Downregulation of the Glo1 Gene Is Associated with Reduced Adiposity and Ectopic Fat Accumulation in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
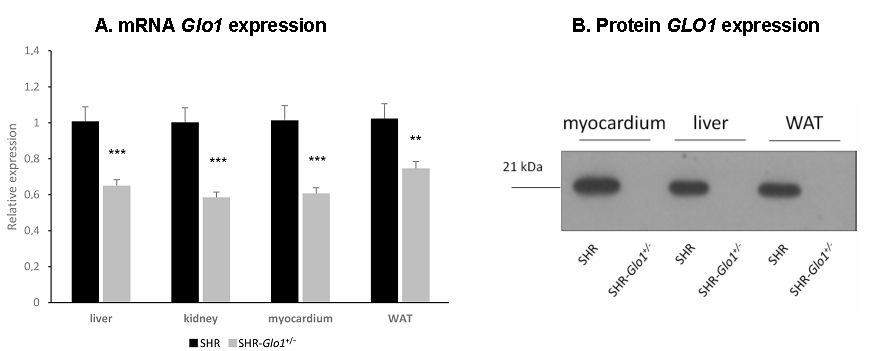
2.2. Generation of Glo1 Knockout SHR Rats
2.3. Glucose Utilization in Epididymal Adipose Tissue
2.4. Tissue Triglyceride and Cholesterol Measurements
2.5. Parameters of Dicarbonyl and Oxidative Stress
2.6. Biochemical Analysis
2.7. Histological Methods
2.8. Gene Expression Determined by Real-Time PCR
2.9. Determination of GLUT4 and AMPK Activity: Tissue Homogenization and Fractionation
2.10. Determination of GLUT4 and AMPK Activity: Electrophoresis and Western Blotting
2.11. Statistical Analysis
3. Results
3.1. Glyoxalase 1 Gene Expression, Enzyme Activity, and Dicarbonyl and Oxidative Stress
3.2. Parameters of Glucose and Lipid Metabolism
3.3. Effect of Glo1 Downregulation on GLUT4 and AMPK Activation
3.4. Microalbuminuria and Histological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Advanced glycation end-products |
| AMPK | AMP-activated protein kinase |
| CAT | Catalase |
| CPM | Crude plasma membranes |
| GL | Glyoxal |
| GLO1 | Glyoxalase 1 |
| GLUT4 | Glucose transporter 4 |
| GSH | Reduced glutathione |
| GSH-Px | Glutathione peroxidase |
| GSSG | Oxidized glutathione |
| LV | Light vesicles |
| MG | Methylglyoxal |
| PNS | Postnuclear supernatant |
| RAGE | Receptor for advanced glycation end-products |
| SOD | Superoxide dismutase |
| TBARS | Thiobarbituric acid reactive substances |
| WAT | White adipose tissue |
References
- Masania, J.; Malczewska-Malec, M.; Razny, U.; Goralska, J.; Zdzienicka, A.; Kiec-Wilk, B.; Gruca, A.; Stancel-Mozwillo, J.; Dembinska-Kiec, A.; Rabbani, N.; et al. Dicarbonyl stress in clinical obesity. Glycoconj. J. 2016, 33, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabbani, N.; Thornalley, P.J. Glyoxalase 1 modulation in obesity and diabetes. Antioxid. Redox. Signal. 2019, 30, 354–374. [Google Scholar] [CrossRef] [PubMed]
- Nigro, C.; Leone, A.; Raciti, G.A.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-glyoxalase 1 balance: The root of vascular damage. Int. J. Mol. Sci. 2017, 18, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Zhou, C.; Huang, M.; Tang, C.; Liu, X.; Yue, Y.; Diao, Q.; Zheng, Z.; Liu, D. Glyoxalase system: A systematic review of its biological activity, related-diseases, screening methods and small molecule regulators. Biomed. Pharmacother. 2020. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Activity, regulation, copy number and function in the glyoxalase system. Biochem. Soc. Trans. 2014, 42, 419–424. [Google Scholar] [CrossRef]
- Pravenec, M.; Zídek, V.; Šimáková, M.; Křen, V.; Křenová, D.; Horký, K.; Jáchymová, M.; Míková, B.; Kazdová, L.; Aitman, T.J.; et al. Genetics of Cd36 and the clustering of multiple cardiovascular risk factors in spontaneous hypertension. J. Clin. Investig. 1999, 103, 1651–1657. [Google Scholar] [CrossRef] [Green Version]
