Abstract
This study determined acteoside and its content in Abeliophyllum distichum via HPLC/UV and LC/ESI-MS to obtain insights into the potential use of this plant as an antioxidant agent. Moreover, 1,1-diphenyl-2-picrylhydrazyl (DPPH), hydroxyl (•OH), and O2− radical scavenging activity assays were performed to assess in vitro antioxidative activity. The DPPH, •OH, and O2− radical scavenging activities of A. distichum leaf EtOH extracts at a 250 μg/mL concentration were 88.32%, 94.48%, and 14.36%, respectively, whereas those of stem extracts at the same concentration were 88.15%, 88.99%, and 15.36%, respectively. The contents of acteoside in A. distichum leaves and stems were 162.11 and 29.68 mg/g, respectively. Acteoside was identified as the main antioxidant compound in A. distichum leaves, which resulted in DPPH, •OH, and O2− radical scavenging activities of 82.84%, 89.46%, and 30.31%, respectively, at a 25 μg/mL concentration. These results indicate that A. distichum leaves and stems containing the antioxidant acteoside can be used as natural ingredients for functional and nutritional supplements.
1. Introduction
Abeliophyllum distichum Nakai is an important plant resource and represents the only species within the genus Abeliophyllum in the world [1]. A. distichum is an endemic plant in Korea and is commonly referred to as white forsythia. Currently, this plant is protected and has been designated as an endangered plant species in Buan-, Goesan-, and Yeongdong-gun, Korea [2,3], with Goesan-gun being the main producer of this resource. Although it has been used as a landscape plant, A. distichum has also been found to possess therapeutic value due to its anti-cancer [4], anti-diabetic (via aldose reductase inhibition) [5], and anti-hypertensive properties [3]. A. distichum is known to contain some glycosides in its leaves including acteoside, isoacteoside, rutin, and hirsutrin [6], responsible for its anti-inflammatory [7], anti-nociceptive activities [8], and antioxidant activity [6,9,10], and it has also been reported to improve sexual function [11]. Acteoside, a commonly identified phenylpropanoid glycoside in plants [12], is a well-known antioxidant and was first isolated from Syringa vulgaris flowers [13]. Moreover, the crude ash contents of A. distichum leaves and stems are 1.32% and 0.91%, respectively, and its fructose and glucose contents have been reported as 32.13 and 56.17 mg/g for the leaves, and 11.38 and 10.59 mg/g for the stems, respectively [14].
Free radicals have been linked to the onset of many adverse health effects such as aging, diabetes, cardiovascular, and neurodegenerative diseases [15]. Free radicals including O2−, hydroxyl (•OH), and singlet oxygen (1O2) are highly reactive in the body and produce reactive oxygen species (ROS) and other free radicals [15]. ROS deteriorate crucial biomolecules for biological functions such as lipids, proteins, DNA, and RNA, thereby leading to cell death and the loss of physiological functions in the body [16]. The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical is a stable free radical that exhibits a characteristic violet color when reduced by antioxidant materials. Therefore, it is widely used in measuring antioxidant activity [17]. The •OH radical is generated from hydrogen peroxide (H2O2) and O2− by Fenton reaction and is more strongly reactive with biological molecules than other radicals, resulting in many diseases [18]. The O2− is generated from molecular oxygen by reduction of one electron in the mitochondrial electron transport chain, endoplasmic reticulum, NADPH oxidase, cytochrome P450, and xanthine oxidase [19]. It is rapidly converted by superoxide dismutase into H2O2, which becomes a highly reactive •OH radical in the presence of transition metals and peroxynitrite with nitric oxide [20].
ROS, also known as oxygen free radicals by characteristics of unpaired valence electrons or unstable bonds, are among the most important factors that lead to aging and other related diseases such as neurodegenerative diseases [15]. In normal cases, ROS are produced via cellular respiration, metabolic byproducts, enzymatic synthesis, and physical and chemical processes in the body, but they can be removed by the body’s antioxidant system such as enzymatic defenses and antioxidant scavengers [21]. However, the incomplete reduction and overproduction of ROS during various physiological processes may lead to oxidative damage of the cells [22]. Therefore, research on antioxidants that can protect living organisms from ROS oxidation is being actively conducted, with a particular focus on naturally occurring antioxidant agents. Among various naturally occurring antioxidants, phenolic compounds are phytochemicals derived from various plants as a result of their secondary metabolism. In particular, many studies demonstrated that phenolic compounds possess various pharmacological properties including anti-aging, anti-neurodegenerative, and anti-cancer activities, by their antioxidant activity counteracts the effects of ROS in the body [23].
In this study, ethanolic (EtOH) extracts were investigated to determine their acteoside distribution and quantify their content in A. distichum via high-performance liquid chromatography (HPLC) coupled with ultraviolet-visible (UV) and electrospray ionization (ESI) ion trap mass spectrometry (MS) detection. Bioactivity of the extracts and acteoside was evaluated by assessing the antioxidant capacity of the A. distichum extracts.
