Assessment of Antioxidants in Selected Plant Rootstocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plants
2.3. Preparation of the Plant Samples for Analyzing Flavonoid, Phenolic Acids and Aldehydes, Catechin and Procyanidin Compounds
2.4. Analysis of the Plant Sample-Extracts Using LC/MS
2.4.1. Separation of Flavonoid Compounds
2.4.2. Separation of Phenolic Acids and Aldehydes
2.4.3. Separation of Catechin and Procyanidin Compounds
2.5. Statistics Methodology
3. Results
3.1. LC/MS-Based Profile of the Test Plant Extracts
3.1.1. Occurrence and Contents of Selected Flavonoid Compounds
(A) Hyperoside (Q-3-galactoside)
(B) Isoquercitrin (Q-3-glucoside)
(C) Rutin (Q-3-rutinoside)
3.1.2. Contents of Phenolic Acids and Aldehydes
(A) Protocatechuic acid
(B) 3,4-Dihydroxybenzaldehyde
(C) Syringic acid, vanilic acid and vanilin
3.1.3. Contents of Selected Catechin and Procyanidin Compounds
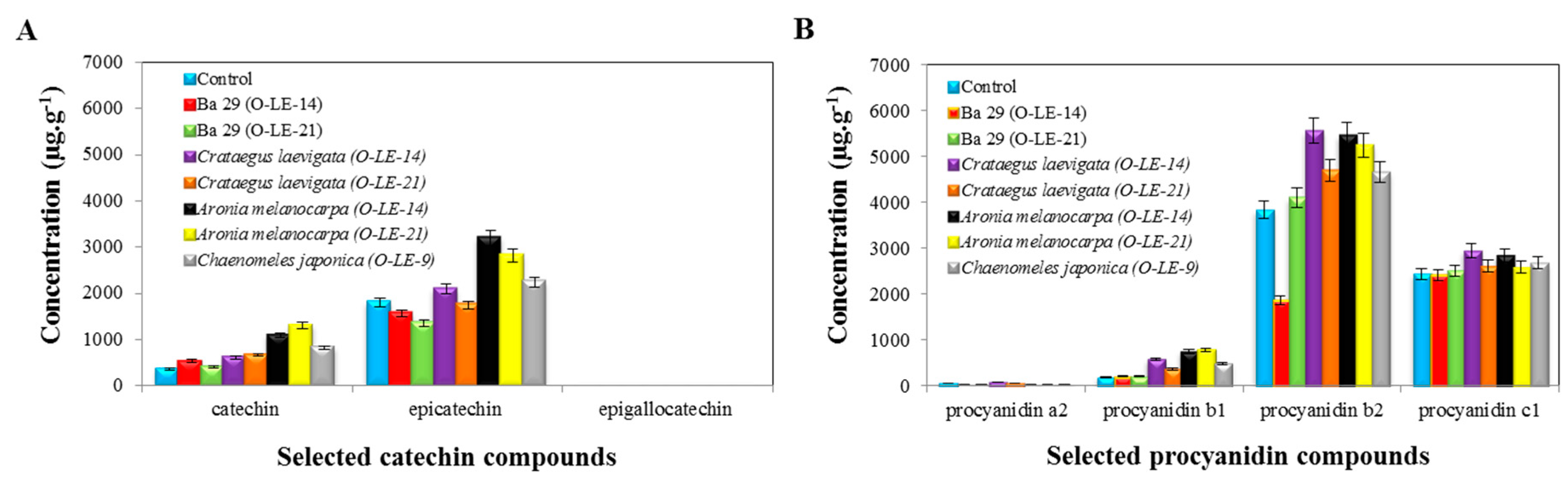
(A) Catechin Compounds
(B) Procyanidin Compounds
3.2. Statistical Analysis
4. Discussion
4.1. An Occurrence and Contents of Selected Flavonoid Compounds
4.2. An Occurrence and Contents of Selected Phenolic Acids and Aldehydes
4.3. An Occurrence and Contents of Selected Catechin and Procyanidin Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shalaby, S.; Horwitz, B.A. Plant phenolic compounds and oxidative stress: Integrated signals in fungal-plant interactions. Curr. Genet. 2015, 61, 347–357. [Google Scholar] [CrossRef] [PubMed]
- McKeen, L.W. The Effect of Long Term Thermal Exposure on Plastics and Elastomers, Chapter Introduction to the Effect of Heat Aging on Plastics; Elsevier: Amsterdam, The Netherlands, 2014; pp. 17–42. [Google Scholar]
- Miltonprabu, S. Quercetin: A Flavonol with Versatile Therapeutic Applications and Its Interactions with Other Drugs; Academic Press Ltd-Elsevier Science Ltd.: London, UK, 2019; pp. 75–83. [Google Scholar]
- Esselen, M.; Barth, S. Food-borne topoisomerase inhibitors: Risk or benefit. Adv. Mol. Toxicol. 2014, 8, 123–171. [Google Scholar]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.A.; da Silva, M.A.; Ortega, M.G.; Cabrera, J.L.; Paraje, M.G. Antifungal activity of a prenylated flavonoid from Dalea elegans against Candida albicans biofilms. Phytomedicine 2015, 22, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- McKeen, L.W. The Effect of Long Term Thermal Exposure on Plastics and Elastomers, Chapter Introduction to the Physical, Mechanical, and Thermal Properties of Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 43–71. [Google Scholar]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Shao, Y.F.; Bao, J.S. Rice Phenolics and Other Natural Products; Woodhead Publ Ltd.: Cambridge, UK, 2019; pp. 221–271. [Google Scholar]
