Plant-Derived Natural Antioxidants in Meat and Meat Products
Abstract
:1. Introduction
2. Lipid and Protein Oxidation of Meat
3. Antioxidants Used in Meat Systems
3.1. Phenols
3.1.1. Tannins
3.1.2. Flavonoids
3.1.3. Lignans
3.1.4. Stilbenes
3.2. Vitamin E
3.3. Carotenoids
3.4. Vitamin C
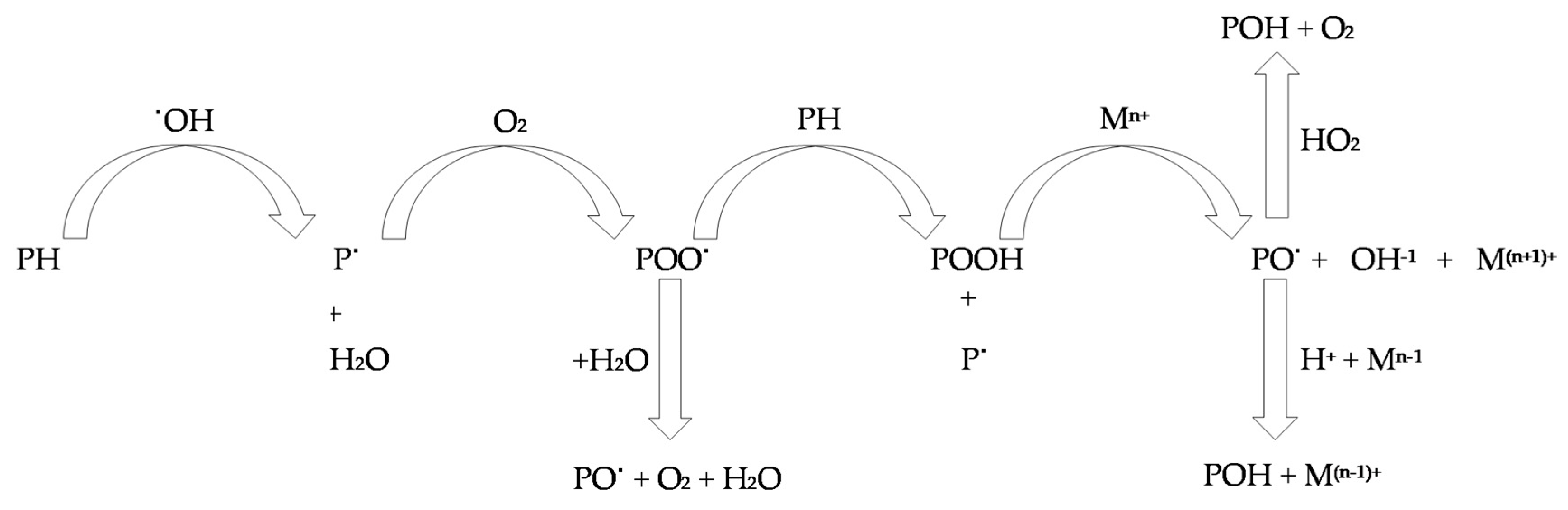
4. Course of Action of Antioxidants
5. Assays Used to Assess Antioxidant Capacity of Meat and Its Products
6. Plant-Derived Antioxidants
6.1. Spice- and Herb-Derived Antioxidants
6.1.1. Oregano
6.1.2. Rosemary
6.1.3. Sage
6.1.4. Thyme
6.1.5. Tea
6.1.6. Black Pepper
6.1.7. Other Herbs and Spices
6.2. Fruit-Derived Antioxidants
6.2.1. Grapes
6.2.2. Plums
6.2.3. Bearberries
6.2.4. Cranberries
6.2.5. Strawberries
6.2.6. Pomegranates
6.2.7. Other Fruits
7. Legislative Framework for Antioxidants in Meat and Meat Products
8. Application of Natural Antioxidants in Meat Industry
9. Future Challenges for and Limitations of Natural Antioxidant Use in Meat Systems
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Xiao, S.; Samaraweera, H.; Joo, E.; Ahn, D.U. Improving functional value of meat products. Meat Sci. 2010, 86, 15–31. [Google Scholar] [CrossRef]
- Bohrer, B.M. Review: Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 2017, 65, 103–112. [Google Scholar] [CrossRef]
- Whitnall, T.; Pitts, N. Global trends in meat consumption. Agric. Commod. 2019, 96–99. [Google Scholar]
- Shahbandeh, M. Global Meat Industry Value, 2018 & 2023|Statista 2019. Available online: https://www.statista.com/statistics/502286/global-meat-and-seafood-market-value/ (accessed on 31 October 2020).
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef] [Green Version]
- Rahman, U.; Sahar, A.; Ishaq, A.; Aadil, R.M.; Zahoor, T.; Ahmad, M.H. Advanced meat preservation methods: A mini review. J. Food Saf. 2018, 38, e12467. [Google Scholar] [CrossRef]
- Pereira, R.N.; Vicente, A.A. Environmental impact of novel thermal and non-thermal technologies in food processing. Food Res. Int. 2010, 43, 1936–1943. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2019.Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Mercier, Y.; Gatellier, P.; Renerre, M. Lipid and protein oxidation in vitro, and antioxidant potential in meat from Charolais cows finished on pasture or mixed diet. Meat Sci. 2004, 66, 467–473. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.P.F.; Garcia, C.C.M.; Sena, F.; Freitas, F.P.; Miyamoto, S.; Medeiros, M.H.G.; Di Mascio, P. Lipid hydroperoxide-induced and hemoglobin-enhanced oxidative damage to colon cancer cells. Free Radic. Biol. Med. 2011, 51, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Broncano, J.M.; Petrón, M.J.; Parra, V.; Timón, M.L. Effect of different cooking methods on lipid oxidation and formation of free cholesterol oxidation products (COPs) in Latissimus dorsi muscle of Iberian pigs. Meat Sci. 2009, 83, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Leonarduzzi, G.; Testa, G.; Gargiulo, S.; Poli, G.; Biasi, F. Lipid Oxidation Derived Aldehydes and Oxysterols Between Health and Disease. Eur. J. Lipid Sci. Technol. 2019, 121, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef]
- Kamala Kumari, P.V.; Akhila, S.; Srinivasa Rao, Y.; Rama Devi, B. Alternative to artificial preservatives. Syst. Rev. Pharm. 2019, 10, S13–S16. [Google Scholar] [CrossRef]
- Pokorný, J. Are natural antioxidants better-and safer-Than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Moylan, S.; Berk, M.; Dean, O.M.; Samuni, Y.; Williams, L.J.; O’Neil, A.; Hayley, A.C.; Pasco, J.A.; Anderson, G.; Jacka, F.N.; et al. Oxidative & nitrosative stress in depression: Why so much stress? Neurosci. Biobehav. Rev. 2014, 45, 46–62. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Insani, E.M.; Biolatto, A.; Sancho, A.M.; García, P.T.; Pensel, N.A.; Josifovich, J.A. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beef. Meat Sci. 2005, 70, 35–44. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of Oxidative Processes in Meat and Toxicity Induced by Postprandial Degradation Products: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Guyon, C.; Meynier, A.; de Lamballerie, M. Protein and lipid oxidation in meat: A review with emphasis on high-pressure treatments. Trends Food Sci. Technol. 2016, 50, 131–143. [Google Scholar] [CrossRef]
- Vieira, S.A.; Zhang, G.; Decker, E.A. Biological Implications of Lipid Oxidation Products. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 339–351. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Kamal Eldin, A. Methods to Determine the Extent of Lipid Oxidation in Foods; Woodhead Publishing Limited: Sawston, UK, 2010; ISBN 9781845696481. [Google Scholar]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef]
- Halliwell, B. Vitamin C: Antioxidant or pro-oxidant in vivo? Free Radic. Res. 1996, 25, 439–454. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H. Bin Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef]
- Swanson, B.G. Tannins and Polyphenols. Encycl. Food Sci. Nutr. 2003, 5729–5733. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. In Phytochemistry: Advances in Research; Research Signpost: Trivandrum, India, 2015; Volume 661, ISBN 8130800349. [Google Scholar]
- Dayan, F.E.; Watson, S.B.; Nanayakkara, N.P.D. Biosynthesis of lipid resorcinols and benzoquinones in isolated secretory plant root hairs. J. Exp. Bot. 2007, 58, 3263–3272. [Google Scholar] [CrossRef]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- Osorio, M.E.; Quiroz, K.A.; Carvajal, M.A.; Vergara, A.P.; Sánchez, E.Y.; González, C.E.; Catalán, K.S. Synthesis, anti-phytopathogenic and DPPH radical scavenging activities of C-prenylated acetophenones and benzaldehydes. J. Chil. Chem. Soc. 2016, 61, 3095–3101. [Google Scholar] [CrossRef] [Green Version]
