Discovery of Spilanthol Endoperoxide as a Redox Natural Compound Active against Mammalian Prx3 and Chlamydia trachomatis Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Chemical Synthesis of SPLE
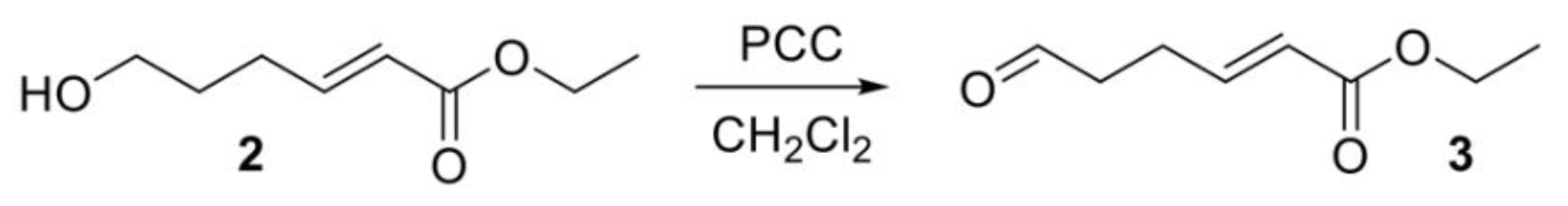
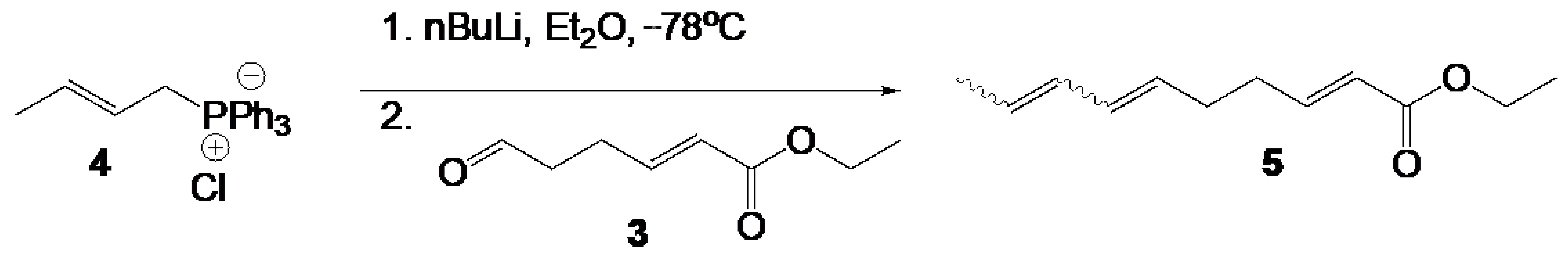
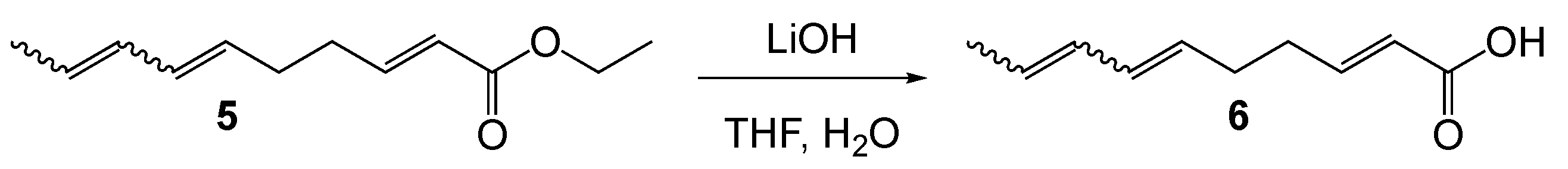
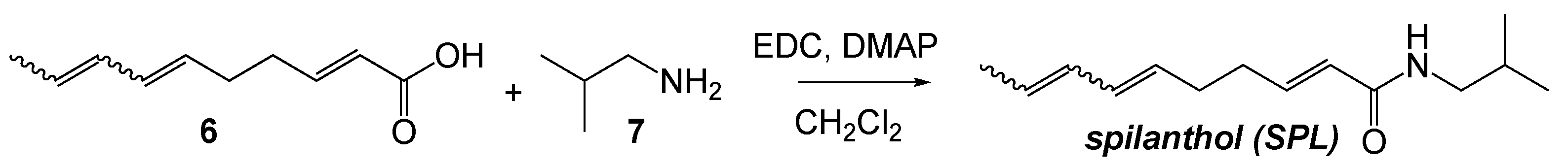

2.3. Cell Culture
2.4. Formulation of Chlamydia Trachomatis Elementary Body (EB) Stock Solution
2.5. Chlamydia Trachomatis Infection
2.6. SPL, SPLE, and MitoPQ (MitoParaquat) Treatment of Infected Cells
2.7. Cell Proliferation Assay
2.8. Confocal Imaging
2.9. Analysis of Mitochondrial Protein Sulfenylation by Flow Cytometry
2.10. Transmission Electron Microscopy Imaging
2.11. Recombinant Proteins and Electrospray Ionization Time-of-Flight Mass Spectrometry (ESI-TOF MS)
2.12. Data Analysis
3. Results
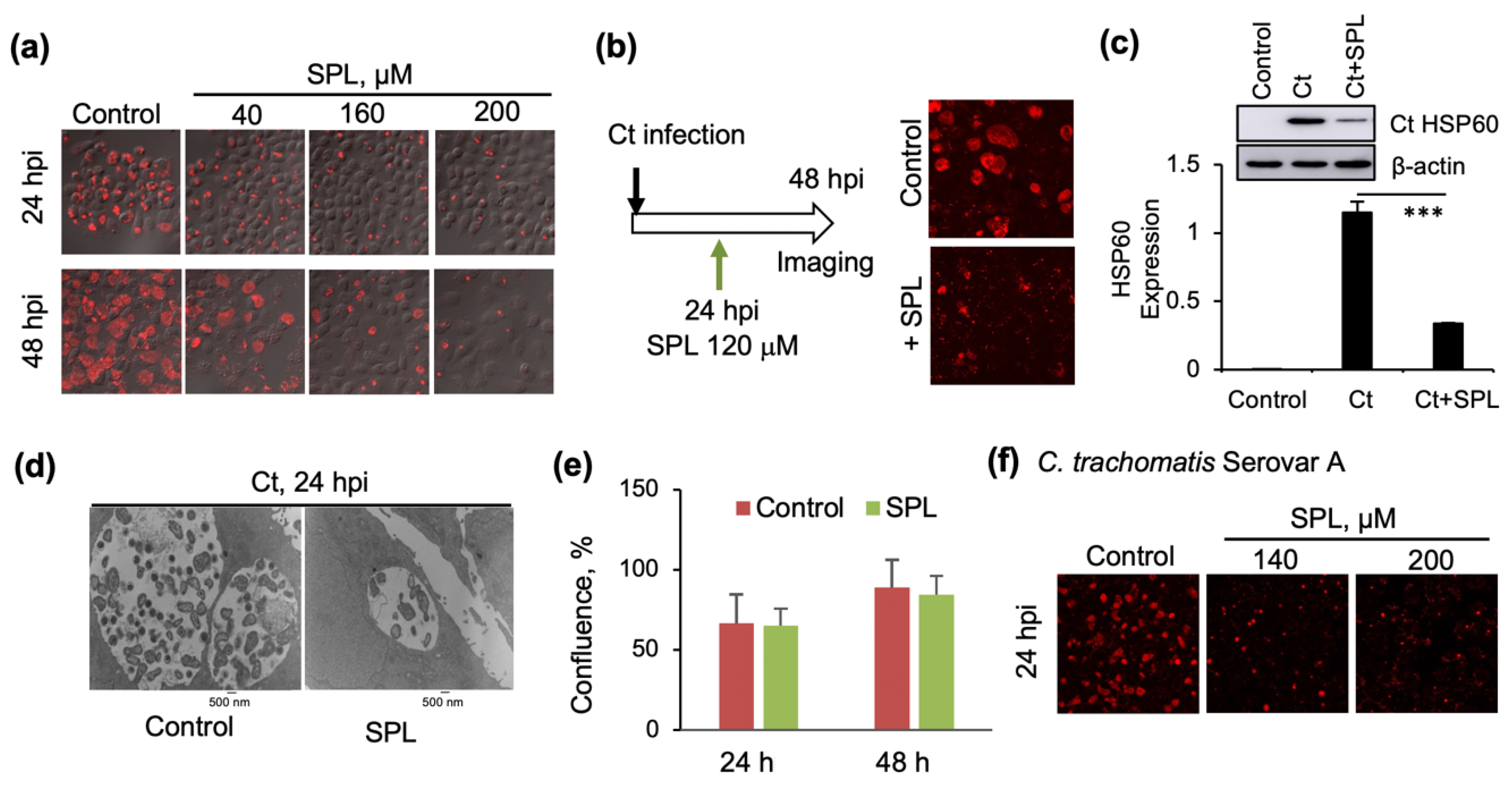
3.1. SPL Inhibits Intracellular Ct Development
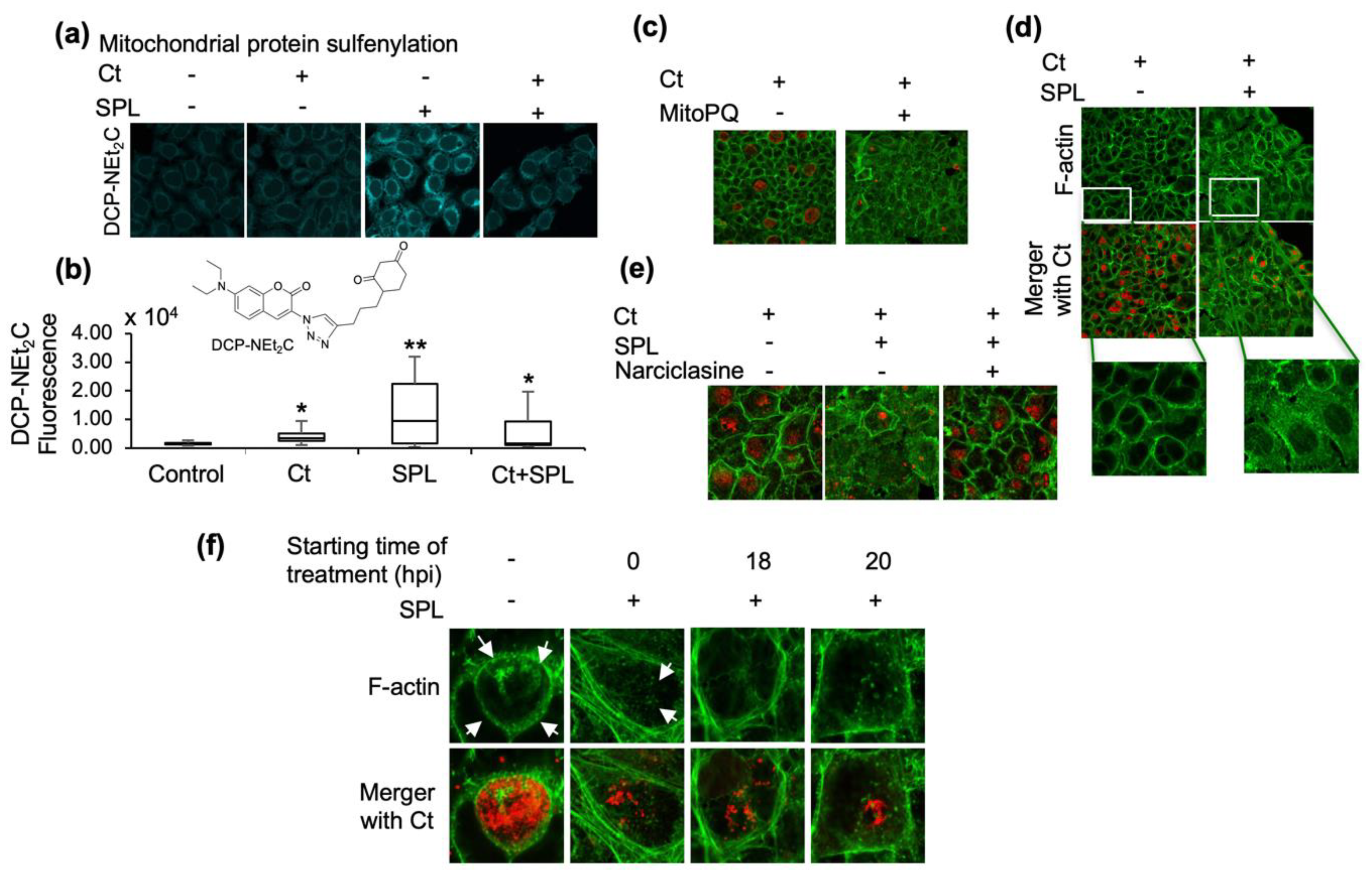
3.2. SPL Increases Mitochondrial Oxidative State and Disrupts the F-Actin Ring Supporting the Ct Inclusion
3.3. The Endoperoxide Derivative of SPL (SPLE) Is a More Potent Inhibitor of Ct Infections and Similarly Interferes with the Organization of the F-Actin Ring
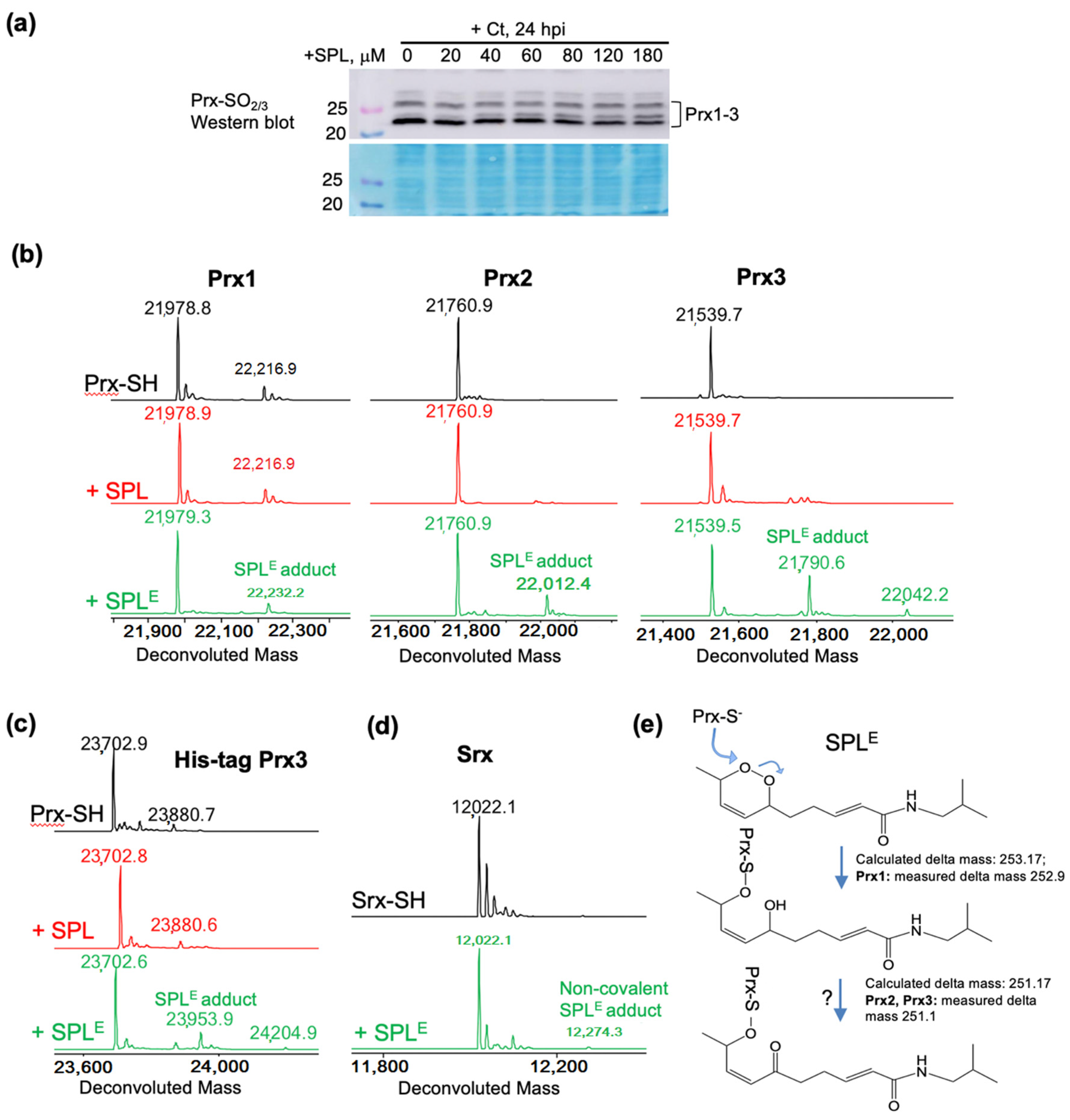
3.4. SPLE Reacts Predominantly with Mitochondrial Antioxidant Protein Peroxiredoxin (Prx3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, L.; Rowley, J.; Vander Hoorn, S.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Stevens, G.; Gottlieb, S.; Kiarie, J.; Temmerman, M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS ONE 2015, 10, e0143304. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, M.; Sood, S.; Mukherjee, A.; Muralidhar, S.; Bala, M. Genital Chlamydia trachomatis: An update. Indian J. Med. Res. 2013, 138, 303–316. [Google Scholar] [PubMed]
