Optimization of the Extraction of Bioactive Compounds from Walnut (Juglans major 209 x Juglans regia) Leaves: Antioxidant Capacity and Phenolic Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Materials
2.3. Extraction
2.4. Box-Behnken Experimental Design
2.5. Analytical Techniques
3. Results and Discussion
3.1. Effect of the Solid/Liquid Ratio on Extraction Yield and Extract Antioxidant Activity
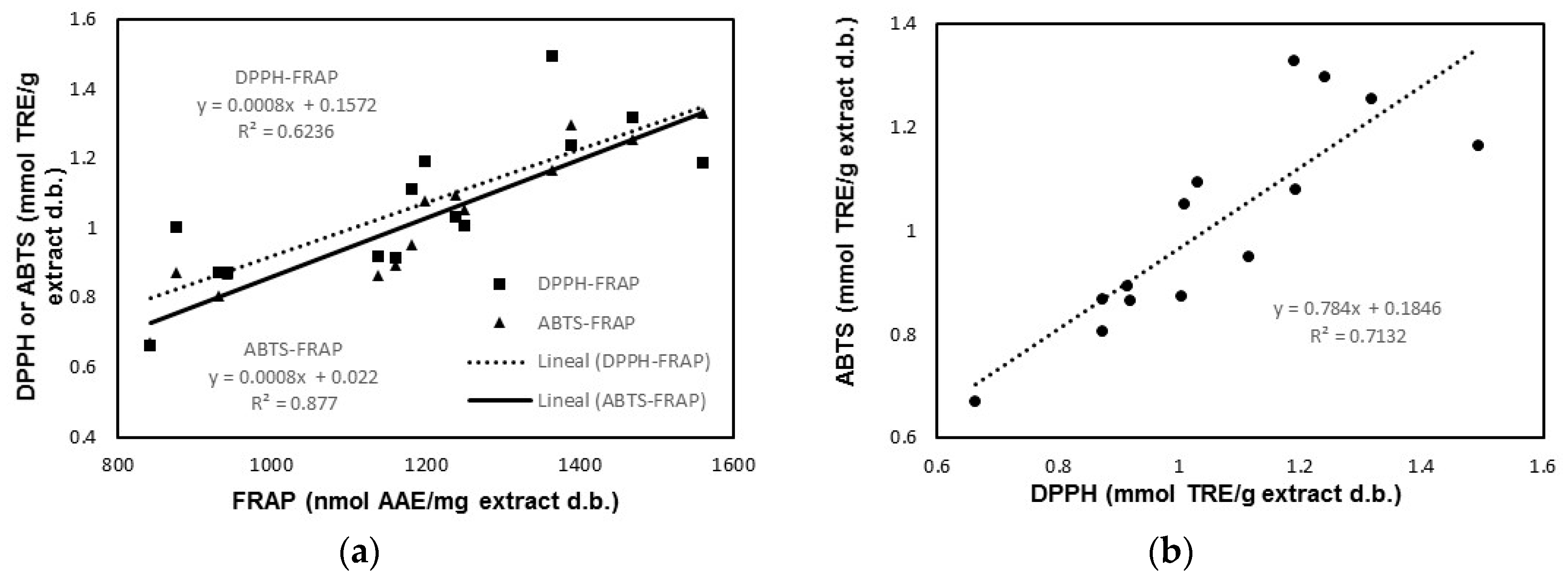
3.2. Relationship between Extract Antioxidant Properties
3.3. Optimization of the Extraction Conditions by a Box-Behnken Design
3.4. Characterization of the Optimum Walnut Leaf Extract
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentao, P.; Andrade, P.B.; Ferreira, I.C.F.R.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.F.; Fernandes, E.; Lima, J.L.F.C.; Costa, P.C.; Bahia, M.F. Walnut (Juglans regia) leaf extracts are strong scavengers of pro-oxidant reactive species. Food Chem. 2008, 106, 1014–1020. [Google Scholar] [CrossRef]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jerónimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Barros, L.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Leaves and decoction of Juglans regia L.: Different performances regarding bioactive compounds and in vitro antioxidant andantitumor effects. Ind. Crops Prod. 2013, 51, 430–436. [Google Scholar] [CrossRef]
- Amaral, J.S.; Seabra, R.M.; Andrade, P.B.; Valentão, P.; Pereira, J.A.; Ferreres, F. Phenolic profile in the quality control of walnut (Juglans regia L.) leaves. Food Chem. 2004, 88, 373–379. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.P.; Ferreira, O.; Ferreira, I.C.F.R. Optimization and comparison of maceration and microwave extraction systems for the production of phenolic compounds from Juglans regia L. for the valorization of walnut leaves. Ind. Crops Prod. 2017, 107, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Piwowarska, N.; González-Alvarez, J. Extraction of antioxidants from forestry biomass: Kinetics and optimization of extraction conditions. Biomass Bioenerg. 2012, 43, 42–51. [Google Scholar] [CrossRef]
- Vázquez, G.; Fernández-Agulló, A.; Gómez-Castro, C.; Freire, M.S.; Antorrena, G.; González-Álvarez, J. Response surface optimization of antioxidants extraction from chestnut (Castanea sativa) bur. Ind. Crops Prod. 2012, 35, 126–134. [Google Scholar] [CrossRef]
