Redox–Oligomeric State of Peroxiredoxin-2 and Glyceraldehyde-3-Phosphate Dehydrogenase in Obstructive Sleep Apnea Red Blood Cells under Positive Airway Pressure Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
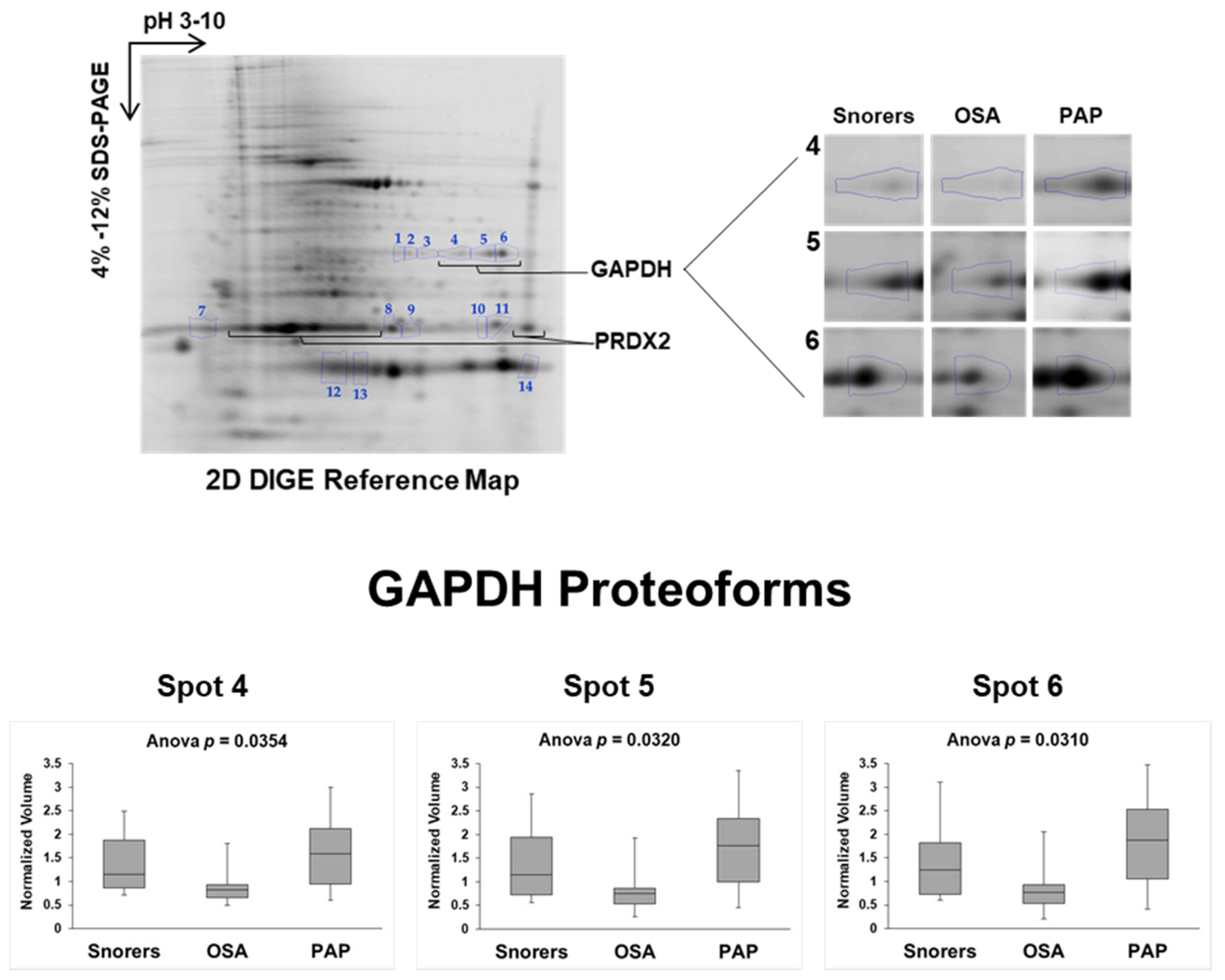
2.2. Discovery Phase: 2D-DIGE Proteomics
2.3. Protein Identification by Mass Spectrometry
2.4. Protein Annotation and Classification
2.5. Validation Phase: Western Blotting Analysis
2.6. Statistical Analysis
3. Results
3.1. Patients: Clinical, Biochemical, and Metabolic Characteristics
3.2. PAP Treatment Induces Changes in the RBC Proteome
3.3. GAPDH and PRDX2 Redox–Oligoforms in OSA before and after PAP Treatment
3.4. GAPDH and PRDX2 Correlation before and after PAP
3.5. Correlation between GAPDH and PRDX2 with OSA Clinical Parameters before and after PAP
3.6. GAPDH and PRDX2 Logistic Regression Models and ROC Curve Analysis for Predicting OSA or OSA Severity
3.7. Clinical Response in PAP-Induced PRDX2 SO2/3 Multimer
4. Discussion
4.1. RBC GAPDH as Predictor of OSA
4.2. PAP-Induced Sulfonylated GAPDH Tetramer/Oligomer in RBC May Be Related with GAPDH Peroxidase Activity and/or Eryptosis Associated “Gain of Function”
4.3. RBC PRDX2 as a Predictor of OSA Severity
4.4. PRDX2 SO2/3 Multimer May Protect RBC from PAP-Mediated Eryptosis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADR | Adrenaline |
| AHI | Apnea-hypopnea index |
| BMI | Body mass index |
| DA | Dopamine |
| EPW | Epworth sleepiness scale |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GLC | Glucose |
| Hb | Hemoglobin |
| HbA1C | Hemoglobin glycated |
| HCT | Hematocrit |
| HCY | Homocysteine |
| HOMA-IR | Model assessment of insulin resistance |
| INS | Insulin |
| MCV | Mean corpuscular volume |
| NAd | Nor-Adrenaline |
| ODI | Oxygen desaturation index |
| OSA | Obstructive sleep apnea |
| PAP | Positive airway pressure |
| PLT | Platelets |
| PRDX2 | Peroxiredoxin-2 |
| RBC | Red blood cell(s) |
| RDI | Respiratory disturbance index |
| RDW | Red cell distribution width |
| TC | Total cholesterol |
| TG | Triglycerides |
| t0 | Before PAP treatment |
| t6 | After six month of PAP treatment |
References
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef]
- Feliciano, A.; Torres, V.M.; Vaz, F.; Carvalho, A.S.; Matthiesen, R.; Pinto, P.; Malhotra, A.; Barbara, C.; Penque, D. Overview of proteomics studies in obstructive sleep apnea. Sleep Med. 2015, 16, 437–445. [Google Scholar] [CrossRef]
- Franklin, K.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population—A review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar]
- Knauert, M.P.; Naik, S.; Gillespie, M.B.; Kryger, M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J. Otorhinolaryngol. Head Neck Surg. 2015, 1, 17–27. [Google Scholar] [CrossRef]
- Toraldo, D.; Passali, D.; Sanna, A.; De Nuccio, F.; Conte, L.; De Benedetto, M. Cost-effectiveness strategies in OSAS management: A short review. Acta Otorhinolaryngol. Ital. 2017, 37, 447–453. [Google Scholar] [PubMed]
- Donovan, L.M.; Boeder, S.; Malhotra, A.; Patel, S.R. New developments in the use of positive airway pressure for obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 1323–1342. [Google Scholar] [PubMed]
- Virk, J.S.; Kotecha, B. When continuous positive airway pressure (CPAP) fails. J. Thorac. Dis. 2016, 8, E1112–E1121. [Google Scholar] [CrossRef] [PubMed]