- Pravenec, M.; Kožich, V.; Krijt, J.; Sokolová, J.; Zídek, V.; Landa, V.; Šimáková, M.; Mlejnek, P.; Šilhavý, J.; Oliyarnyk, O.; et al. Folate deficiency is associated with oxidative stress, increased blood pressure, and insulin resistance in spontaneously hypertensive rats. Am. J. Hypertens. 2013, 26, 135–140. [Google Scholar] [CrossRef]
- Pravenec, M.; Kazdová, L.; Landa, V.; Zídek, V.; Mlejnek, P.; Jansa, P.; Wang, J.; Qi, N.; Kurtz, T.W. Transgenic and recombinant resistin impair skeletal muscle glucose metabolism in the spontaneously hypertensive rat. J. Biol. Chem. 2003, 278, 45209–45515. [Google Scholar] [CrossRef] [Green Version]
- Pravenec, M.; Kajiya, T.; Zídek, V.; Landa, V.; Mlejnek, P.; Šimáková, M.; Šilhavý, J.; Malínská, H.; Oliyarnyk, O.; Kazdová, L.; et al. Effects of human C-reactive protein on pathogenesis of features of the metabolic syndrome. Hypertension 2011, 57, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Arai, M.; Nihonmatsu-Kikuchi, N.; Itokawa, M.; Rabbani, N.; Thornalley, P.J. Measurement of glyoxalase activities. Biochem. Soc. Trans. 2014, 42, 491–494. [Google Scholar] [CrossRef]
- Bryant, N.J.; Govers, R.; James, D.E. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell. Biol. 2002, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Jessen, N.; Goodyear, L.J. AMP-activated protein kinase and the regulation of glucose transport. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E867–E877. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, T.; Sugimoto, K.; Takahashi, T.; Yasunobe, Y.; Xie, K.; Tanaka, M.; Ohnishi, Y.; Yoshida, S.; Kurinami, H.; Akasaka, H.; et al. Overexpression of interleukin-15 exhibits improved glucose tolerance and promotes GLUT4 translocation via AMP-activated protein kinase pathway in skeletal muscle. Biochem. Biophys. Res. Commun. 2019, 509, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef]
- Schumacher, D.; Morgenstern, J.; Oguchi, Y.; Volk, N.; Kopf, S.; Groener, J.B.; Nawroth, P.P.; Fleming, T.; Freichel, M. Compensatory mechanisms for methylglyoxal detoxification in experimental and clinical diabetes. Mol. Metab. 2018, 18, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Flachs, P.; Adamcová, K.; Zouhar, P.; Marques, C.; Janovská, P.; Viegas, I.; Jones, J.G.; Bardová, K.; Svobodová, M.; Hansíková, J.; et al. Induction of lipogenesis in white fat during cold exposure in mice: Link to lean phenotype. Int. J. Obes. 2017, 41, 372–380. [Google Scholar] [CrossRef]
- De Arriba, S.G.; Stuchbury, G.; Yarin, J.; Burnell, J.; Loske, C.; Münch, G. Methylglyoxal impairs glucose metabolism and leads to energy depletion in neuronal cells--protection by carbonyl scavengers. Neurobiol. Aging 2007, 28, 1044–1050. [Google Scholar] [CrossRef]
- Muise, E.S.; Guan, H.-P.; Liu, J.; Nawrocki, A.R.; Yang, X.; Wang, C.; Rodríguez, C.G.; Zhou, D.; Gorski, J.N.; Kurtz, M.M.; et al. Pharmacological AMPK activation induces transcriptional responses congruent to exercise in skeletal and cardiac muscle, adipose tissues and liver. PLoS ONE 2019, 14, e0211568. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wu, L. Methylglyoxal-induced mitochondrial dysfunction in vascular smooth muscle cells. Biochem. Pharmacol. 2009, 77, 1709–1716. [Google Scholar] [CrossRef]