- Parent, G.J.; Giguère, I.; Mageroy, M.; Bohlmann, J.; MacKay, J.J. Evolution of the biosynthesis of two hydroxyacetophenones in plants. Plant Cell Environ. 2018, 41, 620–629. [Google Scholar] [CrossRef]
- Cook, S.D. An Historical Review of Phenylacetic Acid. Plant Cell Physiol. 2019, 60, 243–254. [Google Scholar] [CrossRef]
- Guerrero, E.D.; Chinnici, F.; Natali, N.; Marín, R.N.; Riponi, C. Solid-phase extraction method for determination of volatile compounds in traditional balsamic vinegar. J. Sep. Sci. 2008, 31, 3030–3036. [Google Scholar] [CrossRef]
- Krings, U.; Zelena, K.; Wu, S.; Berger, R.G. Thin-layer high-vacuum distillation to isolate volatile flavour compounds of cocoa powder. Eur. Food Res. Technol. 2006, 223, 675–681. [Google Scholar] [CrossRef]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Singh, P.; Rawat, M.S.M.; Joshi, G.P. Naturally Occurring Xanthones: Chemistry and Biology. J. Appl. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Han, Y.S.; Van Der Heijden, R.; Verpoorte, R. Biosynthesis of anthraquinones in cell cultures of the Rubiaceae. Plant Cell. Tissue Organ Cult. 2001, 67, 201–220. [Google Scholar] [CrossRef]
- Nahak, G.; Sahu, R.K. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. J. Appl. Pharm. Sci. 2011, 1, 153–157. [Google Scholar]
- Zitterman, A. DIETARY FIBER|Bran. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1844–1850. ISBN 9780080552323. [Google Scholar]
- Zálešák, F.; Bon, D.J.Y.D.; Pospíšil, J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Vinardell, M.P.; Ugartondo, V.; Mitjans, M. Potential applications of antioxidant lignins from different sources. Ind. Crops Prod. 2008, 27, 220–223. [Google Scholar] [CrossRef]
- Gellerstedt, G.L.F.; Henriksson, E.G. Lignins: Major sources, structure and properties. Monomers Polym. Compos. Renew. Resour. 2008, 201–224. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Natural Antioxidants of Plant Origin, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 90. [Google Scholar]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. The increase in human plasma antioxidant capacity after apple consumption is due to the metabolic effect of fructose on urate, not apple-derived antioxidant flavonoids. Free Radic. Biol. Med. 2004, 37, 251–258. [Google Scholar] [CrossRef]
- Gülçin, I.; Elias, R.; Gepdiremen, A.; Boyer, L. Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur. Food Res. Technol. 2006, 223, 759–767. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Prasad, K. Hypocholesterolemic and antiatherosclerotic effect of flax lignan complex isolated from flaxseed. Atherosclerosis 2005, 179, 269–275. [Google Scholar] [CrossRef]
- Adlercreutz, H. Lignans and human health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.N.; Lou, H.X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Cassidy, A.; Hanley, B.; Lamuela-Raventos, R.M. Isoflavones, lignans and stilbenes-Origins, metabolism and potential importance to human health. J. Sci. Food Agric. 2000, 80, 1044–1062. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. CRC. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [Green Version]
- DellaPenna, D.; Pogson, B.J. Vitamin synthesis in plants: Tocopherols and carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [Green Version]
- Azzi, A.; Stocker, A. Vitamin E: Non-antioxidant roles. Prog. Lipid Res. 2000, 39, 231–255. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Russell, R.M.; Paiva, S.A.R. β-Carotene and Other Carotenoids as Antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta-Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Levine, M.; Dutta, A.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C: Biosynthesis, recycling and degradation in mammals. Fed. Eur. Biochem. Soc. J. 2007, 274, 1–22. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Duarte, T.L.; Lunec, J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Hudson, B.J. Food Antioxidants; Hudson, B.J.F., Ed.; Springer: Dordrecht, The Netherlands, 1990; Volume 41, ISBN 978-94-010-6824-6. [Google Scholar]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Bohm, V.; Muller, L. Methods to Measure the Antioxidant Capacity of Meat Products. In Handbook of Processed Meats and Poultry Analysis, 1st ed.; Nollet, L.M.L., Toldra, F., Eds.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9780429148262. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Jung, S.; Nam, K.C.; Jo, C. Detection of malondialdehyde in processed meat products without interference from the ingredients. Food Chem. 2016, 209, 90–94. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 1–11. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.J.; Romero, M.P. Recent Advances in Biologically Active Compounds in Herbs and Spices: A Review of the Most Effective Antioxidant and Anti-Inflammatory Active Principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Jo, C. Potential Application of Essential Oils as Natural Antioxidants in Meat and Meat Products: A Review. Food Rev. Int. 2014, 30, 71–90. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’ Ana, A.S.; Khaneghahc, A.M.; Mohsen, G.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Fernandes, R.D.P.; Trindade, M.A.; Melo, M.P. De Natural Antioxidants and Food Applications: Healthy Perspectives; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128114469. [Google Scholar]