- Fleming, W.E.; Holty, J.-E.C.; Bogan, R.K.; Hwang, D.; Ferouz-Colborn, A.S.; Budhiraja, R.; Redline, S.; Mensah-Osman, E.; Osman, N.I.; Li, Q.; et al. Use of blood biomarkers to screen for obstructive sleep apnea. Nat. Sci. Sleep 2018, 10, 159–167. [Google Scholar] [CrossRef]
- Light, M.; Owens, R.L.; Schmickl, C.N.; Malhotra, A. Precision Medicine for Obstructive Sleep Apnea. Sleep Med. Clin. 2019, 14, 391–398. [Google Scholar] [CrossRef]
- Feliciano, A.; Vaz, F.; Torres, V.M.; Valentim-Coelho, C.; Silva, R.; Prosinecki, V.; Alexandre, B.M.; Carvalho, A.S.; Matthiesen, R.; Malhotra, A.; et al. Evening and morning peroxiredoxin-2 redox/oligomeric state changes in obstructive sleep apnea red blood cells: Correlation with polysomnographic and metabolic parameters. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2017, 1863, 621–629. [Google Scholar] [CrossRef]
- Feliciano, A.; Vaz, F.; Valentim-Coelho, C.; Torres, V.M.; Silva, R.; Prosinecki, V.; Alexandre, B.M.; Almeida, A.; Almeida-Marques, C.; Carvalho, A.S.; et al. Evening and morning alterations in Obstructive Sleep Apnea red blood cell proteome. Data Brief 2017, 11, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zappulla, D. Environmental Stress, Erythrocyte Dysfunctions, Inflammation, and the Metabolic Syndrome: Adaptations to CO2Increases? J. CardioMetabolic Syndr. 2008, 3, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, A.; Oliveira, M.; Cysneiros, A.; Martinho, C.; Reis, R.; Penque, D.; Pinto, P.; Bã¡rbara, C. Effects of positive airway pressure therapy on cardiovascular and metabolic markers in males with obstructive sleep apnea. Rev. Port. Pneumol. Engl. Ed. 2017, 23, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, A.; Linhas, R.; Marçôa, R.; Cysneiros, A.; Martinho, C.; Reis, R.; Penque, D.; Pinto, P.; Barbara, C. Hematological evaluation in males with obstructive sleep apnea before and after positive airway pressure. Rev. Port. Pneumol. Engl. Ed. 2017, 23, 71–78. [Google Scholar] [CrossRef]
- Pacheco, S.A.; Aguiar, F.; Ruivo, P.; Proença, M.C.; Sekera, M.; Penque, D.; Simões, T. Occupational exposure to environmental tobacco smoke: A study in Lisbon restaurants. J. Toxicol. Environ. Heal. Part A 2012, 75, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, B.M.; Charro, N.; Blonder, J.; Lopes, C.; Azevedo, P.; De Almeida, A.B.; Chan, K.C.; Prieto, D.A.; Issaq, H.; Veenstra, T.D.; et al. Profiling the erythrocyte membrane proteome isolated from patients diagnosed with chronic obstructive pulmonary disease. J. Proteom. 2012, 76, 259–269. [Google Scholar] [CrossRef]
- Charro, N.; Hood, B.L.; Faria, D.; Pacheco, P.; Azevedo, P.; Lopes, C.; De Almeida, A.B.; Couto, F.M.; Conrads, T.P.; Penque, D. Serum proteomics signature of Cystic Fibrosis patients: A complementary 2-DE and LC–MS/MS approach. J. Proteom. 2011, 74, 110–126. [Google Scholar] [CrossRef]
- Branquinho, R.; Sousa, C.; Lopes, J.; Pintado, M.E.; Peixe, L.V.; Osório, H. Differentiation of Bacillus pumilus and Bacillus safensis Using MALDI-TOF-MS. PLoS ONE 2014, 9, e110127. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Jeong, J.; Jung, Y.; Na, S.; Jeong, J.; Lee, E.; Kim, M.-S.; Choi, S.; Shin, D.-H.; Paek, E.; Lee, H.-Y.; et al. Novel Oxidative Modifications in Redox-Active Cysteine Residues. Mol. Cell. Proteom. 2010, 10, 10. [Google Scholar] [CrossRef]
