Antibacterial Activity of Synthetic Cationic Iron Porphyrins
Abstract
:1. Introduction
2. Materials and Methods
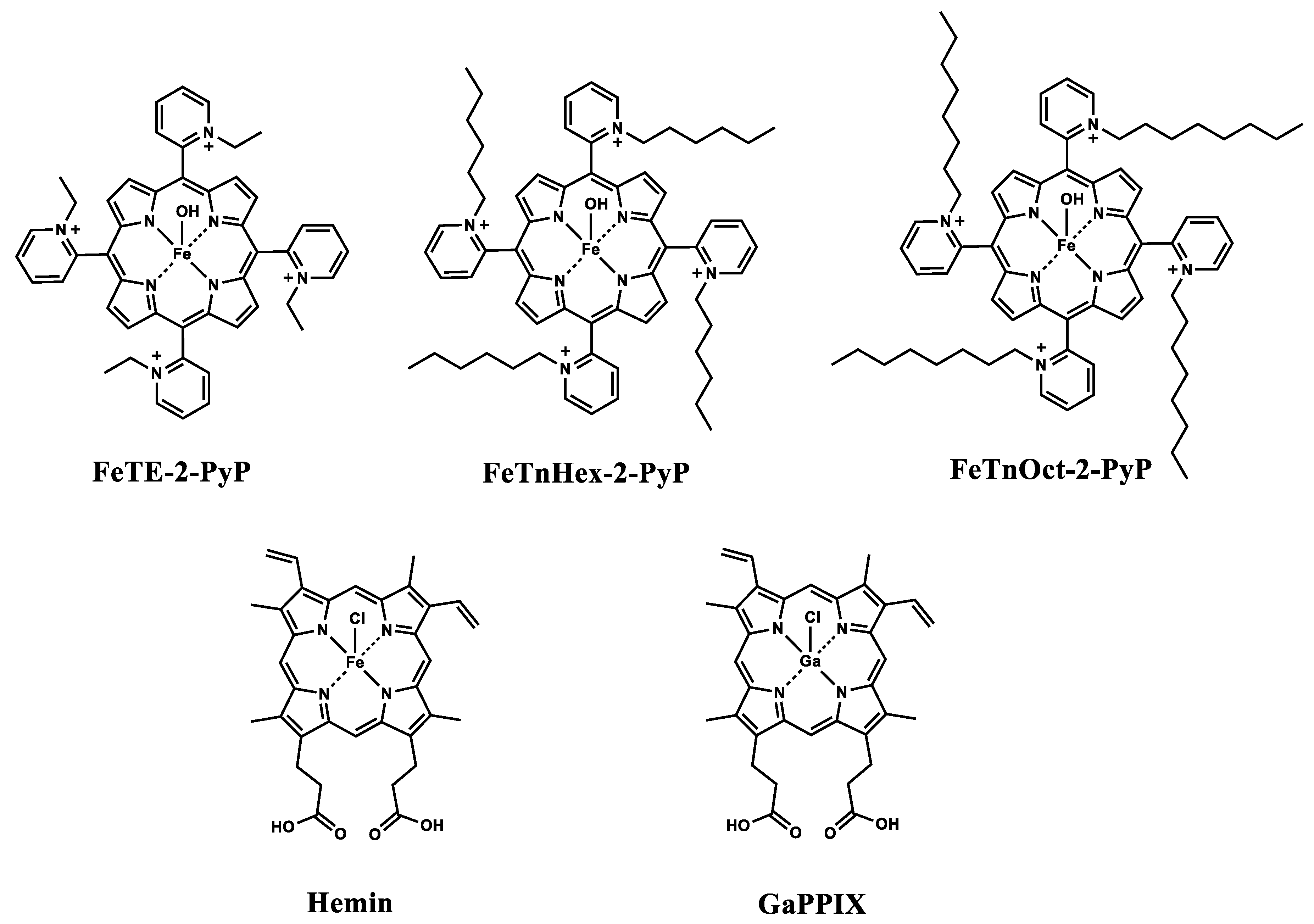
2.1. Metalloporphyrins
2.2. Strains and Growth Conditions
2.3. Uptake and Accumulation of FePs in E. coli
2.4. Oxygen Consumption by Bacterial Suspensions
2.5. Data Analysis
3. Results
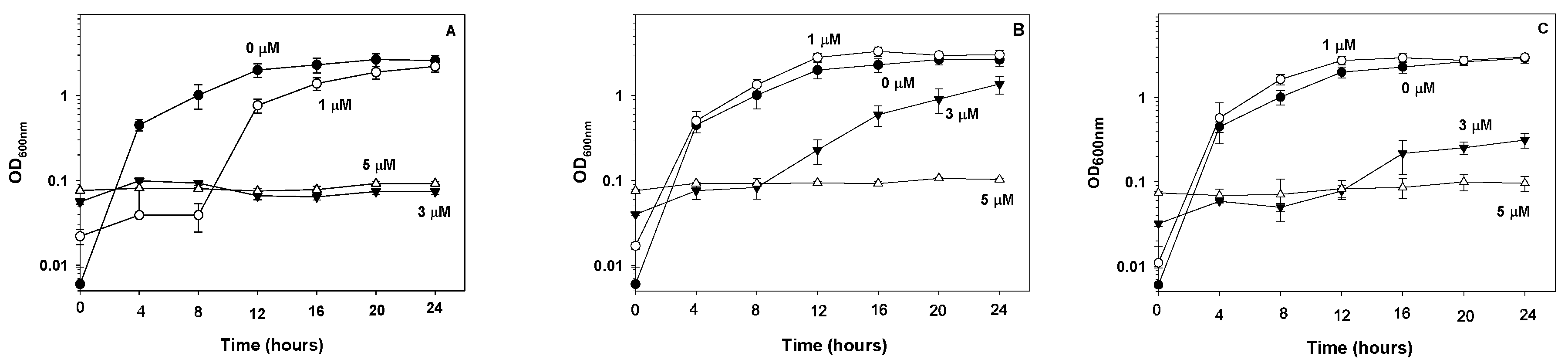
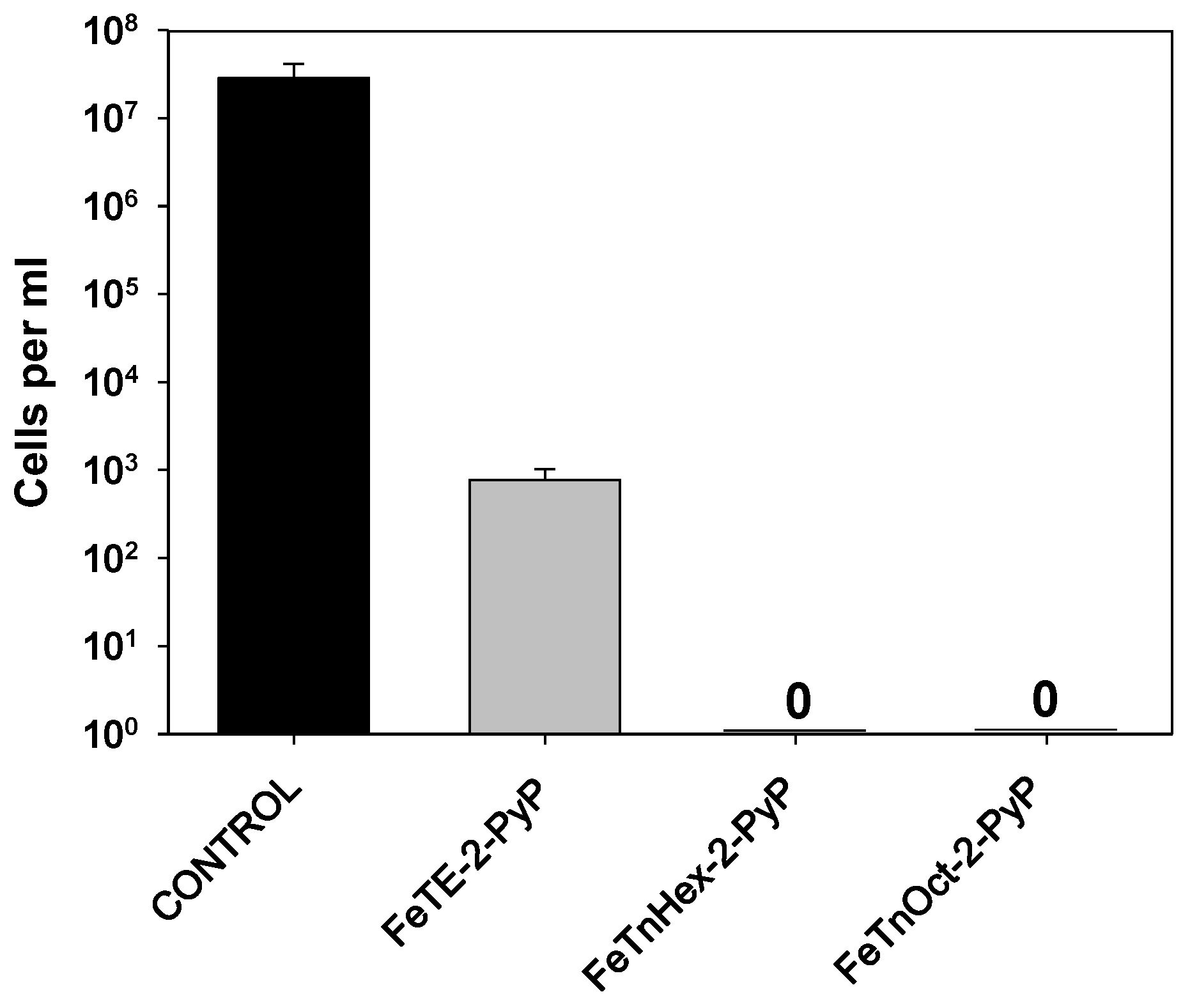
3.1. Effect of FePs on E. coli Proliferation and Viability
3.2. Comparison of FeTnHex-2-PyP with GaPPIX and Hemin
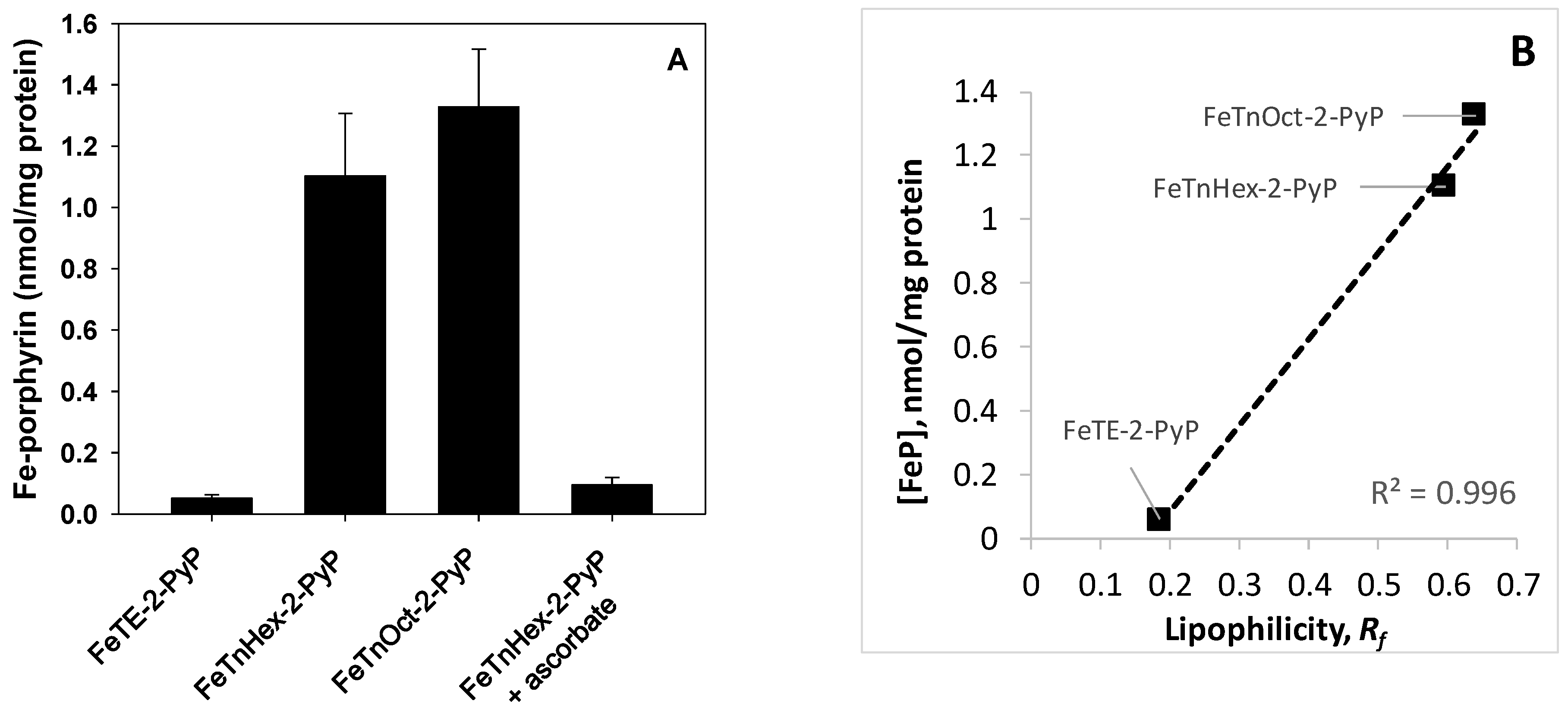
3.3. Cellular Uptake of Fe-Porphyrins
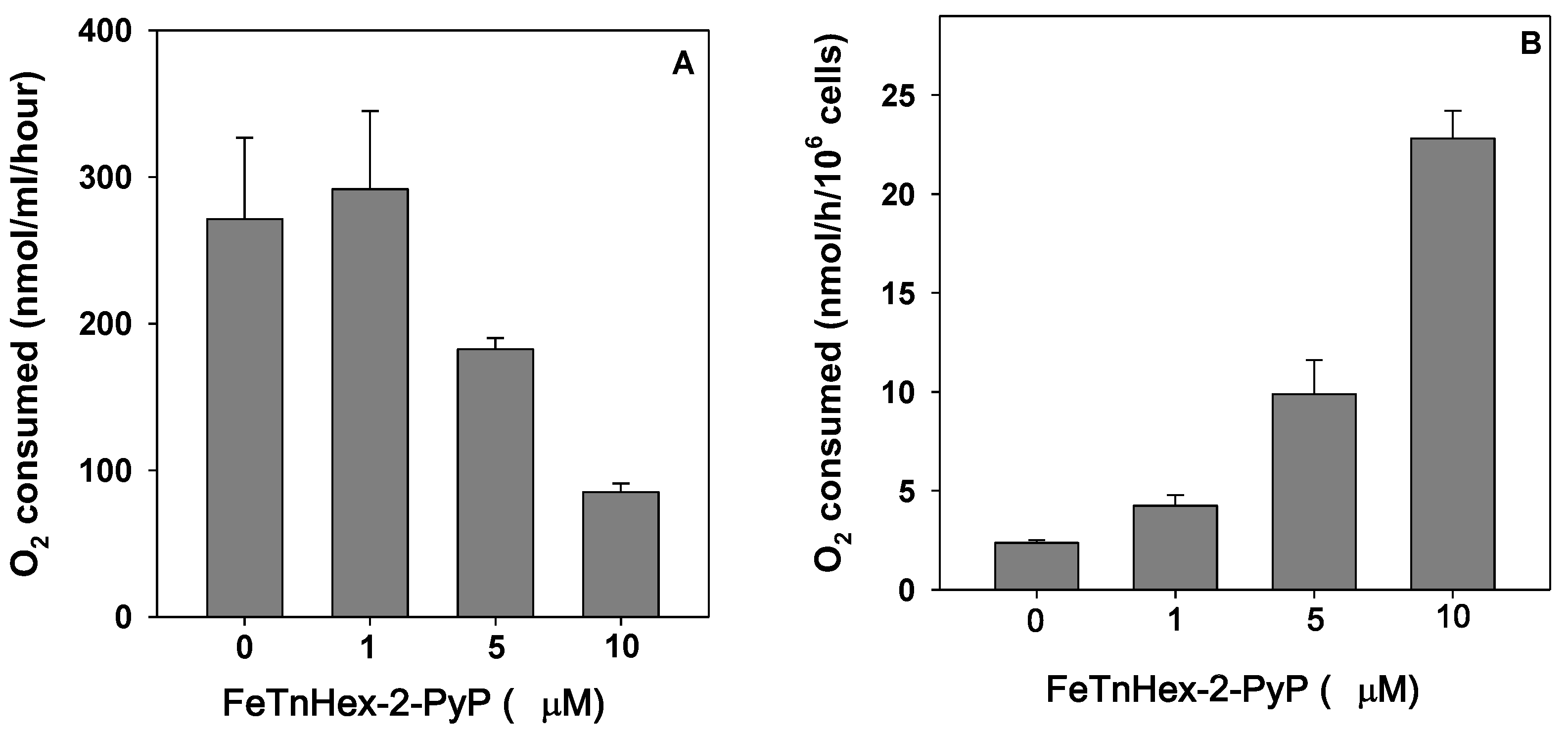
3.4. Effect of FeTnHex-2-PyP on Oxygen Consumption
3.5. Superoxide Radical, Hydrogen Peroxide, and FeP Decomposition
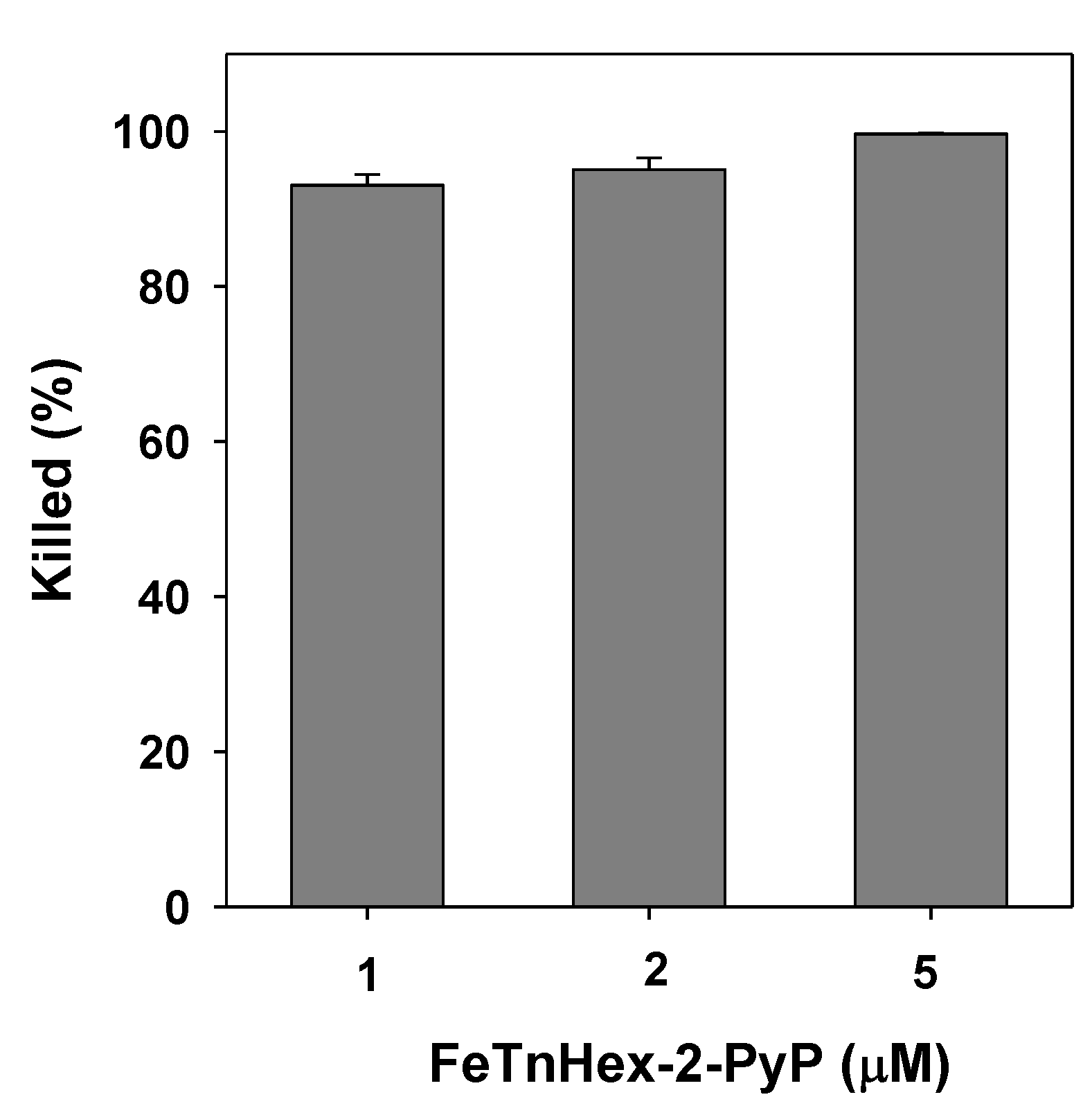
3.6. Bactericidal Action of FeTnHex-2-PyP against S. aureus
3.7. Resistance to Antibiotics and FeP Bactericidal Effect
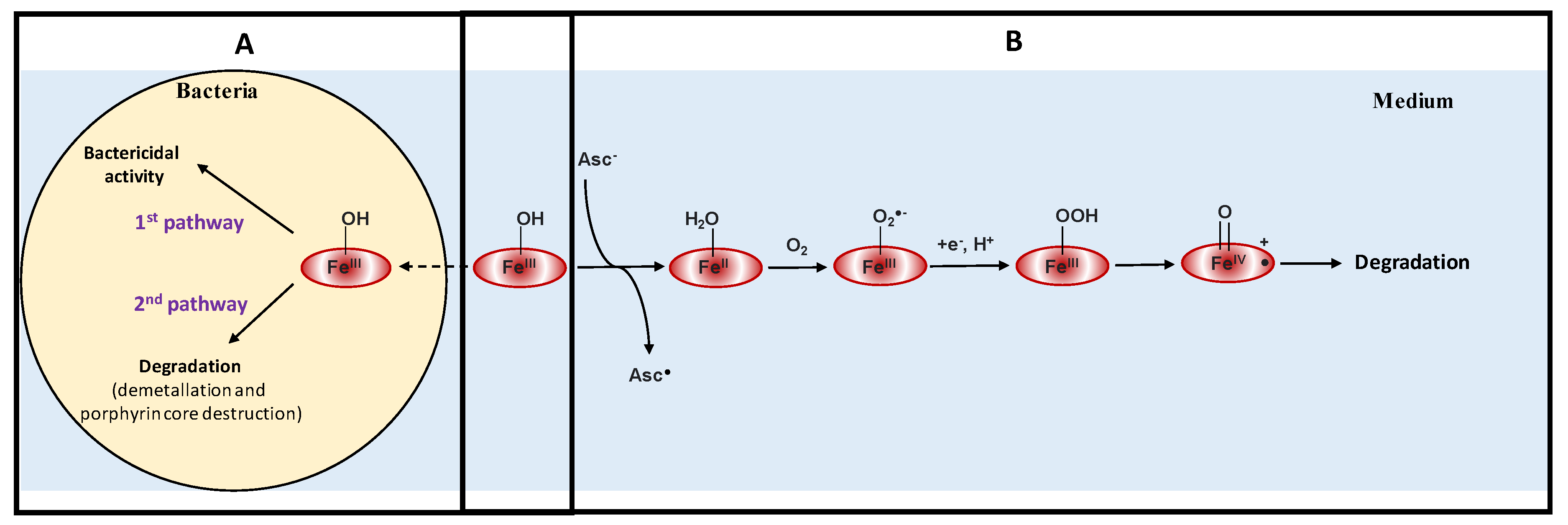
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Antimicrobial Resistance: Global Report on Surveillance 2014; WHO Press: Geneva, Switzerlad, 2014; ISBN 978 92 4 156474 8. [Google Scholar]
- Coates, A.R.M.; Hu, Y. Novel approaches to developing new antibiotics for bacterial infections. Br. J. Pharmacol. 2007, 152, 1147–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J., Jr. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the infectious diseases society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Tillotson, G.S. Antibiotic development: A victim of market forces? IDrugs 2008, 11, 340–345. [Google Scholar] [PubMed]
