Changes in Lipid Profile of Keratinocytes from Rat Skin Exposed to Chronic UVA or UVB Radiation and Topical Application of Cannabidiol
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents/Chemicals
2.2. Animals and Experimental Design
- Control group: rats were treated with non-toxic hydrophilic petrolatum applied topically on the back for 20 min every 12 h for 4 weeks;
- CBD group: rats were treated with CBD (2.5%; w/w in petrolatum) applied topically on the back for 20 min every 12 h for 4 weeks;
- UVA group: the skin of the backs of the rats was irradiated with UVA (365 nm, increasing dose from 0.5 to 5 J/cm2) every 48 h for 4 weeks;
- UVA + CBD group: the skin of the backs of the rats was irradiated with UVA every 48 h as in the UVA group and every 12 h, the backs of the rats were treated with CBD as in the CBD group;
- UVB group: the skin of the backs of the rats was irradiated with UVB (312 nm, increasing doses from 0.02 to 2 J/cm2) every 48 h for 4 weeks;
- UVB + CBD group: the skin of the backs of the rats was irradiated with UVB every 48 h as in to UVB group and every 12 h, the backs of the rats were additionally treated with CBD as in the CBD group.
2.3. Lipidomic Analysis
2.3.1. Lipid Extraction and Quantification of Phospholipid Content
2.3.2. UPLC-ESI-MS and MS/MS Analysis of Phospholipids
2.3.3. RPLC-ESI-MS and MS/MS Analysis of CERs
2.3.4. Data Processing
2.3.5. Statistical Analysis
2.4. Enzymatic Analysis
2.4.1. Measurement of PLA2 Activity
2.4.2. Measurement of Neutral SMase Activity
2.4.3. Statistical Analysis
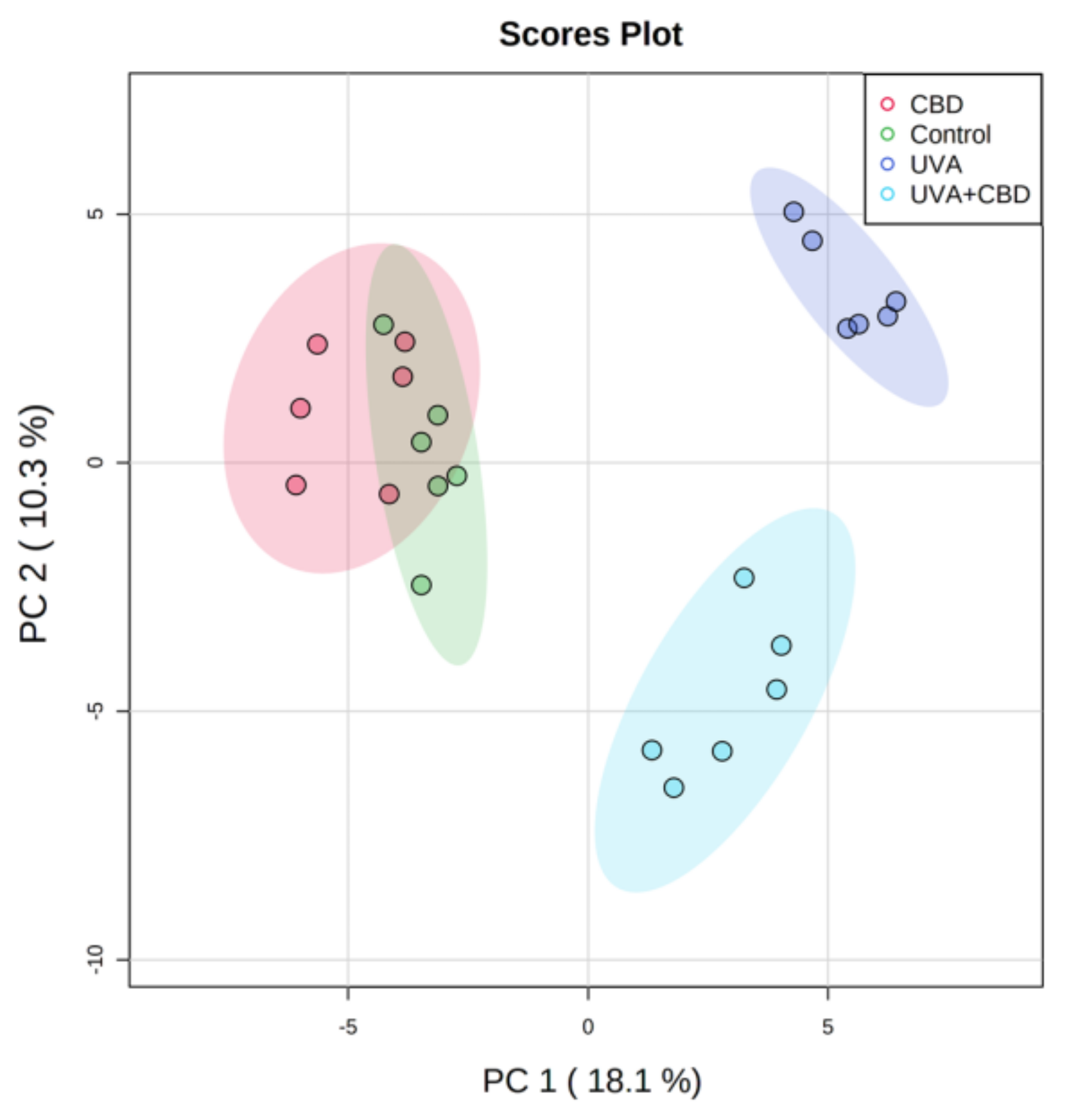
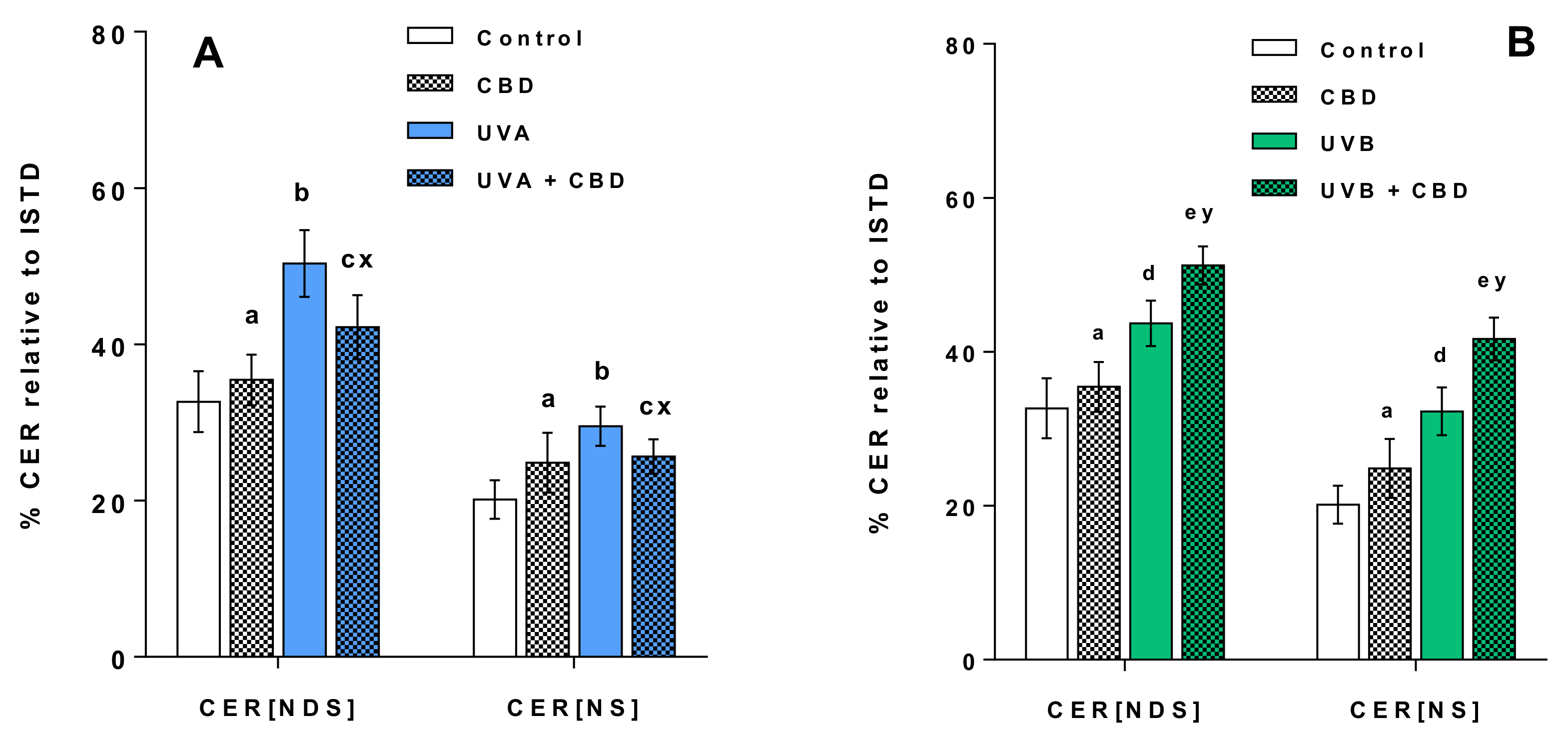
3. Results
Changes in the Lipid Profile of Rat Keratinocytes after Topical Application of CBD
4. Discussion
4.1. Effects of UVA and UVB on the Lipid Profile of Rat Keratinocytes
4.2. Changes in the Lipid Profile of Rat Keratinocytes after Topical Application of CBD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CBD | Cannabidiol |
| CER | Ceramide |
| CER[NDS] | Ceramides containing non-hydroxy fatty acids and dihydrosphingosine |
| CER[NS] | Ceramides containing non-hydroxy fatty acids and sphingosine |
| DDA | Data-dependent acquisition mode |
| DTNB | 5,5′-dithio-bis-(2-nitrobenzoic acid) |
| ESI | Electrospray ionization |
| HILIC | Hydrophilic interaction liquid chromatography |
| UPLC | Ultra performance liquid chromatography |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphoethanolamine |
| PBS | Phosphate buffered saline |