- Poynton, R.A.; Hampton, M.B. Peroxiredoxins as biomarkers of oxidative stress. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.-Y.; Zeger, S.L. Longitudinal data analysis using generalized linear models. Biometrics 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Gower, J.C. A General Coefficient of Similarity and Some of Its Properties. Biometrics 1971, 27, 857. [Google Scholar] [CrossRef]
- Hildebrandt, T.; Knuesting, J.; Berndt, C.; Morgan, B.; Scheibe, R. Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol. Chem. 2015, 396, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Woo, H.A.; Kil, I.S.; Bae, S.H. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J. Biol. Chem. 2012, 287, 4403–4410. [Google Scholar] [CrossRef]
- Peskin, A.V.; Dickerhof, N.; Poynton, R.A.; Paton, L.N.; Pace, P.E.; Hampton, M.B.; Winterbourn, C.C. Hyperoxidation of peroxiredoxins 2 and 3: Rate constants for the reactions of the sulfenic acid of the peroxidatic cysteine. J. Biol. Chem. 2013, 288, 14170–14177. [Google Scholar] [CrossRef]
- Cho, C.-S.; Yoon, H.J.; Kim, J.Y.; Woo, H.A.; Rhee, S.G. Circadian rhythm of hyperoxidized peroxiredoxin II is determined by hemoglobin autoxidation and the 20S proteasome in red blood cells. Proc. Natl. Acad. Sci. USA 2014, 111, 1–6. [Google Scholar] [CrossRef]
- Sirover, M.A. Structural analysis of glyceraldehyde-3-phosphate dehydrogenase functional diversity. Int. J. Biochem. Cell Biol. 2014, 57, 20–26. [Google Scholar] [CrossRef]
- Kunjithapatham, R.; Geschwind, J.F.; Devine, L.; Boronina, T.N.; Omeally, R.N.; Cole, R.N.; Torbenson, M.S.; Ganapathy-Kanniappan, S. Occurrence of a multimeric high-molecular-weight glyceraldehyde-3-phosphate dehydrogenase in human serum. J. Proteome Res. 2015, 14, 1645–1656. [Google Scholar] [CrossRef]
- Sirover, M.A. Moonlighting glyceraldehyde-3-phosphate dehydrogenase: posttranslational modification, protein and nucleic acid interactions in normal cells and in human pathology. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 1–18. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Guzhova, I.V.; Margulis, B.A. Glyceraldehyde-3-phosphate dehydrogenase is a multifaceted therapeutic target. Pharmaceutics 2020, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- White, M.R.; Garcin, E.D. D-Glyceraldehyde-3-Phosphate Dehydrogenase Structure and Function. Subcell. Biochem. 2017, 83, 413–453. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, A.; Emmert, H.; Soehle, J.; Winnefeld, M.; Fischer, F.; Wenck, H.; Gallinat, S.; Terstegen, L.; Lucius, R.; Hildebrand, J.; et al. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol. Cell 2015, 59, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Dick, T.P.; Ralser, M. Metabolic Remodeling in Times of Stress: Who Shoots Faster than His Shadow? Mol. Cell 2015, 59, 519–521. [Google Scholar] [CrossRef]
- Campanella, M.E.; Chu, H.; Low, P.S. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc. Natl. Acad. Sci. USA 2005, 102, 2402–2407. [Google Scholar] [CrossRef]
- Rogers, S.C.; Said, A.; Corcuera, D.; McLaughlin, D.; Kell, P.; Doctor, A. Hypoxia limits antioxidant capacity in red blood cells by altering glycolytic pathway dominance. FASEB J. 2009, 23, 3159–3170. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Sun, K.; Liu, H.; Song, A.; Monte, A.A.; Subudhi, A.W.; Lovering, A.T.; Dvorkin, D.; Julian, C.G.; et al. AltitudeOmics: Red Blood Cell Metabolic Adaptation to High Altitude Hypoxia. J. Proteome Res. 2016, 15, 3883–3895. [Google Scholar] [CrossRef]