2. Materials and Methods
2.1. Plant Materials and Isolation of Acteoside
A. distichum leaves and stems were obtained from Miseonnamu Products Co., Goesan, Korea. A voucher specimen (No. LEE19-01) was deposited at the Department of Plant Science and Technology Herbarium, Chung-Ang University, Korea. Dried leaves (300 g) of A. distichum were extracted with EtOH for 3 h under reflux and were repeated 3 times. The EtOH extract (120 g) was concentrated, suspended in distilled water, and sequentially partitioned with n-hexane (8 g), chloroform (11 g), ethyl acetate (29 g), and n-butanol (75 g). A portion of the n-butanol fraction (20 g) was subjected to open column chromatography. The column was eluted with a stepwise gradient of chloroform and methanol. The sub-fractions were analyzed by TLC, and the dried residue was further purified with Sephadex LH-20 column chromatography. Fractions of similar composition as determined by TLC were pooled and acteoside (yield, 0.8%) (Figure 1) was obtained by methanol (MeOH) recrystallization. TLC was carried out on pre-coated silica gel 60 F254 plates (Merck), developed with chloroform–methanol–water (5.5:4.5:0.2). Open column chromatography was performed on a silica gel column (60–200 mesh, Zeochem) and Sephadex LH-20 (Sigma Aldrich). The purity of acteoside was 98% as determined by HPLC analysis.
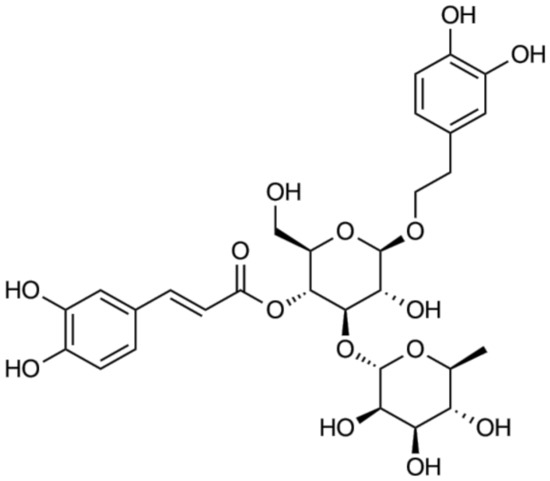
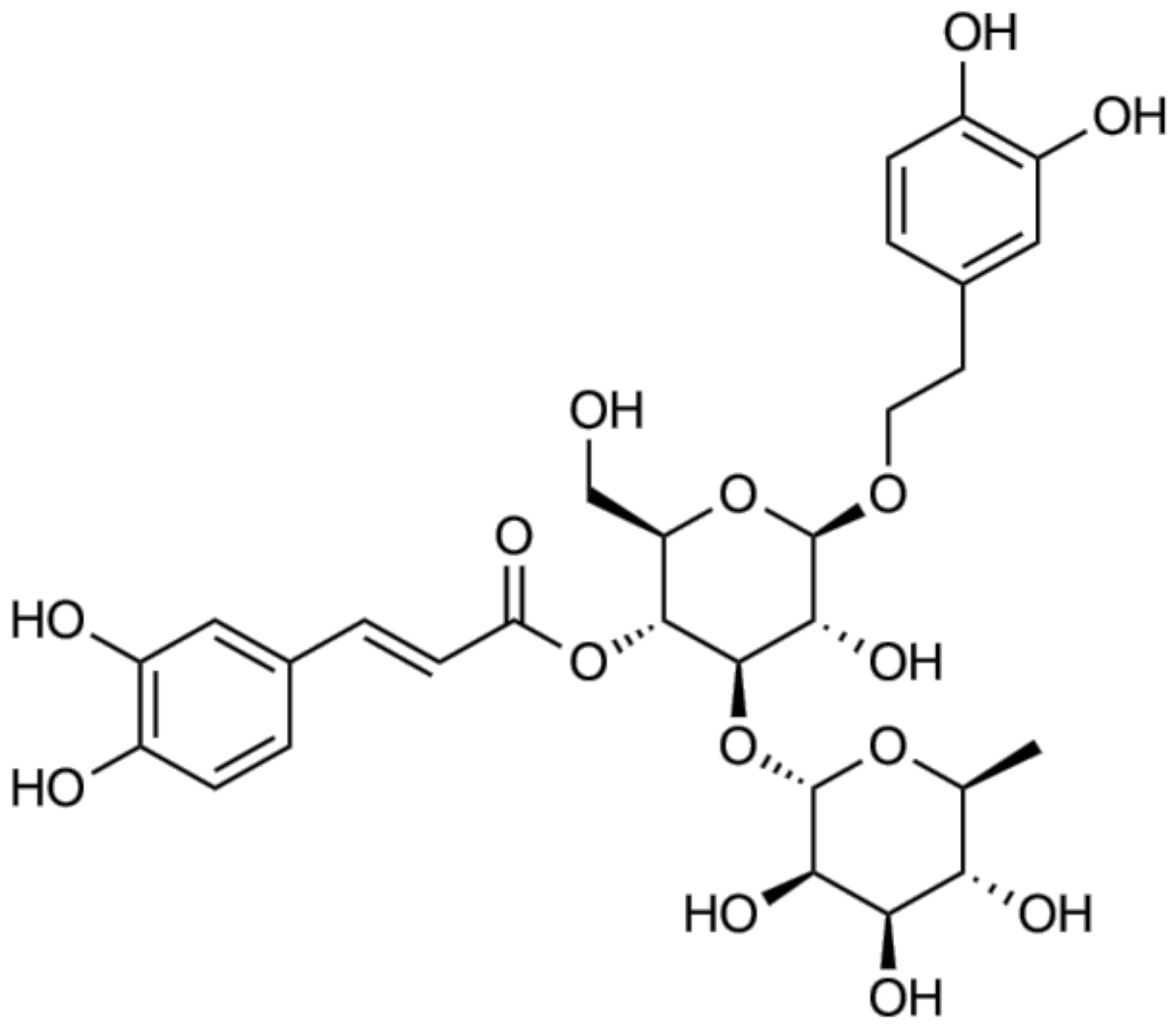
Figure 1.