| PCA | Principal component analysis |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PEo | Ether-linked phosphoethanolamine |
| PI | Phosphatidylinositol |
| PLA2 | Phospholipase A2 |
| PS | Phosphatidylserines |
| QTOF | Quadrupole time of flight mass spectrometer |
| ROS | Reactive oxygen species |
| RP | Reversed-phase |
| SM | Sphingomyelin |
| SMase | Sphingomyelinase |
| UPLC | Ultra performance liquid chromatography |
References
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [PubMed]
- Stellavato, A.; Pirozzi, A.V.A.; Donato, S.; Scognamiglio, I.; Reale, S.; Di Pardo, A.; Filosa, S.; Vassallo, V.; Bellia, G.; De Rosa, M.; et al. Positive Effects against UV-A Induced Damage and Oxidative Stress on an In Vitro Cell Model Using a Hyaluronic Acid Based Formulation Containing Amino Acids, Vitamins, and Minerals. Available online: https://www.hindawi.com/journals/bmri/2018/8481243/ (accessed on 7 October 2020).
- Dalmau, N.; Andrieu-Abadie, N.; Tauler, R.; Bedia, C. Phenotypic and lipidomic characterization of primary human epidermal keratinocytes exposed to simulated solar UV radiation. J. Dermatol. Sci. 2018, 92, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, M.X. A clinical review of phototherapy for psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F. The Skin Lipidome Under Environmental Stress—Technological Platforms, Molecular Pathways and Translational Opportunities. In Skin Stress Response Pathways; Springer: New York, NY, USA, 2016; pp. 1–27. [Google Scholar]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Duchnik, E. Oxidative stress and skin diseases: Possible role of physical activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef]
- Kim, W.B.; Jerome, D.; Yeung, J. Diagnosis and management of psoriasis. Can. Fam Physician 2017, 63, 278–285. [Google Scholar]
- Morales, P.; Reggio, P.H.; Jagerovic, N. An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol. Front. Pharm. 2017, 8. [Google Scholar] [CrossRef]
- Rong, C.; Lee, Y.; Carmona, N.E.; Cha, D.S.; Ragguett, R.-M.; Rosenblat, J.D.; Mansur, R.B.; Ho, R.C.; McIntyre, R.S. Cannabidiol in medical marijuana: Research vistas and potential opportunities. Pharmacol. Res. 2017, 121, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.F.; Lima, A.C.; Hallak, J.E.; Crippa, J.A.; Silva, R.H.; Abílio, V.C. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front. Pharmacol. 2018, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Batista, J.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honório, K.M.; da Silva, A.B.F. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005, 314, 780–788. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The potential role of cannabinoids in dermatology. J. Dermatol. Treat. 2019, 1–7. [Google Scholar] [CrossRef]