- Xavier, L.; Freire, M.S.; González-Álvarez, J. Modeling and optimizing the solid–liquid extraction of phenolic compounds from lignocellulosic subproduct. Biomass Conv. Bioref. 2019, 9, 737–747. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Szöllösi, R.; Szöllösi-Varga, I. Total antioxidant power in some species of Labiatae (adaptation of FRAP method). Acta Biol. Szeged. 2002, 46, 125–127. [Google Scholar]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Xiao, L.; Wu, M.; Apaliya, M.T. Impact of extraction parameters and their optimization on the nutraceuticals and antioxidant properties of aqueous extract mulberry leaf. Int. J. Food Prop. 2018, 21, 717–732. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Li, D.; Wang, L.-J.; Ozkan, N.; Chen, X.D.; Mao, Z.-H.; Yang, H.-Z. Optimization of ethanol–water extraction of lignans from flaxseed. Sep. Purif. Technol. 2007, 57, 17–24. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Ho, S.K.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [Google Scholar] [CrossRef]
- Jokic, S.; Velic, D.; Bilic, M.; Bucic-Kojic, A.; Planinic, M.; Tomas, S. Modelling of the process of solid- liquid extraction of total polyphenols from soybeans. Czech J. Food Sci. 2010, 28, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentao, P.; Andrade, P.B.; González-Álvarez, J.; Pereira, J.A. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crops Prod. 2013, 42, 126–132. [Google Scholar]
- Valentová, K.; Vrba, J.; Bancírˇová, M.; Ulrichová, J.; Krˇen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, A.L.; Berti, F.; Navarini, L.; Monteiro, A.; Resmini, M.; Forzato, C. Synthesis of p-coumaroylquinic acids and analysis of their interconversion. Tetrahedron Asymmetry 2017, 28, 419–427. [Google Scholar] [CrossRef]
- Jin, U.H.; Lee, J.Y.; Kang, S.K.; Kim, J.K.; Park, W.H.; Kim, J.G.; Moon, S.K.; Kim, C.H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of Euonymus. Life Sci. 2005, 77, 2760–2769. [Google Scholar] [CrossRef]






| Exp. | S/L Ratio (g/mL) | Extraction Yield (%) | FRAP (nmol AAE/mg Extract) |
|---|---|---|---|
| A | 1/5 | 25.82 ± 0.25 a | 1246 ± 48 a |
| B | 1/7.5 | 27.04 ± 0.42 b | 1350 ± 53 b |
| C | 1/10 | 27.86 ± 0.04 b | 1512 ± 61 c |
| Exp | Y1 exp | Y1 pred | Y2 exp | Y2 pred | Y3 exp | Y3 pred | Y4 exp | Y4 pred | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 21.03 ± 0.89 | 22.35 | 1181 ± 39 | 1262 | 1.114 ± 0.026 | 1.050 | 0.951 ± 0.026 | 0.964 |
| 2 | −1 | 0 | −1 | 20.35 ± 0.18 | 19.86 | 842 ± 46 | 908 | 0.663 ± 0.044 | 0.666 | 0.671 ± 0.027 | 0.774 |
| 3 | −1 | 0 | 1 | 19.15 ± 2.13 | 18.71 | 930 ± 47 | 908 | 0.873 ± 0.013 | 0.852 | 0.807 ± 0.017 | 0.774 |
| 4 | −1 | 1 | 0 | 23.89 ± 0.28 | 24.16 | 1250 ± 58 | 1262 | 1.008 ± 0.159 | 1.050 | 1.053 ± 0.049 | 0.964 |
| 5 | 0 | −1 | −1 | 23.13 ± 0.27 | 22.85 | 1160 ± 37 | 1041 | 0.913 ± 0.024 | 0.932 | 0.893 ± 0.041 | 0.875 |
| 6 | 0 | −1 | 1 | 20.03 ± 1.16 | 19.96 | 1138 ± 17 | 1041 | 0.919 ± 0.039 | 0.932 | 0.866 ± 0.050 | 0.875 |