Chemical structure of acteoside.
2.2. Instruments, Chemicals and Reagents
DPPH and 2-deoxy-ribose were purchased from Sigma (St. Louis, MO, USA), FeSO4•7H2O-EDTA was purchased from Daejung Chemicals and Metals Co. Ltd. (Siheung, Korea), and H2O2 was obtained from Junsei (Tokyo, Japan). EDTA disodium salt dehydrate was obtained from Samchun Pure Chemical Co. Ltd. (Pyeongtaek, Korea), thiobarbituric acid (TBA) was purchased from Acros Organics (Fair Lawn, NJ, USA), and trichloroacetic acid (TCA) was purchased from Kanto Chemical Co. Inc (Tokyo, Japan). To measure O2− radical scavenging activity, Tris was purchased from LPS Solution (Daejeon, Korea), and phenazine methosulfate (PMS), NADH disodium salt, and nitrotetrazolium blue chloride (NBT) were purchased from Bio Basic Co. (Toronto, Canada). Chromatographic analysis was performed using an HPLC system (Agilent 1260 Infinity II Quat Pump, Santa Clara, CA, USA), equipped with a pump, auto-sampler, and diode array detector (DAD WR detector, Arcade, NY, USA). An Ultimate 3000RS system (Thermo Fisher Scientific Inc., Waltham, MA, USA), equipped with an autosampler and PDA-UV detector was used. Mass spectrometric analyses were performed using a Thermo Finnigan LTQ XL ion trap mass spectrometer, with an electrospray ionization (ESI) interface. HPLC grade solvents such as H2O, MeOH, and acetonitrile (ACN) were obtained from J. T. Baker (Phillipsburg, PA, USA). Acetic acid (99.7%) was obtained from Samchun Pure Chemicals (Pyeongtaek, Korea).
2.3. Preparation of Sample and Standard Solutions for HPLC
The crude EtOH extract (20 mg) of A. distichum leaves and stems were dissolved in 1 mL MeOH and filtered using a syringe filter (0.45 μm). A stock solution of the standard compound was prepared by dissolving 1 mg of acteoside in 1 mL MeOH. To construct an acteoside calibration curve, working solutions were prepared by diluting the stock solution to the desired concentrations.
2.4. DPPH Radical Scavenging Activity
DPPH radical scavenging activity was measured as described by Hatano et al. [24]. The A. distichum EtOH extract and acteoside were first dissolved in EtOH. The samples were then mixed into a 60-μM DPPH solution in 96-well plates, then incubated in the dark at room temperature. After 30 min, absorbance at 540 nm was measured with a microplate reader using l-ascorbic acid as a positive control. DPPH radical scavenging activity was calculated as follows:
DPPH radical scavenging activity (%) = (Absc − Abss)/Absc × 100
Absc: Control absorbance, Abss: Sample absorbance.
2.5. •OH Radical Scavenging Activity
•OH radical scavenging activity was measured via Fenton reaction [25]. The A. distichum EtOH extract and acteoside were dissolved in phosphate-buffered saline, then mixed with 10 mM FeSO4•7H2O-EDTA, 10 mM 2-deoxyribose, and 10 mM H2O2. The mixtures were incubated at 37 ºC in the dark for 4 h, after which 1% TBA and 2.8% TCA solutions were added, and the mixtures were heated to 100 ºC for 20 min. After cooling, the absorbance was measured at 490 nm using a microplate reader. l-ascorbic acid was used as a positive control. •OH radical scavenging activity was calculated as follows:
•OH radical scavenging activity (%) = (Absc − Abss)/Absc × 100
Absc: Control absorbance, Abss: Sample absorbance.
2.6. O2− Radical Scavenging Activity
O2− radical scavenging activity was assessed according to Ewing and Janero et al. [26]. A. distichum samples and acteoside diluted in H2O were mixed with 0.1 M Tris-HCl (pH 7.4), 100 PMS, 500 NBT, and 500 μM NADH, and incubated at room temperature without light. After 10 min, the absorbance was measured at 560 nm using a microplate reader. l-ascorbic acid was used as a positive control. O2- radical scavenging activity was calculated as follows:
O2− radical scavenging activity (%) = (Absc − Abss)/Absc × 100
Absc: Control absorbance, Abss: Sample absorbance.
2.7. HPLC/UV and LC/ESI-MS Conditions
Quantitative analysis of acteoside was performed using a reverse-phase HPLC system with an INNO C18 column (25 cm × 4.6 mm, 5 μm). The injection volume was 10 μL and was monitored at 330 nm. The column temperature was maintained at 25 °C and the flow rate was set at 0.7 mL/min. A gradient elution system of the mobile phase was composed of 0.5% acetic acid in H2O (A) and ACN (B). The elutions were conducted as follows: 90% A at 0 min, followed by 80% A from 0 to 10 min, then 70% A from 10 to 15 min, 50% A from 15 to 20 min, and 0% A from 20 to 30 min, then maintained for 35 min, increased to 90% A from 35 to 40 min, and maintained for 45 min. Regarding the LC/ESI-MS analyses, a Cortecs UPLC T3 column (15 cm × 2.1 mm, 1.6 μm) was used for chromatographic separations. The injection volume was 5 μL and the flow rate was set at 250 μL/min. The mobile phase consisted of 0.1% formic acid in H2O (A) and ACN (B). The elutions were conducted as follows: 90% A at 0 min, followed by 80% A from 0 to 10 min, then 70% A from 10 to 15 min, 50% A from 15 to 20 min, 0% A from 20 to 25 min and maintained for 26 min, then increased to 90% A between 26 and 26.5 min and maintained for 30 min. Ionization of analytes was conducted using a negative mode of ESI. The capillary temperature was maintained at 320 °C; the ion source voltage was set at 3.5 kV, and the sheath gas was set at 42 arb. The capillary voltage was set at 10 V in negative mode. The average scan time was 0.01 min while the average time to change polarity was 0.02 min. The collision energy was generally chosen to maintain an approximately 35% abundance of the precursor ion.