- Łuczaj, W.; Dobrzyńska, I.; Wroński, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Cannabidiol-Mediated Changes to the Phospholipid Profile of UVB-Irradiated Keratinocytes from Psoriatic Patients. Int. J. Mol. Sci. 2020, 21, 6592. [Google Scholar] [CrossRef]
- Reich, A.; Schwudke, D.; Meurer, M.; Lehmann, B.; Shevchenko, A. Lipidome of narrow-band ultraviolet B irradiated keratinocytes shows apoptotic hallmarks. Exp. Dermatol. 2010, 19, e103–e110. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.L.; Lipman, N.S. Feed and Bedding. In Management of Animal Care and Use Programs in Research, Education, and Testing; Weichbrod, R.H., Thompson, G.A., Norton, J.N., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; ISBN 978-1-4987-4844-5. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, E.M.; Lewis, D.H. Spectrophotometric determination of phosphate esters in the presence and absence of orthophosphate. Anal. Biochem. 1970, 36, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Wroński, A.; Domingues, P.; Domingues, M.R.; Skrzydlewska, E. Lipidomic Analysis Reveals Specific Differences between Fibroblast and Keratinocyte Ceramide Profile of Patients with Psoriasis Vulgaris. Molecules 2020, 25, 630. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar]
- Reynolds, L.J.; Hughes, L.L.; Yu, L.; Dennis, E.A. 1-Hexadecyl-2-arachidonoylthio-2-deoxy-sn-glycero-3-phosphorylcholine as a substrate for the microtiterplate assay of human cytosolic phospholipase A2. Anal. Biochem. 1994, 217, 25–32. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- DeLeo, V.; Scheide, S.; Meshulam, J.; Hanson, D.; Cardullo, A. Ultraviolet Radiation Alters Choline Phospholipid Metabolism in Human Keratinocytes. J. Investig. Dermatol. 1988, 91, 303–308. [Google Scholar] [CrossRef]
- Gęgotek, A.; Biernacki, M.; Ambrożewicz, E.; Surażyński, A.; Wroński, A.; Skrzydlewska, E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Zaręba, I.; Surażyński, A.; Skrzydlewska, E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch. Dermatol. Res. 2017, 309, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Gresham, A.; Masferrer, J.; Chen, X.; Leal-Khouri, S.; Pentland, A.P. Increased synthesis of high-molecular-weight cPLA2 mediates early UV-induced PGE2 in human skin. Am. J. Physiol. 