Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning
Abstract
1. Oxidative Damage in the Inner Ear
1.1. Noise-Induced Hearing Loss
1.2. Age-Related Hearing Loss
1.3. Ototoxicity
1.4. Sudden Hearing Loss and Immune-Mediated Hearing Loss
2. Antioxidant Therapies for ROS-Induced Inner Ear Damage
2.1. N-Acetylcysteine (NAC)
2.2. Sodium Thiosulfate (STS)
2.3. d-Methionine
2.4. Alpha-Lipoic Acid
2.5. Amifostine
2.6. Ebselen
2.7. Flunarizine
2.8. Other Antioxidants
3. Antioxidant Therapeutic Mechanisms in the Inner Ear
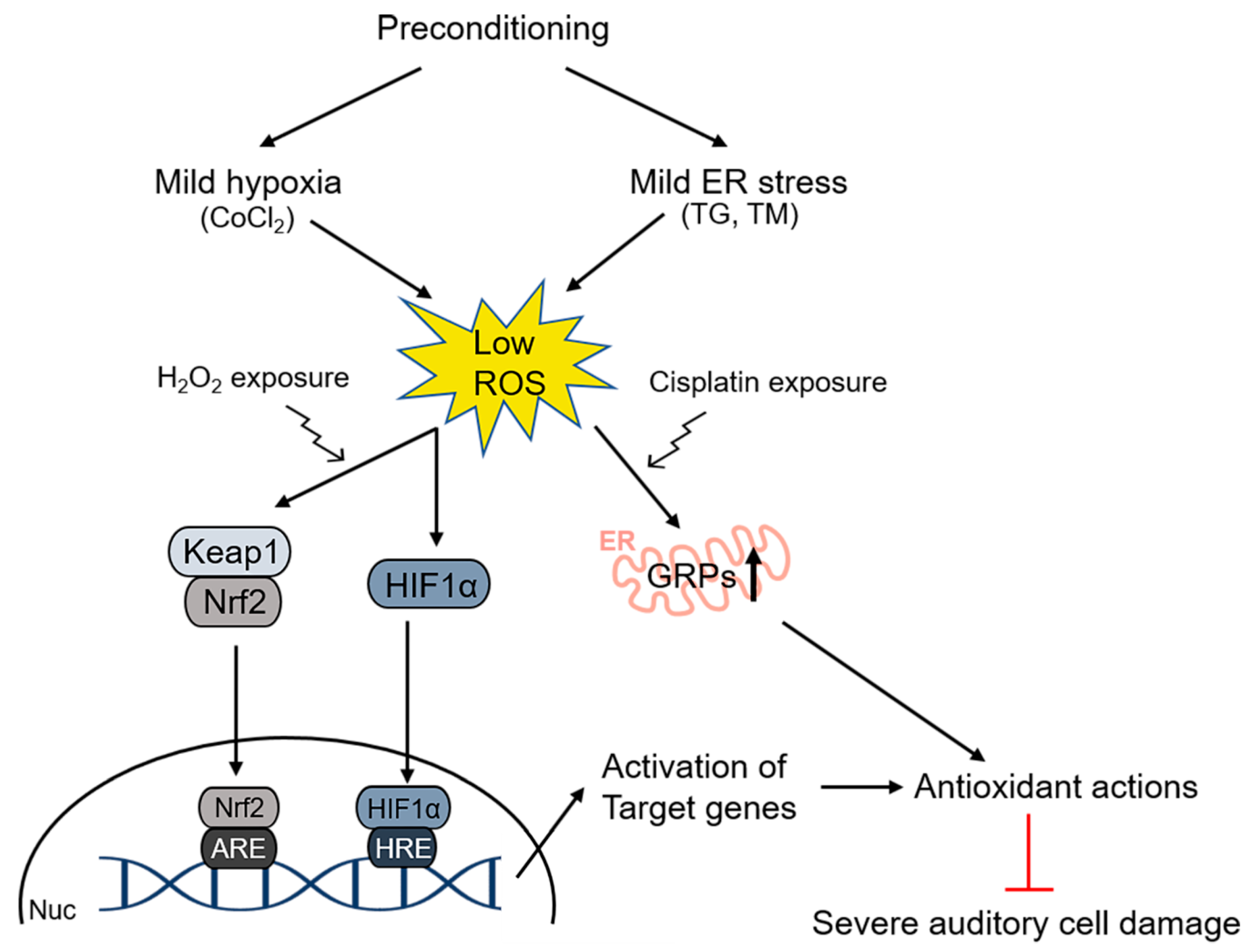
4. Preconditioning Effects in the Inner Ear
4.1. Preconditioning by Sound in Noise-Induced Hearing Loss
4.2. Preconditioning by Hyperthermia and Restraint
4.3. Hypoxic Preconditioning
4.4. Unfolded Protein Response (UPR)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Im, G.J.; Ahn, J.H.; Lee, J.H.; do Han, K.; Lee, S.H.; Kim, J.-S.; Jang, H.; Chung, J.W. Prevalence of severe-profound hearing loss in South Korea: A nationwide population-based study to analyse a 10-year trend (2006–2015). Sci. Rep. 2018, 8, 9940. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Lee, D.; Lim, H.J.; Park, M.K.; Suh, M.W.; Lee, J.H.; Hong, Y.-C.; Oh, S.-H. Hearing loss in Korean adolescents: The prevalence thereof and its association with leisure noise exposure. PLoS ONE 2019, 14, e0209254. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Oh, S.-H.; Jang, H.; Lee, J.-B.; Chung, J.W. Impact of hearing loss on the performance of auditory processing measured by questionnaires in Korean adolescents. Sci. Rep. 2020, 10, 10118. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Predoehl, F.; Starr, A. Review of hair cell synapse defects in sensorineural hearing impairment. Otol. Neurotol. 2013, 34, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Kang, H.H.; Shin, J.E.; Kim, J.U. Accumulation of hypoxia-inducible factor-1α in mouse inner ear by noise stimulation. Neuroreport 2004, 15, 2353–2356. [Google Scholar] [CrossRef]
- Lamm, K.; Arnold, W. Noise-induced cochlear hypoxia is intensity dependent, correlates with hearing loss and precedes reduction of cochlear blood flow. Audiol. Neurotol. 1996, 1, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Lamm, K.; Arnold, W. The effect of blood flow promoting drugs on cochlear blood flow, perilymphatic pO2 and auditory function in the normal and noise-damaged hypoxic and ischemic guinea pig inner ear. Hear. Res. 2000, 141, 199–219. [Google Scholar] [CrossRef]
- Pujol, R.; Puel, J.-L.; D’aldin, C.G.; Eybalin, M. Pathophysiology of the glutamatergic synapses in the cochlea. Acta Oto-Laryngol. 1993, 113, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.B.; Moore, D.R.; Reinke, L.A.; Fechter, L.D. Free radical generation in the cochlea during combined exposure to noise and carbon monoxide: An electrophysiological and an EPR study. Hear. Res. 2001, 161, 113–122. [Google Scholar] [CrossRef]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Oto-Rhino-Laryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef]
- Kamogashira, T.; Fujimoto, C.; Yamasoba, T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ohinata, Y.; Miller, J.M.; Altschuler, R.A.; Schacht, J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000, 878, 163–173. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Wright, J.S.; Dugan, L.L. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol. Neurotol. 1999, 4, 229–236. [Google Scholar] [CrossRef]
- Kopke, R.; Bielefeld, E.; Liu, J.; Zheng, J.; Jackson, R.; Henderson, D.; Coleman, J.K. Prevention of impulse noise-induced hearing loss with antioxidants. Acta Oto-Laryngol. 2005, 125, 235–243. [Google Scholar] [CrossRef]
- Molina, S.J.; Miceli, M.; Guelman, L.R. Noise exposure and oxidative balance in auditory and extra-auditory structures in adult and developing animals. Pharmacological approaches aimed to minimize its effects. Pharmacol. Res. 2016, 109, 86–91. [Google Scholar] [CrossRef]
- Ohinata, Y.; Yamasoba, T.; Schacht, J.; Miller, J.M. Glutathione limits noise-induced hearing loss. Hear. Res. 2000, 146, 28–34. [Google Scholar] [CrossRef]
- Han, C.; Someya, S. Maintaining good hearing: Calorie restriction, Sirt3, and glutathione. Exp. Gerontol. 2013, 48, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Matsuo, T.; Sagara, Y.; Ohba, K.; Ohyama, K.; Sakai, H. A mini-review of reactive oxygen species in urological cancer: Correlation with NADPH oxidases, angiogenesis, and apoptosis. Int. J. Mol. Sci. 2017, 18, 2214. [Google Scholar] [CrossRef]