- Liu, C.; Liu, B.; Zhang, E.-L.; Liao, W.-T.; Liu, J.; Sun, B.-D.; Xu, G.; Chen, J.; Gao, Y.-Q. Elevated pentose phosphate pathway is involved in the recovery of hypoxia-induced erythrocytosis. Mol. Med. Rep. 2017, 16, 9441–9448. [Google Scholar] [CrossRef]
- Zhang, J.; Veasey, S. Making Sense of Oxidative Stress in Obstructive Sleep Apnea: Mediator or Distracter? Front. Neurol. 2012, 3, 179. [Google Scholar] [CrossRef]
- Cekerevac, I.; Jakovljevic, V.; Zivkovic, V.; Petrovic, M.; Cupurdija, V.; Novkovic, L. Impact of severity of obstructive sleep apnea (OSA) and body composition on redox status in OSA patients. Vojn. Pregl. 2018, 75, 1089–1093. [Google Scholar] [CrossRef]
- Mullarky, E.; Cantley, L.C. Diverting Glycolysis to Combat Oxidative Stress. In Innovative Medicine; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–23. [Google Scholar]
- Alonso-Fernández, A.; Río, F.G.; Arias, M.A.; Hernanz, Á.; De La Peña, M.; Piérola, J.; Barceló, A.; Collazo, E.L.; Agusti, A. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: A randomised trial. Thorax 2009, 64, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Gangaraju, R.; Sundar, K.M.; Song, J.; Prchal, J.T. Polycythemia Is Rarely Caused By Obstructive Sleep Apnea. Blood 2016, 128, 2444. [Google Scholar] [CrossRef]
- Sökücü, S.N.; Özdemir, C.; Dalar, L.; Karasulu, L.; Aydın, Ş.; Altın, S. Complete Blood Count Alterations after Six Months of Continuous Positive Airway Pressure Treatment in Patients with Severe Obstructive Sleep Apnea. J. Clin. Sleep Med. 2014, 10, 873–878. [Google Scholar] [CrossRef][Green Version]
- Krieger, J.; Sforza, E.; Delanoe, C.; Petiau, C. Decrease in haematocrit with continuous positive airway pressure treatment in obstructive sleep apnoea patients. Eur. Respir. J 1992, 5, 228–233. [Google Scholar] [PubMed]
- Khan, A.M.; Ashizawa, S.; Hlebowicz, V.; Appel, D.W. Anemia of aging and obstructive sleep apnea. Sleep Breath. 2010, 15, 29–34. [Google Scholar] [CrossRef]
- Sirover, M.A. GAPDH and Hypoxia. In Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH); Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 155–165. [Google Scholar]
- Gupta, V.; Carroll, K.S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 847–875. [Google Scholar] [CrossRef]
- Lia, A.; Dowle, A.; Taylor, C.; Santino, A.; Roversi, P. Partial catalytic Cys oxidation of human GAPDH to Cys-sulfonic acid. Wellcome Open Res. 2020, 5, 114. [Google Scholar] [CrossRef]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell. Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef]
- Repsold, L.; Joubert, A.M. Eryptosis: An Erythrocyte’s Suicidal Type of Cell Death. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Kaneda, M.; Takeuchi, K.-I.; Inoue, K.; Umeda, M. Localization of the phosphatidylserine-binding site of glyceraldehyde-3-phosphate dehydrogenase responsible for membrane fusion. J. Biochem. 1997, 122, 1233–1240. [Google Scholar] [CrossRef]
- Turpin, C.; Catan, A.; Guerin-Dubourg, A.; Debussche, X.; Bravo, S.B.; Castro, A.I.; Elsen, J.V.D.; Meilhac, O.; Rondeau, P.; Bourdon, E. Enhanced oxidative stress and damage in glycated erythrocytes. PLoS ONE 2020, 15, e0235335. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.C.; Jehle, D. The red blood cell distribution width. J. Emerg. Med. 1991, 9, 71–74. [Google Scholar] [CrossRef]
- Tapan, O.O.; Gorgulu, B.; Tertemiz, K.C.; Alpaydin, A.O.; Oztura, I.; Itil, O.; Baklan, B. Effect of Continuous Positive Airway Pressure Treatment on Hemograms of Patients with Severe Obstructive Sleep Apnea in the Lack of Comorbidities. J. Sleep Disord. Ther. 2017, 6. [Google Scholar] [CrossRef]