- Sieniawska, E.; Baj, T. Tannins; Academic Press Ltd-Elsevier Science Ltd.: London, UK, 2017; pp. 199–232. [Google Scholar]
- Qi, Y.J.; Zhang, H.; Wu, G.C.; Gu, L.W.; Wang, L.; Qian, H.F.; Qi, X.G. Mitigation effects of proanthocyanidins with different structures on acrylamide formation in chemical and fried potato crisp models. Food Chem. 2018, 250, 98–104. [Google Scholar] [CrossRef]
- Xu, C.M.; Yagiz, Y.; Marshall, S.; Li, Z.; Simonne, A.; Lu, J.; Marshall, M.R. Application of muscadine grape (Vitis rotundifolia Michx.) pomace extract to reduce carcinogenic acrylamide. Food Chem. 2015, 182, 200–208. [Google Scholar]
- Prabpree, A.; Sangsil, P.; Nualsri, C.; Nakkanong, K. Expression profile of phenylalanine ammonia-lyase (pal) and phenolic content during early stages of graft development in bud grafted Hevea brasiliensis. Biocatal. Agric. Biotechnol. 2018, 14, 88–95. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds; IntechOpen: London, UK, 2019; pp. 1–16. [Google Scholar]
- Sengupta, G.; Gaurav, A.; Tiwari, S. Substituting Medicinal Plants through Drug Synthesis; Elsevier Science Bv: Amsterdam, The Netherlands, 2018; pp. 47–74. [Google Scholar]
- Close, D.C.; McArthur, C. Rethinking the role of many plant phenolics—Protection from photodamage not herbivores? Oikos 2002, 99, 166–172. [Google Scholar] [CrossRef]
- Liang, Y.; Wen, Z. 18—Bio-based nutraceuticals from biorefining. In Advances in Biorefineries; Waldron, K., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 596–623. [Google Scholar]
- Sapienza, C.; Issa, J.-P. Diet, nutrition, and cancer epigenetics. Annu. Rev. Nutr. 2016, 36, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Liu-Smith, F.; Meyskens, F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of citrus flavonolds: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.J.; Wang, J.; Hochstetter, D.; Gao, Y.Y.; Xu, P.; Wang, Y.F. Epigallocatechin-3-gallate functions as a physiological regulator by modulating the jasmonic acid pathway. Physiol. Plant. 2015, 153, 432–439. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Hudina, M.; Orazem, P.; Jakopic, J.; Stampar, F. The phenolic content and its involvement in the graft incompatibility process of various pear rootstocks (Pyrus communis L.). J. Plant Physiol. 2014, 171, 76–84. [Google Scholar] [CrossRef]
- Musacchi, S.; Pagliuca, G.; Kindt, M.; Piretti, M.V.; Sansavini, S. Flavonoids as markers for pear-quince graft incompatibility. J. Appl. Bot.-Angew. Bot. 2000, 74, 206–211. [Google Scholar]
- Hakmaoui, A.; Perez-Bueno, M.L.; Garcia-Fontana, B.; Camejo, D.; Jimenez, A.; Sevilla, F.; Baron, M. Analysis of the antioxidant response of Nicotiana benthamiana to infection with two strains of pepper mild mottle virus. J. Exp. Bot. 2012, 63, 5487–5496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preedy, V.R. Tea in Health and Disease Prevention; Elsevier Academic Press Inc.: San Diego, CA, USA, 2013; pp. 1–1573. [Google Scholar]