1996, 270, C1037–C1050. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, Y.; Han, S.; Kim, Y.; Nam, T.; Ahn, D. Lysophosphatidylcholine Increases Ca2+ Current via Activation of Protein Kinase C in Rabbit Portal Vein Smooth Muscle Cells. Korean J. Physiol. Pharm. 2008, 12, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ryborg, A.K.; Johansen, C.; Iversen, L.; Kragballe, K. Lysophosphatidylcholine induces keratinocyte differentiation and upregulation of AP-1- and NF-kappaB DNA-binding activity. Acta Dermatol. Venereol. 2004, 84, 433–438. [Google Scholar] [CrossRef]
- Chakravarthy, M.V.; Lodhi, I.J.; Yin, L.; Malapaka, R.R.V.; Xu, H.E.; Turk, J.; Semenkovich, C.F. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell 2009, 138, 476–488. [Google Scholar] [CrossRef]
- Straus, D.S.; Glass, C.K. Anti-inflammatory actions of PPAR ligands: New insights on cellular and molecular mechanisms. Trends Immunol. 2007, 28, 551–558. [Google Scholar] [CrossRef]
- Kagan, V.E.; Bayir, H.; Tyurina, Y.Y.; Bolevich, S.B.; Maguire, J.J.; Fadeel, B.; Balasubramanian, K. Elimination of the Unnecessary: Intra- and Extracellular Signaling by Anionic Phospholipids. Biochem. Biophys. Res. Commun. 2017, 482, 482–490. [Google Scholar] [CrossRef]
- Metral, E.; Bechetoille, N.; Demarne, F.; Damour, O.; Rachidi, W. Keratinocyte stem cells are more resistant to UVA radiation than their direct progeny. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef]
- Uchida, Y.; Hara, M.; Nishio, H.; Sidransky, E.; Inoue, S.; Otsuka, F.; Suzuki, A.; Elias, P.M.; Holleran, W.M.; Hamanaka, S. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J. Lipid Res. 2000, 41, 2071–2082. [Google Scholar] [PubMed]
- Dai, Q.; Liu, J.; Chen, J.; Durrant, D.; McIntyre, T.M.; Lee, R.M. Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin. Oncogene 2004, 23, 3650–3658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magnoni, C.; Euclidi, E.; Benassi, L.; Bertazzoni, G.; Cossarizza, A.; Seidenari, S.; Giannetti, A. Ultraviolet B radiation induces activation of neutral and acidic sphingomyelinases and ceramide generation in cultured normal human keratinocytes. Toxicol. Vitr. 2002, 16, 349–355. [Google Scholar] [CrossRef]
- Wefers, H.; Melnik, B.C.; Flür, M.; Bluhm, C.; Lehmann, P.; Plewig, G. Influence of UV Irradiation on the Composition of Human Stratum Corneum Lipids. J. Investig. Dermatol. 1990, 96, 959–962. [Google Scholar] [CrossRef]