- Bo, J.; Xie, S.; Guo, Y.; Zhang, C.; Guan, Y.; Li, C.; Lu, J.; Meng, Q.H. Methylglyoxal impairs insulin secretion of pancreatic β-cells through increased production of ROS and mitochondrial dysfunction mediated by upregulation of UCP2 and MAPKs. J. Diabetes Res. 2016, 2016, 2029854. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.L.; Kim, S.J.; Lee, K.T.; Kim, J.; Mu, J.; Birnbaum, M.J.; Kim, S.; Ha, J. The regulation of AMP-activated protein kinase by H2O2. Biochem. Biophys. Res. Commun. 2001, 287, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Dafre, A.L.; Schmitz, A.E.; Maher, P. Methylglyoxal-induced AMPK activation leads to autophagic degradation of thioredoxin 1 and glyoxalase 2 in HT22 nerve cells. Free Radic. Biol. Med. 2017, 108, 270–279. [Google Scholar] [CrossRef]
- Blackburn, N.J.R.; Vulesevic, B.; McNeill, B.; Cimenci, C.E.; Ahmadi, A.; Gonzalez-Gomez, M.; Ostojic, A.; Zhong, Z.; Brownlee, M.; Beisswenger, P.J.; et al. Methylglyoxal-derived advanced glycation end products contribute to negative cardiac remodeling and dysfunction post-myocardial infarction. Basic Res. Cardiol. 2017, 112, 57. [Google Scholar] [CrossRef] [PubMed]
- Vulesevic, B.; McNeill, B.; Giacco, F.; Maeda, K.; Blackburn, N.J.; Brownlee, M.; Milne, R.W.; Suuronen, E.J. Methylglyoxal-induced endothelial cell loss and inflammation contribute to the development of diabetic cardiomyopathy. Diabetes 2016, 65, 1699–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisostomo, J.; Matafome, P.; Santos-Silva, D.; Rodrigues, L.; Sena, C.M.; Pereira, P.; Seica, R. Methylglyoxal chronic administration promotes diabetes-like cardiac ischaemia disease in Wistar normal rats. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, M.; Hakimi, M.; Fleming, T.; Peters, A.S.; Sijmonsma, T.P.; Herzig, S.; Nawroth, P.P.; Böckler, D.; Dihlmann, S. A glyoxalase-1 knockdown does not have major short term effects on energy expenditure and atherosclerosis in mice. J. Diabetes Res. 2016, 2016, 2981639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanssen, N.M.; Brouwers, O.; Gijbels, M.J.; Wouters, K.; Wijnands, E.; Cleutjens, J.P.; De Mey, J.G.; Miyata, T.; Biessen, E.A.; Stehouwer, C.D.; et al. Glyoxalase 1 overexpression does not affect atherosclerotic lesion size and severity in ApoE−/− mice with or without diabetes. Cardiovasc. Res. 2014, 104, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Engelbrecht, B.; Stratmann, B.; Hess, C.; Tschoepe, D.; Gawlowski, T. Impact of GLO1 knock down on GLUT4 trafficking and glucose uptake in L6 myoblasts. PLoS ONE 2013, 8, e65195. [Google Scholar] [CrossRef] [Green Version]
- Riboulet-Chavey, A.; Pierron, A.; Durand, I.; Murdaca, J.; Giudicelli, J.; Van Obberghen, E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes 2006, 55, 1289–1299. [Google Scholar] [CrossRef] [Green Version]
