Oxidized Low-Density Lipoprotein Associates with Ventricular Stress in Young Adults and Triggers Intracellular Ca2+ Alterations in Adult Ventricular Cardiomyocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Study
2.2. Experimental Study
2.2.1. Cardiomyocyte Isolation
2.2.2. Intracellular Ca2+ Imaging
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. CRP, NT-proBNP and oxLDL Levels in Young Adults
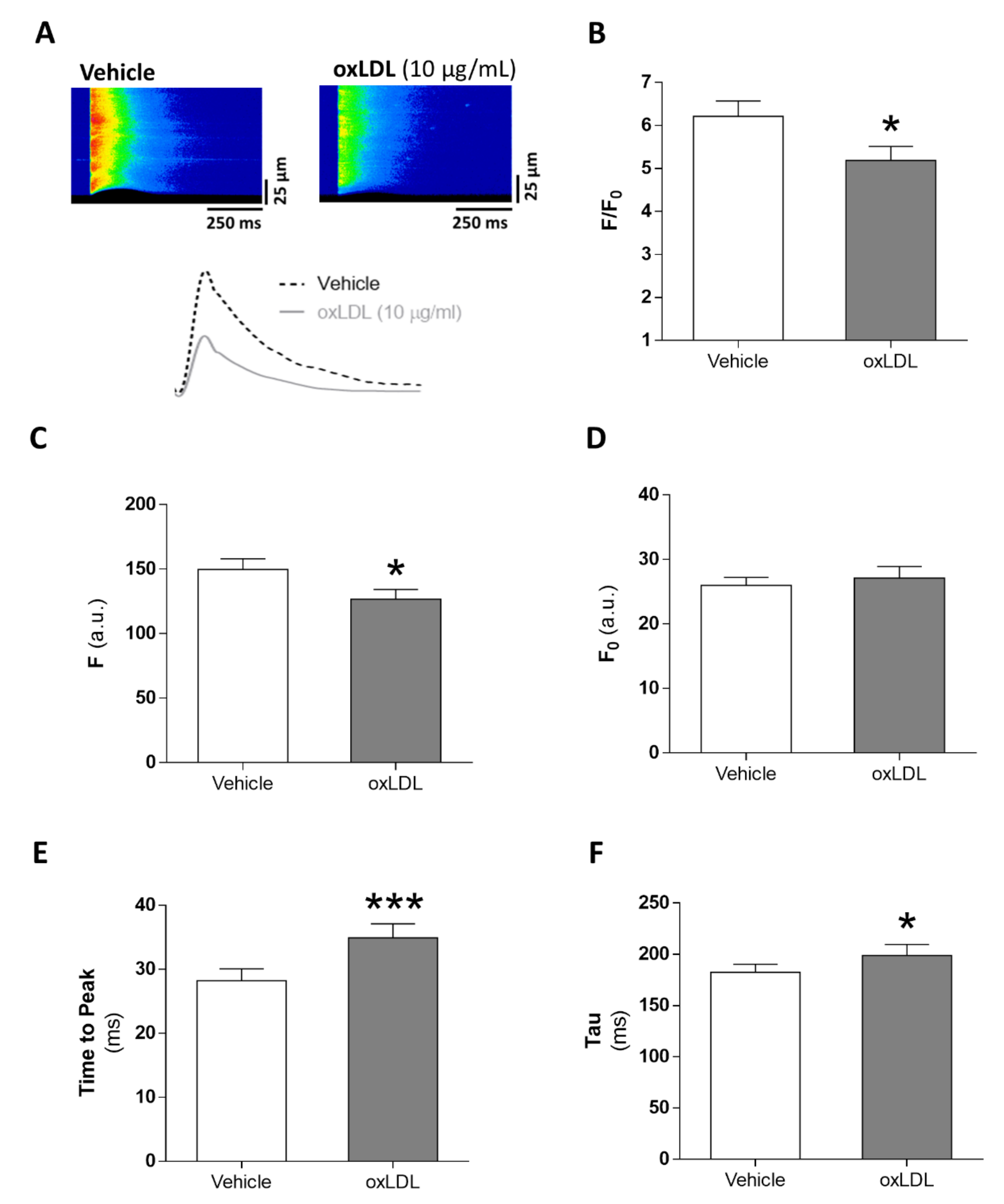
3.3. Acute Exposure to oxLDL Alters Systolic Ca2+ Release in Isolated Rat Cardiomyocytes
3.4. Acute Exposure to oxLDL Induces Diastolic SR-Ca2+ Leak and a Pro-Arrhythmogenic Profile in the Form of Diastolic Ca2+ Waves
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CRP | C-reactive protein |
| CVR | cardiovascular risk |
| EC | excitation–contraction |
| LDL | low-density lipoprotein |
| NCX | Na+-Ca2+ exchanger |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| oxLDL | oxidized LDL |
| ROS | reactive oxygen species |
| SCAD | stable coronary artery disease |
| SERCA | sarcoplasmic reticulum Ca2+-adenosine triphosphatase 2a |
| SR | sarcoplasmic reticulum |
References
- Hajri, T. Effects of oxidized lipids and lipoproteins on cardiac function. Front. Biosci. 2018, 23, 1822–1847. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma Oxidized Low-Density Lipoprotein, a Strong Predictor for Acute Coronary Heart Disease Events in Apparently Healthy, Middle-Aged Men from the General Population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association Between Circulating Oxidized LDL and Atherosclerotic Cardiovascular Disease: A Meta-analysis of Observational Studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef]
- Fabiato, A.; Fabiato, F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J. Physiol. 1975, 249, 469–495. [Google Scholar] [CrossRef] [Green Version]
- Bers, D.M. Altered Cardiac Myocyte Ca Regulation in Heart Failure. Physiology 2006, 21, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Nikolaienko, R.; Bovo, E.; Zima, A.V. Redox Dependent Modifications of Ryanodine Receptor: Basic Mechanisms and Implications in Heart Diseases. Front. Physiol. 2018, 9, 1775. [Google Scholar] [CrossRef] [Green Version]
- Schlüter, K.-D.; Wolf, A.; Weber, M.; Schreckenberg, R.; Schulz, R. Oxidized low-density lipoprotein (oxLDL) affects load-free cell shortening of cardiomyocytes in a proprotein convertase subtilisin/kexin 9 (PCSK9)-dependent way. Basic Res. Cardiol. 2017, 112, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Li, H.-Z.; Zhang, W.-H.; Wang, L.-C.; Wang, L.-N.; Zhang, L.; Li, G.-W.; Yang, B.; Wu, L.; Wang, R.; et al. Increased expression of calcium-sensing receptors induced by ox-LDL amplifies apoptosis of cardiomyocytes during simulated ischaemia-reperfusion. Clin. Exp. Pharmacol. Physiol. 2010, 37, e128–e135. [Google Scholar] [CrossRef]