- Rockenfeller, P.; Carmona-Gutierrez, D.; Pietrocola, F.; Kroemer, G.; Madeo, F. Ethanolamine: A novel anti-aging agent. Mol. Cell Oncol. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidant 2019, 9, 21. [Google Scholar] [CrossRef]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J. Alzheimers Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef]
- Vance, J.E. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J. Lipid Res. 2008, 49, 1377–1387. [Google Scholar] [CrossRef]
- Yang, L.; Rozenfeld, R.; Wu, D.; Devi, L.A.; Zhang, Z.; Cederbaum, A. Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Radic. Biol. Med. 2014, 68, 260–267. [Google Scholar] [CrossRef]
- Evans, A.T.; Formukong, E.; Evans, F.J. Activation of phospholipase A2 by cannabinoids. Lack of correlation with CNS effects. FEBS Lett. 1987, 211, 119–122. [Google Scholar] [CrossRef]
- Lee, H.; Pyo, M.-J.; Bae, S.K.; Heo, Y.; Kim, C.G.; Kang, C.; Kim, E. Improved Therapeutic Profiles of PLA2-Free Bee Venom Prepared by Ultrafiltration Method. Toxicol. Res. 2015, 31, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S.; Hunter, S.A.; Renzulli, L. Stimulation of sphingomyelin hydrolysis by cannabidiol in fibroblasts from a Niemann-Pick patient. Biochem. Biophys. Res. Commun. 1984, 121, 168–173. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łuczaj, W.; Domingues, M.d.R.; Domingues, P.; Skrzydlewska, E. Changes in Lipid Profile of Keratinocytes from Rat Skin Exposed to Chronic UVA or UVB Radiation and Topical Application of Cannabidiol. Antioxidants 2020, 9, 1178. https://doi.org/10.3390/antiox9121178
Łuczaj W, Domingues MdR, Domingues P, Skrzydlewska E. Changes in Lipid Profile of Keratinocytes from Rat Skin Exposed to Chronic UVA or UVB Radiation and Topical Application of Cannabidiol. Antioxidants. 2020; 9(12):1178. https://doi.org/10.3390/antiox9121178
Chicago/Turabian StyleŁuczaj, Wojciech, Maria do Rosário Domingues, Pedro Domingues, and Elżbieta Skrzydlewska. 2020. "Changes in Lipid Profile of Keratinocytes from Rat Skin Exposed to Chronic UVA or UVB Radiation and Topical Application of Cannabidiol" Antioxidants 9, no. 12: 1178. https://doi.org/10.3390/antiox9121178
APA StyleŁuczaj, W., Domingues, M. d. R., Domingues, P., & Skrzydlewska, E. (2020). Changes in Lipid Profile of Keratinocytes from Rat Skin Exposed to Chronic UVA or UVB Radiation and Topical Application of Cannabidiol. Antioxidants, 9(12), 1178. https://doi.org/10.3390/antiox9121178





