Effects of Allicin on Pathophysiological Mechanisms during the Progression of Nephropathy Associated to Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Design
2.3. Ethics Statement
2.4. Allicin Synthesis
2.5. Blood Samples
2.6. Systolic Blood Pressure Record (SBP)
2.7. Plasma Biochemistry
2.8. Renal Function
2.9. Evaluation of α-SMA, CTGF, Epo, Epo-R, HIF-1α, Keap1, Nephrin, KIM-1, Nrf2, TGF-β1, and VEGF Expression in Renal Cortex
2.10. Histological Assessment of Renal Injury
2.11. Evaluation of Oxidative Stress
Measurement of the Total Antioxidant Status (TAS)
2.12. Plasma Insulin Levels
2.13. Expression of GLUT4, IRS-1 and IRS-2 in Skeletal Muscle
2.14. Statistical Analysis
3. Results
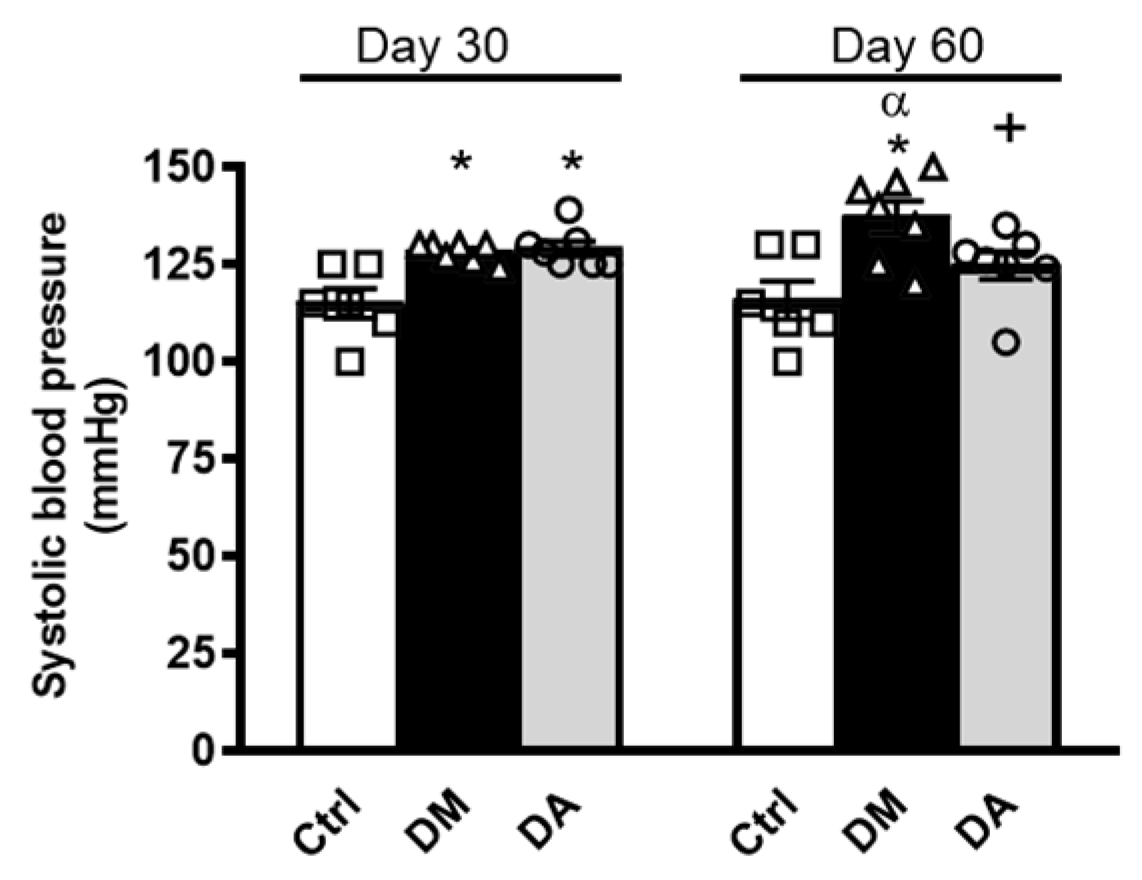
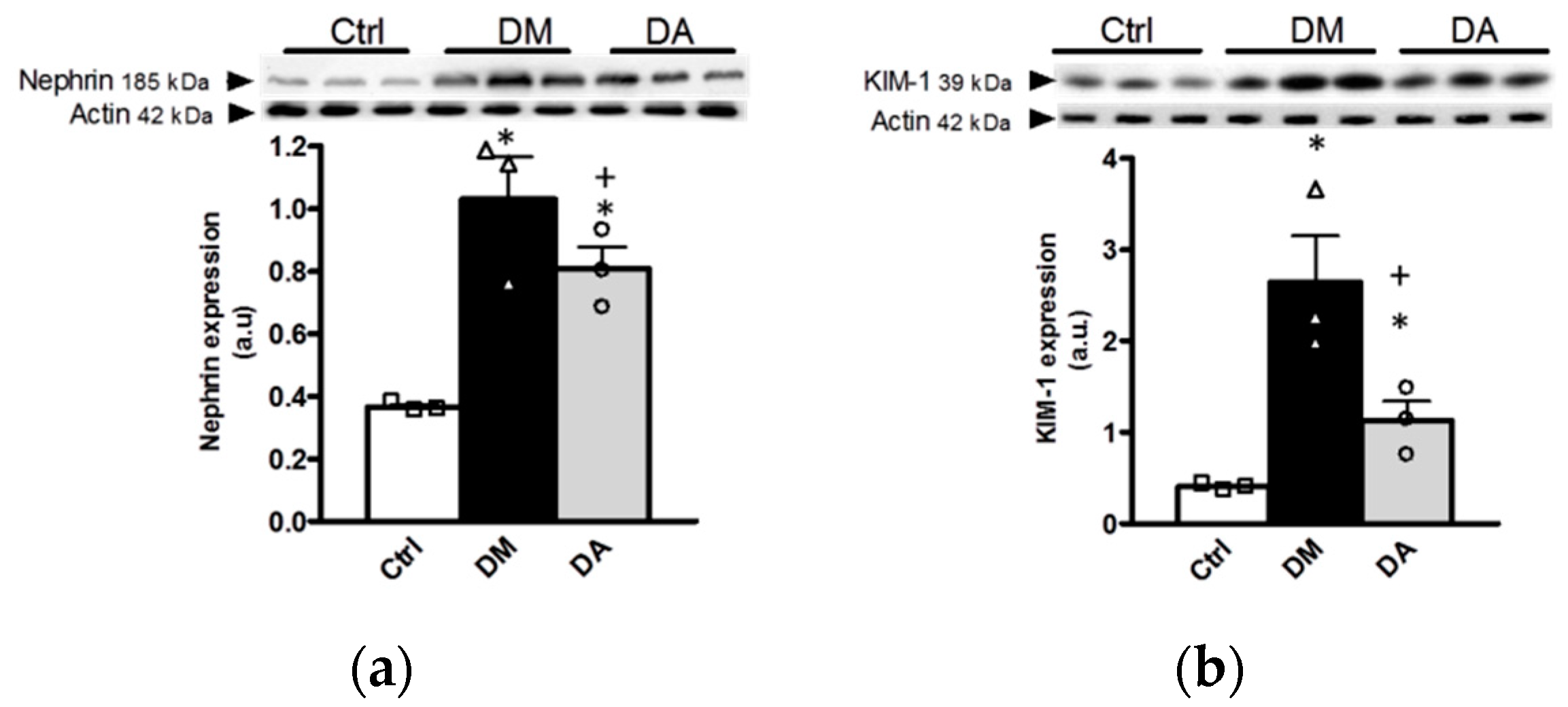
3.1. Allicin Delays the Progression of Diabetic Nephropathy
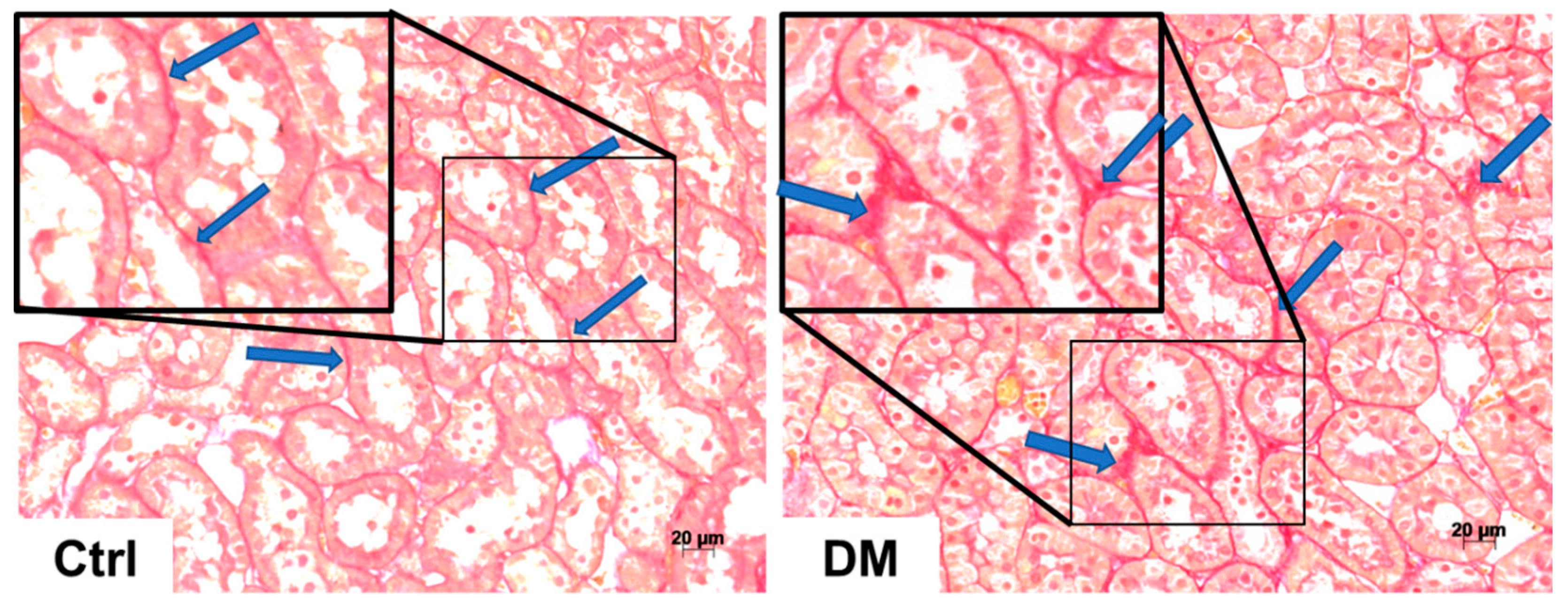
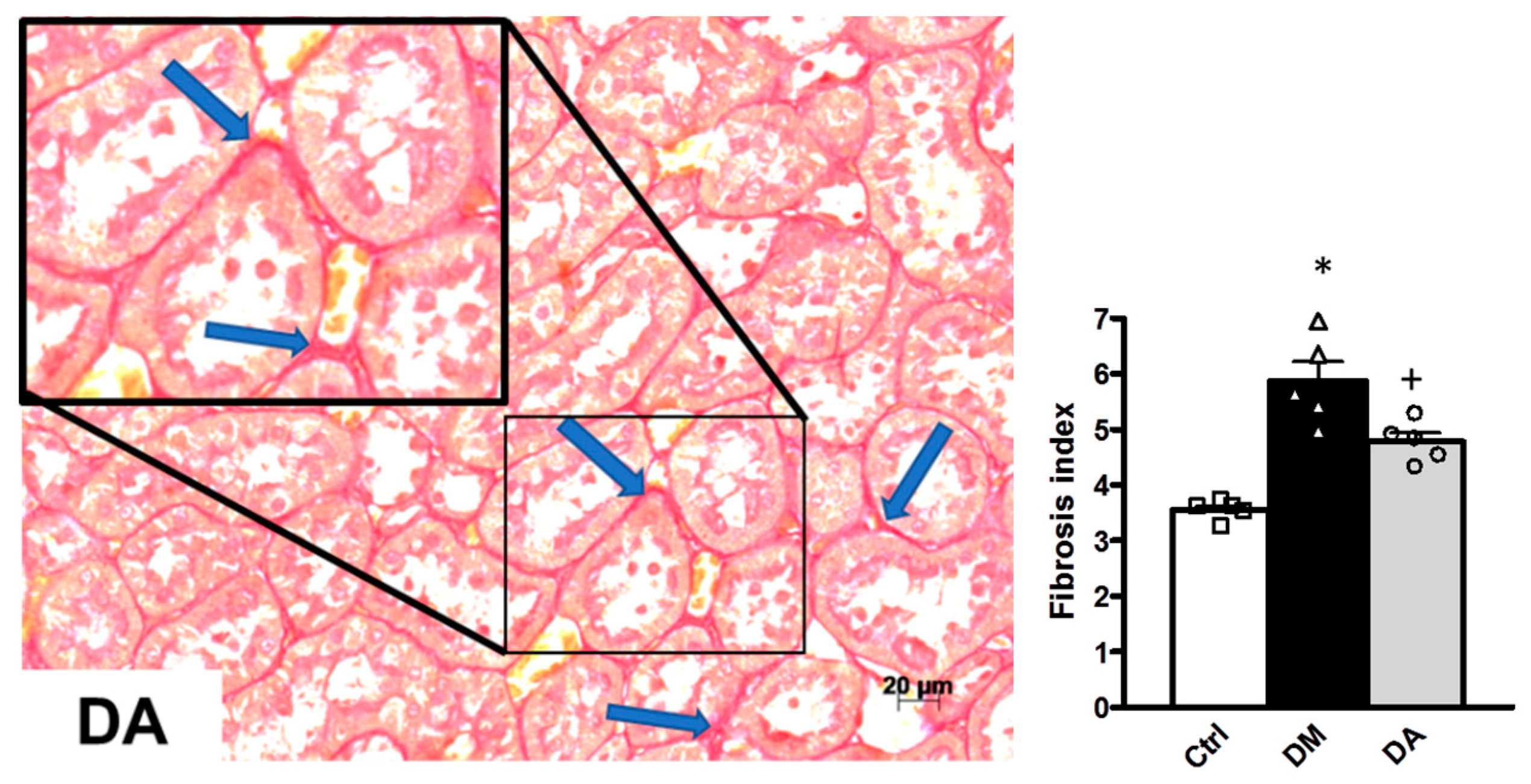
3.2. Histopathological Analysis
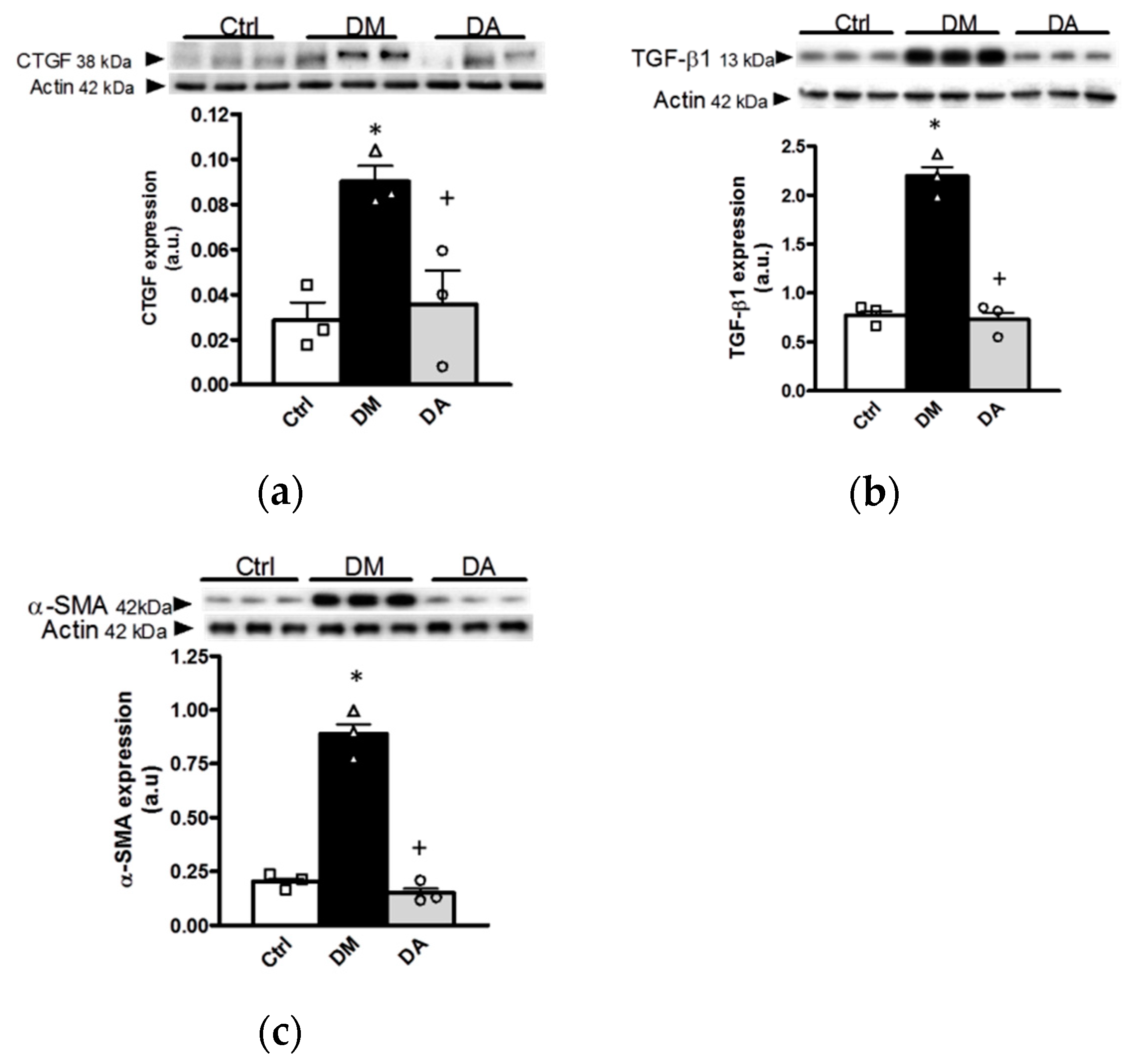
3.3. Mechanisms Associated With the Beneficial Effect Exerted by Allicin in Diabetic Nephropathy
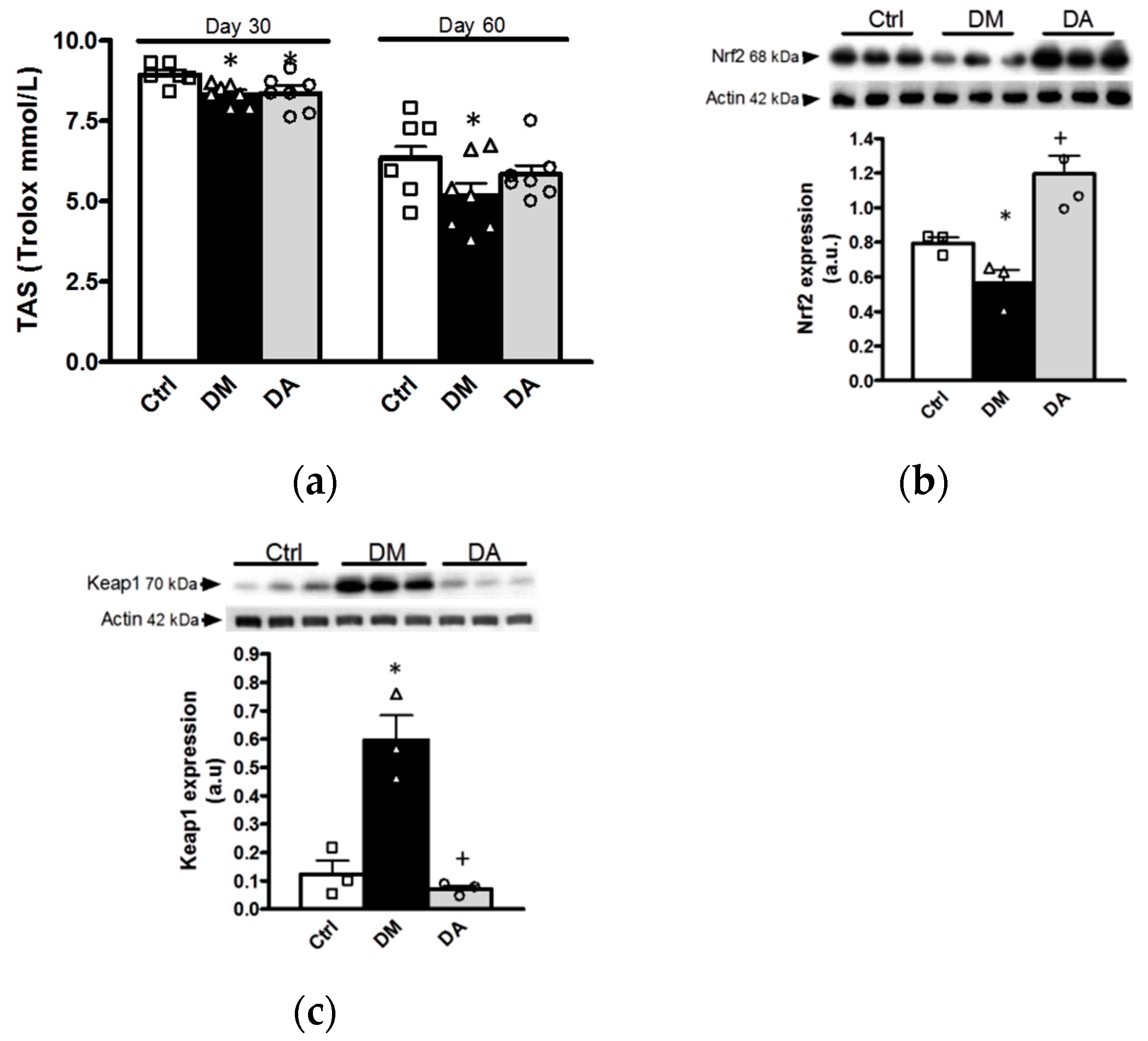
3.3.1. Effects of Allicin on Oxidative Stress
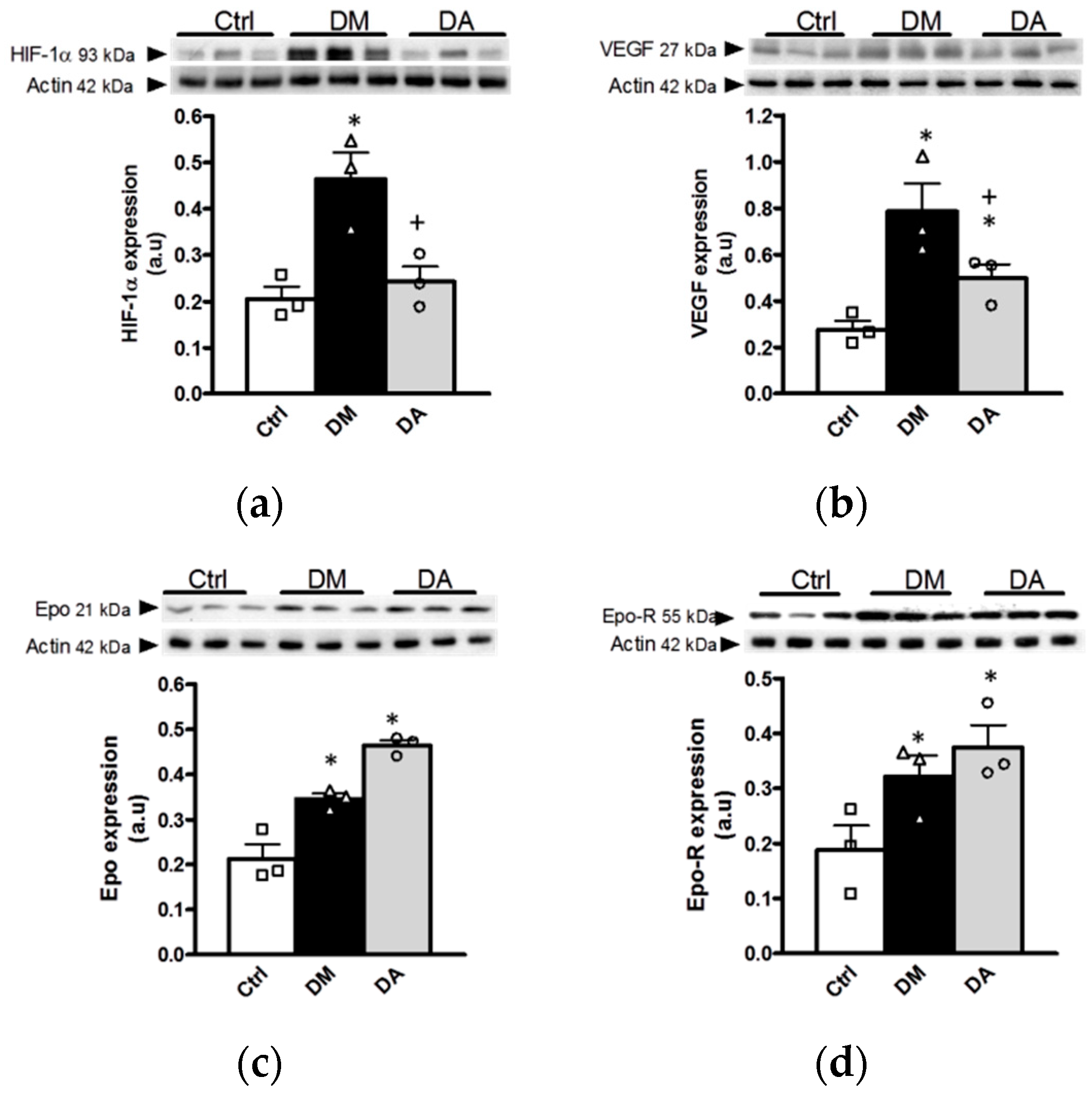
3.3.2. Effect of Allicin on Markers of Hypoxia in Renal Cortex
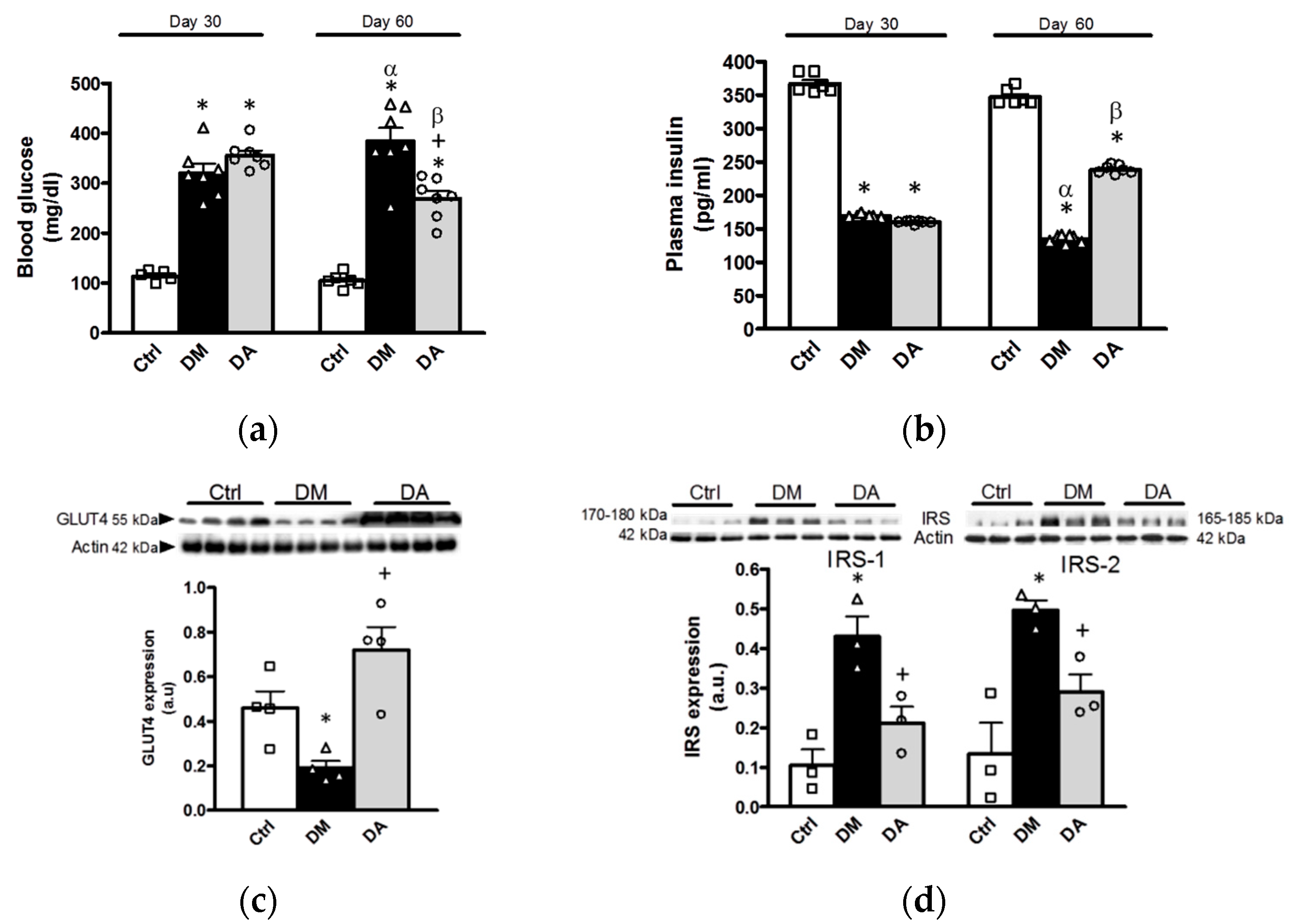
3.4. Effects of Allicin on Glucose Homeostasis
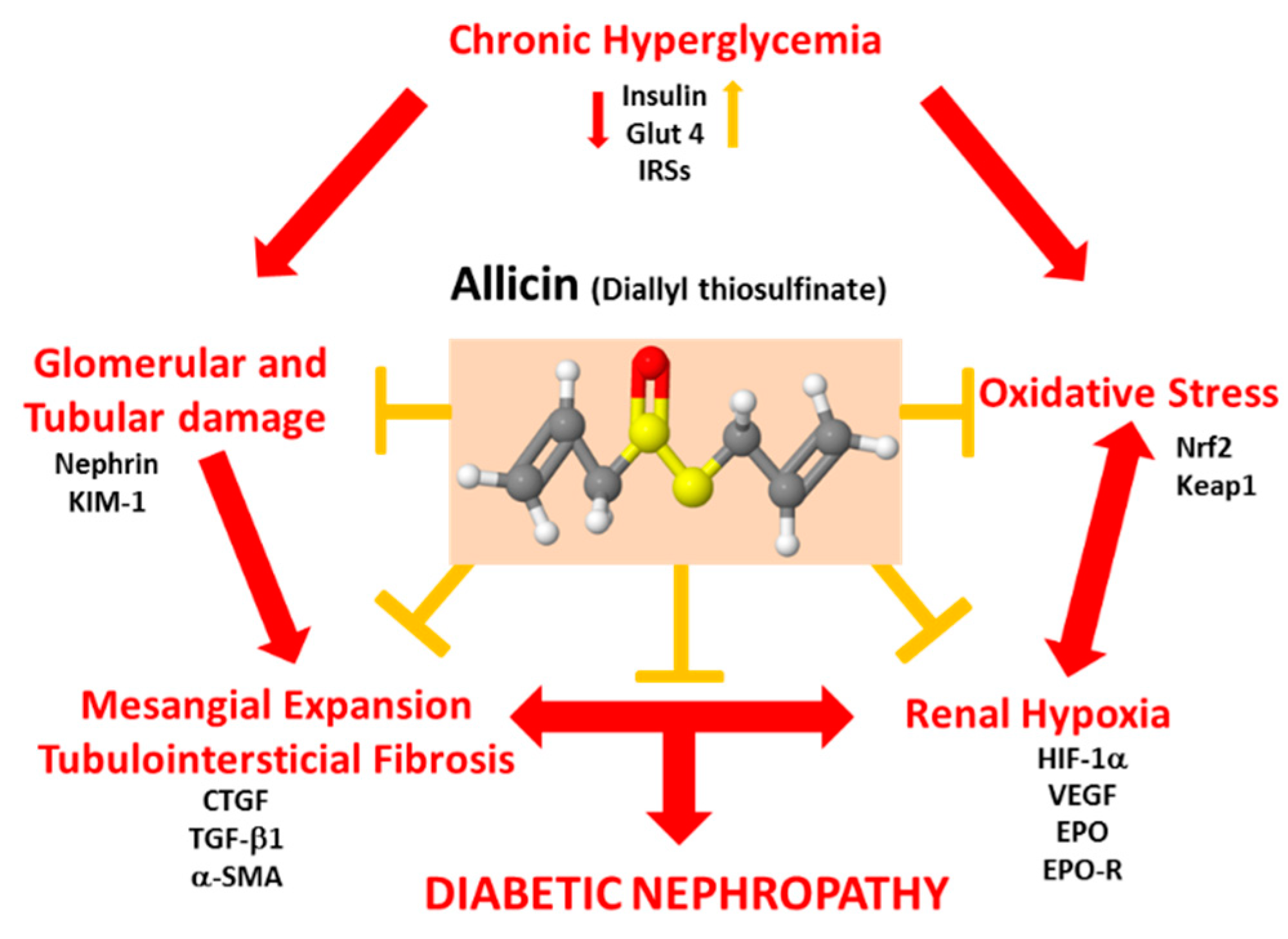
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maqbool, M.; Cooper, M.E.; Jandeleit-Dahm, K. Cardiovascular Disease and Diabetic Kidney Disease. Semin. Nephrol. 2018, 38, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Ghaderian, S.B.; Hayati, F.; Shayanpour, S.; Beladi Mousavi, S.S. Diabetes and end-stage renal disease; a review article on new concepts. J. Ren. Inj. Prev. 2015, 4, 28–33. [Google Scholar] [PubMed]
- Alicic, R.Z.; Tuttle, K.R. Diabetic Kidney Disease Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Medina-Urrutia, A.X.; Martínez-Sánchez, F.D.; Posadas-Romero, C.; Jorge-Galarza, E.; Martínez-Alvarado, M.; González-Salazar, M.; Osorio-Alonso, H.; Juárez-Rojas, J.G. Metabolic control achievement in a population with premature coronary artery disease: Results of the genetics of atherosclerotic disease study. Ther. Adv. Endocrinol. Metab. 2020, 11. [Google Scholar] [CrossRef]