- Liu, K.; Massaeli, H.; Pierce, G.N. The action of oxidized low density lipoprotein on calcium transients in isolated rabbit cardiomyocytes. J. Biol. Chem. 1993, 268, 4145–4151. [Google Scholar]
- Val-Blasco, A.; Piedras, M.J.G.; Ruiz-Hurtado, G.; Suarez, N.; Prieto, P.; Gonzalez-Ramos, S.; Gómez-Hurtado, N.; Delgado, C.; Pereira, L.; Benito, G.; et al. Role of NOD1 in Heart Failure Progression via Regulation of Ca 2+ Handling. J. Am. Coll. Cardiol. 2017, 69, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chaparro, M.A.; Román-García, J.; Calvo-Bonacho, E.; Gómez-Larios, T.; Fernández-Meseguer, A.; Sáinz-Gutiérrez, J.C.; Cabrera-Sierra, M.; García-García, A.; Rueda-Vicente, J.; Gálvez-Moraleda, A.; et al. Prevalence of Cardiovascular Risk Factors in the Spanish Working Population. Revista Española de Cardiología 2006, 59, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.; Robson, J.; Brindle, P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: Cohort study using QResearch database. BMJ 2010, 341, c6624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Sánchez, E.; García, J.A.N.; Aceves-Ripoll, J.; González-Lafuente, L.; Corbacho-Alonso, N.; Martinez, P.; Calvo-Bonacho, E.; Alvarez-Llamas, G.; Barderas, M.G.; Ruilope, L.M.; et al. Lifetime cardiovascular risk is associated with a multimarker score of systemic oxidative status in young adults independently of traditional risk factors. Transl. Res. 2019, 212, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 2014, 52, 70–85. [Google Scholar] [CrossRef] [PubMed]
- García, J.A.N.; Delgado, C.; Fernández-Velasco, M.; Val-Blasco, A.; Rodríguez-Sánchez, E.; Aceves-Ripoll, J.; Gómez-Hurtado, N.; Bada-Bosch, T.; Mérida-Herrero, E.; Hernández, E.; et al. Fibroblast growth factor-23 promotes rhythm alterations and contractile dysfunction in adult ventricular cardiomyocytes. Nephrol. Dial. Transplant. 2019, 34, 1864–1875. [Google Scholar] [CrossRef]
- Ruiz-Hurtado, G.; Gómez-Hurtado, N.; Fernández-Velasco, M.; Calderón, E.; Smani, T.; Ordoñez, A.; Cachofeiro, V.; Boscá, L.; Díez, J.; Gómez, A.M.; et al. Cardiotrophin-1 induces sarcoplasmic reticulum Ca2+ leak and arrhythmogenesis in adult rat ventricular myocytes. Cardiovasc. Res. 2012, 96, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Berry, J.D.; Dyer, A.; Cai, X.; Garside, D.B.; Ning, H.; Thomas, A.; Greenland, P.; Van Horn, L.; Tracy, R.P.; Lloyd-Jones, D.M. Lifetime Risks of Cardiovascular Disease. N. Engl. J. Med. 2012, 366, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Hear. J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Sørensen, A.L.; Hasselbalch, H.C.; Nielsen, C.H.; Poulsen, H.E.; Ellervik, C. Statin treatment, oxidative stress and inflammation in a Danish population. Redox Biol. 2019, 21, 101088. