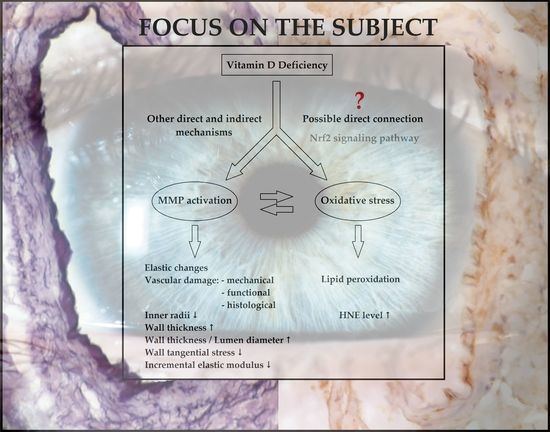
Vitamin D Deficiency Induces Elevated Oxidative and Biomechanical Damage in Coronary Arterioles in Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Vitro Pressure Microarteriography of Intramural Coronary Arterioles
2.3. Biomechanical Calculations
- -
- Outer radius/Ro (μm):
- -
- Inner radius/Ri (μm):
- -
- Wall thickness/h (μm):h = Ro − Ri
- -
- Wall thickness/Lumen diameter ratio:where Di is the lumen or inner diameter.
- -
- Wall cross-sectional area/Aw (μm2):
- -
- Tangential stress/σTang (kPa):where p is the intraluminal pressure.
- -
- Incremental elastic modulus/EInc:where ΔP is the change in intraluminal pressure and ΔRo is the outer radius change in response to ΔP.
- -
- Distensibility/D:where ΔV is the change in lumen volume relative to the initial volume V in response to pressure change (ΔP).
- -
- Myogenic tone (%):
2.4. Histology and Immunohistochemistry of Coronary Arterioles
2.5. Statistical Analysis
3. Results
3.1. Physiological Parameters
3.2. Geometry of Coronary Arterioles
3.3. Elasticity of Coronary Arterioles
3.4. Myogenic Tone of Coronary Arterioles
3.5. Histology and Immunohistochemistry of Coronary Arterioles
4. Discussion, Limitations, and Strengths
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid. Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.; Carlberg, C. Vitamin D and evolution: Pharmacologic implications. Biochem. Pharmacol. 2020, 173, 113595. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Extraskeletal actions of vitamin D. Ann. N. Y. Acad. Sci. 2016, 1376, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Thuesen, B.H.; Linneberg, A. Vitamin D, Cardiovascular Disease and Risk Factors. Adv. Exp. Med. Biol. 2017, 996, 221–230. [Google Scholar]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Trehan, N.; Afonso, L.; Levine, D.L.; Levy, P.D. Vitamin D Deficiency, Supplementation, and Cardiovascular Health. Crit. Pathways Cardiol. 2017, 16, 109–118. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Annweiler, C.; Duval, G.; Karras, S.; Tirabassi, G.; Salvio, G.; Balercia, G.; Kimball, S.; Kotsa, K.; Mascitelli, L.; et al. Vitamin D and cardiovascular disease: From atherosclerosis to myocardial infarction and stroke. Int. J. Cardiol. 2017, 230, 577–584. [Google Scholar] [CrossRef]
- Mozos, I.; Marginean, O. Links between Vitamin D Deficiency and Cardiovascular Diseases. Biomed. Res. Int. 2015, 2015, 109275. [Google Scholar] [CrossRef]
- Beveridge, L.A.; Khan, F.; Struthers, A.D.; Armitage, J.; Barchetta, I.; Bressendorff, I.; Cavallo, M.G.; Clarke, R.; Dalan, R.; Dreyer, G.; et al. Effect of Vitamin D Supplementation on Markers of Vascular Function: A Systematic Review and Individual Participant Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008273. [Google Scholar] [CrossRef] [PubMed]
- Matrai, M.; Mericli, M.; Nadasy, G.L.; Szekeres, M.; Varbiro, S.; Bánhidy, F.; Ács, N.; Monos, E.; Szekacs, B. Gender differences in biomechanical properties of intramural coronary resistance arteries of rats, an in vitro microarteriographic study. J. Biomech. 2007, 40, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Matrai, M.; Hetthéssy, J.; Nadasy, G.L.; Monos, E.; Szekacs, B.; Varbiro, S. Sex differences in the biomechanics and contractility of intramural coronary arteries in angiotensin II-induced hypertension. Gend. Med. 2012, 9, 548–556. [Google Scholar] [CrossRef]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef]