- Jorge-Galarza, E.; Martínez-Sánchez, F.D.; Javier-Montiel, C.I.; Medina-Urrutia, A.X.; Posadas-Romero, C.; González-Salazar, M.C.; Osorio-Alonso, H.; Arellano-Buendía, A.S.; Juárez-Rojas, J.G. Control of blood pressure levels in patients with premature coronary artery disease: Results from the Genetics of Atherosclerotic Disease study. J. Clin. Hypertens. 2020, 22, 1253–1262. [Google Scholar] [CrossRef]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.H.; Stevens, G.A.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- Abubakar, I.I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos PMFNogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Buendía, A.S.; Tostado-González, M.; García-Arroyo, F.E.; Cristóbal-García, M.; Loredo-Mendoza, M.L.; Tapia, E.; Sanchez-Lozada, L.G.; Osorio-Alonso, H. Anti-Inflammatory Therapy Modulates Nrf2-Keap1 in Kidney from Rats with Diabetes. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.; Al-Waili, H.; Al-Waili, T.; Salom, K. Natural antioxidants in the treatment and prevention of diabetic nephropathy; a potential approach that warrants clinical trials. Redox Rep. 2017, 22, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Gioscia-Ryan, R.A.; LaRocca, T.J.; Sindler, A.L.; Zigler, M.C.; Murphy, M.P.; Seals, D.R. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol. 2014, 592, 2549–2561. [Google Scholar] [CrossRef]
- Palm, F.; Cederberg, J.; Hansell, P.; Liss, P.; Carlsson, P.-O. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 2003, 46, 1153–1160. [Google Scholar] [CrossRef]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The role of oxidative stress and hypoxia in renal disease. Kidney Res. Clin. Pract. 2019, 38, 414–426. [Google Scholar] [CrossRef]
- Fine, L.G.; Bandyopadhay, D.; Norman, J. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int. Suppl. 2000, 75, S22–S26. [Google Scholar] [CrossRef]
- Siervo, M.; Riley, H.L.; Fernandez, B.O.; Leckstrom, C.A.; Martin, D.S.; Mitchell, K.; Levett, D.Z.; Montgomery, H.; Mythen, M.G.; Grocott, M.P.W.; et al. Effects of prolonged exposure to hypobaric hypoxia on oxidative stress, inflammation and gluco-insular regulation: The not-so-sweet price for good regulation. PLoS ONE 2014, 9, e94915. [Google Scholar] [CrossRef]
- Jiao, Y.; Lu, H.; Yang, Y.; Zhang, Y.; Zhang, K.; Liu, H. Deficiency of hypoxia inducible factor 1α promoted progression of diabetic nephropathy with hypertension. Exp. Ther. Med. 2018, 16, 3658–3662. [Google Scholar] [CrossRef]