- Nagababu, E.; Mohanty, J.G.; Friedman, J.S.; Rifkind, J.M. Role of peroxiredoxin-2 in protecting RBCs from hydrogen peroxide-induced oxidative stress. Free Radic. Res. 2013, 47, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.A.; Underwood, Z.E.; Tomalin, L.E.; Morgan, B.A.; Pillay, C.S. Hyperoxidation of Peroxiredoxins: Gain or Loss of Function? Antioxid. Redox Signal. 2018, 28, 574–590. [Google Scholar] [CrossRef]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; Van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Erratum: Corrigendum: Peroxiredoxins are conserved markers of circadian rhythms. Nat. Cell Biol. 2012, 489, 590. [Google Scholar] [CrossRef]
- Rey, G.; Valekunja, U.K.; Feeney, K.A.; Wulund, L.; Milev, N.B.; Stangherlin, A.; Ansel-Bollepalli, L.; Velagapudi, V.; O’Neill, J.S.; Reddy, A.B. The Pentose Phosphate Pathway Regulates the Circadian Clock. Cell Metab. 2016, 24, 462–473. [Google Scholar] [CrossRef]
- Osada-Oka, M.; Hashiba, Y.; Akiba, S.; Imaoka, S.; Sato, T. Glucose is necessary for stabilization of hypoxia-inducible factor-1α under hypoxia: Contribution of the pentose phosphate pathway to this stabilization. FEBS Lett. 2010, 584, 3073–3079. [Google Scholar] [CrossRef]
- Jaspers, T.; Morrell, M.; Simonds, A.; Adcock, I.M.; Durham, A.L. The role of hypoxia and the circadian rhythm in sleep apnoea. Sleep Control Breath. 2015, 46, OA298. [Google Scholar] [CrossRef]
- Von Allmen, D.C.; Francey, L.J.; Rogers, G.M.; Ruben, M.D.; Cohen, A.P.; Wu, G.; Schmidt, R.E.; Ishman, S.L.; Amin, R.S.; HogenEsch, J.B.; et al. Circadian Dysregulation: The Next Frontier in Obstructive Sleep Apnea Research. Otolaryngol. Neck Surg. 2018, 159, 948–955. [Google Scholar] [CrossRef]
- Perkins, A.; Poole, L.B.; Karplus, P.A. Tuning of Peroxiredoxin Catalysis for Various Physiological Roles. Biochemistry 2014, 53, 7693–7705. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Piszczek, G.; Rhee, S.G.; Chock, P.B. Glutathionylation of Peroxiredoxin I Induces Decamer to Dimers Dissociation with Concomitant Loss of Chaperone Activity. Biochemistry 2011, 50, 3204–3210. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Suzuki, Y.; Tateishi, N.; Maeda, N. Effects of dietary triglycerides on rheological properties of human red blood cells (abstract). Clin. Hemorheol. Microcirc. 2004, 30, 301–305. [Google Scholar] [PubMed]
- De Man, F.H.; Nieuwland, R.; Van Der Laarse, A.; Romijn, F.; Smelt, A.H.; Leuven, J.A.G.; Sturk, A. Activated platelets in patients with severe hypertriglyceridemia: Effects of triglyceride-lowering therapy. Atherosclerosis 2000, 152, 407–414. [Google Scholar] [CrossRef]
- Peled, N.; Kassirer, M.; Kramer, M.R.; Rogowski, O.; Shlomi, D.; Fox, B.; Berliner, A.S.; Shitrit, D. Increased erythrocyte adhesiveness and aggregation in obstructive sleep apnea syndrome. Thromb. Res. 2008, 121, 631–636. [Google Scholar] [CrossRef]
- Sinnapah, S.; Cadelis, G.; Waltz, X.; Lamarre, Y.; Connes, P. Overweight explains the increased red blood cell aggregation in patients with obstructive sleep apnea. Clin. Hemorheol. Microcirc. 2015, 59, 17–26. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Q. The protective effect of peroxiredoxin II on oxidative stress induced apoptosis in pancreatic β-cells. Cell Biosci. 2012, 2, 22. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kim, S.-U.; Kwon, T.; Lee, D.-S.; Ha, H.-L.; Park, D.-S.; Woo, E.-J.; Lee, S.Y.; Kim, J.-M.; Chae, H.-B.; et al. Peroxiredoxin II is essential for preventing hemolytic anemia from oxidative stress through maintaining hemoglobin stability. Biochem. Biophys. Res. Commun. 2012, 426, 427–432. [Google Scholar] [CrossRef]