- Ahn, J.H.; Joo, H.S.; Suh, J.K.; Kim, H.; So, H.S.; Chung, J.W. Effects of cigarette smoking on hearing recovery from noise-induced temporary hearing threshold shifts in mice. Otol. Neurotol. 2011, 32, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, N.; Yajima, I.; Iida, M.; Li, X.; Oshino, R.; Kumasaka, M.Y.; Kato, M. Manganese-mediated acceleration of age-related hearing loss in mice. Sci. Rep. 2016, 6, 36306. [Google Scholar] [CrossRef]
- Katsumi, S.; Sahin, M.I.; Lewis, R.M.; Iyer, J.S.; Landegger, L.D.; Stankovic, K.M. Intracochlear perfusion of tumor necrosis factor-alpha induces sensorineural hearing loss and synaptic degeneration in guinea pigs. Front. Neurol. 2019, 10, 1353. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.A.; Liberman, M.C.; Nadol, J.B., Jr. Morphometric analysis of age-related changes in the human basilar membrane. Ann. Otol. Rhinol. Laryngol. 2001, 110, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Seidman, M.D.; Ahmad, N.; Joshi, D.; Seidman, J.; Thawani, S.; Quirk, W.S. Age-related hearing loss and its association with reactive oxygen species and mitochondrial DNA damage. Acta Oto-Laryngol. 2004, 124, 16–24. [Google Scholar] [CrossRef]
- Ünal, M.; Tamer, L.; Doğruer, Z.N.; Yildirim, H.; Vayisoğlu, Y.; Çamdeviren, H. N-acetyltransferase 2 gene polymorphism and presbycusis. Laryngoscope 2005, 115, 2238–2241. [Google Scholar] [PubMed]
- Ando, K.; Fujita, T. Metabolic syndrome and oxidative stress. Free Radic. Biol. Med. 2009, 47, 213–218. [Google Scholar] [CrossRef]
- Kim, T.S.; Park, S.W.; Kim, D.Y.; Kim, E.B.; Chung, J.W.; So, H.S. Visceral adipose tissue is significantly associated with hearing thresholds in adult women. Clin. Endocrinol. 2014, 80, 368–375. [Google Scholar] [CrossRef]
- Kim, T.S.; Kim, E.H.; Chung, J.W. The association between age-related hearing impairment and metabolic syndrome in Korean women: 5-year follow-up observational study. Metab. Syndr. Relat. Disord. 2017, 15, 240–245. [Google Scholar] [CrossRef]
- Shibata, R.; Murohara, T.; Ouchi, N. Protective role of adiponectin in cardiovascular disease. Curr. Med. Chem. 2012, 19, 5459–5466. [Google Scholar] [CrossRef]
- Hwang, J.H.; Hsu, C.J.; Liu, T.C.; Yang, W.S. Association of plasma adiponectin levels with hearing thresholds in adults. Clin. Endocrinol. 2011, 75, 614–620. [Google Scholar] [CrossRef]
- Tanigawa, T.; Shibata, R.; Ouchi, N.; Kondo, K.; Ishii, M.; Katahira, N.; Kambara, T.; Inoue, Y.; Takahashi, R.; Ikeda, N. Adiponectin deficiency exacerbates age-related hearing impairment. Cell Death Dis. 2014, 5, e1189. [Google Scholar] [CrossRef]
- Chu, S.H.; Lee, M.K.; Ahn, K.Y.; Im, J.-A.; Park, M.S.; Lee, D.-C.; Jeon, J.Y.; Lee, J.W. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS ONE 2012, 7, e34710. [Google Scholar] [CrossRef] [PubMed]
- Clerici, W.J.; Yang, L. Direct effects of intraperilymphatic reactive oxygen species generation on cochlear function. Hear. Res. 1996, 101, 14–22. [Google Scholar] [CrossRef]
- Ravi, R.; Somani, S.M.; Rybak, L.P. Mechanism of cisplatin ototoxicity: Antioxidant system. Pharmacol. Toxicol. 1995, 76, 386–394. [Google Scholar] [CrossRef]
- Church, M.W.; Kaltenbach, J.A.; Blakley, B.W.; Burgio, D.L. The comparative effects of sodium thiosulfate, diethyldithiocarbamate, fosfomycin and WR-2721 on ameliorating cisplatin-induced ototoxicity. Hear. Res. 1995, 86, 195–203. [Google Scholar] [CrossRef]
- Rybak, L.P.; Ravi, R.; Somani, S.M. Mechanism of protection by diethyldithiocarbamate against cisplatin ototoxicity: Antioxidant system. Fundam. Appl. Toxicol. 1995, 26, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front. Cell. Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef]
- Sha, S.-H.; Schacht, J. Formation of reactive oxygen species following bioactivation of gentamicin. Free Radic. Biol. Med. 1999, 26, 341–347. [Google Scholar] [CrossRef]
- Wu, W.-J.; Sha, S.-H.; Schacht, J. Recent advances in understanding aminoglycoside ototoxicity and its prevention. Audiol. Neurotol. 2002, 7, 171–174. [Google Scholar] [CrossRef]
- Alexander, T.H.; Harris, J.P. Incidence of sudden sensorineural hearing loss. Otol. Neurotol. 2013, 34, 1586–1589. [Google Scholar] [CrossRef]
- Bovo, R.; Aimoni, C.; Martini, A. Immune-mediated inner ear disease. Acta Oto-Laryngol. 2006, 126, 1012–1021. [Google Scholar] [CrossRef]