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.; Park, I.B.; Lee, K.; Ahn, T.H.; Bin Park, W.; Kim, J.H.; Ahn, Y.; Jeong, M.H.; Lee, D.H. Statin has more protective effects in AMI patients with higher plasma BNP or NT-proBNP level, but not with lower left ventricular ejection fraction. J. Cardiol. 2018, 71, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Node, K.; Fujita, M.; Kitakaze, M.; Hori, M.; Liao, J.K. Short-Term Statin Therapy Improves Cardiac Function and Symptoms in Patients with Idiopathic Dilated Cardiomyopathy. Circulation 2003, 108, 839–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stypmann, J.; Schubert, A.; Welp, H.; Schulte, H.; Assmann, G.; Breithardt, G.; Nofer, J.-R. Atorvastatin Therapy Is Associated with Reduced Levels of N-terminal Prohormone Brain Natriuretic Peptide and Improved Cardiac Function in Patients with Heart Failure. Clin. Cardiol. 2008, 31, 478–481. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Omland, T.; Wolf, P.A.; Vasan, R.S. Plasma Natriuretic Peptide Levels and the Risk of Cardiovascular Events and Death. N. Engl. J. Med. 2004, 350, 655–663. [Google Scholar] [CrossRef]
- Willeit, P.; Kaptoge, S.; Welsh, P.; Butterworth, A.S.; Chowdhury, R.; Spackman, S.A.; Pennells, L.; Gao, P.; Burgess, S.; Freitag, D.F.; et al. Natriuretic peptides and integrated risk assessment for cardiovascular disease: An individual-participant-data meta-analysis. Lancet Diabetes Endocrinol. 2016, 4, 840–849. [Google Scholar] [CrossRef] [Green Version]
- Rietzschel, E.-R.; Langlois, M.; De Buyzere, M.L.; Segers, P.; De Bacquer, D.; Bekaert, S.; Cooman, L.; Van Oostveldt, P.; Verdonck, P.; De Backer, G.; et al. Oxidized Low-Density Lipoprotein Cholesterol Is Associated with Decreases in Cardiac Function Independent of Vascular Alterations. Hypertension 2008, 52, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, T.; Tsutamoto, T.; Wada, A.; Maeda, K.; Mabuchi, N.; Hayashi, M.; Ohnishi, M.; Kinoshita, M. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J. Am. Coll. Cardiol. 2002, 39, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, O.; Mohanty, B.D.; Martin, S.S.; Joshi, P.H.; Blaha, M.J.; Nasir, K.; Blumenthal, R.S.; Budoff, M.J. High-Sensitivity C-Reactive Protein and Cardiovascular Disease. J. Am. Coll. Cardiol. 2013, 62, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Daniels, L.B.; Maisel, A.S. Natriuretic Peptides. J. Am. Coll. Cardiol. 2007, 50, 2357–2368. [Google Scholar] [CrossRef] [Green Version]