- Qiu, Y.; Huang, X.; He, W. The regulatory role of HIF-1 in tubular epithelial cells in response to kidney injury. Histol. Histopathol. 2020, 35, 321–330. [Google Scholar]
- Elkayam, A.; Peleg, E.; Grossman, E.; Shabtay, Z.; Sharabi, Y. Effects of Allicin on Cardiovascular Risk Factors in Spontaneously Hypertensive Rats. Isr. Med. Assoc. J. 2013, 115, 170–173. [Google Scholar]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- García-Trejo, E.M.A.; Arellano-Buendía, A.S.; Argüello-García, R.; Loredo-Mendoza, M.L.; García-Arroyo, F.E.; Arellano-Mendoza, M.G.; Castillo-Hernández, M.C.; Guevara-Balcázar, G.; Tapia, E.; Sánchez-Lozada, L.G.; et al. Effects of Allicin on Hypertension and Cardiac Function in Chronic Kidney Disease. Oxid. Med. Cell Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Trejo, E.M.; Ángel, G.; Buendía, A.S.A.; Reyes, O.S.; García-Arroyo, F.E.; García, R.A.; Mendoza, M.L.L.; Tapia, E.; Sanchez-Lozada, L.G.; Alonso, H.O. The Beneficial Effects of Allicin in Chronic Kidney Disease Are Comparable to Losartan. Int. J. Mol. Sci. 2017, 18, 1980. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.-Y.; Yuen, A.C.-Y.; Chan, R.Y.-K.; Chan, S.-W. A review of the cardiovascular benefits and antioxidant properties of allicin. Phytother. Res. 2013, 27, 637–646. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, Y.; Mao, G.; Yuan, F.; Zheng, H.; Ruan, Y.; Wu, T. Protective effects of allicin on streptozotocin-induced diabetic nephropathy in rats. J. Sci. Food Agric. 2017, 97, 1359–1366. [Google Scholar] [CrossRef]
- Buendía, A.S.A.; González, M.T.; Reyes, O.S.; García-Arroyo, F.E.; García, R.A.; Tapia, E.; Sanchez-Lozada, L.G.; Alonso, H.O. Immunomodulatory Effects of the Nutraceutical Garlic Derivative Allicin in the Progression of Diabetic Nephropathy. Int. J. Mol. Sci. 2018, 19, 3107. [Google Scholar] [CrossRef]
- Argüello-García, R.; Pérez-Hernández, N.; Pedraza-Chaverrí, J.; Ortega-Pierres, G. Hypoclorous acid scavenging activities of thioallyl compound from garlic. J. Agric. Food Chem. 2010, 58, 11226–11233. [Google Scholar] [CrossRef]
- Encarnacion, M.M.D.; Griffin, M.D.; Slezak, J.M.; Bergstralh, E.J.; Stegall, M.D.; Velosa, J.A.; Grande, J.P. Correlation of quantitative digital image analysis with the glomerular filtration rate in chronic allograft nephropathy. Am. J. Transpl. 2004, 4, 248–256. [Google Scholar] [CrossRef]