- Maragakis, L.L.; Perencevich, E.N.; Cosgrove, S.E. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 2008, 6, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. Resistance patterns among nosocomial pathogens: Trends over the past few years. Chest 2001, 119, 397S–404S. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, I.; Evavold, B.D.; Kumar, V. Antimicrobial properties of porphyrins. Expert Opin. Investig. Drugs 2001, 10, 309–320. [Google Scholar] [CrossRef]
- Thomas, M.; Craik, J.D.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L.T. Amphiphilic cationic Zn-porphyrins with high photodynamic antimicrobial activity. Future Microbiol. 2015, 10, 709–724. [Google Scholar] [CrossRef]
- Awad, M.M.; Tovmasyan, A.; Craik, J.D.; Batinic-Haberle, I.; Benov, L.T. Important cellular targets for antimicrobial photodynamic therapy. Appl. Microbiol. Biot. 2016, 100, 7679–7688. [Google Scholar] [CrossRef]
- Alenezi, K.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L.T. Optimizing Zn porphyrin-based photosensitizers for efficient antibacterial photodynamic therapy. Photodiagn. Photodyn. Ther. 2017, 17, 154–159. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Kashef, N.; Huang, Y.Y.; Hamblin, M.R. Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Stojiljkovic, I.; Kumar, V.; Srinivasan, N. Non-iron metalloporphyrins: Potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol. Microbiol. 1999, 31, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Bozja, J.; Yi, K.; Shafer, W.M.; Stojiljkovic, I. Porphyrin-based compounds exert antibacterial action against the sexually transmitted pathogens Neisseria gonorrhoeae and Haemophilus ducreyi. Int. J. Antimicrob. Agents 2004, 24, 578–584. [Google Scholar] [CrossRef]