- Daniels, L.B.; Clopton, P.; Jiang, K.; Greenberg, B.; Maisel, A.S. Prognosis of Stage A or B Heart Failure Patients with Elevated B-type Natriuretic Peptide Levels. J. Card. Fail. 2010, 16, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Shomanova, Z.; Ohnewein, B.; Schernthaner, C.; Höfer, K.; Pogoda, C.A.; Frommeyer, G.; Wernly, B.; Brandt, M.C.; Dieplinger, A.M.; Reinecke, H.; et al. Classic and Novel Biomarkers as Potential Predictors of Ventricular Arrhythmias and Sudden Cardiac Death. J. Clin. Med. 2020, 9, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggers, K.M.; Lindahl, B. Prognostic Biomarkers in Acute Coronary Syndromes: Risk Stratification beyond Cardiac Troponins. Curr. Cardiol. Rep. 2017, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Morrow, D.A.; Jablonski, K.A.; Rice, M.M.; Warnica, J.W.; Domanski, M.J.; Hsia, J.; Gersh, B.J.; Rifai, N.; Ridker, P.M.; et al. Prognostic Significance of the Centers for Disease Control/American Heart Association High-Sensitivity C-Reactive Protein Cut Points for Cardiovascular and Other Outcomes in Patients with Stable Coronary Artery Disease. Circulation 2007, 115, 1528–1536. [Google Scholar] [CrossRef] [Green Version]
- Leistner, D.M.; Klotsche, J.; Pieper, L.; Palm, S.; Stalla, G.K.; Lehnert, H.; Silber, S.; März, W.; Wittchen, H.-U.; Zeiher, A.M. Prognostic value of NT-pro-BNP and hs-CRP for risk stratification in primary care: Results from the population-based DETECT study. Clin. Res. Cardiol. 2013, 102, 259–268. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Blacher, J.; De Edelenyi, F.S.; Faure, P.; Julia, C.; Hercberg, S.; Galan, P. Prognostic value of multiple emerging biomarkers in cardiovascular risk prediction in patients with stable cardiovascular disease. Atherosclerosis 2013, 228, 478–484. [Google Scholar] [CrossRef]
- Magnussen, C.; Blankenberg, S. Biomarkers for heart failure: Small molecules with high clinical relevance. J. Intern. Med. 2018, 283, 530–543. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sun, Y.; Yang, H.; Lu, Y.; Li, L. Oxidized Low-Density Lipoprotein Induces Apoptosis in Cultured Neonatal Rat Cardiomyocytes by Modulating the TLR4/NF-κB Pathway. Sci. Rep. 2016, 6, 27866. [Google Scholar] [CrossRef]
- Iwai-Kanai, E.; Hasegawa, K.; Sawamura, T.; Fujita, M.; Yanazume, T.; Toyokuni, S.; Adachi, S.; Kihara, Y.; Sasayama, S. Activation of lectin-like oxidized low-density lipoprotein receptor-1 induces apoptosis in cultured neonatal rat cardiac myocytes. Circulation 2001, 104, 2948–2954. [Google Scholar] [CrossRef] [Green Version]