- Dhar, A.; Dhar, I.; Jiang, B.; Desai, K.M.; Wu, L. Chronic methylglyoxal infusion by minipump causes pancreatic β-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes 2011, 60, 899–908. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Wu, L. Accumulation of endogenous methylglyoxal impaired insulin signaling in adipose tissue of fructose-fed rats. Mol. Cell. Biochem. 2007, 306, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Fiory, F.; Lombardi, A.; Miele, C.; Giudicelli, J.; Beguinot, F.; Van Obberghen, E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia 2011, 54, 2941–2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marková, I.; Hüttl, M.; Oliyarnyk, O.; Kačerová, T.; Haluzík, M.; Kačer, P.; Šeda, O.; Malínská, H. The effect of dicarbonyl stress on the development of kidney dysfunction in metabolic syndrome—A transcriptomic and proteomic approach. Nutr. Metab. 2019, 16, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Mori, T.; Jiang, Y.; Hu, C.; Osaki, Y.; Yoneki, Y.; Sun, Y.; Hosoya, T.; Kawamata, A.; Ogawa, S.; et al. Methylglyoxal contributes to the development of insulin resistance and salt sensitivity in Sprague-Dawley rats. J. Hypertens. 2009, 27, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Nigro, C.; Raciti, G.; Leone, A.; Fleming, T.; Longo, M.; Prevenzano, I.; Fiory, F.; Mirra, P.; D’Esposito, V.; Ulianich, L.; et al. Methylglyoxal impairs endothelial insulin sensitivity both in vitro and in vivo. Diabetologia 2014, 57, 1485–1494. [Google Scholar] [CrossRef]
- Zunkel, K.; Simm, A.; Bartling, B. Long-term intake of the reactive metabolite methylglyoxal is not toxic in mice. Food Chem. Toxicol. 2020. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Despres, J.P. Cardiovascular and metabolic heterogeneity of obesity: Clinical challenges and implications for management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Lee, J.J.; Pedley, A.; Hoffmann, U.; Massaro, J.M.; Levy, D.; Long, M.T. Visceral and intrahepatic fat are associated with cardiometabolic risk factors above other ectopic fat depots: The Framingham Heart Study. Am. J. Med. 2018, 131, 684–692. [Google Scholar] [CrossRef]
- Marchington, J.M.; Mattacks, C.A.; Pond, C.M. Adipose tissue in the mammalian heart and pericardium: Structure, foetal develop ment and biochemical properties. Comp. Biochem. Physiol. B 1989, 94, 225–232. [Google Scholar] [CrossRef]
- Gaborit, B.; Kober, F.; Jacquier, A.; Moro, P.J.; Cuisset, T.; Boullu, S.; Dutour, A. Assessment of epicardial fat volume and myocardial triglyceride content in severely obese subjects: Relationship to metabolic profile, cardiac function and visceral fat. Int. J. Obes. 2012, 36, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Nasarre, L.; Juan-Babot, O.; Gastelurrutia, P.; Llucia-Valldeperas, A.; Badimon, L.; Bayes-Genis, A.; Llorente-Cortes, V. Low density lipoprotein receptor-related protein 1 is upregulated in epicardial fat from type 2 diabetes mellitus patients and correlates with glucose and triglyceride plasma levels. Acta Diabetol. 