2.8. Calibration Curve
Calibration curves were constructed by plotting the concentrations of the standard solutions with their respective peak areas. The linearity of the calibration curve was determined based on the correlation coefficient (r2), after which the acteoside concentrations in the samples were calculated from the calibration curve. The calibration functions were determined based on the peak area (Y), concentration (X, mg/mL), and mean values (n = 5) ± standard deviation (SD).
2.9. Statistical Analysis
All results were reported as the mean ± SD. Statistical significance (p < 0.05) was determined via analysis of variance (ANOVA) followed by Duncan’s multiple test using the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA) program.
3. Results and Discussion
The body has natural antioxidant systems such as antioxidant enzymes and antioxidant scavengers [27]. Antioxidant enzymes such as superoxide dismutase, glutathione peroxidases, and catalase have an antioxidant defense by detoxifying ROS [28]. In addition, antioxidant scavengers from dietary origin include tocopherol, ascorbic acid, and polyphenols, which play an important role in ROS detoxification [27]. Therefore, many researchers have focused on the development and identification of natural antioxidant products. A number of studies have reported antioxidant activity of natural products such as flavonoids by measuring their in vitro DPPH, •OH, and O2- radical scavenging activity [29,30]. In this study, we identified a flavonoid from A. distichum and also evaluated the in vitro antioxidant activities of its extracts as well as its active compound, acteoside.
Table 1 summarizes the DPPH, •OH, and O2- radical scavenging activity of the EtOH extracts from A. distichum leaves and stems at various concentrations (5–250 μg/mL). A. distichum extract treatments increased the DPPH radical scavenging activity in a dose-dependent manner. The leaves and stems of A. distichum at a 50 μg/mL concentration exhibited 84.50% and 67.30% activities, respectively. In addition, the IC50 values against DPPH of leaves and stems from A. distichum were 19.03 and 21.77 μg/mL, respectively, indicating that the leaves of A. distichum possessed higher DPPH radical scavenging activity than its stems. Moreover, the •OH radical scavenging activity of A. distichum leaves and stems exceeded 80% in all concentrations. Particularly, leaf extracts of A. distichum exceeding 50 μg/mL concentrations exhibited •OH radical scavenging activity higher than 90%, which was higher than that of the stem extracts. Previous studies reported that leaf extracts exerted stronger in vitro antioxidant activity than that of stem extracts [29,31]. The O2− radical scavenging activity of A. distichum leaves and stems was lower than that of the DPPH and •OH radicals. A. distichum leaves and stem extracts exhibited O2− radical scavenging activity at 100 and 50 μg/mL, respectively.