- Ni, Y. Metalloporphyrins and functional analogues as MRI contrast agents. Curr. Med. Imaging Rev. 2008, 4, 96–112. [Google Scholar] [CrossRef] [Green Version]
- Crow, J.P. Catalytic antioxidants to treat amyotropic lateral sclerosis. Expert Opin. Investig. Drugs 2006, 15, 1383–1393. [Google Scholar] [CrossRef]
- Orrell, R.W. AEOL-10150 Aeolus. Curr. Opin. Investig. Drugs 2006, 7, 70–80. [Google Scholar]
- Kinnula, V.L. Production and degradation of oxygen metabolites during inflammatory states in the human lung. Curr. Drug Targets Inflamm. Allergy 2005, 4, 465–470. [Google Scholar] [CrossRef]
- Dennery, P.A. Metalloporphyrins for the treatment of neonatal jaundice. Curr. Opin. Pediatrics 2005, 17, 167–169. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Spasojevic, I. 25 years of development of Mn porphyrins-from mimics of superoxide dismutase enzymes to thiol signaling to clinical trials: The story of our life in the USA. J. Porphyr. Phthalocyanines 2019, 23, 1326–1335. [Google Scholar] [CrossRef] [Green Version]
- Peters, K.; Kirkpatrick, J.; Batinic-Haberle, I.; Affronti, M.; Woodring, S.; Iden, D.; Panta, S.; Lipp, E.; Healy, P.; Herndon, J.; et al. ACTR-28. PHASE 1 Dose Escalation Trial Of The Safety Of Bmx-001 Concurrent With Radiation Therapy And Temozolomide In Newly Diagnosed Patients With High-Grade Gliomas. Neuro-Oncology 2018, 20, vi17. [Google Scholar] [CrossRef]