- Aminzare, M.; Hashemi, M.; Ansarian, E.; Bimkar, M.; Azar, H.H.; Mehrasbi, M.R.; Daneshamooz, S.; Raeisi, M.; Jannat, B.; Afshari, A. Using Natural Antioxidants in Meat and Meat Products as Preservatives: A Review. Adv. Anim. Vet. Sci. 2019, 7, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Kalogianni, A.I.; Lazou, T.; Bossis, I.; Gelasakis, A.I. Natural phenolic compounds for the control of oxidation, bacterial spoilage, and foodborne pathogens in meat. Foods 2020, 9, 794. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Chouliara, E.; Karatapanis, A.; Savvaidis, I.N.; Kontominas, M.G.Ã. Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4oC. Food Microbiol. 2007, 24, 607–617. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Hasapidou, A.; Savvaidis, I.N. The effects of modified atmosphere packaging, EDTA and oregano oil on the quality of chicken liver meat. Food Res. Int. 2011, 44, 2751–2756. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT-Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Pugine, S.M.P.; Lima, C.G.; Lorenzo, J.M.; Melo, M.P. De Evaluation of oxidative stability of lamb burger with Origanum vulgare extract. Food Chem. 2017, 233, 101–109. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Lorenzo, J.M.; Melo, M.P. De Assessment of the stability of sheep sausages with the addition of di ff erent concentrations of Origanum vulgare extract during storage. Meat Sci. 2018, 137, 244–257. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Florou-Paneri, P.; Christaki, E.; Giannenas, I.; Spais, A.B. Performance of rabbits and oxidative stability of muscle tissues as affected by dietary supplementation with oregano essential oil. Arch. Anim. Nutr. 2004, 58, 209–218. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Deligeorgis, S.G.; Bizelis, J.A.; Dardamani, A.; Theodosiou, I.; Fegeros, K. Effect of dietary oregano oil supplementation on lamb meat characteristics. Meat Sci. 2008, 79, 217–223. [Google Scholar] [CrossRef]
- Ranucci, D.; Beghelli, D.; Trabalza-Marinucci, M.; Branciari, R.; Forte, C.; Olivieri, O.; Badillo Pazmay, G.V.; Cavallucci, C.; Acuti, G. Dietary effects of a mix derived from oregano (Origanum vulgare L.) essential oil and sweet chestnut (Castanea sativa Mill.) wood extract on pig performance, oxidative status and pork quality traits. Meat Sci. 2015, 100, 319–326. [Google Scholar] [CrossRef]
- Zou, Y.; Xiang, Q.; Wang, J.; Wei, H.; Peng, J. Effects of oregano essential oil or quercetin supplementation on body weight loss, carcass characteristics, meat quality and antioxidant status in finishing pigs under transport stress. Livest. Sci. 2016, 192, 33–38. [Google Scholar] [CrossRef]
- Haak, L.; Raes, K.; Smet, S. De Effect of plant phenolics, tocopherol and ascorbic acid on oxidative stability of pork patties. J. Sci. Food Agric. 2009, 1360–1365. [Google Scholar] [CrossRef]
- Lara, M.S.; Gutierrez, J.I.; Timón, M.; Andrés, A.I. Evaluation of two natural extracts (Rosmarinus officinalis L. and Melissa officinalis L.) as antioxidants in cooked pork patties packed in MAP. Meat Sci. 2011, 88, 481–488. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; True, A.D.; Zhou, L.; Xiong, Y.L. Inhibition of Lipid Oxidation and Rancidity in Precooked Pork Patties by Radical-Scavenging Licorice (Glycyrrhiza glabra) Extract. J. Food Sci. 2013, 78. [Google Scholar] [CrossRef]
- Armenteros, M.; Morcuende, D.; Ventanas, J.; Estévez, M. The application of natural antioxidants via brine injection protects Iberian cooked hams against lipid and protein oxidation. Meat Sci. 2016, 116, 253–259. [Google Scholar] [CrossRef]
- Jongberg, S.; Tørngren, M.A.; Gunvig, A.; Skibsted, L.H.; Lund, M.N. Effect of green tea or rosemary extract on protein oxidation in Bologna type sausages prepared from oxidatively stressed pork. Meat Sci. 2013, 93, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Aurrekoetxea, G.P.; Angulo, I.; Paseiro-Losada, P.; Cruz, J.M. Development of new active packaging fi lms coated with natural phenolic compounds to improve the oxidative stability of beef. Meat Sci. 2014, 97, 249–254. [Google Scholar] [CrossRef]
- Rižnar, K.; Čelan, Š.; Knez, Ž.; Škerget, M.; Bauman, D.; Glaser, R. Antioxidant and antimicrobial activity of rosemary extract in chicken frankfurters. J. Food Sci. 2006, 71, 425–429. [Google Scholar] [CrossRef]
- Pizzale, L.; Bortolomeazzi, R.; Vichi, S.; Überegger, E.; Conte, L.S. Antioxidant activity of sage (Salvia officinalis and S fruticosa) and oregano (Origanum onites and O indercedens) extracts related to their phenolic compound content. J. Sci. Food Agric. 2002, 82, 1645–1651. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Estévez, M.; Ramírez, R.; Ventanas, S.; Cava, R. Sage and rosemary essential oils versus BHT for the inhibition of lipid oxidative reactions in liver pâté. LWT-Food Sci. Technol. 2007, 40, 58–65. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.H.; Leng, X.J.; Huang, M.; Zhou, G.H. Effect of sage (Salvia officinalis) on the oxidative stability of Chinese-style sausage during refrigerated storage. Meat Sci. 2013, 95, 145–150. [Google Scholar] [CrossRef]
- Akcan, T.; Estévez, M.; Serdaroğlu, M. Antioxidant protection of cooked meatballs during frozen storage by whey protein edible films with phytochemicals from Laurus nobilis L. and Salvia officinalis. LWT-Food Sci. Technol. 2017, 77, 323–331. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Zeković, Z.; Tomović, V.; Ikonić, P.; Kocić-Tanackov, S.; Džinić, N. The effect of essential oil and extract from sage (Salvia officinalis L.) herbal dust (food industry by-product) on the oxidative and microbiological stability of fresh pork sausages. LWT-Food Sci. Technol. 2018, 89, 749–755. [Google Scholar] [CrossRef]