- Jha, J.C.; Banal, C.; Chow, B.S.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid. Redox Signal. 2016, 25, 657–684. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Xu, W.; Lu, N.; Cao, J.; Yu, S. Effects of intensive blood pressure lowering on mortality and cardiovascular and renal outcomes in type 2 diabetic patients: A meta-analysis. PLoS ONE 2019, 14, e0215362. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.G.; de Souza, M.L.; da Silva, V.D.; Dos Santos, M.; da Silva, W.; Itaquy, T.P.; Garbin, H.I.; Veronese, F.V. Markers of renal fibrosis: How do they correlate with podocyte damage in glomerular diseases? PLoS ONE 2019, 14, e0217585. [Google Scholar] [CrossRef] [PubMed]
- Torres, Á.; Muñoz, K.; Nahuelpán, Y.R.; Saez, A.P.; Mendoza, P.; Jara, C.; Cappelli, C.; Suarez, R.; Oyarzún, C.; Quezada, C.; et al. Intraglomerular Monocyte/Macrophage Infiltration and Macrophage-Myofibroblast Transition during Diabetic Nephropathy Is Regulated by the A2B Adenosine Receptor. Cells 2020, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zheng, F.; Dong, X.; Wu, F.; Wu, T.; Li, H. Allicin inhibits tubular epithelial-myofibroblast transdifferentiation under high glucose conditions in vitro. Exp. Ther. Med. 2017, 13, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Okada, H.; Inoue, T.; Kanno, Y.; Suzuki, H. Tubular expression of connective tissue growth factor correlates with interstitial fibrosis in type 2 diabetic nephropathy. Nephrol. Dial. Transplant. 2006, 21, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Koop, K.; Eikmans, M.; Baelde, H.J.; Kawachi, H.; De Heer, E.; Paul, L.C.; Bruijn, J.A. Expression of podocyte-associated molecules in acquired human kidney diseases. J. Am. Soc. Nephrol. 2003, 14, 2063–2071. [Google Scholar] [CrossRef]
- Jia, J.; Ding, G.; Zhu, J.; Chen, C.; Liang, W.; Franki, N.; Singhal, P.C. Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am. J. Nephrol. 2008, 28, 500–507. [Google Scholar] [CrossRef]
- Forbes, J.M.; Bonnet, F.; Russo, L.M.; Burns, W.C.; Cao, Z.; Candido, R.; Kawachi, H.; Allen, T.J.; Cooper, M.E.; Jerums, G.; et al. Modulation of nephrin in the diabetic kidney: Association with systemic hypertension and increasing albuminuria. J. Hypertens. 2002, 20, 985–992. [Google Scholar] [CrossRef]