- Peters, K.B.; Kirkpatrick, J.P.; Batinic-Haberle, I.; Affronti, M.L.; Woodring, S.; Iden, D.; Lipp, E.S.; Boyd, K.; Healy, P.; Herndon, J.; et al. First in Human Clinical Trial of a Metalloporphyrin Dual Radioprotectant and Radiosensitizer, BMX-001, in Newly Diagnosed High-Grade Glioma Undergoing Concurrent Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, E106. [Google Scholar] [CrossRef] [Green Version]
- Tovmasyan, A.; Weitner, T.; Sheng, H.X.; Lu, M.M.; Rajic, Z.; Warner, D.S.; Spasojevic, I.; Reboucas, J.S.; Benov, L.; Batinic-Haberle, I. Differential Coordination Demands in Fe versus Mn Water-Soluble Cationic Metalloporphyrins Translate into Remarkably Different Aqueous Redox Chemistry and Biology. Inorg. Chem. 2013, 52, 5677–5691. [Google Scholar] [CrossRef] [Green Version]
- Batinić-Haberle, I.; Spasojević, I.; Hambright, P.; Benov, L.; Cmmbliss, A.L.; Fridovich, I. Relationship among redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vivo and in vitro superoxide dismutating activities of manganese(III) and iron(III) water-soluble porphyrins. Inorg. Chem. 1999, 38, 4011–4022. [Google Scholar] [CrossRef]
- Ohse, T.; Nagaoka, S.; Arakawa, Y.; Kawakami, H.; Nakamura, K. Cell death by reactive oxygen species generated from water-soluble cationic metalloporphyrins as superoxide dismutase mimics. J. Inorg. Biochem. 2001, 85, 201–208. [Google Scholar] [CrossRef]