- Dilwali, S.; Landegger, L.D.; Soares, V.Y.; Deschler, D.G.; Stankovic, K.M. Secreted factors from human vestibular schwannomas can cause cochlear damage. Sci. Rep. 2015, 5, 18599. [Google Scholar] [CrossRef] [PubMed]
- Vambutas, A.; Pathak, S. AAO: Autoimmune and autoinflammatory (disease) in otology: What is new in immune-mediated hearing loss. Laryngoscope Investig. Otolaryngol. 2016, 1, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, M.H.; Storper, I.S.; Spitzer, J.B. Diagnosis and treatment of sudden-onset sensorineural hearing loss: A study of 51 patients. Otolaryngol. Head Neck Surg. 2003, 128, 92–98. [Google Scholar] [CrossRef]
- Tsinaslanidou, Z.; Tsaligopoulos, M.; Angouridakis, N.; Vital, V.; Kekes, G.; Constantinidis, J. The expression of TNFα, IL-6, IL-2 and IL-8 in the serum of patients with idiopathic sudden sensorineural hearing loss: Possible prognostic factors of response to corticosteroid treatment. Audiol. Neurotol. Extra 2016, 6, 9–19. [Google Scholar] [CrossRef]
- Dayal, V.S.; Ellman, M.; Sweiss, N. Autoimmune inner ear disease: Clinical and laboratory findings and treatment outcome. J. Otolaryngol. Head Neck Surg. 2008, 37, 591–596. [Google Scholar] [PubMed]
- Riva, C.; Donadieu, E.; Magnan, J.; Lavieille, J.-P. Age-related hearing loss in CD/1 mice is associated to ROS formation and HIF target proteins up-regulation in the cochlea. Exp. Gerontol. 2007, 42, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Aminpour, S.; Tinling, S.P.; Brodie, H.A. Role of tumor necrosis factor-α in sensorineural hearing loss after bacterial meningitis. Otol. Neurotol. 2005, 26, 602–609. [Google Scholar] [CrossRef]
- Fujioka, M.; Kanzaki, S.; Okano, H.J.; Masuda, M.; Ogawa, K.; Okano, H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res. 2006, 83, 575–583. [Google Scholar] [CrossRef]
- So, H.; Kim, H.; Lee, J.-H.; Park, C.; Kim, Y.; Kim, E.; Kim, J.-K.; Yun, K.-J.; Lee, K.-M.; Lee, H.-Y. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-κB. J. Assoc. Res. Otolaryngol. 2007, 8, 338–355. [Google Scholar] [CrossRef]
- Mata-Castro, N.; Sanz-López, L.; Varillas-Delgado, D.; García-Fernández, A. Intratympanic infliximab is a safe and effective rescue therapy for refractory immune-mediated hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 393–400. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.S.; Zinsmaier, A.K.; Patterson, G.; Leptich, E.J.; Shoemaker, S.L.; Yatskievych, T.A.; Gibboni, R.; Pace, E.; Luo, H. Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLoS Biol. 2019, 17, e3000307. [Google Scholar] [CrossRef] [PubMed]
- Dhukhwa, A.; Bhatta, P.; Sheth, S.; Korrapati, K.; Tieu, C.; Mamillapalli, C.K.; Ramkumar, V.; Mukherjea, D. Targeting Inflammatory Processes Mediated by TRPV1 and TNF-α for Treating Noise-Induced Hearing Loss. Front. Cell. Neurosci. 2019, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Darrat, I.; Ahmad, N.; Seidman, K.; Seidman, M.D. Auditory research involving antioxidants. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 358–363. [Google Scholar] [CrossRef]
- Frisina, R.D.; Frisina, D.R. Physiological and neurobiological bases of age-related hearing loss: Biotherapeutic implications. Am. J. Audiol. 2013. [Google Scholar] [CrossRef]
- Erdem, T.; Bayindir, T.; Filiz, A.; Iraz, M.; Selimoglu, E. The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 2185–2188. [Google Scholar] [CrossRef] [PubMed]
- Seidman, M.D. Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope 2000, 110, 727–738. [Google Scholar] [CrossRef]
- Hazlitt, R.A.; Min, J.; Zuo, J. Progress in the Development of Preventative Drugs for Cisplatin-Induced Hearing Loss: Miniperspective. J. Med. Chem. 2018, 61, 5512–5524. [Google Scholar] [CrossRef]
- Le, T.N.; Straatman, L.V.; Lea, J.; Westerberg, B. Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol.-Head Neck Surg. 2017, 46, 41. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Ralli, M.; Sergi, B.; Parrilla, C.; Troiani, D.; Paludetti, G. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol. Ital. 2009, 29, 70. [Google Scholar]
- Bielefeld, E.C.; Kopke, R.D.; Jackson, R.L.; Coleman, J.K.; Liu, J.; Henderson, D. Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Oto-Laryngol. 2007, 127, 914–919. [Google Scholar] [CrossRef]
- Lorito, G.; Giordano, P.; Petruccelli, J.; Martini, A.; Hatzopoulos, S. Different strategies in treating noise-induced hearing loss with N-acetylcysteine. Med. Sci. Monit. 2008, 14, BR159–BR164. [Google Scholar]