- Langham, R.G.; Kelly, D.J.; Cox, A.J.; Gow, R.M.; Holthofer, H.; Gilbert, R.E. Angiotensin II-induced proteinuria and expression of the podocyte slit pore membrane protein, nephrin. Nephrol. Dial. Transplant. 2004, 19, 262–263. [Google Scholar] [CrossRef]
- Jim, B.; Ghanta, M.; Qipo, A.; Fan, Y.; Chuang, P.Y.; Cohen, H.W.; Abadi, M.; Thomas, D.B.; He, J.C. Dysregulated nephrin in diabetic nephropathy of type 2 diabetes: A cross sectional study. PLoS ONE 2012, 7, e36041. [Google Scholar] [CrossRef]
- Kostovska, I.; Tosheska-Trajkovska, K.; Topuzovska, S.; Cekovska, S.; Spasovski, G.; Kostovski, O.; Labudovic, D. Urinary nephrin is earlier, more sensitive and specific marker of diabetic nephropathy than microalbuminuria. J. Med. Biochem. 2020, 39, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Horev-Azaria, L.; Eliav, S.; Izigov, N.; Pri-Chen, S.; Mirelman, D.; Miron, T.; Rabinkov, A.; Wilchek, M.; Jacob-Hirsch, J.; Amariglio, N.; et al. Allicin up-regulates cellular glutathione level in vascular endothelial cells. Eur. J. Nutr. 2009, 48, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Li, C.-Y.; Xiang, Z.-G.; Hu, J.-J.; Lu, J.-M.; Tian, R.-B.; Jia, W. Allicin Ameliorates Cardiac Hypertrophy and Fibrosis through Enhancing of Nrf2 Antioxidant Signaling Pathways. Cardiovasc. Drugs Ther. 2012, 26, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.; Wilchek, M.; Sharp, A.; Nakagawa, Y.; Naoi, M.; Nozawa, Y.; Akao, Y. Allicin inhibits cell growth and induces apoptosis through the mitochondrial pathway in HL60 and U937 cells. J. Nutr. Biochem. 2008, 19, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Glozman, S.; Yavin, E.; Weiner, L. S-Allylmercaptoglutathione: The reaction product of allicin with glutahione possesses SH-Modifying and antioxidant properties. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2000, 1499, 144–153. [Google Scholar] [CrossRef]
- Okada, Y.; Tanaka, K.; Sato, E.; Okajima, H. Kinetic and mechanistic studies of allicin as an antioxidant. Org. Biomol. Chem. 2006, 4, 4113–4117. [Google Scholar] [CrossRef] [PubMed]
- Rabinkov, A.; Miron, T.; Konstantinovski, L.; Wilchek, M.; Mirelman, D.; Weiner, L. The mode of action of allicin: Trapping of radicals andd interaction with thiol containning proteins. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1998, 1379, 233–244. [Google Scholar] [CrossRef]