Table 1.
DPPH, •OH, and O2− radical scavenging activities of A. distichum leaf and stem EtOH extracts.
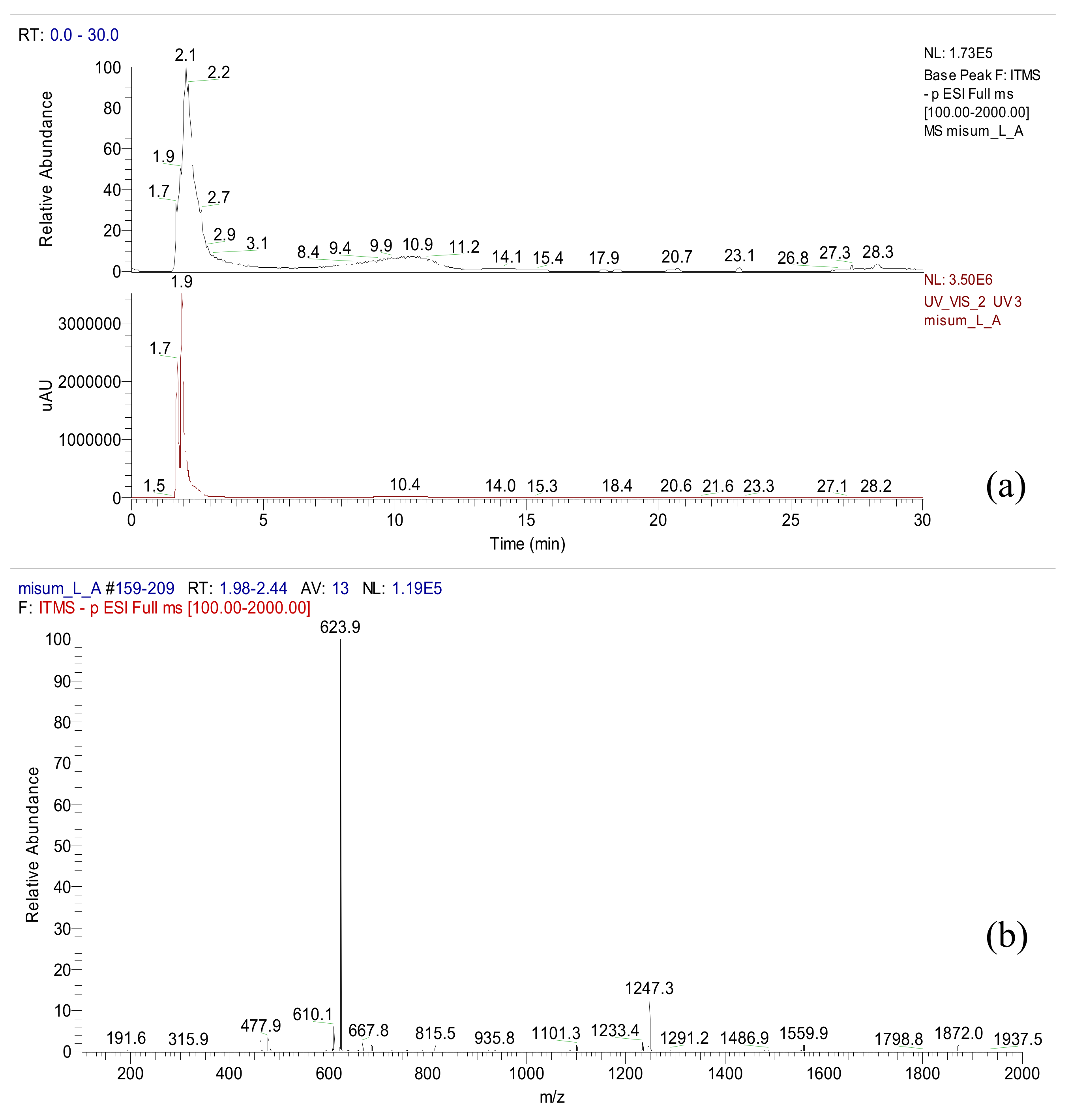
Acteoside was isolated from the n-butanol fraction of A. distichum. As depicted in the 1H-NMR spectrum, typical patterns of 3,4-dihydroxyphenyl (6.17–7.47 ppm) and rhamnosyl (0.95 ppm) moieties of acteoside were observed (NMR not shown). A total ionization chromatogram in the negative mode revealed the presence of acteoside (m/z 624) at a retention time of 2.1 min in A. distichum leaves as a major metabolite (Figure 2).
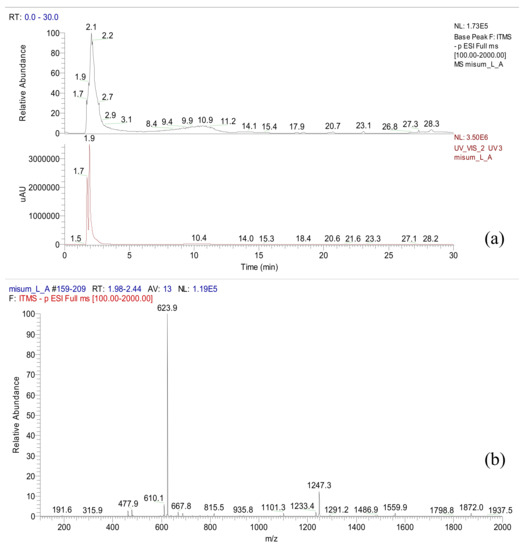
Figure 2.
LC/ESI-MS data of A. distichum (LC chromatogram (a) and ESI-MS data (b)).
As shown in Table 2, the in vitro antioxidant activity of acteoside derived from A. distichum and l-ascorbic acid (1–25 μg/mL) were characterized by measuring their DPPH, •OH, and O2− radical scavenging activities. Acteoside significantly increased DPPH radical scavenging activity in a dose-dependent manner. Particularly, an acteoside concentration of 25 μg/mL resulted in a DPPH radical scavenging activity of 82.83%. The IC50 values for acteoside and l-ascorbic acid were 4.28 and 0.16 μg/mL, respectively, in DPPH radical scavenging activity. Moreover, the •OH radical scavenging activities for acteoside and l-ascorbic acid at 2.5 μg/mL were 89.46% and 89.95%, respectively. Meanwhile, the IC50 values of acteoside and l-ascorbic acid were 0.22 and 0.48 μg/mL, respectively, indicating that A. distichum-derived acteoside possessed a strong •OH radical scavenging activity. Furthermore, acteoside from A. distichum also dose-dependently enhanced O2− radical scavenging activity. Moreover, acteoside and l-ascorbic acid at 25 μg/mL exhibited O2− radical scavenging activities of 30.31% and 17.68%, respectively, indicating a strong acteoside-mediated O2− radical scavenging activity. A previous study reported that the antioxidant properties of acteoside were likely due to its hydroxyphenylethyl and caffeoyl moieties [32]. It was found to decrease oxidative stress by inhibiting free radicals and lipid peroxidation [30,32]. Furthermore, acteoside attenuated oxidative stress-induced neuronal apoptosis via inhibition of ROS levels and activation of the Nrf2 pathway [33,34]. In previous studies [35,36], compounds such as phenolic glucosides having similar structures to that of acteoside exhibited similar antioxidant activities.

Table 2.
DPPH, •OH, and O2− radical scavenging activities of A. distichum-derived acteoside.