- Buckner, L.R.; Amedee, A.M.; Albritton, H.L.; Kozlowski, P.A.; Lacour, N.; McGowin, C.L.; Schust, D.J.; Quayle, A.J. Chlamydia trachomatis Infection of Endocervical Epithelial Cells Enhances Early HIV Transmission Events. PLoS ONE 2016, 11, e0146663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koskela, P.; Anttila, T.; Bjørge, T.; Brunsvig, A.; Dillner, J.; Hakama, M.; Hakulinen, T.; Jellum, E.; Lehtinen, M.; Lenner, P. Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. Int. J. Cancer 2000, 85, 35–39. [Google Scholar] [CrossRef]
- Samoff, E.; Koumans, E.H.; Markowitz, L.E.; Sternberg, M.; Sawyer, M.K.; Swan, D.; Papp, J.R.; Black, C.M.; Unger, E.R. Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am. J. Epidemiol. 2005, 162, 668–675. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.L.; Chen, X.; Wood, S.T.; Stuart, E.S.; Arcaro, K.F.; Molina, D.P.; Petrovic, S.; Furdui, C.M.; Tsang, A.W. Activation of epidermal growth factor receptor is required for Chlamydia trachomatis development. BMC Microbiol. 2014, 14, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial intracellular survival strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef] [Green Version]
- Hybiske, K.; Stephens, R.S. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc. Natl. Acad. Sci. USA 2007, 104, 11430–11435. [Google Scholar] [CrossRef] [Green Version]
- Moulder, J.W. Inhibition of onset of overt multiplication of Chlamydia psittaci in persistently infected mouse fibroblasts (L cells). Infect. Immun. 1983, 39, 898–907. [Google Scholar] [CrossRef] [Green Version]
- Galasso, G.J.; Manire, G.P. Effect of antiserum and antibiotics on persistent infection of HeLa cells with meningopneumonitis virus. J. Immunol. 1961, 86, 382–385. [Google Scholar]