- Prasad, K.; Laxdal, V.A.; Yu, M.; Raney, B.L. Antioxidant activity of allicin, an active principle in garlic. Mol. Cell. Biochem. 1995, 148, 183–189. [Google Scholar] [CrossRef]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Seki, T.; Ariga, T. Thermostability of allicin determined by chemical and biological assays. Biosci. Biotechnol. Biochem. 2008, 72, 2877–2883. [Google Scholar] [CrossRef]
- Rosenberger, C.; Khamaisi, M.; Abassi, Z.; Shilo, V.; Weksler-Zangen, S.; Goldfarb, M.T.; Shina, A.; Zibertrest, F.; Eckardt, K.; Rosen, S.; et al. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int. 2008, 73, 34–42. [Google Scholar] [CrossRef]
- Arjamaa, O.; Nikinmaa, M. Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors. Exp. Eye Res. 2006, 83, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Bahlmann, F.H.; Boehm, S.M.; Mengel, M.; von Wasielewski, R.; Lindschau, C.; Kirsch, T.; de Groot, K.; Laudeley, R.; Niemczyk, E.; Güler, F.; et al. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failure. Circulation 2004, 110, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Eto, N.; Inagi, R.; Takano, H.; Shimizu, A.; Kato, H.; Kurihara, H.; Kawachi, H.; Shankland, S.J.; Fujita, T.; Nangaku, M. Podocyte protection by darbepoetin: Preservation of the cytoskeleton and nephrin expression. Kidney Int. 2007, 72, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Katavetin, P.; Inagi, R.; Miyata, T.; Shao, J.; Sassa, R.; Adler, S.; Eto, N.; Kato, H.; Fujita, T.; Nangaku, M. Erythropoietin induces heme oxygenase-1 expression and attenuates oxidative stress. Biochem. Biophys. Res. Commun. 2007, 359, 928–934. [Google Scholar] [CrossRef]
- Fischer, C.; Deininger, N.; Wolf, G.; Loeffler, I. CERA attenuates kidney fibrogenesis in the db/db mouse by influencing the renal myofibroblast generation. J. Clin. Med. 2018, 7, 15. [Google Scholar] [CrossRef]
- Loeffler, I.; Rüster, C.; Franke, S.; Liebisch, M.; Wolf, G. Erythropoietin ameliorates podocyte injury in advanced diabetic nephropathy in the db/db mouse. Am. J. Physiol. Ren. Physiol. 2013, 305, F911–F918. [Google Scholar] [CrossRef]
- Menne, J.; Park, J.-K.; Shushakova, N.; Mengel, M.; Meier, M.; Fliser, D. The continuous erythropoietin receptor activator affects different pathways of diabetic renal injury. J. Am. Soc. Nephrol. 2007, 18, 2046–2053. [Google Scholar] [CrossRef]