| 7 | 0 | 1 | −1 | 24.52 ± 0.11 | 24.66 | 943 ± 65 | 1041 | 0.873 ± 0.037 | 0.932 | 0.869 ± 0.030 | 0.875 |
| 8 | 0 | 1 | 1 | 21.93 ± 1.76 | 21.77 | 876 ± 18 | 1041 | 1.003 ± 0.036 | 0.932 | 0.875 ± 0.028 | 0.875 |
| 9 | 1 | −1 | 0 | 29.08 ± 1.43 | 28.40 | 1364 ± 42 | 1529 | 1.493 ± 0.106 | 1.396 | 1.166 ± 0.047 | 1.250 |
| 10 | 1 | 0 | −1 | 27.01 ± 0.30 | 27.64 | 1199 ± 50 | 1174 | 1.193 ± 0.021 | 1.198 | 1.089 ± 0.035 | 1.060 |
| 11 | 1 | 0 | 1 | 22.35 ± 0.88 | 23.02 | 1238 ± 32 | 1174 | 1.031 ± 0.023 | 1.012 | 1.095 ± 0.032 | 1.060 |
| 12 | 1 | 1 | 0 | 30.17 ± 0.09 | 30.21 | 1468 ± 20 | 1529 | 1.318 ± 0.049 | 1.396 | 1.256 ± 0.017 | 1.250 |
| 13 | 0 | 0 | 0 | 25.85 ± 0.05 | 26.28 | 1389 ± 78 | 1395 | 1.239 ± 0.043 | 1.223 | 1.299 ± 0.049 | 1.315 |
| 14 | 0 | 0 | 0 | 27.19 ± 0.51 | 26.28 | 1560 ± 49 | 1395 | 1.190 ± 0.031 | 1.223 | 1.330 ± 0.032 | 1.315 |
| Independent Variables | Levels | ||||||||||
| −1 | 0 | +1 | |||||||||
| x1, Temperature (°C) | 25 | 50 | 75 | ||||||||
| x2, Time (min) | 30 | 75 | 120 | ||||||||
| x3, % EtOH | 10 | 50 | 90 | ||||||||
| Compound | Linear Range (mg/L) | Calibration Curve | R2 |
|---|---|---|---|
| (–)-Gallocatechin | 1–200 | y = 8068x + 17,932 | 0.9941 |
| Catechin hydrate | 1–200 | y = 15,461x + 52,943 | 0.9958 |
| Chlorogenic acid | 1–1000 | y = 8181x + 22,563 | 0.9936 |
| Ellagic acid | 1–1000 | y = 9647x + 38,987 | 0.9959 |
| Epicatechin | 1–1000 | y = 9780x + 17,102 | 0.9976 |
| Ferulic acid | 1–200 | y = 3455x + 22,085 | 0.9936 |
| Gallic acid | 1–1000 | y = 3814x + 4250 | 0.9977 |
| Isorharmnetin | 1–200 | y = 61,453x + 78,552 | 0.9915 |
| Kaempferol | 1–200 | y = 61,712x + 75,935 | 0.9923 |
| Neochlorogenic acid | 1–200 | y = 10,675x + 15,125 | 0.9989 |
| p-Coumaric acid | 1–200 | y = 4442x + 4535 | 0.9972 |
| Procyanidin B2 | 1–200 | y = 4014x + 7252 | 0.9925 |
| Quercetin | 1–1000 | y = 45,006x + 111,541 | 0.9922 |
| Quercetin 3-β-d-glucoside | 1–1000 | y = 13,239x + 42,498 | 0.9836 |
| Taxifolin | 1–200 | y = 21,398x + 28,956 | 0.9903 |
| Y1 | Y2 | Y3 | Y4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coeff. | SE | p | Coeff | SE | p | Coeff. | SE | p | Coeff. | SE | p | |
| a0 | 26.281 | 0.274 | 0.000 | 1395.250 | 40.672 | 0.000 | 1.223 | 0.019 | 0.000 | 1.315 | 0.028 | 0.000 |
| a1 | 3.024 | 0.274 | 0.000 | 13.25 | 40.672 | 0.006 | 0.173 | 0.019 | 0.000 | 0.143 | 0.019 | 0.000 |
| a2 | 0.905 | 0.274 | 0.008 | - | - | NS | - | - | NS | - | - | NS |
| a3 | −1.444 | 0.274 | 0.000 | - | - | NS | - | - | NS | - | - | NS |
| a12 | - | - | NS | - | - | NS | - | - | NS | - | - | NS |
| a13 | −0.865 | 0.387 | 0.049 | - | - | NS | −0.093 | 0.027 | 0.005 | - | - | NS |