- Stahl-Biskup, E.; Saez, F. Thyme. In Handbook of Herbs and Spices, Volume 2; CRC Press: Boca Raton, FL, USA, 2004; ISBN 0849325358. [Google Scholar]
- Youdim, K.A.; Deans, S.G.; Finlayson, H.J. The antioxidant properties of thyme (Thymus zygis L.) essential oil: An inhibitor of lipid peroxidation and a free radical scavenger. J. Essent. Oil Res. 2002, 14, 210–215. [Google Scholar] [CrossRef]
- Fratianni, F.; De Martino, L.; Melone, A.; De Feo, V.; Coppola, R.; Nazzaro, F. Preservation of chicken breast meat treated with thyme and balm essential oils. J. Food Sci. 2010, 75. [Google Scholar] [CrossRef] [PubMed]
- Zengin, H.; Baysal, A.H. Antioxidant and Antimicrobial Activities of Thyme and Clove Essential Oils and Application in Minced Beef. J. Food Process. Preserv. 2015, 39, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.; Mendiratta, S.K.; Agrawal, R.K.; Gurunathan, K.; Kumar, S.; Singh, T.P. Use of Various Essential Oils as Bio Preservatives and Their Effect on the Quality of Vacuum Packaged Fresh Chicken Sausages under Frozen Conditions; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 81, ISBN 9189305159. [Google Scholar]
- Yen, G.C.; Chen, H.Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Gramza, A.; Khokhar, S.; Yoko, S.; Gliszczynska-Swiglo, A.; Hes, M.; Korczak, J. Antioxidant activity of tea extracts in lipids and correlation with polyphenol content. Eur. J. Lipid Sci. Technol. 2006, 108, 351–362. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Ochi, H.; Lee, K.G.; Shibamoto, T. Antioxidative Activities of Volatile Extracts from Green Tea, Oolong Tea, and Black Tea. J. Agric. Food Chem. 2003, 51, 7396–7401. [Google Scholar] [CrossRef]
- Bozkurt, H. Utilization of natural antioxidants: Green tea extract and Thymbra spicata oil in Turkish dry-fermented sausage. Meat Sci. 2006, 73, 442–450. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Yang, J.Y.; Lu, H.B.; Wang, S.S.; Yang, J.; Yang, X.C.; Chai, M.; Li, L.; Cao, J.X. Effect of chitosan film incorporated with tea polyphenol on quality and shelf life of pork meat patties. Int. J. Biol. Macromol. 2013, 61, 312–316. [Google Scholar] [CrossRef]
- Pateiro, M.; Lorenzo, J.M.; Amado, I.R.; Franco, D. Effect of addition of green tea, chestnut and grape extract on the shelf-life of pig liver pâté. Food Chem. 2014, 147, 386–394. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhuang, H.; Li, L.; Chen, X.; Zhang, J. Effects of Plant Polyphenols and α -Tocopherol on Lipid Oxidation, Microbiological Characteristics, and Biogenic Amines Formation in Dry-Cured Bacons. J. Food Sci. 2015, 80, 547–555. [Google Scholar] [CrossRef]
- Alirezalu, K.; Hesari, J.; Eskandari, M.H.; Valizadeh, H.; Sirousazar, M. Effect of Green Tea, Stinging Nettle and Olive Leaves Extracts on the Quality and Shelf Life Stability of Frankfurter Type Sausage. J. Food Process. Preserv. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Jayawardana, B.C.; Warnasooriya, V.B.; Thotawattage, G.H.; Dharmasena, V.A.K.I.; Liyanage, R. Black and green tea (Camellia sinensis L.) extracts as natural antioxidants in uncured pork sausages. J. Food Process. Preserv. 2019, 43, 1–8. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Pan, D.D.; Cao, J.X.; Shao, X.F.; Chen, Y.J.; Sun, Y.Y.; Ou, C.R. Effect of black pepper essential oil on the quality of fresh pork during storage. Meat Sci. 2016, 117, 130–136. [Google Scholar] [CrossRef]
- Vasavada, M.N.; Dwivedi, S.; Cornforth, D. Evaluation of garam masala spices and phosphates as antioxidants in cooked ground beef. J. Food Sci. 2006, 71, 292–297. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Gokulakrishnan, P.; Giriprasad, R.; Yatoo, M.A. Fruit-based Natural Antioxidants in Meat and Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1503–1513. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zhang, Z.H.; Li, L.; Xiong, W.; Shi, J.Y.; Zhao, T.R.; Fan, J. Antioxidant effect of pomegranate rind powder extract, pomegranate juice, and pomegranate seed powder extract as antioxidants in raw ground pork meat. Food Sci. Biotechnol. 2013, 22, 1063–1069. [Google Scholar] [CrossRef]
- Lee, C.H.; Reed, J.D.; Richards, M.P. Ability of various polyphenolic classes from cranberry to inhibit lipid oxidation in mechanically separated turkey and cooked ground pork. J. Muscle Foods 2006, 17, 248–266. [Google Scholar] [CrossRef]
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT-Food Sci. Technol. 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modi fi ed atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Jia, N.; Kong, B.; Liu, Q.; Diao, X.; Xia, X. Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Sci. 2012, 91, 533–539. [Google Scholar] [CrossRef]
- Carpenter, R.; O’Grady, M.N.; O’Callaghan, Y.C.; O’Brien, N.M.; Kerry, J.P. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 2007, 76, 604–610. [Google Scholar] [CrossRef]