- Clifford, R.E.; Coleman, J.K.; Balough, B.J.; Liu, J.; Kopke, R.D.; Jackson, R.L. Low-dose d-methionine and N-acetyl-l-cysteine for protection from permanent noise-induced hearing loss in chinchillas. Otolaryngol.-Head Neck Surg. 2011, 145, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.; Huang, X.; Liu, J.; Kopke, R.; Jackson, R. Dosing study on the effectiveness of salicylate/N-acetylcysteine for prevention of noise-induced hearing loss. Noise Health 2010, 12, 159. [Google Scholar]
- Doosti, A.; Lotfi, Y.; Moossavi, A.; Bakhshi, E.; Talasaz, A.H.; Hoorzad, A. Comparison of the effects of N-acetyl-cysteine and ginseng in prevention of noise induced hearing loss in male textile workers. Noise Health 2014, 16, 223. [Google Scholar] [PubMed]
- Kopke, R.; Slade, M.D.; Jackson, R.; Hammill, T.; Fausti, S.; Lonsbury-Martin, B.; Sanderson, A.; Dreisbach, L.; Rabinowitz, P.; Torre, P., III. Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: A randomized clinical trial. Hear. Res. 2015, 323, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.; Dreisbach, L.; Lockwood, J.; Baldwin, K.; Kopke, R.; Scranton, S.; O’Leary, M. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J. Am. Acad. Audiol. 2006, 17, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Kranzer, K.; Elamin, W.F.; Cox, H.; Seddon, J.A.; Ford, N.; Drobniewski, F. A systematic review and meta-analysis of the efficacy and safety of N-acetylcysteine in preventing aminoglycoside-induced ototoxicity: Implications for the treatment of multidrug-resistant TB. Thorax 2015, 70, 1070–1077. [Google Scholar] [CrossRef]
- Choe, W.-T.; Chinosornvatana, N.; Chang, K.W. Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol. Neurotol. 2004, 25, 910–915. [Google Scholar] [CrossRef]
- Dickey, D.T.; Wu, Y.J.; Muldoon, L.L.; Neuwelt, E.A. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J. Pharmacol. Exp. Ther. 2005, 314, 1052–1058. [Google Scholar] [CrossRef]
- Brock, P.R.; Maibach, R.; Childs, M.; Rajput, K.; Roebuck, D.; Sullivan, M.J.; Laithier, V.; Ronghe, M.; Dall’Igna, P.; Hiyama, E. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N. Engl. J. Med. 2018, 378, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, Y.; Chen, X.; Zhang, P.; Su, D.; Wang, L.; Yang, F.; Yang, J. Effects of d-methionine in mice with noise-induced hearing loss mice. J. Int. Med. Res. 2019, 47, 3874–3885. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.-C.; Liao, L.-J.; Wang, C.-T.; Young, Y.-H.; Chang, Y.-L.; Cheng, P.-W. Dose-dependent effects of d-methionine for rescuing noise-induced permanent threshold shift in guinea-pigs. Neuroscience 2013, 254, 222–229. [Google Scholar] [CrossRef]
- Campbell, K.; Nayar, R.; Borgonha, S.; Hughes, L.; Rehemtulla, A.; Ross, B.; Sunkara, P. Oral d-methionine (MRX-1024) significantly protects against cisplatin-induced hearing loss: A phase II study in humans. In Proceedings of the IX European Federation of Audiology Societies (EFAS) Congress, Tenerife, Spain, 21–24 June 2009. [Google Scholar]
- Kim, K.-H.; Lee, B.; Kim, Y.-R.; Kim, M.-A.; Ryu, N.; Kim, U.-K.; Baek, J.-I.; Lee, K.-Y. Evaluating protective and therapeutic effects of alpha-lipoic acid on cisplatin-induced ototoxicity. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hou, N.; Bao, D.; Liu, S.; Xu, T. Mechanism of alpha-lipoic acid in attenuating kanamycin-induced ototoxicity. Neural Regen. Res. 2012, 7, 2793. [Google Scholar]
- Huang, S.; Xu, A.; Sun, X.; Shang, W.; Zhou, B.; Xie, Y.; Zhao, M.; Li, P.; Lu, P.; Liu, T. Otoprotective Effects of α-lipoic Acid on A/J Mice With Age-related Hearing Loss. Otol. Neurotol. 2020, 41, e648–e654. [Google Scholar] [CrossRef]
- Quaranta, N.; Dicorato, A.; Matera, V.; D’Elia, A.; Quaranta, A. The effect of alpha-lipoic acid on temporary threshold shift in humans: A preliminary study. Acta Otorhinolaryngol. Ital. 2012, 32, 380. [Google Scholar]
- Hyppolito, M.A.; Oliveira, A.A.d.; Lessa, R.M.; Rossato, M. Amifostine otoprotection to cisplatin ototoxicity: A guinea pig study using otoacoustic emission distortion products (DPOEA) and scanning electron microscopy. Rev. Bras. Otorrinolaringol. 2005, 71, 268–273. [Google Scholar] [CrossRef]