2014, 51, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Burgeiro, A.; Fuhrmann, A.; Cherian, S.; Espinoza, D.; Jarak, I.; Carvalho, R.A.; Loureiro, M.; Patrício, M.; Antunes, M.; Carvalho, E. Glucose uptake and lipid metabolism are impaired in epicardial adipose tissue from heart failure patients with or without diabetes. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E550–E564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated levels of the reactive metabolite methylglyoxal recapitulate progression of type 2 diabetes. Cell. Metab. 2018, 27, 926–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandran, M.; Priebe, S.; Grigolon, G.; Rozanov, L.; Groth, M.; Laube, B.; Guthke, R.; Platzer, M.; Zarse, K.; Ristow, M. Impairing L-threonine catabolism promotes healthspan through methylglyoxal-mediated proteohormesis. Cell. Metab. 2018, 27, 914–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Trait | SHR | SHR-Glo1+/− |
|---|---|---|
| Plasma or Blood | ||
| Glyoxal (nmol/mL) (plasma) | 0.097 ± 0.009 | 0.157 ± 0.012 * |
| Methylglyoxal (nmol/mL) (plasma) | 0.291 ± 0.011 | 0.726 ± 0.048 ** |
| GSH (μmol/L) (blood) | 778.8 ± 5.8 | 718.1 ± 16.5 * |
| GSSG (μmol/L) (blood) | 53.8 ± 4.9 | 53.9 ± 8.5 |
| GSH/GSSG ratio | 14.9 ± 1.2 | 13.6 ± 1.6 |
| Glo1 activity (μmol/min/μg Hb) (erytrocytes) | 0.406 ± 0.019 | 0.192 ± 0.007 ** |
| White Adipose Tissue | ||
| Glyoxal (nmol/mg) | 0.165 ± 0.003 | 0.199 ± 0.019 |
| Methylglyoxal (nmol/mg) | 2.159 ± 0.301 | 2.567 ± 0.186 |
| Glo1 activity (μmol/min/μg protein) | 0.831 ± 0.070 | 0.707 ± 0.069 |
| Myocardium | ||
| Glyoxal (nmol/mg) | 0.380 ± 0.019 | 0.960 ± 0.074 ** |
| Methylglyoxal (nmol/mg) | 1.054 ± 0.170 | 2.159 ± 0.102 ** |
| GSH (μmol/mg protein) | 12.2 ± 0.8 | 10.1 ± 0.7 |
| GSSG (μmol/mg protein) | 2.8 ± 0.3 | 2.3 ± 0.2 |
| GSH/GSSG ratio | 4.5 ± 0.7 | 4.4 ± 0.2 |
| Glo1 activity (μmol/min/μg protein) | 0.685 ± 0.055 | 0.453 ± 0.061 * |
| Kidney | ||
| Glyoxal (nmol/mg) | 0.626 ± 0.017 | 0.717 ± 0.009 |
| Methylglyoxal (nmol/mg) | 2.206 ± 0.179 | 2.114 ± 0.105 |
| GSH (μmol/mg protein) | 42.1 ± 2.2 | 27.0 ± 1.5 ** |
| GSSG (μmol/mg protein) | 4.3 ± 0.3 | 3.2 ± 0.1 ** |
| GSH/GSSG ratio | 9.9 ± 1.5 | 8.6 ± 0.6 |
| Liver | ||
| Glyoxal (nmol/mg) | 0.728 ± 0.095 | 0.772 ± 0.095 |
| Methylglyoxal (nmol/mg) | 3.093 ± 0.179 | 3.314 ± 0.371 |
| GSH (μmol/mg protein) | 93.5 ± 6.1 | 68.6 ± 1.1 ** |
| GSSG (μmol/mg protein) | 4.7 ± 0.1 | 4.7 ± 0.2 |
| GSH/GSSG ratio | 19.8 ± 0.9 | 15.0 ± 0.6 ** |
| Glo1 activity (μmol/min/μg protein) | 0.562 ± 0.021 | 0.285 ± 0.011 ** |
| Trait | SHR | SHR-Glo1+/− |
|---|---|---|
| Myocardium | ||
| SOD (U/mg protein) | 0.075 ± 0.006 | 0.054 ± 0.004 ** |
| GSH-Px (μM NADPH/min/mg protein) | 131 ± 12 | 138 ± 11 |
| GR (μM NADPH/min/mg protein) | 125 ± 8 | 114 ± 8 |
| CAT (mM H2O2/min/mg protein) | 594 ± 26 | 530 ± 31 |