- Chandrakala, A.N.; Sukul, D.; Selvarajan, K.; Sai-Sudhakar, C.; Sun, B.; Parthasarathy, S. Induction of brain natriuretic peptide and monocyte chemotactic protein-1 gene expression by oxidized low-density lipoprotein: Relevance to ischemic heart failure. Am. J. Physiol. Physiol. 2012, 302, C165–C177. [Google Scholar] [CrossRef]
- Sunman, H.; Özkan, A.; Yorgun, H.; Canpolat, U.; Karabulut, E.; Bayrak, T.; Kaya, E.B.; Tokgözoğlu, L.; Ozer, N.; Özkara, A.; et al. Evaluating the effects of cardiac resynchronization therapy on pathophysiological pathways of heart failure using surrogate biomarkers. Cardiol. J. 2018, 25, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hurtado, G.; Li, L.; Fernández-Velasco, M.; Rueda, A.; Lefebvre, F.; Wang, Y.; Mateo, P.; Cassan, C.; Gellen, B.; Benitah, J.P.; et al. Reconciling depressed Ca2+ sparks occurrence with enhanced RyR2 activity in failing mice cardiomyocytes. J. Gen. Physiol. 2015, 146, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorn-Pauly, K.; Schaffer, P.; Pelzmann, B.; Bernhart, E.; Wei, G.; Lang, P.; Ledinski, G.; Greilberger, J.; Koidl, B.; Jürgens, G. Oxidized LDL induces ventricular myocyte damage and abnormal electrical activity?role of lipid hydroperoxides. Cardiovasc. Res. 2005, 66, 74–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.S.; Di Li, T.; Zeng, Z.H. Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids Health Dis. 2020, 19, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zipes, D.; Wellens, H.J.J. Sudden Cardiac Death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef]
- Hayashi, M.; Shimizu, W.; Albert, C.M. The Spectrum of Epidemiology Underlying Sudden Cardiac Death. Circ. Res. 2015, 116, 1887–1906. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Castellanos, A.; Myerburg, R.J. Sudden Death Due to Cardiac Arrhythmias. N. Engl. J. Med. 2001, 345, 1473–1482. [Google Scholar] [CrossRef]
- Benchimol, D.; Dubroca, B.; Bernard, V.; Lavie, J.; Paviot, B.; Benchimol, H.; Couffinhal, T.; Pillois, X.; Dartigues, J.-F.; Bonnet, J. Short- and long-term risk factors for sudden death in patients with stable angina. Int. J. Cardiol. 2000, 76, 147–156. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Zaccardi, F.; Karppi, J.; Kurl, S.; Laukkanen, J.A. Is High Serum LDL/HDL Cholesterol Ratio an Emerging Risk Factor for Sudden Cardiac Death? Findings from the KIHD Study. J. Atheroscler. Thromb. 2017, 24, 600–608. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-B.; Wu, C.-C.; Lee, C.-M.; Chen, W.-J.; Wang, T.-D.; Lee, Y.-T.; Chen, P.-S. Dyslipidemia is Associated with Ventricular Tachyarrhythmia in Patients with Acute ST-Segment Elevation Myocardial Infarction. J. Formos. Med Assoc. 