- Nuñez De Gonzalez, M.T.; Boleman, R.M.; Miller, R.K.; Keeton, J.T.; Rhee, K.S. Antioxidant properties of dried plum ingredients in raw and precooked pork sausage. J. Food Sci. 2008, 73. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Kubota, E.H.; da Silva, C.G.; dos Santos Alves, J.; Hautrive, T.P.; Rodrigues, G.S.; Campagnol, P.C.B. Banana inflorescences: A cheap raw material with great potential to be used as a natural antioxidant in meat products. Meat Sci. 2020, 161, 107991. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; González-Rodríguez, R.M.; Sánchez, M.; Amado, I.R.; Franco, D. Effects of natural (grape seed and chestnut extract) and synthetic antioxidants (buthylatedhydroxytoluene, BHT) on the physical, chemical, microbiological and sensory characteristics of dry cured sausage “chorizo”. Food Res. Int. 2013, 54, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Nuñez de Gonzalez, M.T.; Hafley, B.S.; Boleman, R.M.; Miller, R.M.; Rhee, K.S.; Keeton, J.T. Qualitative effects of fresh and dried plum ingredients on vacuum-packaged, sliced hams. Meat Sci. 2009, 83, 74–81. [Google Scholar] [CrossRef]
- Nuñez de Gonzalez, M.T.; Hafley, B.S.; Boleman, R.M.; Miller, R.K.; Rhee, K.S.; Keeton, J.T. Antioxidant properties of plum concentrates and powder in precooked roast beef to reduce lipid oxidation. Meat Sci. 2008, 80, 997–1004. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U.; Fernando, L.N. Antioxidant properties of natural plant extracts containing polyphenolic compounds in cooked ground beef. J. Food Sci. 2002, 67, 1364–1369. [Google Scholar] [CrossRef]
- Rojas, M.C.; Brewer, M.S. Effect of natural antioxidants on oxidative stability of frozen, vacuum-packaged beef and pork. J. Food Qual. 2008, 31, 173–188. [Google Scholar] [CrossRef]
- Yldz-Turp, G.; Serdaroglu, M. Effects of using plum puree on some properties of low fat beef patties. Meat Sci. 2010, 86, 896–900. [Google Scholar] [CrossRef]
- Movileanu, I.; Núñez De González, M.T.; Hafley, B.; Miller, R.K.; Keeton, J.T. Comparison of dried plum puree, rosemary extract, and BHA/BHT as antioxidants in irradiated ground beef patties. Int. J. Food Sci. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- García-Lomillo, J.; Gonzalez-SanJose, M.L.; Del Pino-García, R.; Ortega-Heras, M.; Muñiz-Rodríguez, P. Antioxidant effect of seasonings derived from wine pomace on lipid oxidation in refrigerated and frozen beef patties. LWT-Food Sci. Technol. 2017, 77, 85–91. [Google Scholar] [CrossRef]
- Morsy, M.K.; Mekawi, E.; Elsabagh, R. Impact of pomegranate peel nanoparticles on quality attributes of meatballs during refrigerated storage. LWT-Food Sci. Technol. 2018, 89, 489–495. [Google Scholar] [CrossRef]
- Turgut, S.S.; Işıkçı, F.; Soyer, A. Antioxidant activity of pomegranate peel extract on lipid and protein oxidation in beef meatballs during frozen storage. Meat Sci. 2017, 129, 111–119. [Google Scholar] [CrossRef]
- Kulkarni, S.; Desantos, F.A.; Kattamuri, S.; Rossi, S.J.; Brewer, M.S. Effect of grape seed extract on oxidative, color and sensory stability of a pre-cooked, frozen, re-heated beef sausage model system. Meat Sci. 2011, 88, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Selani, M.M.; Contreras-Castillo, C.J.; Shirahigue, L.D.; Gallo, C.R.; Plata-Oviedo, M.; Montes-Villanueva, N.D. Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci. 2011, 88, 397–403. [Google Scholar] [CrossRef]
- Vaithiyanathan, S.; Naveena, B.M.; Muthukumar, M.; Girish, P.S.; Kondaiah, N. Effect of dipping in pomegranate (Punica granatum) fruit juice phenolic solution on the shelf life of chicken meat under refrigerated storage (4 °C). Meat Sci. 2011, 88, 409–414. [Google Scholar] [CrossRef]
- Jo, S.C.; Nam, K.C.; Min, B.R.; Ahn, D.U.; Cho, S.H.; Park, W.P.; Lee, S.C. Antioxidant activity of Prunus mume extract in cooked chicken breast meat. Int. J. Food Sci. Technol. 2006, 41, 15–19. [Google Scholar] [CrossRef]
- Biswas, A.K.; Beura, C.K.; Yadav, A.S.; Pandey, N.K.; Mendiratta, S.K.; Kataria, J.M. Influence of novel bioactive compounds from selected fruit by-products and plant materials on the quality and storability of microwave-assisted cooked poultry meat wafer during ambient temperature storage. LWT-Food Sci. Technol. 2015, 62, 727–733. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Brenes, A.; Goñi, I. Effect of grape antioxidant dietary fiber on the lipid oxidation of raw and cooked chicken hamburgers. LWT-Food Sci. Technol. 2009, 42, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Brannan, R.G. Effect of grape seed extract on physicochemical properties of ground, salted, chicken thigh meat during refrigerated storage at different relative humidity levels. J. Food Sci. 2008, 73, 36–40. [Google Scholar] [CrossRef]
- Naveena, B.M.; Sen, A.R.; Vaithiyanathan, S.; Babji, Y.; Kondaiah, N. Comparative efficacy of pomegranate juice, pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Sci. 2008, 80, 1304–1308. [Google Scholar] [CrossRef]
- Sharma, P.; Yadav, S. Effect of incorporation of pomegranate peel and bagasse powder and their extracts on quality characteristics of chicken meat patties. Food Sci. Anim. Resour. 2020, 40, 388–400. [Google Scholar] [CrossRef] [Green Version]
- Basanta, M.F.; Rizzo, S.A.; Szerman, N.; Vaudagna, S.R.; Descalzo, A.M.; Gerschenson, L.N.; Pérez, C.D.; Rojas, A.M. Plum (Prunus salicina) peel and pulp microparticles as natural antioxidant additives in breast chicken patties. Food Res. Int. 2018, 106, 1086–1094. [Google Scholar] [CrossRef] [Green Version]
- Chandralekha, S.; Jagadeesh Babu, A.; Moorthy, P.R.S.; Karthikeyan, B. Studies on the Effect of Pomegranate Rind Powder Extract as Natural Antioxidant in Chicken Meat Balls During Refrigerated Storage. J. Adv. Vet. Res. 2012, 2, 107–112. [Google Scholar]
- Mielnik, M.B.; Olsen, E.; Vogt, G.; Adeline, D.; Skrede, G. Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT-Food Sci. Technol. 2006, 39, 191–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, I.; Bridges, W.C.; Dawson, P.L. Peach skin powder inhibits oxidation in cooked Turkey meat. Poult. Sci. 2016, 95, 2435–2440. [Google Scholar] [CrossRef]
