| APX | PcAPX | Populus tomentosa | Tobacco | Increased AsA content; NADP+/NADPH ratio; Decreased lipid peroxidation and H2O2 contents; Increased salt and drought stress tolerance | [230] |
| APX | CaAPX | Camellia azalea | Tobacco | Enhanced MDHAR and DHAR activity; Regulated ROS generation; Enhanced cold and HT tolerances | [238] |
| APX | ScAPX6 | Sugarcane | Nicotiana benthamiana | Hormonal regulation; Lower ROS generation; Enhanced tolerance to Cu stress | [239] |
| APX | OgCytAPX1 | Oncidium | Arabidopsis | Efficient ROS scavenging capacity; Maintained redox homeostasis and increased GPX activities which resulted in lower H2O2 | [240] |
| MDHAR | Am-MDAR | Avicennia marina | A. marina | Increase in MDHAR and GR activity; Increased AsA content; Decreased lipid peroxidation; Improved salt-induced oxidative stress | [241] |
| MDHAR | MgMDHAR | Malpighia glabra | Tobacco | DHAR activity increased by 1.8–2.1 fold; AsA/DHA increased by 81–84%; Lipid peroxidation decreased by 41–62% | [232] |
| MDHAR | BrMDHAR | Rapeseed | Arabidopsis | AsA/DHA ratio increased by 7%; Decrease of H2O2 content by 55%; Radical scavenging was 16% higher | [233] |
| DHAR | DHAR | Human | Tobacco | No changes in AsA content; Enhanced tolerance to chilling and salt stress | [242] |
| DHAR | DHAR1 | Rice | Arabidopsis | AsA content increased by 1.4 fold; Enhanced tolerance to salt | [243] |
| DHAR | DHAR | Human | Tobacco | Increase in AsA content; Increased the activities of SOD and APX; Enhanced salt tolerance | [244] |
| DHAR | DHAR | Arabidopsis | Arabidopsis | AsA content increased by 2.0–4.25 fold; Enhanced tolerance to HT stress | [245] |
| DHAR | DHAR | Rice | Tobacco | AsA content increased by 1.6-fold; Improved tolerance to salinity and chilling | [246] |
| DHAR | BrDHAR | Rapeseed | Arabidopsis | AsA/DHA ratio increased by 11%; H2O2 content decreased by 56%; Radical scavenging was 16% higher | [233] |
| GR | AtGR1 | Arabidopsis | Arabidopsis | Enhanced GSH content and GR activity (0.4- to 1.0-fold higher); H2O2 content reduced by 26% | {234] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. https://doi.org/10.3390/antiox8090384
Hasanuzzaman M, Bhuyan MHMB, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants. 2019; 8(9):384. https://doi.org/10.3390/antiox8090384
Chicago/Turabian StyleHasanuzzaman, Mirza, M. H. M. Borhannuddin Bhuyan, Taufika Islam Anee, Khursheda Parvin, Kamrun Nahar, Jubayer Al Mahmud, and Masayuki Fujita. 2019. "Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress" Antioxidants 8, no. 9: 384. https://doi.org/10.3390/antiox8090384
APA StyleHasanuzzaman, M., Bhuyan, M. H. M. B., Anee, T. I., Parvin, K., Nahar, K., Mahmud, J. A., & Fujita, M. (2019). Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants, 8(9), 384. https://doi.org/10.3390/antiox8090384