- Poston, T.B.; Gottlieb, S.L.; Darville, T. Status of vaccine research and development of vaccines for Chlamydia trachomatis infection. Vaccine 2017, 7289–7294. [Google Scholar] [CrossRef]
- Somani, J.; Bhullar, V.B.; Workowski, K.A.; Farshy, C.E.; Black, C.M. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J. Infect. Dis. 2000, 181, 1421–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potroz, M.G.; Cho, N.J. Natural products for the treatment of trachoma and Chlamydia trachomatis. Molecules 2015, 20, 4180–4203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saez, D.; Dushime, R.; Wu, H.; Ramos Cordova, L.B.; Shukla, K.; Brown-Harding, H.; Furdui, C.M.; Tsang, A.W. Sulforaphane promotes chlamydial infection by suppressing mitochondrial protein oxidation and activation of complement C3. Protein Sci. 2019, 28, 216–227. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, L.C.; Hu, S.; Hsiao, C.Y.; Wu, S.J. Spilanthol inhibits TNFalphainduced ICAM1 expression and proinflammatory responses by inducing heme oxygenase1 expression and suppressing pJNK in HaCaT keratinocytes. Mol. Med. Rep. 2018, 18, 2987–2994. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-C.; Wu, L.-Y.; Hu, S.; Wu, S.-J. Spilanthol Inhibits COX-2 and ICAM-1 Expression via Suppression of NF-κB and MAPK Signaling in Interleukin-1β-Stimulated Human Lung Epithelial Cells. Inflammation 2018, 41, 1934–1944. [Google Scholar] [CrossRef]
- Hong, F.; Freeman, M.L.; Liebler, D.C. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem. Res. Toxicol. 2005, 18, 1917–1926. [Google Scholar] [CrossRef]
- Paulraj, J.; Govindarajan, R.; Palpu, P. The genus spilanthes ethnopharmacology, phytochemistry, and pharmacological properties: A review. Adv. Pharm. Sci. 2013, 2013, 510298. [Google Scholar] [CrossRef] [Green Version]