- Andrés, A.I.; Petrón, M.J.; Adámez, J.D.; López, M.; Timón, M.L. Food by-products as potential antioxidant and antimicrobial additives in chill stored raw lamb patties. Meat Sci. 2017, 129, 62–70. [Google Scholar] [CrossRef]
- Das, A.K.; Rajkumar, V.; Nanda, P.K.; Chauhan, P.; Pradhan, S.R.; Biswas, S. Antioxidant efficacy of litchi (Litchi chinensis sonn.) pericarp extract in sheep meat nuggets. Antioxidants 2016, 5, 16. [Google Scholar] [CrossRef]
- Rababah, T.M.; Ereifej, K.I.; Alhamad, M.N.; Al-Qudah, K.M.; Rousan, L.M.; Al-Mahasneh, M.A.; Al-U’Datt, M.H.; Yang, W. Effects of green tea and grape seed and TBHQ on physicochemical properties of baladi goat meats. Int. J. Food Prop. 2011, 14, 1208–1216. [Google Scholar] [CrossRef]
- Devatkal, S.K.; Thorat, P.; Manjunatha, M. Effect of vacuum packaging and pomegranate peel extract on quality aspects of ground goat meat and nuggets. J. Food Sci. Technol. 2014, 51, 2685–2691. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; Siddiqui, I.A.; El-Abd, S.; Mukhtar, H.; Ahmad, N. Combination chemoprevention with grape antioxidants. Mol. Nutr. Food Res. 2016, 60, 1406–1415. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Raffoul, J.J. Potential anticancer properties of grape antioxidants. J. Oncol. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Bioactive non-coloured polyphenols content of grapes, wines and vinification by-products: Evaluation of the antioxidant activities of their extracts. Food Res. Int. 2010, 43, 805–813. [Google Scholar] [CrossRef]
- Bertelli, A.A.A.; Das, D.K. Grapes, Wines, Resveratrol, and Heart Health. J. Cardiovasc. Pharmacol. 2009, 54, 468–476. [Google Scholar] [CrossRef]
- Reham, A.A.; Shimaa, N.E. Grape Seed Extract as Natural Antioxidant and Antibacterial in Minced Beef. PSM Biol. Res. 2017, 2, 89–96. [Google Scholar]
- Rupasinghe, H.P.V.; Jayasankar, S.; Lay, W. Variation in total phenolics and antioxidant capacity among European plum genotypes. Sci. Hortic. (Amsterdam) 2006, 108, 243–246. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Changes in hydrophilic and lipophilic antioxidant activity and related bioactive compounds during postharvest storage of yellow and purple plum cultivars. Postharvest Biol. Technol. 2009, 51, 354–363. [Google Scholar] [CrossRef]
- Lee, Y.S.; Han, C.H.; Kang, S.H.; Lee, S.-J.; Kim, S.W.; Shin, O.R.; Sim, Y.-C.; Lee, S.-J.; Cho, Y.-H. Synergistic effect between catechin and ciprofloxacin on chronic bacterial prostatitis rat model. Int. J. Urol. 2005, 12, 383–389. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Gallego, M.G.; Segovia, F.; Abdullah, S.; Shaarani, S.M.; Almajano Pablos, M.P. Study of the properties of bearberry leaf extract as a natural antioxidant in model foods. Antioxidants 2016, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Pegg, R.B.; Amarowicz, R.; Naczk, M. Antioxidant Activity of Polyphenolics from a Bearberry-Leaf (Arctostaphylos uva-ursi L. Sprengel) Extract in Meat Systems. In Phenolic Compounds in Foods and Natural Health Products; ACS Publications: Washington, DC, USA, 2005; pp. 67–82. [Google Scholar] [CrossRef]
- Vattem, D.A.; Ghaedian, R.; Shetty, K. Enhancing health benefits of berries through phenolic antioxidant enrichment: Focus on cranberry. Asia Pac. J. Clin. Nutr. 2005, 14, 120–130. [Google Scholar]
- Çelik, H.; Özgen, M.; Serçe, S.; Kaya, C. Phytochemical accumulation and antioxidant capacity at four maturity stages of cranberry fruit. Sci. Hortic. (Amsterdam) 2008, 117, 345–348. [Google Scholar] [CrossRef]
- Kathirvel, P.; Gong, Y.; Richards, M.P. Identification of the compound in a potent cranberry juice extract that inhibits lipid oxidation in comminuted muscle. Food Chem. 2009, 115, 924–932. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT-Food Sci. Technol. 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-Lóaez, J.; Pérez-álvarez, J.A. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C. Pomegranate in human health: An overview. Bioact. Foods Promot. Health 2010, 551–563. [Google Scholar] [CrossRef]
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem. Toxicol. 2013, 55, 470–475. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Trends in Food Science & Technology Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Zhong, H.J.; Ambigaipalan, P. Antioxidants: Regulatory Status. Bailey’s Ind. Oil Fat Prod. 2020, 1–21. [Google Scholar] [CrossRef]
- European Parliament and the Concil of the European Union Regulation (EC) No 1333/2008 of the European Parliament ans of the Council of 16 December 2998 on food additives. Off. J. Eur. Union 2008, 16–33.
- European Parliament and Council Commission Regulation (EU) No 1129/2011 of 11 November 2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives. Off. J. Eur. Union 2011, L295, 1–177. [CrossRef]
- FDA. Food Additive Status List|FDA. U.S Food Drug Adm. 2019. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (accessed on 31 October 2020).
- Codex Alimentarius Commission GSFA Food Additives. 2019. Available online: www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/gsfa/en/ (accessed on 31 October 2020).
- Cunha, L.C.M.; Monteiro, M.L.G.; Lorenzo, J.M.; Munekata, P.E.S.; Muchenje, V.; de Carvalho, F.A.L.; Conte-Junior, C.A. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Res. Int. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.; Qazi, I.M.; Pavase, T.R.; Lv, L. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- European Commission Commission Regulation (EC) No. 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, L 135, 3–11.