- Fisher, M.J.; Lange, B.J.; Needle, M.N.; Janss, A.J.; Shu, H.K.G.; Adamson, P.C.; Phillips, P.C. Amifostine for children with medulloblastoma treated with cisplatin-based chemotherapy. Pediatr. Blood Cancer 2004, 43, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.G.; Bass, J.K.; Onar-Thomas, A.; Huang, J.; Chintagumpala, M.; Bouffet, E.; Hassall, T.; Gururangan, S.; Heath, J.A.; Kellie, S. Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma. Neuro-Oncology 2014, 16, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Kemp, G.; Rose, P.; Lurain, J.; Berman, M.; Manetta, A.; Roullet, B.; Homesley, H.; Belpomme, D.; Glick, J. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: Results of a randomized control trial in patients with advanced ovarian cancer. J. Clin. Oncol. 1996, 14, 2101–2112. [Google Scholar] [CrossRef]
- Lynch, E.D.; Gu, R.; Pierce, C.; Kil, J. Combined oral delivery of ebselen and allopurinol reduces multiple cisplatin toxicities in rat breast and ovarian cancer models while enhancing anti-tumor activity. Anti-Cancer Drugs 2005, 16, 569–579. [Google Scholar] [CrossRef]
- Yamasoba, T.; Pourbakht, A.; Sakamoto, T.; Suzuki, M. Ebselen prevents noise-induced excitotoxicity and temporary threshold shift. Neurosci. Lett. 2005, 380, 234–238. [Google Scholar] [CrossRef]
- Kil, J.; Lobarinas, E.; Spankovich, C.; Griffiths, S.K.; Antonelli, P.J.; Lynch, E.D.; Le Prell, C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 390, 969–979. [Google Scholar] [CrossRef]
- Kang, W.S.; Chung, J.W. Ingestion of Korean Red ginseng after noise exposure can potentiate rapid recovery of hearing in mice. J. Ginseng Res. 2010, 34, 336–341. [Google Scholar] [CrossRef]
- Choung, Y.H.; Kim, S.W.; Tian, C.; Min, J.Y.; Lee, H.K.; Park, S.N.; Lee, J.B.; Park, K. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope 2011, 121, 1294–1302. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Piacentini, R.; Fiorita, A.; Paludetti, G.; Troiani, D. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL). Brain Res. 2009, 1257, 108–116. [Google Scholar] [CrossRef]
- Staffa, P.; Cambi, J.; Mezzedimi, C.; Passali, D.; Bellussi, L. Activity of coenzyme Q 10 (Q-Ter multicomposite) on recovery time in noise-induced hearing loss. Noise Health 2014, 16, 265. [Google Scholar] [PubMed]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef]
- Bijarnia, R.K.; Bachtler, M.; Chandak, P.G.; van Goor, H.; Pasch, A. Sodium thiosulfate ameliorates oxidative stress and preserves renal function in hyperoxaluric rats. PLoS ONE 2015, 10, e0124881. [Google Scholar] [CrossRef]
- Lo, W.-C.; Chang, C.-M.; Liao, L.-J.; Wang, C.-T.; Young, Y.-H.; Chang, Y.-L.; Cheng, P.-W. Assessment of d-methionine protecting cisplatin-induced otolith toxicity by vestibular-evoked myogenic potential tests, ATPase activities and oxidative state in guinea pigs. Neurotoxicol. Teratol. 2015, 51, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of hepatic glutathione synthesis. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: New York, NY, USA, 1998; pp. 331–343. [Google Scholar]
- Fernández-Checa, J.C.; Kaplowitz, N.; García-Ruiz, C.; Colell, A. Mitochondrial glutathione: Importance and transport. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: New York, NY, USA, 1998; pp. 389–401. [Google Scholar]
- Campbell, K.C.; Meech, R.P.; Klemens, J.J.; Gerberi, M.T.; Dyrstad, S.S.; Larsen, D.L.; Mitchell, D.L.; El-Azizi, M.; Verhulst, S.J.; Hughes, L.F. Prevention of noise-and drug-induced hearing loss with d-methionine. Hear. Res. 2007, 226, 92–103. [Google Scholar] [CrossRef]
- Kaltenbach, J.A.; Church, M.W.; Blakley, B.W.; McCASLIN, D.L.; Burgio, D.L. Comparison of five agents in protecting the cochlea against the ototoxic effects of cisplatin in the hamster. Otolaryngol. Head Neck Surg. 1997, 117, 493–500. [Google Scholar] [CrossRef]
- Kil, J.; Pierce, C.; Tran, H.; Gu, R.; Lynch, E.D. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear. Res. 2007, 226, 44–51. [Google Scholar] [CrossRef]
- Kim, S.-J.; Park, C.; Han, A.L.; Youn, M.-J.; Lee, J.-H.; Kim, Y.; Kim, E.-S.; Kim, H.-J.; Kim, J.-K.; Lee, H.-K. Ebselen attenuates cisplatin-induced ROS generation through Nrf2 activation in auditory cells. Hear. Res. 2009, 251, 70–82. [Google Scholar] [CrossRef]
- So, H.; Kim, H.; Kim, Y.; Kim, E.; Pae, H.-O.; Chung, H.-T.; Kim, H.-J.; Kwon, K.-B.; Lee, K.-M.; Lee, H.-Y. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J. Assoc. Res. Otolaryngol. 2008, 9, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Elimadi, A.; Bouillot, L.; Sapena, R.; Tillement, J.-P.; Morin, D. Dose-related inversion of cinnarizine and flunarizine effects on mitochondrial permeability transition. Eur. J. Pharmacol. 1998, 348, 115–121. [Google Scholar] [CrossRef]