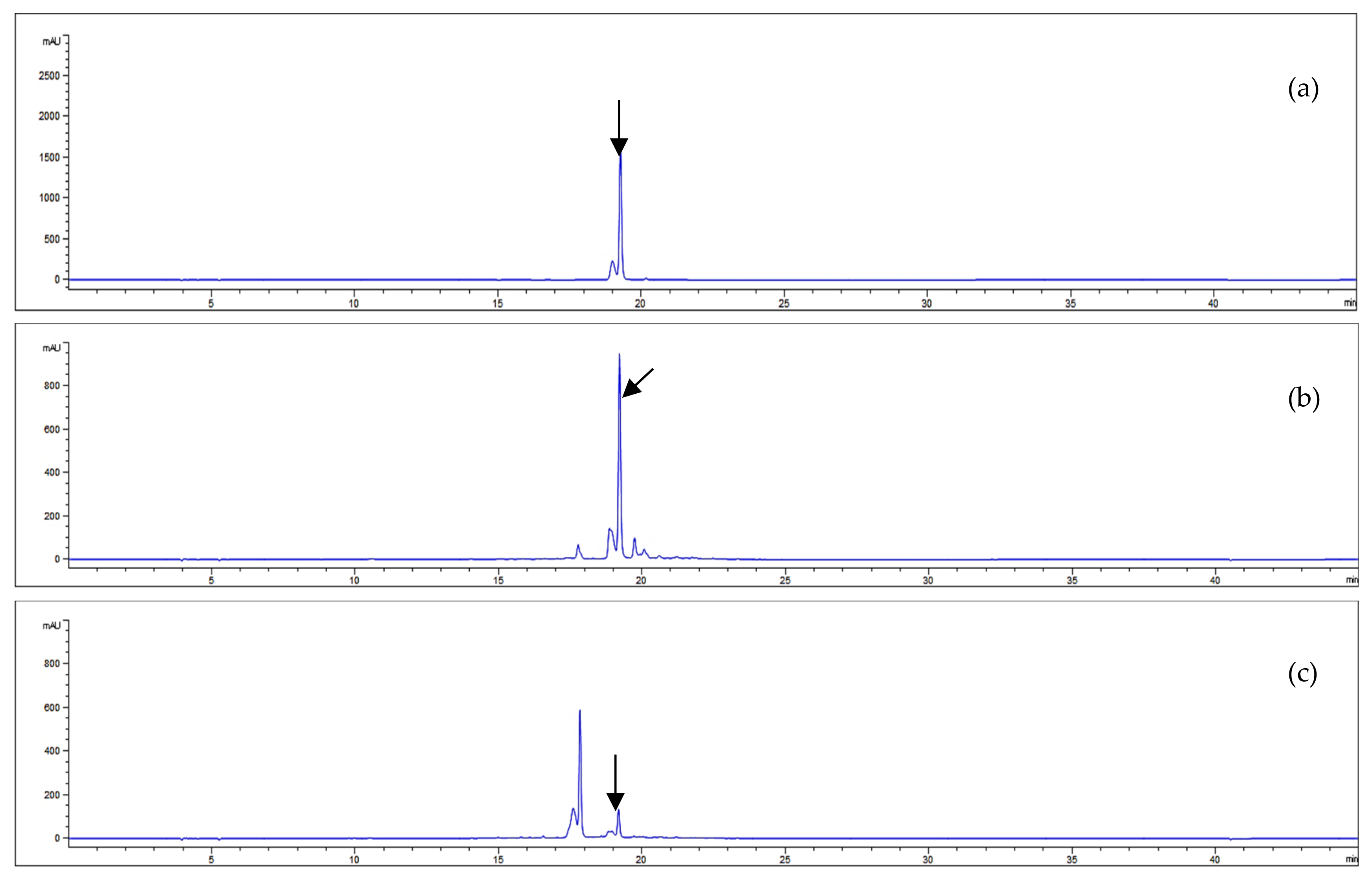
Our study investigated the acteoside contents of A. distichum leaves and stems via HPLC-UV analysis. Good separations were observed in the HPLC chromatogram with retention time detected at 19.26 min. The HPLC conditions and the results of acteoside quantification are illustrated in Figure 3.
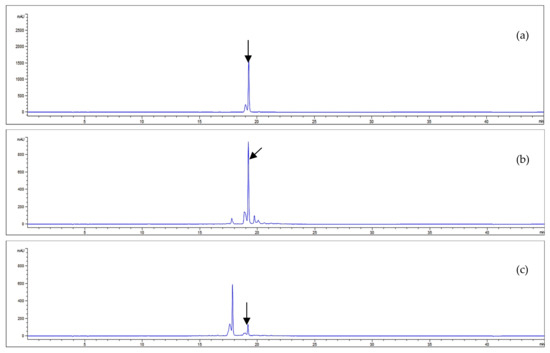
Figure 3.
HPLC chromatograms of acteoside (a) and the EtOH extracts from A. distichum leaves (b) and stems (c) (330 nm).
The equation of the standard curve linear calibration was Y = 34,674X − 50,661, where Y represents a given peak area and X is the corresponding acteoside concentration. The analytical method used showed good linearity with a correlation coefficient (r2) greater than 0.999 (Table 3). The amount of acteoside present in each sample was calculated from the calibration curve. Figure 3 illustrates the chromatographic separation of acteoside and the EtOH extract of A. distichum and the results of the quantitative analyses are summarized in Table 3.

Table 3.
Calibration curve and content of acteoside.