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- McCarthy, T.L.; Kerry, J.P.; Kerry, J.F.; Lynch, P.B.; Buckley, D.J. Evaluation of the antioxidant potential of natural food/plant extracts as compared with synthetic antioxidants and vitamin e in raw and cooked pork patties. Meat Sci. 2001, 58, 45–52. [Google Scholar] [CrossRef]
- Nieto, G.; Estrada, M.; Jordán, M.J.; Garrido, M.D.; Bañón, S. Effects in ewe diet of rosemary by-product on lipid oxidation and the eating quality of cooked lamb under retail display conditions. Food Chem. 2011, 124, 1423–1429. [Google Scholar] [CrossRef]
- Serrano, R.; Jordán, M.J.; Bañón, S. Use of dietary rosemary extract in ewe and lamb to extend the shelf life of raw and cooked meat. Small Rumin. Res. 2014, 116, 144–152. [Google Scholar] [CrossRef]
- Nieto, G.; Díaz, P.; Bañón, S.; Garrido, M.D. Effect on lamb meat quality of including thyme (Thymus zygis ssp. gracilis) leaves in ewes’ diet. Meat Sci. 2010, 85, 82–88. [Google Scholar] [CrossRef]
- Kotsampasi, B.; Christodoulou, V.; Zotos, A.; Liakopoulou-Kyriakides, M.; Goulas, P.; Petrotos, K.; Natas, P.; Bampidis, V.A. Effects of dietary pomegranate byproduct silage supplementation on performance, carcass characteristics and meat quality of growing lambs. Anim. Feed Sci. Technol. 2014, 197, 92–102. [Google Scholar] [CrossRef]
- Jerónimo, E.; Alfaia, C.M.M.; Alves, S.P.; Dentinho, M.T.P.; Prates, J.A.M.; Vasta, V.; Santos-Silva, J.; Bessa, R.J.B. Effect of dietary grape seed extract and Cistus ladanifer L. in combination with vegetable oil supplementation on lamb meat quality. Meat Sci. 2012, 92, 841–847. [Google Scholar] [CrossRef]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Kokka, E.; Kouka, P.; Terzopoulou, Z.; Gerasopoulos, K.; Stagos, D.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. (Berl) 2017, 101, e108–e121. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, H.; Liu, K.; Jia, H.; Chen, Y.; Wang, Z. Antioxidant effects of liquorice (Glycyrrhiza uralensis) extract during aging of longissimus thoracis muscle in Tan sheep. Meat Sci. 2015, 105, 38–45. [Google Scholar] [CrossRef]
- Botsoglou, E.; Govaris, A.; Ambrosiadis, I.; Fletouris, D. Lipid and protein oxidation of α-linolenic acid-enriched pork during refrigerated storage as influenced by diet supplementation with olive leaves (Olea europea L.) or α-tocopheryl acetate. Meat Sci. 2012, 92, 525–532. [Google Scholar] [CrossRef]
- Rossi, R.; Pastorelli, G.; Cannata, S.; Tavaniello, S.; Maiorano, G.; Corino, C. Effect of long term dietary supplementation with plant extract on carcass characteristics meat quality and oxidative stability in pork. Meat Sci. 2013, 95, 542–548. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Brenes, A.; Viveros, A.; Goñi, I. Antioxidative effect of dietary grape pomace concentrate on lipid oxidation of chilled and long-term frozen stored chicken patties. Meat Sci. 2009, 83, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Nkukwana, T.T.; Muchenje, V.; Masika, P.J.; Hoffman, L.C.; Dzama, K.; Descalzo, A.M. Fatty acid composition and oxidative stability of breast meat from broiler chickens supplemented with Moringa oleifera leaf meal over a period of refrigeration. Food Chem. 2014, 142, 255–261. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, D.; Tong, J.; Vaddella, V. Influence of chestnut tannins on welfare, carcass characteristics, meat quality, and lipid oxidation in rabbits under high ambient temperature. Meat Sci. 2012, 90, 164–169. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Gerencsér, Z.; Szendro, Z.; Mugnai, C.; Cullere, M.; Kovàcs, M.; Ruggeri, S.; Mattioli, S.; Castellini, C.; Dalle Zotte, A. Effect of dietary supplementation of Spirulina (Arthrospira platensis) and Thyme (Thymus vulgaris) on rabbit meat appearance, oxidative stability and fatty acid profile during retail display. Meat Sci. 2014, 96, 114–119. [Google Scholar] [CrossRef]
- Gobert, M.; Gruffat, D.; Habeanu, M.; Parafita, E.; Bauchart, D.; Durand, D. Plant extracts combined with vitamin E in PUFA-rich diets of cull cows protect processed beef against lipid oxidation. Meat Sci. 2010, 85, 676–683. [Google Scholar] [CrossRef]
- Pokorný, J.; Yanishlieva, N.; Gordon, M.H. (Eds.) Antioxidants in Food: Practical Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]

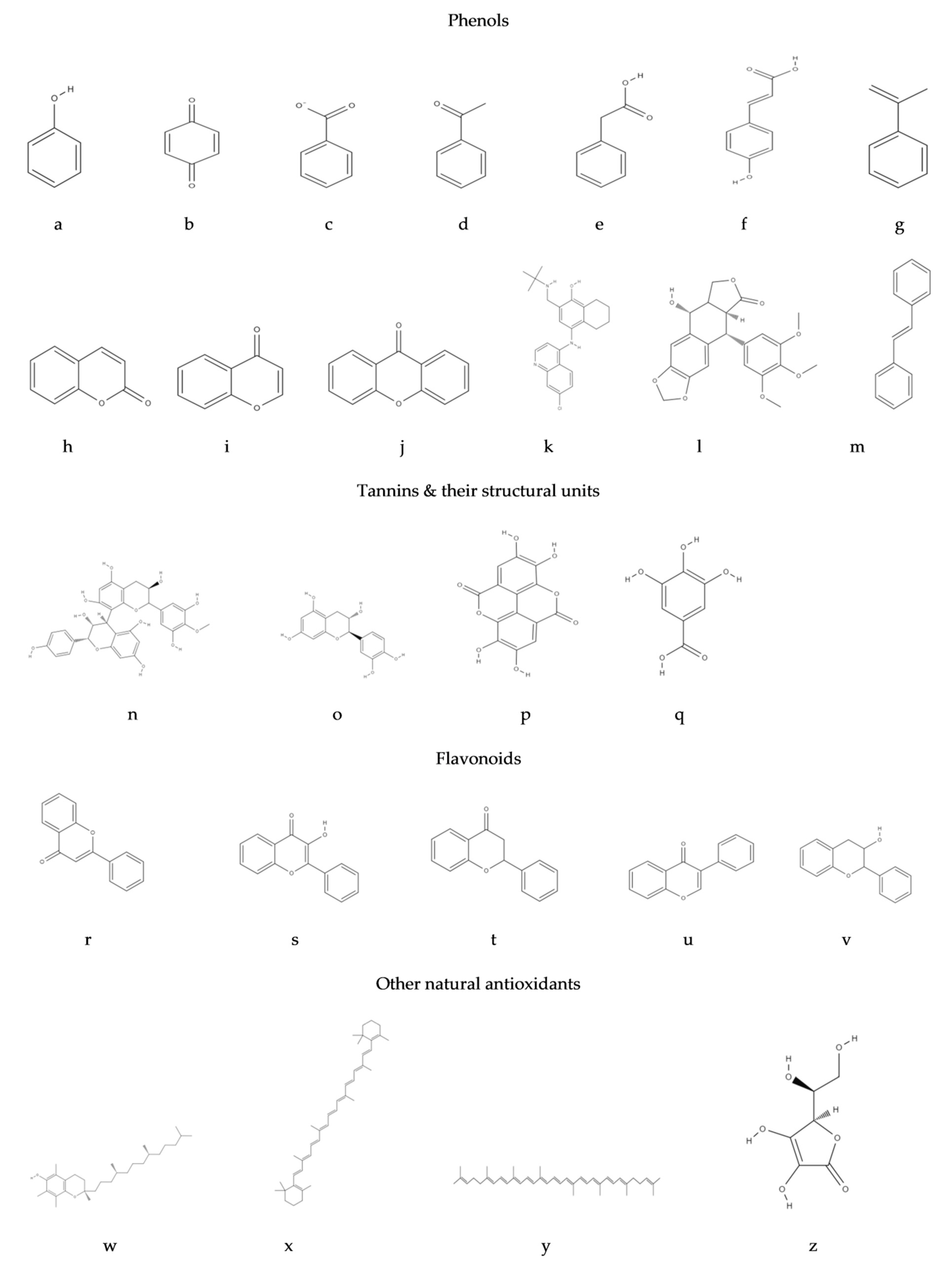
| Class | Basic Skeleton | Plant Sources |
|---|---|---|
| Phenols and benzoquinones | C6 | Primula obconica [37], sorghum [38], berries, fruit wines, olive oil [39] |
| Phenolic acids | C6–C1 | blueberries, blackberries, persimmon, apple juice, cider, cherry laurels, canola meal, oranges, rye [39] |
| Acetophenones and phenylacetic acids | C6–C2 | root bark of Derris indica, Ageratina pichinchensis [40], spruce, pine [41], pisum, nicotiana, phaseolus, triticum, trapoleum, lycopersicon, avena [42], balsamic vinegar [43], cocoa powder [44] |
| Hydroxycinnamic acids, phenylpropenes, coumarins-isocoumarins and chromones | C6–C3 | berries, pomes, herbs, seeds, cereal grains, leafy greens, asparagus, cinnamon, cloves and potatoes [45] |
| Naphthoquinones | C6–C4 | oranges, Plumbaginaceae, Droseraceae, Ebenaceae [37] |
| Xanthones | C6–C1–C6 | Gentianna ottonis [39], Guttiferae, Moraceae, Clusiaceae, and Polygalaceae [46] |
| Stilbenes and anthraquinones | C6–C2–C6 | grapes, pine, peanuts, sorghum, rheum [47], Rubiaceae [48], black pepper [49] |
| Flavonoids (flavones, flavonols, flavanones, flavan-3-ols, isoflavones, anthocyanidin compounds) | C6–C3–C6 | celery, parsley, red prickly pears, olives, acerola, litchis, avocadoes, green and black tea, cherries, raspberries, strawberries, grapes and red wine [45] |