2006, 105, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-B.; Wu, C.-C.; Lu, L.-S.; Su, M.-J.; Lin, C.-W.; Cheng, J.; Chen, L.S.; Fishbein, M.C.; Chen, P.-S.; Lee, Y.-T. Sympathetic Nerve Sprouting, Electrical Remodeling, and Increased Vulnerability to Ventricular Fibrillation in Hypercholesterolemic Rabbits. Circ. Res. 2003, 92, 1145–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, P.; Quan, D.; Huang, Y.; Huang, H. CaMKII Activation Promotes Cardiac Electrical Remodeling and Increases the Susceptibility to Arrhythmia Induction in High-fat Diet–Fed Mice with Hyperlipidemia Conditions. J. Cardiovasc. Pharmacol. 2017, 70, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, T.-Y.; Su, M.-J.; Yang, Y.-F.; Liu, Y.-B.; Liang, H.-C.; Wu, C.-C.; Lee, Y.-T. Effect of hypercholesterolemia on myocardial function in New Zealand white rabbits. J. Biomed. Sci. 2004, 11, 829–837. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, N.M.; Wang, L.; Herren, A.W.; Wang, J.; Shenasa, F.; Bers, D.M.; Lindsey, M.L.; Ripplinger, C.M. Atherosclerosis exacerbates arrhythmia following myocardial infarction: Role of myocardial inflammation. Hear. Rhythm. 2015, 12, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.A.; Russo, V.; Ceraso, S.; Gupta, D.; Barrett-Jolley, R. Anti-arrhythmic properties of non-antiarrhythmic medications. Pharmacol. Res. 2020, 156, 104762. [Google Scholar] [CrossRef] [PubMed]
- Pilote, L.; Dasgupta, K.; Guru, V.; Humphries, K.H.; McGrath, J.; Norris, C.; Rabi, D.; Tremblay, J.; Alamian, A.; Barnett, T.; et al. A comprehensive view of sex-specific issues related to cardiovascular disease. Can. Med. Assoc. J. 2007, 176, S1–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Low-Lifetime CVR (n = 22) | High-Lifetime CVR (n = 21) | SCAD (n = 23) | |
|---|---|---|---|
| Age (years) | 44.1 ± 4.0 | 43.9 ± 5.3 | 44.8 ± 4.4 |
| Males (n, %) | 6 (27) | 16 (76) ** | 19 (83) *** |
| BMI (kg/m2) | 23.4 ± 2.6 | 29.8 ± 4.4 *** | 28.3 ± 5.7 ** |
| Hypertension (n, %) | 0 (0) | 13 (62) *** | 9 (39) ** |
| SPB (mmHg) | 111.7 ± 9.1 | 137.3 ± 12.3 *** | 122.8 ± 20.0 *,## |
| DBP (mmHg) | 71.1 ± 9.9 | 88.3 ± 9.8 *** | 76.3 ± 12.3 ### |
| Antihypertensive treatment (n, %) | 0 (0) | 8 (38) ** | 8 (35) ** |
| Dyslipidemia (n, %) | 0 (0) | 9 (43) *** | 3 (13) # |
| Total cholesterol (mg/dL) | 188.1 ± 29.7 | 210.7 ± 38.9 * | 143.5 ± 41.6 ***,### |
| LDL-cholesterol (mg/dL) | 99.4 ± 23.2 | 137.2 ± 35.8 *** | 78.5 ± 39.4 *,### |
| HDL-cholesterol (mg/dL) | 72.7 ± 16.7 | 41.2 ± 11.5 *** | 39.3 ± 9.5 *** |
| Triglycerides (mg/dL) | 75.5 ± 28.4 | 207.8 ± 100.8 *** | 116.2 ± 56.4 ### |
| Statin treatment (n, %) | 0 (0) | 5 (24) * | 23 (100) ***,### |
| Renal function | |||