- Spelman, K.; Depoix, D.; McCray, M.; Mouray, E.; Grellier, P. The traditional medicine Spilanthes acmella, and the alkylamides spilanthol and undeca-2e-ene-8, 10-diynoic acid isobutylamide, demonstrate in vitro and in vivo antimalarial activity. Phytother. Res. 2011, 25, 1098–1101. [Google Scholar] [CrossRef] [Green Version]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. High therapeutic potential of Spilanthes acmella: A review. EXCLI J. 2013, 12, 291–312. [Google Scholar]
- Silveira, N.; Saar, J.; Santos, A.D.; Barison, A.; Sandjo, L.P.; Kaiser, M.; Schmidt, T.J.; Biavatti, M.W. A New Alkamide with an Endoperoxide Structure from Acmella ciliata (Asteraceae) and Its in Vitro Antiplasmodial Activity. Molecules 2016, 21, 765. [Google Scholar] [CrossRef]
- Jagadeeswaran, R.; Surawska, H.; Krishnaswamy, S.; Janamanchi, V.; Mackinnon, A.C.; Seiwert, T.Y.; Loganathan, S.; Kanteti, R.; Reichman, T.; Nallasura, V.; et al. Paxillin is a target for somatic mutations in lung cancer: Implications for cell growth and invasion. Cancer Res. 2008, 68, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Narayan, R.S.; Schomaker, J.M.; Borhan, B. One-pot regio- and stereoselective cyclization of 1,2,n-triols. J. Am. Chem. Soc. 2005, 127, 6946–6947. [Google Scholar] [CrossRef]
- Hoye, T.R.; Eklov, B.M.; Jeon, J.; Khoroosi, M. Sequencing of three-component olefin metatheses: Total synthesis of either (+)-gigantecin or (+)-14-deoxy-9-oxygigantecin. Org. Lett. 2006, 8, 3383–3386. [Google Scholar] [CrossRef]