- Xu, Z.; Wei, L.H.; Ge, Z.Z.; Zhu, W.; Li, C.M. Comparison of the degradation kinetics of a-type and b-type proanthocyanidins dimers as a function of pH and temperature. Eur. Food Res. Technol. 2015, 240, 707–717. [Google Scholar] [CrossRef]
- Thomas, H.R.; Frank, M.H. Connecting the pieces: Uncovering the molecular basis for long-distance communication through plant grafting. New Phytol. 2019, 223, 582–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irisarri, P.; Zhebentyayeva, T.N.; Errea, P.; Pina, A. Inheritance of self- and graft-incompatibility traits in an f1 apricot progeny. PLoS ONE 2019, 14, e0216371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutkowska, M.; Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Owczarek, A. Sorbus domestica leaf extracts and their activity markers: Antioxidant potential and synergy effects in scavenging assays of multiple oxidants. Molecules 2019, 24, 2289. [Google Scholar] [CrossRef] [Green Version]
- Mohtia, H.; Taviano, M.F.; Cacciola, F.; Dugo, P.; Mondello, L.; Zaid, A.; Cavo, E.; Miceli, N. Silene vulgaris subsp. macrocarpa leaves and roots from morocco: Assessment of the efficiency of different extraction techniques and solvents on their antioxidant capacity, brine shrimp toxicity and phenolic characterization. Plant Biosyst. 2019, 1–8. [Google Scholar] [CrossRef]
- Berezina, E.V.; Brilkina, A.A.; Veselov, A.P. Content of phenolic compounds, ascorbic acid, and photosynthetic pigments in Vaccinium macrocarpon Ait. dependent on seasonal plant development stages and age (the example of introduction in Russia). Sci. Hortic. 2017, 218, 139–146. [Google Scholar] [CrossRef]
- Coelho, E.M.; De Azevedo, L.C.; Correa, L.C.; Bordignon-Luiz, M.T.; Lima, M.D. Phenolic profile, organic acids and antioxidant activity of frozen pulp and juice of the jambolan (Syzygium cumini). J. Food Biochem. 2016, 40, 211–219. [Google Scholar] [CrossRef]
- Grases, F.; Prieto, R.M.; Fernandez-Cabot, R.A.; Costa-Bauza, A.; Sanchez, A.M.; Prodanov, M. Effect of consuming a grape seed supplement with abundant phenolic compounds on the oxidative status of healthy human volunteers. Nutr. J. 2015, 14, 94. [Google Scholar] [CrossRef] [Green Version]
- Errea, P.; Garay, L.; Marin, J.A. Early detection of graft incompatibility in apricot (Prunus armeniaca) using in vitro techniques. Physiol. Plant. 2001, 112, 135–141. [Google Scholar] [CrossRef]
- Zarrouk, O.; Testillano, P.S.; Risueno, M.C.; Moreno, M.A.; Gogorcena, Y. Changes in cell/tissue organization and peroxidase activity as markers for early detection of graft incompatibility in peach/plum combinations. J. Am. Soc. Hortic. Sci. 2010, 135, 9–17. [Google Scholar] [CrossRef]