- Shim, H.J.; Kang, H.H.; Ahn, J.H.; Chung, J.W. Retinoic acid applied after noise exposure can recover the noise-induced hearing loss in mice. Acta Oto-Laryngol. 2009, 129, 233–238. [Google Scholar] [CrossRef]
- McFadden, S.L.; Woo, J.M.; Michalak, N.; Ding, D. Dietary vitamin C supplementation reduces noise-induced hearing loss in guinea pigs. Hear. Res. 2005, 202, 200–208. [Google Scholar] [CrossRef]
- Kapoor, N.; Mani, K.V.; Shyam, R.; Sharma, R.K.; Singh, A.P.; Selvamurthy, W. Effect of vitamin E supplementation on carbogen-induced amelioration of noise induced hearing loss in man. Noise Health 2011, 13, 452. [Google Scholar] [CrossRef]
- Quaranta, A.; Scaringi, A.; Bartoli, R.; Margarito, M.A.; Quaranta, N. The effects of ‘supra-physiological’vitamin B12 administration on temporary threshold shift: Efectos de la administración “supra-fisiológica” de vitamina B12 sobre el cambio temporal de umbrales. Int. J. Audiol. 2004, 43, 162–165. [Google Scholar] [CrossRef]
- Le Prell, C.G.; Hughes, L.F.; Miller, J.M. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic. Biol. Med. 2007, 42, 1454–1463. [Google Scholar] [CrossRef]
- Dirain, C.O.; Ng, M.R.A.V.; Bailey Milne-Davies, J.K.J.; Antonelli, P.J. Evaluation of mitoquinone for protecting against amikacin-induced ototoxicity in guinea pigs. Otol. Neurotol. 2018, 39, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.-H.; Qiu, J.-H.; Schacht, J. Aspirin to prevent gentamicin-induced hearing loss. N. Engl. J. Med. 2006, 354, 1856–1857. [Google Scholar] [CrossRef] [PubMed]
- Kyle, M.E.; Wang, J.C.; Shin, J.J. Ubiquitous aspirin: A systematic review of its impact on sensorineural hearing loss. Otolaryngol.-Head Neck Surg. 2015, 152, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Crabb, S.J.; Martin, K.; Abab, J.; Ratcliffe, I.; Thornton, R.; Lineton, B.; Ellis, M.; Moody, R.; Stanton, L.; Galanopoulou, A. COAST (Cisplatin ototoxicity attenuated by aspirin trial): A phase II double-blind, randomised controlled trial to establish if aspirin reduces cisplatin induced hearing-loss. Eur. J. Cancer 2017, 87, 75–83. [Google Scholar] [CrossRef]
- Pourbakht, A.; Yamasoba, T. Ebselen attenuates cochlear damage caused by acoustic trauma. Hear. Res. 2003, 181, 100–108. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kang, H.H.; Kim, Y.J.; Chung, J.W. Anti-apoptotic role of retinoic acid in the inner ear of noise-exposed mice. Biochem. Biophys. Res. Commun. 2005, 335, 485–490. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kang, H.H.; Kim, T.Y.; Shin, J.E.; Chung, J.W. Lipoic acid rescues DBA mice from early-onset age-related hearing impairment. Neuroreport 2008, 19, 1265–1269. [Google Scholar] [CrossRef]
- Coleman, J.K.; Kopke, R.D.; Liu, J.; Ge, X.; Harper, E.A.; Jones, G.E.; Cater, T.L.; Jackson, R.L. Pharmacological rescue of noise induced hearing loss using N-acetylcysteine and acetyl-L-carnitine. Hear. Res. 2007, 226, 104–113. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- Fisher, A.B. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox Signal. 2011, 15, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tavanai, E.; Mohammadkhani, G. Role of antioxidants in prevention of age-related hearing loss: A review of literature. Eur. Arch. Otorhinolaryngol. 2017, 274, 1821–1834. [Google Scholar] [CrossRef]
- Ahn, J.H.; Shin, J.E.; Chung, B.Y.; Lee, H.M.; Kang, H.H.; Chung, J.W.; Pak, J.H. Involvement of retinoic acid-induced peroxiredoxin 6 expression in recovery of noise-induced temporary hearing threshold shifts. Environ. Toxicol. Pharmacol. 2013, 36, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Kristiansen, A.; Liberman, M.C. Heat stress and protection from permanent acoustic injury in mice. J. Neurosci. 1999, 19, 10116–10124. [Google Scholar] [CrossRef]
- Wang, Y.; Liberman, M.C. Restraint stress and protection from acoustic injury in mice. Hear. Res. 2002, 165, 96–102. [Google Scholar] [CrossRef]
- Chung, J.W.; Shin, J.-E.; Han, K.W.; Ahn, J.H.; Kim, Y.-J.; Park, J.-W.; So, H.-S. Up-regulation of hypoxia-inducible factor-1 alpha by cobalt chloride prevents hearing loss in noise-exposed mice. Environ. Toxicol. Pharmacol. 2011, 31, 153–159. [Google Scholar] [CrossRef]
- Gagnon, P.M.; Simmons, D.D.; Bao, J.; Lei, D.; Ortmann, A.J.; Ohlemiller, K.K. Temporal and genetic influences on protection against noise-induced hearing loss by hypoxic preconditioning in mice. Hear. Res. 2007, 226, 79–91. [Google Scholar] [CrossRef]