- Luo, R.; Zhang, W.; Zhao, C.; Zhang, Y.; Wu, H.; Jin, J.; Zhang, W.; Grenz, A.; Eltzschig, H.K.; Tao, L.; et al. Elevated Endothelial Hypoxia-Inducible Factor-1α Contributes to Glomerular Injury and Promotes Hypertensive Chronic Kidney Disease. Hypertension 2015, 66, 75–84. [Google Scholar] [CrossRef]
- Kimura, K.; Iwano, M.; Higgins, D.F.; Yamaguchi, Y.; Nakatani, K.; Harada, K.; Kubo, A.; Akai, Y.; Rankin, E.B.; Neilson, E.G.; et al. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am. J. Physiol. Ren. Physiol. 2008, 295, F1023–F1029. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, H.; Wang, Y.; Wu, M.; Cao, Y.; Huang, W.; Li, L.; Ji, Z.; Sun, H. Allicin protects against myocardial apoptosis and fibrosis in streptozotocin-induced diabetic rats. Phytomedicine 2012, 19, 693–698. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Huang, L.; Zhu, X.; Sha, J.; Li, G.; Ma, G.; Zhang, W.; Gu, M.; Guo, Y.; et al. Nrf2 signaling attenuates epithelial-to-mesenchymal transition and renal interstitial fibrosis via PI3K/Akt signaling pathways. Exp. Mol. Pathol. 2019, 111, 104296. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Wang, S.; Lin, Y.; Zhao, Y.; Yang, S.; Song, J.; Xie, T.; Tian, J.; Wu, S.; Du, G. Antihyperuricemic effect of mangiferin aglycon derivative J99745 by inhibiting xanthine oxidase activity and urate transporter 1 expression in mice. Acta Pharm. Sin. B 2017, 8, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, X.; Chu, X.; Wang, J.; Xie, B.; Ge, J.; Guo, Y.; Li, X.; Yang, G. Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota. Nutrients 2019, 11, 2909. [Google Scholar] [CrossRef] [PubMed]
- Padiya, R. Garlic as an anti-diabetic agent: Recent progress and patent reviews. Recent Patents Food Nutr. Agric. 2013, 5, 105–127. [Google Scholar] [CrossRef]
- Melino, S.; Leo, S.; Toska Papajani, V. Natural Hydrogen Sulfide Donors from Allium sp. as a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy. Nutrients 2019, 11, 1581. [Google Scholar] [CrossRef]
- Reynaldi, M.A.; Riza, H.; Luliana, S. Docking study of allicin with sulfonylurea receptor 1, complex 1 and PPARγ receptor on insulin resistance. Int. J. Pharm. Pharm. Sci. 2018, 10, 130–133. [Google Scholar] [CrossRef][Green Version]