- Errea, P.; Treutter, D.; Feucht, W. Scion-rootstock effects on the content of flavan-3-ols in the union of heterografts consisting of apricots and diverse prunus rootstocks. Gartenbauwissenschaft 1992, 57, 134–138. [Google Scholar]
- Assuncao, M.; Pinheiro, J.; Cruz, S.; Brazao, J.; Queiroz, J.; Dias, J.E.E.; Canas, S. Gallic acid, sinapic acid and catechin as potential chemical markers of vitis graft success. Sci. Hortic. 2019, 246, 129–135. [Google Scholar] [CrossRef]
- Prodhomme, D.; Fonayet, J.V.; Hevin, C.; Franc, C.; Hilbert, G.; de Revel, G.; Richard, T.; Ollat, N.; Cookson, S.J. Metabolite profiling during graft union formation reveals the reprogramming of primary metabolism and the induction of stilbene synthesis at the graft interface in grapevine. BMC Plant Biol. 2019, 19, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canas, S.; Assuncao, M.; Brazao, J.; Zanol, G.; Eiras-Dias, J.E. Phenolic compounds involved in grafting incompatibility of vitis spp: Development and validation of an analytical method for their quantification. Phytochem. Anal. 2015, 26, 1–7. [Google Scholar] [CrossRef]
- Soltana, H.; De Rosso, M.; Lazreg, H.; Dalla Vedova, A.; Hammami, M.; Flamini, R. Lc-qtof characterization of non-anthocyanic flavonoids in four Tunisian fig varieties. J. Mass Spectrom. 2018, 53, 817–823. [Google Scholar] [CrossRef]
- Zhang, M.; Swarts, S.G.; Yin, L.J.; Liu, C.M.; Tian, Y.P.; Cao, Y.B.; Swarts, M.; Yang, S.M.; Zhang, S.B.; Zhang, K.Z.; et al. Antioxidant properties of quercetin. In Oxygen Transport to Tissue XXXII; LaManna, J.C., Puchowicz, M.A., Xu, K., Harrison, D.K., Bruley, D.F., Eds.; Springer: Berlin, Germany, 2011; Volume 701, pp. 283–289. [Google Scholar]
- Geissman, T.A. The isolation of eriodictyol and homoeriodictyol. An improved procedure. J. Am. Chem. Soc. 1940, 62, 3258–3259. [Google Scholar] [CrossRef]
- Usenik, V.; Krska, B.; Vican, M.; Stampar, F. Early detection of graft incompatibility in apricot (Prunus armeniaca L.) using phenol analyses. Sci. Hortic. 2006, 109, 332–338. [Google Scholar] [CrossRef]



| Compound Name | Precursor Ion | Product Ion | Fragmentation Voltage [V] | Collision Energy [V] | Polarity |
|---|---|---|---|---|---|
| Dihydrokaempherol | 287 | 259 | 130 | 4 | Negative |
| Eriodictyol | 287 | 151 | 106 | 0 | Negative |
| Hyperoside * (Q-3-galactoside) | 463 | 300 | 150 | 20 | Negative |
| Isoquercitrin ** (Q-3-glucoside) | 255 | 119 | 100 | 16 | Negative |
| Isovitexin | 431.1 | 311 | 140 | 20 | Negative |
| Naringeninchalcone | 271 | 151 | 104 | 4 | Negative |
| Pentahydroxychalcone | 287 | 151 | 96 | 8 | Negative |
| Quercetin | 301 | 151 | 208 | 8 | Negative |