- Canlon, B.; Borg, E.; Flock, Å. Protection against noise trauma by pre-exposure to a low level acoustic stimulus. Hear. Res. 1988, 34, 197–200. [Google Scholar] [CrossRef]
- Harris, K.C.; Bielefeld, E.; Hu, B.H.; Henderson, D. Increased resistance to free radical damage induced by low-level sound conditioning. Hear. Res. 2006, 213, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Tahera, Y.; Canlon, B. Protection against acoustic trauma by forward and backward sound conditioning. Audiol. Neurotol. 2004, 9, 265–273. [Google Scholar] [CrossRef]
- Jacono, A.A.; Hu, B.; Kopke, R.D.; Henderson, D.; Van De Water, T.R.; Steinman, H.M. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear. Res. 1998, 117, 31–38. [Google Scholar] [CrossRef]
- Niu, X.; Shao, R.; Canlon, B. Suppression of apoptosis occurs in the cochlea by sound conditioning. Neuroreport 2003, 14, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Canlon, B. Protective mechanisms of sound conditioning. Adv. Otorhinolaryngol. 2002, 59, 96–105. [Google Scholar] [PubMed]
- Tahera, Y.; Meltser, I.; Johansson, P.; Salman, H.; Canlon, B. Sound conditioning protects hearing by activating the hypothalamic–pituitary–adrenal axis. Neurobiol. Dis. 2007, 25, 189–197. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Conditioning-related protection from acoustic injury: Effects of chronic deefferentation and sham surgery. J. Neurophysiol. 1997, 78, 3095–3106. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ahn, J.H.; Chung, J.W. Effect of Prednisolone on Acoustic Trauma Applied in the Inactive Phase of the Hypothalamic-Pituitary-Adrenal Axis in Mice. Korean J. Audiol. 2009, 13, 156–159. [Google Scholar]
- Kim, J.Y.; Kang, H.H.; Ahn, J.H.; Chung, J.W. Circadian changes in serum corticosterone levels affect hearing in mice exposed to noise. Neuroreport 2008, 19, 1373–1376. [Google Scholar] [CrossRef]
- Song, H.M.; Lim, G.C.; Lim, H.W.; Kim, M.J.; Choi, S.H.; Chung, J.W. Changes of Serum Aldosterone Concentration after Noise Exposure in Mice. Korean J. Audiol. 2011, 15, 137–140. [Google Scholar]
- Kubo, T.; Maezawa, N.; Osada, M.; Katsumura, S.; Funae, Y.; Imaoka, S. Bisphenol A, an environmental endocrine-disrupting chemical, inhibits hypoxic response via degradation of hypoxia-inducible factor 1α (HIF-1α): Structural requirement of bisphenol A for degradation of HIF-1α. Biochem. Biophys. Res. Commun. 2004, 318, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.H.; Yi, J.; Ryu, S.; Kim, I.K.; Kim, J.-W.; Baek, H.; Chung, J.W. Induction of Redox-Active Gene Expression by CoCl2 Ameliorates Oxidative Stress-Mediated Injury of Murine Auditory Cells. Antioxidants 2019, 8, 399. [Google Scholar] [CrossRef]
- Yi, J.; Kim, T.; Pak, J.; Chung, J. Protective Effects of Glucose-Related Protein 78 and 94 on Cisplatin-Mediated Ototoxicity. Antioxidants 2020, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Kozutsumi, Y.; Segal, M.; Normington, K.; Gething, M.J.; & Sambrook, J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 1988, 332, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Kaufman, R.J. ER stress and the unfolded protein response. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2005, 569, 29–63. [Google Scholar]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Lin, J.H.; Li, H.; Yasumura, D.; Cohen, H.R.; Zhang, C.; Panning, B.; Shokat, K.M.; LaVail, M.M.; Walter, P. IRE1 signaling affects cell fate during the unfolded protein response. Science 2007, 318, 944–949. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Bertolotti, A.; Zeng, H.; Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 2000, 5, 897–904. [Google Scholar] [CrossRef]
- Okada, T.; Yoshida, H.; Akazawa, R.; Negishi, M.; Mori, K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem. J. 2002, 366, 585–594. [Google Scholar] [CrossRef]
- Lee, K.; Tirasophon, W.; Shen, X.; Michalak, M.; Prywes, R.; Okada, T.; Yoshida, H.; Mori, K.; Kaufman, R.J. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002, 16, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Li, L.; Sun, Y.; Feng, B. Three common GJB2 mutations causing nonsyndromic hearing loss in Chinese populations are retained in the endoplasmic reticulum. Acta Oto-Laryngol. 2010, 130, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, Y.; Mutai, H.; Kamiya, K.; Mizutari, K.; Fujii, M.; Matsunaga, T. Enhanced expression of C/EBP homologous protein (CHOP) precedes degeneration of fibrocytes in the lateral wall after acute cochlear mitochondrial dysfunction induced by 3-nitropropionic acid. Neurochem. Int. 2010, 56, 487–494. [Google Scholar] [CrossRef]