- Li, L.; Tovmasyan, A.; Sheng, H.; Xu, B.; Sampaio, R.S.; Reboucas, J.S.; Warner, D.S.; Batinic-Haberle, I.; Spasojevic, I. Fe Porphyrin-Based SOD Mimic and Redox-Active Compound, (OH)FeTnHex-2-PyP4+, in a Rodent Ischemic Stroke (MCAO) Model: Efficacy and Pharmacokinetics as Compared to Its Mn Analogue, (H2O)MnTnHex-2-PyP5+. Antioxidants 2020, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.I.; Benov, L.; Touati, D.; Fridovich, I. Induction of the soxRS regulon of Escherichia coli by superoxide. J. Biol. Chem. 1999, 274, 9479–9481. [Google Scholar] [CrossRef] [Green Version]
- Varghese, S.; Wu, A.; Park, S.; Imlay, K.R.C.; Imlay, J.A. Submicromolar hydrogen peroxide disrupts the ability of fur protein to control free-iron levels in Escherichia coli. Mol. Microbiol. 2007, 64, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Seaver, L.C.; Imlay, J.A. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J. Biol. Chem. 2004, 279, 48742–48750. [Google Scholar] [CrossRef] [Green Version]
- Udo, E.E.; Jacob, L.E.; Mathew, B. A cadmium resistance plasmid, pXU5, in Staphylococcus aureus, strain ATCC25923. FEMS Microbiol. Lett. 2000, 189, 79–80. [Google Scholar] [CrossRef]
- Tovmasyan, A.; Reboucas, J.S.; Benov, L. Simple biological systems for assessing the activity of superoxide dismutase mimics. Antioxid. Redox Signal. 2014, 20, 2416–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kos, I.; Benov, L.; Spasojević, I.; Rebouças, J.S.; Batinić-Haberle, I. High lipophilicity of meta Mn(III) N-alkylpyridylporphyrin-based superoxide dismutase mimics compensates for their lower antioxidant potency and makes them as effective as ortho analogues in protecting superoxide