Our results revealed that the content of phenolic glycosides in A. distichum extracts varied depending on the anatomical structure analyzed. Specifically, the leaf acteoside content (162.11 mg/g) was higher than that in the stems (Table 3). There are similar reports about acteoside content of A. distichum from H2O extract (171.3 mg/g), 50% prethanol A extract (240.1 mg/g), 70% prethanol A extract (269.4 mg/g), and 100% prethanol A extract (326.1 mg/g) [37]. Moreover, the total phenolic compounds and flavonoid contents of A. distichum were 50.64 and 96.47 mg/g in leaves and 13.53 and 18.53 mg/g in stems, respectively [14]. Phenolic glycosides, which belong to a group of natural substances with variable phenolic structure, are found in fruits, bark roots, grains, vegetables, and wine. A. distichum contains various active glycosides, such as acteoside, eutigoside B, isoacteoside, rutin, hirsutism, and cornoside [5]. In a previous study, a phenolic glycoside identified as acteoside was found as the main compound present in A. distichum leaves [3]. The said compound is reported to possess various biological activities [38]. Our study utilized different assays to measure the antioxidant activities of different parts of A. distichum. The design of the study further assessed whether antioxidant activities could vary depending on the plant part containing the active compound.
The previous studies reported the antioxidant activity of A. distichum. The callus and flowers of A. distichum showed antioxidant activity by radical scavenging activities [39]. In addition, A. distichum protected DNA from oxidative stress in the oxidative damage-induced skin fibroblast cells [40]. Furthermore, acteoside isolated from A. distichum alleviated oxidative stress-induced cellular damage by decreasing the levels of phosphorylated p53 and γ-H2AX in skin fibroblast cells [41]. A. distichum leaves and stems were found to be particularly valuable due to their high content of acteoside, which has therapeutic qualities. Therefore, A. distichum could be potentially used as a novel health supplement or in natural medicinal products and antioxidant beverages.
4. Conclusions
The antioxidant activity of A. distichum EtOH extract and its bioactive compound acteoside were evaluated. Screening of plants containing antioxidants is very important to widen the knowledge of possible sources that can counteract the effects of ROS. This will help prevent the occurrence of ROS-induced diseases such as aging, cancer, and other related diseases. The DPPH, •OH, and O2− radical scavenging activities of A. distichum leaf EtOH extracts at a 250 μg/mL concentration were 88.32%, 94.48%, and 14.36%, respectively, whereas those of stem extracts at the same concentration were 88.15%, 88.99%, and 15.36%, respectively. The contents of acteoside in A. distichum leaves and stems were 162.11 and 29.68 mg/g, respectively. Acteoside was identified as the main antioxidant compound in A. distichum leaves, which resulted in DPPH, •OH, and O2− radical scavenging activities of 82.84%, 89.46%, and 30.31%, respectively, at a 25 μg/mL concentration. The results in our study demonstrated that A. distichum extract has a potent antioxidant activity which can be attributed to its high acteoside content. Moreover, our analyses have shown that the content of phenolic glycosides varies depending on the plant part analyzed. This study established the antioxidant qualities of the leaves and stems of A. distichium as an endemic plant to Korea that could be used a basis for developing therapeutic and nutritional products. Therefore, A. distichum showed possible use as an effective natural antioxidant for the prevention and treatment of oxidative stress-related diseases such as aging, cardiovascular, and neurodegenerative diseases.
Author Contributions
Analysis of A. distichum by HPLC/UV and LC-ESI/MS, H.-D.L.; antioxidant experiments, J.H.K. and Q.Q.P.; experimental materials and acteoside content, P.-M.J.; writing and editing, E.J.C.; acteoside preparation and writing, S.L. All authors have read and agreed to the published version of this manuscript.
Funding
This study was supported by a grant from the Natural Product Institute of Science and Technology (NIST-20-001), Anseong 17546, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, J.H. Antioxidant activities and inhibitory effect on oxidative DNA damage of extracts from Abeliophylli distichi Folium. Korea J. Herbol. 2011, 26, 95–99. [Google Scholar]
- Kang, U.C.; Chang, C.S.; Kim, Y.S. Genetic structure and conservation considerations of rare endemic Abeliophyllum distichum Nakai (Oleaceae) in Korea. J. Plant Res. 2000, 113, 127–138. [Google Scholar] [CrossRef]
- Oh, H.; Kang, D.G.; Kwon, T.O.; Jang, K.K.; Chai, K.Y.; Yun, Y.G.; Chung, H.T.; Lee, H.S. Four glycosides from the leaves of Abeliophyllum distichum with inhibitory effects on angiotensin converting enzyme. Phytother. Res. 2003, 17, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Park, G.H.; Park, J.H.; Eo, H.J.; Song, H.M.; Woo, S.H.; Kim, M.K.; Lee, J.W.; Lee, M.H.; Lee, J.R.; Koo, J.S.; et al. The induction of activating transcription factor 3 (ATF3) contributes to anti-cancer activity of Abeliophyllum distichum Nakai in human colorectal cancer cells. BMC Complement Altern. Med. 2014, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Kim, J.K.; Jang, J.M.; Cui, C.B.; Lim, S.S. Analysis of the inhibitory activity of Abeliophyllum distichum leaf constituents against aldose reductase by using high-speed counter current chromatography. Arch. Pharm. Res. 2013, 36, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Kadota, S.; Tani, T.; Namba, T. Antioxidative effects of phenylethanoids from Cistanche deserticola. Biol. Pharm. Bull. 1996, 19, 1580–1585. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef]