- Sun, Q.; Shi, L.; Rimm, E.; Giovanucci, E.; Hu, F.; Manson, J.; Rexrode, K.M. Vitamin D intake and risk of cardiovascular disease in US men and women. Am. J. Clin. Nutr. 2011, 94, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Kienreich, K.; Tomaschitz, A.; Verheyen, N.; Pieber, T.; Gaksch, M.; Grübler, M.R.; Pilz, S. Vitamin D and cardiovascular disease. Nutrients 2013, 5, 3005–3021. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- Hadjadj, L.; Várbíró, S.; Horváth, E.M.; Monori-Kiss, A.; Pál, É.; Karvaly, G.B.; Heinzlmann, A.; Magyar, A.; Szabo, I.; Sziva, R.E.; et al. Insulin resistance in an animal model of polycystic ovary disease is aggravated by vitamin D deficiency: Vascular consequences. Diab. Vasc. Dis. Res. 2018, 15, 294–301. [Google Scholar] [CrossRef]
- Pál, É.; Hadjadj, L.; Fontanyi, Z.; Monori-Kiss, A.; Mezei, Z.; Lippai, N.; Magyar, A.; Heinzlmann, A.; Karvaly, G.; Monos, E.; et al. Vitamin D deficiency causes inward hypertrophic remodeling and alters vascular reactivity of rat cerebral arterioles. PLoS ONE 2018, 13, e0192480. [Google Scholar] [CrossRef]
- Nadasy, G.L.; Szekeres, M.; Dézsi, L.; Varbiro, S.; Szekacs, B.; Monos, E. Preparation of intramural small coronary artery and arteriole segments and resistance artery networks from the rat heart for microarteriography and for in situ perfusion video mapping. Microvasc. Res. 2001, 61, 282–286. [Google Scholar] [CrossRef]
- Mulvany, M.J. Mechanical and other factors involved in vascular injury related to hypertension. Blood Press. Suppl. 1994, 1, 11–17. [Google Scholar] [PubMed]
- Mulvany, M.J.; Hansen, O.K.; Aalkjaer, C. Direct evidence that the greater contractility of resistance vessels in spontaneously hypertensive rats is associated with a narrowed lumen, a thickened media, and an increased number of smooth muscle cell layers. Circ. Res. 1978, 43, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, L.; Monori-Kiss, A.; Horváth, E.M.; Heinzlmann, A.; Magyar, A.; Sziva, R.E.; Miklós, Z.; Pál, É.; Gál, J.; Szabó, I.; et al. Geometric, elastic and contractile-relaxation changes in coronary arterioles induced by Vitamin D deficiency in normal and hyperandrogenic female rats. Microvasc. Res. 2019, 122, 78–84. [Google Scholar] [CrossRef]
- Li, K.; Tay, F.R.; Yiu, C.K.Y. The past, present and future perspectives of matrix metalloproteinase inhibitors. Pharmacol. Ther. 2020, 207, 107465. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef]
- Nsengiyumva, V.; Krishna, S.M.; Moran, C.S.; Moxon, J.V.; Morton, S.; Clarke, M.; Seto, S.-W.; Golledge, J. Vitamin D deficiency promotes large rupture-prone abdominal aortic aneurysms and cholecalciferol supplementation limits progression of aneurysms in a mouse model. Clin. Sci. 2020, 134, 2521–2534. [Google Scholar] [CrossRef] [PubMed]
- Aliashrafi, S.; Ebrahimi-Mameghani, M.; Jafarabadi, M.A.; Lotfi-Dizaji, L.; Vaghef-Mehrabany, E.; Arefhosseini, S. Effect of high-dose vitamin D supplementation in combination with weight loss diet on glucose homeostasis, insulin resistance, and matrix metalloproteinases in obese subjects with vitamin D deficiency: A double-blind, placebo-controlled, randomized clinical trial. Appl. Physiol. Nutr. Metab. 2019, 45, 1–7. [Google Scholar]
- Henriet, P.; Emonard, H. Matrix metalloproteinase-2: Not. (just) a “hero” of the past. Biochimie 2019, 166, 223–232. [Google Scholar] [CrossRef]
- Ebrahim, H.F.; Hamid, F.F.A.; A Haykal, M.; Soliman, A.F. Cyclophilin A and matrix metalloproteinase-9: Their relationship, association with, and diagnostic relevance in stable coronary artery disease. Vascular 2020, 28, 212–221. [Google Scholar] [CrossRef]
- Guizani, I.; Zidi, W.; Zayani, Y.; Boudiche, S.; Hadj-Taieb, S.; Sanhaji, H.; Zaroui, A.; Mechmeche, R.; Mourali, M.S.; Feki, M.; et al. Matrix metalloproteinase-3 predicts clinical cardiovascular outcomes in patients with coronary artery disease: A 5 years cohort study. Mol. Biol. Rep. 2019, 46, 4699–4707. [Google Scholar] [CrossRef]