| Lignans and neolignans | C6–C3 | wheat, oats, rye, barley [50], berries [51] |
| Lignins | (C6–C3)n | Arabidopsis thaliana, Pinus radiata [52], sugar cane [53], spruce, wattle, birch, rice, eucalyptus, pine [54] |
| Meat/Meat-Based Product | Treatment (Main Antioxidant Compound) | LA 1 | PA 2 | COL 3 | FL/TA 4 | FA 5 | TBARS 6 | Ref 7 |
|---|---|---|---|---|---|---|---|---|
| Pork meat and meat-based products | ||||||||
| Ground meat | Pomegranate rind powder extract | ++ | N/A | 0 | + | BHT (+) | ++ | [149] |
| Pomegranate juice, seed powder extract | + | N/A | 0 | + | BHT (+) | + | ||
| Cranberry powder (phenolic acids, anthocyanins, flavanols and proanthocyanidins) | ++ | N/A | N/A | N/A | N/A | ++ | [150] | |
| Patties | Red grape pomace extracts | ++ | N/A | + | N/A | N/A | ++ | [151] |
| Grape seed extract (N/A) | ++ | N/A | ++ | N/A | ΒHΤ (−/0) | ++ | [152] | |
| Blackcurrant (Ribes nigrum L.) extract (anthocyanins) | ++ | ++ | 0 | N/A | BHA (0) | ++ | [153] | |
| Grape seed extract (N/A) | ++ | N/A | 0 | 0 | Ν/A | ++ | [154] | |
| Bearberry extract (N/A) | ++ | N/A | 0 | 0 | Ν/A | ++ | ||
| Liver pâté | Grape extract (N/A) | ++ | N/A | 0 | N/A | ΒHΤ (−) | ++ | [141] |
| Sausages | Plum puree | ++ | N/A | 0 | −/0 | BHA/BHT | ++ | [155] |
| Plum and apple puree | ++ | N/A | 0 | −/0 | BHA/BHT | ++ | ||
| Banana male flower extract (flavonoids) | ++ | N/A | 0 | 0 | N/A | ++ | [156] | |
| Grape seed extract (N/A) | ++ | Ν/A | + | + | BHT (−) | ++ | [157] | |
| Ham | Plum juice concentrate, plum powder | 0 | N/A | 0 | −/0 | N/A | 0 | [158] |
| Beef meat and meat-based products | ||||||||
| Roast beef | Plum juice concentrate, plum powder | ++ | N/A | 0 | 0 | N/A | ++ | [159] |
| Ground beef | Commercial grape seed extract | + | N/A | N/A | 0 | BHA/BHT (−) | + | [160] |
| Patties | Grape seed extract | + | N/A | 0 | 0 | N/A | + | [161] |
| Plum puree | + | N/A | 0 | −/0 | N/A | + | [162] | |
| Dried plum puree | ++ | N/A | N/A | N/A | BHA/BHT (+) | ++ | [163] | |
| Seasonings derived from wine pomace (N/A) | ++ | N/A | N/A | N/A | Sulfites (−) | [164] | ||
| Meatballs | Lyophilized pomegranate peel nanoparticles | ++ | N/A | + | + | BHT (−) | ++ | [165] |
| Pomegranate peel extract (N/A) | ++ | ++ | + | 0 | BHT (−) | [166] | ||
| Sausages | Grape seed extract (N/A) | ++ | N/A | + | + | AA, PG (−) | ++ | [167] |
| Poultry meat and meat-based products | ||||||||
| Fresh meat | Grape seed and peel extracts | + | N/A | (−/0) | (−/0) | BHT (0) | + | [168] |
| Dipping in pomegranate juice phenolic solution | + | + | N/A | + | N/A | + | [169] | |
| Cooked chicken breast meat | Prunus mume (Japanese apricot) methanolic extracts | ++ | N/A | 0 | N/A | N/A | ++ | [170] |
| Chicken meat wafer | Apple peel (N/A) | ++ | N/A | + | + | N/A | ++ | [171] |
| Banana peel (N/A) | ++ | N/A | + | + | N/A | ++ | ||
| Chicken patties | Grape dietary fiber | ++ | N/A | 0 | + | N/A | ++ | [172] |
| Grape seed extract + NaCl 1% | ++ | N/A | N/A | N/A | N/A | ++ | [173] | |
| Pomegranate juice, pomegranate rind powder extract (N/A) | ++ | N/A | 0 | 0 | BHT (−) | ++ | [174] | |
| Pomegranate peel powder and extract | ++ | Ν/A | − | N/A | BHT (−) | ++ | [175] | |
| Plum peel and pulp fiber microparticles (β-carotene, lutein, α- and γ-tocopherols, polyphenols) | ++ | Ν/A | 0 | 0 | Ν/A | ++ | [176] | |
| Chicken meatballs | Pomegranate rind powder extract | + | N/A | (0/+) | (0/+) | BHA/BHT (−) | + | [177] |
| Turkey meat | Grape seed extract | ++ | N/A | N/A | N/A | N/A | ++ | [178] |
| Cranberry powder (phenolic acids, anthocyanins, flavanols and proanthocyanidins) | ++ | N/A | N/A | N/A | N/A | ++ | [150] | |
| Peach skin powder | ++ | N/A | N/A | N/A | BHA (0) | N/A | [179] | |
| Lamb meat and meat-based products | ||||||||
| Patties | Red grape by-product extract (N/A) | ++ | ++ | + | N/A | SA (−) | ++ | [180] |
| Pomegranate by-product extract (N/A) | 0 | 0 | 0 | N/A | SA (0) | 0 | ||
| Sheep meat nuggets | Litchi pericarp extract | + | N/A | N/A | 0 | BHT (0) | + | [181] |
| Goat meat and meat-based products | ||||||||
| Ground meat | Grape seed extract | + | N/A | + | N/A | TBHQ (+) | + | [182] |
| Tea extract | + | N/A | − | N/A | TBHQ (+) | + | ||
| Pomegranate peel extract + vacuum packaging | + | N/A | 0 | 0 | N/A | + | [183] | |
| Goat meat nuggets | Pomegranate peel extract + vacuum packaging | + | N/A | 0 | 0 | N/A | + | [183] |
| Food Category | E-Number | Name | Maximum Level (mg/kg) | Restrictions/Exceptions |
|---|---|---|---|---|
| Non-heat-treated and heat-treated processed meat | E 160a | Carotenes | 20 | Only sausages, pâtés and terrines |
| E 392 | Extracts of rosemary | 100 | Only dried sausages | |
| 150 | Excluding dried sausages | |||
| 150 | Only dehydrated meat |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. https://doi.org/10.3390/antiox9121215
Manessis G, Kalogianni AI, Lazou T, Moschovas M, Bossis I, Gelasakis AI. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants. 2020; 9(12):1215. https://doi.org/10.3390/antiox9121215
Chicago/Turabian StyleManessis, Georgios, Aphrodite I. Kalogianni, Thomai Lazou, Marios Moschovas, Ioannis Bossis, and Athanasios I. Gelasakis. 2020. "Plant-Derived Natural Antioxidants in Meat and Meat Products" Antioxidants 9, no. 12: 1215. https://doi.org/10.3390/antiox9121215
APA StyleManessis, G., Kalogianni, A. I., Lazou, T., Moschovas, M., Bossis, I., & Gelasakis, A. I. (2020). Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants, 9(12), 1215. https://doi.org/10.3390/antiox9121215