| Demographic, Polysomnographic, and Analytical Characterization | |||||
|---|---|---|---|---|---|
| Demographic and PSG Parameters | Screened Subjects | ||||
| Mean (Standard Deviation) | p Value (<0.05) | ||||
| Snorers (n = 10) | OSA (n = 10) | PAP (n = 10) | Snorers vs. OSA | OSA vs. PAP | |
| Age (years) | 45.6 (10.9) | 46.4 (6.5) | - | NS | n/a |
| Habits | |||||
| Current Smoking (n) | 2 | 1 | - | - | - |
| EPW Score | 9.7 (6.5) | 7.7 (2.9) | 4.7 (3.8) | NS | NS |
| Observational features | |||||
| Morning arterial pressure (mmHg) * | 132.5 (17.0)/80.9 (11.0) | 138.4 (12.6)/90.7 (10.4) | - | n/a | n/a |
| BMI (kg/m2) | 26.7 (1.6) | 30.1 (2.9) | - | 0.006 | n/a |
| Abdominal perimeter (cm) | 95.5 (4.4) | 106.1 (10.4) | - | 0.012 | n/a |
| Comorbidities | |||||
| Hypertension (n) | 3 | 7 | - | - | - |
| Respiratory diseases (n) | 0 | 0 | - | - | - |
| Dyslipidemia (n) | 3 | 6 | - | - | - |
| Diabetes (n) | 0 | 0 | - | - | - |
| Polysomnographic parameters | |||||
| Mild/Moderate/Severe (n) | - | -/-/10 | - | n/a | n/a |
| RDI (events/h) | 3.1 (1.2) | 53.7 (17.3) | - | 0.011 | n/a |
| ODI (events/h) | 3.3 (4.7) | 46.2 (22.9) | - | <0.001 | n/a |
| Sleep efficiency (%) | 78.4 (15.2) | 73.6 (12.7) | - | n/a | n/a |
| Arousal index (%) | 14.7 (6.7) | 38.2 (15.3) | - | <0.001 | n/a |
| Minimum Arterial Saturation (%) | 89.3 (0.03) | 78.2 (0.08) | - | <0.001 | n/a |
| PAP record | |||||
| Number of days without use | - | - | 53.2 (64.9) | - | - |
| Total of recording days | - | - | 291.6 (158.7) | - | - |
| Residual AHI | - | - | 1.9 (1.5) | - | - |
| Analytical parameters | |||||
| Glycemic profile | |||||
| Glucose (70–110 mg/dL) | 96.7 (9.4) | 96.2 (11.5) | 92.9 (14.9) | NS | NS |
| HbA1C (4–6%) | 5.7 (0.3) | 5.8 (0.4) | 5.6 (0.3) | NS | 0.027 |
| Insulin (3–25 mU/L) | 13.1 (6.7) | 23.6 (13.5) | 41.1 (43.3) | 0.047 | NS |
| HOMA-IR (<2.15) | 3.1 (1.6) | 5.7 (3.2) | 9.9 (11.2) | 0.041 | NS |
| Lipid profile | |||||
| Cholesterol (<190 mg/dL) | 202.8 (37.1) | 189.7 (29.9) | 177.2 (35.6 | NS | NS |
| Triglycerides (<150 mg/dL) | 127.3 (66.7) | 155.6 (92.2) | 141.4 (75.2) | NS | NS |
| Cardiovascular marker | |||||
| Homocysteine (3.7–13.9 µmol/L) | 14.8 (3.0) | 16.0 (3.4) | 16.6 (3.3) | NS | NS |
| Urinary catecholamines | |||||
| Adrenaline (1.7–22.4 µg/24h) | 20.9 (13.7) | 61.2 (142.8) | 15.3 (7.3) | NS | NS |
| Nor-adrenaline (12.1–85.5 µg/24 h) | 54.6 (16.5) | 252.9 (565.5) | 62.5 (25.5) | NS | NS |
| Dopamine (0–498 μg/24 h) | 297.3 (94.9) | 1066.9 (2403.7) | 372.4 (146.9) | NS | NS |
| Complete Hemogram | |||||
| RBC (4.5–5.9 × 1012/L) | 5.1 (0.3) | 5.3 (0.3) | 5.2 (0.3) | NS | NS |
| Hemoglobin (13–17.5 g/dL) | 15.3 (0.8) | 16.0 (0.9) | 15.4 (1.1) | NS | 0.007 |
| Hematocrit (40–50%) | 45.1 (2.9) | 47.0 (3.01) | 45.9 (2.8) | NS | NS |
| MCV (80–97 fL) | 89.3 (4.7) | 89.1 (4.7) | 88.8 (4.9) | NS | NS |
| RDW (11.5–14.5%) | 13.4 (0.6) | 13.9 (0.8) | 13.7 (0.6) | NS | NS |
| Platelets (150–450 × 103µL) | 230.7 (33.6) | 217.0 (48.9) | 194.1 (46.5) | NS | NS |
| Demographic. Polysomnographic and Analytical Characterization | |||||
|---|---|---|---|---|---|