- Bhowmick, S.; D’Mello, V.; Caruso, D.; Abdul-Muneer, P. Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J. Mol. Med. 2019, 97, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Gongora, E. Oxidative Stress and Cardiovascular Aging: Interaction Between NRF-2 and ADMA. Curr. Cardiol. Rev. 2017, 13, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.K.; Goh, B.H.; Yap, W.H. Oxidative Stress in Cardiovascular Diseases: Involvement of Nrf2 Antioxidant Redox Signaling in Macrophage Foam Cells Formation. Int. J. Mol. Sci. 2017, 18, 2336. [Google Scholar] [CrossRef] [PubMed]
- Steed, M.M.; Tyagi, S.C. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid. Redox Signal 2011, 15, 1927–1943. [Google Scholar] [CrossRef]
- Saad El-Din, S.; Rashed, L.; Medhat, E.; Aboulhoda, B.E.; Badawy, A.D.; ShamsEldeen, A.M.; Abdelgwad, M. Active form of vitamin D analogue mitigates neurodegenerative changes in Alzheimer’s disease in rats by targeting Keap1/Nrf2 and MAPK-38p/ERK signaling pathways. Steroids 2020, 156, 108586. [Google Scholar] [CrossRef]
- Yao, B.; He, J.; Yin, X.; Shi, Y.; Wan, J.; Tian, Z. The protective effect of lithocholic acid on the intestinal epithelial barrier is mediated by the vitamin D receptor via a SIRT1/Nrf2 and NF-kappaB dependent mechanism in Caco-2 cells. Toxicol. Lett. 2019, 316, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, L.; Pál, É.; Monori-Kiss, A.; Sziva, R.E.; Korsós-Novák, Á.; Horváth, E.M.; Benkő, R.; Magyar, A.; Magyar, P.; Benyó, Z.; et al. Vitamin D deficiency and androgen excess result eutrophic remodeling and reduced myogenic adaptation in small cerebral arterioles in female rats. Gynecol. Endocrinol. 2019, 35, 529–534. [Google Scholar] [CrossRef]
- Garcia, S.R.; Izzard, A.S.; Heagerty, A.; Bund, S.J. Myogenic tone in coronary arteries from spontaneously hypertensive rats. J. Vasc. Res. 1997, 34, 109–116. [Google Scholar] [CrossRef]
- DiBona, G.F.; Mark, A.L. Bjorn Folkow. Hypertension 2013, 61, 4. [Google Scholar] [CrossRef][Green Version]
- Folkow, B. “Structural factor” in primary and secondary hypertension. Hypertension 1990, 16, 89–101. [Google Scholar] [CrossRef]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Pál, E.; Hadjadj, L.; Fontányi, Z.; Monori-Kiss, A.; Lippai, N.; Horváth, E.M.; Magyar, A.; Monos, E.; Nádasy, G.L.; Benyó, Z.; et al. Gender, hyperandrogenism and vitamin D deficiency related functional and morphological alterations of rat cerebral arteries. PLoS ONE 2019, 14, e0216951. [Google Scholar] [CrossRef]
- Mirhosseini, N.Z.; Knaus, S.J.; Bohaychuk, K.; Singh, J.; Vatanparast, H.A.; Weber, L.P. Both high and low plasma levels of 25-hydroxy vitamin D increase blood pressure in a normal rat model. Br. J. Nutr. 2016, 116, 1889–1900. [Google Scholar] [CrossRef]
- Gou, S.H.; Liu, B.-J.; Han, X.-F.; Wang, L.; Zhong, C.; Liang, S.; Liu, H.; Qiang, Y.; Zhang, Y.; Ni, J.M. Anti-atherosclerotic effect of Fermentum Rubrum and Gynostemma pentaphyllum mixture in high-fat emulsion- and vitamin D3-induced atherosclerotic rats. J. Chin. Med. Assoc. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Pang, J.; Xu, Q.; Xu, X.; Yin, H.; Xu, R.; Guo, S.; Hao, W.; Wang, L.; Chen, C.; Cao, J.-M. Hexarelin suppresses high lipid diet and vitamin D3-induced atherosclerosis in the rat. Peptides 2010, 31, 630–638. [Google Scholar] [CrossRef]
- Si, J.; Li, K.; Shan, P.; Hu, W. The combined presence of hypertension and vitamin D deficiency increased the probability of the occurrence of small vessel disease in China. BMC Neurol. 2019, 19, 164. [Google Scholar] [CrossRef]