| eGFR (mL/min/1.73m2) | 98.0 ± 11.0 | 96.5 ± 11.0 | 97.8 ± 16.3 |
| Serum creatinine (mg/dL) | 0.797 ± 0.115 | 0.905 ± 0.136 | 0.892 ± 0.191 |
| Cardiovascular risk | |||
| QRisk-lifetime (%) | 21.3 ± 2.7 | 42.9 ± 9.2 *** |
| B | CI | p-Value | |
|---|---|---|---|
| oxLDL | 0.459 | 0.11–0.81 | 0.011 |
| LDL | −0.078 | −0.21–0.05 | 0.227 |
| Sex | 7.223 | −1.21–15.66 | 0.092 |
| BMI | −0.831 | −1.80–0.14 | 0.092 |
| SBP | 0.353 | −0.097–0.61 | 0.008 |
| CRP | −0.313 | −2.39–1.76 | 0.763 |
| Treatment | Peak (F/F0) | Full Width at Half Maximum (µm) | Full Duration at Half Maximum (ms) | N (Sparks/Cells/Rats) |
|---|---|---|---|---|
| Vehicle | 1.75 ± 0.18 | 3.73 ± 0.57 | 29.5 ± 4.7 | 1014/21/5 |
| oxLDL | 1.06 ± 0.16 * | 3.57 ± 0.46 | 31.6 ± 8.1 | 1325/21/5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sánchez, E.; Navarro-García, J.A.; González-Lafuente, L.; Aceves-Ripoll, J.; Vázquez-Sánchez, S.; Poveda, J.; Mercado-García, E.; Corbacho-Alonso, N.; Calvo-Bonacho, E.; Fernández-Velasco, M.; et al. Oxidized Low-Density Lipoprotein Associates with Ventricular Stress in Young Adults and Triggers Intracellular Ca2+ Alterations in Adult Ventricular Cardiomyocytes. Antioxidants 2020, 9, 1213. https://doi.org/10.3390/antiox9121213
Rodríguez-Sánchez E, Navarro-García JA, González-Lafuente L, Aceves-Ripoll J, Vázquez-Sánchez S, Poveda J, Mercado-García E, Corbacho-Alonso N, Calvo-Bonacho E, Fernández-Velasco M, et al. Oxidized Low-Density Lipoprotein Associates with Ventricular Stress in Young Adults and Triggers Intracellular Ca2+ Alterations in Adult Ventricular Cardiomyocytes. Antioxidants. 2020; 9(12):1213. https://doi.org/10.3390/antiox9121213
Chicago/Turabian StyleRodríguez-Sánchez, Elena, José Alberto Navarro-García, Laura González-Lafuente, Jennifer Aceves-Ripoll, Sara Vázquez-Sánchez, Jonay Poveda, Elisa Mercado-García, Nerea Corbacho-Alonso, Eva Calvo-Bonacho, María Fernández-Velasco, and et al. 2020. "Oxidized Low-Density Lipoprotein Associates with Ventricular Stress in Young Adults and Triggers Intracellular Ca2+ Alterations in Adult Ventricular Cardiomyocytes" Antioxidants 9, no. 12: 1213. https://doi.org/10.3390/antiox9121213
APA StyleRodríguez-Sánchez, E., Navarro-García, J. A., González-Lafuente, L., Aceves-Ripoll, J., Vázquez-Sánchez, S., Poveda, J., Mercado-García, E., Corbacho-Alonso, N., Calvo-Bonacho, E., Fernández-Velasco, M., Álvarez-Llamas, G., Barderas, M. G., Ruilope, L. M., & Ruiz-Hurtado, G. (2020). Oxidized Low-Density Lipoprotein Associates with Ventricular Stress in Young Adults and Triggers Intracellular Ca2+ Alterations in Adult Ventricular Cardiomyocytes. Antioxidants, 9(12), 1213. https://doi.org/10.3390/antiox9121213