dismutase-deficient Escherichia coli. J. Med. Chem. 2009, 52, 7868–7872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Benov, L. Effect of growth media on the MTT colorimetric assay in bacteria. PLoS ONE 2019, 14, e0219713. [Google Scholar] [CrossRef] [PubMed]
- Moghnie, S.; Tovmasyan, A.; Craik, J.; Batinic-Haberle, I.; Benov, L. Cationic amphiphilic Zn-porphyrin with high antifungal photodynamic potency. Photochem. Photobiol. Sci. 2017, 16, 1709–1716. [Google Scholar] [CrossRef]
- Tovmasyan, A.G.; Rajic, Z.; Spasojevic, I.; Reboucas, J.S.; Chen, X.; Salvemini, D.; Sheng, H.; Warner, D.S.; Benov, L.; Batinic-Haberle, I. Methoxy-derivatization of alkyl chains increases the in vivo efficacy of cationic Mn porphyrins. Synthesis, characterization, SOD-like activity, and SOD-deficient E. coli study of meta Mn(iii) N-methoxyalkylpyridylporphyrins. Dalton Trans. 2011, 40, 4111–4121. [Google Scholar] [CrossRef]
- Batinić-Haberle, I.; Rajić, Z.; Benov, L. A combination of two antioxidants (an SOD mimic and ascorbate) produces a pro-oxidative effect Forcing Escherichia coli to adapt via induction of oxyR regulon. Anti-Cancer Agents Med. Chem. 2011, 11, 329–340. [Google Scholar]
- Liochev, S.I. The role of iron-sulfur clusters in in vivo hydroxyl radical production. Free Radic. Res. 1996, 25, 369–384. [Google Scholar] [CrossRef]
- Anzaldi, L.L.; Skaar, E.P. Overcoming the Heme Paradox: Heme Toxicity and Tolerance in Bacterial Pathogens. Infect. Immun. 2010, 78, 4977–4989. [Google Scholar] [CrossRef] [Green Version]