- Matovic, N.; Matthias, A.; Gertsch, J.; Raduner, S.; Bone, K.M.; Lehmann, R.P.; Devoss, J.J. Stereoselective synthesis, natural occurrence and CB(2) receptor binding affinities of alkylamides from herbal medicines such as Echinacea sp. Org. Biomol. Chem. 2007, 5, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Mimaki, K.; Tanigami, K.I.; Maegawa, T. An Improved and Practical Method for Synthesizing of alpha-Sanshools and Spilanthol. Front. Chem. 2020, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.G.; Kim, S.Y.; Brunmeir, R.; Sinnakannu, J.R.; Ge, X.; Li, H.; Ma, W.; Yaligar, J.; Kn, B.P.; Velan, S.S.; et al. Narciclasine attenuates diet-induced obesity by promoting oxidative metabolism in skeletal muscle. PLoS Biol. 2017, 15, e1002597. [Google Scholar] [CrossRef] [Green Version]
- Holmila, R.J.; Vance, S.A.; Chen, X.; Wu, H.; Shukla, K.; Bharadwaj, M.S.; Mims, J.; Wary, Z.; Marrs, G.; Singh, R.; et al. Mitochondria-targeted Probes for Imaging Protein Sulfenylation. Sci. Rep. 2018, 8, 6635. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Bolduc, J.A.; Nelson, K.J.; Haynes, A.C.; Lee, J.; Reisz, J.A.; Graff, A.H.; Clodfelter, J.E.; Parsonage, D.; Poole, L.B.; Furdui, C.M.; et al. Novel hyperoxidation resistance motifs in 2-Cys peroxiredoxins. J. Biol. Chem. 2018, 293, 11901–11912. [Google Scholar] [CrossRef] [Green Version]
- Haynes, A.C.; Qian, J.; Reisz, J.A.; Furdui, C.M.; Lowther, W.T. Molecular basis for the resistance of human mitochondrial 2-Cys peroxiredoxin 3 to hyperoxidation. J. Biol. Chem. 2013, 288, 29714–29723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, T.J.; Johnson, L.C.; Lowther, W.T. Structure of the sulphiredoxin-peroxiredoxin complex reveals an essential repair embrace. Nature 2008, 451, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forshaw, T.E.; Holmila, R.; Nelson, K.J.; Lewis, J.E.; Kemp, M.L.; Tsang, A.W.; Poole, L.B.; Lowther, W.T.; Furdui, C.M. Peroxiredoxins in Cancer and Response to Radiation Therapies. Antioxidants 2019, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Roszak, A.W.; Gourlay, L.J.; Lindsay, J.G.; Isaacs, N.W. Bovine mitochondrial peroxiredoxin III forms a two-ring catenane. Structure 2005, 13, 1661–1664. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Abe, H.; Tsuruta, S.; Chiba, S.; Fujii-Kuriyama, Y.; Sekiya, T.; Morita, R.; Yoshimura, A. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int. Immunol. 2014, 26, 209–220. [Google Scholar] [CrossRef]
- Subramaniam, R.; Barnes, P.F.; Fletcher, K.; Boggaram, V.; Hillberry, Z.; Neuenschwander, P.; Shams, H. Protecting against post-influenza bacterial pneumonia by increasing phagocyte recruitment and ROS production. J. Infect. Dis. 2014, 209, 1827–1836. [Google Scholar] [CrossRef] [Green Version]
- Azenabor, A.A.; Mahony, J.B. Generation of reactive oxygen species and formation and membrane lipid peroxides in cells infected with Chlamydia trachomatis. Int. J. Infect. Dis. 2000, 4, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Boncompain, G.; Schneider, B.; Delevoye, C.; Kellermann, O.; Dautry-Varsat, A.; Subtil, A. Production of reactive oxygen species is turned on and rapidly shut down in epithelial cells infected with Chlamydia trachomatis. Infect. Immun. 2010, 78, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Kading, N.; Kaufhold, I.; Muller, C.; Szaszak, M.; Shima, K.; Weinmaier, T.; Lomas, R.; Conesa, A.; Schmitt-Kopplin, P.; Rattei, T.; et al. Growth of Chlamydia pneumoniae Is Enhanced in Cells with Impaired Mitochondrial Function. Front. Cell. Infect. Microbiol. 2017, 7, 499. [Google Scholar] [CrossRef]