- Xie, J.H.; Wu, C.F. Effect of ethanolic extract of Cistanche deserticola on the contents of monoamine neurotransmitters in rat brain. Zhong Cao Yao 1993, 24, 417–419. [Google Scholar]
- He, Z.D.; Lau, K.M.; Xu, H.X.; Li, P.C.; Pui-Hay, B.P. Antioxidant activity of phenylethanoid glycosides from Brandisia hancei. J. Ethnopharmacol. 2000, 71, 483–486. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.F.; Zheng, R.; Liu, Z.M.; Jia, Z. Protection of phenylpropanoid glycosides from Pedicularis against oxidative hemolysis in vitro. J. Med. Plant Nat. Prod. Res. 1993, 59, 315–317. [Google Scholar]
- Zong, G.; He, W.; Wu, G.; Chen, M.; Shen, X.; Shi, M. Comparison between Cistanche deserticola Y. C. Ma and C. tubulosa (Shenk) Wight on some pharmacological actions. Zhongguo Zhong Yao Za Zhi 1996, 21, 436–437. [Google Scholar] [PubMed]
- Pu, X.; Song, Z.; Li, Y.; Tu, P.; Li, H. Acteoside from Cistanche salsa inhibits apoptosis by 1-methyl-4-phenylpyridinium ion in cerebellum granule neurons. Planta Med. 2003, 69, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Birkofer, L.; Kaiser, C.; Thomas, U. Acteoside and neoacteoside: Zuckerester aus Syringa vulgaris. Z. Naturforsch. B 1968, 23, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.B.; Kang, H.J.; Kim, M.J.; Kim, J.H.; Shin, H.S.; Kim, K.S. Analysis on the components and safety evaluation of Abeliophyllum distichum Nakai leaves and stems. Korean J. Environ. Health 2014, 40, 234–244. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Floyd, R.A.; Carney, J.M. Free radical damage to protein and DNA: Mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann. Neurol. 1992, 32, 22–27. [Google Scholar] [CrossRef]
- Okawa, M.; Kinjo, J.; Nohara, T.; Ono, M. DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol. Pharm. Bull. 2001, 24, 1202–1205. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Afanas’ev, I. Superoxide and nitric oxide in senescence and aging. Front. Biosci. 2009, 14, 3899–3912. [Google Scholar] [CrossRef][Green Version]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.R. Prospects for the use of antioxidant therapies. Drugs 1995, 49, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshica, T.; Okuda, T. Effects of the interaction of tannins with co-existing substances, VI. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 1989, 37, 2016–2021. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem. J. 1987, 243, 709–714. [Google Scholar] [CrossRef]
- Ewing, J.F.; Janero, D.R. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef]
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of ageing and role of dietary antioxidants. Biomed. Res. Int. 2014, 2014, 831841. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; de Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Wang, W.; Zu, Y.; Fu, Y.; Efferth, T. In vitro antioxidant and antimicrobial activity of extracts from Morus alba L. leaves, stems and fruits. Am. J. Chin. Med. 2012, 40, 349–356. [Google Scholar] [CrossRef]
- Chiou, W.F.; Lin, L.C.; Chen, C.F. Acteoside protects endothelial cells against free radical-induced oxidative stress. J. Pharm. Pharmacol. 2004, 56, 743–748. [Google Scholar] [CrossRef]
- Cvetanović, A.; Zengin, G.; Zeković, Z.; Švarc-Gajić, J.; Ražić, S.; Damjanović, A.; Mašković, P.; Mitić, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Hori, H. Antioxidant action of acteoside and its analogs on lipid peroxidation. Redox Rep. 1996, 2, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, F.; Xu, T.; Song, H.; Lu, B. Acteoside protects against 6-OHDA-induced dopaminergic neuron damage via Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2018, 119, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhang, Z.; Zhao, Y. Acteoside attenuates oxidative stress and neuronal apoptosis in rats with focal cerebral ischemia-reperfusion injury. Biol. Pharm. Bull. 2018, 41, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Ding, H.Y.; Chan, L.P.; Liang, J.Y.; Liang, C.H. Novel phenolic glucoside, origanoside, protects against oxidative damage and modulates antioxidant enzyme activity. Food Res. Int. 2011, 44, 1496–1503. [Google Scholar] [CrossRef]
- Li, H.L.; Chai, Z.; Shen, G.X.; Li, C.Y. Polyphenol profiles and antioxidant properties of ethanol extracts from Osmanthus fragrans (Thunb.) Lour. flowers. Pol. J. Food Nutr. Sci. 2017, 67, 317–325. [Google Scholar] [CrossRef]
- Jang, T.W.; Choi, J.S.; Kim, H.K.; Lee, E.J.; Han, M.W.; Lee, K.B.; Kim, D.W.; Park, J.H. Whitening activity of Abeliophyllum distichum Nakai leaves according to the ratio of prethanol A in the extracts. Korean J. Plant Res. 2018, 31, 667–674. [Google Scholar]
- Chen, C.H.; Lin, Y.S.; Chien, M.Y. Antioxidant and antihypertensive activities of acteoside and its analogs. Bot. Stud. 2012, 53, 421–429. [Google Scholar]
- Jang, T.W.; Park, J.H. Antioxidant activity and inhibitory effects on oxidative DNA damage of callus from Abeliophyllum distichum Nakai. Korean J. Plant Res. 2018, 31, 228–236. [Google Scholar]
- Ahn, J.; Park, J.H. Effects of Abeliophyllum distichum Nakai flower extracts on antioxidative activities and inhibition of DNA damage. Korean J. Plant Res. 2013, 26, 355–361. [Google Scholar] [CrossRef][Green Version]
- Jang, T.W.; Choi, J.S.; Park, J.H. Protective and inhibitory effects of acteoside from Abeliophyllum distichum Nakai against oxidative DNA damage. Mol. Med. Rep. 2020, 22, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).