- Steffensen, I.L.; Dirven, H.; Couderq, S.; David, A.; D’Cruz, S.C.; Fernández, M.F.; Mustieles, V.; Rodríguez-Carrillo, A.; Hofer, T. Bisphenols and Oxidative Stress Biomarkers-Associations Found. in Human Studies, Evaluation of Methods Used, and Strengths and Weaknesses of the Biomarkers. Int. J. Environ. Res. Public Health 2020, 17, 3609. [Google Scholar] [CrossRef]
- Keeney, J.T.R.; Förster, S.; Sultana, R.; Brewer, L.D.; Latimer, C.S.; Cai, J.; Klein, J.B.; Porter, N.M.; Butterfield, D.A. Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: Implications for low vitamin D-dependent age-related cognitive decline. Free Radic. Biol. Med. 2013, 65, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Lajtai, K.; Nagy, C.T.; Tarszabó, R.; Benkő, R.; Hadjadj, L.; Sziva, R.E.; Gerszi, D.; Bányai, B.; Ferdinandy, P.; Nadasy, G.L.; et al. Effects of Vitamin D Deficiency on Proliferation and Autophagy of Ovarian and Liver Tissues in a Rat Model. of Polycystic Ovary Syndrome. Biomolecules 2019, 9, 471. [Google Scholar] [CrossRef]
- Luczaj, W.; Gegotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Coccia, R.; Perluigi, M.; Pupo, G.; Schininà, M.E.; Giorgi, A.; Blarzino, C.; Nappi, F.; Sutherland, F.W.; Chello, M.; et al. Redox proteomic analysis of serum from aortic anerurysm patients: Insights on oxidation of specific protein target. Mol. Biosyst. 2016, 12, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Argacha, J.-F.; Egrise, D.; Pochet, S.; Fontaine, D.; Lefort, A.; Libert, F.; Goldman, S.; Van De Borne, P.; Berkenboom, G.; Moreno-Reyes, R. Vitamin D deficiency-induced hypertension is associated with vascular oxidative stress and altered heart gene expression. J. Cardiovasc. Pharmacol. 2011, 58, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Chapple, S.J.; Cheng, X.; Mann, G.E. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013, 1, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ouyang, P.; De Boer, I.H.; Lutsey, P.L.; Farag, Y.M.; Guallar, E.; Siscovick, D.S.; Post, W.S.; Kalyani, R.R.; Billups, K.L.; et al. Serum vitamin D and sex hormones levels in men and women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2017, 96, 95–102. [Google Scholar] [CrossRef] [PubMed][Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sziva, R.E.; Fontányi, Z.; Pál, É.; Hadjadj, L.; Monori-Kiss, A.; Horváth, E.M.; Benkő, R.; Magyar, A.; Heinzlmann, A.; Benyó, Z.; et al. Vitamin D Deficiency Induces Elevated Oxidative and Biomechanical Damage in Coronary Arterioles in Male Rats. Antioxidants 2020, 9, 997. https://doi.org/10.3390/antiox9100997
Sziva RE, Fontányi Z, Pál É, Hadjadj L, Monori-Kiss A, Horváth EM, Benkő R, Magyar A, Heinzlmann A, Benyó Z, et al. Vitamin D Deficiency Induces Elevated Oxidative and Biomechanical Damage in Coronary Arterioles in Male Rats. Antioxidants. 2020; 9(10):997. https://doi.org/10.3390/antiox9100997
Chicago/Turabian StyleSziva, Réka Eszter, Zoltán Fontányi, Éva Pál, Leila Hadjadj, Anna Monori-Kiss, Eszter Mária Horváth, Rita Benkő, Attila Magyar, Andrea Heinzlmann, Zoltán Benyó, and et al. 2020. "Vitamin D Deficiency Induces Elevated Oxidative and Biomechanical Damage in Coronary Arterioles in Male Rats" Antioxidants 9, no. 10: 997. https://doi.org/10.3390/antiox9100997
APA StyleSziva, R. E., Fontányi, Z., Pál, É., Hadjadj, L., Monori-Kiss, A., Horváth, E. M., Benkő, R., Magyar, A., Heinzlmann, A., Benyó, Z., Nádasy, G. L., & Várbíró, S. (2020). Vitamin D Deficiency Induces Elevated Oxidative and Biomechanical Damage in Coronary Arterioles in Male Rats. Antioxidants, 9(10), 997. https://doi.org/10.3390/antiox9100997