- Abdul-Sater, A.A.; Saïd-Sadier, N.; Lam, V.M.; Singh, B.; Pettengill, M.A.; Soares, F.; Tattoli, I.; Lipinski, S.; Girardin, S.E.; Rosenstiel, P. Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member, NLRX1. J. Biol. Chem. 2010, 285, 41637–41645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Schwarzer, C.; Hybiske, K.; Machen, T.E.; Stephens, R.S. Developmental stage oxidoreductive states of Chlamydia and infected host cells. mBio 2014, 5, e01924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardhan, H.; Bhengraj, A.R.; Jha, R.; Srivastava, P.; Jha, H.C.; Mittal, A. Higher expression of ferritin protects Chlamydia trachomatis infected HeLa 229 cells from reactive oxygen species mediated cell death. Biochem. Cell. Biol. 2010, 88, 835–842. [Google Scholar] [CrossRef]
- Wongsawatkul, O.; Prachayasittikul, S.; Isarankura-Na-Ayudhya, C.; Satayavivad, J.; Ruchirawat, S.; Prachayasittikul, V. Vasorelaxant and antioxidant activities of Spilanthes acmella Murr. Int. J. Mol. Sci 2008, 9, 2724–2744. [Google Scholar] [CrossRef]
- Poynton, R.A.; Peskin, A.V.; Haynes, A.C.; Lowther, W.T.; Hampton, M.B.; Winterbourn, C.C. Kinetic analysis of structural influences on the susceptibility of peroxiredoxins 2 and 3 to hyperoxidation. Biochem. J. 2016, 473, 411–421. [Google Scholar] [CrossRef]
- Ali, H.M.; Homeida, M.M.; Abdeen, M.A. “Drug dumping” in donations to Sudan. Lancet 1988, 1, 538–539. [Google Scholar] [CrossRef]
- Berckmans, P.; Dawans, V.; Schmets, G.; Vandenbergh, D.; Autier, P. Inappropriate drug-donation practices in Bosnia and Herzegovina, 1992 to 1996. New Engl. J. Med. 1997, 337, 1842–1845. [Google Scholar] [CrossRef]
- Gustafsson, L.L.; Wide, K. Marketing of obsolete antibiotics in Central America. Lancet 1981, 1, 31–33. [Google Scholar] [CrossRef]
- WHO Executive Board. Implementation of the global strategy for health for all by the year 2000. Second evaluation. Eighth report on the world health situation. WHO Reg. Publ. Eur. Ser. 1994, 52, 1–289. [Google Scholar]
- Horton, R. The infected metropolis. Lancet 1996, 347, 134–135. [Google Scholar] [CrossRef]