| TBARS (nM/mg protein) | 0.561 ± 0.026 | 0.651 ± 0.021 * |
| Kidney cortex | ||
| SOD (U/mg protein) | 0.024 ± 0.002 | 0.026 ± 0.002 |
| GSH-Px (μM NADPH/min/mg protein) | 73 ± 6 | 68 ± 7 |
| GR (μM NADPH/min/mg protein) | 28 ± 3 | 39 ± 5 |
| CAT (mM H2O2/min/mg protein) | 451 ± 26 | 430 ± 27 |
| TBARS (nM/mg protein) | 0.338 ± 0.021 | 0.391 ± 0.008 * |
| Liver | ||
| SOD (U/mg protein) | 0.142 ± 0.009 | 0.115 ± 0.008 * |
| GSH-Px (μM NADPH/min/mg protein) | 292 ± 17 | 245 ± 20 |
| GR (μM NADPH/min/mg protein) | 139 ± 13 | 154 ± 23 |
| CAT (mM H2O2/min/mg protein) | 1515 ± 126 | 1707 ± 154 |
| TBARS (nM/mg protein) | 1.759 ± 0.173 | 1.867 ± 0.128 |
| Trait | SHR | SHR-Glo1+/− |
|---|---|---|
| Body weight (g) | 362 ± 4 | 344 ± 6 * |
| Relative weight of epididymal fat (g/100 g BW) | 0.98 ± 0.02 | 0.88 ± 0.03 * |
| Relative liver weight (g/100 g BW) | 3.24 ± 0.04 | 3.11 ± 0.03 * |
| Non-fasting glucose (mmol/L) | 6.3 ± 0.2 | 6.2 ± 0.1 |
| Insulin (μmol/L) | 0.275 ± 0.023 | 0.188 ± 0.017 * |
| NEFA (mmol/L) | 0.38 ± 0.02 | 0.42 ± 0.02 |
| Serum triglycerides (mmol/L) | 0.48 ± 0.03 | 0.34 ± 0.01 * |
| Serum cholesterol (mmol/L) | 1.17 ± 0.04 | 1.10 ± 0.03 |
| Triglycerides in the liver (μmol/g) | 6.1 ± 0.5 | 4.1 ± 0.4 ** |
| Cholesterol in the liver (μmol/g) | 10.2 ± 0.5 | 7.6 ± 0.3 ** |
| Triglycerides in the myocardium (μmol/g) | 0.56 ± 0.08 | 0.32 ± 0.06 * |
| Basal lipogenesis (nmol glucose/g/2 h) | 354 ± 12 | 505 ± 34 ** |
| Insulin-stimulated lipogenesis (nmol glucose/g/2 h) | 734 ± 37 | 1123 ± 73 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šilhavý, J.; Malínská, H.; Hüttl, M.; Marková, I.; Oliyarnyk, O.; Mlejnek, P.; Šimáková, M.; Liška, F.; Kazdová, L.; Moravcová, R.; et al. Downregulation of the Glo1 Gene Is Associated with Reduced Adiposity and Ectopic Fat Accumulation in Spontaneously Hypertensive Rats. Antioxidants 2020, 9, 1179. https://doi.org/10.3390/antiox9121179
Šilhavý J, Malínská H, Hüttl M, Marková I, Oliyarnyk O, Mlejnek P, Šimáková M, Liška F, Kazdová L, Moravcová R, et al. Downregulation of the Glo1 Gene Is Associated with Reduced Adiposity and Ectopic Fat Accumulation in Spontaneously Hypertensive Rats. Antioxidants. 2020; 9(12):1179. https://doi.org/10.3390/antiox9121179
Chicago/Turabian StyleŠilhavý, Jan, Hana Malínská, Martina Hüttl, Irena Marková, Olena Oliyarnyk, Petr Mlejnek, Miroslava Šimáková, František Liška, Ludmila Kazdová, Radka Moravcová, and et al. 2020. "Downregulation of the Glo1 Gene Is Associated with Reduced Adiposity and Ectopic Fat Accumulation in Spontaneously Hypertensive Rats" Antioxidants 9, no. 12: 1179. https://doi.org/10.3390/antiox9121179
APA StyleŠilhavý, J., Malínská, H., Hüttl, M., Marková, I., Oliyarnyk, O., Mlejnek, P., Šimáková, M., Liška, F., Kazdová, L., Moravcová, R., Novotný, J., & Pravenec, M. (2020). Downregulation of the Glo1 Gene Is Associated with Reduced Adiposity and Ectopic Fat Accumulation in Spontaneously Hypertensive Rats. Antioxidants, 9(12), 1179. https://doi.org/10.3390/antiox9121179