| a23 | - | - | NS | - | - | NS | - | - | NS | - | - | NS |
| a11 | - | - | NS | - | - | NS | - | - | NS | −0.083 | 0.028 | 0.012 |
| a22 | - | - | NS | - | - | NS | - | - | NS | −0.125 | 0.028 | 0.001 |
| a33 | −3.973 | 0.387 | 0.000 | −354.500 | 57.519 | 0.000 | −0.291 | 0.027 | 0.000 | −0.315 | 0.028 | 0.000 |
| R2 | 0.964 | 0.789 | 0.947 | 0.951 | ||||||||
| R2corr. | 0.947 | 0.757 | 0.934 | 0.933 | ||||||||
| SE | 0.774 | 115.038 | 0.053 | 0.055 | ||||||||
| p | 0.000 | 0.000 | 0.000 | 0.000 | ||||||||
| Compound | Retention Time (min) | m/z | ng/mg Extract d.b. | mg/100 g Leaves d.b. |
|---|---|---|---|---|
| (–)-Gallocatechin | 2.4 | 305.06 | 14.17 | 0.45 |
| Catechin hydrate | 2.6 | 289.07 | 55.83 | 1.78 |
| Chlorogenic acid | 2.7 | 353.09 | 5737.50 | 183.14 |
| Ellagic Acid | 4 | 300.99 | 68.92 | 2.20 |
| Epicatechin | 2.8 | 289.07 | 12.08 | 0.39 |
| Ferulic acid | 3.6 | 193.04 | 158.58 | 5.06 |
| Gallic acid | 2.3 | 169.01 | 432.58 | 13.81 |
| Isorharmnetin | 5.4 | 315.05 | 3.17 | 0.10 |
| Kaempferol | 5.3 | 285.04 | 14.25 | 0.45 |
| Neochlorogenic acid | 2.5 | 353.08 | 9125.00 | 291.27 |
| p-Coumaric ccid | 3.6 | 163.04 | 10.83 | 0.35 |
| Procyanidin B2 | 2.6 | 577.14 | 45.50 | 1.45 |
| Quercetin | 4.8 | 301.04 | 163.50 | 5.22 |
| Quercetin 3-β-d-glucoside | 3.8 | 463.09 | 15,441.67 | 492.90 |
| Taxifolin | 3.4 | 303.05 | 4.50 | 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Agulló, A.; Castro-Iglesias, A.; Freire, M.S.; González-Álvarez, J. Optimization of the Extraction of Bioactive Compounds from Walnut (Juglans major 209 x Juglans regia) Leaves: Antioxidant Capacity and Phenolic Profile. Antioxidants 2020, 9, 18. https://doi.org/10.3390/antiox9010018
Fernández-Agulló A, Castro-Iglesias A, Freire MS, González-Álvarez J. Optimization of the Extraction of Bioactive Compounds from Walnut (Juglans major 209 x Juglans regia) Leaves: Antioxidant Capacity and Phenolic Profile. Antioxidants. 2020; 9(1):18. https://doi.org/10.3390/antiox9010018
Chicago/Turabian StyleFernández-Agulló, Adela, Aída Castro-Iglesias, María Sonia Freire, and Julia González-Álvarez. 2020. "Optimization of the Extraction of Bioactive Compounds from Walnut (Juglans major 209 x Juglans regia) Leaves: Antioxidant Capacity and Phenolic Profile" Antioxidants 9, no. 1: 18. https://doi.org/10.3390/antiox9010018
APA StyleFernández-Agulló, A., Castro-Iglesias, A., Freire, M. S., & González-Álvarez, J. (2020). Optimization of the Extraction of Bioactive Compounds from Walnut (Juglans major 209 x Juglans regia) Leaves: Antioxidant Capacity and Phenolic Profile. Antioxidants, 9(1), 18. https://doi.org/10.3390/antiox9010018