- Korte, R.; Rehle, T.; Merkle, A. Strategies to maintain health in the Third World. Tropical medicine and parasitology: Official organ of Deutsche Tropenmedizinische Gesellschaft and of Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ). Eur. PMC 1991, 42, 428–432. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dushime, R.; Zhu, Y.; Wu, H.; Saez, D.; Shukla, K.; Brown-Harding, H.; Biavatti, M.W.; Nelson, K.J.; Poole, L.B.; Lowther, W.T.; et al. Discovery of Spilanthol Endoperoxide as a Redox Natural Compound Active against Mammalian Prx3 and Chlamydia trachomatis Infection. Antioxidants 2020, 9, 1220. https://doi.org/10.3390/antiox9121220
Dushime R, Zhu Y, Wu H, Saez D, Shukla K, Brown-Harding H, Biavatti MW, Nelson KJ, Poole LB, Lowther WT, et al. Discovery of Spilanthol Endoperoxide as a Redox Natural Compound Active against Mammalian Prx3 and Chlamydia trachomatis Infection. Antioxidants. 2020; 9(12):1220. https://doi.org/10.3390/antiox9121220
Chicago/Turabian StyleDushime, Rosine, Yunhuang Zhu, Hanzhi Wu, Daniel Saez, Kirtikar Shukla, Heather Brown-Harding, Maique W. Biavatti, Kimberly J. Nelson, Leslie B. Poole, William T. Lowther, and et al. 2020. "Discovery of Spilanthol Endoperoxide as a Redox Natural Compound Active against Mammalian Prx3 and Chlamydia trachomatis Infection" Antioxidants 9, no. 12: 1220. https://doi.org/10.3390/antiox9121220
APA StyleDushime, R., Zhu, Y., Wu, H., Saez, D., Shukla, K., Brown-Harding, H., Biavatti, M. W., Nelson, K. J., Poole, L. B., Lowther, W. T., Jones, P. B., Furdui, C. M., & Tsang, A. W. (2020). Discovery of Spilanthol Endoperoxide as a Redox Natural Compound Active against Mammalian Prx3 and Chlamydia trachomatis Infection. Antioxidants, 9(12), 1220. https://doi.org/10.3390/antiox9121220