| Quercitrin *** (Q-3-rhamnoside) | 447.1 | 300 | 158 | 16 | Negative |
| Rutin **** (Q-3-rutinoside) | 609 | 300 | 220 | 35 | Negative |
| Vitexin | 431.1 | 311 | 142 | 20 | Negative |
| Vitexin-2-O-rhamnoside | 431 | 268 | 170 | 32 | Negative |
| Compound Name. | Precursor Ion | Product Ion | Fragmentation Voltage [V] | Collision Energy [V] | Polarity |
|---|---|---|---|---|---|
| 3,4-Dihydroxybenzaldehyde | 137 | 108 | 120 | 20 | Negative |
| Caffeic acid | 179 | 135 | 100 | 10 | Negative |
| Gallic acid | 169 | 125 | 100 | 10 | Negative |
| p-Coumaric acid | 163 | 119 | 66 | 12 | Negative |
| p-Hydroxybenzaldehyde | 121 | 92 | 120 | 20 | Negative |
| p-Hydroxybenzoic acid | 137 | 93 | 100 | 10 | Negative |
| Protocatechuic acid | 153 | 109 | 100 | 10 | Negative |
| Salicylic acid | 137 | 93 | 100 | 10 | Negative |
| Syringic acid | 197 | 182 | 80 | 10 | Negative |
| Vanilic acid | 167 | 152 | 80 | 10 | Negative |
| Vanilin | 151 | 136 | 80 | 8 | Negative |
| Chlorogenic acid | 353 | 191 | 100 | 10 | Negative |
| Cryptochlorogenic acid | 353 | 191 | 105 | 10 | Negative |
| Compound Name | Precursor Ion | Product Ion | Fragmentation Voltage [V] | Collision Energy [V] | Polarity |
|---|---|---|---|---|---|
| Catechin | 289 | 109 | 100 | 20 | Negative |
| Epicatechin | 289 | 245 | 146 | 4 | Negative |
| Epigallocatechin | 305 | 125 | 146 | 12 | Negative |
| Procyanidin a2 | 575 | 285 | 170 | 28 | Negative |
| Procyanidin b1 | 577.5 | 407 | 170 | 20 | Negative |
| Procyanidin b2 | 577.5 | 407 | 170 | 16 | Negative |
| Procyanidin c1 | 865 | 407 | 160 | 36 | Negative |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnus, S.; Gazdik, F.; Anjum, N.A.; Kadlecova, E.; Lackova, Z.; Cernei, N.; Brtnicky, M.; Kynicky, J.; Klejdus, B.; Necas, T.; et al. Assessment of Antioxidants in Selected Plant Rootstocks. Antioxidants 2020, 9, 209. https://doi.org/10.3390/antiox9030209
Magnus S, Gazdik F, Anjum NA, Kadlecova E, Lackova Z, Cernei N, Brtnicky M, Kynicky J, Klejdus B, Necas T, et al. Assessment of Antioxidants in Selected Plant Rootstocks. Antioxidants. 2020; 9(3):209. https://doi.org/10.3390/antiox9030209
Chicago/Turabian StyleMagnus, Samuel, Filip Gazdik, Naser A. Anjum, Eliska Kadlecova, Zuzana Lackova, Natalia Cernei, Martin Brtnicky, Jindrich Kynicky, Borivoj Klejdus, Tomas Necas, and et al. 2020. "Assessment of Antioxidants in Selected Plant Rootstocks" Antioxidants 9, no. 3: 209. https://doi.org/10.3390/antiox9030209
APA StyleMagnus, S., Gazdik, F., Anjum, N. A., Kadlecova, E., Lackova, Z., Cernei, N., Brtnicky, M., Kynicky, J., Klejdus, B., Necas, T., & Zitka, O. (2020). Assessment of Antioxidants in Selected Plant Rootstocks. Antioxidants, 9(3), 209. https://doi.org/10.3390/antiox9030209