| Demographic and PSG Parameters | Screened Subjects | ||||
| Mean (Standard Deviation) | p Value (<0.05) | ||||
| Snorers (n = 23) | OSA (n = 36) | PAP (n = 36) | Snorers vs. OSA | OSA vs. PAP | |
| Age (years) | 44.8 (9.6) | 47.1 (7.5) | - | NS | n/a |
| Habits | |||||
| Current Smoking (n) | 9 | 6 | - | NS | n/a |
| EPW Score | 9.5 (4.8) | 10.8 (4.9) | 6.2 (4.4) | NS | <0.001 |
| Observational features | |||||
| Morning arterial pressure (mmHg) * | 134.9 (17.2)/83.4 (11.9) | 131.0 (16.9)/83.7 (11.9) | - | n/a | n/a |
| BMI (kg/m2) | 27.2 (3.2) | 30.2 (2.9) | - | <0.001 | n/a |
| Abdominal perimeter (cm) | 97.5 (7.7) | 106.0 (8.1) | - | <0.001 | n/a |
| Comorbidities | |||||
| Hypertension (n) | 6 | 23 | - | - | - |
| Respiratory diseases (n) | 0 | 0 | - | - | - |
| Dyslipidemia (n) | 9 | 18 | - | - | - |
| Diabetes (n) | 0 | 0 | - | - | - |
| Polysomnographic parameters | |||||
| Mild/Moderate/Severe (n) | - | 16/3/17 | - | n/a | n/a |
| RDI (events/h) | 2.7 (1.4) | 31.7 (25.2) | - | <0.001 | n/a |
| ODI (events/h) | 2.2 (3.3) | 26.3 (25.4) | - | <0.001 | n/a |
| Sleep efficiency (%) | 78.1 (12.2) | 74.6 (16.8) | - | NS | n/a |
| Arousal index (%) | 14.2 (6.0) | 28.3 (17.8) | - | <0.001 | n/a |
| Minimum Arterial Saturation (%) | 89.3 (2.9) | 82.6 (6.3) | - | <0.001 | n/a |
| PAP record | |||||
| Number of days without use | - | - | 42.8 (47.5) | - | - |
| Total of recording days | - | - | 275.6 (118.2) | - | - |
| Residual AHI | - | - | 1.7 (1.2) | - | - |
| Analytical parameters | |||||
| Glycemic profile | |||||
| Glucose (70–110 mg/dL) | 92.9 (7.9) | 95.7 (12.2) | 93.9 (13.7) | NS | NS |
| HbA1C (4–6%) | 5.5 (0.4) | 5.6 (0.4) | 5.6 (0.6) | NS | NS |
| Insulin (3–25 mU/L) | 12.4 (6.1) | 16.4 (10.1) | 24.6 (31.0) | NS | NS |
| HOMA-IR (<2.15) | 2.9 (1.5) | 3.9 (2.6) | 6.2 (8.4) | 0.040 | NS |
| Lipid profile | |||||
| Cholesterol (<190 mg/dL) | 190.3 (37.0) | 186.7 (39.5) | 182.3 (31.6) | NS | NS |
| Triglycerides (<150 mg/dL) | 118.9 (62.9) | 134.1 (66.1) | 139.6 (83.4) | NS | NS |
| Cardiovascular marker | |||||
| Homocysteine (3.7–13.9 µmol/L) | 15.4 (3.8) | 16.2 (6.7) | 17.1 (5.6) | NS | NS |
| Urinary catecholamines | |||||
| Adrenaline (1.7–22.4 µg/24 h) | 20.4 (17.7) | 28.6 (76.1) | 17.2 (9.4) | NS | NS |
| Nor-adrenaline (12.1–85.5 µg/24 h) | 64.0 (29.4) | 117.9 (300.5) | 55.0 (20.9) | NS | NS |
| Dopamine (0–498 μg/24 h) | 375.5 (201.0) | 547.1 (1273.6) | 313.8 (133.0) | NS | NS |
| Complete Hemogram | |||||
| RBC (4.5–5.9 × 1012/L) | 5.1 (0.4) | 5.1 (0.3) | 5.0 (0.3) | NS | <0.001 |
| Hemoglobin (13–17.5 g/dL) | 15.3 (0.8) | 15.6 (1.0) | 15.1 (0.9) | NS | <0.001 |
| Hematocrit (40–50%) | 45.0 (2.1) | 45.6 (2.9) | 44.3 (2.7) | NS | <0.001 |
| MCV (80–97 fL) | 89.1 (5.6) | 88.6 (3.8) | 88.5 (3.4) | NS | NS |
| RDW (11.5–14.5%) | 13.5 (0.5) | 13.4 (0.7) | 13.7 (0.6) | NS | NS |
| Platelets (150–450 × 103µL) | 232.2 (47.5) | 226.2 (48.6) | 204.6 (46.4) | NS | <0.001 |
| GAPDH Correlate | OSA | PAP | ||||
|---|---|---|---|---|---|---|
| Pearson r Value | p Value | Pearson r Value | p Value | |||
| PRDX2 | S-S/S-S Dimer | Tetramer | −0.512 * | 0.025 | - | - |
| SO3 Tetramer | −0.483 * | 0.036 | - | - | ||
| SO3 Oligomers | −0.473 * | 0.041 | - | - | ||
| SO2/3 Monomer | Monomer | - | - | −0.551 * | 0.015 | |
| Tetramer | - | - | −0.551 * | 0.015 | ||
| Oligomers | - | - | −0.485 * | 0.035 | ||
| SO3 Tetramer | - | - | −0.506 * | 0.027 | ||
| SO3 Oligomers | - | - | −0.516 * | 0.024 | ||