- Schultz, C.R.; Gruhlke, M.C.H.; Slusarenko, A.J.; Bachmann, A.S. Allicin, a Potent New Ornithine Decarboxylase Inhibitor in Neuroblastoma Cells. J. Nat. Prod. 2020, 83, 2518–2527. [Google Scholar] [CrossRef]

| Parameter/Group | Ctrl | DM | DA |
|---|---|---|---|
| Urinary volume (mL/24 h) | 16.44 ± 2.10 | 44.67 ± 6.97 * | 33.18 ± 2.53 *+ |
| sCr (mg/dL) | 0.69 ± 0.01 | 0.73 ± 0.04 | 0.73 ± 0.01 |
| BUN (mg/dL) | 22.83 ± 1.108 | 30.44 ± 0.88 * | 27.60 ± 1.22 * |
| uCr (mg/mL) | 7.44 ± 0.69 | 28.88 ± 5.96 * | 22.50 ± 2.72 * |
| uUrea (mg/mL) | 160.6 ± 12.77 | 323.4 ± 21.36 * | 256.5 ± 18.83 *,+ |
| CrCl (ml/min) | 0.75 ± 0.016 | 2.66 ± 0.20 * | 2.15 ± 0.08 *,+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arellano-Buendía, A.S.; Castañeda-Lara, L.G.; Loredo-Mendoza, M.L.; García-Arroyo, F.E.; Rojas-Morales, P.; Argüello-García, R.; Juárez-Rojas, J.G.; Tapia, E.; Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; et al. Effects of Allicin on Pathophysiological Mechanisms during the Progression of Nephropathy Associated to Diabetes. Antioxidants 2020, 9, 1134. https://doi.org/10.3390/antiox9111134
Arellano-Buendía AS, Castañeda-Lara LG, Loredo-Mendoza ML, García-Arroyo FE, Rojas-Morales P, Argüello-García R, Juárez-Rojas JG, Tapia E, Pedraza-Chaverri J, Sánchez-Lozada LG, et al. Effects of Allicin on Pathophysiological Mechanisms during the Progression of Nephropathy Associated to Diabetes. Antioxidants. 2020; 9(11):1134. https://doi.org/10.3390/antiox9111134
Chicago/Turabian StyleArellano-Buendía, Abraham Said, Luis Gerardo Castañeda-Lara, María L. Loredo-Mendoza, Fernando E. García-Arroyo, Pedro Rojas-Morales, Raúl Argüello-García, Juan G. Juárez-Rojas, Edilia Tapia, José Pedraza-Chaverri, Laura Gabriela Sánchez-Lozada, and et al. 2020. "Effects of Allicin on Pathophysiological Mechanisms during the Progression of Nephropathy Associated to Diabetes" Antioxidants 9, no. 11: 1134. https://doi.org/10.3390/antiox9111134
APA StyleArellano-Buendía, A. S., Castañeda-Lara, L. G., Loredo-Mendoza, M. L., García-Arroyo, F. E., Rojas-Morales, P., Argüello-García, R., Juárez-Rojas, J. G., Tapia, E., Pedraza-Chaverri, J., Sánchez-Lozada, L. G., & Osorio-Alonso, H. (2020). Effects of Allicin on Pathophysiological Mechanisms during the Progression of Nephropathy Associated to Diabetes. Antioxidants, 9(11), 1134. https://doi.org/10.3390/antiox9111134