- Atamna, H.; Ginsburg, H. Heme degradation in the presence of glutathione. A proposed mechanism to account for the high levels of non-heme iron found in the membranes of hemoglobinopathic red blood cells. J. Biol. Chem. 1995, 270, 24876–24883. [Google Scholar] [CrossRef] [Green Version]
- Tovmasyan, A.; Maia, C.G.C.; Weitner, T.; Carballal, S.; Sampaio, R.S.; Lieb, D.; Ghazaryan, R.; Ivanovic-Burmazovic, I.; Ferrer-Sueta, G.; Radi, R.; et al. A comprehensive evaluation of catalase-like activity of different classes of redox-active therapeutics. Free Radic. Biol. Med. 2015, 86, 308–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovmasyan, A.; Bueno-Janice, J.C.; Jaramillo, M.C.; Sampaio, R.S.; Reboucas, J.S.; Kyui, N.; Benov, L.; Deng, B.; Huang, T.T.; Tome, M.E.; et al. Radiation-Mediated Tumor Growth Inhibition Is Significantly Enhanced with Redox-Active Compounds That Cycle with Ascorbate. Antioxid. Redox Signal. 2018, 29, 1196–1214. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Tome, M.E. Thiol regulation by Mn porphyrins, commonly known as SOD mimics. Redox Biol. 2019, 25, 101139. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Rifkind, J.M. Heme degradation by reactive oxygen species. Antioxid. Redox Signal. 2004, 6, 967–978. [Google Scholar]
- Han, A.R.; Jin Jeong, Y.; Kang, Y.; Yoon Lee, J.; Sook Seo, M.; Nam, W. Direct evidence for an iron(IV)-oxo porphyrin π-cation radical as an active oxidant in catalytic oxygenation reactions. Chem. Commun. 2008, 1076–1078. [Google Scholar] [CrossRef]