| SO2/3 Multimer | Monomer | - | - | 0.526 * | 0.021 | |
| Tetramer | - | - | 0.777 *** | <0.001 | ||
| Oligomers | - | - | 0.712 *** | 0.001 | ||
| SO3 Tetramer | - | - | 0.838 *** | <0.001 | ||
| SO3 Oligomers | - | - | 0.787 *** | <0.001 | ||
| Protein | Oligomers | Correlate | OSA | PAP | ||
|---|---|---|---|---|---|---|
| Pearson r Value | p Value | Pearson r Value | p Value | |||
| GAPDH | Monomer | RBC | 0.389 * | 0.019 | - | - |
| Hb | 0.392 * | 0.018 | - | - | ||
| RDW | - | - | 0.363 * | 0.029 | ||
| RDI | 0.375 * | 0.024 | - | - | ||
| Tetramer | HbA1C | - | - | 0.336 * | 0.045 | |
| MCV | −0.359 * | 0.032 | - | - | ||
| ADR | - | - | 0.490 ** | 0.002 | ||
| Oligomers | HbA1C | −0.337 * | 0.044 | - | - | |
| MCV | −0.339 * | 0.043 | - | - | ||
| ADR | - | - | 0.436 ** | 0.008 | ||
| EPW | - | - | 0.335 * | 0.046 | ||
| SO3 Tetramer | HbA1C | −0.359 * | 0.031 | 0.421 * | 0.010 | |
| TG | - | - | 0.341 * | 0.042 | ||
| ADR | - | - | 0.553 *** | <0.001 | ||
| HCY | - | - | 0.355 * | 0.034 | ||
| SO3 Oligomers | HbA1C | −0.354 * | 0.034 | 0.362 * | 0.030 | |
| ADR | - | - | 0.479 ** | 0.003 | ||
| PRDX2 | Monomer | TG | −0.593 ** | 0.007 | - | - |
| EPW | - | - | 0.557 * | 0.013 | ||
| HCY | 0.469 * | 0.043 | - | - | ||
| S-S/S-S Dimer | RDW | −0.577 ** | 0.010 | - | - | |
| PLT | 0.510 * | 0.026 | - | - | ||
| S-S Dimer | INS | −0.462 * | 0.047 | - | - | |
| HOMA-IR | −0.476 * | 0.040 | - | - | ||
| RDW | −0.457 * | 0.049 | - | - | ||
| PLT | 0.552 * | 0.014 | - | - | ||
| EPW | 0.523 * | 0.022 | - | - | ||
| RDI | −0.570* | 0.011 | - | - | ||
| SO2/3 Monomer | GLC | - | - | −0.601 ** | 0.007 | |
| ADR | - | - | −0.456 * | 0.050 | ||
| S-S/SO2/3 Dimer | HbA1C | - | - | 0.549 * | 0.015 | |
| RDW | −0.465 * | 0.045 | - | - | ||
| PLT | 0.508 * | 0.026 | - | - | ||
| SO2/3 Multimer | TG | - | - | 0.479 * | 0.038 | |
| ADR | - | - | 0.772 *** | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentim-Coelho, C.; Vaz, F.; Antunes, M.; Neves, S.; Martins, I.L.; Osório, H.; Feliciano, A.; Pinto, P.; Bárbara, C.; Penque, D. Redox–Oligomeric State of Peroxiredoxin-2 and Glyceraldehyde-3-Phosphate Dehydrogenase in Obstructive Sleep Apnea Red Blood Cells under Positive Airway Pressure Therapy. Antioxidants 2020, 9, 1184. https://doi.org/10.3390/antiox9121184
Valentim-Coelho C, Vaz F, Antunes M, Neves S, Martins IL, Osório H, Feliciano A, Pinto P, Bárbara C, Penque D. Redox–Oligomeric State of Peroxiredoxin-2 and Glyceraldehyde-3-Phosphate Dehydrogenase in Obstructive Sleep Apnea Red Blood Cells under Positive Airway Pressure Therapy. Antioxidants. 2020; 9(12):1184. https://doi.org/10.3390/antiox9121184
Chicago/Turabian StyleValentim-Coelho, Cristina, Fátima Vaz, Marília Antunes, Sofia Neves, Inês L. Martins, Hugo Osório, Amélia Feliciano, Paula Pinto, Cristina Bárbara, and Deborah Penque. 2020. "Redox–Oligomeric State of Peroxiredoxin-2 and Glyceraldehyde-3-Phosphate Dehydrogenase in Obstructive Sleep Apnea Red Blood Cells under Positive Airway Pressure Therapy" Antioxidants 9, no. 12: 1184. https://doi.org/10.3390/antiox9121184
APA StyleValentim-Coelho, C., Vaz, F., Antunes, M., Neves, S., Martins, I. L., Osório, H., Feliciano, A., Pinto, P., Bárbara, C., & Penque, D. (2020). Redox–Oligomeric State of Peroxiredoxin-2 and Glyceraldehyde-3-Phosphate Dehydrogenase in Obstructive Sleep Apnea Red Blood Cells under Positive Airway Pressure Therapy. Antioxidants, 9(12), 1184. https://doi.org/10.3390/antiox9121184