- Bonventre, J.V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 2003, 14, S55–S61. [Google Scholar] [CrossRef]
- Peyrou, M.; Hanna, P.E.; Cribb, A.E. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol. Sci. 2007, 99, 346–353. [Google Scholar] [CrossRef]
- Inagi, R.; Kumagai, T.; Nishi, H.; Kawakami, T.; Miyata, T.; Fujita, T.; Nangaku, M. Preconditioning with endoplasmic reticulum stress ameliorates mesangioproliferative glomerulonephritis. J. Am. Soc. Nephrol. 2008, 19, 915–922. [Google Scholar] [CrossRef]
- Mahfoudh-Boussaid, A.; Zaouali, M.A.; Hadj-Ayed, K.; Miled, A.-H.; Saidane-Mosbahi, D.; Rosello-Catafau, J.; Abdennebi, H.B. Ischemic preconditioning reduces endoplasmic reticulum stress and upregulates hypoxia inducible factor-1α in ischemic kidney: The role of nitric oxide. J. Biomed. Sci. 2012, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Rutkowski, D.T.; Dubois, M.; Swathirajan, J.; Saunders, T.; Wang, J.; Song, B.; Yau, G.D.-Y.; Kaufman, R.J. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 2007, 13, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Pallepati, P.; Averill-Bates, D.A. Activation of ER stress and apoptosis by hydrogen peroxide in HeLa cells: Protective role of mild heat preconditioning at 40 C. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2011, 1813, 1987–1999. [Google Scholar] [CrossRef]

| Name | Study Subjects | Condition | Outcome Regarding Hearing Loss | Reference | |
|---|---|---|---|---|---|
| Animal Model | Clinical Study | ||||
| N-acetyl cysteine | Guinea pig | Noise | Protection | [59] | |
| Chinchilla | Noise | Protection | [60] | ||
| Rat | Noise | Protection | [61] | ||
| Combined with d-methionine | Chinchilla | Noise | Protection | [62] | |
| Combined with salicylate | Chinchilla | Noise | Protection | [63] | |
| 48 textile workers, RCT, phase 2 | Noise | Protection | [64] | ||
| 634 military population during weapon training, phase 2 | Noise | Partial effect (post-hoc analysis, handedness) | [65] | ||
| 31 normal-hearing participants, phase 2 | Noise | No effect in this study setting | [66] | ||
| Meta-analysis, 3 studies, 146 patients with end-stage renal disease | Aminoglycoside | Protection | [67] | ||
| Guinea pig | Cisplatin | Protection | [68] | ||
| Sodium thiosulfate | Rat | Cisplatin | Rescue | [69] | |
| 109 pediatric patients with hepatoblastoma, RCT, phase 3 | Cisplatin | Rescue | [70] | ||
| d-methionine | Mouse | Noise | Rescue | [71] | |
| Guinea pig | Noise | Rescue | [72] | ||
| Rat | Noise | Protection | [73] | ||
| Lipoic acid | Mouse | Cisplatin | Protection, rescue | [74] | |
| Mouse | Kanamycin | Protection | [75] | ||
| Mouse | Aging | Protection | [76] | ||
| 30 normal-hearing participants | Noise | Protection | [77] | ||
| Amifostine | Guinea pig | Cisplatin | Protection | [78] | |
| 9 pediatric patients with medulloblastoma | Cisplatin | No effect in this study setting | [79] | ||
| 379 children with medulloblastoma, not randomized | Cisplatin | Protection from serious hearing loss in average-risk patients | [80] | ||
| 242 ovarian cancer patients, RCT, phase 3 | Cisplatin | No effect in this study setting | [81] | ||
| Ebselen | Rat | Cisplatin | Protection | [82] | |
| Guinea pig | Noise | Protection | [83] | ||
| 83 normal-hearing participants, RCT, phase 2 trial | Noise | Protection | [84] | ||
| Korean red ginseng | Mouse | Noise | Rescue | [85] | |
| Rat | Gentamicin | Protection | [86] | ||
| Coenzyme Q10 | Guinea pig | Noise | Protection | [87] | |
| 30 normal-hearing participants | Noise | Protection | [88] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pak, J.H.; Kim, Y.; Yi, J.; Chung, J.W. Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning. Antioxidants 2020, 9, 1076. https://doi.org/10.3390/antiox9111076
Pak JH, Kim Y, Yi J, Chung JW. Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning. Antioxidants. 2020; 9(11):1076. https://doi.org/10.3390/antiox9111076
Chicago/Turabian StylePak, Jhang Ho, Yehree Kim, Junyeong Yi, and Jong Woo Chung. 2020. "Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning" Antioxidants 9, no. 11: 1076. https://doi.org/10.3390/antiox9111076
APA StylePak, J. H., Kim, Y., Yi, J., & Chung, J. W. (2020). Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning. Antioxidants, 9(11), 1076. https://doi.org/10.3390/antiox9111076