- Spasojevic, I.; Colvin, O.M.; Warshany, K.R.; Batinic-Haberle, I. New approach to the activation of anti-cancer pro-drugs by metalloporphyrin-based cytochrome P450 mimics in all-aqueous biologically relevant system. J. Inorg. Biochem. 2006, 100, 1897–1902. [Google Scholar] [CrossRef]
- Scheidt, W.R.; Reed, C.A. Spin-State/Stereochemical Relationships in Iron Porphyrins: Implications for the Hemoproteins. Chem. Rev. 1981, 81, 543–555. [Google Scholar] [CrossRef]
- McIntosh, J.A.; Heel, T.; Buller, A.R.; Chio, L.; Arnold, F.H. Structural Adaptability Facilitates Histidine Heme Ligation in a Cytochrome P450. J. Am. Chem. Soc. 2015, 137, 13861–13865. [Google Scholar] [CrossRef]
- Li, T.; Bonkovsky, H.L.; Guo, J.T. Structural analysis of heme proteins: Implications for design and prediction. BMC Struct. Biol. 2011, 11, 13. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tovmasyan, A.; Batinic-Haberle, I.; Benov, L. Antibacterial Activity of Synthetic Cationic Iron Porphyrins. Antioxidants 2020, 9, 972. https://doi.org/10.3390/antiox9100972
Tovmasyan A, Batinic-Haberle I, Benov L. Antibacterial Activity of Synthetic Cationic Iron Porphyrins. Antioxidants. 2020; 9(10):972. https://doi.org/10.3390/antiox9100972
Chicago/Turabian StyleTovmasyan, Artak, Ines Batinic-Haberle, and Ludmil Benov. 2020. "Antibacterial Activity of Synthetic Cationic Iron Porphyrins" Antioxidants 9, no. 10: 972. https://doi.org/10.3390/antiox9100972
APA StyleTovmasyan, A., Batinic-Haberle, I., & Benov, L. (2020). Antibacterial Activity of Synthetic Cationic Iron Porphyrins. Antioxidants, 9(10), 972. https://doi.org/10.3390/antiox9100972






